ABSTRACT
Production of psychrophilic enzymes in the commonly used mesophilic expression systems is hampered by low intrinsic stability of the recombinant enzymes at the optimal host growth temperatures. Unless strategies for low-temperature expression are advanced, research on psychrophilic enzymes may end up being biased toward those that can be stably produced in commonly used mesophilic host systems. Two main strategies are currently being explored for the development of low-temperature expression in bacterial hosts: (i) low-temperature adaption of existing mesophilic expression systems, and (ii) development of new psychrophilic hosts. These developments include genetic engineering of the expression cassettes to optimize the promoter/operator systems that regulate heterologous expression. In this addendum we present our efforts in the development of such low-temperature expression systems, and speculate about future advancements in the field and potential applications.
KEYWORDS: AraC/PBAD, cold-adapted, cspA promoter, Heterologous expression, psychrophilic, Pseudomonas, T7 promoter, T7 RNA polymerase, XylS/Pm
Our latest work involves the investigation of low-temperature expression of putatively cold-adapted (psychrophilic) gene candidates.1 Psychrophilic enzymes present significant economic potential for industrial applications. For example, introduction of psychrophilic proteases, lipases, cellulases and amylases into laundry detergents can decrease the demand for both chemical additives and enzyme concentrations during production, but can also reduce the need for heating and the water consumption for end-users.2,3 In 2014, DuPont and P&G received the 2014 Sustainable Bio Award for their innovative protein modification producing a protease that has optimal performance at temperatures as low as 15°C. Although this was an engineered protease, basic research on psychrophilic proteases, such as subtilisins from Antarctic Bacillus species,4,5 have contributed to their economic value.
Bioprospecting, the process of exploring the biodiversity of our planet as a potential source of products, has in recent decades turned to cold habitats such as the polar regions, glaciers and oceans to discover cold-acting enzymes that can be exploited for industrial applications. These enzymes originate from psychrophiles that have optimal growth temperatures below 15°C.6 The intrinsic instability of psychrophilic enzymes makes their heterologous expression in commonly used bacterial hosts such as E. coli, where growth limits to around 15°C, particularly challenging, and presents a significant barrier to their exploitation in biotechnology.7 In cases where psychrophilic enzymes must be produced as zymogens, such as proteases, activation or autoprocessing may also need to be addressed in the chosen expression systems.8 Unless strategies for recombinant protein expression at low temperatures are advanced, research on psychrophilic enzymes may end up being biased by production in mesophilic host systems, such as the commonly used E. coli (Gram-negative bacteria) and Bacillus subtilis (Gram-positive bacteria). This may limit the diversity of possible enzyme products to those that can be stably produced in these systems.
Psychrophilic enzymes are generally less stable compared to their mesophilic homologues, having high structural flexibility which enables them to function at low temperatures and imparts a decreased thermal stability.2 Their active sites are even less thermostable than the whole protein structure due to high local flexibility. The properties of psychrophilic enzymes that are useful in industrial applications include their high activity at low and moderate temperatures as lower enzyme concentrations are required to achieve the same performance compared to their higher-temperature acting homologues.2 Furthermore, their heat-lability can be exploited to selectively inactivate the enzyme by heat treatment.2 The use of the alkaline phosphatase and nuclease from the Arctic shrimp Pandalus borealis in molecular biology applications9,10 provide 2 examples of psychrophilic enzymes that can be stably produced by recombinant technologies, both of which are commercially available.
Successful protein production systems for expression of psychrophilic enzymes will depend on being able to produce functional proteins at low enough temperatures to ensure correct folding and to maintain their structural integrity. This presents 2 possible bioengineering strategies for cell-based production: (i) low-temperature adaptations of existing mesophilic expression systems, and (ii) development of new psychrophilic hosts. The first strategy includes engineering the genetic tools in the mesophilic expression systems to improve low-temperature growth and to promote correct folding of recombinant proteins at low temperature. The best-known example of such a strategy is the co-expression of cold-adapted chaperonines to enable correct folding of recombinant proteins. Co-expression of cold-adapted homologues of the E. coli GroELS chaperonines, Cpn60 and Cpn10 from Oleispira antarctica, allows the E. coli host to have an operational folding system at 4–12°C.11 This resulted in improvement of host growth at low temperatures and enhanced the solubility of the resulting recombinant proteins. In our case, co-expression of cold-adapted chaperonines was not sufficient for soluble expression of genes derived from Arctic-sources metagenomes in combination with the commonly used T7 expression system (T7 RNA polymerase/T7 promoter).1 We therefore targeted optimization of low-temperature expression at the transcription level by investigating the utility of a cold-shock inducible promoter for low-temperature expression.1 In this E. coli based system, expression of recombinant proteins is regulated by a temperature-inducible promoter, the E. coli cold shock protein A (cspA) promoter, which allows induction by a downshift in growth temperature.12,13 Analogous systems have been investigated for Bacillus subtilis where the cold-inducible promoter of the desaturase encoding gene (des) in combination with other 5′ UTR regulatory elements was shown to generate higher expression levels.14,15 Although the success of both expression systems had previously been demonstrated for mesophilic proteins, from bacteria to plants and humans,16,17 their potential for psychrophilic enzymes had not been systematically explored. By expressing the putatively psychrophilic genes in this E. coli system, we found that expression levels were generally high, and comparable to the commonly used T7 system which functions optimally at 37°C.1 In contrast to the T7 system some soluble proteins were detected, however in most cases the yield was still too low for further experimentation. We therefore optimized cspA-driven expression by fusing the maltose-binding protein (MBP), thioredoxin (TRX), the small ubiquitin-like modifier (SUMO) and trigger factor (TF) N-terminally to these enzymes, in an attempt to improve solubility. Consistent with a recent report,17 we found that combining low-temperature induction with these solubility partners improved solubility. This has recently allowed us to perform preliminary characterization of the selected enzymes. For example, activity assays on the His-SUMO fused chitinase described in our paper1 suggests that the enzyme is truly psychrophilic with an optimal temperature around 20°C (Fig. 1). Adding this result to our previous data1 indicates that the E. coli/cspA expression system is a viable approach for production of psychrophilic chitinase, which was not feasible with the T7 system.
Figure 1.
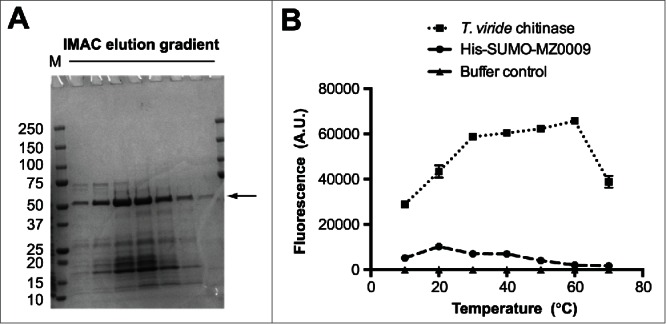
Temperature optimum of a recombinant chitinase (MZ0009) of Arctic origin. (A) Recombinant His-SUMO fusion of the chitinase crudely purified by immobilized affinity chromatography (IMAC). The arrow points to the recombinant protein (49kDa), which was subsequently identified by mass spectrometry. The chitinase part of the fusion protein is 36kDa; note that the His-SUMO partner is known to migrate slower (about 20kDa) than its corresponding monomeric mass of 13 kDa, thus generating a larger shift as a fusion protein. (B) The activity of the crudely purified His-SUMO-MZ0009 is expressed as the degree of fluorescence (arbitrary units) from hydrolysis of a synthetic chitin analog substrate, 4MU-β-D-N,N′,N″-triacetylchitotriose, and compared to equal amounts of the commercially available Trichoderma viride chitinase. Error bars show the variation between 2 parallel samples in one experiment.
Although the commonly used MBP and SUMO fusion proteins were utilized successfully in the E. coli/cspA system, we and others found that the TRX fusion partner negatively impacted cell viability.1,17 In the course of developing low-temperature expression systems, attention should be given to new solubility partners that may be more compatible to psychrophilic target proteins. With this in mind, we also investigated the utility of the TF protein from the psychrophilic Pseudoalteromonas haloplanktis as an alternative fusion partner. The TF from P. haloplanktis has a low-temperature activity and is strongly upregulated in the native host at 4°C in conditions where protein folding is a rate-limiting step for growth.18 We assumed that the psychrophilic TF fusion partner would ensure a faster and more efficient folding assistance of newly synthesized polypeptides during low-temperature expression in E. coli, however we were unable to observe any difference in the solubility of our target enzymes using the 2 different TF partners (data not shown). As we reported, removal of the E. coli TF fusion partner was ineffective,1 and we again want to highlight the need for a systematic study of optimal linker length which has not, to our knowledge, been carried out for fusion proteins with the TF as a fusion partner. This is required to exploit its potential as a solubility and co-folding partner in recombinant protein expression. As an alternative to the fusion strategy, co-expression of the TF from P. haloplanktis in E. coli may be applied to improve solubility of psychrophilic enzymes, analogous to the co-expression of the chaperonines from Oleispira antarctica.11
As mentioned, co-expression of cold-adapted chaperonines was not sufficient for production of soluble enzymes with the T7 system.1 In line with another report,19 regulation of protein expression by the cspA promoter in combination with the co-expression of cold-adapted chaperonines was successful in producing soluble psychrophilic enzymes in E. coli.1 Taken together, our data indicate that engineering of E. coli is an attractive route for soluble low-temperature expression, but further advances in the genetic tools for protein expression will be required to improve its performance as a production host for psychrophilic enzymes.
The second strategy we propose to ensure correct folding of functional psychrophilic enzymes is based on the development of psychrophiles as production hosts. The first reported and most-studied psychrophilic host is the Gram-negative Pseudoalteromonas haloplanktis TAC125,20–22 which grows at low temperatures (4–25°C). With the availability of the complete genomic sequence23 it is possible to inactivate or delete genes selectively, but the Pseudoalteromonas host systems currently lack effective protocols for gene engineering and transformation.24 In the absence of better tools, conjugation is currently used to introduce foreign DNA into the host, and the most commonly used donor organism for conjugation is the mesophilic E. coli. During the conjugative mating a mixture of both donor and recipient must be incubated on a non-selective agar surface, which may limit the success of conjugation if the recipient (psychrophilic) organism require growth conditions (such as high salt or low growth temperature) that is not preferred by E. coli.24 A recently developed host-independent plasmid system, pTA-Mob,25 may eliminate these conjugation challenges as this plasmid carries all necessary conjugative functions on a pBBR1-based broad-host-range replicon. Utilization of this plasmid, given that the plasmid is replicative in the chosen host, will allow any bacterial host to function as a donor in conjugative mating. By choosing a donor host with similar growth preferences to the recipient psychrophilic host, conjugation efficiency can be increased. A continued effort in genetic manipulations is however still needed to address problems with restriction of recombinant DNA, protease-degradation of the encoded proteins and multidrug resistance.24 Although the Pseudoalteromonas system has been successful in producing recombinant proteins where E. coli failed, it is still limited by low cell densities.26 As a recent example, the production yield of 2 recombinant proteins in Pseudoalteromonas sp. SM20429 was shown to be more than 5 times lower than in E. coli.8
Alongside our explorations of the E. coli/cspA system, we pursued engineering of new hosts for low-temperature expression. As part of this work, more than 70 isolates of psychrophilic Pseudomonas spp. isolates originating from the marine Arctic environment were investigated for their growth at 4-30°C; in most cases the shortest generation time was achieved at around 20°C. Two of the isolates were further explored for their potential as psychrophilic hosts in combination with both the XylS/Pm and AraC/PBAD transcription regulator/promoter system on a broad-host-range mini-RK2 replicon (manuscript in preparation). As proof of principle, several proteins, including the above-mentioned TF protein from P. haloplanktis (by AraC/PBAD) and the Arctic-sourced chitinase (by XylS/Pm), could be expressed from a mini-RK2 plasmid in Pseudomonas at a higher level than in the E. coli/T7-system (data not shown). Subsequently, we also introduced the T7 system into 2 Pseudomonas spp. strains by regulating the expression of a T7 bacteriophage gene 1 (T7 RNA polymerase) from the chromosome under the control of the XylS/Pm promoter. This system was used to express a red fluorescent reporter protein (mCherry) in the mini-RK2 plasmid under the control of T7 promoter with a lac operator sequence (Fig. 2A and B). As the system was found to be leaky, the XylS/Pm system was further modified in the 5′ UTR region, based on an earlier finding,27 to reduce the leakiness of the system. This modification led to a tightly regulated system in these Pseudomonas hosts as exemplified by the heterologous expression of mCherry (Fig. 2B). As these expression systems were carried out at the fastest growing temperature, 20°C, further studies are needed to investigate their performance at lower temperature.
Figure 2.
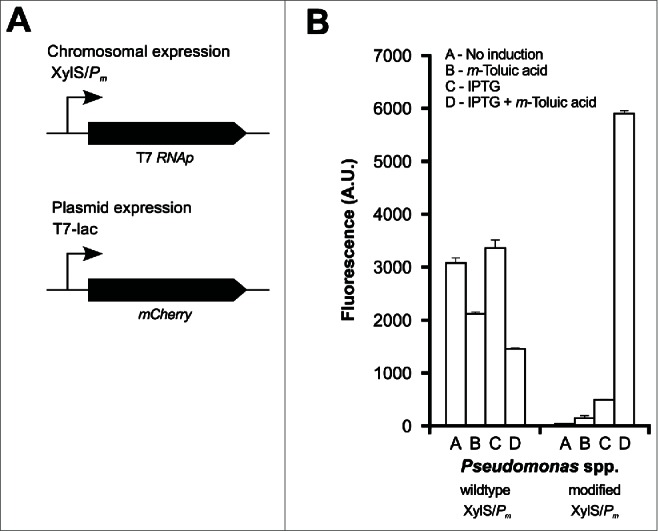
Expression of mCherry using the T7 system in Arctic marine Pseudomonas. (A) The figure depicts the organization of the relevant expression cassettes. The chromosomal expression of T7 RNA polymerase T7 RNAp is driven by the XylS/Pm system, and the expression of the mCherry reporter protein is driven by the T7 promoter with the lac operator sequence (T7-lac). (B) Degree of fluorescence (arbitrary units) from mCherry expression at 20°C in the wildtype and the modified XylS/Pm system in Pseudomonas. Isopropyl β-D-1-thiogalactopyranoside (IPTG) serves as the inducer of the T7 promoter, whereas m-Toluic acid is the inducer of the XylS/Pm system.
To conclude, advances in generating efficient low-temperature expression systems is required for the continued effort in both basic research and industrial exploration of psychrophilic enzymes. Currently, 2 main strategies are being explored for development of such low-temperature systems in bacterial hosts: (i) low-temperature adaptations of existing mesophilic expression systems, and (ii) development of new psychrophilic hosts. Besides benefiting the exploitation of psychrophilic enzymes, a low-temperature expression system may also be advantageous for mesophilic and thermophilic proteins. In particular, expression at lower temperature may be beneficial where properties of the enzymes are deleterious to the host cell growth. In the case of potent meso- and thermophilic proteases a low-temperature expression system, where the thermal stability is retained but temperature condition is sub-optimal for the activation of the zymogens, could be advantageous to prevent short half-life due to autoproteolysis.28-30
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The experimental work conducted in this addendum was funded by the Norwegian Research Council (project ID: 192123).
Authors' contributions
GEKB and RL performed the experiments for the presented data. GEKB coordinated and drafted the manuscript. All authors edited the manuscript and approved the final version.
References
- 1.Bjerga GEK, Williamson AK. Cold shock induction of recombinant Arctic environmental genes. BMC Biotechnol 2015; 15:78; PMID:26286037; http://dx.doi.org/ 10.1186/s12896-015-0185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feller G. Psychrophilic enzymes: from folding to function and biotechnology. Scientifica (Cairo) 2013; 2013:512840; PMID:24278781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan F, Shah AA, Javed S, Hameed A. Enzymes used in detergents: Lipases. 2010; 9:4836-44; PMID:2115027721150277 [Google Scholar]
- 4.Davail S, Feller G, Narinx E, Gerday C. Cold adaptation of proteins. Purification, characterization, and sequence of the heat-labile subtilisin from the Antarctic psychrophile Bacillus TA41. J Biol Chem 1994; 269:17448-53; PMID:8021248 [PubMed] [Google Scholar]
- 5.Narinx E, Baise E, Gerday C. Subtilisin from psychrophilic antarctic bacteria: characterization and site-directed mutagenesis of residues possibly involved in the adaptation to cold. Protein Eng 1997; 10:1271-9; PMID:9514115; http://dx.doi.org/ 10.1093/protein/10.11.1271 [DOI] [PubMed] [Google Scholar]
- 6.Morita RY. Psychrophilic bacteria. Bacteriol Rev 1975; 39:144-67; PMID:1095004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Struvay C, Feller G. Optimization to low temperature activity in psychrophilic enzymes. Int J Mol Sci 2012; 13:11643-65; PMID:23109875; http://dx.doi.org/ 10.3390/ijms130911643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Z-C, Tang B-L, Zhao D-L, Pang X, Qin Q-L, Zhou B-C, Zhang X-Y, Chen X-L, Zhang Y-Z. Development of a cold-adapted pseudoalteromonas expression system for the pseudoalteromonas proteins intractable for the escherichia coli system. PLoS One 2015; 10:e0137384; PMID:26333173; http://dx.doi.org/ 10.1371/journal.pone.0137384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Rha E, Yeom S-J, Lee D-H, Choi E-S, Lee S-G, 2013. Generating in vivo cloning vectors for parallel cloning of large gene clusters by homologous recombination. PLoS One 8, e79979 Doi: 10.1371/journal.pone.0079979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson A, Pedersen H. Recombinant expression and purification of an ATP-dependent DNA ligase from Aliivibrio salmonicida. Protein Expr Purif 2014; 97:29-36; PMID:24582823; http://dx.doi.org/ 10.1016/j.pep.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 11.Ferrer, M., Chernikova, T.N., Yakimov, M.M., Golyshin, P.N., Timmis, K.N. , 2003. Chaperonins govern growth of Escherichia coli at low temperatures. Nat. Biotechnol. 21, 1266–7. doi: 10.1038/nbt1103-1266 [DOI] [PubMed] [Google Scholar]
- 12.Vasina JA, Baneyx F. Recombinant protein expression at low temperatures under the transcriptional control of the major Escherichia coli cold shock promoter cspA. Appl Environ Microbiol 1996; 62:1444-7; PMID:8919809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanabe H, Goldstein J, Yang M, Inouye M. Identification of the promoter region of the Escherichia coli major cold shock gene, cspA. J Bacteriol 1992; 174:3867-73; PMID:1597410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsch, N., Homuth, G., Schweder, T., 2015. Stepwise optimization of a low-temperature Bacillus subtilis expression system for “difficult to express” proteins. Appl. Microbiol. Biotechnol. 99, 6363-76. [DOI] [PubMed] [Google Scholar]
- 15.Le ATT, Schumann W, Thuy Le AT, Schumann W, Le ATT, Schumann W. A novel cold-inducible expression system for Bacillus subtilis. Protein Expr Purif 2007; 53:264-9; PMID:17307364; http://dx.doi.org/ 10.1016/j.pep.2006.12.023 [DOI] [PubMed] [Google Scholar]
- 16.Qing G, Ma L-C, Khorchid A, Swapna GVT, Mal TK, Takayama MM, Xia B, Phadtare S, Ke H, Acton T, et al.. Cold-shock induced high-yield protein production in Escherichia coli. Nat Biotechnol 2004; 22:877-82; PMID:15195104; http://dx.doi.org/ 10.1038/nbt984 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Kojima C. Efficient protein production method for NMR using soluble protein tags with cold shock expression vector. J Biomol NMR 2010; 48:147-55; PMID:20844927; http://dx.doi.org/ 10.1007/s10858-010-9445-5 [DOI] [PubMed] [Google Scholar]
- 18.Piette F, D'Amico S, Struvay C, Mazzucchelli G, Renaut J, Tutino ML, Danchin A, Leprince P, Feller G. Proteomics of life at low temperatures: trigger factor is the primary chaperone in the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125. Mol Microbiol 2010; 76:120-32; PMID:20199592; http://dx.doi.org/ 10.1111/j.1365-2958.2010.07084.x [DOI] [PubMed] [Google Scholar]
- 19.Ueda M, Ito A, Nakazawa M, Miyatake K, Sakaguchi M, Inouye K. Cloning and expression of the cold-adapted endo-1,4-β-glucanase gene from Eisenia fetida. Carbohydr Polym 2014; 101:511-6; PMID:24299806; http://dx.doi.org/ 10.1016/j.carbpol.2013.09.057 [DOI] [PubMed] [Google Scholar]
- 20.Parrilli E, De Vizio D, Cirulli C, Tutino ML. Development of an improved Pseudoalteromonas haloplanktis TAC125 strain for recombinant protein secretion at low temperature. Microb Cell Fact 2008; 7:2; PMID:18257924; http://dx.doi.org/ 10.1186/1475-2859-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papa R, Rippa V, Sannia G, Marino G, Duilio A. An effective cold inducible expression system developed in Pseudoalteromonas haloplanktis TAC125. J Biotechnol 2007; 127:199-210; PMID:16959351; http://dx.doi.org/ 10.1016/j.jbiotec.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 22.Cusano AM, Parrilli E, Marino G, Tutino ML. A novel genetic system for recombinant protein secretion in the Antarctic Pseudoalteromonas haloplanktis TAC125. Microb Cell Fact 2006; 5:40; PMID:17169153; http://dx.doi.org/ 10.1186/1475-2859-5-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Médigue C, Krin E, Pascal G, Barbe V, Bernsel A, Bertin PN, Cheung F, Cruveiller S, D'Amico S, Duilio A, et al.. Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res 2005; 15:1325-35; http://dx.doi.org/ 10.1101/gr.4126905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang P, Yu Z, Li B, Cai X, Zeng Z, Chen X, Wang X. Development of an efficient conjugation-based genetic manipulation system for Pseudoalteromonas. Microb Cell Fact 2015; 14:1-11; PMID:25567661; http://dx.doi.org/ 10.1186/s12934-014-0183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strand TA, Lale R, Degnes KF, Lando M, Valla S. A new and improved host-independent plasmid system for RK2-based conjugal transfer. PLoS One 2014; 9:1-6; http://dx.doi.org/ 10.1371/journal.pone.0090372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilmes B, Hartung A, Lalk M, Liebeke M, Schweder T, Neubauer P. Fed-batch process for the psychrotolerant marine bacterium Pseudoalteromonas haloplanktis. Microb Cell Fact 2010; 9:72; PMID:20858251; http://dx.doi.org/ 10.1186/1475-2859-9-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lale R, Berg L, Stüttgen F, Netzer R, Stafsnes M, Brautaset T, Aune TEV, Valla S. Continuous control of the flow in biochemical pathways through 5′ untranslated region sequence modifications in mRNA expressed from the broad-host-range promoter Pm. Appl Environ Microbiol 2011; 77:2648-55; PMID:21335387; http://dx.doi.org/ 10.1128/AEM.02091-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan AR, James MNG. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci 1998; 7:815-36; PMID:9568890; http://dx.doi.org/ 10.1002/pro.5560070401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y-R, Zhu H, Fang N, Liang X, Zhong C-Q, Tang X-F, Shen P, Tang B. Cold-adapted maturation of thermophilic WF146 protease by mimicking the propeptide binding interactions of psychrophilic subtilisin S41. FEBS Lett 2008; 582:2620-6; PMID:18586033; http://dx.doi.org/ 10.1016/j.febslet.2008.06.041 [DOI] [PubMed] [Google Scholar]
- 30.Fornbacke M, Clarsund M. Cold-adapted proteases as an emerging class of therapeutics. Infect Dis Ther 2013; 2:15-26; PMID:25135820; http://dx.doi.org/ 10.1007/s40121-013-0002-x [DOI] [PMC free article] [PubMed] [Google Scholar]


