Combined therapy with autologous adipose-derived mesenchymal stem cells and ciprofloxacin was found to be superior to either therapy alone in improving the outcome in a rodent sepsis model.
Keywords: Sepsis syndrome, Adipose-derived mesenchymal stem cells, Antibiotics, Inflammation, Urogenital organ damage
Abstract
We hypothesized that combined treatment with autologous adipose-derived mesenchymal stem cell (ADMSC) and ciprofloxacin is superior to ciprofloxacin only in reducing sepsis-induced urogenital organ damage and mortality in rat sepsis syndrome (SS) caused by intrapelvic injection of cecal bacteria (1.0 × 104 cells per milliliter; total, 5.0 ml). Male Sprague-Dawley rats (n = 60) equally divided into group 1 (sham-control), group 2 (SS), group 3 (SS-ADMSC [5.0 × 105 intravenously at 0.5, 6, and 18 hours after sepsis induction]), group 4 (SS-ciprofloxacin [3.0 mg/kg, b.i.d.] for 5 days), and group 5 (SS-ADMSC-ciprofloxacin) were sacrificed by day 5. Mortality rate and creatinine level were highest in group 2 and lowest in group 1 and significantly higher in groups 3 and 4 than those in group 5, but there was no difference between groups 3 and 4 (all p < .005). The kidney injury score, inflammatory biomarker expressions at protein (tumor necrosis factor-1α, nuclear factor-κB, matrix metallopeptidase-9, regulated on activation, normal T-cell expressed and secreted, interleukin-1β) and cellular (CD14+, migratory inhibitor factor positive, CD68+) levels in kidneys and urinary bladder were lowest in group 1 and highest in group 2, higher in group 4 than in groups 3 and 5, and higher in group 3 than in group 5 (all p < .001). Protein expressions of apoptosis (Bax, cleaved caspase 3 and poly[ADP-ribose] polymerase 1, p21 protein [Cdc42/Rac]-activated kinase 2) and oxidative stress (oxidized protein, NADPH oxidase (NOX)-1, NOX-2) in these organs showed an identical pattern compared with that of inflammation in all groups (all p < .001). In conclusion, ADMSC-assisted ciprofloxacin therapy offered an additional benefit by reducing acute urogenital organ damage in rat.
Significance
Autologous adipose-derived mesenchymal stem cell-assisted ciprofloxacin therapy offered an additional benefit by reducing acute urogenital organ damage in rats.
Introduction
Despite state-of-the-art treatment strategies, advanced antibiotic development, and optimal health care, acute sepsis syndrome (SS) remains a global threat and the leading cause of death in hospitalized patients [1–4]. Sepsis is also associated with elevated in-hospital mortality in the elderly, ranging from 30% to 60% [5–7].
Death from SS is caused by a complicated process that involves myriad factors [8–10]. Of these factors, overwhelming immune response (i.e., host response), generations of oxidative stress and reactive oxygen species (ROS), and over-compensatory inflammatory reaction [4, 8, 9] are the major contributors to direct or indirect assaults to the vital organs [11–14]. Additionally, studies have shown that SS-related organ damage, especially in the settings of acute lung injury and acute kidney injury commonly encountered in critically ill patients [13], were strongly associated with untoward prognostic outcomes [9–17]. Although use of antibiotics is the gold standard treatment of SS [1, 2], the in-hospital mortality rate of SS is still unacceptably high. This could be mainly due to the fact that antibiotics cannot effectively control the overwhelming immune response and vigorous inflammatory reaction. Therefore, in addition to the appropriate choice of antibiotics [1, 2], control of the overwhelming immune response and vigorous inflammatory reaction that contribute to damage of vital and vulnerable organs may be crucial to reduce sepsis-induced morbidity and mortality [8–10].
Not only has growing evidence demonstrated that stem cells, especially adipose-derived mesenchymal stem cells (ADMSCs), possess the intrinsic capacity of immunomodulation [18, 19], but stem cell therapy has also been shown to attenuate inflammation and immune responses [18–20]. Of particular interest, limited experimental studies suggest that, in the setting of sepsis syndrome, stem cell treatment could reduce mortality through clearance of bacteria, regulation of overwhelming immune response, and alleviation of sepsis-induced inflammatory reactions and the generation of oxidative stress/reactive oxygen species [21–23].
However, data on stem cell treatment of SS remain too limited to allow adequate evaluation of its therapeutic potential. Moreover, in the setting of SS, the effect of stem cells compared with that of antibiotics, as well as that when the two are combined, remains unclear. Accordingly, this study tested the hypothesis that ADMSC-supported ciprofloxacin would be superior to ciprofloxacin monotherapy in reducing bacterial sepsis-induced urogenital organ damage and mortality.
Materials and Methods
Ethics
All animal experimental procedures were approved by the Institute of Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital (Affidavit of Approval of Animal Use Protocol No. 2008121108) and performed in accordance with the Guide for the Care and Use of Laboratory Animals [24].
Estimation of Sample Size for Different Groups
On the basis of our previous work [25], it was estimated that mortality in rats at 72 hours after SS induction without treatment would be about 40%–50%. In addition, we calculated that at least eight surviving rats would be required in any one group for statistical significance to be reached at day 5 after sepsis induction. Accordingly, 16 rats were randomly assigned to each group. Survival of the animals was recorded.
Animal Grouping, Sepsis Syndrome Induction, and Rationale of Regimen of ADMSC and Ciprofloxacin
Pathogen-free, adult male Sprague-Dawley (SD) rats weighing 350–375 g (Charles River Technology, BioLASCO, Taiwan, http://www.biolasco.com.tw) were randomly assigned and equally divided into group 1 (sham control, intrapelvic injection [IPI] of normal saline, 1 ml; n = 16), group 2 (SS: IPI of cecal ligation puncture [CLP]-derived bacteria only [1.0 × 104 mixed bacteria/ml; total, 5.0 ml abdominal fluid per rat]; n = 16), group 3 (SS + autologous ADMSC [5.0 × 105 intravenously at 30 minutes, 6 hours, and 18 hours after SS induction procedure]; n = 16), group 4 (SS + ciprofloxacin [3.0 mg/kg b.i.d. for 5 days]; n = 16), and group 5 (SS + ADMSC + ciprofloxacin; n = 16).
The dosage of 5.0 × 105 intravenously at 30 minutes, 6 hours, and 18 hours after SS induction procedure was based on our previous report [25], with great modification because we found that the high dose of ADMSC administration to the animals in the previous study (i.e., 1.2 × 106 intravenously at 30 minutes, 6 hours, and 18 hours after sepsis syndrome) might induce adverse effects, including a higher mortality rate.
The animals were sacrificed at day 5 after SS induction. The blood sample was collected for measuring the creatinine level at day 5 before the animals were sacrificed. The kidney and urinary bladder tissue specimens were collected for individual study.
In the present study, SS induction mimicked the clinical phenomenon of sepsis from a perforated alimentary tract. Additionally, the time course of ADMSC therapy mimicked the clinical schedule of antibiotic treatment for patients with sepsis syndrome (i.e., every 8 hours) and was based on our recent report with minimal modifications [26, 27]. Furthermore, to elucidate the optimal effect and safety of ciprofloxacin treatment, different regimens of ciprofloxacin (i.e., 1 mg/kg per day [lowest dose], 3 mg/kg per day [intermediate dose], and 6 mg/kg per day [highest dose]) were given to six additional SD rats (one regimen for two animals). Seventy-two hours after SS induction, blood samples were drawn for white blood cell count and differential count. The results showed that the white blood cell count was notably higher in animals receiving the lowest dose than in those receiving the intermediate and highest doses, without significant difference between the latter two groups (12,000 vs. 8,800 vs. 8,600 cells per microliter, respectively). Of importance is that no adverse effect or unstable condition was observed in animals receiving intermediate dose. Therefore, ciprofloxacin, 3.0 mg/kg per day, was used in the present study.
Preparation of Abdominal-Derived Bacteria Using CLP
Five additional SD rats were anesthetized with inhalational 2.0% isoflurane and placed in a supine position on a warming pad at 37°C with the abdomen shaved. Under sterile conditions, the abdominal skin and muscle were opened and the cecum exposed. In the experimental CLP animals, the cecum was ligated by polypropylene sutures over its distal portion (i.e., distal ligation) and the cecum distal to the ligature was punctured twice with an 18-gauge needle to allow the cecal contents to be expressed intraperitoneally, as previously described [25, 26]. The abdominal wound is closed and the animal was allowed to recover from anesthesia. Thirty-six hours after the CLP procedure, the abdomens of the five animals were opened again and the ascitic fluid, which contained colon-derived bacteria (mixed bacteria), was collected and pooled for IPI in the experimental groups (except for the sham controls). The ascitic fluid was collected for quantitative analysis of the amount of bacteria using counting bacteria colony on high-power field (HPF) and Gram stain.
Isolation of Adipose-Derived Mesenchymal Stem Cells
At day 14 before SS induction, isolation of adipose tissue for culturing the ADMSC was performed in groups 3 and 5 (n = 16 for each group). Adipose tissue surrounding the epididymis was carefully dissected, excised, and prepared on the basis of our recent reports [25–28]. Then, 200–300 μl of sterile saline was added to every 0.5 g of adipose tissue to prevent dehydration. The tissue was cut into <1-mm3 pieces using a pair of sharp, sterile surgical scissors. Sterile saline (37°C) was added to the homogenized adipose tissue in a ratio of 3:1 (saline:adipose tissue), followed by the addition of stock collagenase solution to a final concentration of 0.5 units/ml. The centrifuge tubes, with the contents, were placed and secured on a Thermaline shaker (Thermaline, Auburn, WA, https://www.thermaline.com) and incubated with constant agitation for 60 ± 15 minutes at 37°C. After 40 minutes of incubation, the content was triturated with a 25-ml pipette for 2–3 minutes.
The cells obtained were placed back on the rocker for incubation. The contents of the flask were transferred to 50-ml tubes after digestion, followed by centrifugation at 600 g for 5 minutes at room temperature. The fatty layer and saline supernatant from the tube were poured out gently in one smooth motion or removed using vacuum suction. The cell pellet thus obtained was resuspended in 40 ml saline and then centrifuged again at 600 g for 5 minutes at room temperature.
After being resuspended again in 5 ml saline, the cell suspension was filtered through a 100-μm filter into a 50-ml conical tube to which 2 ml of saline was added to rinse the remaining cells through the filter. The flow-through was pipetted into a new 50-ml conical tube through a 40-μm filter. The tubes were centrifuged for a third time at 600 g for 5 minutes at room temperature. The cells were resuspended in saline. An aliquot of cell suspension was then removed for cell culture in Dulbecco’s modified Eagle's medium/low-glucose medium containing 10% fetal bovine serum for 14 days. Approximately 2.0–3.0 × 106 ADMSCs were obtained from each rat. Flow cytometric analysis was performed to identify cellular characteristics after cell labeling with appropriate antibodies on day 14 before transfusion.
Histopathology Scoring of Kidney Injury at Day 5 After the SS Induction
Histopathology scoring was determined in a blinded fashion, as we previously reported [26, 27]. Briefly, the kidney specimens from all animals were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E) for light microscopy. The scoring system reflecting the grading of tubular necrosis, loss of brush border, cast formation, and tubular dilatation in 10 randomly chosen, nonoverlapping fields (200×) for each animal was as follows: 0 (none), 1 (≤10%), 2 (11%–25%), 3 (26%–45%), 4 (46%–75%), and 5 (≥76%).
Immunohistochemical and Immunofluorescent Studies
The procedures and protocols for immunohistochemical (IHC) and immunofluorescent (IF) examinations were also based on our recent studies [23, 26, 28]. Briefly, for IHC staining, rehydrated paraffin sections were first treated with 3% H2O2 for 30 minutes and incubated with Immuno-Block reagent (Bio SB Inc., Goleta, CA, http://www.biosb.com) for 30 minutes at room temperature. Sections were then incubated with primary antibodies specifically against CD14 (1:300, Bio SB Inc.) at 4°C overnight. Irrelevant antibody (p53 [1:500; Abcam, Cambridge, UK, http://www.abcam.com] and mouse control IgG [Abcam]) served as controls in the current study. IF staining was performed for the examinations of γ-H2AX (1:500; Abcam), macrophage migratory inhibitor factor (MIF) (1:200; Abcam), CD68 (1:100; Abcam), and COX-2 (1:100; Abcam) using respective primary antibody; irrelevant antibodies were used as controls. Three sections of kidney specimens were analyzed in each rat. For quantification, three randomly selected HPFs (×200 or ×400 for IHC and IF studies) were analyzed in each section. The mean number per HPF for each animal was then determined by summation of all numbers divided by 9.
An IHC-based scoring system was adopted for semiquantitative analyses of CD14 in the kidney and bladder as a percentage of positive cells in blinded fashion (score of positively stained cell for CD14: 0 = negative staining; 1= <15%; 2 = 15%–25%; 3 = 25%–50%; 4 = 50%–75%; 5= >75%–100% per HPF).
Western Blot Analysis of Kidney and Urinary Bladder Specimens
Equal amounts (10–30 μg) of protein extracts from the kidneys were loaded and separated by SDS-polyacrylamide gel electrophoresis using 8%–10% acrylamide gradients. After electrophoresis, the separated proteins were transferred electrophoretically to a polyvinylidene difluoride membrane (Amersham Biosciences, GE Healthcare Life Sciences, Pittsburgh, PA, http://www.gelifesciences.com). Nonspecific proteins were blocked by incubating the membrane in blocking buffer (5% nonfat dry milk in Tris-buffered saline and Tween 20 containing 0.05% Tween 20) overnight. The membranes were incubated with monoclonal antibodies against polyclonal antibodies against tumor necrotic factor (TNF)-α (1:1,000; Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com/); nuclear factor (NF)-κB (1:250; Abcam); matrix metalloproteinase (MMP)-9 (1:3,000; Abcam); interleukin (IL)-1β (1:500; Abcam); RANTES (regulated on activation, normal T cell expressed and secreted) (1:1,000; Cell Signaling Technology); mitochondrial Bax (1:1,000; Abcam); caspases 1, 2, and 3 (1:1,000; Cell Signaling Technology); poly(ADP-ribose) polymerase (PARP) (1:1,000; Cell Signaling Technology); p21 protein (Cdc42/Rac)-activated kinase 2 (PAK2) (1:1,000; Abcam), NOX-1 (NADPH oxidase 1) (1:1,500; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), and NOX-2 (NADPH oxidase 2) (1:750; Sigma-Aldrich). Signals were detected with horseradish peroxidase-conjugated goat anti-mouse, goat anti-rat, or goat anti-rabbit IgG.
We used the Oxyblot Oxidized Protein Detection Kit (S7150; Chemicon, EMD Millipore, Darmstadt, Germany, https://www.emdmillipore.com) The procedure of 2,4-dinitrophenylhydrazine (DNPH) derivatization was carried out on 6 μg of protein for 15 minutes according to manufacturer’s instructions. One-dimensional electrophoresis was carried out on 12% SDS/polyacrylamide gel after DNPH derivatization. Proteins were transferred to nitrocellulose membranes, which were then incubated in the primary antibody solution (anti-DNP, 1:150) for 2 hours, followed by incubation with the secondary antibody solution (1:300) for 1 hour at room temperature. The washing procedure was repeated 8 times within 40 minutes
Immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham Biosciences, GE Healthcare Life Sciences), which was then exposed to Biomax L film (Kodak, Rochester, NY, http://www.kodak.com). For quantification, ECL signals were digitized by using Labworks software (UVP, Upland, CA, http://www.uvp.com). For Oxyblot protein analysis, a standard control was loaded on each gel.
Statistical Analysis
Quantitative data are expressed as mean ± SD. Statistical analysis was performed by analysis of variance, followed by Bonferroni multiple-comparison post hoc test. All analyses were conducted by using SAS statistical software for Windows, version 8.2 (SAS Institute, Cary, NC, https://www.sas.com/). A p value <.05 was considered to indicate a statistically significant difference.
Results
Flow Cytometric Results, Mortality Rate, and Circulating Level of Creatinine Among the Five Groups of Animals After SS Induction
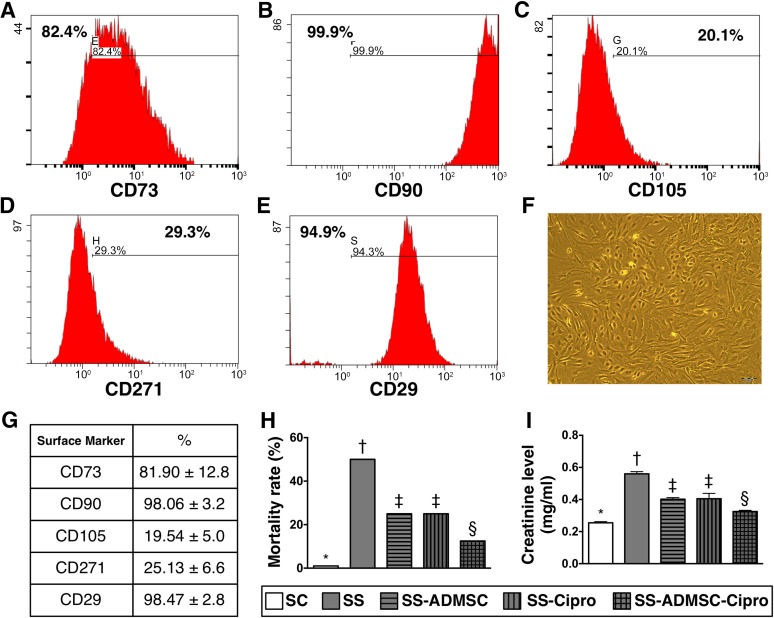
By day 14 after cell culturing, the flow cytometric analysis (Fig. 1A–1E) showed that the CD90+ and CD29+ surface markers of ADMSCs were the two highest ADMSC population (Fig. 1G). The spindle-shape picture, a typical appearance of ADMSC, was present in the culture plate (Fig. 1F).
Figure 1.
The flow cytometric analysis, mortality rate, and circulating level of creatinine by day 5 after SS induction (n = 16). (A–E): Flow cytometric analysis by day 14 after cell culturing. (F): Spindle-shape picture of the cells in culture plate, a typical appearance of mesenchymal stem cells (original magnification, ×100). (G): CD90+ and CD29+ cells were the two highest populations of ADMSCs. (H): Statistical analysis of mortality rate among five groups. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡). (I): Statistical analysis of circulating creatinine level; ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §). All statistical analyses were performed by one-way analysis of variance, followed by Bonferroni multiple-comparison post hoc test (n = 10 for group 1; n = 8 for group 2; n = 12 for groups 3 and 4; n = 14 for group 5). Symbols (∗, †, ‡, §) indicate significance (at the .05 level). Abbreviations: ADMSC, adipose-derived mesenchymal stem cell; Cipro, ciprofloxacin; SC, sham control; SS, sepsis syndrome.
By day 5, the mortality rate for sepsis syndrome was highest in group 2 and lowest in group 1 and significantly higher in groups 3 and 4 than those in group 5, but there was no difference between groups 3 and 4 (Fig. 1H).
By day 5 after SS induction, the circulating level of creatinine, an indicator of renal function, showed an identical pattern as mortality rate among the five groups (0.25 ± 0.02 vs. 0.57 ± 0.03 vs. 0.41 ± 0.02 vs. 0.41 ± 0.06 vs. 0.33 ± 0.02 mg/ml, p < .001) (Fig. 1I). Combined treatment with ciprofloxacin and ADMSC significantly reduced sepsis syndrome-associated mortality and kidney injury compared with treatment with either one.
Protein Expressions of Inflammatory Biomarkers in Kidneys and Urinary Bladder
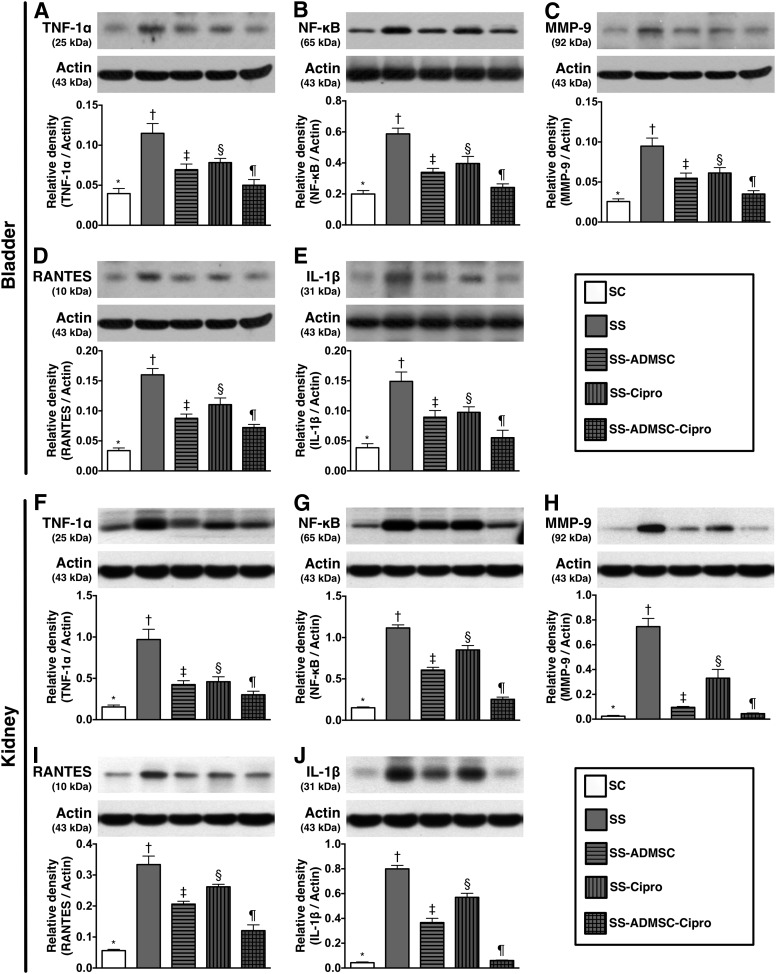
The protein expressions of TNF-1α, NF-κB, MMP-9, RANTES, and IL-1β (five proinflammation markers in the kidney and urinary bladder) were lowest in group 1, highest in group 2, significantly higher in group 3 and group 4 than in group 5, and significantly higher in group 4 than in group 3 (Fig. 2).
Figure 2.
The protein expressions of inflammatory biomarkers in urinary bladder and kidney parenchyma by day 5 after sepsis induction (n = 8). Upper panel (protein expressions in bladder): (A): Protein expression of TNF-1α, ∗, p < .001 compared with other groups with different symbols (∗, †, ‡, §, ¶). (B): Protein expression of NF-κB. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶) . (C): Protein expression MMP-9. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (D): Protein expression of RANTES. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (E): Protein expression of IL-1β. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). Lower panel (protein expression in kidney parenchyma): (F): Protein expression of TNF-1α. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (G): Protein expression of NF-κB. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (H): Protein expression of MMP-9. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (I): Protein expression of RANTES. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (J): Protein expression of IL-1β. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). All statistical analyses were performed by one-way analysis of variance, followed by Bonferroni multiple-comparison post hoc test. Symbols (∗, †, ‡, §, ¶) indicate significance (at the .05 level). Abbreviations: ADMSC, adipose-derived mesenchymal stem cell; Cipro, ciprofloxacin; IL, interleukin; MMP-9, matrix metalloproteinase-9; NF, nuclear factor; RANTES, regulated on activation, normal T-cell expressed and secreted; SC, sham control; SS, sepsis syndrome; TNF, tumor necrosis factor. The error bars are defined as standard error of the mean.
IHC and IF Staining for Identification of Inflammatory Cells in Kidneys and Urinary Bladder by Day 5 After Sepsis Induction
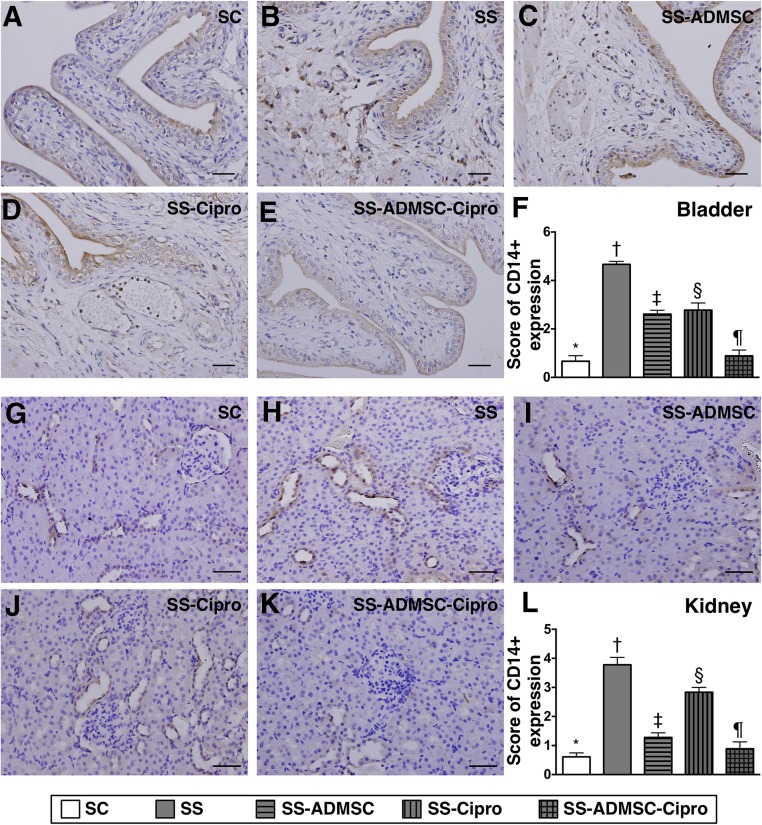
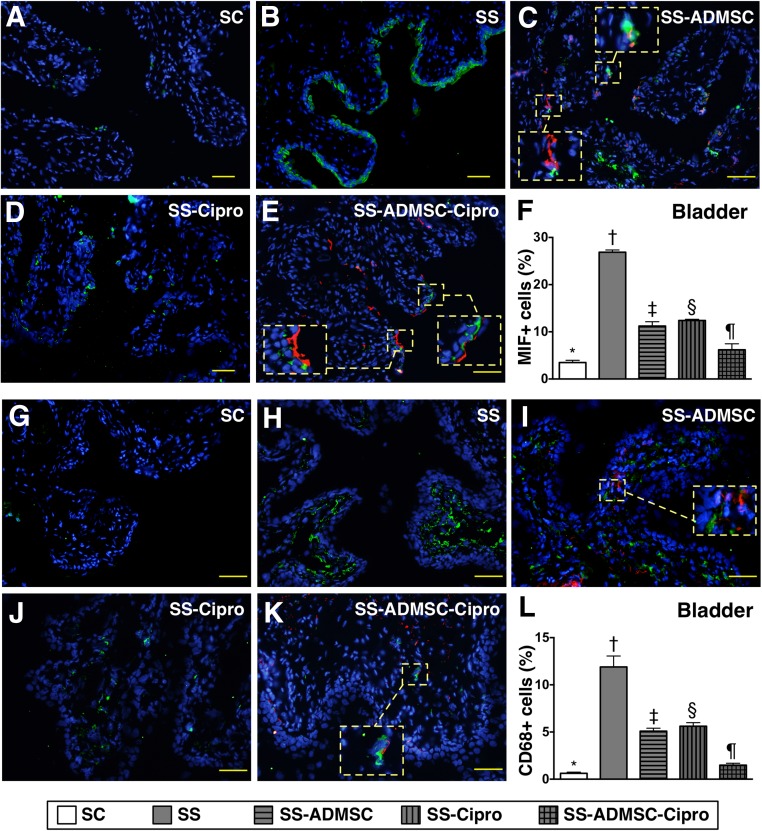
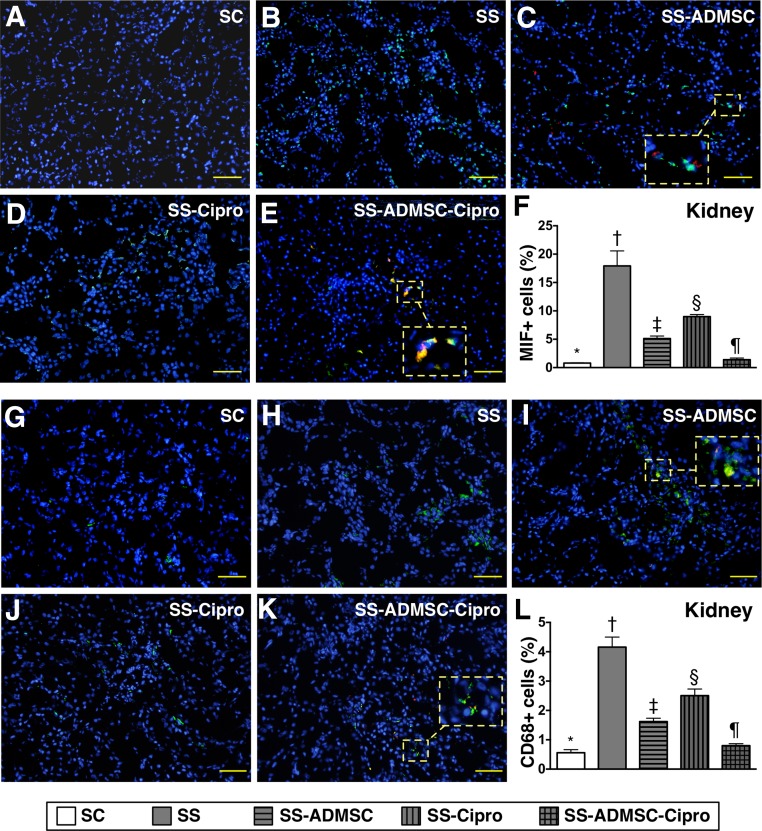
IHC findings demonstrated that the cellular expressions of CD14, one indicator of inflammation in the kidneys and urinary bladder, was highest in group 2 and lowest in group 1, significantly higher in groups 3 and 4 than that in group 5, and significantly higher in group 4 than in group 3 (Fig. 3). Moreover, IF microscopy revealed that the cellular expressions of MIF and CD68 (two other inflammatory cell biomarkers) in the two organs showed an identical pattern compared with that of IHC staining among the five groups (Figs. 4, 5).
Figure 3.
Immunohistochemical (IHC) microscopic findings of inflammatory cells in urogenital organ by day 5 after sepsis induction (n = 8) (A–E): Microscopic finding (original magnification, ×200) of CD14+ cells in urinary bladder. (F): Score of CD14+ expression in bladder. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (G–K): IHC microscopic finding (original magnification, ×200) of CD14+ cells in kidney parenchyma. (L) Score of CD14+ in kidney. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶) . The scale bars in right lower corner represent 50 µm. All statistical analyses were performed by one-way analysis of variance, followed by Bonferroni multiple-comparison post hoc test. Symbols (∗, †, ‡, §, ¶) indicate significance (at the .05 level). Abbreviations: ADMSC, adipose-derived mesenchymal stem cell; Cipro, ciprofloxacin; SC, sham control; SS, sepsis syndrome.
Figure 4.
Immunofluorescent (IF) microscopic identification of inflammatory cells in urinary bladder by day 5 after sepsis induction. (A–E): IF microscopic (original magnification, ×200) finding of macrophage MIF+ cells in urinary bladder. The MIF+ cells (green) and implanted ADMSCs (red) in smaller dotted-line square were magnified to larger dotted-line square in (C) and (E). (F): Percentage of MIF+ cells in bladder. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (G–K): IF microscopic (original magnification, ×200) finding of CD68+ cells in urinary bladder. The CD68+ cells (green) and implanted ADMSCs (red) in smaller dotted-line square were magnified to larger dotted-line square in (I) and (K). (L): Percentage of CD68+ cells in bladder. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). The scale bars in right lower corner represent 50 µm. All statistical analyses were performed by one-way analysis of variance, followed by Bonferroni multiple-comparison post hoc test. Symbols (∗, †, ‡, §, ¶) indicate significance (at the .05 level). Abbreviations: ADMSC, adipose-derived mesenchymal stem cell; Cipro, ciprofloxacin; MIF, migratory inhibitor factor; SC, sham control; SS, sepsis syndrome.
Figure 5.
Immunofluorescent (IF) microscopic identification of inflammatory cells in kidney parenchyma by day 5 after sepsis induction. (A–E): IF microscopic (original magnification, ×200) finding of macrophage MIF+ cells in kidney parenchyma. The MIF+ cells (green) and implanted ADMSCs (red) in smaller dotted-line square were magnified to larger dotted- line square in (C) and (E). (F): Percentage of MIF+ cells in kidney. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (G–K): IF microscopic (original magnification, ×200) finding of CD68+ cells in kidney parenchyma. The CD68+ cells (green) and implanted ADMSCs (red) in smaller dotted-line square were magnified to larger dotted-line square in (I) and (K). (L): Percentage of CD68+ cells in kidney. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). The scale bars in right lower corner represent 50 µm. All statistical analyses were performed by one-way analysis of variance, followed by Bonferroni multiple-comparison post hoc test. Symbols (∗, †, ‡, §, ¶) indicate significance (at the .05 level). Abbreviations: ADMSC, adipose-derived mesenchymal stem cell; Cipro, ciprofloxacin; MIF, migratory inhibitor factor; SC, sham control; SS, sepsis syndrome.
Protein Expressions of Apoptotic and Oxidative Stress Biomarkers in Kidneys and Urinary Bladder by Day 5 After Sepsis Syndrome Induction
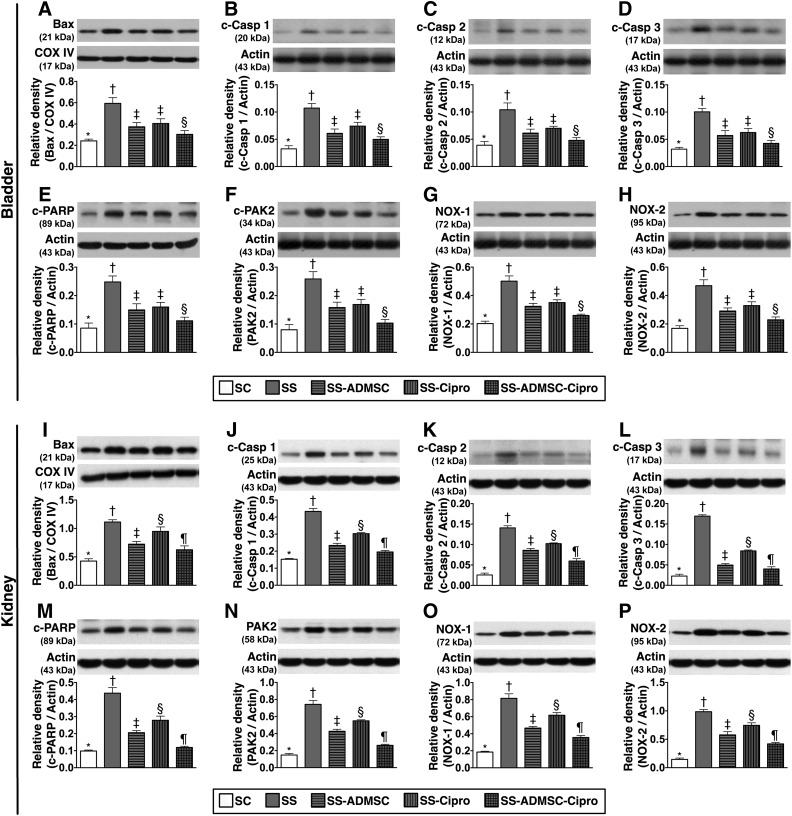
The protein expressions of mitochondrial Bax cleaved caspases 1, 2, and 3; and PARP and PAK2 (six indicators of apoptosis) were highest in group 2 and lowest in group 1, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3 in kidneys; there was no significant difference between groups 3 and 4 in the bladder (Fig. 6). Furthermore, the protein expression of NOX-1 and NOX-2, two indicators of reactive oxygen species, displayed an identical pattern compared with that of apoptosis among the five groups.
Figure 6.
The protein expressions of apoptosis and reactive oxygen species in urinary bladder and kidney parenchyma by day 5 after sepsis induction. Upper panel (protein expressions in the bladder): (A): Protein expression of mitochondrial Bax. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (B–D): Protein expressions of c-Casp 1, 2, and 3. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (E): Protein expression of c-PARP. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (F): Protein expression of PAK2. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (G): Protein expression of NOX-1. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (H): Protein expression of NOX-2. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). Lower panel (protein expressions in kidney parenchyma): (I): Protein expression of mitochondrial Bax. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (J–L): Protein expressions of c-Casp 1, 2, and 3. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (M): Protein expression of c-PARP, ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (N): Protein expression of PAK2. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (O): Protein expression of NOX-1. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (P): Protein expression of NOX-2. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). All statistical analyses were performed by one-way analysis of variance, followed by Bonferroni multiple-comparison post hoc test. Symbols (∗, †, ‡, §, ¶) indicate significance (at the .05 level). ADMSC, adipose-derived mesenchymal stem cell; c-Casp, cleaved caspases; c-PAK2, cleaved p21 activated kinase 2; c-PARP, cleaved poly(ADP-ribose) polymerase; Cipro, ciprofloxacin; COX IV, cytochrome c oxidase subunit IV; NOX, NADPH oxidase; SC, sham control; SS, sepsis syndrome. The error bars are defined as standard error of the mean.
Pathological Assessment of the Kidney Parenchyma and Oxidized Protein Expression in Kidney and Bladder by Day 5 After Sepsis Syndrome Induction
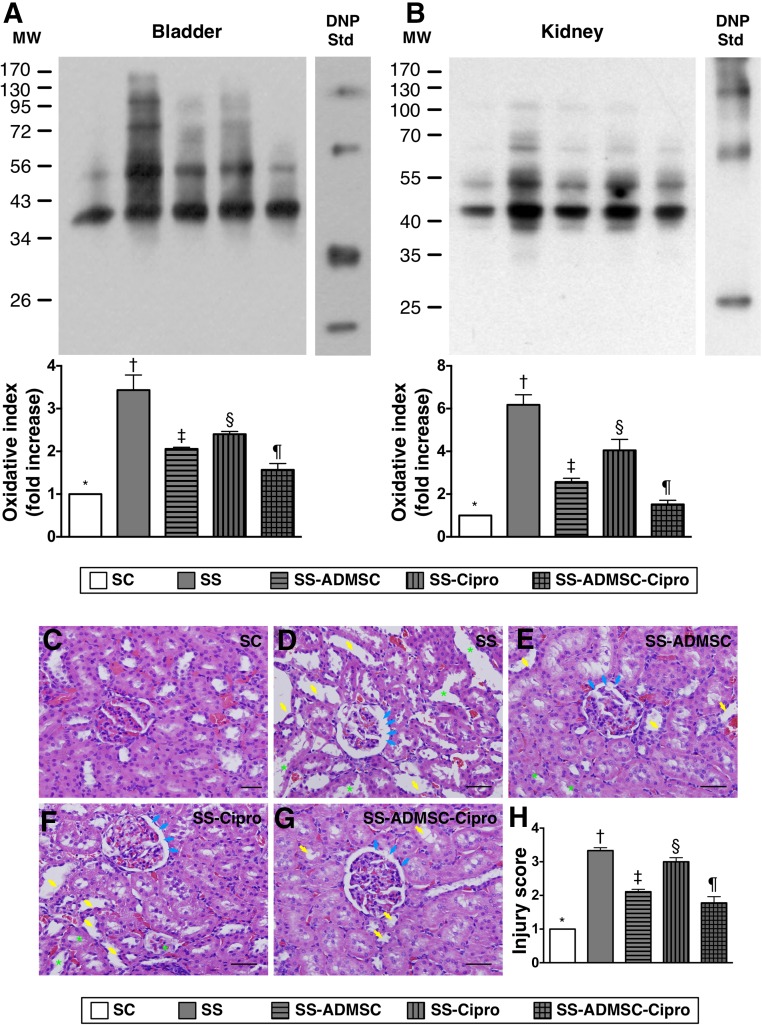
The oxidized protein expression, an indicator of oxidative stress, was highest in group 2 and lowest in group 1, significantly higher in groups 3 and 4 than in group 5, and significantly higher in group 4 than in group 3 (Fig. 7). Additionally, H&E staining of kidney sections revealed that kidney injury score exhibited an identical pattern as oxidative index among the five groups.
Figure 7.
The protein expression of oxidative stress in urogenital organs and pathological assessment of the kidney parenchyma by day 5 after sepsis induction. (A): Protein expression of oxidized protein (i.e., oxidative index) in urinary bladder. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (B): Protein expression of oxidized protein in kidney parenchyma. ∗, p < .0001 vs. other groups with different symbols (∗, †, ‡, §, ¶). (Note: left and right lanes shown in the upper panel represent protein MW marker and control oxidized molecular protein Std, respectively). (C–G): Hematoxylin and eosin stain (original magnification, ×200) demonstrating significantly higher degree of loss of brush border in renal tubules (yellow arrows), tubular necrosis (green asterisk), and dilatation of Bowman capsule (blue arrows) in SS group than in other groups. (H): Analytical result of kidney injury score. ∗, p < .001 vs. other groups with different symbols (∗, †, ‡, §, ¶). All statistical analyses were performed by one-way analysis of variance, followed by Bonferroni multiple-comparison post hoc test. Symbols (∗, †, ‡, §, ¶) indicate significance (at the .05 level). ADMSC, adipose-derived mesenchymal stem cell; Cipro, ciprofloxacin; DNP, 1-3 dinitrophenylhydrazone; MW, molecular weight; SC, sham control; SS, sepsis syndrome; Std, standard.
IF for Identifying DNA Damaged and Inflammatory Markers of the Kidney Parenchyma at Day 5 After Sepsis Syndrome Procedure
The IF microscopic findings showed that the number of γ-H2AX+ cells, a DNA-damaged marker, and the number of COX-2+ cells, an indicator of inflammation, displayed an identical pattern as kidney injury score among the five groups (supplemental online Fig. 1).
Discussion
This study, which investigated the therapeutic effect of ADMSC-ciprofloxacin on SS-induced organ injury, yielded several striking implications. First, the mortality rate was notably increased in animals without treatment, suggesting that the SS animal model was successfully created. Second, both ADMSC and ciprofloxacin were equally effective for protecting against SS-induced urogenital organ damage. Third, combined therapy with ADMSC-ciprofloxacin was superior to either alone in reducing urogenital organ damage, inflammation, and mortality. The results of this study highlight the potential use of this combined regimen in patients with severe sepsis who responded poorly to conventional medical treatment.
Despite the state-of-the-art treatment strategy with advanced pharmacological regimens and in-depth understanding of the underlying mechanism, abundant data have shown that severe SS results in multiple organ failure and a high rate of mortality, especially in elderly patients with comorbidities [1–7]. Consistently, the most important finding in the present study is that pelvic injection of mixed bacteria not only involved urogenital organ damage but also caused remarkably higher mortality in SS animals without treatment. In this way, our findings corroborated those of previous clinical observational studies [1–7]. It is not surprising that, compared with SS-only animals, ciprofloxacin therapy remarkably reduced the mortality and urogenital organ damage in animals after SS induction. Of importance is that ADMSC therapy helped suppress mortality and urogenital organ damage, similar to the effect of ciprofloxacin in this experimental setting. Intriguingly, our previous studies [25, 26] have also demonstrated that ADMSC treatment significantly reduced major organ damage and mortality in rats with CLP-induced SS. Therefore, the results of the present study reinforced the findings of our previous studies [25, 26]. One distinctively important finding in the present study is that combined therapy with ciprofloxacin and ADMSC was superior to either one alone in reducing the mortality and urogenital organ damage in the setting of SS. Accordingly, our findings, in addition to strengthening those of our previous studies [25, 26], also highlight that the combined regimen may be of clinical therapeutic potential for treating high-risk patients with severe SS and poor response to conventional antibiotic therapy.
The underlying mechanisms of SS have been extensively investigated. The high in-hospital mortality caused by the overwhelming infection is mainly attributable to the inappropriate hyperactive immune response (i.e., host response), oxidative stress through overproduction of ROS, and overcompensatory inflammatory reaction [4, 8, 9], which, in turn, contribute to the direct or indirect assaults on the vital organs [11–14]. An essential finding in the present study is that the protein and cellular expressions of inflammatory biomarkers were substantially increased in SS animals. Additionally, the generation of ROS and the resulting oxidative stress were strongly upregulated in animals after sepsis induction. Therefore, our findings corroborated those of previous studies [4, 8, 9, 11–14]. Another interesting finding in the present study is that ADMSC or ciprofloxacin therapy significantly reduced the inflammatory responses, the generation of ROS, and oxidative stress. In particular, combined therapy with ADMSC and ciprofloxacin offered significant benefit in reducing the expressions of these parameters. These findings may explain the superiority of the combined regimen to monotherapy in attenuating urogenital organ damage and mortality.
We propose that the role of ADMSC therapy in reducing the untoward outcomes of SS could be due to multiple mechanisms. Intriguingly, our recent experimental study [29] showed that ADMSC therapy on protecting the organ from ischemia-reperfusion injury was not only through suppressing the inflammatory and immune responses but also through an increase in paracrine effects (i.e., upregulating the secretions of anti-inflammatory chemokines, such as IL-4, IL-10, and prostaglandin E2). The recent study [29], therefore, supports our proposal and the results of the current study.
An association between inflammatory reaction, oxidative stress, and cellular apoptosis was previously identified [25–27]. Clinically, the link between cellular apoptosis and unfavorable outcome has also been clearly delineated [30]. A principal finding in the present study is that the protein (in kidneys and urinary bladder) expressions of proapoptosis markers were significantly increased in animals after induction of sepsis compared with those in sham controls. The degree of apoptosis was significantly reduced after ADMSC or ciprofloxacin treatment and more significantly reduced in the combined treatment group. Our findings could, at least in part, explain the most notable suppression of urogenital organ damage and mortality in animals with sepsis after receiving the combined regimen.
This study has limitations. First, we did not test the optimal dosage of ADMSC for the treatment of SS. Second, we remain uncertain about whether combined treatment with ADMSC and ciprofloxacin offered synergic or merely additional therapeutic benefit in the setting of SS. Third, although abundant data support the effect of combined therapy on improving outcome in rat with SS, this study did not identify the exact underlying mechanisms of this therapy. The proposed mechanisms underlying the observed protection of ADMSC + ciprofloxacin treatment against acute SS based on our findings have been summarized in supplemental online Fig 2. Fourth, although the circulating level of creatinine was measured, the urine level of creatinine, albumin, and total protein were not measured in the present study. Accordingly, we did not provide the data on the ratio of urine protein to urine creatine, an indicator of kidney function. Finally, the study period was relative shorter (i.e., only 5 days from SS induction to euthanasia of the animals); therefore, we did not provide relevant information regarding the long-term outcome as well as kidney function, which would have further strengthened the importance of our study.
Conclusion
Combined therapy with ADMSC and ciprofloxacin was superior to either therapy alone in improving the outcome in a rodent sepsis model.
Supplementary Material
Acknowledgments
This study was supported by a program grant from Chang Gung Memorial Hospital, Chang Gung University (Grant CMRPG8C0301).
Author Contributions
P.-H.S. and H.-J.C.: study design, data acquisition, analysis, drafting of manuscript; C.-H.C., Y.-L.C., T.-H.H., Y.-Y.Z., and M.-W.C: laboratory assay and troubleshooting; C.-F.L., S.-Y.C., Y.-L.C., and H.-T.C: data acquisition, analysis, and interpretation; C.-K.S. and H.-K.Y.: study conception and design, coordination, drafting of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 2.Cronshaw HL, Daniels R, Bleetman A, et al. Impact of the Surviving Sepsis Campaign on the recognition and management of severe sepsis in the emergency department: are we failing? Emerg Med J. 2011;28:670–675. doi: 10.1136/emj.2009.089581. [DOI] [PubMed] [Google Scholar]
- 3.Tipler PS, Pamplin J, Mysliwiec V, et al. Use of a protocolized approach to the management of sepsis can improve time to first dose of antibiotics. J Crit Care. 2013;28:148–151. doi: 10.1016/j.jcrc.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Grozdanovski K, Milenkovic Z, Demiri I, et al. Prediction of outcome from community-acquired severe sepsis and septic shock in tertiary-care university hospital in a developing country. Crit Care Res Pract. 2012;2012:182324. doi: 10.1155/2012/182324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Schäfer ST, Gessner S, Scherag A, et al. Hydrocortisone fails to abolish NF-κB1 protein nuclear translocation in deletion allele carriers of the NFKB1 promoter polymorphism (-94ins/delATTG) and is associated with increased 30-day mortality in septic shock. PLoS One. 2014;9:e104953. doi: 10.1371/journal.pone.0104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn JM, Le T, Angus D, et al. The epidemiology of chronic critical illness in the United States. Crit Care Med. 2015;43:282–287. doi: 10.1097/CCM.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz M, Jacob A, Yang WL, et al. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93:329–342. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonçalves GM, Zamboni DS, Câmara NO. The role of innate immunity in septic acute kidney injuries. Shock. 2010;34(suppl 1):22–26. doi: 10.1097/SHK.0b013e3181e7e69e. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda A, Jacob A, Wu R, et al. Novel therapeutic targets for sepsis: Regulation of exaggerated inflammatory responses. J Nippon Med Sch. 2012;79:4–18. doi: 10.1272/jnms.79.4. [DOI] [PubMed] [Google Scholar]
- 11.Nesseler N, Launey Y, Aninat C, et al. Clinical review: The liver in sepsis. Crit Care. 2012;16:235. doi: 10.1186/cc11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selby NM, Kolhe NV, McIntyre CW, et al. Defining the cause of death in hospitalised patients with acute kidney injury. PLoS One. 2012;7:e48580. doi: 10.1371/journal.pone.0048580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent JL. Acute kidney injury, acute lung injury and septic shock: how does mortality compare? Contrib Nephrol. 2011;174:71–77. doi: 10.1159/000329238. [DOI] [PubMed] [Google Scholar]
- 14.Clark E, Wald R, Levin A, et al. Timing the initiation of renal replacement therapy for acute kidney injury in Canadian intensive care units: A multicentre observational study. Can J Anaesth. 2012;59:861–870. doi: 10.1007/s12630-012-9750-4. [DOI] [PubMed] [Google Scholar]
- 15.Zeyed YF, Bastarache JA, Matthay MA, et al. The severity of shock is associated with impaired rates of net alveolar fluid clearance in clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L550–L555. doi: 10.1152/ajplung.00190.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janisch T, Wendt J, Hoffmann R, et al. Expected and observed mortality in critically ill patients receiving initial antibiotic therapy. Wien Klin Wochenschr. 2012;124:775–781. doi: 10.1007/s00508-012-0276-0. [DOI] [PubMed] [Google Scholar]
- 17.Belkhouja K, Ben Romdhane K, Ghariani A, et al. Severe pneumococcal community-acquired pneumonia admitted to medical Tunisian ICU. J Infect Chemother. 2012;18:324–331. doi: 10.1007/s10156-011-0337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun CK, Yen CH, Lin YC, et al. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J Transl Med. 2011;9:118. doi: 10.1186/1479-5876-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YT, Sun CK, Lin YC, et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weil BR, Herrmann JL, Abarbanell AM, et al. Intravenous infusion of mesenchymal stem cells is associated with improved myocardial function during endotoxemia. Shock. 2011;36:235–241. doi: 10.1097/SHK.0b013e318225f6ae. [DOI] [PubMed] [Google Scholar]
- 22.Krasnodembskaya A, Samarani G, Song Y, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the Care and Use of Laboratory Animals. NIH Publication No. 85-23. Washington, DC: National Academy Press, 1996.
- 25.Chang CL, Leu S, Sung HC, et al. Impact of apoptotic adipose-derived mesenchymal stem cells on attenuating organ damage and reducing mortality in rat sepsis syndrome induced by cecal puncture and ligation. J Transl Med. 2012;10:244. doi: 10.1186/1479-5876-10-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung PH, Chang CL, Tsai TH, et al. Apoptotic adipose-derived mesenchymal stem cell therapy protects against lung and kidney injury in sepsis syndrome caused by cecal ligation puncture in rats. Stem Cell Res Ther. 2013;4:155. doi: 10.1186/scrt385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HH, Lin KC, Wallace CG, et al. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J Pineal Res. 2014;57:16–32. doi: 10.1111/jpi.12140. [DOI] [PubMed] [Google Scholar]
- 28.Yip HK, Chang YC, Wallace CG, et al. Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J Pineal Res. 2013;54:207–221. doi: 10.1111/jpi.12020. [DOI] [PubMed] [Google Scholar]
- 29.Sun CK, Leu S, Hsu SY, et al. Mixed serum-deprived and normal adipose-derived mesenchymal stem cells against acute lung ischemia-reperfusion injury in rats. Am J Transl Res. 2015;7:209–231. [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai TH, Lin YC, Sun CK, et al. Prognostic value of circulating dead monocytes in patients with acute st-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiology. 2010;117:131–139. doi: 10.1159/000320208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.