This study investigated the role of hypoxic-conditioned media (HCM) from adipose-derived stem cells (ASCs). Hypoxic preconditioning of ASCs increased expression of mediators promoting anti-inflammatory and regenerative responses. The liver regenerative effects of HCM appear to be mediated by persistent and uninhibited expression of signal transducer and activator of transcription 3 in the liver, which results from decreased expression of suppressor of cytokine signaling 3.
Keywords: Liver regeneration, Hypoxia, SOCS3 protein, STAT3 transcription factor, Secretome, Adipose tissue-derived stem cell, Mesenchymal stem cell
Abstract
Adipose-derived stem cells (ASCs) mainly exert their function by secreting materials that are collectively termed the secretome. Despite recent attention to the secretome as an alternative to stem cell therapy, the culture conditions for generating optimal secretome contents have not been determined. Therefore, we investigated the role of hypoxic-conditioned media (HCM) from ASCs. Normoxic-conditioned media (NCM) and HCM were obtained after culturing ASCs in 20% O2 or 1% O2 for 24 hours, respectively. Subsequently, partially hepatectomized mice were infused with saline, control medium, NCM, or HCM, and then sera and liver specimens were obtained for analyses. Hypoxia (1% O2) significantly increased mRNA expression of mediators from ASCs, including interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF). HCM infusion significantly increased the number of Ki67-positive cells in the liver (p < .05). HCM infusion significantly increased phospho-signal transducer and activator of transcription 3 (STAT3) and decreased suppressor of cytokine signaling 3 (SOCS3) expression in the liver (p < .05). To determine the role of IL-6 in liver regeneration, we then performed IL-6 RNA interference study. Conditioned media (CM) obtained from ASCs, which were transfected with either siIL-6 or siControl, were administered to partially hepatectomized mice. The siIL-6 CM groups exhibited lower liver proliferation (Ki67-positive cells) and markers of regeneration (protein expression of proliferating cell nuclear antigen, p-STAT3, HGF, and VEGF and liver weights) than the siControl CM groups (p < .05). Taken together, hypoxic preconditioning of ASCs increased expression of mediators promoting anti-inflammatory and regenerative responses. The liver regenerative effects of HCM appear to be mediated by persistent and uninhibited expression of STAT3 in the liver, which results from decreased expression of SOCS3.
Significance
In this study, it was found that treatment with the medium from hypoxic-preconditioned adipose-derived stem cells (ASCs) increased the viability of hepatotoxic hepatocytes and enhance liver regeneration in partially hepatectomized mice. In addition, the researchers first revealed that the hepatoprotective effects of hypoxic-conditioned media are mediated by persistent and uninhibited expression of signal transducer and activator of transcription 3 in the liver, which result from a decreased expression of suppressor of cytokine signaling 3. Therefore, the hypoxic preconditioning of ASCs is expected to play a crucial role in regenerative medicine by optimizing the production of a highly effective secretome from ASCs.
Introduction
Optimizing liver regeneration following resection is a prerequisite for avoiding hepatic insufficiency and subsequent morbidity and mortality. Recent research suggests promise for enhancing liver regeneration using stem cells. Among various stem cell sources, mesenchymal stem cells (MSCs) are widely used because they are easily obtained, but they have moderate potential for self-renewal and differentiation [1]. MSCs can be obtained from diverse tissues such as the bone marrow (BM), connective tissue, muscle, and adipose [2]. MSCs derived from adipose tissue are termed adipose-derived stem cells (ASCs). Recently, ASC-based research has been increasing because of the abundance of fat tissue, ease of processing, long in vitro maintenance, and higher proliferative ability than BM-derived MSC [3].
Despite many potential benefits, clinical applications of stem cells have several limitations. One of the major limitations is the short residence of stem cells after transplantation; most are lost within only a few days [4–7]. Moreover, stem cells possess the potential for malignant transformation [8, 9]. Recently, to overcome such limitations, the use of the stem cell secretome has been actively investigated [10–15]. The secretome refers to the rich, complex set of molecules secreted by stem cells into the surrounding extracellular space [16]. These secreted molecules are released from ASCs either solitarily or in the form of extracellular vesicles (EVs). The EVs include exosome (diameter, 50–100 nm) and the larger microvesicle (diameter, 50–100 nm) [17]. Of note, EVs can transfer proteins and genetic materials, such as functional RNAs (mRNAs and microRNAs), to other cells [18–20]. The use of the secretome instead of stem cells is supported by the evidence that some principal mechanisms of action of stem cells are mediated by the secretome [1, 16, 21]. Conditioned medium (CM) obtained from MSCs contains high levels of cytokines and growth factors that help enhance tissue repair and regeneration in vitro and in vivo in experimental models [11, 22, 23].
Although ASCs are typically cultured in room air oxygen (20% oxygen) during in vitro experiments, the physiological oxygen tension in the stem cell niche is recognized to be considerably lower than 20% [24]. Reduced oxygen tension was reported to be a key determinant in maintaining stem cells at a primitive stage [25, 26]. Moreover, recent evidence suggests that hypoxic-conditioned MSCs improve angiogenesis and tissue regeneration [27, 28]. We previously demonstrated the therapeutic effects of the ASC secretome in various hepatic-failure models [12–14]. To extend this work, we investigated the advantages of hypoxic preconditioning of ASCs for the purpose of obtaining a secretome that exhibits increased therapeutic potential. Our investigations could help determine the optimal culturing conditions for obtaining a high-yield secretome that is most beneficial for liver recovery.
Methods
Hypoxic Preconditioning of ASCs
ASCs were fed with serum-free low-glucose Dulbecco’s modified Eagle’s medium. ASCs were then cultured under either normoxic or hypoxic conditions for 24 hours. Hypoxic preconditioning was achieved by placing the ASCs in a hypoxic chamber (MIC-101; Billups-Rothenberg Inc., San Diego, CA, http://www.brincubator.com) with a mixture of 1% O2, 5% CO2, and balanced N2 at 37°C for 24 hours. Each CM was then concentrated by 25-fold, using ultrafiltration units (Amicon Ultra-PL 3; Millipore, Bedford, MA, http://www.emdmillipore.com) with a 3-kDa cutoff. Concentrated CM, obtained under either normoxic or hypoxic culturing conditions, are herein termed normoxic-conditioned media (NCM) and hypoxic-conditioned media (HCM), respectively. NCM and HCM were stored at −80°C until use.
Partial Hepatectomy and Infusions of NCM or HCM
Animal studies were carried out in compliance with the guidelines of the Institute for Laboratory Animal Research in Korea. Eight-week-old male BALB/c mice (Damool Science, Daejeon, Korea) were used in this study. A 70% partial hepatectomy (PH) was performed [29].
Additional Experimental Procedures
More detailed and additional information on experimental procedures is given in the supplemental online data.
Results
Identification of ASC Characteristics
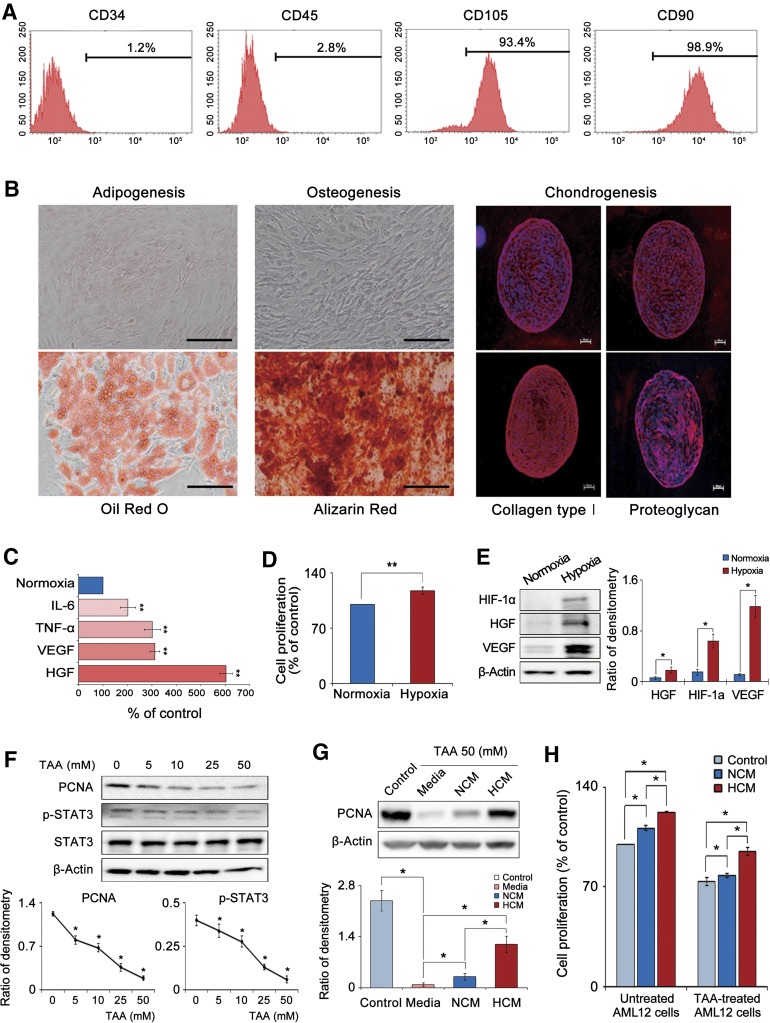
Cultured cells were assessed for expression of stem cell markers by flow cytometry and were verified to be negative for CD34 and CD45 (hematopoietic stem cell-associated markers) and positive for CD105 and CD90 (MSC-associated markers) (Fig. 1A). We next examined the multilineage differentiation potential of ASCs by inducing ASCs to differentiate into adipocytes, osteocytes, or chondrocytes. ASCs were successfully differentiated into these three cell types, confirming the multilineage differentiation potential of the cells (Fig. 1B).
Figure 1.
Characterization of adipose-derived stem cells (ASCs) and in vitro effect of HCM on mouse hepatocyte AML12 cells. (A): Characterization of ASCs using flow cytometry. Our cultured cells were negative for CD34 and CD45 (hematopoietic stem cell-associated markers) and positive for CD105 and CD90 (mesenchymal stem cell-associated markers). (B): Photomicrographs showing successful differentiation of ASCs into adipocytes, osteocytes, and chondrocytes; the differentiated cells were identified using four distinct staining methods (Oil Red O, Alizarin Red, collagen type 1, and proteoglycan). Scale bars = 100 μm. (C): Quantitative reverse transcription polymerase chain reaction analysis showing that hypoxic culturing of ASCs (1% O2) increased the mRNA expression of IL-6, TNF-α, HGF, and VEGF relative to the normoxic culturing (20% O2) (p < .05). (D): Cell proliferation assay showing that hypoxic culturing of ASCs significantly increased ASC proliferation (p < .05). (E): Western blot results showing that hypoxic culturing of ASCs significantly increased the protein levels of HIF-1α, HGF, and VEGF (p < .05). (F): Western blot results showing that TAA, a hepatotoxin, dose-dependently decreased the expression of PCNA, p-STAT, and STAT3. (G): Effect of HCM on the expression of a proliferation marker (PCNA) in the TAA-treated AML12 cells. Addition of the secretome (particularly, HCM) significantly increased PCNA expression (p < .05). (H): Effect of HCM on the proliferation of TAA-treated or untreated AML12 cells. HCM treatment significantly increased cell proliferations in both kinds of AML12 cells (p < .05). Values represent means ± SD of three independent experiments. ∗, p < .05; ∗∗, p < .01. Abbreviations: HCM, hypoxic-conditioned media; HGF, hepatocyte growth factor; HIF-1α, hypoxia-inducible factor-1α; IL-6, interleukin-6; NCM, normoxic-conditioned media; PCNA, proliferating cell nuclear antigen; p-STAT3, phospho-signal transducer and activator of transcription 3; STAT3, signal transducer and activator of transcription 3; TAA, thioacetamide; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Effects of Hypoxia on ASC Expression of Cytokines and Growth Factors
We investigated how hypoxia affected the expression of representative proinflammatory cytokines (interleukin-6 [IL-6] and tumor necrosis factor α [TNF-α]) and hepatic mitogens (hepatocyte growth factor [HGF] and vascular endothelial growth factor [VEGF]) in ASCs [30]. Real-time quantitative polymerase chain reaction (RT-qPCR) analysis revealed that hypoxia increased the mRNA expression of IL-6, TNF-α, HGF, and VEGF; mean fold changes were 2.0, 3.0, 3.1, and 6.0 relative to the control, respectively (p < .05) (Fig. 1C). Hypoxia also increased ASC proliferation 1.17-fold (p < .05) (Fig. 1D). Subsequently, Western blot results showed that after hypoxic culturing of ASCs, the protein levels of hypoxia-inducible factor (HIF)-1α, HGF, and VEGF were significantly higher than those under 20% O2 conditions (p < .05) (Fig. 1E).
HCM Effects on the Expression of a Cell Proliferation Marker (Proliferating Cell Nuclear Antigen) and AML12 Cell Proliferation
We collected and concentrated the CM from ASCs following either 20% or 1% O2 preconditioning. Next, NCM or HCM was added to AML12 cells that were undamaged or damaged (using the thioacetamide [TAA]). The AML12 cell line is the mouse (CD1 strain, line MT42) hepatocyte line, transgenic for human transforming growth factor α (a potent hepatocyte mitogen) facilitating long-term maintenance of the cells [31].
First, we investigated the dose-dependent effects of TAA on the expressions of proliferating cell nuclear antigen (PCNA), p-STAT3, and STAT3 in the AML12 cells (Fig. 1F). Based on this result and our previous investigation [12], 50 mM TAA, which reduced the expression of PCNA and p-STAT3 considerably, was used to establish the damaged AML12 cell model. Compared with control cells, damaged AML12 cells expressed lower levels of PCNA; however, addition of the secretome (particularly HCM) markedly increased PCNA expression (Fig. 1G). Moreover, HCM treatment significantly increased cell proliferations in both undamaged and damaged AML12 cells (p < .05) (Fig. 1H).
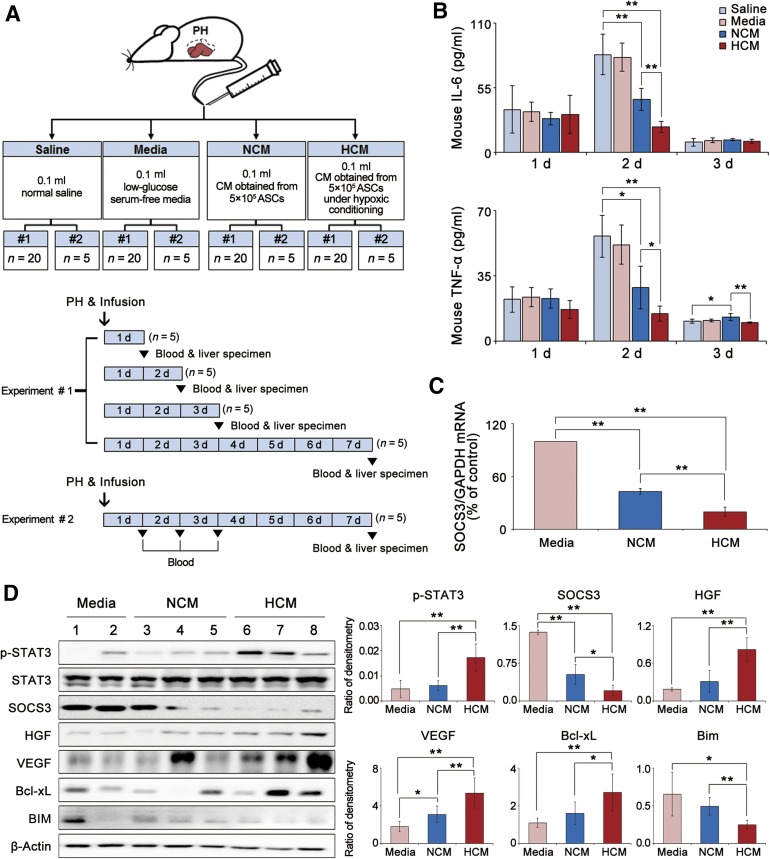
HCM Effects on Inflammation and Protein Expression in the Liver of Partially Hepatectomized Mice
Partially hepatectomized mice were infused with saline, medium, NCM, or HCM (Fig. 2A). We first investigated the effects of HCM infusion on the serum levels of proinflammatory cytokines (IL-6 and TNF-α). Enzyme-linked immunosorbent assay (ELISA) showed that secretome infusions (particularly HCM infusion) significantly lowered the concentrations of these cytokines than did saline or medium infusion, particularly at 2 days after infusion (p < .05) (Fig. 2B).
Figure 2.
HCM effects on the serum cytokines and protein expression in the liver of partially hepatectomized mice. (A): Partially hepatectomized mice were infused with saline, medium, NCM, or HCM. Each group included 25 mice (total, 100 mice). Our study comprised two sets (1 and 2) of experiments. In the first set (1; n = 20 in each group), both blood and liver specimens were simultaneously obtained after sacrificing on postoperative days 1, 2, 3, and 7. In the second set (2; n = 5 in each group), serum samples were consecutively obtained on postoperative days 1, 2, 3, and 7 to determine the changes of aspartate transaminase and alanine transaminase, and liver specimens were obtained on postoperative day 7. (B): Enzyme-linked immunosorbent assay analysis for the serum levels of IL-6 and TNF-α in each group on 1, 2, and 3 days after infusion. Secretome infusions (particularly HCM infusion) significantly lowered these concentrations than did saline or medium infusion, particularly on 2 days after infusion (p < .05). (C): Quantitative reverse transcription polymerase chain reaction analysis for the mRNA levels SOCS3 in the liver specimens 1 day after infusion. HCM infusion significantly reduced SOCS3 mRNA expression in the liver specimens than did control medium infusion (p < .05). (D): Left: Western blot analysis of the markers of STAT3 signaling (p-STAT3 and STAT3), liver regeneration (HGF and VEGF), and apoptosis (Bcl-xL and BIM) in the liver specimens 2 days after infusion. Right: Relative densities of these markers in each group. HCM infusion significantly increased the levels of p-STAT3, HGF, VEGF, and Bcl-xL, and decreased the levels of SOCS3 and BIM (all p < .05). Values represent means ± SD. ∗, p < .05; ∗∗, p < .01. Abbreviations: ASC, adipose-derived stem cell; Bcl-xL, B-cell lymphoma-extra large; BIM, Bcl-2-like protein 11; CM, conditioned media; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HCM, hypoxic-conditioned media; HGF, hepatocyte growth factor; interleukin-6, IL-6; NCM, normoxic-conditioned media; PH, partial hepatectomy; p-STAT3, phospho-signal transducer and activator of transcription 3; SOCS3, suppressor of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Next, we measured the expression of signaling intermediates that are known to be associated with liver regeneration or cell proliferation [30]. Liver specimens obtained at 24 and 48 hours after infusion were used for the analyses. RT-qPCR results demonstrated that HCM infusion reduced SOCS3 mRNA expression in the liver specimens to a greater extent than did control medium infusion at 24 hours after infusion (p < .05) (Fig. 2C). In Western blot assays, we investigated the expression of growth factors (HGF and VEGF) and markers for STAT3 signaling (STAT3, p-STAT3, and SOCS3), antiapoptosis (B-cell lymphoma-extra large [Bcl-xL]), and apoptosis (Bcl-2-like protein 11 [BIM]) in the liver specimens (Fig. 2D). HCM infusion significantly increased the phosphorylation of STAT3 and decreased SOCS3 at 48 hours after infusion (p < .05). HCM infusion also increased the expression of HGF, VEGF, and Bcl-xL, and it decreased the expression of BIM (p < .05).
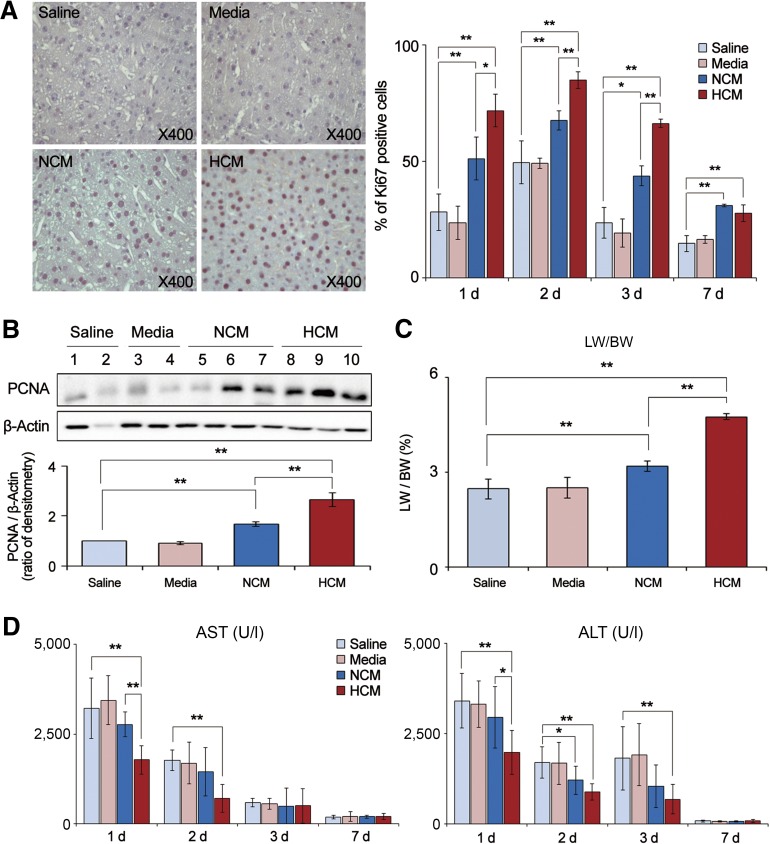
HCM Effects on Liver Regeneration and Hepatic Function in Partially Hepatectomized Mice
We investigated the effects of HCM infusion on liver regeneration, which was estimated by means of (a) Ki67 immunohistochemistry, (b) PCNA Western blot analysis, and (c) calculation of the ratio of liver weight to body weight (LW/BW) [32]. Cells labeled for Ki67 are considered to be proliferative [33]. The number of Ki67-positive cells in the HCM group was significantly higher than that in the NCM group on days 1, 2, and 3 after infusion (Fig. 3A) (p < .05). Next, Western blot analysis of liver specimens revealed that the HCM group expressed the highest levels of PCNA, again followed by the NCM group (p < .05) (Fig. 3B). Finally, the LW/BW value was also highest for the HCM group, reflecting the greatest liver regeneration at 7 days after infusion (p < .05) (Fig. 3C).
Figure 3.
HCM effects on liver regeneration and hepatic function in partially hepatectomized mice. (A): Left: Immunohistochemical stain of Ki67 (a proliferation marker) in the liver specimens of each group 1 day after infusion. Right: Percentage of Ki67 positive cells in each group on 1, 2, 3 and 7 days after infusion. HCM group showed the highest number of Ki67-positive cells, which was followed by the NCM group on days 1, 2, and 3 after infusion (p < .05). (B): Western blot analysis for expression of PCNA (a proliferation marker) on 2 days after infusion. HCM group expressed the highest levels of PCNA, again followed by the NCM group (p < .05). (C): Comparison of LW/BW of each group 7 days after infusion. The LW/BW value was also highest for the HCM group, reflecting the greatest liver regeneration 7 days after infusion (p < .05). (D): Serology tests of AST and ALT in each group. HCM infusion significantly decreased the serum levels of both (AST and ALT) 1 and 2 days after infusion (p < .05) and also significantly decreased the ALT level 3 days after infusion (p < .05). In the experiment presented in (A–C), each group included 20 mice at each time point (80 mice in total) and in the serologic tests. Each group included 5 mice (20 mice in total). Values represent means ± SD. ∗, p < .05; ∗∗, p < .01. Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; HCM, hypoxic-conditioned media; LW/BW, liver weight to body weight; NCM, normoxic-conditioned media; PCNA, proliferating cell nuclear antigen.
We next evaluated the effects of HCM infusion on two serum biochemical parameters (aspartate transaminase [AST] and alanine transaminase [ALT] levels) known to reflect hepatic function (Fig. 3D). HCM infusion significantly decreased the serum levels of both (AST and ALT) 1 and 2 days after infusion (p < .05) and also significantly decreased the ALT level 3 days after infusion (p < .05).
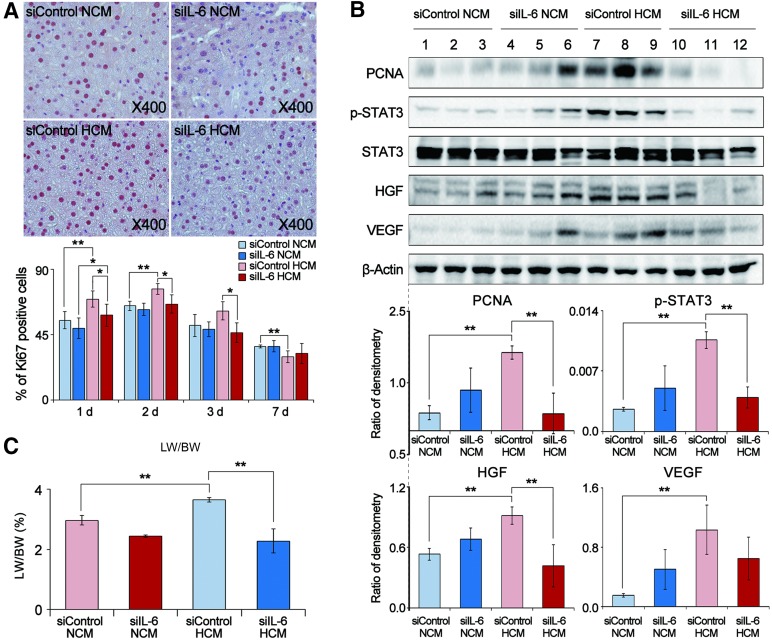
IL-6 RNA Interference Study Using IL-6-Knockdown ASCs in the Partially Hepatectomized Mice
IL-6 plays an essential role in liver regeneration and mediates its function via by STAT3 activation [34]. We thus investigated whether and how HCM enhances liver regeneration through IL-6, using an IL-6 RNA interference study. ASCs were transfected with either negative-control siRNA or IL-6 siRNA and then cultured under either 20% or 1% O2 conditions. Subsequently, partially hepatectomized mice were administrated the CM obtained from these ASCs (siControl NCM, siIL-6 NCM, siControl HCM, and siIL-6 HCM). Liver specimens were investigated by Ki67 immunohistochemistry, Western blot analysis, and liver weight analyses.
The siIL-6 HCM (or NCM) group showed lower numbers of Ki67-positive cells than did the siContrl HCM (or NCM) group (p < .05) (Fig. 4A). Western blot results demonstrated that the levels of PCNA, p-STAT3, HGF, and VEGF were lower in the siIL-6 HCM (or NCM) group than in the siControl HCM (or NCM) group (p < .05) (Fig. 4B). Finally, the LW/BW value was lower for the siIL-6 HCM (or NCM) group than for the siControl HCM (or NCM) group (p < .05) (Fig. 4C). Collectively, these results support the hypothesis that IL-6 plays a crucial role in mediating the secretome effects on liver regeneration by way of the Janus kinase (JAK)/STAT3 signaling pathway activation (Fig. 5).
Figure 4.
Effects of HCM obtained from IL-6-knockdown adipose-derived stem cells (ASCs) on liver regeneration in partially hepatectomized mice. (A): Top: Effect of IL-6 knockdown ASC for immunohistochemical stain of Ki67 (a proliferation marker) in the liver specimens of each group 1 day after infusion. Bottom: Percentage of Ki67 positive cells in each group 1, 2, 3, and 7 days after infusion. The siIL-6 HCM (or NCM) group showed lower numbers of Ki67-positive cells than did the siContrl HCM (or NCM) group. (B): Top: Western blot results showing the effect of IL-6 knockdown ASCs for expression of various markers in the liver specimens 2 days after infusion. Bottom: Relative densities of these markers in each group. The levels of PCNA, p-STAT3, HGF, and VEGF were lower in the siIL-6 HCM (or NCM) group than those in the siControl HCM (or NCM) group. (C): Comparison of LW/BW of each group 7 days after infusion; in this set of experiments, each group included 20 mice (total 80 mice). The LW/BW value was lower for the siIL-6 HCM (or NCM) group than for the siControl HCM (or NCM) group (p < .05). Values represent means ± SD. ∗, p < .05; ∗∗, p < .01. Abbreviations: HCM, hypoxic-conditioned media; HGF, hepatocyte growth factor; IL-6, interleukin-6; LW/BW, liver weight to body weight; NCM, normoxic-conditioned media; PCNA, proliferating cell nuclear antigen; p-STAT3, phospho-signal transducer and activator of transcription 3; STAT3, signal transducer and activator of transcription 3; VEGF, vascular endothelial growth factor.
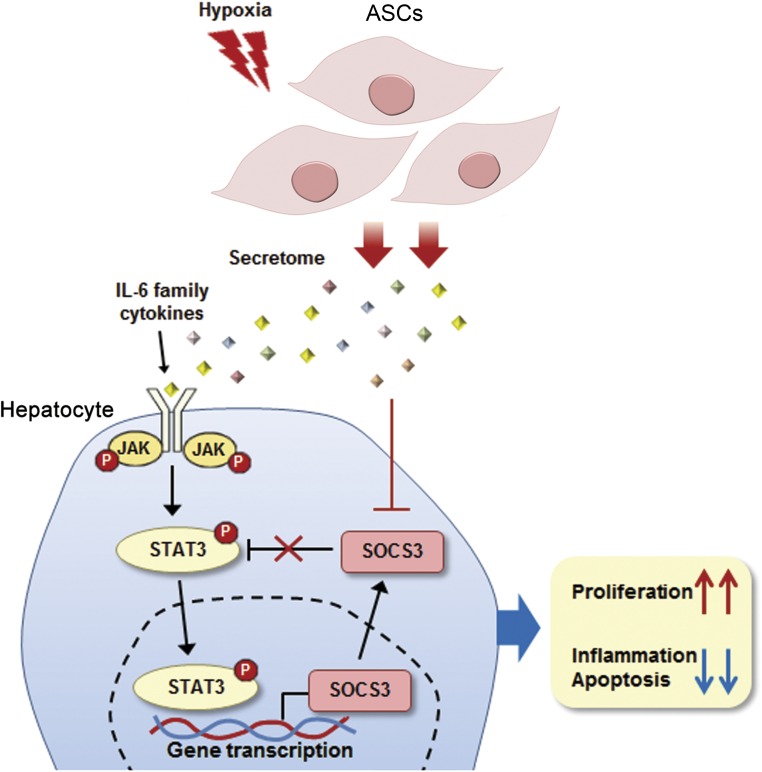
Figure 5.
Proposed mechanisms by which hypoxic-conditioned media (HCM) promotes regeneration and reduces inflammation and apoptosis of liver cells. After partial hepatectomy, the STAT3 signaling pathway plays a pivotal role in regulating the proliferation of hepatocytes. Activated STAT3 also induces the expression of SOCS3, which terminates the STAT3 signaling cascade and forms a negative-feedback loop. We propose that HCM infusion could induce persistent and uninhibited expression of STAT3 by decreasing the expression of SOCS3 in the liver. Abbreviations: ASCs, adipose-derived stem cells; IL-6, interleukin-6; JAK, Janus kinase; STAT3, signal transducer and activator of transcription; SOCS3, suppressor of cytokine signaling 3.
Discussion
In this study, we showed that hypoxic preconditioning led to substantial alterations in the expression of various molecules in ASCs, including an increase in the expression of proinflammatory markers (IL-6 and TNF-α) and hepatocyte mitogens (HGF and VEGF). In the in vitro experiment using TAA-treated AML12 cells, hypoxic culturing of ASCs induced higher cell proliferation and PCNA expression than normoxic culturing. In the in vivo experiment using partially hepatectomized mice, HCM infusion provided higher therapeutic potential in terms of (a) promoting liver regeneration, (b) inhibiting the proinflammatory cytokines, and (c) reducing the abnormally elevated liver enzymes. HCM infusion significantly promoted STAT3 phosphorylation and suppressed SOCS3 expression in the liver after hepatectomy. In addition, IL-6 RNA interference study demonstrated that the inhibition of JAK/STAT3 signaling in ASCs by siIL-6 significantly attenuated the beneficial effect of HCM on liver regeneration. We also found that secretome (particularly HCM) administration significantly reduces the expression of SOCS3 in liver cells. Taken altogether, our study suggests that HCM treatment increases the liver’s regenerative potential in part through the induction of persistent and uninhibited STAT3 activation.
The secretome can include a diverse array of materials, such as serum proteins, growth factors, angiogenic factors, hormones, cytokines, extracellular matrix proteins, extracellular matrix proteases, hormones, lipid mediators, and genetic materials [35, 36]. The secretome used in this study was the 25-fold concentrated CM, which had been generated by centrifugation (at 100,000 g for 2 hours) using ultrafiltration units with a 3-kDa-molecular-weight cutoff. Because cells and larger particles are removed by the centrifugal forces, smaller molecules and exosomes are precipitated and incorporated into the secretome content. Exosomes contain a number of proteins, mRNAs, microRNAs, and lipid molecules important for intercellular communication [37]. Once secreted, exosomes can either be taken up by nearby target cells or travel to more distant sites through the blood and possibly other biological fluids. Although we did not identify exosomes, we believe our secretome contains a substantial amount of exosomes because our way of collecting secretome is in line with the way of obtaining exosome [17, 37].
Intriguingly, although hypoxia increased the expression of IL-6 in ASCs, HCM infusion significantly reduced the serum level of IL-6 in the partially hepatectomized mice. PH induces systemic inflammation, which involves the rise of proinflammatory cytokines, such as IL-6 and TNF-α, most of which are released from the liver cells (i.e., Kupffer cells) [38]. ASCs exhibit anti-inflammatory and immunomodulatory properties via a paracrine mechanism [39]. In response to inflammatory signals, ASCs secrete an array of growth factors and anti-inflammatory proteins, which mediate complex feedback mechanisms with various host immune cells [40–43]. The key immunomodulatory cytokines include prostaglandin 2, transforming growth factor β1, HGF, stromal cell-derived factor 1, nitrous oxide, and soluble TNF-α [39]. The findings of previous studies suggest that although individual mediators in the secretome perform diverse functions, their collective effects promote anti-inflammatory, immunomodulatory, and tissue-reparative responses [1, 15, 16, 44–46]. Therefore, it appears that the proinflammatory IL-6, which had been increased during PH, was decreased by the anti-inflammatory and immunomodulatory effect of HCM.
In addition, IL-6 is a pleiotropic cytokine that can exert either proinflammatory or anti-inflammatory effects [47]. The former is mediated by coupling with soluble IL-6R (sIL-6R), and the latter is mediated by membrane-bound IL-6 receptor (mIL-6R) [47, 48]. Whereas proinflammatory sIL-6R is widely present in body fluids, such as blood or urine, anti-inflammatory mIL-6R is expressed only by a few cell types in the body, including hepatocytes and certain leukocytes. Anti-inflammatory activities of IL-6 include STAT3-dependent regulation of hepatocyte proliferation and the induction of the hepatic acute-phase response [47, 48]. In our experiment, HCM infusion increased STAT3 phosphorylation in the liver. Besides IL-6, other IL-6 family cytokines similarly activate liver regeneration via JAK/STAT3 signaling pathway [49–51]. IL-6 family cytokines include IL-6, IL-11, leukemia inhibitory factor, oncostatin M, ciliary inhibitory factor, cardiotropin-1, cardiotrophin-like related cytokine and stimulating neurotrophin-1/B-cell stimulating factor 3, neuropoietin, IL-27, and IL-31 [50]. Further study is required to determine the levels of other IL-6 family cytokines in HCM.
Of note, we found that secretome (particularly HCM) administration significantly reduces the SOCS3 expression in liver cells. Because the JAK/STAT signaling pathway has considerable plasticity, its deregulatory process could lead to chronic inflammation diseases or tumorigenesis. SOCS3 protein is not only a negative regulator of the JAK/STAT signaling pathway, but it also regulates other signaling pathways via cross-talk [52]. SOCS3 can directly interact with the phosphorylated JAKs and inhibit JAK kinases activation [53]. Activated STAT3 induces the expression of SOCS3, which, in turn, terminates the JAK/STAT signaling cascade, thus forming a negative-feedback loop that allows the cell to return to its basal (unstimulated) state [54]. Therefore, it appears that secretome (particularly HCM) infusion increases the SOCS3 expression and, thus, the persistent and uninhibited expression of STAT3 by increased SOCS3 effectively ameliorates liver injury by promoting liver regeneration and decreasing inflammation and apoptosis.
Our study has several limitations. First, because our research focused primarily on the HCM effect on liver regeneration, our results cannot be applied to all the liver failures of various etiologies. Although our research includes an in vitro toxin-induced hepatic failure model, it does not include the validation of HCM effect in the in vivo toxic hepatic failure model. Accordingly, our investigation can be potentially applied to the clinical situations, such as split or living donor liver transplantation or liver resection, where acquiring sufficient liver volume is crucial. Next, we did not identify the components of secretome that have the liver regenerative potential. The identifying task would be difficult and time-consuming because it requires investigation of a diverse array of substances, including soluble molecules, exosomes, and microRNAs. Further in-depth studies are necessary to identify the specific factors related to liver regeneration and hepatic recovery in the secretome.
Conclusion
Our study showed that hypoxic culturing caused substantial changes in the expression of molecules in ASCs, including an increase in the markers for proinflammation and regeneration (HGF and VEGF). HCM infusion exerted a stronger therapeutic effect in partially hepatectomized mice, which was reflected by (a) improved liver regeneration, (b) diminished serum concentrations of proinflammatory cytokines (IL-6 and TNF-α), and (c) reduced levels of upregulated liver enzymes (AST and ALT). These effects of HCM appear to be mediated by the persistent and uninhibited expression of STAT3, which resulted from a reduction of SOCS3 expression in the liver. Therefore, we expect hypoxic preconditioning of ASCs to play a crucial role in future strategies used in regenerative medicine by serving as an approach for optimizing the production of a highly effective secretome from ASCs.
Supplementary Material
Acknowledgments
We thank Hurim BioCell Company for providing human ASCs. We are grateful to Ok-Hee Kim and Woo Joo Jeong for technical assistance. We thank Professor Jong-Hoon Kim and his colleagues at Korea University for their helpful discussions regarding our experiments. This work was supported by The Catholic University of Korea, Daejeon St. Mary’s Hospital, through a Clinical Research Institute Grant (CMCDJ-P-2013-005).
Author Contributions
S.C.L.: collection and/or assembly of data, data analysis and interpretation, and manuscript writing; H.J.J.: design and help with in vitro and in vivo experiments, data analysis and interpretation; S.K.L.: design and help with in vitro and in vivo experiments, collection and/or assembly of data, data analysis and interpretation; S.-J.K.: conception and design, data analysis and interpretation, financial support, and final approval of manuscript.
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banas A, Teratani T, Yamamoto Y, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70–77. doi: 10.1111/j.1440-1746.2008.05496.x. [DOI] [PubMed] [Google Scholar]
- 3.Puglisi MA, Saulnier N, Piscaglia AC, et al. Adipose tissue-derived mesenchymal stem cells and hepatic differentiation: Old concepts and future perspectives. Eur Rev Med Pharmacol Sci. 2011;15:355–364. [PubMed] [Google Scholar]
- 4.Assmus B, Honold J, Schächinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 5.Lee TJ, Bhang SH, Yang HS, et al. Enhancement of long-term angiogenic efficacy of adipose stem cells by delivery of FGF2. Microvasc Res. 2012;84:1–8. doi: 10.1016/j.mvr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K, Murtuza B, Beauchamp JR, et al. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004;18:1153–1155. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 7.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 8.Rubio D, Garcia S, Paz MF, et al. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS One. 2008;3:e1398. doi: 10.1371/journal.pone.0001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio D, Garcia-Castro J, Martín MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing [retracted in: Cancer Res 2010:81:6682] PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouraschen SM, Pan Q, de Ruiter PE, et al. Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev. 2012;21:2410–2419. doi: 10.1089/scd.2011.0560. [DOI] [PubMed] [Google Scholar]
- 12.Lee SC, Jeong HJ, Lee SK, et al. Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome. Stem Cell Res Ther. 2015;6:75. doi: 10.1186/s13287-015-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SC, Kim JO, Kim SJ. Secretome from human adipose-derived stem cells protects mouse liver from hepatic ischemia-reperfusion injury. Surgery. 2015;157:934–943. doi: 10.1016/j.surg.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Lee SK, Lee SC, Kim SJ. A novel cell-free strategy for promoting mouse liver regeneration: Utilization of a conditioned medium from adipose-derived stem cells. Hepatol Int. 2015;9:310–320. doi: 10.1007/s12072-014-9599-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee SM, Lee SC, Kim SJ. Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res. 2014;188:280–289. doi: 10.1016/j.jss.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 16.Makridakis M, Roubelakis MG, Vlahou A. Stem cells: Insights into the secretome. Biochim Biophys Acta. 2013;1834:2380–2384. doi: 10.1016/j.bbapap.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Lavoie JR, Rosu-Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie. 2013;95:2212–2221. doi: 10.1016/j.biochi.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 21.Yoon BS, Moon J-H, Jun EK, et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:887–902. doi: 10.1089/scd.2009.0138. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 23.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanovic Z, Belloc F, Faucher JL, et al. Hypoxia maintains and interleukin-3 reduces the pre-colony-forming cell potential of dividing CD34(+) murine bone marrow cells. Exp Hematol. 2002;30:67–73. doi: 10.1016/s0301-472x(01)00765-2. [DOI] [PubMed] [Google Scholar]
- 25.Ivanović Z, Dello Sbarba P, Trimoreau F, et al. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion. 2000;40:1482–1488. doi: 10.1046/j.1537-2995.2000.40121482.x. [DOI] [PubMed] [Google Scholar]
- 26.Valorani MG, Montelatici E, Germani A, et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012;45:225–238. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Gao J, Yuan Y, et al. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. 2013;37:551–560. doi: 10.1002/cbin.10097. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Yin S, Zhang W, et al. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013;4:83. doi: 10.1186/scrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greene AK, Puder M. Partial hepatectomy in the mouse: Technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- 30.Fujiyoshi M, Ozaki M. Molecular mechanisms of liver regeneration and protection for treatment of liver dysfunction and diseases. J Hepatobiliary Pancreat Sci. 2011;18:13–22. doi: 10.1007/s00534-010-0304-2. [DOI] [PubMed] [Google Scholar]
- 31.Wu JC, Merlino G, Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci USA. 1994;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bockhorn M, Goralski M, Prokofiev D, et al. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138:291–299. doi: 10.1016/j.jss.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 33.Bullwinkel J, Baron-Lühr B, Lüdemann A, et al. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206:624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 34.Streetz KL, Luedde T, Manns MP, et al. Interleukin 6 and liver regeneration. Gut. 2000;47:309–312. doi: 10.1136/gut.47.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsuda T, Kosaka N, Takeshita F, et al. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 36.Ranganath SH, Levy O, Inamdar MS, et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196–2211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Drucker C, Gewiese J, Malchow S, et al. Impact of interleukin-6 classic- and trans-signaling on liver damage and regeneration. J Autoimmun. 2010;34:29–37. doi: 10.1016/j.jaut.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: Novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 41.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 42.Weiss DJ, Bertoncello I, Borok Z, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yagi H, Soto-Gutierrez A, Kitagawa Y, et al. Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant. 2010;19:823–830. doi: 10.3727/096368910X508942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 45.Atoui R, Chiu RC. Concise review: Immunomodulatory properties of mesenchymal stem cells in cellular transplantation: Update, controversies, and unknowns. Stem Cells Transl Med. 2012;1:200–205. doi: 10.5966/sctm.2011-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trento C, Dazzi F. Mesenchymal stem cells and innate tolerance: Biology and clinical applications. Swiss Med Wkly. 2010;140:w13121. doi: 10.4414/smw.2010.13121. [DOI] [PubMed] [Google Scholar]
- 47.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 49.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27(suppl 2):89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein C, Wüstefeld T, Assmus U, et al. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J Clin Invest. 2005;115:860–869. doi: 10.1172/JCI200523640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam SP, Luk JM, Man K, et al. Activation of interleukin-6-induced glycoprotein 130/signal transducer and activator of transcription 3 pathway in mesenchymal stem cells enhances hepatic differentiation, proliferation, and liver regeneration. Liver Transpl. 2010;16:1195–1206. doi: 10.1002/lt.22136. [DOI] [PubMed] [Google Scholar]
- 52.Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol. 2008;19:414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin Y, Liu W, Dai Y. SOCS3 and its role in associated diseases. Hum Immunol. 2015;76:775–780. doi: 10.1016/j.humimm.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 54.Babon JJ, Varghese LN, Nicola NA. Inhibition of IL-6 family cytokines by SOCS3. Semin Immunol. 2014;26:13–19. doi: 10.1016/j.smim.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.