The protective effects of mesenchymal stromal cell-derived extracellular vesicles (MSC-EVs) were studied in an ovine preclinical model of preterm hypoxic-ischemic brain injury. Systemic in utero administration of MSC-EVs improved brain function by reducing the total number and duration of seizures and by preserving baroreceptor reflex sensitivity. These functional protections were accompanied by a tendency to prevent hypomyelination. Cerebral inflammation remained unaffected by the MSC-EV treatment.
Keywords: Hypoxia-ischemia, Brain injury, Preterm, Mesenchymal stromal cells, Extracellular vesicles, Exosomes
Abstract
Preterm neonates are susceptible to perinatal hypoxic-ischemic brain injury, for which no treatment is available. In a preclinical animal model of hypoxic-ischemic brain injury in ovine fetuses, we have demonstrated the neuroprotective potential of systemically administered mesenchymal stromal cells (MSCs). The mechanism of MSC treatment is unclear but suggested to be paracrine, through secretion of extracellular vesicles (EVs). Therefore, we investigated in this study the protective effects of mesenchymal stromal cell-derived extracellular vesicles (MSC-EVs) in a preclinical model of preterm hypoxic-ischemic brain injury. Ovine fetuses were subjected to global hypoxia-ischemia by transient umbilical cord occlusion, followed by in utero intravenous administration of MSC-EVs. The therapeutic effects of MSC-EV administration were assessed by analysis of electrophysiological parameters and histology of the brain. Systemic administration of MSC-EVs improved brain function by reducing the total number and duration of seizures, and by preserving baroreceptor reflex sensitivity. These functional protections were accompanied by a tendency to prevent hypomyelination. Cerebral inflammation remained unaffected by the MSC-EV treatment. Our data demonstrate that MSC-EV treatment might provide a novel strategy to reduce the neurological sequelae following hypoxic-ischemic injury of the preterm brain. Our study results suggest that a cell-free preparation comprising neuroprotective MSC-EVs could substitute MSCs in the treatment of preterm neonates with hypoxic-ischemic brain injury, thereby circumventing the potential risks of systemic administration of living cells.
Significance
Bone marrow-derived mesenchymal stromal cells (MSCs) show promise in treating hypoxic-ischemic injury of the preterm brain. Study results suggest administration of extracellular vesicles, rather than intact MSCs, is sufficient to exert therapeutic effects and avoids potential concerns associated with administration of living cells. The therapeutic efficacy of systemically administered mesenchymal stromal cell-derived extracellular vesicles (MSC-EVs) on hypoxia-ischemia-induced injury was assessed in the preterm ovine brain. Impaired function and structural injury of the fetal brain was improved following global hypoxia-ischemia. A cell-free preparation of MSC-EVs could substitute for the cellular counterpart in the treatment of preterm neonates with hypoxic-ischemic brain injury. This may open new clinical applications for “off-the-shelf” interventions with MSC-EVs.
Introduction
Hypoxia-ischemia (HI) in the developing brain has been strongly correlated with morbidity and mortality in premature and full-term infants [1]. One of the induced pathologies is hypoxic ischemic encephalopathy (HIE), which is associated with adverse neurodevelopmental outcomes, resulting in enormous physical, psychological, and economic burdens [1, 2]. Currently, therapeutic intervention strategies for HIE are limited. Term and late-preterm neonates with moderate or severe HIE are eligible for 72 hours of therapeutic cooling, which has been shown to improve outcomes [1, 3, 4]. Preterm neonates born before 35 weeks’ gestational age, however, are still excluded from this therapy [5]. Thus, new treatment strategies are urgently needed.
In a preclinical animal model of HIE, we have demonstrated that systemic administration of mesenchymal stromal cells (MSCs) promoted functional recovery and prevented structural injury in the preterm brain after global hypoxia-ischemia [6]. These effects were, in part, attributable to reduction of neuroinflammatory processes coordinated by a splenic response [6]. However, the exact mechanisms of action of the exogenously delivered MSCs are not clear.
Initially, MSCs were considered to home to affected tissues and substitute lost cell types. Increasing evidence, however, indicates that, instead, MSCs exert their therapeutic effects in a paracrine manner via secretion of small extracellular vesicles (EVs) (i.e., exosomes 70–150 nm in diameter) and microvesicles (100–1,000 nm) [7–14], suggesting that administration of intact MSCs is not mandated to exert therapeutic effects. In comparison with MSCs, mesenchymal stromal cell-derived extracellular vesicles (MSC-EVs) have been shown to elicit similar biological effects after administration in various preclinical disease models, including models of kidney, cardiac, and brain injury [9, 15–21]. Recently, a therapy-refractory patient with graft-versus-host disease was successfully treated with allogeneic MSC-EVs with immune suppressive effects in vitro and in vivo without showing any side effects [22].
Thus, the aim of this study was to assess the therapeutic efficacy of MSC-EVs in preterm brain injury. We hypothesized that systemic administration of MSC-EVs, the paracrine mediators of MSCs, would be neuroprotective in hypoxic-ischemic injury in the preterm brain. We tested this hypothesis in a well-established preclinical animal model in which hypoxic-ischemic brain injury was induced by transient umbilical cord occlusion (UCO) in the preterm ovine fetus [23]. The therapeutic effects of systemic administration of MSC-EVs on brain function were determined by evaluation of seizure burden, a measure for cortical function, and by calculating baroreflex-mediated heart rate response (baroreceptor reflex sensitivity), which is needed to regulate blood pressure fluctuations, as a measurement for function of the brainstem. Structural injury was investigated by histological examination of the subcortical white matter. Anti-inflammatory effects were assessed using histological detection of microglia in the hippocampus and subcortical white matter, and of cerebral infiltration of T lymphocytes.
Materials and Methods
Study Approval
Experimental procedures and the study design were in line with institutional guidelines for animal experiments and approved by the Animal Ethics Committee of Maastricht University, The Netherlands.
Randomization and Blinding
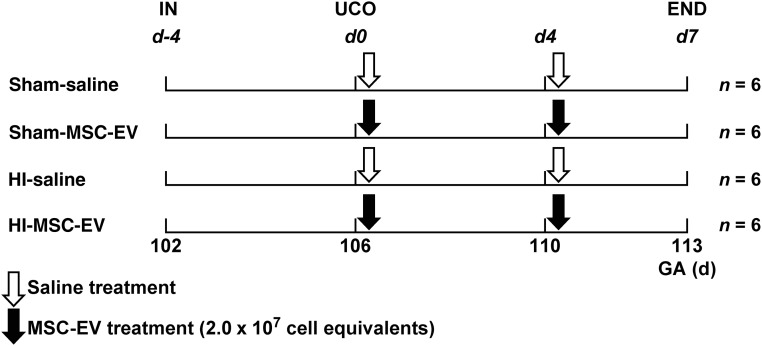
Singleton fetuses (n = 24) of time-mated Texel ewes were randomly assigned to 4 experimental groups: (a) sham umbilical cord occlusion, saline treatment (sham-SAL; n = 6), (b) sham umbilical cord occlusion, MSC-EV treatment (sham-MSC-EV; n = 6), (c) umbilical cord occlusion, saline treatment (HI-SAL; n = 6), and (d) umbilical cord occlusion, MSC-EV treatment (HI-MSC-EV; n = 6) (Fig. 1). The investigator performing the umbilical cord occlusions, including the sham occlusions, was blinded to treatment allocation. Tissue sampling and postmortem analyses were conducted in a blinded fashion.
Figure 1.
Study design. Fetuses were instrumented at gestational age 102 days. After a recovery period of 4 days, fetuses were subjected to 25 minutes of umbilical cord occlusion or sham occlusion (d0). One hour and 4 days (d4) after umbilical cord occlusion or sham occlusion, fetuses received either intravenous MSC-EVs (2.0 × 107 cell equivalents; closed arrow) or saline 0.9% (open arrow). After a 7-day reperfusion period, brain tissue was collected. Abbreviations: d, day; END, end of experiment; GA, gestational age; HI, hypoxia-ischemia; IN, instrumentation; MSC-EV, mesenchymal stem cell‐derived extracellular vesicle; UCO, umbilical cord occlusion.
A dropout of 16% (n = 4) was observed which was primarily restricted to the sham-MSC-EV group: (i.e., 3 animals of the sham-MSC-EV group and 1 animal of the HI-MSC-EV). Importantly, autopsy of these animals revealed that fetal death was exclusively caused by a single technical reason namely arterial catheter not in situ resulting in exsanguination and subsequent fetal death, thereby excluding any other cause.
Animals and Surgery
Singleton fetuses were surgically instrumented at 102 days of gestational age (term is approximately 147 days), as described previously [23] (Fig. 1). In short, fetuses were exposed through a midline laparotomy of the ewe. Umbilical vessel catheters (1.2 mm; Medtronic, Mansfield, MA, http://www.medtronic.com/covidien) were inserted in the femoral artery and brachial vein for blood pressure recordings, blood sampling, and administration of MSC-EVs. Three electrocardiogram (ECG) electrodes were attached to the fetal chest for cardiologic monitoring. Two pairs of custom-made, shielded, silver-tipped electroencephalogram (EEG) electrodes (Cooner Wire Co., Chatsworth, CA, http://www.coonerwire.com/) were placed bilaterally on the dura over the parasagittal cortex. An additional reference electrode was placed in the neck [23].
An inflatable vascular occluder (OCD16HD, 16 mm; In Vivo Metric, Healdsburg, CA, http://www.invivometric.com) was placed around the umbilical cord for induction of transient global HI.
An additional catheter was placed in the amniotic sac for recordings of amniotic pressure. All leads were exteriorized through a trocar hole in the maternal flank. Postoperatively, the sheep were housed individually with access to food and water ad libitum.
Experimental Design
After a 4-day recovery period, the fetuses (gestational age: 106 days; experimental day 0) were subjected to 25 minutes of sham or actual umbilical cord occlusion by rapid inflation of the vascular occluder with a predefined volume of sterile saline (Fig. 1).
Occlusion was confirmed by an acute drop in heart rate and a gradual decline of blood pressure. Furthermore, global hypoxia-ischemia was monitored with subsequent arterial blood gas analysis (data not shown).
Animals that were randomized to receive MSC-EV treatment received 1 aliquot of 4.0 × 107 cell equivalents divided into two boluses of 2.0 × 107 cell equivalents. The first bolus of 2.0 × 107 cell equivalents was administered 1 hour following sham or actual umbilical cord occlusion (UCO). The second bolus was administered 4 days after the hypoxic-ischemic insult. Control animals received an equal volume of sterile 0.9% NaCl intravenously at the designated time points. The fetuses were killed 7 days after sham or actual UCO and prepared for tissue sampling.
Data Acquisition and Analysis
Blood pressure, amniotic pressure, ECG, and EEG data were acquired and digitized by a custom-made Maastricht-Programmable Acquisition System unit (Maastricht Instruments BV, Maastricht, The Netherlands, http://www.maastrichtinstruments.nl) with IDEEQ software (Maastricht Instruments) and stored for off-line analysis, as described in detail previously [6, 23]. Briefly, following detection and removal of high-voltage (EEG signal >1,000 μV) and flat-line artifacts, raw signals were converted into amplitude-integrated EEG (aEEG) traces. The aEEG processing include an asymmetric band-pass filter that strongly attenuates activity below 2 Hz and above 15 Hz, semilogarithmic amplitude compression, and time compression. As in the neonatal intensive care unit, these aEEG traces were used to detect electrographic seizure activity, which is characterized by an abrupt rise in the lower and upper margin amplitude of the aEEG. Electrographic seizure activity with a duration of at least 10 seconds was annotated using aEEG/EEG traces and were performed by a neonatologist experienced in neonatal aEEG interpretation.
To quantify baroreflex-mediated heart rate response (baroreceptor reflex sensitivity), the ratio between the standard deviation of the mean heart rate variability and that of the mean systolic blood pressure was calculated, as reported previously [24, 25].
MSC-EVs
Human bone marrow-derived MSCs were raised from bone marrow from donors after informed consent according to the Declaration of Helsinki, as described previously [22]. Briefly, MSCs were expanded in MSC basal media (Pan Biotech, Aidenbach, Germany, http://www.pan-biotech.de) supplemented with 10% human thrombocyte lysate, 1% glutamine, and 1% penicillin-streptomycin (Thermo Fisher Scientific, Darmstadt, Germany, https://www.thermofisher.com). Their MSC nature was confirmed by flow cytometry with fluorescent labeled anti-CD44, anti-CD73, anti-CD90, anti-CD105, and anti-CD146 antibodies as positive markers, as well as with by anti-CD14, anti-CD31, and anti-CD45 antibodies as negative controls. In addition, their osteogenic and adipogenic differentiation potential was confirmed in conventional MSC differentiation assays. MSC-EVs were harvested from MSC-conditioned media from passage 3 onward, as described previously [22]. Briefly, media exchanges were performed every 48 hours. MSC-conditioned media were passed through a 0.22-µm filter membrane (TPP, Trasadingen, Switzerland, http://www.tpp.ch) and stored at −20°C until use. Upon processing, all conditioned media samples where thawed and processed at once.
After adding polyethylene glycol (PEG) and NaCl to a final concentration of 10% PEG 6000 volume per volume and 75 mM NaCl overnight incubation at 4°C, MSC-EVs were concentrated by low-speed centrifugation (30 minutes at 1,500 g). Pellets were washed in 0.9% NaCl twice and resuspended in 0.9% NaCl. MSC-EVs were stored as 1-ml aliquots, each containing the MSC-EV equivalents harvested from the 48-hour conditioned media of 4 × 107 cells. Aliquots were stored at −80°C until use. The obtained MSC-EV fraction was characterized as presented by Kordelas et al. [22] by nanoparticle tracking analyses (NTA) [26] and Western blot analysis (supplemental online Fig. 1), as described previously [22]. Briefly, protein concentrations were determined by the micro-BCA assay (Thermo Fisher Scientific). Concentrated MSC-EVs (5 µg) were treated with sample buffer (dithiothreitol, 0.1% SDS, 0.1 M Tris HCl, pH 7.0) and boiled for 5 minutes at 95°C. Samples were separated on 12% SDS-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes (Merck Millipore, Darmstadt, Germany, http://www.merckmillipore.com). Membranes were blocked in 5% skim milk in phosphate-buffered saline (PBS) followed by incubation with antibodies recognizing Tsg101 (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) or CD81 (Becton Dickinson, Franklin Lakes, NJ, http://www.bd.com). Subsequently, membranes were incubated with appropriate horseradish-peroxidase-conjugated secondary antibodies (Dianova, Hamburg, Germany, http://www.dianova.com). Visualization was accomplished by enhanced chemiluminescence (Thermo Fisher Scientific). Furthermore, test results showed MSC-EVs were negative for the presence of bacteria, viruses, and endotoxins.
Immunohistochemistry in the Brain
Right-side cerebral hemispheres underwent immersion fixation in 4% paraformaldehyde and subsequently were embedded in gelatin. Serial coronal sections (50 μm) were cut on a Leica VT 1200S vibrating microtome (Leica Biosystems, Nussloch, Germany, http://www.leicabiosystems.com). Free-floating sections at the level of midthalamus and posterior hippocampus were stained for ionized calcium binding adaptor molecule (IBA-1) (Wako Pure Chemical Industries, Osaka, Japan, http://www.wako-chem.co.jp) for microglia or for myelin basic protein (MBP) (Merck Millipore) for white matter and cleaved caspase-3 for apoptosis (Cell Signaling Technology, Boston, MA, http://www.cellsignal.com), as previously described [23, 27].
Paraffin embedded brain coronal sections at the level of the midthalamus and posterior hippocampus were stained for T cells with CD3 (Dako A0452; Dako, Glostrup, Denmark, http://www.dako.com), as reported previously [6]. Briefly, endogenous peroxidase was inactivated by incubation with 0.3% H2O2 dissolved in PBS. Before blocking of nonspecific binding with 5% bovine serum albumin and incubation with the primary CD3 antibody, antigen retrieval was performed in sodium citrate buffer (pH 6.0) using a microwave oven. Following overnight incubation with the primary antibody, sections were incubated with the appropriate secondary biotin-labeled antibody. Immunostaining was enhanced with Vectastain ABC peroxidase elite kit (PK-6200; Vector Laboratories, Burlingame, CA, https://www.vectorlabs.com) followed by nickel-intensified diaminobenzidine chromagen staining and counterstaining with 0.1% nuclear fast red.
Analysis Immunohistochemistry
Brain
Digital images of the hippocampus (IBA-1; magnification, ×20) and subcortical white matter (SCWM) (IBA-1, MBP, and caspase-3; magnification, ×100) were acquired on an Olympus AX-70 microscope (Olympus, Tokyo, Japan, http://www.olympus-global.com) equipped with a black-white digital camera.
Areal fractions of IBA-1 and MBP immunoreactivity, expressed as the percentage immunoreactivity of the total area using a standard threshold, were determined with Leica Qwin Pro V 3.5.1 software (Leica, Rijswijk, The Netherlands, http://www.leica-microsystems.com), as described previously [23].
Numbers of caspase-3-positive cells were determined with Leica Qwin Pro V 3.5.1 software and averaged to obtain cell numbers for caspase-3 per section.
For the analysis of CD3 staining in the SCWM, two coronal sections per animal were studied at the midthalamus and posterior hippocampus level. CD3-positive cells were counted at ×200 magnification in 10 fields of view on a Leica DM2000 microscope equipped with a digital camera, focused on cerebral vasculature. Averages per animal were expressed as cells per field of view.
Statistical Analysis
Animal characteristics and histological outcome parameters are shown as means with 95% confidence interval (CI). Comparisons between groups of all parameters were drawn with analysis of variance or with random intercept models in case of repeated measurements per animal (e.g., different sections per brain), as described previously [28]. Data or variables whose distributions were positively skewed were log-transformed for statistical testing. For interpretation purposes, averages on the log scale were transformed back to original scale and are presented as geometric means and corresponding 95% confidence intervals [6].
Seizure data, cumulative throughout the entire experiment, showed right-skewness due to the absence of seizures in non-HI groups that could not be corrected by log-transformation. Therefore, group comparisons were performed with Mann-Whitney tests. Seizure burden is represented as median and interquartile range (IQR). A value of p < .05 was considered statistically significant. All statistical analyses were performed with SPSS Statistics version 22 (IBM Corp., Armonk, NY, http://www-01.ibm.com).
Statistical analysis of baroreflex sensitivity was performed using a Bayesian multilevel model [29]. Statistical time series analysis was conducted using the Stan for R package, version 2.6.0, in R version 3.1.1 (https://www.r-project.org).
Results
Animal Characteristics
To assess the neuroprotective capacities of MSC-EVs, we randomized 24 preterm ovine fetuses in different experimental groups (Fig. 1). Fetuses were subjected to 25 minutes of global HI through UCO or sham UCO. MSC-EVs were administered 1 hour and 4 days after UCO (Fig. 1).
Brain to body weight ratios did not differ significantly between the experimental groups. Splenic weight relative to body weight, indicative of activation of the splenic inflammatory response [23], was significantly reduced after global HI (sham-SAL vs. HI-SAL; p < .05) and indicative of splenic involution, which is an indication of activation of the peripheral immune system [23, 30]. MSC-EVs prevented HI-induced splenic involution (HI-SAL vs. HI-MSC-EV; p < .05) (Table 1).
Table 1.
Animal characteristics
MSC-EVs Prevent Loss of Cortical Function and Baroreflex Sensitivity After Global HI
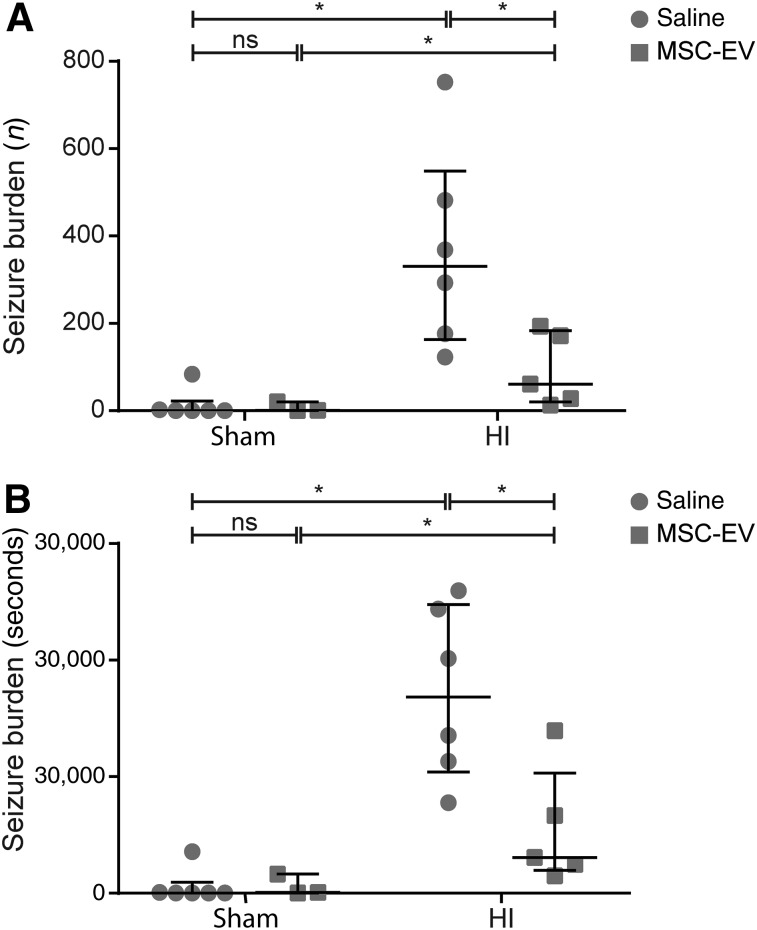
Electrographic seizure burden was assessed as a measure of cortical function and was investigated by aEEG for total numbers of seizures (Fig. 2A) and total amount of time-of-seizure activity (Fig. 2B). Global HI induced a profound increase in the cumulative number of seizures (sham-SAL vs. HI-SAL; p = .003) and the cumulative duration (seconds) of seizure activity (sham-SAL vs. HI-SAL; p = .002), which were reduced by MSC-EV treatment (cumulative number: HI-SAL vs. HI-MSC-EV, p = .021; cumulative duration: HI-SAL vs. HI-MSC-EV; p = .029). No differences in seizure burden between the sham conditions could by detected.
Figure 2.
MSC-EV treatment induced functional neuroprotection after global HI. Global HI caused a significant seizure burden, indicated by an increased total number (A) and duration (B) of seizures compared with sham-occluded animals. Administration of MSC-EVs reduced electrographic seizure number and duration compared with saline-treated animals. Medians ± interquartile ranges and levels of significance, which were calculated by Mann‐Whitney test, are depicted. ∗, p ≤ .05. Abbreviations: HI, hypoxia‐ischemia; MSC-EV, mesenchymal stem cell‐derived extracellular vesicle; ns, not significant.
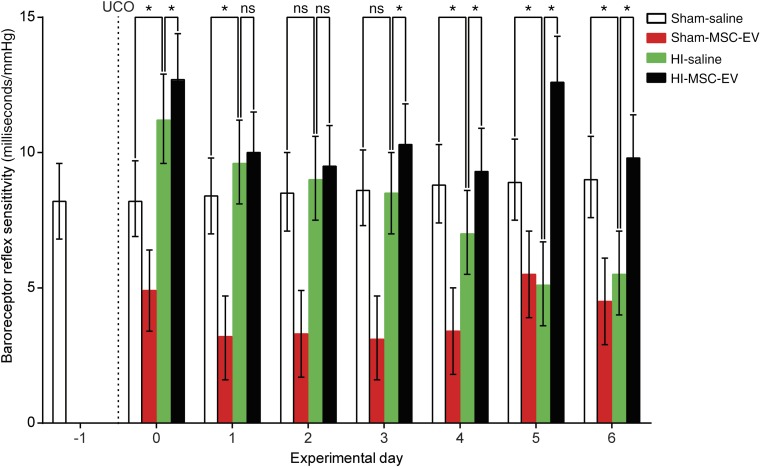
Brain stem function was assessed by analysis of baroreflex sensitivity (Fig. 3). The baroreflex is a vital part of the vascular autoregulatory system, ensuring adequate perfusion of the fetal brain upon disturbance of homeostasis [24]. Baroreceptor reflex sensitivity was reduced at experimental day 4 following global HI when compared with controls and remained compromised throughout the experiment. MSC-EV treatment prevented HI-induced compromise of the baroreceptor reflex sensitivity from experimental day 3 onward. Surprisingly, MSC-EV treatment significantly reduced baroreflex sensitivity in healthy controls.
Figure 3.
MSC-EVs prevented loss of baroreflex sensitivity. Global HI (green bars) caused a significant gradual decline of baroreflex sensitivity over time, which was prevented by MSC-EV treatment (black bars). Means ± 95% confidence intervals and levels of significance of the treatment effect (HI‐saline vs. HI‐MSC-EV) are depicted and were calculated by the Bayesian multilevel model. MSC-EV treatment significantly compromised baroreflex sensitivity in healthy controls (red bars). ∗, p ≤ .05. Abbreviations: HI, hypoxia‐ischemia; MSC-EV, mesenchymal stem cell‐derived extracellular vesicle; ns, not significant; UCO, umbilical cord occlusion.
MSC-EVs Partially Protect Against HI-Induced Hypomyelination, but Not Against Apoptosis
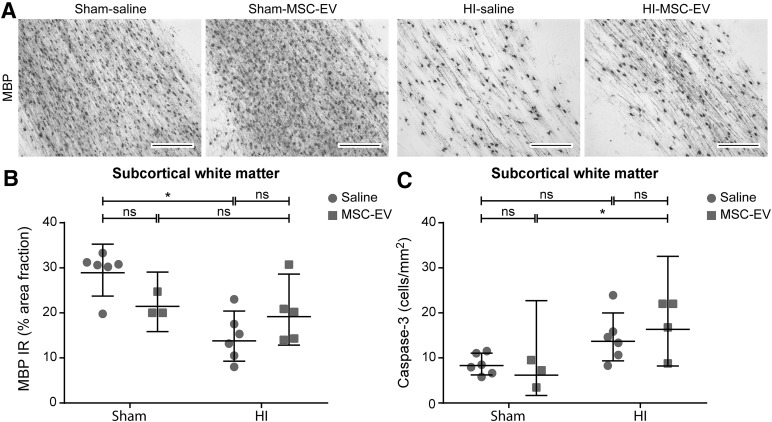
MBP immunoreactivity, which was analyzed to determine white matter injury, was assessed in the SCWM (Fig. 4A, 4B). Global HI resulted in marked hypomyelination in the SCWM, as indicated by a significant decrease of MBP immunoreactivity (sham-SAL vs. HI-SAL; p = .001). MSC-EV treatment showed a mild trend toward protection against hypomyelination; however, statistical significance was not reached (HI-SAL vs. HI-MSC-EV; p = .100).
Figure 4.
MSC-EVs reduced white matter injury after global HI but did not prevent HI-induced apoptosis. (A): Immunohistochemical MBP staining in the subcortical white matter of all four experimental groups. Magnification, ×100. Global HI induced marked hypomyelination in the subcortical white matter. MSC-EVs tended to prevent the decrease in MBP reactivity after global HI. The area fraction of MBP was similar in sham conditions. (B): Graphic representation of MBP immunoreactivity in the subcortical white matter. (C): Graphic presentation of caspase-3 cell density in the subcortical white matter. A mild trend in increase of caspase-3 cell density was found following global HI. MSC-EV treatment did not affect apoptosis in the subcortical white matter. Levels of significance are depicted, which were calculated by the random intercept model with all repeated measures (i.e., brain sections) per animal (sham-saline, n = 6; sham-MSC-EV, n = 3; HI-saline, n = 6; HI-MSC-EV, n = 5). Because of positive skewing, data were log-transformed for statistical testing. For graphic presentation and interpretation, averages on the log scale were transformed to the original scale and presented as geometric means with corresponding 95% confidence intervals. Scale bars = 200 µm. ∗, p ≤ .05. Abbreviations: HI, hypoxia‐ischemia; IR, immunoreactivity; MBP, myelin basic protein 1; MSC-EV, mesenchymal stem cell‐derived extracellular vesicle; ns, not significant.
Assessment of cleaved caspase-3 in the SCWM was performed as a marker for apoptotic cell death. The numbers of caspase-3-positive cells after global HI tended to be higher at 7 days in comparison with controls (p = .070). There was no effect on the numbers of caspase -3-positive cells after hypoxia-ischemia by MSC-EV treatment (HI-SAL vs. HI-MSC-EV; p = .457).
MSC-EVs Did Not Protect Against HI-Induced Neuroinflammation
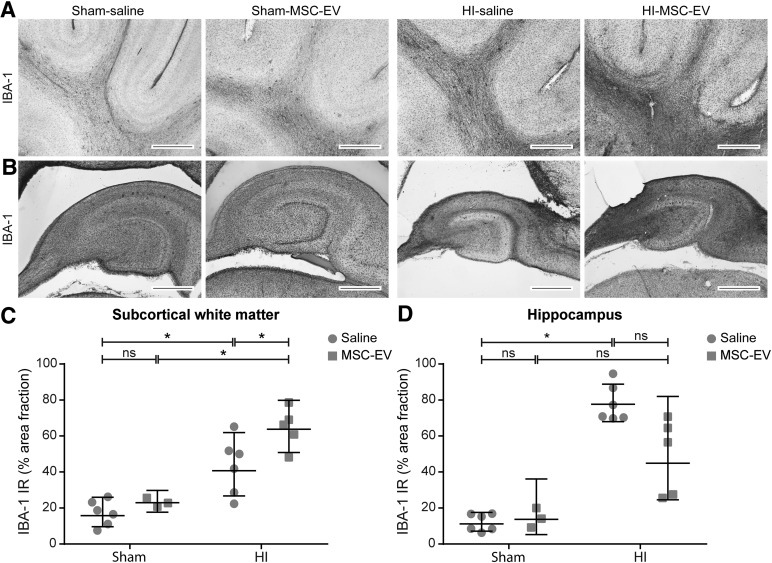
We assessed neuroinflammation in the SCWM (Fig. 5A–5C) and the hippocampus (Fig. 5B–5D) by analyzing immunoreactivity of IBA-1. Global HI induced a marked increase of IBA-1 immunoreactivity in the SCWM (sham-SAL vs. HI-SAL; p = .001) and the hippocampus (sham-SAL vs. HI-SAL; p < .0001), indicative of profound microglial activation and proliferation. MSC-EV treatment had no effects on hippocampal IBA-1 immunoreactivity (HI-SAL vs. HI-MSC-EV; p = .398) but increased IBA-1 immunoreactivity in the SCWM (HI-SAL vs. HI-MSC-EV; p = .041). MSC-EV treatment had no effects on IBA-1 immunoreactivity in either the SCWM or hippocampus in sham-occluded animals compared with controls.
Figure 5.
MSC-EVs do not reduce cerebral inflammation in the subcortical white matter and hippocampus. (A, B): Immunohistochemical IBA-1 staining. Global HI induced a significant increase of IBA-1 immunoreactivity in (A) the subcortical white matter (magnification, ×100) and (B) the hippocampus (magnification, ×20), which was not attenuated by MSC-EV treatment. (C, D): Graphic presentation of the area fraction of IBA‐1 immunoreactivity in (C) the subcortical white matter and (D) the hippocampus. Levels of significance are depicted, which were calculated by the random intercept model with all repeated measures (i.e., brain sections) per animal (sham-saline, n = 5; sham-MSC-EV, n = 3; HI-saline, n = 6; HI-MSC-EV, n = 5). Because of positive skewing, data were log-transformed for statistical testing. For graphic presentation and interpretation, averages on the log scale were transformed to the original scale and presented as geometric means with corresponding 95% confidence intervals. Scale bars = 1,000 µm. ∗, p ≤ .05. Abbreviations: HI = hypoxia‐ischemia; IBA‐1, ionized calcium binding adaptor molecule 1; IR, immunoreactivity; MSC-EV, mesenchymal stem cell-derived extracellular vesicles; ns, not significant.
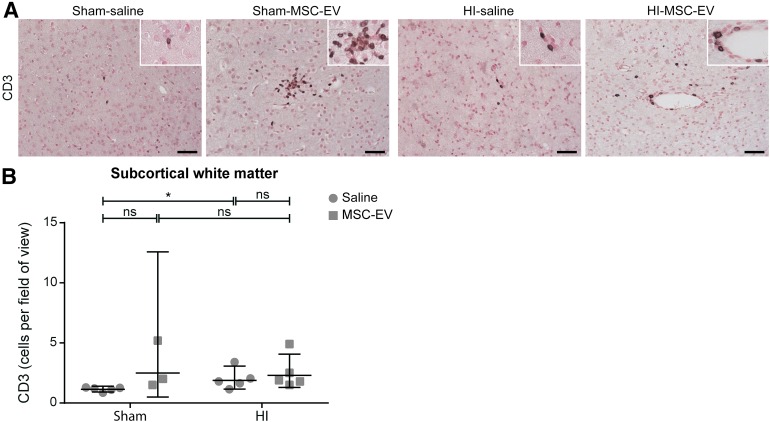
Finally, we assessed the influx of T lymphocytes in the fetal brain by immunohistochemical analysis for CD3 in the SCWM (Fig. 6). Cerebral influx of CD3-positive cells tended to increase following global HI (sham-SAL vs. HI-SAL; p = .078). Remarkably, MSC-EV treatment resulted in an increase of CD3-positive cells in the SCWM of sham-occluded animals (sham-SAL vs. sham-MSC-EV; p = .013).
Figure 6.
MSC-EVs increase cerebral influx of T‐effector cells. (A): Immunohistochemical staining for CD3-positive T lymphocytes in the subcortical white matter of all four experimental groups. Magnification, ×200; inset, ×400. (B): Graphic representation of CD3-positive cells per field of view in the subcortical white matter. MSC-EV treatment increased numbers of CD3-positive T lymphocytes in the subcortical white matter compared with saline-treated animals. Levels of significance are depicted, which were calculated by the random intercept model with all repeated measures (i.e., brain sections) per animal (sham-saline, n = 5; sham-MSC-EV, n = 3; HI-saline, n = 5; HI-MSC-EV, n = 5). Because of positive skewing, data were log-transformed for statistical testing. For graphic presentation and interpretation, averages on the log scale were transformed to the original scale and presented as geometric means with corresponding 95% confidence intervals. Scale bars = 100 µm. ∗, p ≤ .05. Abbreviations: HI, hypoxia‐ischemia; MSC-EV = mesenchymal stem cell‐derived extracellular vesicle; ns = not significant.
Discussion
In this study, we showed that intravenous administration of MSC-EVs prevented functional impairment and showed a tendency to protect against structural injury of the preterm brain after global HI, apparently without reducing cerebral inflammation. These findings are clinically highly relevant because postischemic seizure activity is associated with adverse neurodevelopmental outcomes [31–34]. The baroreceptor reflex buffers short-term changes in blood pressure by adapting systemic vascular resistance, myocardial contractility, and heart rate. The clinical relevance of an impaired baroreceptor reflex function relates to the increased risk of developing additional brain injury by exposing the vulnerable developing cerebral vascular network to large fluctuations in blood pressure.
In preterm infants, fluctuations associated with high blood pressure may disrupt the cerebral capillaries in the germinal matrix, whereas periods of low blood pressure may cause localized hypoxia-ischemia of the watershed areas [35]. Moreover, preterm infants display fluctuating pressure passivity between systemic blood pressure and cerebral blood flow, representing a considerably increased risk for cerebral hemorrhage or hypoxia [36]. We have shown that HI reduces the baroreceptor reflex-mediated heart rate response [24].
In this report, we demonstrate for the first time that MSC-EV treatment results in preservation of baroreceptor reflex sensitivity after exposure to global HI. Our data suggest a widespread functional protection by MSC-EVs of the central nervous system after global HI. Remarkably, we found that MSC-EV treatment reduced baroreceptor reflex sensitivity in healthy controls, which suggests that MSC-EV treatment should be given with care to avoid adverse effects and warrants further analyses of the effects of MSC-EVs on the developing brain in preclinical animal models before introducing MSC-EVs into clinical trials. However, clinical data in an adult patient with graft-versus-host disease demonstrated safe intravenous administration of MSC-EVs without adverse cardiovascular and hemodynamic outcomes [22].
Consistent with improved electrocortical function and preservation of baroreceptor reflex sensitivity, we demonstrated that intravenous administration of MSC-EVs tended to protect against HI-induced white matter injury, which is the clinical hallmark of neonatal HI brain injury [37]. This study suggests that the functional neuroprotective effects of MSC-EVs are not primarily caused by anti-inflammatory mechanisms. This is in contrast to our previous results in which the neuroprotective effects of intravenously administered MSCs could be, at least in part, attributed to anti-inflammatory capacities of MSCs [6]. Although prevention of splenic involution [6] might indicate inhibition of systemic immune activation [30], this effect was not paralleled by anti-inflammatory modifications of the ischemic fetal brain. This latter finding is in contrast to results from our previous study in which splenic involution was associated with prevention of cerebral inflammation after systemic MSC administration. This difference in anti-inflammatory properties of MSCs-EVs in the current study and of MSCs in the previous study could be explained by two reasons: First, the immune modulatory properties of MSCs and their EVs differ between independent MSC preparations [22]. Second, in contrast to MSC-EVs, MSCs can sense microenvironmental conditions to which they are exposed (e.g., licensing); in a proinflammatory environment, MSCs stimulate polarization toward an anti-inflammatory phenotype, whereas alternative stimulation has been reported to propagate a proinflammatory phenotype instead [38–45]. This may affect their secretion of therapeutically active immune modulatory components. However, we compared the therapeutic effect of MSC-EVs and that of corresponding MSCs in a murine ischemic stroke model and did not recognize any differences [21]. Although, such a side-by-side comparison was not performed in our model, this latter study suggests that differences between the two studies cannot be attributed to licensing.
The concept of inflammation-independent effects as neuroprotective mechanisms of MSC-EVs therapy is supported by several studies showing that MSC-EVs can prevent apoptosis and stimulate angiogenesis after hypoxia-ischemia [16, 20, 46–50]. However, no effects on angiogenic markers (mRNA levels of vascular endothelial growth factor [VEGF]-A and VEGF-receptor 2; data not shown) or on the number of apoptotic cells after global HI with MSC-EV treatment were found in our study, suggesting that the neuroprotective potential of MSC-EVs cannot be attributed to antiapoptotic or angiogenic properties.
Despite the identified discrepancies in therapeutic effects between MSCs and MSC-EVs used in this study, the application of EVs offers several advantages over the administration of MSCs: (a) The risk for malignant transformation is greatly reduced because EVs are nonself-replicating [51]. (b) Lacking an own metabolism, the EVs' activity can hardly be influenced by the in vivo environment in patients, thus allowing for a much better characterization of their functional properties. (c) Owing to their small size, EVs are less likely to generate emboli upon intravenous administration, as may be the case with MSCs. (d) In addition, EVs can be sterilized by filtration. Thus, from a regulatory point of view, the production, and especially the quality control of EV fractions for clinical treatment application, is less complicated than for a cellular therapeutic of in vitro expanded cells [52]. (e) Last but not least, EVs can be developed independently of the original donor because they are derived from MSCs from unrelated donors and thus offer the opportunity to be turned into an “off-the-shelf” product.
We have chosen this well-established preclinical sheep model because it enables us to accurately mimic the etiology of hypoxic-ischemic injury of the developing preterm brain, including continuous registration of clinical parameters with strong clinical relevance and predictive values [6, 53, 54]. Nevertheless, our study has several shortcomings. We have no information on the dynamics of the effects of MSC-EVs nor have we studied different doses. Time-course information is thus desirable in our model. In future experiments, back-to-back comparison of MSCs and their corresponding EV fraction will provide crucial mechanistic insights into the mode of action of MSCs and their EVs. The scope of this study, however, was assessing the feasibility of MSC-EV treatment to improve brain function after hypoxia-ischemia.
Conclusion
We have demonstrated in a preclinical animal model that MSC-EVs harbor neuroprotective potential as shown by improved functional and structural outcomes of the preterm brain following global HI, which is in line with previous reports [16, 17, 19, 22, 55, 56]. Future studies to determine the optimal EV contents and dosing strategy should be performed to establish a clinically safe, cell-free therapy for preterm babies after hypoxia-ischemia.
Supplementary Material
Acknowledgments
We thank Maria Nikiforou, Hellen Steinbusch, Lilian Kessels, and Nico Kloosterboer for their excellent technical support. We thank Dr. Lambros Kordelas and the Stem Cell Department of Red Cross Blood Service West for kindly providing bone marrow samples to use to raise MSCs. This work was supported by the Dutch Brain Foundation (R.K.J., T.G.A.M.W., B.W.K.) and the Volkswagen Foundation (B.G.).
Author Contributions
D.R.M.G.O.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, animal experiments, final approval of manuscript; T.G.A.M.W., R.K.J., and B.W.K.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript; A.Z.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; P.A. and T.D.: data analysis and interpretation, manuscript writing, final approval of manuscript; A.-K.L. and S.R.: provision of study material or patients, final approval of manuscript; V.P. and L.J.: collection and/or assembly of data, final approval of manuscript; B.G.: conception and design, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Fleiss B, Tann CJ, Degos V, et al. Inflammation-induced sensitization of the brain in term infants. Dev Med Child Neurol. 2015;57(suppl 3):17–28. doi: 10.1111/dmcn.12723. [DOI] [PubMed] [Google Scholar]
- 2.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzopardi D, Robertson NJ, Cowan FM, et al. Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics. 2000;106:684–694. doi: 10.1542/peds.106.4.684. [DOI] [PubMed] [Google Scholar]
- 5.Briatore E, Ferrari F, Pomero G, et al. EEG findings in cooled asphyxiated newborns and correlation with site and severity of brain damage. Brain Dev. 2013;35:420–426. doi: 10.1016/j.braindev.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Jellema RK, Wolfs TG, Lima Passos V, et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One. 2013;8:e73031. doi: 10.1371/journal.pone.0073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanotti L, Sarukhan A, Dander E, et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia. 2013;27:500–503. doi: 10.1038/leu.2012.202. [DOI] [PubMed] [Google Scholar]
- 9.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (Amst) 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res (Amst) 2007;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 12.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 13.Katsuda T, Kosaka N, Takeshita F, et al. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 14.Fleiss B, Guillot PV, Titomanlio L, et al. Stem cell therapy for neonatal brain injury. Clin Perinatol. 2014;41:133–148. doi: 10.1016/j.clp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin H, Li Y, Cui Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 18.Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doeppner TR, Herz J, Görgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Translational Medicine. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 23.Jellema RK, Lima Passos V, Zwanenburg A, et al. Cerebral inflammation and mobilization of the peripheral immune system following global hypoxia-ischemia in preterm sheep. J Neuroinflammation. 2013;10:13. doi: 10.1186/1742-2094-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwanenburg A, Jellema RK, Jennekens W, et al. Heart rate-mediated blood pressure control in preterm fetal sheep under normal and hypoxic-ischemic conditions. Pediatr Res. 2013;73:420–426. doi: 10.1038/pr.2013.15. [DOI] [PubMed] [Google Scholar]
- 25.Andriessen P, Koolen AM, Berendsen RC, et al. Cardiovascular fluctuations and transfer function analysis in stable preterm infants. Pediatr Res. 2003;53:89–97. doi: 10.1203/00006450-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Sokolova V, Ludwig AK, Hornung S, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. 2011;87:146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Kuypers E, Jellema RK, Ophelders DR, et al. Effects of intra-amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep. PLoS One. 2013;8:e81644. doi: 10.1371/journal.pone.0081644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jellema RK, Lima Passos V, Ophelders DR, et al. Systemic G-CSF attenuates cerebral inflammation and hypomyelination but does not reduce seizure burden in preterm sheep exposed to global hypoxia-ischemia. Exp Neurol. 2013;250:293–303. doi: 10.1016/j.expneurol.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Gelman A, Carlin JB, Stern HS, et al. Boca Raton, FL: CRC Press; 2014. Bayesian Data Analysis. [Google Scholar]
- 30.Ajmo CT, Jr, Vernon DO, Collier L, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 32.Glass HC, Glidden D, Jeremy RJ, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- 34.de Vries LS, Toet MC. Amplitude integrated electroencephalography in the full-term newborn. Clin Perinatol. 2006;33:619–632. doi: 10.1016/j.clp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Perlman JM. The relationship between systemic hemodynamic perturbations and periventricular-intraventricular hemorrhage—A historical perspective. Semin Pediatr Neurolo. 2009;16:191–199. doi: 10.1016/j.spen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Fyfe KL, Yiallourou SR, Wong FY, et al. The development of cardiovascular and cerebral vascular control in preterm infants. Sleep Med Rev. 2014;18:299–310. doi: 10.1016/j.smrv.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Volpe JJ. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: A key player in ‘innate tolerance’? Immunology. 2012;137:206–213. doi: 10.1111/j.1365-2567.2012.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Ren G, Huang Y, et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemeda H, Jakob M, Ludwig AK, et al. Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19:693–706. doi: 10.1089/scd.2009.0365. [DOI] [PubMed] [Google Scholar]
- 43.Ranganath SH, Levy O, Inamdar MS, et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): Controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental” niches” in culture: A two-stage hypothesis for regulation of MSC fate. Sci STKE. 2005;2005:pe37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- 46.Salomon C, Ryan J, Sobrevia L, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8:e68451. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Wu X, Zhang X, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Translational Medicine. 2015;4:513–522. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Liu Z, Hong MM, et al. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS One. 2014;9:e115316. doi: 10.1371/journal.pone.0115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Döppner TR, Herz J, Görgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent post-ischemic immunosuppression. Stem Cells Translational Medicine. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dlouhy BJ, Awe O, Rao RC, et al. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J Neurosurg Spine. 2014;21:618–622. doi: 10.3171/2014.5.SPINE13992. [DOI] [PubMed] [Google Scholar]
- 52.Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Back SA, Riddle A, Dean J, et al. The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics. 2012;9:359–370. doi: 10.1007/s13311-012-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunn AJ, Bennet L. Fetal hypoxia insults and patterns of brain injury: Insights from animal models. Clin Perinatol. 2009;36:579–593. doi: 10.1016/j.clp.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin H, Li Y, Buller B, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.