Mesenchymal stem cell (MSC)-based therapies are under investigation for tissue repair but suffer from poor cell persistence and engraftment upon transplantation. When entrapped in an adhesive biomaterial, MSC spheroids exhibited improved survival and proangiogenic growth factor secretion in vitro and bone formation in vivo compared with cells in nonadhesive hydrogels. These findings demonstrate the value of deploying MSC spheroids in instructive biomaterials to improve cell function.
Keywords: Mesenchymal stem cell, Spheroid, Alginate, Osteogenesis, Adhesion
Abstract
Mesenchymal stem cell (MSC)-based therapies are under broad investigation for applications in tissue repair but suffer from poor cell persistence and engraftment upon transplantation. MSC spheroids exhibit improved survival, anti-inflammatory, and angiogenic potential in vitro, while also promoting vascularization when implanted in vivo. However, these benefits are lost once cells engage the tissue extracellular matrix and migrate from the aggregate. The efficacy of cell therapy is consistently improved when using engineered materials, motivating the need to investigate the role of biomaterials to instruct spheroid function. In order to assess the contribution of adhesivity on spheroid activity in engineered materials and promote the bone-forming potential of MSCs, we compared the function of MSC spheroids when entrapped in Arg-Gly-Asp (RGD)-modified alginate hydrogels to nonfouling unmodified alginate. Regardless of material, MSC spheroids exhibited reduced caspase activity and greater vascular endothelial growth factor (VEGF) secretion compared with equal numbers of dissociated cells. MSC spheroids in RGD-modified hydrogels demonstrated significantly greater cell survival than spheroids in unmodified alginate. After 5 days in culture, spheroids in RGD-modified gels had similar levels of apoptosis, but more than a twofold increase in VEGF secretion compared with spheroids in unmodified gels. All gels contained mineralized tissue 8 weeks after subcutaneous implantation, and cells entrapped in RGD-modified alginate exhibited greater mineralization versus cells in unmodified gels. Immunohistochemistry confirmed more diffuse osteocalcin staining in gels containing spheroids compared with dissociated controls. This study demonstrates the promise of cell-instructive biomaterials to direct survival and function of MSC spheroids for bone tissue engineering applications.
Significance
Mesenchymal stem cell (MSC) spheroids exhibit improved therapeutic potential in vitro compared with dissociated MSCs, yet spheroids are directly injected into tissues, ceding control of cell function to the extracellular matrix and potentially limiting the duration of improvement. Cell delivery using adhesive biomaterials promotes cell retention and function. These studies explored the role of adhesion to the surrounding matrix on spheroid function. When entrapped in an adhesive biomaterial, MSC spheroids exhibited improved survival and proangiogenic growth factor secretion in vitro and bone formation in vivo compared with cells in nonadhesive hydrogels. These findings demonstrate the value of deploying MSC spheroids in instructive biomaterials to improve cell function.
Introduction
The delivery of mesenchymal stem cells (MSCs) into hypoxic, poorly vascularized tissue defects commonly induces cellular apoptosis, translating to poor cell survival or impaired engraftment [1, 2]. Thus, there is a compelling need for improved methods to promote cell survival and function. The vast majority of cell therapies deploy dissociated cells expanded in monolayer, trypsinized from the culture dish, and injected or seeded on a biomaterial. However, trypsinization of the cell monolayer disrupts critical cell-cell communication established during culture. Although cell-cell interactions are crucial in spheroid culture, the cell-extracellular matrix (cell-ECM) interaction is equally important. MSCs are anchorage-dependent cells that require cell adhesion to matrix proteins via integrin engagement to prevent anoikis [3]. Furthermore, cell-cell contact by cadherin binding activates other mechanosensitive transmembrane proteins that can participate in cell and tissue homeostasis [4].
Cells cultured as three-dimensional (3D) aggregates, also known as spheroids, yield a multicellular mass mediated by cadherins that more accurately mimics the physiological cellular niche [5, 6]. Others have hypothesized that spheroid culture preconditions cells residing in the inner core to an ischemic environment, making them more resilient when implanted into hypoxic conditions [7] and enhancing endogenous secretion of growth factors and cytokines necessary for cell survival [8], angiogenesis [1, 9–12], and immunomodulation [13, 14]. Despite the benefits observed when cells are formed as aggregates, spheroids are commonly injected directly into the tissue [1, 7, 15, 16]. Thus, cells are allowed to immediately interact with adhesion sites within the native (ECM) and migrate from the aggregate, potentially limiting the benefit conferred by 3D culture.
Engineered biomaterials are valuable platforms for interrogating the role of integrin activation and cell adhesion, and they may serve as cell carriers for use in tissue engineering. Alginate hydrogels are particularly promising because of their biocompatibility, high water content, low cost, and tailorability [17–19]. In addition, the nonfouling native alginate is easily modified to present specific binding sites for cell adhesion. The cell recognition motif arginine-glycine-aspartic acid (RGD), a tripeptide found in fibronectin and other adhesive ECM proteins, is one of the most widely used peptide sequences for triggering integrin-stimulated cell adhesion within biomaterials [20]. Cell spreading and formation of focal adhesions initiate the survival and proliferative pathways of anchorage-dependent cells [21, 22]. Therefore, alginate is an ideal material for probing the role of cell adhesion in spheroid culture.
This study sought to address two important questions. (a) Would MSC spheroids exhibit improved functionality compared with an equal number of dissociated MSCs once entrapped in a cytocompatible material designed as an implant? (b) Do MSC spheroids function differently when allowed to adhere to the material? We hypothesized that MSC spheroids would exhibit increased survival and enhanced osteogenesis when entrapped in RGD-modified alginate gels compared with unmodified alginate lacking adhesion motifs. To test this hypothesis, we entrapped MSC spheroids in RGD-modified or unmodified alginate gels and compared their survival and function to equal numbers of dissociated cells in the same materials. We then characterized the capacity of spheroids entrapped in these gels to create bone in a murine ectopic site.
Materials and Methods
Cell Culture
Human bone marrow-derived MSCs from a single donor were purchased from Lonza (Walkersville, MD, http://www.lonza.com) and used without further characterization. Cells were cultured in α-modified Eagle’s medium (α-MEM) (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with 10% fetal bovine serum (JR Scientific, Woodland, CA, http://www.jrscientific.com), 100 units/ml penicillin, and 100 μg/ml streptomycin (Mediatech, Manassas, VA, http://www.cellgro.com) under standard culture conditions until use at passage 6.
Fabrication of MSC Spheroids
MSC spheroids were formed by using the hanging-drop technique with 15,000 MSCs in a 25-μl droplet as described [11]. Droplets were added to a nontissue culture-treated petri dish (CellTreat Scientific, Shirley, MA, https://www.celltreat.com) by using a multichannel pipette, and phosphate-buffered saline was added to the bottom of the dish to prevent evaporative loss. After MSCs were allowed to aggregate for 48 hours, spheroids were collected via pipette for entrapment in alginate.
Preparation of RGD-Modified Alginate
RGD-modified alginate was prepared as described previously [23–25]. Briefly, G4RGDSP (Commonwealth Biotechnologies, Richmond, VA, http://www.cbi-biotech.com) was covalently coupled to irradiated UltraPure MVG sodium alginate (ProNova, Lysaker, Norway, http://pronovasolutions.com) by using standard carbodiimide chemistry, yielding hydrogels with 0.32 mM RGD. The resulting RGD-alginate was sterile-filtered and lyophilized for 4 days. Lyophilized alginate was reconstituted in serum-free α-MEM to obtain a 2% (wt/vol) solution. Unmodified alginate was reconstituted in serum free α-MEM to obtain a 2% alginate solution and sterile-filtered.
Entrapment of Spheroids Within Alginate Gels
Current methods that use CaSO4 to cross-link alginate hydrogels involve mixing components with two syringes, causing loss of polymeric and cellular material for a specific volume. To ensure we entrapped equivalent numbers of MSCs as dissociated cells or spheroids, we applied a diffusion-mediated method of alginate cross-linking similar to that described for entrapping individual spheroids [26]. Briefly, alginate gels containing a known spheroid concentration were pipetted into wells of a silicone mold adhered to a glass plate to yield 2% alginate gels with a consistent volume of 80 µl. A dialysis membrane (MWCO 3500, Spectrum Laboratories, Rancho Dominguez, CA, http://www.spectrumlabs.com) was overlaid and flattened onto the mold. Molds were then immersed in CaCl2 solution to cross-link the alginate. The optimal parameters for alginate cross-linking, specifically CaCl2 concentration and exposure time, were determined by characterizing changes in rheological properties and viability of entrapped cells. Subsequently, all remaining studies were conducted with a single CaCl2 concentration and exposure duration.
Assessment of Hydrogel Mechanical Properties
We measured the viscoelastic properties of spheroid-containing alginate gels cross-linked in varying CaCl2 concentrations and exposure durations by using a Discovery HR2 Hybrid Rheometer (TA Instruments, New Castle, DE, http://www.tainstruments.com). An 8.0-mm-diameter peltier plate geometry was used for 80-µL gels with corresponding 8.0-mm diameter. Gels were cross-linked and then maintained in medium under standard culture conditions for 24 hours before measurement. An oscillatory strain sweep ranging from 0.004% to 4% strain was performed on each gel to obtain the linear viscoelastic region (LVR) before gel failure. A minimum of 10 data points were collected for the LVR and averaged to obtain gel shear storage modulus.
Live/Dead Staining of MSCs Within Alginate Gels
Calcein acetoxymethyl (calcein AM) ester and propidium iodide (Invitrogen) were added to α-MEM to create a 2 µM and 5 µM solution of each reagent, respectively. Gels were fully immersed for 30 minutes before imaging. Gels collected for confocal microscopy were processed in the same manner and imaged by using an Olympus Fluoview FV1000 confocal laser scanning system with a ×20 objective (Olympus, Tokyo, Japan, http://www.olympus.co.jp).
Biochemical Analysis of MSC Spheroid Function Within Alginate Gels
MSCs, either dissociated cells or spheroids, were entrapped in alginate gels at a concentration of 40 × 106 cells per milliliter. Gels were cultured in α-MEM under standard culture conditions for 5 days, and collected, minced, and lysed in passive lysis buffer (Promega, Madison, WI, http://www.promega.com). Apoptosis was quantitatively measured by analyzing 100 μl of lysate per sample using a Caspase-Glo 3/7 assay (Promega) as we reported [27, 28]. Luminescence was detected on a microplate reader and normalized to DNA content determined from the same lysate by using the Quant-iT PicoGreen DNA Assay Kit (Invitrogen). To measure vascular endothelial growth factor (VEGF) secretion, medium was refreshed 24 hours before collection, and VEGF concentration was determined by using a human-specific VEGF enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, https://www.rndsystems.com) according to the manufacturer’s instructions [11]. Quantification of proangiogenic proteins within conditioned medium was performed by using a Human Angiogenesis Antibody Array (RayBiotech, Norcross, GA, http://www.raybiotech.com) [27, 29]. Protein quantification was performed with a GenePix 4000B scanner and associated GenePix Pro software (Molecular Devices, Sunnyvale, CA, http://www.moleculardevices.com) to calculate median minus background fluorescent intensity at 532 nm for each cytokine. Data are normalized to internal positive controls.
To quantify osteogenic potential of entrapped spheroids, gels prepared as described above were cultured in α-MEM overnight and then moved to osteogenic medium containing standard osteogenic supplements (10 mM β-glycerophosphate, 50 μg/ml ascorbate-2-phosphate, and 100 nM dexamethasone; all from Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). Osteogenic potential was assessed by quantifying intracellular alkaline phosphatase (ALP) activity from a p-nitrophenyl phosphate assay, osteocalcin secretion via ELISA, and calcium deposition by o-cresolphthalein assay [24, 30, 31].
In Vivo Subcutaneous Ectopic Bone Assay
Treatment of all experimental animals was in accordance with the University of California, Davis animal care guidelines and all National Institutes of Health animal handling protocols. Eight-week-old nonobese diabetic/severe combined immunodeficient γ (NSG, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice (Jackson Laboratory, Bar Harbor, ME, https://www.jax.org) were anesthetized and maintained under a 2% isoflurane/O2 mixture delivered through a nose cone. Two longitudinal incisions were made in the dorsum, and subcutaneous pockets were created on each side of the incision to create four spaces for biomaterial implantation, after which surgical sites were closed with staples. Animals were euthanized after 8 weeks by CO2 inhalation. Alginate gels were explanted and fixed in phosphate-buffered formalin for 24 hours, then maintained in 70% ethanol (n = 4 per group) for analysis.
Analysis of Bone Formation in Gel Explants
The formation of mineralized tissue was detected in explants by radiography at a voltage of 22 kVp for 2 minutes. Explants were subsequently demineralized in Calci-Clear Rapid (National Diagnostics, Atlanta, GA, https://www.nationaldiagnostics.com), processed, paraffin-embedded, and sectioned at 5-μm thickness. Sections were stained with hematoxylin and eosin (H&E) and imaged by using a Nikon Eclipse TE2000U microscope (Nikon, Tokyo, Japan, http://www.nikon.com) and Andor Zyla 5.5 scientific complementary metal-oxide semiconductor (sCMOS) digital camera (Andor, Belfast, Northern Ireland, http://www.andor.com). To visualize cells undergoing osteogenic differentiation, we performed immunohistochemistry (IHC) on sections by using a primary antibody against osteocalcin (1:200, ab13420, Abcam, Cambridge, MA, http://www.abcam.com) [32] and a mouse-specific horseradish peroxidase/3,3′-diaminobenzidine detection kit (ab64259, Abcam).
Statistical Analysis
Data are presented as means ± SD. Statistical analysis was performed by using a two-way analysis of variance with Bonferroni correction for multiple comparisons in Prism 6 software (GraphPad Software Inc., La Jolla, CA, http://www.graphpad.com); p < .05 was considered statistically significant. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, whereas groups with significance do not share the same letters.
Results
Cross-Linking Alginate Hydrogels by Dialysis for Entrapping MSC Spheroids
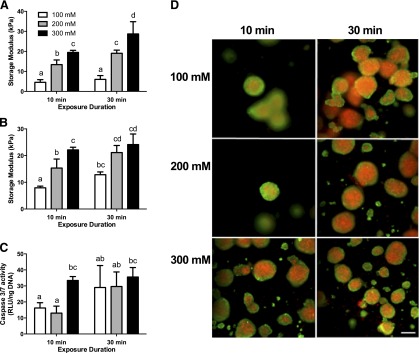
The rheological properties of alginate hydrogels were initially characterized without cells to directly ascertain the role of CaCl2 concentration and duration. Alginate hydrogels cross-linked by CaCl2 diffusion exhibited increasing shear storage modulus with increasing calcium concentration (Fig. 1A). Additionally, shear storage modulus increased when prolonging the exposure duration to 30 minutes for gels exposed to 200 and 300 mM CaCl2. When spheroids were entrapped in alginate hydrogels, we observed similar relationships between shear storage modulus, CaCl2 concentration, and duration of exposure (Fig. 1B). Compared with acellular gels, the entrapment of MSC spheroids resulted in increased storage modulus for most conditions. Quantification of cell apoptosis was performed in tandem with Live/dead imaging by using inverted fluorescence microscopy. A concentration of 300 mM CaCl2 at 10 minutes of exposure significantly increased caspase 3/7 activity compared with 100 and 200 mM concentrations (Fig. 1C). The viability of entrapped spheroids was dependent upon the calcium concentration and exposure time (Fig. 1D). We observed increasing cell death as CaCl2 concentration increased after 10 minutes of exposure. After 30 minutes of exposure, all groups exhibited high levels of cell death, regardless of CaCl2 concentration. Similar trends were observed after 4 and 7 days in culture (data not shown). In order to achieve the biomaterial with the highest mechanical properties while limiting cell death, we cross-linked alginate gels in subsequent experiments with 200 mM CaCl2 for 10 minutes.
Figure 1.
Rheological properties and survival of entrapped mesenchymal stem cell spheroids correlate with calcium concentration and duration of exposure. (A): Shear storage moduli of acellular arginine-glycine-aspartic acid (RGD)-modified gels at day 1 (n = 3 per group). (B): Shear storage moduli of RGD-modified gels containing spheroids (n = 3 per group). (C): Caspase-3/7 activity as an indicator of apoptosis. (D): Live/dead staining of spheroids entrapped in RGD-modified gels cross-linked with varying CaCl2 concentrations and for 10 and 30 minutes at day 1. Live/dead images at ×10 magnification using inverted fluorescence microscopy; scale bar = 200 µm. Different letters above bars indicate significance of at least p < .05. Bars sharing the same letters indicate no significance. Abbreviation: RLU, relative luminescence units.
MSCs From Spheroids Adhere, Spread, and Exhibit Enhanced Survival in RGD-Modified Alginate
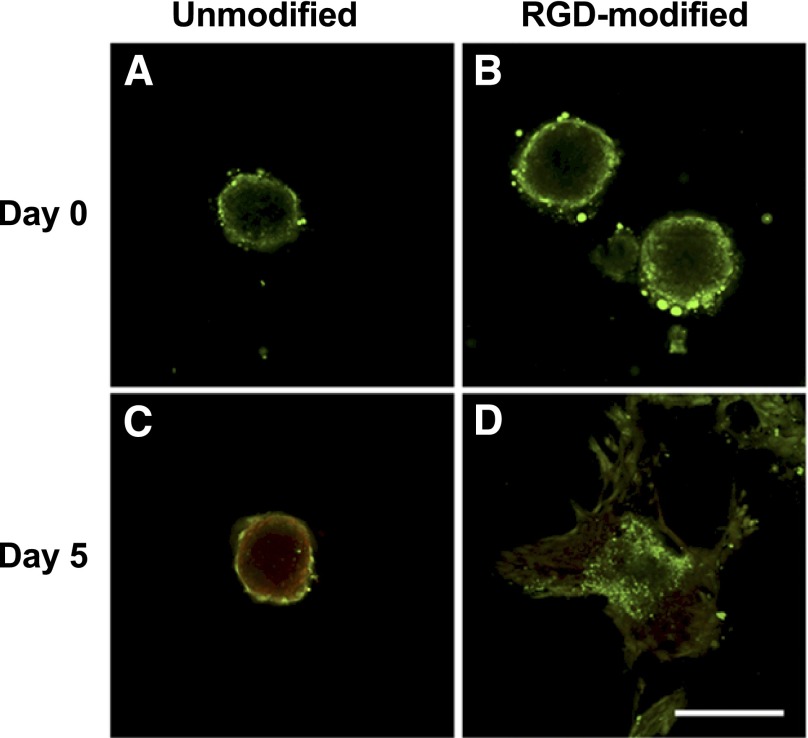
We used confocal microscopy to discern changes in spheroid morphology and cell survival over time. Immediately after entrapment at day 0, spheroids exhibited similar, spherical morphology with high viability in unmodified or RGD-modified alginate gels (Fig. 2A, 2B). After 5 days in culture, spheroid morphology in unmodified alginate remained compact and spherical, but with significant cell death (Fig. 2C). However, spheroids entrapped in RGD-modified gels demonstrated a loss in spherical structure, with cells migrating from the spheroid and becoming more elongated (Fig. 2D). Furthermore, we observed a significant increase in cell viability for spheroids in RGD-modified gels compared with cells entrapped in unmodified alginate.
Figure 2.
Mesenchymal stem cell spheroids entrapped in RGD-modified gels exhibit increased cell viability and migration. (A, B): Live/dead stain reveals comparable viability and spherical morphology by confocal microscopy for spheroids at day 0 in unmodified (A) and RGD-modified (B) gels when visualized with confocal microscopy. (C, D): Live/dead stain demonstrates increase in dead cells and retained spherical morphology for spheroids at day 5 in unmodified alginate (C), whereas spheroids in RGD-modified alginate gels have increased viability and migration from the aggregate (D). Scale bar = 200 µm. Abbreviation: RGD, arginine-glycine-aspartic acid.
MSCs in RGD-Modified Gels Demonstrate Reduced Apoptosis and Increased VEGF Secretion
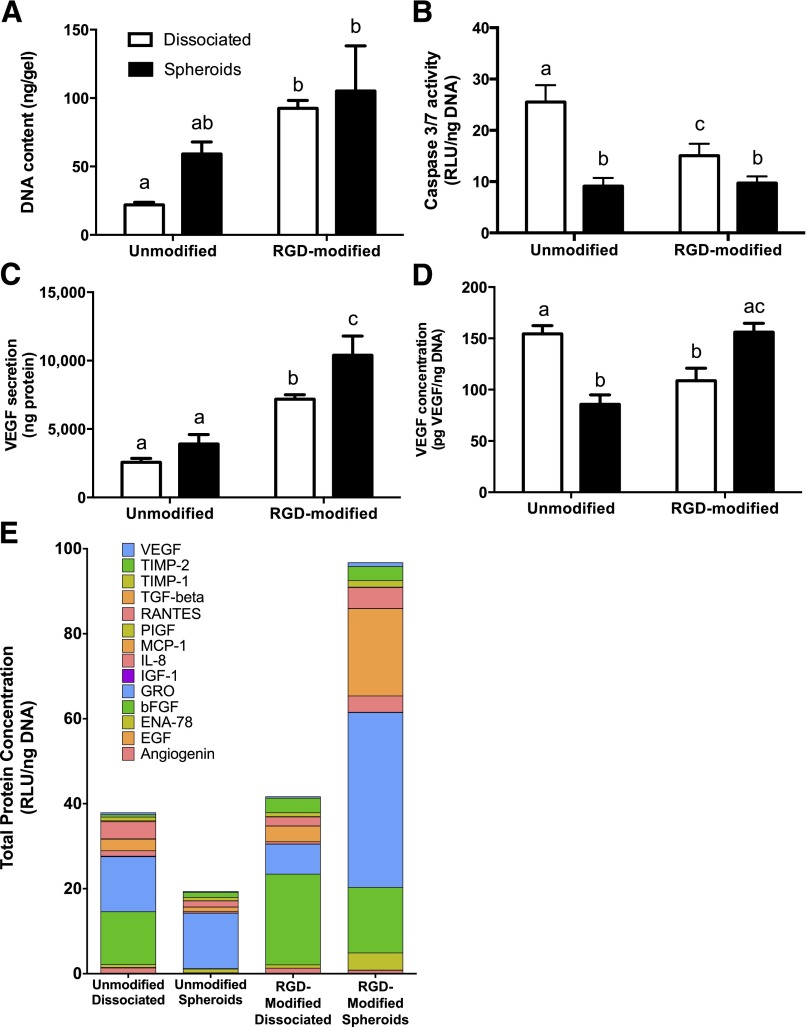
We entrapped equal numbers of MSCs in both gel formulations to assess the role of adhesivity on cell proliferation, apoptosis, and VEGF secretion in dissociated cells and MSC spheroids. We detected increased DNA content, an indicator of cell number, when entrapping MSCs in RGD-modified alginate gels compared with cells in unmodified gels. The entrapment of dissociated MSCs in RGD-modified gels resulted in a 4.2-fold increase in DNA content compared with cells in unmodified gels (Fig. 3A). We measured significant reductions in caspase-3/7 activity, an indicator of apoptosis, for MSCs entrapped as spheroids versus dissociated cells, regardless of gel formulation (Fig. 3B). Spheroids in unmodified gels exhibited a 2.8-fold reduction in caspase activity compared with dissociated controls, while caspase-3/7 activity in dissociated cells was reduced in RGD-modified gels. MSC spheroids secreted more total VEGF than an equivalent number of dissociated MSCs in each alginate formulation (Fig. 3C). VEGF secretion was significantly increased for MSC spheroids and dissociated cells when entrapped in RGD-modified versus unmodified alginate (2.7- and 2.8-fold increase, respectively). Furthermore, MSC spheroids suspended in RGD-modified gels secreted 1.4-fold more VEGF compared with dissociated controls. These increases in VEGF secretion were not solely a function of cell proliferation, because MSC spheroids in RGD-modified gels secreted more normalized VEGF compared with dissociated cells or MSC spheroids in unmodified alginate (1.4- and 1.8-fold more, respectively) (Fig. 3D). The angiogenic microarray demonstrated the greatest protein secretion from spheroids in RGD-modified alginate and the least in unmodified groups (Fig. 3E). MSC spheroids in RGD-modified alginate secreted more protein than both dissociated cell and unmodified controls (2.5- and 5-fold increase, respectively). Of interest, spheroids in RGD-modified alginate exhibited an upregulation in growth-regulated oncogene (GRO), monocyte chemoattractant protein 1 (MCP-1), and interleukin 8 (IL-8), potent stimulators of angiogenesis, compared with both dissociated and unmodified control groups.
Figure 3.
Mesenchymal stem cell (MSC) spheroids entrapped in RGD-modified alginate hydrogels have increased survival and proangiogenic potential compared with dissociated MSCs. (A): Total DNA content in gel scaffold. (B): Caspase-3/7 activity as an indicator of apoptosis. (C): VEGF secretion measured by enzyme-linked immunosorbent assay (ELISA). (D): Secreted VEGF normalized to DNA. Gels loaded with an equal number of dissociated cells are represented by open bars, and hydrogels entrapping spheroids are represented by filled bars (n = 4 for all groups). (E): Total angiogenic proteins normalized to DNA measured by angiogenic microarray. Different letters above bars indicate significance of at least p < .05. Bars sharing the same letters indicate no significance. Abbreviations: RGD, arginine-glycine-aspartic acid; RLU, relative luminescence units; VEGF, vascular endothelial growth factor.
Spheroids in RGD-Modified Alginate Undergo Osteogenic Differentiation
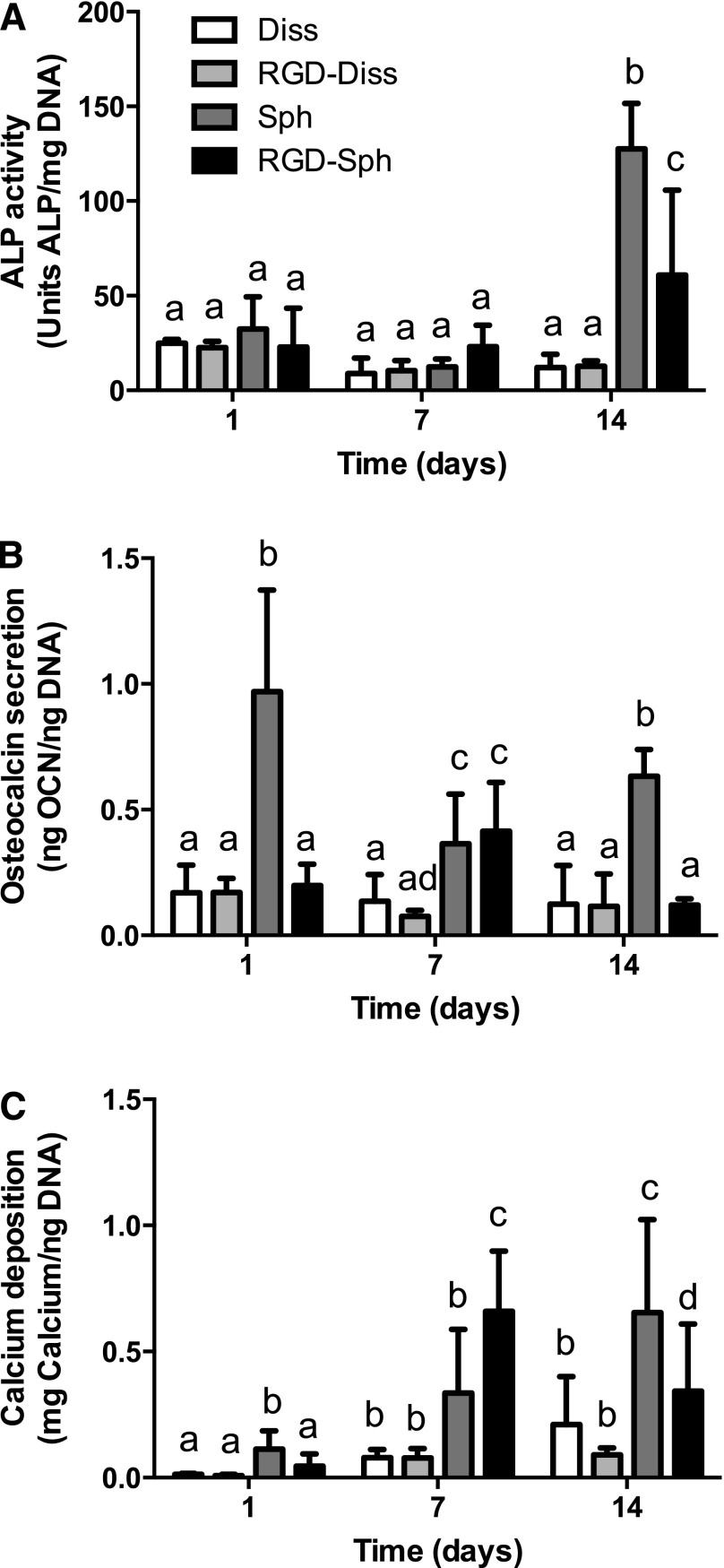
We analyzed the osteogenic response of MSC spheroids to unmodified or RGD-modified alginate when cultured under osteogenic conditions. ALP activity was relatively constant among all groups over the first 7 days. After 14 days, MSC spheroids in RGD-modified gels exhibited a 4.8-fold increase in ALP activity compared with dissociated controls (Fig. 4A), and spheroids in unmodified alginate demonstrated further increases. Spheroids entrapped in unmodified alginate demonstrated elevated osteocalcin levels compared with dissociated controls at all time points, while spheroids in RGD-modified gels exhibited a significant increase only at 7 days (Fig. 4B). We quantified cell-secreted calcium, a late stage marker of osteogenic differentiation, to determine mineralization of the scaffold. At days 7 and 14, we observed higher calcium content in all spheroid groups compared with dissociated controls (Fig. 4C), demonstrating that MSC spheroids can undergo osteogenic differentiation when entrapped in alginate gels.
Figure 4.
Mesenchymal stem cell (MSC) spheroids exhibit enhanced osteogenic potential in RGD-modified alginate gels over 14 days in culture compared with dissociated MSCs. (A): Intracellular ALP activity. (B): Secreted osteocalcin. (C): Secreted calcium in gels. n = 5 per group. Different letters above bars indicate significance of at least p < .05. Bars sharing the same letters indicate no significance. Abbreviations: ALP, alkaline phosphatase; Diss, dissociated; OCN, osteocalcin; RGD, arginine-glycine-aspartic acid; RGD-Diss, arginine-glycine-aspartic acid-dissociated; Sph, spheroids; RGD-Sph, arginine-glycine-aspartic acid-spheroid.
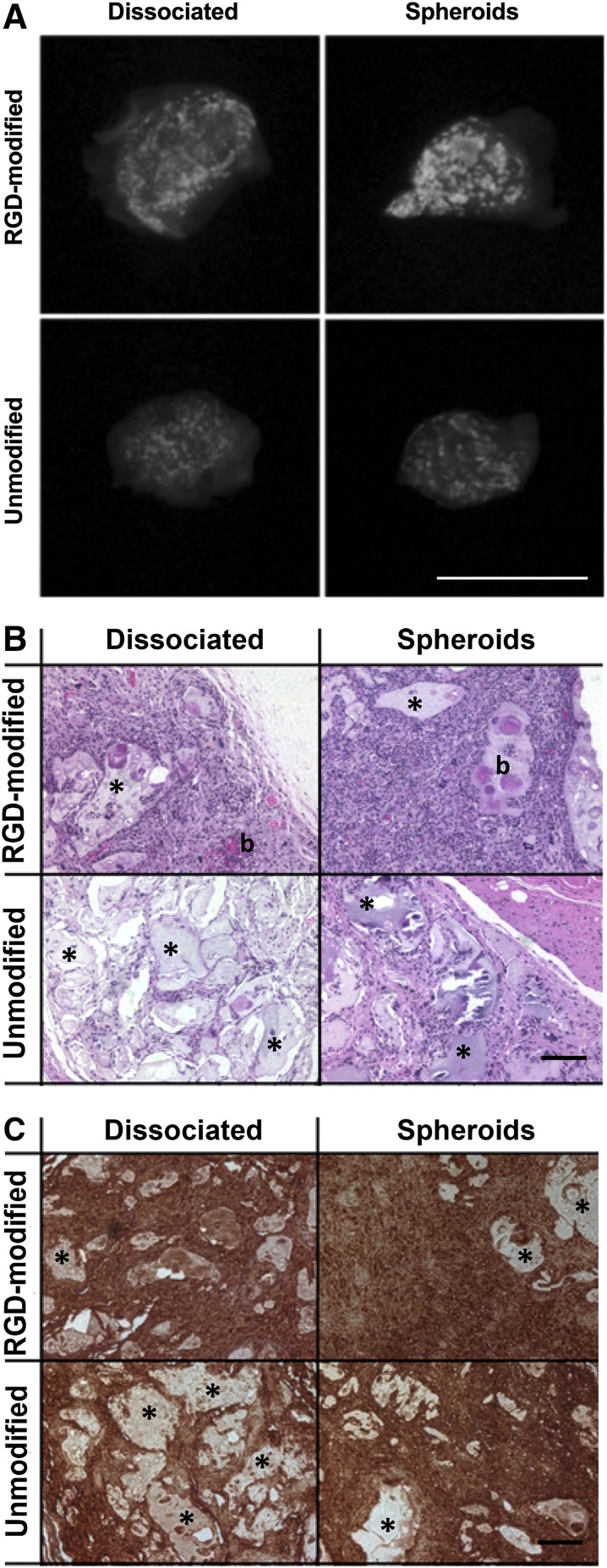
Spheroids in RGD-Modified Alginate Exhibit Enhanced Osteogenesis In Vivo
We analyzed the presence and distribution of mineral within explants containing MSC spheroids and dissociated MSCs after 8 weeks. Representative radiographs confirmed widespread mineralization in all groups (Fig. 5A), but mineralization appeared substantially more intense in the RGD-modified groups. Radiograph data indicated that spheroids retain their capacity to form bone when compared with dissociated controls. Furthermore, dissociated MSCs delivered in RGD-modified alginate gels yielded markedly more mineralized tissue compared with unmodified alginate hydrogels seeded with an equal number of dissociated cells. As revealed by H&E staining, alginate gels containing dissociated MSCs had markedly reduced cellularity with larger clumps of alginate remaining compared with MSC spheroids in either formulation (Fig. 5B). Additionally, hydrogels containing MSC spheroids reveal more diffuse and homogeneous osteocalcin staining compared with dissociated controls (Fig. 5C), confirming osteogenic differentiation of cells entrapped in alginate gels.
Figure 5.
Mesenchymal stem cell spheroids exhibit robust bone-forming potential when deployed in RGD-modified alginate. (A): Representative radiograph illustrating mineral deposition in explants after 8 weeks. Image is not magnified; scale bar = 8 mm. (B): Representative H&E staining of explants at 8 weeks. Blue indicates cell nuclei, pink indicates matrix proteins, asterisk indicates residual alginate, and b represents new bone tissue. (C): Representative images of immunohistochemical staining of explants for osteocalcin. Asterisk indicates residual alginate. All stained images were taken under brightfield microscopy at a magnification of ×10; scale bars = 100 µm. Abbreviation: RGD, arginine-glycine-aspartic acid.
Discussion
Cell therapies consist of transplanting dissociated or dispersed cell populations, either in the presence or absence of a biomaterial, to stimulate tissue repair. Once implanted, cells exhibit poor survival and increased apoptosis in vivo, perhaps due to the loss of cell-ECM and cell-cell contacts established during cell culture. Previous studies reported the proangiogenic and antiapoptotic advantages of spheroids compared with dissociated cells [1, 9, 11, 13]. However, spheroids are commonly transplanted through direct injection into the tissue site [1, 7, 15, 16], thereby ceding control of this improved cell function to the surrounding tissue ECM as cells migrate from the spheroid into the tissue in an uncontrolled manner. Hence, there is a critical need to understand the effect of controllable presentation of adhesion sites external to the spheroid to develop improved delivery vehicles for preserving spheroid survival and function.
Alginate hydrogels can be cross-linked with the addition of divalent cations, commonly achieved via the addition of a supersaturated solution of CaSO4. This calcium solution enables slow and relatively homogenous gelation, but suffers from unpredictable gelation kinetics; distribution of large, potentially cytotoxic calcium nodules because of low solubility [18, 33]; and unpredictable loss of spheroids in the residual material. In these studies, we needed to entrap an exact number of spheroids to compare with equal numbers of dissociated MSCs, thus motivating the application of an alternative gelation method. CaCl2 is widely used to rapidly cross-link alginate beads and cure gels [34, 35], and previous reports have used a similar diffusion-based strategy to entrap individual spheroids [26, 36]. We successfully engineered robust alginate gels by cross-linking with 200 mM CaCl2 for 10 minutes, resulting in hydrogels with similar or greater mechanical properties as others used in bone formation [37] while retaining cell viability. This concentration is comparable to concentrations used to generate alginate microbeads [18] and higher than other dialyzing methods, but is used over shorter durations [38, 39].
These data confirm that alginate hydrogels seeded with equal numbers of MSCs as spheroids possess greater proangiogenic potential and decreased apoptosis compared with dissociated cells, data that are in agreement with other comparisons lacking a biomaterial [1, 9, 11, 13]. For dissociated MSCs, binding to the RGD ligand enhances MSC attachment and survival [40] and promotes osteogenic differentiation in a dose-dependent manner [41]. We selected a single, commonly used RGD concentration and polymer concentration to evaluate the response of MSC spheroids to adhesivity in alginate gels. In light of other studies reporting the interplay of RGD concentration and substrate stiffness on cell migration [42] and osteogenic differentiation [43], the role of polymer and RGD concentration on spheroid response to the delivery vehicle merits further investigation. Herein, we demonstrate that spheroids exhibit greater osteogenic differentiation compared with dissociated MSCs, regardless of adhesion to the gel. The underlying reason for why spheroids in unmodified gels exhibit higher levels of osteoblastic markers is unclear and merits further examination. This phenomenon may be due to relative differences in the contribution of cells within spheroids engaging adhesion ligands within the gel versus cells engaging endogenous matrix within the spheroid. These data suggest that cell-cell interactions have a similarly critical role as cell-ECM engagement to drive differentiation. Differences in osteogenic differentiation of spheroids with respect to RGD modification can be attributed to MSC population heterogeneity and differences in timing of cellular response. Although RGD improves cell engagement with the biomaterial, previous studies demonstrate that it does not affect angiogenesis in dissociated MSCs [44]. However, MSC spheroids entrapped in RGD-modified gels exhibited increased VEGF secretion and improved survival compared with hydrogels seeded with an equal number of dissociated MSCs or cells entrapped in unmodified alginate gels. Beyond VEGF, MSC spheroids in RGD-modified alginate secrete the most total proangiogenic factors. Of note, GRO, MCP-1, and IL-8 are important cytokines most highly upregulated in spheroids entrapped in RGD-modified alginate. These factors have critical angiogenic functions and, in addition to being secreted by MSCs, are also produced by cells of the innate immune system such as macrophages, neutrophils, and dendritic cells. These data indicate that RGD modification enhances the proangiogenic potential of MSC spheroids in a manner previously unrecognized with dissociated cells, and manipulation of RGD concentration may further enhance the proangiogenic potential of entrapped spheroids. Together, these data motivate the use of RGD-modified alginate as a biomaterial vehicle to effectively balance survival, proangiogenic potential, and osteogenic differentiation of entrapped MSC spheroids.
When used as cell-delivery vehicles, all tested biomaterials facilitated mineralization in situ. MSCs in RGD-modified alginate, whether entrapped as dissociated cells or spheroids, exhibited more robust mineralization than either group in unmodified gels. Regardless of biomaterial, the implantation of spheroid-containing gels resulted in more diffuse osteocalcin staining compared with dissociated controls. Importantly, these data confirm that MSCs deployed as spheroids in alginate gels exhibit increased cell survival and promote host cell infiltration without compromising their potential for bone formation. We utilized a high cell density (40 × 106 cells per milliliter) of MSCs, facilitating mineralization even in unmodified alginate. The capacity of RGD-modified gels to instruct spheroid function and promote bone formation with lower cell concentrations merits further investigation to reduce the total number of required cells for therapy.
These studies demonstrate that MSC spheroids entrapped in an engineered biomaterial retain their advantages in survival and function compared with dissociated MSCs when used for bone tissue engineering. To our knowledge, this is the first report characterizing the response of MSC spheroids when entrapped in RGD-modified alginate hydrogels. The use of engineered biomaterials provides an opportunity to prolong the proangiogenic and antiapoptotic advantages of spheroidal culture when cells are transplanted in vivo. Furthermore, increases in VEGF secretion observed with MSC spheroids in RGD-modified gels can be applied to any tissue-engineering strategy in need of vascularization.
Conclusion
The results of these studies confirm that MSC spheroids can be effectively entrapped in a biomaterial that subsequently enhances MSC survival and secretion of proangiogenic cues compared with dissociated cells. Future efforts to deploy spheroids in vivo should consider the use of biomaterials with desired adhesivity to regulate survival, angiogenesis, and tissue formation.
Acknowledgments
This work was supported by NIH Grant R01-AR067252 (to J.K.L.). S.S.H. was supported by the T32 Animal Models of Infectious Disease Training Program Kirschstein-NRSA (T32 AI060555). K.C.M. was supported by American Heart Association Western States Affiliate Predoctoral Fellowship 15PRE21920010.
Author Contributions
S.S.H. and K.C.M.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; B.Y.K.B. and C.B.V.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.K.L.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
J.K.L. is the president of a company to commercialize a hydrogel formulation from the University of California, Davis. The company is in the fundraising stage, there is no compensation for this work, and he is the inventor of the technology presented above. The other authors indicated no potential conflicts of interest.
References
- 1.Bhang SH, Lee S, Shin JY, et al. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012;18:2138–2147. doi: 10.1089/ten.tea.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci USA. 2006;103:2494–2499. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiarugi P, Giannoni E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fennema E, Rivron N, Rouwkema J, et al. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;31:108–115. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Maître JL, Heisenberg CP. Three functions of cadherins in cell adhesion. Curr Biol. 2013;23:R626–R633. doi: 10.1016/j.cub.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shweiki D, Neeman M, Itin A, et al. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: Implications for tumor angiogenesis. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaedtke L, Thoenes L, Culmsee C, et al. Proteomic analysis reveals differences in protein expression in spheroid versus monolayer cultures of low-passage colon carcinoma cells. J Proteome Res. 2007;6:4111–4118. doi: 10.1021/pr0700596. [DOI] [PubMed] [Google Scholar]
- 9.Bhang SH, Lee S, Lee TJ, et al. Three-dimensional cell grafting enhances the angiogenic efficacy of human umbilical vein endothelial cells. Tissue Eng Part A. 2012;18:310–319. doi: 10.1089/ten.tea.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EJ, Park SJ, Kang SK, et al. Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther. 2012;20:1424–1433. doi: 10.1038/mt.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy KC, Fang SY, Leach JK. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014;357:91–99. doi: 10.1007/s00441-014-1830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CC, Chen CH, Hwang SM, et al. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724–732. doi: 10.1634/stemcells.2008-0944. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann JA, McDevitt TC. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy. 2014;16:331–345. doi: 10.1016/j.jcyt.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Ohtaki H, Ylostalo JH, Foraker JE, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusamori K, Nishikawa M, Mizuno N, et al. Transplantation of insulin-secreting multicellular spheroids for the treatment of type 1 diabetes in mice. J Controlled Release. 2014;173:119–124. doi: 10.1016/j.jconrel.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Uchida S, Itaka K, Nomoto T, et al. An injectable spheroid system with genetic modification for cell transplantation therapy. Biomaterials. 2014;35:2499–2506. doi: 10.1016/j.biomaterials.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 18.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 20.Hersel U, Dahmen C, Kessler H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen CS, Mrksich M, Huang S, et al. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 22.Giancotti FG. Complexity and specificity of integrin signalling. Nat Cell Biol. 2000;2:E13–E14. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- 23.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 24.Bhat A, Hoch AI, Decaris ML, et al. Alginate hydrogels containing cell-interactive beads for bone formation. FASEB J. 2013;27:4844–4852. doi: 10.1096/fj.12-213611. [DOI] [PubMed] [Google Scholar]
- 25.Jose S, Hughbanks ML, Binder BY, et al. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater. 2014;10:1955–1964. doi: 10.1016/j.actbio.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KH, No DY, Kim SH, et al. Diffusion-mediated in situ alginate encapsulation of cell spheroids using microscale concave well and nanoporous membrane. Lab Chip. 2011;11:1168–1173. doi: 10.1039/c0lc00540a. [DOI] [PubMed] [Google Scholar]
- 27.Binder BY, Genetos DC, Leach JK. Lysophosphatidic acid protects human mesenchymal stromal cells from differentiation-dependent vulnerability to apoptosis. Tissue Eng Part A. 2014;20:1156–1164. doi: 10.1089/ten.tea.2013.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis HE, Miller SL, Case EM, et al. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011;7:691–699. doi: 10.1016/j.actbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Hoch AI, Binder BY, Genetos DC, et al. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis HE, Binder BY, Schaecher P, et al. Enhancing osteoconductivity of fibrin gels with apatite-coated polymer microspheres. Tissue Eng Part A. 2013;19:1773–1782. doi: 10.1089/ten.tea.2012.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decaris ML, Binder BY, Soicher MA, et al. Cell-derived matrix coatings for polymeric scaffolds. Tissue Eng Part A. 2012;18:2148–2157. doi: 10.1089/ten.TEA.2011.0677. [DOI] [PubMed] [Google Scholar]
- 32.Shih YR, Hwang Y, Phadke A, et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc Natl Acad Sci USA. 2014;111:990–995. doi: 10.1073/pnas.1321717111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens MM, Qanadilo HF, Langer R, et al. A rapid-curing alginate gel system: Utility in periosteum-derived cartilage tissue engineering. Biomaterials. 2004;25:887–894. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Wilson JL, Najia MA, Saeed R, et al. Alginate encapsulation parameters influence the differentiation of microencapsulated embryonic stem cell aggregates. Biotechnol Bioeng. 2014;111:618–631. doi: 10.1002/bit.25121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BR, Hwang JW, Choi YY, et al. In situ formation and collagen-alginate composite encapsulation of pancreatic islet spheroids. Biomaterials. 2012;33:837–845. doi: 10.1016/j.biomaterials.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Jeon O, Alt DS, Ahmed SM, et al. The effect of oxidation on the degradation of photocrosslinkable alginate hydrogels. Biomaterials. 2012;33:3503–3514. doi: 10.1016/j.biomaterials.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouhadir KH, Lee KY, Alsberg E, et al. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 39.Gillette BM, Jensen JA, Tang B, et al. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat Mater. 2008;7:636–640. doi: 10.1038/nmat2203. [DOI] [PubMed] [Google Scholar]
- 40.Salinas CN, Anseth KS. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med. 2008;2:296–304. doi: 10.1002/term.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Williams CG, Wang DA, et al. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Caires HR, Gomez-Lazaro M, Oliveira CM, et al. Finding and tracing human MSC in 3D microenvironments with the photoconvertible protein Dendra2. Sci Rep. 2015;5:10079. doi: 10.1038/srep10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huebsch N, Arany PR, Mao AS, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu J, Du KT, Fang Q, et al. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials. 2010;31:7012–7020. doi: 10.1016/j.biomaterials.2010.05.078. [DOI] [PubMed] [Google Scholar]