The profusion of stem cell-based clinical trials registered at ClinicalTrials.gov was mapped to explore the diversity of the fields of application and the temporal complexity of the domain. Mapping may reveal a lack of global strategy despite the regulations and the related costs associated with good manufacturing practices.
Keywords: Mesenchymal stromal cells, Stem cells, Regenerative medicine, Data mining, Clinical trials as topic
Abstract
We aim to provide an innovative, comprehensive way of mapping the profusion of stem cell-based clinical trials registered at ClinicalTrials.gov to explore the diversity of the fields of application and the temporal complexity of the domain. We used a chord diagram and phylogenetic-like tree visualizations to assist in data mining and knowledge discovery. The search strategy used the following terms: “stromal OR stem OR mesenchymal OR progenitor.” The Medical Subject Headings (MeSH) thesaurus was used to more finely classify diseases treated by stem cells, from large fields of application to specific diseases. Of the 5,788 trials screened, 939 were included, 51.1% of which were related to mesenchymal stem cells (MSCs). No real specificity emerged as to the therapeutic uses of the different types of stem cells. More than half the MSC studies concerned allogeneic MSCs and received more support from industry than autologous MSC studies (p < .001). Over time, the uses of cultured cells have increased greatly, particularly since 2009. Cells derived from adipose tissue are also increasingly used in trials compared with bone marrow cells. The use of adipose-derived stromal cells was predominantly autologous (p < .001), restricted to European countries (p < .01), and supported by industry (p = .02) compared with other MSCs. Details about MeSH keywords are available at http://multireview.perso.sfr.fr/. In conclusion, mapping may reveal a lack of global strategy despite the regulations and the related costs associated with good manufacturing practices. A systematic approach to preclinical data, intended to objectively and robustly reveal the most appropriate fields with the most efficient cells, is needed. Repeated exchanges between the bench and the bedside are necessary.
Significance
Except for a few trials concerning specific tissue stem cells used in their corresponding tissues, this global analysis revealed no real specificity of stem cell uses (including mesenchymal stromal cells). This raised the question of the physiopathological rationale for these uses and the lack of a global strategy despite the regulations and the related costs associated with good manufacturing practices. This original method, leading to the development of new concepts from already available data, would help policymakers to optimize resources and investments in terms of public health priorities. Such an approach should draw parallels between in vitro, in vivo, and human data. Exchanges in both directions between preclinical and clinical research could optimize the parameters of clinical trials step by step.
Introduction
In recent decades, many advances have been made in the area of stem cell therapies, revolutionizing our knowledge of tissue development, function, and physiopathology, as analyzed by the National Institutes of Health (NIH) [1, 2]. In fact, the broad development of bone marrow (BM) transplantation serves as the proof of concept for the use of adult stem cells [3, 4]. This demonstration also highlighted another immature cell closely associated with hematopoietic stem cells (HSCs) through its niche: the mesenchymal stem cell (MSC). First discovered by Friedenstein in the 1970s [5], MSCs are multipotent but also play a key supportive role via the secretion of numerous paracrine factors, including immunosuppressive molecules [6]. The identification of stem cells in almost all tissues [7] has paved the way for a large amount of preclinical data and numerous clinical trials—to such an extent that it has become difficult to have a global view of current trends and future directions in this still young and continuously growing field of research [8, 9]. Heterogeneous cell populations, sometimes characterized as “stem cells” (e.g., mononuclear cells from bone marrow [BMMNCs] and stromal vascular fraction from adipose tissue [AT; SVF]) or the adherent and expanded counterpart that corresponds to MSCs (bone marrow-MSCs [BM-MSCs] and adipose tissue-derived MSCs [ASCs], respectively), are used at clinical level. In addition to adult stem cells, embryonic stem cells (ESCs) and induced pluripotent stem cells have given rise to great hopes and are just starting to be investigated at the clinical level [10].
Many doubts about the efficacy and safety of cell therapy led to the need for exhaustive searches of clinical trials [11], critical analysis of their methods (i.e., risk of bias [12]), and investigation of the various parameters taken into account. The comparison between the different sources of stem cells, the use of heterogeneous or purified cells, and the recourse to allogeneic or autologous cell sources are among the many issues that remain unresolved [13, 14]. The scientific and economic implications of stem cells and the associated health care policy decisions make these issues crucial.
Public availability of mass data [15, 16] opens the way to the creation of new, practical knowledge that will help physicians, researchers, and policymakers to identify pertinent areas of intervention [15, 17] and reveal unexpected facts leading to the development of new concepts [18, 19]. We are keen to provide a view of the stem cell area that is both broad and deep by means of a systematic mapping review [18, 20]. This method could help in the interpretation of scientific information while enlarging the field of exploration [21] and providing adequate data visualization [22].
One requirement for knowledge discovery in databases (KDD) is to work with relevant, up-to-date databases associated with low noise and errors [23]. Different sources of data can be used. Analyses of published studies take advantage of the scientific peer-review process, but publication is often delayed and negative trials are less likely to be published. This leads to a retrospective, and potentially biased, point of view. During the 1990s, clinical trial registers were strongly promoted in biomedical research, with the aim of revealing the existence of all trials and eliminating publication bias [24]. The International Committee of Medical Journal Editors required registration of all trials starting enrollment after July 1, 2005, and of ongoing clinical trials that began enrolling patients before that date [24]. Exploration of these registers gives a more up-to-date and representative snapshot of the complexity of the young and constantly evolving field of stem cells [25, 26]. Among the different registers, the ClinicalTrials.gov database (CTD) is one of the best designed for aggregation and analyses [27]. Launched in 2000, concomitantly with the setting up of stem cell related trials, this worldwide register contains detailed, standardized characteristics of each trial, particularly keywords describing disease conditions using the Medical Subject Headings (MeSH) thesaurus, as furnished by the U.S. National Library of Medicine. This provides an in-depth classification and enables computer tools to be used for interpretation at both low (fields of application) and high (specific diseases) granularity.
From this database, we screened 5788 trials and, using computer tools, comprehensively mapped human stem cell-based clinical trials using a chord diagram and phylogenetic-like tree visualizations. This revealed no real specificity in the uses of stem cells, including MSCs, in the different fields of application. The lack of an apparent global strategy despite the regulations (e.g., requirements for advanced-therapy medicinal products in Europe and good tissue practice in the United States [28]) and the costs associated with good manufacturing practices may be hypothesized and are probably linked to the field’s strong intention to reach the market. Increasing interest in cultured and allogeneic cells compared with the heterogeneous fraction and autologous uses, respectively, was revealed.
Materials and Methods
Data Aggregation
Data aggregation was performed using a Perl script developed in the laboratory to minimize errors during the screening process and to computerize data for further analyses (supplemental online Figure 1; supplemental online Table 1).
Dataset Selection, Data Cleaning, and Preprocessing
The search strategy used the keywords “stromal OR stem OR mesenchymal OR progenitor.” All trials were exported into XML format, then parsed into local database. Trials were included if (a) cell therapy was based on enriched/purified/sorted stem cells or expanded stem cells and (b) efficacy or safety of stem cell therapy was stated as an objective of the trial. For each trial included, the original tissue for cells and the donor (autologous or allogeneic) were recorded. Only the “Diseases” branch (C) of the MeSH classification is used by the CTD to describe study conditions. This branch contains 26 sub-branches, corresponding to major disease groupings. If the CTD had failed to attribute MeSH keywords to a trial, two authors (P.M. and J.N.V.) manually added the keywords they found most appropriate by consensus. The last search was performed on June 28, 2015.
Data Reduction and Projection
The C23 (Pathological Conditions, Signs and Symptoms) sub-branch was removed for lack of specificity. For chord diagrams, MeSH redundancy was assumed: One trial could be classified in several fields of application. For tree rendering, the final custom MeSH structure used is described in supplemental online File 1 (selected terms in bold); desired duplicates were identified to indicate to the reader that there were several occurrences.
Exploratory Analysis, Model, and Hypothesis
The phase and size of the clinical trial, the sex of participants, the clinical trial sites, the blinding and randomization techniques used, the type of stem cells used, the cell donors, and the fields of application were described. Each CTD entry contained information about the lead sponsor and the collaborators declared by the authors, especially if this funding source was considered to derive from “NIH, Industry or Others.” By using the algorithm of Califf et al. [29] on these lead sponsors and collaborators tags, the probable funding source (i.e., the main sponsor) was categorized as “NIH, Industry or Others.” The start dates of trials were considered, as was their clinical phases, as markers of the progress and maturity of a theme.
Data Mining
A phylogenetic-like representation was obtained by using the Interactive Tree of Life (http://itol.embl.de) [30]. Because phylogenetic trees were developed to schematically represent kinship relationships between groups of living beings, we used this type of representation to visualize relationships between diseases described in the MeSH classification. All MeSH descriptor terms extracted from included trials (disease conditions) were represented around a rooted tree. Summary statistics for each term, together with individual lists of the related trials, were provided. Internal divisions of the tree were represented by sublevels of these terms (ancestors). Two major datasets were represented around the tree (online data): trial phases and start dates. Chord diagrams [31] were used to analyze double-entry tables (http://mkweb.bcgsc.ca/tableviewer/). We presented the proportion of trials classed in each of the MeSH sub-branches (fields of application) in relationship with type of stem cells and the start dates of studies or trial phases. An example was furnished (supplemental online Figure 2). Each disease was linked to a website we generated (http://multireview.perso.sfr.fr/) in which all statistics were summarized for each MeSH keyword included.
Results
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is given in supplemental online Figure 3. A majority of records (1,497 [61.5%]) dealt with HSC transplantation after chemotherapy or radiotherapy. We focused our analysis on the remaining 939 trials, of which 51.1% were related to MSCs (supplemental online Table 2).
Temporal Evolution of Stem Cell Use According to the Applications
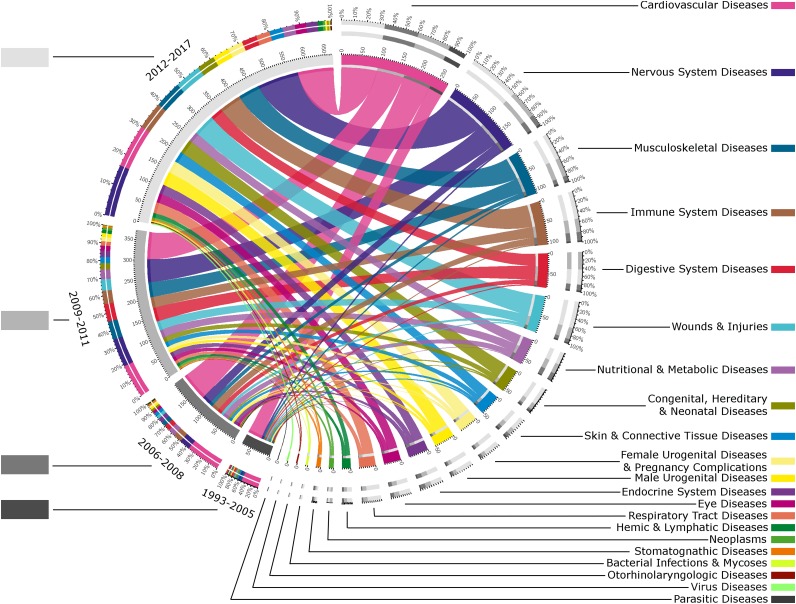
Ultimately, 21 MeSH fields of application were selected, allowing diseases and their consequences to be considered. Figure 1 presents the temporal evolution of these fields. Three important groups of diseases can be extracted according to their representations: (a) cardiovascular system (CVD) and nervous system diseases (NSD); (b) musculoskeletal conditions, immune system disease (ISD), digestive diseases, and wounds and injuries, which really emerged from 2009; and (c) a smaller group with nutritional, skin (and connective tissue), female urogenital (and pregnancy complications), male urogenital, endocrinal, eye, and respiratory diseases. About half the studies starting in 1993–2005 dealt with CVD, and their number remained constant over the years (approximately 80 studies), whereas NSD studies began to gain momentum from 2006 (25 studies in 2006–2008 rising to 100 in 2012–2017).
Figure 1.
Temporal evolution of fields of application for regenerative medicine by stem cells. Studies using hematopoietic stem cell (HSC) transplant (with total-body irradiation, myeloablative or nonmyeloablative regimens, or genetically modified HSCs) were excluded from this figure. This chord diagram represents the proportion of studies dealing with each field of application (branch of the Medical Subject Heading [MeSH] classification [Diseases]) and links them to their respective start dates. The gray levels for the different years (on the left) and the color codes for the different fields of application (on the right) are shown beside their respective labels, listed in order of frequency (this order will be maintained hereafter). The outer ring of the figure contains the proportion of studies and the inner circle shows their absolute numbers. In each ring, the contribution of each field of application in each time category is coded by colored segments, and vice versa. These colored segments are also sorted by importance. For instance, and reading the diagram from MeSH classification, in the field of cardiovascular regeneration, 70 trials started in 2006–2008 (indicated in the inner part of the circular diagram) and made up 36% of the fields in 2006–2008 (indicated on the left of the outer part of the circular diagram). Conversely, 25% of cardiovascular diseases trials were started in 2006–2008 (indicated on the right of the outer part of the circular diagram).
Application Areas According to Stem Cell Types
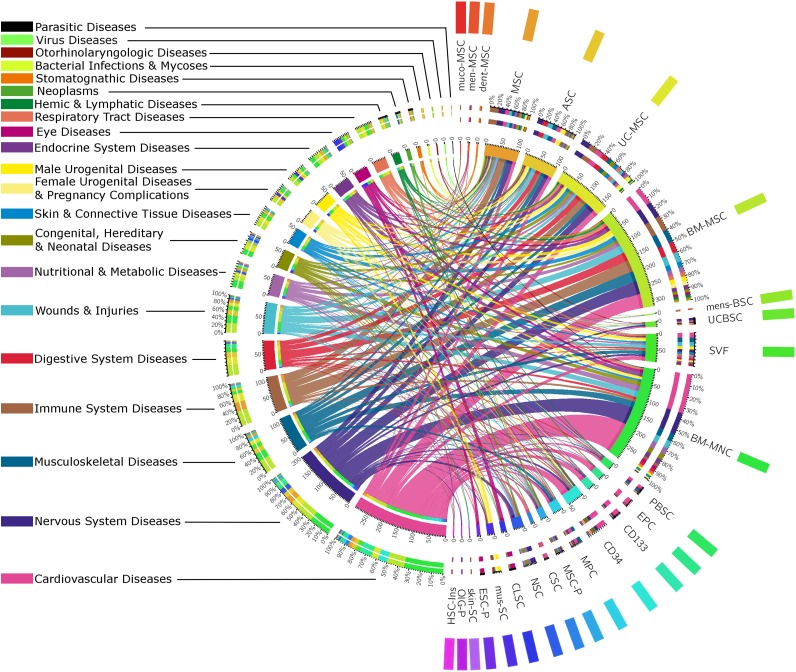
We grouped cells into four categories: MSCs, their respective heterogeneous fractions, other hematopoietic stem cells (PBSCs, EPCs, CD34+ cells, and CD133+ cells), and other stem cells and progenitors (Fig. 2). ESC-derived cells were considered in 10 studies, with the use of ESC-derived retinal pigment epithelial cells in 8 studies for eye diseases. Tissue-specific stem cells were used in the corresponding application field (e.g., cardiac stem cells for CVD and corneal limbal stem cells for eye diseases). The predominant uses of EPCs and CD133+ cells were also in CVD. Nevertheless, these cells were used in few studies and can be considered as exceptions. A glance at the diagram shows that each cell type was addressed by multiple fields of application and, in a mirror analysis, each field of application addressed multiple types of cells. As a whole, Figure 2 reveals no specific application for a specific cell type.
Figure 2.
Uses of the different types of stem cells in regenerative medicine. Studies using hematopoietic stem cell transplant (with total-body irradiation, myeloablative or nonmyeloablative regimens, or genetically modified HSCs) were excluded from this figure. This chord diagram represents the proportion of studies dealing with each field of application, linked to their respective uses of stem cells. The color codes for the different fields of application (on the left) and the color codes for the different types of stem cells (on the right) are shown beside their respective labels. Abbreviations: ASC, adipose tissue-derived mesenchymal stem cell; BMMNC, bone marrow mononuclear cell; BM-MSC, bone marrow mesenchymal stem cell; CD133, CD133+ cells; CD34, CD34+ cells; CLSC, corneal limbal stem cell or retinal progenitor cell; CSC, cardiosphere-derived cells, cardiac stem cells; dent-MSC, mesenchymal stem cell from dental tissues; EPC, endothelial or angiogenic precursor cell; ESC-P, embryonic stem cell-derived cells; ESC-RPE, embryonic stem cell retinal pigment epithelial and other progenitors; HSC-Ins, hematopoietic stem cell-derived cell producing insulin; men-MSC, menstrual mesenchymal stem cell; mens-BSC, menstrual blood stem cell; MPC, mesenchymal precursor cell; MSC, mesenchymal stem cell (probably bone marrow stem cells); MSC-P, mesenchymal stem cell-derived progenitors and cells (osteoprogenitor, mesenchymal stem cell-derived cardiopoietic cell, neuroprogenitor, osteoprogenitor, hepatic cell, endometrium); muco-MSC, mucosal mesenchymal stem cell; mus-SC, muscular stem cell; NSC, neural stem cell; OlG-P, oligodendrocyte or glial progenitor; PBSC, peripheral blood stem cell; skin-SC, skin stem cell; SVF, stromal vascular fraction; UCBSC, umbilical cord blood stem cell; UC-MSC, umbilical-cord or umbilical-cord blood mesenchymal stem cell or Wharton jelly mesenchymal stem cell or placental mesenchymal stem cells.
Description of Studies Using MSCs and BMMNCs/SVF
Because MSCs were involved in half the trials, we next focused on these cells (supplemental online Table 2; Figure 2). Studies using these cells enrolled predominantly small cohorts of patients (median with interquartile range [Q1, Q3] of 25 [12, 55]); 44.7% (213) of them were randomized and 67.4% were open-labeled. The MSCs used were allogeneic for 53% and autologous for 47%. With the website we created (http://multireview.perso.sfr.fr/msc/29163.html), we found 50% of allogeneic use in CVD but 63% and 70% for the subcategories for stroke and myocardial infarction.
When studies using ASCs were compared with those using other sources of MSCs, the number of enrolled patients was smaller in ASC studies (respective medians of 19 [Q1, Q3: 10, 40] and 27 [Q1, Q3: 12, 50], p = .02), more uses were autologous (68.4% and 43.0% of studies, respectively; p < .001) and activity was more restricted to European countries (55.4% and 23.3% of studies, respectively; p < .001). Concerning the main sponsor, industry supported 41.6% and 28.3% of ASC and other MSC studies, respectively (p = .02). Although the number of trials involving ASCs was smaller than for other sources of MSCs, there was no difference in study phases. ASCs were also significantly less tested in ISD (p = .01), were more tested in female urogenital diseases (p = .04), and showed an increasing trend in digestive diseases and wounds and injuries. Nevertheless, for all areas where ASCs were tested, MSCs were also tested.
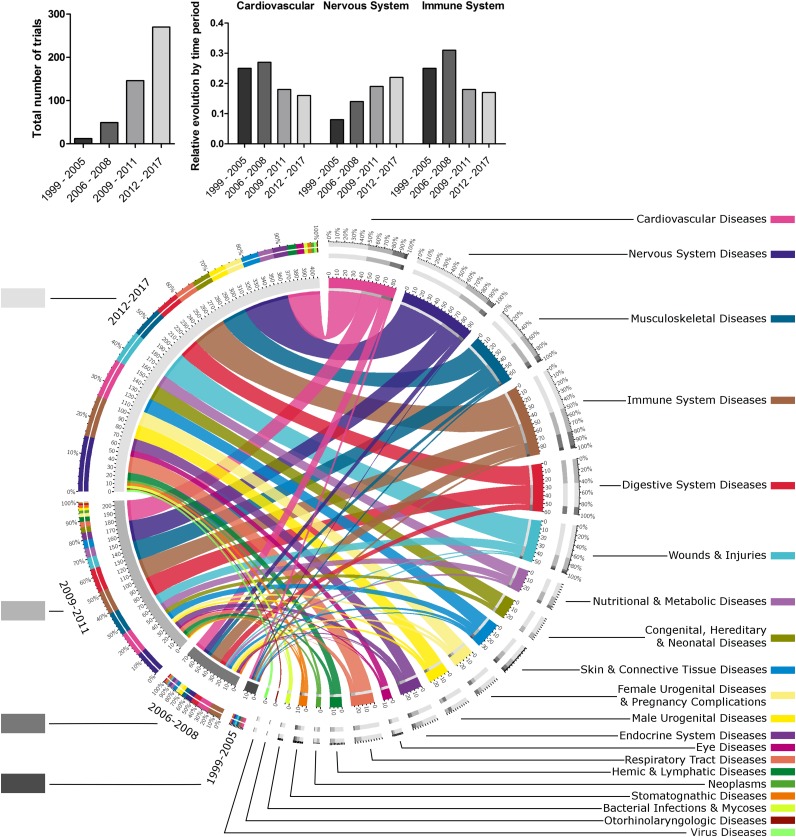
Temporal Evolution of MSC Applications
From 1999 to 2005, 12 trials were set up in 8 fields whereas 270 trials in 20 out of 21 fields are described today (Fig. 3). Until 2008, CVD and ISD (e.g., graft-versus-host disease, autoimmune diseases) were the most important, but their relative importance decreased over time in favor of a diversification of the fields. NSD has increased over time to become the most studied field. Digestive and musculoskeletal investigations also developed at this time. From 2012, trials concerning respiratory and male or female urogenital diseases increased greatly, and a set of otorhinolaryngology themes emerged. Over time, allogeneic MSCs became used more than did autologous ones (6, 15, 81, and 144 vs. 5, 31, 61, and 120 for allogeneic and autologous, respectively). Studies using allogeneic MSCs were also significantly more supported by industry (modest-sized companies [supplemental online Table 3]) than studies using autologous MSCs (37.0% and 22.4%, respectively; p < .001).
Figure 3.
Temporal evolution of fields of application for regenerative medicine by mesenchymal stem cells (MSCs). This chord diagram represents the proportion of studies dealing with each field of application, linked to the corresponding start year of trials. This formally illustrates the burst of cell therapy by MSCs between 1999 and today. The following MSCs were merged: mucosal MSCs, menstrual blood MSCs, MSCs from dental tissues, adipose tissue-derived MSCs, umbilical cord-MSCs, and bone marrow-MSCs. More details about abbreviations for cells are given in the legend of Figure 2. The gray levels for the different years (on the left) and the color codes for the different fields of application (on the right) are shown beside their respective labels. For each time period, the total number of studies, and the relative number of studies for cardiovascular, nervous system and immune system diseases, are represented by a histogram in the upper part of the figure.
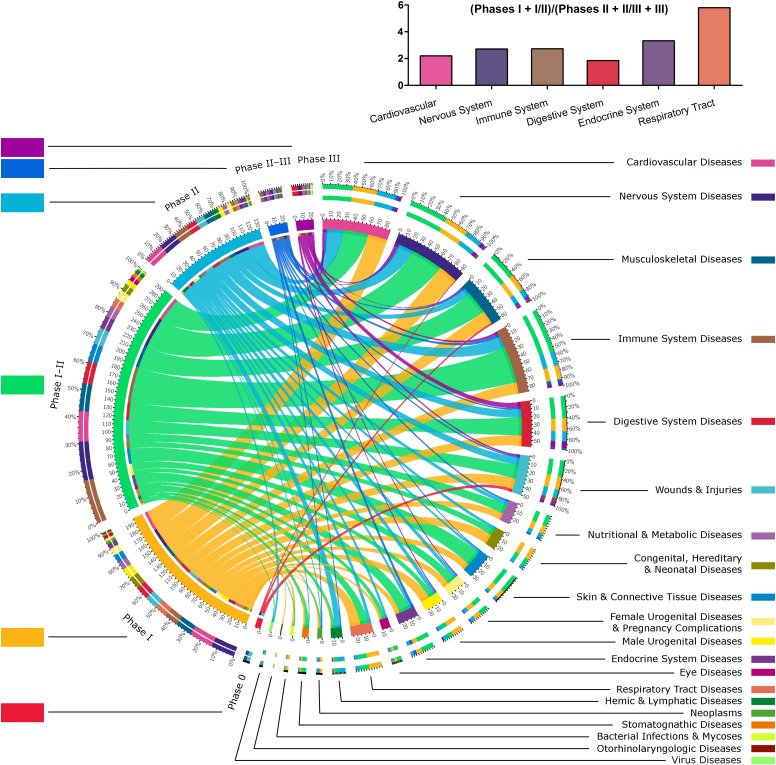
Study Phases for MSCs as a Marker for Maturity of the Field
Phase I/II and phase II studies made up 59.6% of MSC studies. Of 21 fields of application, only 11 fields had phase II/III and 8 had phase III studies registered. Digestive disease then CVD, ISD, and NSD fields of application appeared more mature, with more phase III, II/III, and II studies (Fig. 4). With 50% of phase I studies, the respiratory field appeared to be a younger topic. With both a high number of phase I/II and some phase II/III and III trials, endocrine, male or female urogenital and skin diseases, and wounds and injuries seemed to be in an intermediate state of study. This tendency was confirmed with a higher ratio of phase I and I/II to phase II, II/III, and III trials for the respiratory tract and endocrine system (Fig. 4, histograms). Moreover, some phase II studies (e.g., for stomatognathic, respiratory, or eye diseases) were not represented in phase II/III or phase III.
Figure 4.
State of progress of mesenchymal stem cell (MSC) uses in regenerative medicine. This chord diagram exposes the relationships between application fields and study phases for MSCs, which reveals the enthusiasm for such therapy and the fact that we will soon know the efficacy of these therapies in a large number of fields of application. The following MSCs were merged: mucosal MSCs, menstrual blood MSCs, MSCs from dental tissues, adipose tissue-derived MSCs, umbilical cord-MSCs, and bone marrow-MSCs. For more details about abbreviations for cells, please see the legend of Figure 2. The color codes for the different study phases (on the left) and the color codes for the different fields of application (on the right) are shown beside their respective labels. The ratios of the number of phase I, I/II trials to the number of phase II, II/III, and III trials for cardiovascular, nervous system, immune system, digestive system, respiratory tract, and endocrine system diseases are represented by a histogram in the upper part of the figure.
Expanded Cells Versus Heterogeneous Fraction and BM- versus AT-derived cells
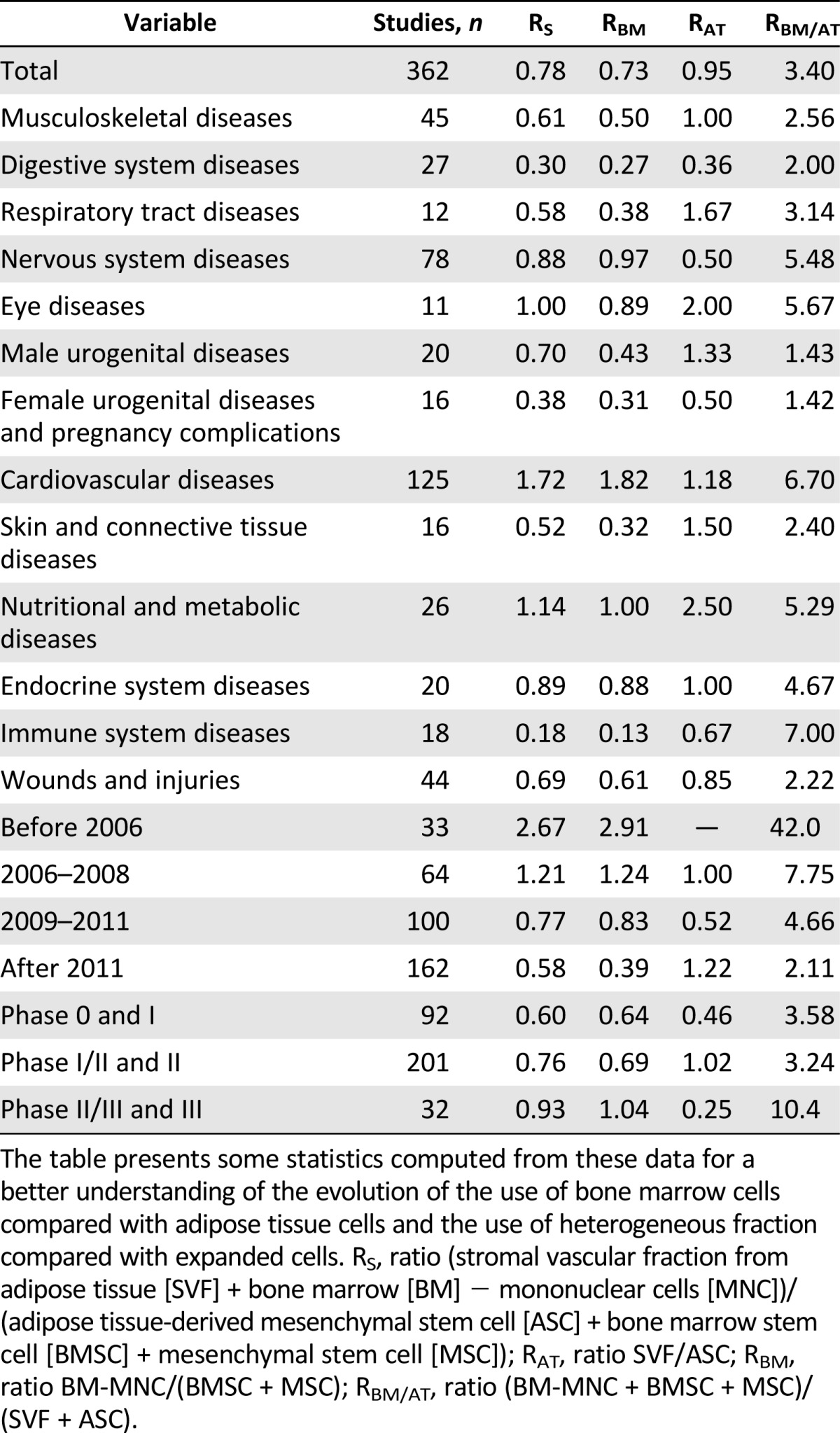
To make these comparisons, we computed different ratios (Table 1; supplemental online Figure 4). The ratio of heterogeneous fraction to expanded cells (RS) decreased over time (RS of 2.67 before 2006, 0.58 after 2011). This meant that heterogeneous fractions tended to be neglected in favor of cultured cells over time. Concerning study phases, heterogeneous fractions concerned more phase II/III and phase III than other phases (RS of 0.93 versus 0.76 and 0.60). Remarkably, three fields of application had lower RS than the others, showing that cultured cells were particularly used for female urogenital, digestive, and immune diseases. In the same way, RBM and RAT ratios compared heterogeneous versus expanded cells in BM and AT, respectively. Some differences can be pointed out between these two ratios: for respiratory, male urogenital, skin, and immune diseases, expanded cells were more used for BM than they were for AT. The BM-to-AT cell ratio (RBM/AT) highlighted disparities between fields of application (e.g., a greater use of BM cells for CVD and ISD and a greater use of AT cells for female or male urogenital diseases compared with other fields). Despite these differences, both heterogeneous and expanded cells, and both BM and AT cells, were used in all main fields of application. BM cells were also more used in phase III studies than were AT cells (RBM/AT was higher in phase II/III/phase III studies than in other phases). But this ratio decreased over time (RBM/AT of 42.0 before 2006, 2.11 after 2011). This meant that cells from AT were increasingly used compared with cells from BM.
Table 1.
Comparison between culture-expanded mesenchymal stem cells and the respective heterogeneous fractions, from bone marrow or adipose tissue
Phylogenetic-Like Tree Representation to Detail Uses of Stem Cells for Each Precise Disease
Start dates and phases of studies were represented at the level of specific diseases, around the tree. Our website (http://multireview.perso.sfr.fr/) was composed of three parts:all stem cells, MSCs, and ASCs. It was created to go deeper into the MeSH architecture and to obtain more details about the clinical trials using stem cells in the context of a specific disease (example for MSCs in cardiovascular diseases in supplemental online Figure 5). The following link provides an enlargeable interactive visualization of all pathologies for which stem cells are used: http://itol.embl.de/shared/paulmonsarrat. For instance, supplemental online Figure 6 gives a precise inventory of the pathologies that may benefit from treatment with ASCs. Again, we observed no real specificity in their use, and, even for each application, a great diversity was observed.
Discussion
The 2000s were marked by great interest in regenerative medicine based on adult stem cells [10, 32–35] and the establishment of financing programs before the isolation of ESCs [36]. Since then, the increasing proliferation of preclinical data has been generating great hopes. The recent translation of this research to clinical trials requires an objective view of these constantly evolving fields. Our innovative approach revealed that, apart from the classic use of hematopoietic stem cells [4], there was no strict specificity in the therapeutic uses of the different types of stem cells. We also highlighted the evolution toward the use of allogenic and cultured and expanded cells at the expense of heterogeneous and autologous fractions. Finally, adipose tissue appears to be a tissue source that is becoming more and more privileged.
The lack of specificity is surprising with regard to the large amount of preclinical data but is symptomatic of the immaturity of this domain [6, 25, 37]. This suggests that most of what emerges at the level of clinical trials is not the logical consequence of a strong, convergent background of basic and preclinical studies but is more related to individual initiatives. This could be considered as the result of a variety of points of view. First, such therapy is thought to treat any disease because of the pluripotency/multipotency and immune regulatory/suppressive action of the putative therapeutic products [38]. This is reinforced by the dream of industrial companies to have a universal cell product that treats many diseases. Second, the therapeutic products (i.e., cells) are not yet well-defined and could correspond to different products, pluripotent or multipotent, heterogeneous nonpurified or purified cells, nonexpanded or cultured, autologous or allogeneic cells, with no clear comparative view, as previously described. Both these standpoints differ from the classic “one product for one target” approach [39].
Third, the lack of a consensual view emerging from the multiplicity of preclinical data leaves many opportunities and cell/application combinations open. This also raises questions about the physiologic relevance of preclinical models to diseases. Furthermore, few preclinical and experimental studies comparing different types of cells have been conducted and published [40, 41]. This point highlights the need for a systematic approach to preclinical data to objectively and robustly reveal the most appropriate fields with the most efficient cells. Exchanges of information back and forth between the bench and the bedside are necessary. Finally, ethical issues associated with the use of ESCs in many countries [10] and the fact that opinion has often been refractory to research on ESCs [42] has led to alternative sources of therapeutic cells that are even less efficient being found.
Our analysis reveals that the cell types most often tested are MSCs, with increasing interest over time in cultured cells compared with their respective heterogeneous fraction. The cardiovascular field was the first to be clearly identified but, since 2005, the number of studies dedicated to such applications has not changed. 2005 was a key year because, from then on, all applications other than cardiovascular ones were increasingly investigated. The heterogeneous fraction is accessible at the bedside within hours and is almost never characterized. In contrast, the expanded fraction needs time, and it is more expensive to comply with US Food and Drug Administration and European regulations [28]; however, this fraction can be more finely characterized and secured. The fewer important resources necessary for the heterogeneous fraction compared with expanded cells may explain why there are still more advanced phases for the former, although several studies have reported modest successes or negative outcomes [43] in chronic heart failure, in contrast to Cochrane meta-analyses [44, 45].
These elements could explain why cardiac regeneration by stem cells is no longer the main attractive area. This discrepancy in result analysis may be related to the design of the control groups [46]. In most trials, control groups did not undergo tissue sampling to isolate cell products and did not have the benefit of putative placebo effects and/or the positive physiologic response induced by sampling in injected patients. This strongly suggests that sham-operated control groups are absolutely required to allow a conclusion about efficacy.
The choice between heterogeneous BMMNCs and BM-MSCs may depend on the application, as demonstrated by the meta-analysis of large animal models of ischemic heart disease that reported significantly reduced efficacy of BMMNCs compared with BM-MSCs [40] but no difference for chronic kidney diseases [41]. Unexplained and recurrent discrepancies with autologous BMMNC in many clinical trials for heart diseases [47], as well as smaller suggested effects of BMMNC compared with BM-MSC for critical limb ischemia and foot ulcers, may have a role in the progressive decline of the use of heterogeneous fraction [48]. This could also explain the relative decline of investigations in the cardiac field. Furthermore, the increasing involvement of the pharmaceutical industry in cell therapy promotes the uses of cultured cells compared with the heterogeneous fraction most supported by the bio-devices industry [8, 49].
For MSCs, two thirds of studies were run on an open-label design, revealing that this field has not yet reached maturity, but it should do so in the next few years given the number of phase II trials in various fields. Among MSC tissue sources, the increasing use of AT is consistent with the fact that it is the richest adult source of MSCs and is easy to sample by liposuction under local anesthesia [50]. BM-MSCs benefit from their longer research history and the associated significant scientific hindsight [5, 51].
The power of our analysis, based on the MeSH ontology, allows us the opportunity to reflect on pathophysiology when focusing on fields of application. This work thus highlights the predominance of cultured cells for immune diseases or their consequences [37]. It is noteworthy that the uses of BM-MSCs for neurologic disorders and ASCs for graft-versus-host disease or rectal fistula were, as expected, far from unrelated to the putative specific biological features because of the initial native environment. This emphasizes the possibility that the biological features of these cells have not been well-characterized and could reveal unexpected physiological features of these cells [51]. Allogeneic MSC trials have increased, and their easy production, in large numbers from selected donors with no systemic pathology, may compensate for the decrease in MSC potentiality with age or pathological conditions, which may interfere with autologous grafts [14, 51].
The limitations of this study concerning the representativeness of this dataset should be considered. Other sources could have been consulted, such as the International Clinical Trials Registry Platform (ICTRP), which provides access to trials from several worldwide databases, including CTD (which accounts for about 60%–80% [52, 53]). Unfortunately, ICTRP displays great disparities in data quality among the different registries, particularly for important elements, such as primary outcomes and intervention details; this makes it difficult to conduct a systematic review [52]. CTD was chosen because it has more detailed and standardized exportable characteristics and registers a majority of trials. For instance, a search performed in ICTRP with the keywords “mesenchymal stem cells” indicated that at least 408 of 562 trials (73%) were registered in CTD. Furthermore, disease misclassification, although variable according to clinical specialties, is low [27], and some registered data even better reflect reality than the final publications [54]. Another limitation was that registered trials make up only a part of all existing trials [24]. It has been suggested that only 50% of clinical studies indexed in PubMed that involved administration of cells for regenerative medicine indicated any clinical trial identifier [53]. In fact, the final dataset is only the tip of the iceberg, and the explosion of cell therapy outside the framework of academic research strongly concerns the scientific community. All around the world, private clinics already offer stem cell tourism, routinely injecting MSCs provided by some companies with nonvalidated or unproven procedures [55–58]. Because these clinics often use registries to gain legitimacy, an important implication of this work would be to carefully analyze final publications, especially from these companies, to assess the quality of and transparency of cell culture/procedures, cell controls, study design, reporting, placebo-controlled efficacy, and transparency about cell culture/processing and ethical considerations [25, 59, 60].
Another limitation is semantic. The frequent confusion between expanded cells and heterogeneous fraction (particularly the term “stem” for CVD) does not facilitate analysis of this field. For instance, in NCT01788059, “injection [of] mesenchymal stem cell” was used, whereas the authors described the isolation of BMMNCs using Ficoll gradient. Similarly, the term "adipose-derived stem cells" has been used by some authors to describe the injection of stromal vascular fraction (e.g., NCT02216630 and NCT01586715).
Now, although almost 30% of studies have been terminated or completed, only 1% of the studies have posted results on CTD. For the future and for all cells and applications, the registration of final results will be required to fight against bias in analysis that results from the fact that negative outcomes are rarely published.
Conclusion
Stem cell research is challenged by two contradictory trends: diminished funding from public and private sponsors and increased scientific opportunities [61, 62]. Our work demonstrates the power of wide-reaching analysis, which could reveal unexpected facts and lead to the development of new concepts from already available data. Such an approach should draw parallels between in vitro, in vivo, and human data. Exchanges in both directions between preclinical and clinical research could optimize the parameters of clinical trials step by step. Such optimization would include the best sources of stem cells, the choice between heterogeneous or purified cells, and between allogeneic or autologous cell sources, taking the pathophysiology of diseases and the patients’ characteristics into account.
Supplementary Material
Acknowledgments
This study was supported by funding from the Midi-Pyrenees region, Paul Sabatier University, and the research platform from Toulouse Dental Faculty. We thank Susan Becker for proofreading.
Author Contributions
P.M.: conception and design, collection and assembling of the data, data analysis and interpretation, manuscript writing; J.-N.V.: conception and design, data analysis and interpretation, manuscript writing; V.P.-B. and P.K.: conception and design, manuscript writing, final approval of manuscript; P.R.: conception and design, final approval of manuscript; L.S.: data analysis and interpretation, manuscript writing, final approval of manuscript, language checking; L.C.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript, language checking.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Medvinsky A, Livesey FJ. On human development: lessons from stem cell systems. Development. 2015;142:17–20. doi: 10.1242/dev.114868. [DOI] [PubMed] [Google Scholar]
- 2.Collins FS. Exceptional opportunities in medical science: A view from the National Institutes of Health. JAMA. 2015;313:131–132. doi: 10.1001/jama.2014.16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser SL. Hematopoietic stem cell transplantation for MS: extraordinary evidence still needed. JAMA. 2015;313:251–252. doi: 10.1001/jama.2014.18150. [DOI] [PubMed] [Google Scholar]
- 4.Harousseau JL, Moreau P. Autologous hematopoietic stem-cell transplantation for multiple myeloma. N Engl J Med. 2009;360:2645–2654. doi: 10.1056/NEJMct0805626. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 6.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 8.Breakdown of mesenchymal stem cell clinical trials by phase, geography, sponsors, and more. Bioinformant. Available at http://www.bioinformant.com/global-breakdown-of-mesenchymal-stem-cell-clinical-trials-by-phase/. Accessed March 28, 2016.
- 9.Squillaro T, Peluso G, Galderisi U. Clinical Trials with mesenchymal stem cells: An update. Cell Transplant. 2015 [Epub ahead of print] doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 10.Aldhous P. A world of difference. Nature. 2001;414:838. doi: 10.1038/414838a. [DOI] [PubMed] [Google Scholar]
- 11.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: The Cochrane Collaboration; 2009. [Google Scholar]
- 13.Vonk LA, de Windt TS, Slaper-Cortenbach IC, et al. Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: A concise review. Stem Cell Res Ther. 2015;6:94. doi: 10.1186/s13287-015-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minguell JJ, Allers C, Lasala GP. Mesenchymal stem cells and the treatment of conditions and diseases: The less glittering side of a conspicuous stem cell for basic research. Stem Cells Dev. 2013;22:193–203. doi: 10.1089/scd.2012.0417. [DOI] [PubMed] [Google Scholar]
- 15.Weber GM, Mandl KD, Kohane IS. Finding the missing link for big biomedical data. JAMA. 2014;311:2479–2480. doi: 10.1001/jama.2014.4228. [DOI] [PubMed] [Google Scholar]
- 16.Zaslavsky A, Perera C, Georgakopoulos D. Sensing as a service and big data. Available at http://arxiv.org/ftp/arxiv/papers/1301/1301.0159.pdf. Accessed March 28, 2016.
- 17.Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309:1351–1352. doi: 10.1001/jama.2013.393. [DOI] [PubMed] [Google Scholar]
- 18.Gough D, Thomas J, Oliver S. Clarifying differences between review designs and methods. Syst Rev. 2012;1:28. doi: 10.1186/2046-4053-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trounson A, Thakar RG, Lomax G, et al. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant MJ, Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Y, Hunt RH. Systematic reviews: The good, the bad, and the ugly. Am J Gastroenterol. 2009;104:1086–1092. doi: 10.1038/ajg.2009.118. [DOI] [PubMed] [Google Scholar]
- 22.Wong B. Points of view: Visualizing biological data. Nat Methods. 2012;9:1131–1131. [Google Scholar]
- 23.Fayyad U, Piatetsky-Shapiro G, Smyth. P. From data mining to knowledge discovery in databases. AI Mag. 1996;17:37–54. [Google Scholar]
- 24.Dickersin K, Rennie D. The evolution of trial registries and their use to assess the clinical trial enterprise. JAMA. 2012;307:1861–1864. doi: 10.1001/jama.2012.4230. [DOI] [PubMed] [Google Scholar]
- 25.Sharma RR, Pollock K, Hubel A, et al. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418–1437. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeAngelis CD, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA. 2004;292:1363–1364. doi: 10.1001/jama.292.11.1363. [DOI] [PubMed] [Google Scholar]
- 27.Tasneem A, Aberle L, Ananth H, et al. The database for aggregate analysis of ClinicalTrials.gov (AACT) and subsequent regrouping by clinical specialty. PLoS One. 2012;7:e33677. doi: 10.1371/journal.pone.0033677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sensebé L, Gadelorge M, Fleury-Cappellesso S. Production of mesenchymal stromal/stem cells according to good manufacturing practices: A review. Stem Cell Res Ther. 2013;4:66. doi: 10.1186/scrt217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Califf RM, Zarin DA, Kramer JM, et al. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA. 2012;307:1838–1847. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 30.Letunic I, Bork P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 31.Krzywinski M, Schein J, Birol I, et al. Circos: An information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuehn BM. Advances aim to ease stem cell concerns. JAMA. 2005;294:2557–2558. doi: 10.1001/jama.294.20.2557. [DOI] [PubMed] [Google Scholar]
- 33.Kuehn BM. Stem cells created from somatic cells. JAMA. 2005;294:1475–1476. doi: 10.1001/jama.294.12.1475. [DOI] [PubMed] [Google Scholar]
- 34.Aldhous P. Can they rebuild us? Nature. 2001;410:622–625. doi: 10.1038/35070659. [DOI] [PubMed] [Google Scholar]
- 35.A human stem cell project? Nature. 2002;418:1. doi: 10.1038/418001a. [DOI] [PubMed] [Google Scholar]
- 36.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 37.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 38.Ren G, Chen X, Dong F, et al. Concise review: Mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Translational Medicine. 2012;1:51–58. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilic D. Trends in the stem cell and regenerative medicine industry. Regen Med. 2012;7:645–648. doi: 10.2217/rme.12.52. [DOI] [PubMed] [Google Scholar]
- 40.van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 41.Papazova DA, Oosterhuis NR, Gremmels H, et al. Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech. 2015;8:281–293. doi: 10.1242/dmm.017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nisbet MC. The competition for worldviews: Values, information, and public support for stem cell research. Int J Public Opin Res. 2005;17:90–112. [Google Scholar]
- 43.Pavo N, Charwat S, Nyolczas N, et al. Cell therapy for human ischemic heart diseases: Critical review and summary of the clinical experiences. J Mol Cell Cardiol. 2014;75:12–24. doi: 10.1016/j.yjmcc.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Fisher SA, Brunskill SJ, Doree C, et al. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2014;4:CD007888. doi: 10.1002/14651858.CD007888.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Clifford DM, Fisher SA, Brunskill SJ, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 46.Jeong H, Yim HW, Cho Y, et al. The effect of rigorous study design in the research of autologous bone marrow-derived mononuclear cell transfer in patients with acute myocardial infarction. Stem Cell Res Ther. 2013;4:82. doi: 10.1186/scrt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis DP, Mielewczik M, Zargaran D, et al. Autologous bone marrow-derived stem cell therapy in heart disease: Discrepancies and contradictions. Int J Cardiol. 2013;168:3381–3403. doi: 10.1016/j.ijcard.2013.04.152. [DOI] [PubMed] [Google Scholar]
- 48.Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 49.TiGenix. TiGenix announces Cx601 meets primary endpoint in pivotal phase III trial August 23, 2015. Available at http://globenewswire.com/news-release/2015/08/23/762658/10146900/en/TiGenix-announces-Cx601-meets-primary-endpoint-in-pivotal-Phase-III-trial.html. Accessed March 28, 2016.
- 50.Bourin P, Sensebé L, Planat-Bénard V, et al. Culture and use of mesenchymal stromal cells in phase I and II clinical trials. Stem Cells Int. 2010;2010:503593. doi: 10.4061/2010/503593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 52.Viergever RF, Karam G, Reis A, et al. The quality of registration of clinical trials: Still a problem. PLoS One. 2014;9:e84727. doi: 10.1371/journal.pone.0084727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bersenev A. Cell therapy clinical trials—2014 report. CellTrials blog. January 22, 2015. Available at http://celltrials.info/2015/01/22/2014-report/. Accessed March 28, 2016.
- 54.Riveros C, Dechartres A, Perrodeau E, et al. Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals. PLoS Med. 2013;10:e1001566–; discussion e1001566. doi: 10.1371/journal.pmed.1001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cyranoski D. Stem cells in Texas: Cowboy culture. Nature. 2013;494:166–168. doi: 10.1038/494166a. [DOI] [PubMed] [Google Scholar]
- 56.Cyranoski D. Stem-cell therapy takes off in Texas. Nature. 2012;483:13–14. doi: 10.1038/483013a. [DOI] [PubMed] [Google Scholar]
- 57.Gunter KC, Caplan AL, Mason C, et al. Cell therapy medical tourism: Time for action. Cytotherapy. 2010;12:965–968. doi: 10.3109/14653249.2010.532663. [DOI] [PubMed] [Google Scholar]
- 58.Cyranoski D. Stem-cell therapy faces more scrutiny in China. Nature. 2009;459:146–147. doi: 10.1038/459146a. [DOI] [PubMed] [Google Scholar]
- 59.Isasi R. Registration of stem cell-based clinical trials: A scientific and ethical imperative. World Stem Cell Report. 2009. Available at http://worldstemcellsummit.com/files/2009_report/1-3_2009.pdf. Accessed March 28, 2016.
- 60.Mendicino M, Bailey AM, Wonnacott K, et al. MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 61.Moses H, 3rd, Matheson DH, Cairns-Smith S, et al. The anatomy of medical research: US and international comparisons. JAMA. 2015;313:174–189. doi: 10.1001/jama.2014.15939. [DOI] [PubMed] [Google Scholar]
- 62.Zerhouni EA. Research funding. NIH in the post-doubling era: Realities and strategies. Science. 2006;314:1088–1090. doi: 10.1126/science.1136931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.