Abstract
This paper describes a million-plus granule cell compartmental model of the rat hippocampal dentate gyrus, including excitatory, perforant path input from the entorhinal cortex, and feedforward and feedback inhibitory input from dentate interneurons. The model includes experimentally determined morphological and biophysical properties of granule cells, together with glutamatergic AMPA-like EPSP and GABAergic GABAA-like IPSP synaptic excitatory and inhibitory inputs, respectively. Each granule cell was composed of approximately 200 compartments having passive and active conductances distributed throughout the somatic and dendritic regions. Modeling excitatory input from the entorhinal cortex was guided by axonal transport studies documenting the topographical organization of projections from subregions of the medial and lateral entorhinal cortex, plus other important details of the distribution of glutamatergic inputs to the dentate gyrus.
Results showed that when medial and lateral entorhinal cortical neurons maintained Poisson random firing, dentate granule cells expressed, throughout the million-cell network, a robust, non-random pattern of spiking best described as spatiotemporal “clustering”. To identify the network property or properties responsible for generating such firing “clusters”, we progressively eliminated from the model key mechanisms such as feedforward and feedback inhibition, intrinsic membrane properties underlying rhythmic burst firing, and/or topographical organization of entorhinal afferents. Findings conclusively identified topographical organization of inputs as the key element responsible for generating a spatio-temporal distribution of clustered firing. These results uncover a functional organization of perforant path afferents to the dentate gyrus not previously recognized: topography-dependent clusters of granule cell activity as “functional units” that organize the processing of entorhinal signals.
I. Introduction
Developing large-scale, quantitatively based models of neural systems has become a realizable goal in recent years, largely because of three major developments: (i) data collection over the course of the past several decades has led to substantial databases of anatomical and physiological properties for many neural systems; (ii) the development of sophisticated and parallelizable software systems for representing these anatomical and physiological characteristics; (iii) the continued growth of high performance computing systems capable of sustaining the numerical burden of such large-scale models.
One of the most extensively studied regions of the brain is the hippocampal formation. Numerous anatomical analyses over the course of the last century have documented the classes, numbers, and organization of principal neurons and interneurons in this limbic region. Extensive studies at the electron microscopic level have provided knowledge of the numbers, densities, membrane locations, and neurotransmitter properties of synapse populations. Despite this wealth of knowledge, there have been few detailed, quantitative models of the hippocampal system. Those that have been developed have been limited to subregions of the hippocampus, understandably given the complexity of the system. These initial models have been successful in providing insights into functional properties of the hippocampus at a subsystems level.
Here we describe the first step in an implementation of a full-scale model of the hippocampal formation. We have dealt with the first stage in what has been termed the intrinsic “tri-synaptic pathway” of the hippocampus, i.e., the “perforant path” excitatory projections from the entorhinal cortex (EC) to granule cells of the dentate gyrus (DG), including inhibitory feedback from DG interneurons. Our model is based on the hippocampal formation of the rat, as the majority of quantitative anatomical information available is for the rat species. We have taken into consideration a number of factors concerning the EC-DG projection in an attempt to attain a model that is as biologically realistic as is achievable given current knowledge. In general, these factors include: the number and ratio of layer II EC neurons and dentate granule cells; the ratio of inhibitory interneurons and dentate granule cells; the dendritic morphological structure and morphological variability of granule cells; the terminal field distributions of EC layer II cells; the synaptic density of EC layer II cells onto dentate granule cells; the passive membrane properties of granule cells; both somatic and dendritic active conductances responsible for the action potential and for other voltage-dependent properties.
One other anatomical feature that also is the focus of the present study concerns the topographical organization of EC-DG projections. Topography of anatomical connections, i.e., the point-to-point relation of typically non-uniform synaptic connectivity between any two brain regions, is a property of nearly all mammalian brain systems, and is distinctly different for each. The topography of EC-DG projections in the rat has been studied elegantly and reported previously. The question being asked in the present study is the functional consequence of that topography. To our knowledge, the issue of the functional significance of the topographical organization of a projection system has yet to be addressed quantitatively for any brain system, and is an issue particularly well-suited for a large-scale, structural-functional model. We show here that the topographical characteristics of EC projections to the DG impose quantifiable boundaries on the spatio-temporal properties of granule cell network activity. Findings conclusively identified topographical organization of inputs as the key element responsible for generating a spatio-temporal distribution of clustered firing. As such, these results uncover a functional organization of perforant path afferents to the DG not previously recognized: topography-dependent clusters of granule cell activity as “functional units” or “channels” that organize the processing of EC signals.
II. Methods
A. Model Scale and Features
Models of the EC-DG system were completed according to two scales – one with 1,000,000 granule cells, i.e., equivalent to the number of cells in one hemisphere of the rat hippocampus, and one with 100,000 granule cells, i.e., equivalent to 1:10 scale, to accelerate simulation time. All results reported here were observed at both scales. All networks studies were composed of dentate granule cells and inhibitory interneurons, with excitatory input to granule cells modeled after the organization of layer II EC afferents. In general terms, the models featured complex and variable morphologies for granule cells, multiple active conductances distributed non-uniformly throughout granule cell membranes, interneurons configured (as cell bodies only) to provide both feed-forward and feedback inhibition, and either randomly or topographically organized excitatory input to network neurons. The specifics of these features have been described in previous conference proceedings [1], [2].
III. Results
A. Granule Cell Response to Random Entorhinal Input: Firing in Spatio-Temporal “Clusters”
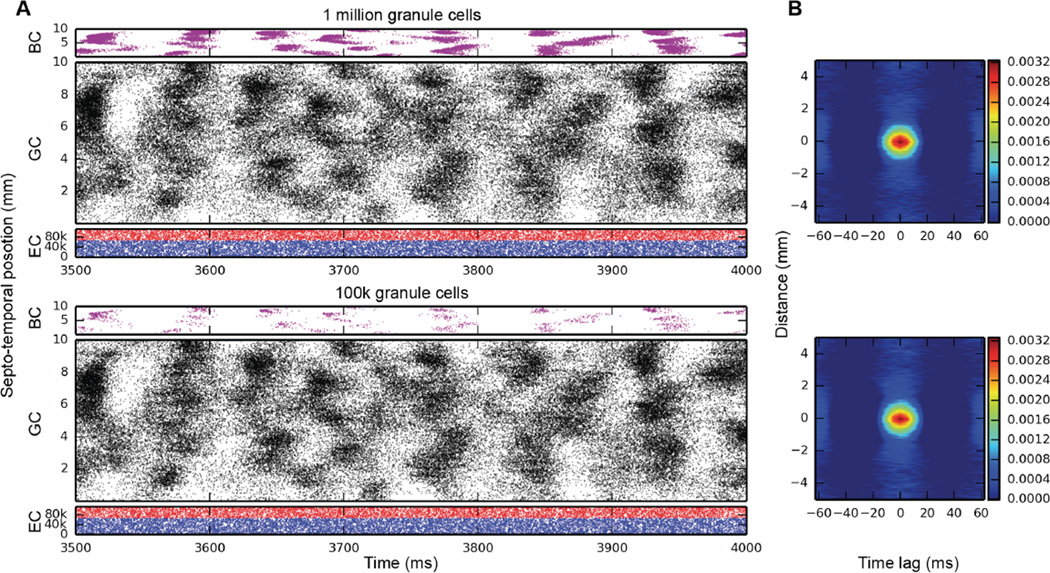
Initial simulations of granule cell network dynamics to EC input involved both medial and lateral entorhinal (MEC & LEC) neurons firing at a mean frequency of 3.0 Hz, accelerated (by design) over the course of approximately the first 1000 ms of the simulation. Four (4.0) seconds of time were simulated in all results presented here. Entorhinal inputs were topographically organized according to the relations described in the Methods. Basket cells were configured to provide both feedforward and feedback inhibition. All active granule cell membrane properties included those described in Table II. Simulation results with a one million granule cell population revealed that despite continued Poisson EC input, granule cells discharged in a decidedly non-random, nonuniform manner. As shown in Fig. 1 (top), granule cells throughout the entire septo-temporal extent of the DG fired in what we call here spatio-temporal “clusters,” i.e., irregular periods of spiking and non-spiking lasting approximately 50–100 ms “on” and 50–75 ms “off”. The granule cells engaged in firing changed spatial location along the septo-temporal axis, as evidenced by the apparent “drift” in patterned firing in Fig. 1A and other similar figures. The appearance of clustered spiking in response to Poisson EC input was not specific to million-granule cell populations, but was equally apparent for simulations involving 100k granule cells as well (Fig. 1, bottom).
Fig. 1.
Simulation results for topographically constrained EC-DG networks with feedforward and feedback inhibition, run at two different scales: 1M granule cells (top), and 100k granule cells (bottom). At both scales, spatio-temporal clusters appear in the granule cell activity, despite the random nature of the EC input. In the million-cell case, only a subset of the full dataset is plotted to keep it from appearing solid black. Column B: 2D autocorrelations confirm the presence of these clusters.
In some cases, the appearance of spatial-temporal clusters did not appear immediately with the onset of EC input, but instead only appeared after a period of highly rhythmic granule cell activity. In those cases, granule cell spiking started after approximately 200 ms, reaching a maximum at approximately 300 ms. At this time point granule cell output was highly synchronous, with granule cells along the entire extent of the septo-temporal axis firing at a high rate for a duration of approximately 100 ms. Approximately midpoint in this initial 100 ms of extended firing, basket cells fired synchronously as well, leading to a termination of extended granule cell firing. After a few more periods of synchronous granule cell discharge alternating with periods of heightened basket cell output the system appears to reach an equilibrium (after 800–900 ms into the simulation). It is at this point that a steady-state of “clustered” spike discharges emerges and continues.
Quantitative analyses verified the existence of clusters of spike firing. 2D autocorrelation allows an analysis of the data in both spatial and temporal dimensions, and was used to analyze most of the results presented here. It was constructed by computing every pairwise cross-correlation of discretized spike trains in a random sample of 10,000 neurons. The spike trains were discretized by counting the number of spikes elicited by a particular neuron within a bin size of 5 ms. The resulting cross-correlations were sorted by the distance between the neuron pairs and were further binned using a resolution of 0.05 mm. The mean cross-correlation within each bin was computed.
The right-hand column of Fig. 1 shows the 2D autocorrelations for the million-cell and 100k-cell simulations. What emerges from the analysis is something that looks like a typical cluster for each of the datasets: in the million-cell case, clusters are roughly elliptical, with a temporal width of approximately 40–50 ms and a spatial height of 1–2 mm. The analysis looks very similar for the 100k-cell simulation, which verifies that clusters exist and are similar at both simulation scales.
Further analysis was performed on the 100k-cell dataset using DENCLUE 2.0, a density-based cluster identification algorithm. For this application, a Gaussian density kernel was used. Analyses show that a) clusters exist, and b) they appear in a wide variety of sizes, though their basic shape remains similar. Statistical analysis of the identified clusters shows that inter-centroid cluster time is 11±12 ms, and that the density of spikes within a cluster is approximately 12±9 spikes/ms-mm.
B. Mechanisms Responsible for Spatio-Temporal Clustered Spiking: Inhibition
Following this initial characterization, we conducted experiments designed to identify the mechanisms underlying granule cell clustered spiking: what was responsible for transforming continuous random spike firing into noncontinuous, non-periodic, clusters of spikes? A first hypothesis concerned a possible role for GABAergic inhibition, given the strong effect of interneuron activity in synchronizing granule cell activity in the early stages of the simulation. Indeed, when feedback inhibition was increased by 4–10 times that used in initial simulations, longer periods of synchronous activity, marked by multiple bands of high activity followed by bands of almost zero activity in the DG followed. This pattern of bands eventually becomes asynchronous, though, giving rise to spatio-temporal clusters similar to the ones we see with less inhibition. When we increase inhibition to a level 20 times greater than normal, however, the synchrony persists for the entire duration of the simulation.
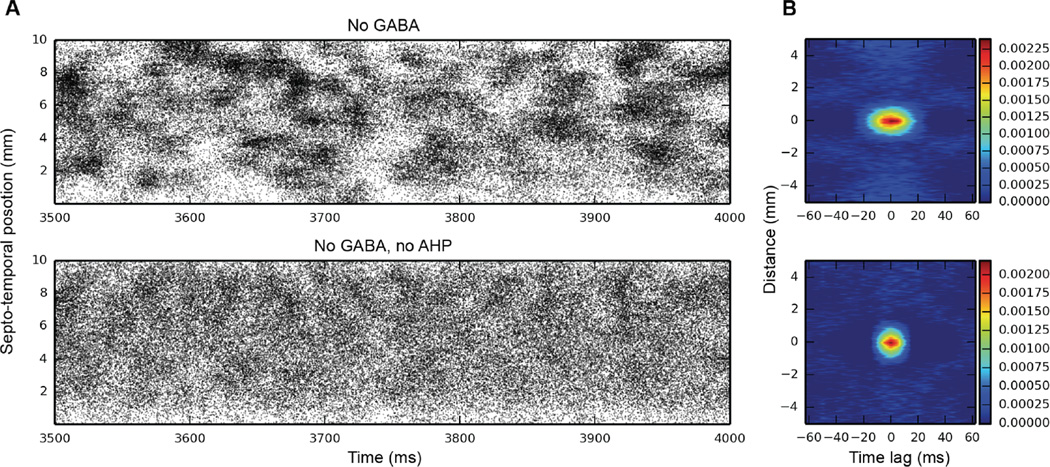
Given the synchrony introduced into granule cell spiking as a result of feedback inhibition, we decided to remove sources of inhibition to see whether the cluster activity would also disappear. There are two sources of inhibition in dentate granule cells: external inhibition, from the basket cell population, and internal inhibition, due to the after hyperpolarization (AHP) that granule cells generate after one or more action potentials (APs). As Fig. 2 illustrates, spatio-temporal clusters persist with one or both of these sources of inhibition absent from the network.
Fig. 2.
Granule cell activity when removing internal and external sources of inhibition. Top: GABAergic inhibition removed; bottom: both AHP and GABA removed. Spatio-temporal clusters persist in both cases, as evidenced by both the raster plots and 2D autocorrelations (B).
C. Mechanisms Responsible for Spatio-Temporal Clustered Spiking: Topographic Organization of Entorhinal Afferents
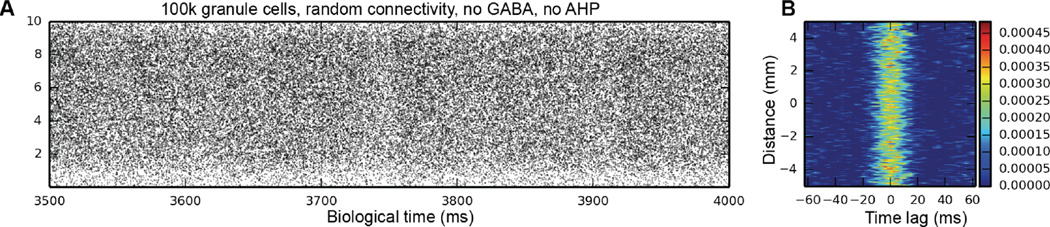
We next turn to topography as the potential source of clustering. As Fig. 3 shows, when the constraints placed on EC-to-DG connectivity are removed, thus allowing a randomly connected network where any EC neuron can synapse onto any dentate granule cell, the clusters that have always been present in the output disappear, and bands of activity appear in their place. The 2D autocorrelation corroborates this: while there is still a small amount of temporal structure in the network activity, it is 8x–10x weaker than in any of the topographically constrained cases, and the spatial component has completely disappeared – what once looked like an elliptical cluster in the 2D autocorrelation has become a band that occupies the full septo-temporal extent of DG. Thus, it is the topography of the EC-DG projection that is responsible for the emergence of spatio-temporal clusters, even when the input to the network is random.
Fig. 3.
Simulation results for a randomly connected EC-DG network. In this simulation, the granule cell AHP was removed, as was GABAergic inhibition. Spatio-temporal clusters are no longer present, having been replaced with bands of activity with a high level of background activity. In the 2D autocorrelation, what looked like a typical cluster is now a vertical band. Thus, while there’s still a temporal variation in granule cell activity, the spatial component is gone.
IV. Discussion
The study described here is based on the creation of a large-scale, compartmental neuron model of the EC-DG projection system of the hippocampal formation. We have intended for the model to be large-scale in the sense of including a total number of dentate granule cells, GABAergic interneurons, and EC axons that are equivalent to those found in one hemisphere of the rat brain. We have intended the model to be biologically realistic with respect to the numbers and ratios of different classes of neurons, granule cell dendritic morphologies, classes and distributions of somatic and dendritic conductances, and the presence of feedforward and feedback GABAergic interneurons. In this its first generation of development and use, the model has uncovered a functional organization of perforant path afferents to the DG not previously recognized: a spatio-temporal clustering of granule cell spiking on the order of 11 ms between clusters and 12 spikes/ms-mm within clusters. These findings are important in several respects. First, these spatio-temporal clusters of active granule cells represent “functional units” or “channels” that organize the processing of EC signals. Second, results of the present study clearly demonstrate that the spatio-temporal clustering property of the EC-DG pathway depends primarily on the topographic organization of perforant path afferents. This is the first time that a functional characteristic of a cortical projection has been linked specifically to topographic features of that projection. Third, this organizational property of the EC-DG pathway is apparent only when structural-functional relations are examined at large-scales. Smaller scale models would not have revealed the clustering phenomenon, and thus, these results point to the importance of large-scale modeling of cortical systems.
Acknowledgments
Computation for the work described in this paper was supported by the University of Southern California Center for High-Performance Computing and Communications (www.usc.edu/hpcc).
Contributor Information
Phillip J. Hendrickson, Email: phendric@usc.edu.
Gene J. Yu, Email: geneyu@usc.edu.
Dong Song, Email: dsong@usc.edu.
Theodore W. Berger, Email: berger@usc.edu.
References
- 1.Hendrickson PJ, Yu GJ, Robinson BS, Song D, Berger TW. Towards a Large-Scale, Biologically Realistic Model of the Hippocampus; 34th Annual International Conference of the IEEE EMBS; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu GJ, Robinson BS, Hendrickson PJ, Song D, Berger TW. Implementation of a Topographically Constrained Connectivity for a Large-Scale Biologically Realistic Model of the Hippocampus; 34th Annual International Conference of the IEEE EMBS; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]