Abstract
Neurogenin-3 (ngn-3) expression is critical for endocrine development in the developing pancreas. We found that when ngn-3 was inhibited in an E11.5 pancreas, using either morpholino antisense or siRNA, it led to a significant decrease in endocrine differentiation after seven days in culture. Endocrine differentiation was rescued when ngn-3 inhibition was withdrawn after three days of culture, suggesting that the embryonic pancreas retains progenitor cells with the ability to differentiate into endocrine cell types when ngn-3 expression recurs. To determine whether the rescue phenomenon observed after withdrawing ngn-3 antisense treatment was the result of the original endocrine-committed cells reinitiating endocrine differentiation, or was instead due to new recruitment of later progenitor cells, we blocked ngn-3 expression for only the last four days of a seven-day culture. Here, insulin-positive differentiation was slightly reduced, but there was a normal number of glucagon-positive cells. In addition, there was an increase in SOX9-positive cells in ngn-3 inhibited, as well as in ngn-3 rescued pancreata, with a significant proportion of these SOX9-positive cells co-localized with DBA, an early ductal marker. This increased number of cells with co-localization of SOX9 and DBA could indicate an increased numbers of endocrine progenitor cells.
INTRODUCTION
The organogenesis of the pancreas is spatio-temporally controlled by the sequential activation of a specific cascade of transcription factors. The homeodomain protein pdx-1 expression in the pre-pancreatic primitive foregut at the 10-12 somite stage (E8.5) [1; 2] is critical for proper pancreatic development [3]. Both mice and humans lacking pdx-1 are born without a pancreas [1; 2]. Pdx-1 expression is followed by the expression of several lineage-regulating molecules including notch-1, neurogenin-3 (ngn-3) and pax-6.
Notch-1 proteins regulate developmental lineage decisions. The activated notch-1 intracellular domain activates transcription of basic helix-loop-helix (bHLH) Hes genes [4], and in the pancreas, Hes-1 suppresses the expression of the pro-endocrine transcription factor ngn-3 [5]. Ngn-3 marks pancreatic cells destined for an endocrine fate [6; 7]. This expression of notch-1 in the early pancreas inhibits expression of neurogenins and prevents differentiation of early pancreatic progenitor cells to their endocrine fate [8]. Without notch-1 signaling, cells may prematurely choose a default lineage pathway. Inhibiting the notch-1 pathway during very early pancreas development leads to premature development of endocrine tissue, with no branching and a complete lack of exocrine tissue [8]. Transgenic over-expression of neurogenin-3 in mice, or ectopic expression of neurogenin-3 in chick embryo endodermal cells led to premature differentiation of only glucagon and somatostatin-producing cells [9]. Ngn-3 null mutant mice lack endocrine cells, and die postnatally between day 1 and 3 due to diabetes [8]. Thus, either inhibiting the notch-1 pathway or transgenic over-expression of ngn-3 in the early pancreas both appear to result in premature commitment of pancreatic progenitor cells to an endocrine fate, depleting residual progenitor cells. These results suggest that the spatio-temporal regulation of ngn-3 is a critical determinant of pancreatic endocrine progenitor cell commitment [10]. Here, we show that blocking ngn-3 expression in the E11.5 embryonic pancreas results in reduced endocrine differentiation, no change in the number of amylase-positive acinar-cells, and an apparent augmentation in the pool of undifferentiated epithelial-ductal cells. We demonstrate that endocrine differentiation can be later rescued in this epithelial-ductal population by restoring ngn-3 expression, thus suggesting that endocrine-committed progenitor cells in the developing pancreas may retain the ability to differentiate into endocrine cells.
Materials and Methods
We blocked ngn-3 expression in cultured pancreas using an antisense protocol described earlier [11]. The details of the reagents and the methods are provided as supplementary data.
RESULTS
Endocrine differentiation in early embryonic pancreas requires neurogenin-3 expression
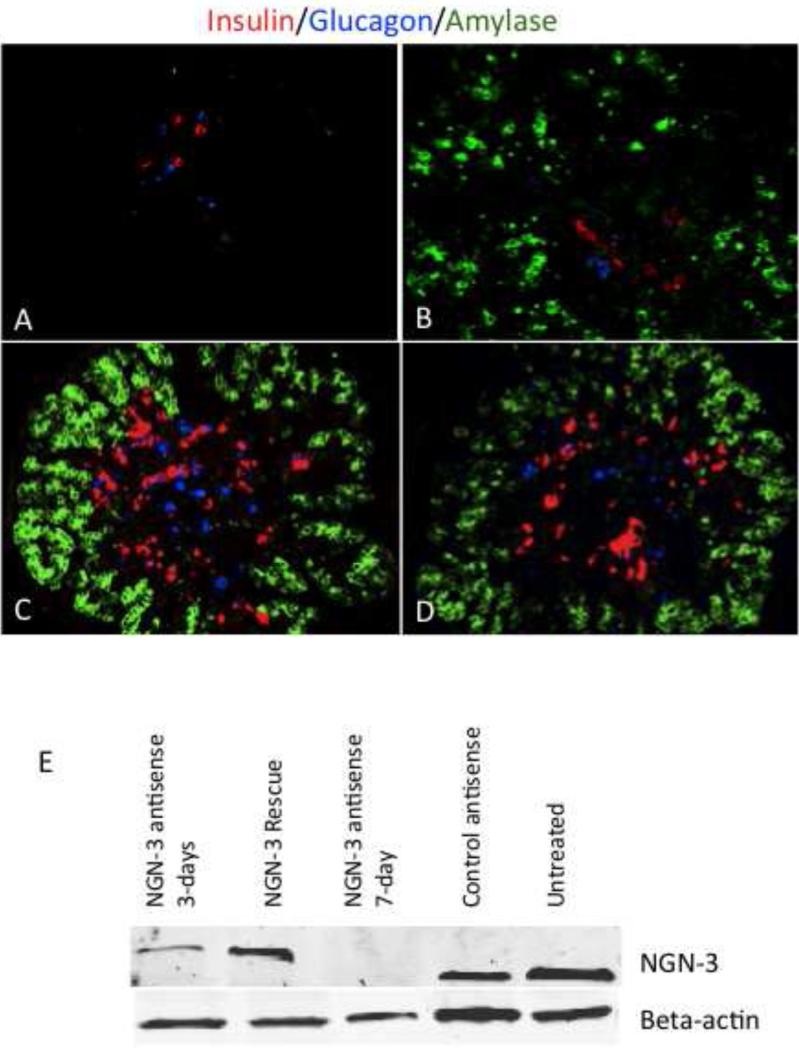
Ngn-3 is a critical regulator of early endocrine commitment during pancreas development [12]. Ngn-3 expression is first seen in the developing pancreas around day 11.5 [13]. To identify whether ngn-3 expression is necessary for further endocrine recruitment after this initial commitment, we blocked ngn-3 expression in vitro in freshly harvested day 11.5 embryonic pancreases by adding either morpholino antisense oligonucleotides, or siRNA against ngn-3. Blocking ngn-3 expression starting at E11.5 with morpholino antisense treatment for either 3 days (Figure 1 A) or 7 days (Figure 1 B) resulted in a significant inhibition of both insulin and glucagon-positive differentiation compared to missense control oligo-treated (Figure 1 C) or untreated wild type pancreases (Figure 1 D). Amylase expression after 7 days of ngn-3 antisense treatment appeared relatively normal (Figure 1 B), though perhaps less robust than controls (Figure 1 C, D). Amylase expression was not expected in cultures after 3 days of antisense treatment (Figure 1 A) since exocrine commitment and amylase expression do not normally initiate until after this stage. Using Western blot (Figure 1E lanes 1 and 3) and immunohistochemistry (Figure 2 F, G, H and J), we confirmed ngn-3 inhibition by morpholino in day 11.5 embryonic pancreas. In addition to the antisense strategy, we also used siRNA to block ngn-3 expression in embryonic pancreas. Semi-quantitative RT-PCR of ngn-3 mRNA levels confirmed the effectiveness of the siRNA knockdown (supplemental figure 3D). siRNA treatment of embryonic pancreas also resulted in a significant reduction in both insulin and glucagon expression after seven days in culture (Supplemental Figures 1 C, D and Supplemental Figure 2). There was a 75 and 33 % reduction of insulin and glucagon-positive area after ngn-3 siRNA treatment, respectively, compared to siRNA controls (data not shown). Similarly, the total number of insulin-positive and glucagon-positive cells was significantly lower in siRNA-treated explants, suggesting that ngn-3 inhibition prevented these cells from entering into an endocrine differentiation pathway (supplemental figure 2). Control siRNA treated pancreases did not show significant changes in insulin or glucagon-positive differentiation on a percentage basis (supplemental figure 2), but did appear to have lower absolute levels than untreated controls (supplemental figure 1 A, B: control siRNA compared with figure 1E,F untreated). We also performed real time semi-quantitative RT-PCR to analyze insulin and glucagon mRNA levels in cultured pancreatic explants treated with ngn-3 or control siRNA. RT-PCR confirmed a significant decrease in relative mRNA levels of both insulin and glucagon (supplemental figure 3 A, B) following siRNA-mediated ngn-3 inhibition, but the relative mRNA levels for amylase was unchanged (supplemental figure 3 C). Together, these morpholino and siRNA results confirm that ngn-3 expression beyond the initial endocrine commitment phase of pancreatic development is still required for normal endocrine differentiation.
Figure 1.
Insulin, glucagon, and amylase immunohistochemistry in day 11 embryonic pancreas after 3 or 7 days of culture with neurogenin-3 antisense or missense control oligonucleotide. Neurogenin-3 antisense treatment for 3-days (A) or 7-days (B) resulted in significant inhibition of insulin and glucagon expression in embryonic day 11.5 pancreases when compared to 7-day untreated or control missense-treated pancreases (C and D). (E.) Western blot was performed on protein lysate isolated from embryonic pancreas harvested at E11.5 and then treated with neurogenin-3 antisense for 3 days, 7 days, or “rescued” after three days of neurogenin-3 antisense with four additional days without antisense. Controls were control antisense or untreated explants harvested after 7 days in culture. Blots were probed with neurogenin-3 or beta-actin antibody as described in the methods. Neurogenin-3 protein expression was significantly lower in 3 day (Lane 1) ngn-3 antisense-treated pancreas and was not detectable in samples treated for 7 days (Lane 3) with antisense in culture, whereas expression was similar among control (Lane 4), untreated (Lane 5), and neurogenin-3 rescued (Lane 2) pancreases. Bottom panel shows beta-actin levels as loading control.
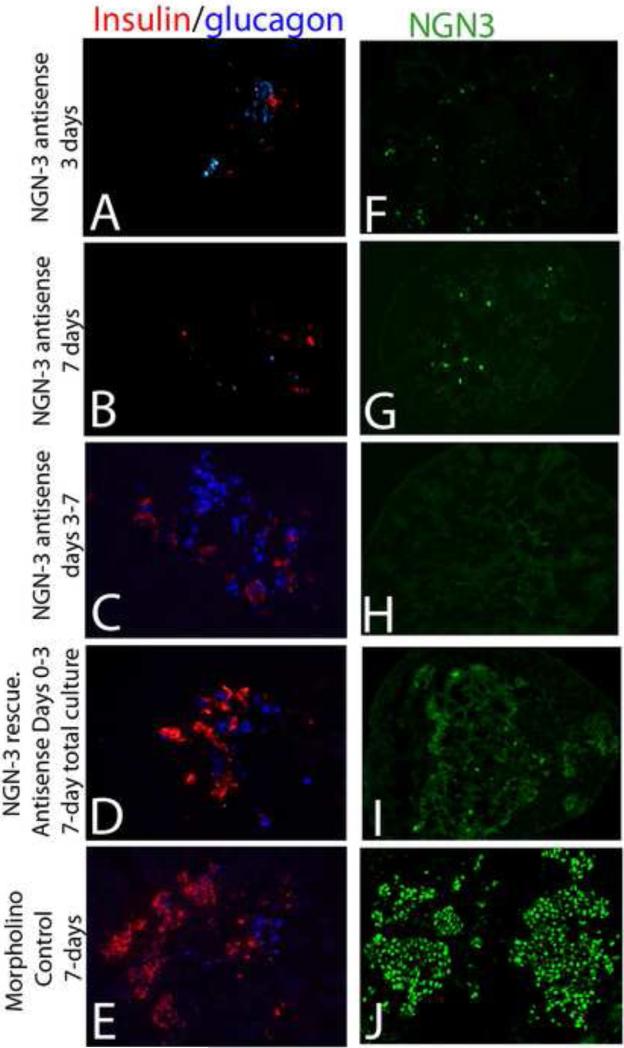
Figure 2.
Reinitiating neurogenin-3 expression restored insulin and glucagon expression. Here, neurogenin-3 antisense or control oligonucleotide-treated pancreases were transferred to normal media after 3 days of antisense treatment, and then cultured for an additional 4 days to allow recovery of ngn-3 expression (D, I). The cultured pancreases were then analyzed for insulin and glucagon expression by immunohistochemistry. The additional 4 days of culture in normal media restored neurogenin-3 expression (I) (P=0.01). Immunohistochemistry on neurogenin-3 rescued pancreases show restored insulin and glucagon expression (D) very similar to that of control antisense treated cultures (E). Neurogenin-3 antisense treatment for 3 days (or 7 days similar to data in Figure 1), showed significant inhibition of insulin and glucagon (A, B) (P=0.001). When neurogenin-3 was blocked only during the last four days of a seven-day culture, there were below-normal numbers of insulin cells, but normal numbers of glucagon cells (C). Immunohistochemistry confirmed effectiveness of neurogenin-3 antisense treatment with significantly reduced neurogenin-3 expression in antisense treated samples (F, G and H). Eliminating antisense treatment from the media after three days restored neurogenin-3 expression (I) when compared with control missense treated samples (J) and neurogenin-3 inhibited cultures (F, G, and H).
Neurogenin-3 re-expression (“rescue”) restores endocrine differentiation in gestational day 11 pancreas
Next we asked whether undifferentiated cells in the early pancreatic epithelium retain the ability to differentiate into endocrine-positive cells after removing the inhibitory effects of ngn-3 antisense treatment. Here E11.5 pancreas after an initial treatment with ngn-3 antisense we continued the culture for an additional four days in normal media to allow recovery of ngn-3 expression. The ngn-3 rescue restored ngn-3 expression (Figure 2 I), and in the ngn-3 rescued pancreas there was a significant restoration of islet hormones, insulin and glucagon (Figure 2D) compared to tissues in which ngn-3 was inhibited (Figure 2 A, B, p=0.001). Control cultured pancreases without any treatment (data not shown) as well as missense antisense-treated pancreases showed normal expression of insulin and glucagon (Figure 3E).
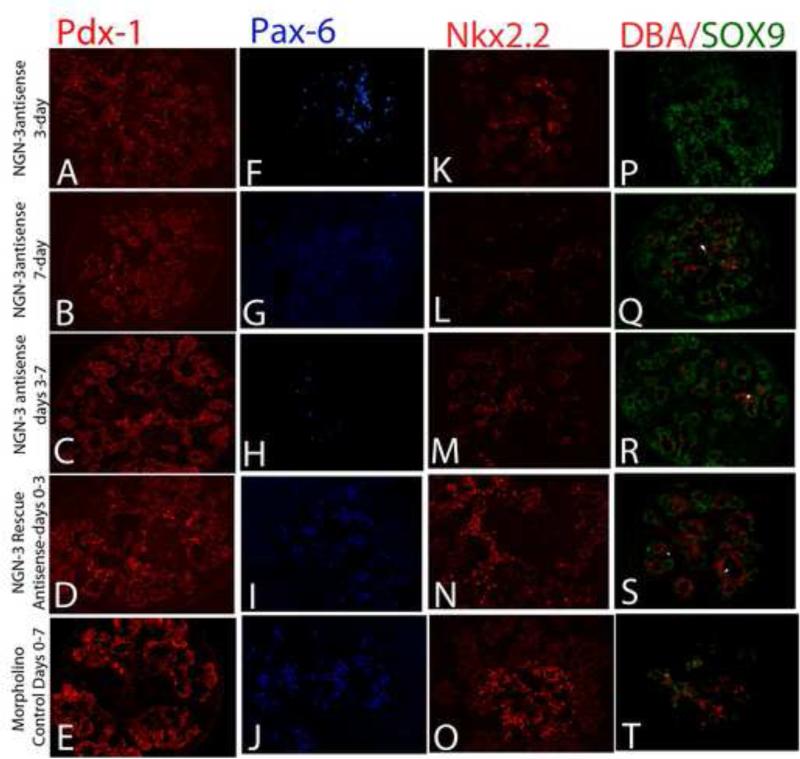
Figure 3.
Immunohistochemistry for pdx-1 was done after pancreases were treated with neurogenin-3 antisense for either three days (A), only the last four days of a 7-day culture (C), or for the entire seven days in culture (B). These cultures were compared to missense control oligo treatment for seven days (E), or after rescue of neurogenin-3 expression (D). Pdx-1 high-expressing cells (arrowhead) were present but reduced after three days of neurogenin-3 antisense treatment (A), however with longer treatment with neurogenin-3 antisense the number of high-expressing cells reduced significantly (B, C), compared to control sections (E), and Pdx-1 high-expressing cells were restored in neurogenin-3 rescued pancreases where antisense treatment was terminated after 3 days and the pancreas transferred to normal media and cultured for an additional 4 days (D).
Pax-6 expression was analyzed in neurogenin-3 antisense treated and rescued samples, as a marker of committed endocrine progenitor cells. Pax-6 expression was nearly absent in tissues in which neurogenin-3 expression was inhibited for a full seven days (G), however with a three day treatment with neurogenin-3 antisense similar to the 3-day ngn-3 inhibition, several pax-6-positive cells were present, suggesting that cells may have still been able to commit to an endocrine fate, though not able to become hormone-positive (F). In control antisense treated samples (J), and in tissues where neurogenin-3 expression was rescued (I), the pax-6 expression was restored to normal levels.
Immunohistochemistry for Nkx2.2 was done after pancreases were treated with neurogenin-3 antisense for either three days (K), only the last four days of a 7-day culture (L), or for the entire seven days in culture (M). Nkx2.2 levels were reduced in 7-day ngn-3 antisense-treated pancreas (M). The levels were also low after 3 days of ngn-3 antisense (K) or in a 7-day culture with only the last four days of culture in ngn-3 antisense (L) compared to control cultures (O). Nkx2.2 expression was restored in neurogenin-3 rescued pancreases (N).
Immunohistochemistry was performed for SOX9 in embryonic pancreases treated with ngn-3 antisense. SOX9 expression was low in the pancreas treated with control antisense for seven days (Figure 3 T) and localized primarily to the DBA-positive ductal/epithelial region, whereas the pancreas treated with ngn-3 antisense only for the first three days of culture (Figure 3 P) or treated for seven days in culture (Figure 3 R) both showed enhancement in the number of SOX9-positive cells. Similarly, tissues treated with ngn-3 antisense only for the last four days of a 7-day culture (Figure 3Q) also showed an increased number of SOX9-positive cells. On the other hand, when ngn-3 expression was rescued the number of SOX9-positive cells was reduced (Figure 3 S).
Dolichos Biflorus Agglutinin (DBA) lectin was used to label undifferentiated epithelial-ductal cells in the cultured embryonic pancreases that were treated with either control or a neurogenin-3 specific antisense. Pancreas cultured for seven days in either control antisense or neurogenin-3 antisense had numerous DBA-positive cells in the pancreas (Q, R, S, T). However, neurogenin-3 antisense treatment for the last four days or for the entire seven days of culture resulted in a significant increase in SOX-9-, DBA- double-positive cells (arrows in Q, and R), compared to control antisense (T). Similarly, neurogenin-3 rescued samples also had several SOX-9-positive cells co-localized with DBA positive cells (arrows in S).
Rather than being truly “rescued” progenitor cells, these cells could instead be cells that normally only commit to becoming ngn-3-positive endocrine cells later in gestation. To differentiate between these two possibilities we cultured day 11. 5 pancreas for the first three days in normal media, followed by four days of ngn-3 antisense treatment. Immunohistochemistry showed significantly reduced ngn-3 expression (Figure 2H), and reduced numbers of insulin-positive cells (Figure 2 C) following antisense treatment from day-3 through day-7. Interestingly, in these pancreases with the later inhibition of ngn-3, we observed normal numbers of alpha cells (Figure 2 C).
Neurogenin-3 inhibition enhanced the number of uncommitted cells in the developing pancreas
Endocrine cells in the developing pancreas originate from pdx-1-high expressing cells [14]. We analyzed pdx-1 expression in ngn-3 morpholino antisense-treated embryonic pancreases and then compared the pattern to control antisense treated embryonic pancreases of the same age. Pancreas treated with control antisense or harvested after three days of ngn-3 antisense treatment had several pdx-1-high-positive cells (Figure 3A, E, supplemental figure 4 A). Whereas with longer ngn-3 antisense-treatment the number of pdx-1-high-expressing cells was significantly reduced, suggesting that blocking ngn-3 in E11.5 pancreas prevented further endocrine differentiation of these pdx-1-high-expressing cells (Figure 3B, C). Similarly, pax6 serves as a general marker of all early endocrine cells in the developing pancreas. We analyzed pax-6 expression in ngn-3 antisense-treated embryonic pancreases using immunohistochemistry (Figure 3 F-J). Ngn-3 antisense treatment for seven days in culture significantly reduced Pax-6 expression (Figure 3 G, supplemental figure 4 B), compared to control oligo treated pancreas (Figure 3 J). Pax-6 was restored to normal levels after the ngn-3 expression was rescued (Figure 3 I).
SOX9 is a multipotent progenitor cell marker in the developing pancreas. It is expressed in a subset of pdx-1 positive epithelial cells that are both notch-responsive and mitotically active, possibly regulating cell proliferation and differentiation of both endocrine and exocrine tissues [15]. SOX9 expression, however, is excluded from committed cells [16]. We therefore analyzed SOX9 expression in embryonic pancreas treated with ngn-3 antisense. The pancreas treated with control antisense for seven days showed the expected low expression of SOX9 (Figure 3 T), mainly in the region of DBA-positive duct/epithelial cells, whereas the pancreas treated with ngn-3 antisense and harvested after three days (Figure 3 P) or seven days (Figure 3 R) in culture showed enhancement in the number of SOX9-positive cells (Figure 3 R, supplemental figure 5 B). Similarly, tissues treated with ngn-3 antisense only for the last four days of culture (Figure 3 Q; supplemental figure 5 B) also showed increased number of SOX9-positive cells. Tissues in which the ngn-3 expression was rescued had a reduced number of SOX9-positive cells (Figure 3 S; supplemental figure 5 B). In addition, ngn-3 antisense treatment for three days followed by rescue of ngn-3 expression resulted in a significant increase in Dolichos Biflorus Agglutinin-positive cells (DBA) (Figure 3 S, T), many of which were also positive for SOX9 expression. A similar co-localization of DBA-positive cells and SOX9 were also found in pancreas treated with ngn-3 antisense for the last four days of a seven day culture (Figure 3 Q), as well as in 7-day antisense treated pancreas (Figure 3 R). Thus, this increase in DBA-, and SOX9-positive cells in ngn-3 antisense treated and rescued tissues suggests an increase in an uncommitted epithelial-ductal cell population in the mid-gestational pancreas in the absence of ngn-3 expression.
Nkx2.2 expression is required for specification alpha and beta cells during pancreatic islet [17; 18] and in its absence the progenitor cells are converted to ghrelin-positive cells [19]. Nkx2.2 expression decreased significantly in the pancreas treated with ngn-3 antisense (Figure 3 K, L M and O; supplemental figure 5A). The effect was most significant when treated for the entire duration of the seven-day culture (Figure 3 M, Supplemental figure 5A). However, a significant decrease in Nkx2.2 expression was still seen when treated with the antisense for the last four days in culture (Figure 3 L; supplemental figure 5 A), as well as in explants cultured with ngn-3 antisense for only three days (Figure 3 K; supplemental figure 5 A). When the ngn-3 expression was rescued we found Nkx2.2 expression at similar levels as control tissues (Figure 3 N, supplemental figure 5 A).
DISCUSSION
The basic HLH transcription factor ngn-3 plays an important role during endocrine pancreas development [12; 13; 21]. Ngn-3 has a bi-phasic expression during pancreas development: the first phase is during E10- E10.5 and the second phase begins at E12.5 [22]. In our study, to determine the role of ngn-3 during pancreas development beyond early time points, we analyzed the expression of some key transcriptional regulators of pancreatic development, as well as the expression of islet hormones in the developing pancreas after blocking ngn-3 expression well after initiation of pancreatic organogenesis, at day 11.5.
Blocking ngn-3 expression in embryonic pancreases in vitro resulted in a significant inhibition of endocrine differentiation, however there was no effect on the number of amylase positive cells suggesting that after an initial endocrine commitment, these “pre-endocrine” cells cannot be “reprogrammed” to become exocrine cells. Lack of an effect of ngn-3 antisense on amylase expression, together with the demonstrated ability to rescue insulin and glucagon expression in pancreases previously treated with ngn-3 antisense, suggest that these pancreatic progenitor cells are already endocrine-committed. Thus, blocking ngn-3 in these cells may only prevent ngn-3-dependent endocrine differentiation. We confirmed this hypothesis (that cells retain a ngn-3-specific differentiation potential) when we rescued the insulin and glucagon-positive differentiation by restoring ngn-3 expression following a transient three-day inhibition of ngn-3 expression. This ability to recover endocrine differentiation suggests that the requirement for ngn-3 expression in endocrine differentiation persists later into development. Specifically, endocrine progenitor cells at day 11.5 in the embryonic pancreas appear to retain their endocrine differentiation potential for at least three days when ngn-3 expression is transiently inhibited. To determine whether these rescued progenitor cells may instead be arising from new endocrine progenitor cells, not from rescued cells in which ngn-3 expression had been first inhibited, we blocked ngn-3 expression only during the last four days of the culture, and then compared the results to cultures in which ngn-3 was inhibited only for the first three days of culture. Here, instead of the complete inhibition of endocrine differentiation that we saw with inhibition only for the first three days, there was instead a normal number of glucagon-positive cells when the ngn-3 expression was blocked for the final four days of culture. This presence of a normal number of glucagon cells in a pancreas in which the ngn-3 expression was blocked for the final four days of culture suggests that the cells that initially expressed ngn-3, but were inhibited by the antisense, had committed toward an endocrine fate, but further expression of neurogenin-3 was required for β-cell differentiation. A continued expression of ngn-3 for proper β-cell differentiation, maturation, and function was also suggested else where [23]. In the absence of continued ngn-3 expression, the committed cells may develop toward an alpha cell fate. A recent study found that pancreatic progenitor cells and alpha cells adopted a beta cell fate after ectopic expression of pax-4, which in turn induced ngn-3 expression and alpha cell neogenesis from progenitor cells in the adult pancreas [24]. In our experiments, blocking ngn-3 expression early during the first three days of culture inhibited endocrine differentiation. Inhibiting ngn-3 expression later, only during the last four days of culture, prevented further expression of ngn-3 in committed cells, and prevented the formation of any new ngn-3-positive cells. Since we saw no enhancement of alpha cells and normal insulin expression in our rescued samples, we assume that the rescued cells are cells in which ngn-3 expression was restored.
Furthermore ngn-3 inhibition in cultured embryonic pancreases led to an increase in SOX9/DBA double-positive cells, markers of undifferentiated progenitor cells and embryonic epithelial-ductal cells, respectively [20]. When ngn-3 was then rescued after the initial blockage, the uncommitted progenitor cells in the ducts may continue to proliferate inappropriately, resulting in an increased number of SOX9/DBA-double-positive cells. This increase in progenitor cells (SOX9/DBA double-positive cells), with the subsequent ability to recover the endocrine phenotype after recovery of ngn-3 expression, supports the possibility that later-gestational epithelium contains endocrine-committed progenitor cells that retain an endocrine differentiation potential later into development.
In the developing pancreas SOX9 maintains multipotent progenitors in an undifferentiated state by regulating cell proliferation and differentiation [15]. SOX9 does not co-localize with ngn-3, and is not expressed in differentiated cells, but is restricted to a subset of pdx-1 positive cells that are both notch-responsive and mitotically active [15]. In a transgenic mouse model in which pancreatic differentiation was blocked, enhanced expression of SOX9 was found throughout the tubular network of undifferentiated pdx-1-positive epithelial cells [15; 25]. In our studies the increase in SOX9 expression, and the fact that several of these cells were also DBA-positive after ngn-3 inhibition, further suggests an increase in the number of undifferentiated progenitor cells. Furthermore, since we did not see any significant change in exocrine development, and we were able to rescue the endocrine development, we believe that these SOX9/DBA double-positive cells are endocrine committed progenitor cells.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the NIH (GKG, RO1 DK064952, RO1 DK083541-01), Pennsylvania State Tobacco Fund (GKG), and the financial support of Children's Hospital of Pittsburgh. The authors would like to thank all GKG lab members for their technical assistance and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–8. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002;12:540–7. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 3.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–95. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 4.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 5.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 6.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–44. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- 7.Smith SB, Watada H, German MS. Neurogenin3 activates the islet differentiation program while repressing its own expression. Mol Endocrinol. 2004;18:142–9. doi: 10.1210/me.2003-0037. [DOI] [PubMed] [Google Scholar]
- 8.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 9.Lammert E, Brown J, Melton DA. Notch gene expression during pancreatic organogenesis. Mech Dev. 2000;94:199–203. doi: 10.1016/s0925-4773(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 10.Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–65. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Prasadan K, Daume E, Preuett B, Spilde T, Bhatia A, Kobayashi H, Hembree M, Manna P, Gittes GK. Glucagon is required for early insulin- positive differentiation in the developing mouse pancreas. Diabetes. 2002;51:3229–36. doi: 10.2337/diabetes.51.11.3229. [DOI] [PubMed] [Google Scholar]
- 12.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–11. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–57. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 14.Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis- regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes & Development. 2006;20:253–66. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–70. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seymour PA, Freude KK, Dubois CL, Shih HP, Patel NA, Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol. 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle MJ, Loomis ZL, Sussel L. Nkx2.2-repressor activity is sufficient to specify alpha-cells and a small number of beta-cells in the pancreatic islet. Development. 2007;134:515–23. doi: 10.1242/dev.02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–40. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 19.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A. 2004;101:2924–9. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi H, Spilde T, Li Z, Marosky J, Bhatia A, Hembree M, Prasadan K, Preuett B, Gittes G. Lectin as a marker for staining and purification of embryonic pancreatic epithelium. Biochem Biophys Res Commun. 2002;293:691–7. doi: 10.1016/S0006-291X(02)00278-4. [DOI] [PubMed] [Google Scholar]
- 21.Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–42. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 22.Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. 2008;237:3270–3279. doi: 10.1002/dvdy.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Jensen JN, Seymour PA, Hsu W, Dor Y, Sander M, Magnuson MA, Serup P, Gu G. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A. 2009;106:9715–20. doi: 10.1073/pnas.0904247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–62. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323–38. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.