Abstract
Constant exposure to new and persisting antigens and the need to replace cellular attrition with newly build cells lead to profound remodeling of the immune system during the second half of life. The impact of the immunosenescence process varies amongst the different functional subsets represented within the immune system, and emerging data suggest that progressive aging significantly affects frequencies, subset distribution and functional competence of regulatory T cells (Treg). Given the central role of Treg cells in immune homeostasis, age-related loss of Treg function would be predicted to render the host susceptible to excessive immunity, encountered in elderly humans as a syndrome of chronic-smoldering inflammation. Conversely, age-dependent gain of Treg activity would expose the host to greater risk of immune failure, such as the rising risk of malignancies and infections in the aging population. Emerging data suggest that some Treg populations, specifically naturally occurring Tregs (nTreg), seem to accumulate with advancing age, whereas inducible Tregs (iTreg) appear to be less available in the older host. More studies are necessary to elucidate functional competence of old Tregs, with emphasis on comparing efficacy of young on old Tregs for defined functional domains. Mechanisms of declining Treg inducibility are not understood, but may provide an opportunity for targeted immunomodulation in the elderly. On the horizon is the potential to develop novel therapeutic interventions that target Tregs to make the elderly more efficient in fighting cancers and infections and dampen the risk for senescence-associated inflammation.
Keywords: naturally occurring regulatory T cells, inducible regulatory T cells, immune aging
Age and T lymphocytes
Just like other organ systems, the immune system is susceptible to age-related changes, overall resulting in a deteriorating immune competence and a shift in the balance between protective and pathogenic immune responses. Paradoxically, immune aging is associated with muted immunity co-occurring with low-grade and chronic inflammation [1–3]. The process of immune aging has detrimental consequences for the aging host: succumbing to otherwise innocuous infectious agents, inability to respond to vaccination [4] and a declining ability to fight cancer [5]. In parallel, advancing age is a risk factor for autoimmunity and is associated with a chronic-smoldering inflammatory syndrome [6]. Cellular senescence has been associated with inflammatory tissue damage through the senescence-associated secretory phenotype (SASP) [7, 8]. While both the innate and adaptive branches of the immune system are affected by age, the changes in the T cell compartment are particularly important and bring considerable challenges to the aging organism. The T cell pool contains a number of well-defined, functionally distinct subsets: CD4+ T cells, CD8+ T cells, γδ T cells, NKT cells and other nonconventional T cells, each of those being further divided into subpopulations [such as naïve versus memory, Th1, Th2, Th17 and regulatory T cells (Tregs)]. While not all T cell compartments are equally affected by age, overall T cell numbers decline with age as thymic involution leads to decreased output of cells. With dwindling thymic T cell production, homeostatic proliferation of peripheral T cells has to compensate and is responsible for maintaining naïve T cell numbers. While this is an effective mechanism, it eventually fails, ultimately resulting in a decrease in the total number of naïve T cells allowing memory and effector T cells to become dominant [9]. In addition to the numerical decline, T cell receptor (TCR) repertoire diversity progressively contracts over time [9, 10], thus skewing further T cell responses. Repertoire contraction and declining T cell input affects functional subsets differentially [11]. For example, it has been shown for CD4+ T cells that Th1 cells numbers decline first, followed later by Th2 cells [12]. It has been proposed that age-related deviations in immune competence may partially be a reflection of changing function in T regulatory cells, profoundly affecting the balance between protective and pathogenic immune responses [13].
In this review, we investigate how regulatory T cells (Tregs) are affected by age both in terms of numbers and function and what the consequences are for the host immune system. While regulatory function has been ascribed to not only CD4+ and CD8+ T cells, but also to γδT cells and NKT cells [14–15], only CD4+ and CD8+ Tregs have been characterized and studied extensively enough to asses the impact of progressive age on their numbers and function. Therefore, we will concentrate on CD4+ and CD8+ Tregs, while acknowledging that aging-induced changes within smaller nonconventional Treg populations could have a critical impact on the whole immune system.
CD4+ Regulatory T cells
CD4+ Tregs, characterized by the expression of CD25 and the transcription factor FOXP3, are thymically-derived, hence termed naturally occurring, T cells with the ability to regulate both adaptive and innate immune responses [16]. The critical role they play in regulating immune responses and controlling inflammation has been demonstrated in a number of murine models as well as by the human FOXP3 mutation, which leads to immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX), characterized by multiple autoimmune conditions [17]. There is ample data to suggest that the numbers of CD4+ Tregs increase with age [18]. More specifically, in aging mice the percentage of CD4+ Treg cells rises in the spleen and the lymph nodes [19–21] (Figure 1, Table 1). While this increase in secondary lymphoid organs is well documented, murine studies have not shown any measurable increase in the percentage of Tregs between aged and young mice in peripheral blood [19, 21]. Human studies assessing peripheral blood of aged versus young individuals seem to concur that the percentage of CD4+ Tregs is increased in older individuals [21–23]. More supporting data comes from human in situ studies showing an increased percentage of CD4+FOXP3+ Tregs in skin biopsies from older individuals compared to younger adults. Interestingly, the increased percentage of Tregs in the skin was observed both pre- and post-antigenic stimulation, suggesting that not only Treg numbers are increased in the steady state in older adults, but those cells are able to expand very potently upon antigenic stimulation. All these data indicate that the CD4+ Treg compartment expands with age, relative to the total CD4+ T cells. However, since a number of CD4+ T cell subsets shrink in the elderly, it is not clear whether this relative increase is due to a loss of naïve CD4+ T cells or a bona fide expansion in absolute Treg numbers. Data from both mice [19, 20] and humans suggest that the increase in Tregs measured in older subjects is not simply a relative increase due to a decrease in other CD4+ T cell subsets, but reflects an increase in absolute Treg cell numbers (Figure 1, Table 1). In addition to an increase in the numbers of CD4+CD25+FOXP3+ Tregs, it has been suggested that CD4+CD25− T cells, when derived from aged mice become hyporesponsive and acquire immunosuppressive properties and are able to suppress allogeneic CD4+CD25− T cells from young mice [24]. This implies that not only Treg numbers increase, but conventional T cells acquire Treg properties, amplifying the general immunosuppressive environment.
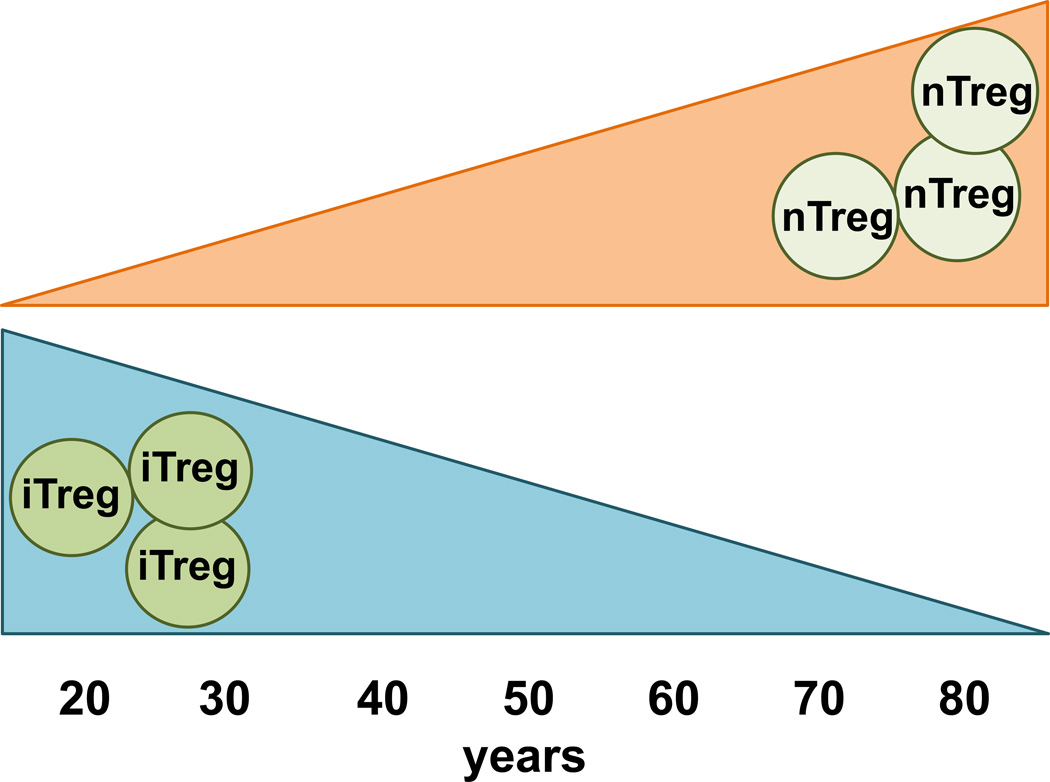
Figure 1. Natural Tregs accumulate with age while inducible Treg numbers decline.
The frequency and absolute number of naturally occurring Tregs (both CD4+ and CD8+) increases with advancing age in both humans and mice. In contrast, the inducibility of Tregs from non-regulatory CD4+ and CD8+ precursor cells declines over time.
Table 1. Regulatory T cell populations and the aging process.
Susceptibility of different Treg subpopulations to aging-induced changes. In the aging host, naturally occurring CD4+ and CD8+ Tregs expand in numbers. In contrast, inducible CD4+ and CD8+ Tregs numbers decline with advancing age. Information on many of the specialized Treg subsets is still missing.
| Changes in Tregs | References |
|---|---|
| Increased with age | |
| CD4+CD25+FOXP3+ natural Tregs | 15–17 (mouse); 17–21 (human) |
| CD8+FOXP3+ Tregs | 16 (mouse); 29 (human) |
| Decreased with age | |
| CD4+CD25+FOXP3+ inducible Tregs | 24 (mouse) |
| CD8+CD44+CD62L+CCR7+ Tregs | 32 (mouse) |
| CD8+CD45RA+CCR7+ Tregs | 30 (human) |
| Unknown | |
| CD8+CD39+FOXP3− Tregs | |
| CD8+CD103+ Tregs | |
| γδ Tregs | |
| NKTregs |
While CD4+FOXP3+ Tregs are thymically derived, cells with a similar phenotype can be induced from CD4+CD25−FOXP3− conventional T cells with TGF-β [25]. These inducible Tregs (iTregs) display the same suppressive function as natural Tregs (nTregs). Studies on iTregs are much more limited, but data suggest that induction of iTregs from conventional CD4+ T cells is impaired in aged mice, compared to younger mice [26] (Figure 1, Table 1). This is in stark contrast to the findings that natural Tregs are increased with age and implies that older individuals are less capable of generating iTregs, when required.
While increased numbers of Tregs could have devastating consequences for the host, it is only physiologically relevant if the suppressive function of the accumulated aged Tregs has remained intact. Phenotypic evaluation of Tregs suggests that the expression of the memory markers CD103 and CD62L is increased in Tregs from aged mice [19, 21] and that the expression of Treg markers CD25, GITR and CD69 is similarly increased in aged CD4+ Tregs [27]. The expression of CTLA-4, which mediates, at least in part, the immunosuppressive capability, remains unchanged [21], indicating that functionality of Tregs in aged mice is most likely neither reduced nor enhanced. In terms of FOXP3, which is the prototypic Treg transcription factor that defines commitment to regulatory function, controversy remains with some studies showing no difference in FOXP3 expression between Tregs from aged and young mice [19, 21], whereas others find an increase in FOXP3 expression in Tregs from aged mice compared to young mice [27, 28]. Human studies corroborate the latter set of studies; human CD4+FOXP3+ Tregs from older individuals show enhanced FOXP3 expression compared to Tregs from young individuals, while the remainder of their phenotypic make-up (GITR, CTLA-4, CD127low) is unchanged [21, 22]. While increased FOXP3 expression is seen as a surrogate marker of enhanced function, the impact on the suppressive function of Tregs needs to be evaluated. Lastly, the Vβ distribution of murine CD4+ Tregs changes with age, albeit in a similar fashion as non-Treg CD4+ T cells [21]. Again, the functional significance of this finding is unclear.
More concrete tests of suppressive capacity have shown that purified Tregs from aged and young mice [21, 28] and humans [22] are capable of suppressing CD4 T cell proliferation in vitro to the same extend (Figure 2). Similarly, CD8 proliferation and IFN-γ production have been shown to be suppressed to the same extend by Tregs derived from aged versus young mice [27]. However, Zhao et al have highlighted an interesting distinction by demonstrating that CD4+ Tregs from aged mice are equally capable of suppressing allogeneic CD4+ T cell proliferation and IFN-γ production, consistent with the rest of the literature, but were less capable of suppressing syngeneic CD4+ T cells [21]. It is possible that the inability of aged Tregs to suppress syngeneic CD4+ T cells is due to changes in the CD4+CD25− non-Treg compartment. More recent data show that although Tregs from aged mice are able to suppress IFN-γ–producing CD4+ T cells, consistent with the previous literature, aged Tregs failed to suppress IL-17–producing CD4+ T cells [29] (Figure 2). The implication of this finding is that although Tregs maintain most of their functional properties, which would be responsible for suppressing immune activation against infection and tumors, they may fail to control autoimmune inflammation. Such an abnormality could explain the apparent paradox observed during aging of aberrant inflammation coexisting with immune hyporesponsiveness to infection, vaccination and tumors.
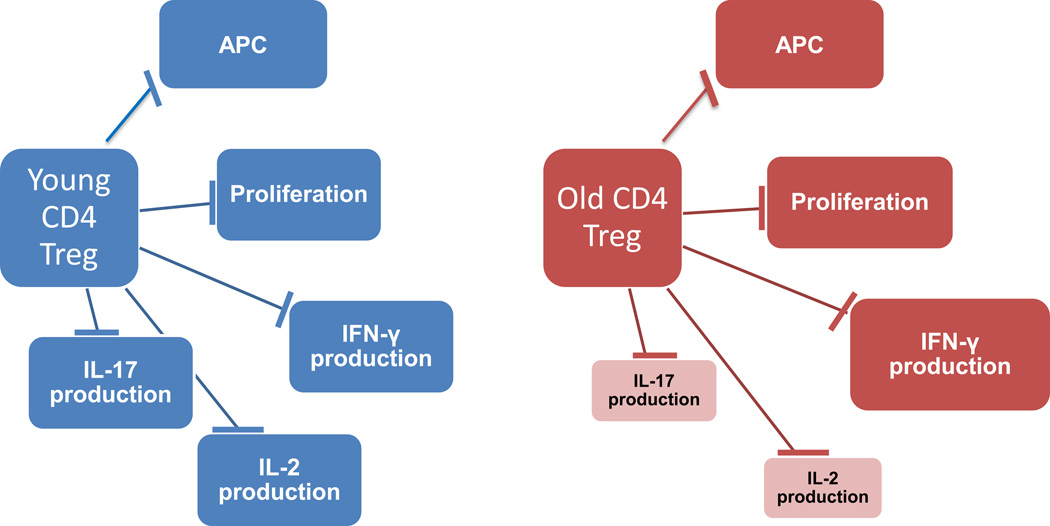
Figure 2. The impact of aging on the different functional domains of Tregs.
Tregs isolated from young hosts are able to efficiently suppress APC activation, T cell proliferation and production of cytokines (IFN-γ, IL-2 and IL-17). Aged Tregs maintain suppressive activity towards APCs, T cell proliferation and IFN-γ production; whereas such aged Tregs are much less efficient in downregulating IL-2 and IL-17 production in neighboring T cells.
One aspect of Treg function lies in regulating the function of antigen presenting cells. Some data suggest that there is downregulation of activation markers (CD40, CD80, CD86) on dendritic cells as a result of age-induced increases in Treg numbers [19]. Thus, aged Tregs may still be functionally capable of suppressing dendritic cells and increased numbers of skin-residing Tregs may play a role in the suppression of cutaneous macrophages in the skin of older individuals (Figure 2).
While most of the data from both mice and humans are in agreement that CD4+ Tregs numbers increase with age, it is still unclear why CD4+ Tregs accumulate with progressing age. Several mechanisms may contribute to the age-induced expansion of Treg frequencies (Table 2): a) increased thymic output of Tregs at the expense of naïve T cells with advancing age, b) changes in the homing of Tregs, c) increased conversion of conventional CD4+ T cells to Treg cells in the periphery and d) better survival of CD4+ Treg cells in the periphery compared to conventional CD4+ T cells. While limited data exist on the topic, a group that has looked into the percentage of CD4+FOXP3+ Tregs in the thymus found that there is no difference in the percentage of Treg cells in the thymus of older mice, therefore increased peripheral numbers of Tregs are probably not due to increased thymic output [26]. Since increased Treg numbers have been measured in circulating blood (at least for humans), it is also unlikely that the increased peripheral tissue numbers of Tregs are due to a redistribution of Tregs from blood to tissue. Induction of Tregs, at least in vitro, appears to be impaired in mice [26], rendering it unlikely that increasing Treg numbers in the elderly are due to increased conversion of non-Treg CD4+ T cells to Tregs. Measurement of in vivo proliferation of Tregs, using BrdU showed no enhanced proliferation of aged Tregs [26] suggesting that increased numbers also do not reflect excessive proliferative expansion. Evidence for reduced apoptosis rates in Treg cells from aged hosts came from Chouget et al who demonstrated reduction of the pro-apoptotic molecule Bim in aged Treg cells [26]. Interestingly, Bim expression is not changed in older non-Treg CD4+ T cells. This suggests that Tregs selectively lose Bim expression with age, which gives them a survival advantage over other CD4+ T cells. Whether Bim is solely responsible for this phenotype or whether there are other mechanisms involved remains to be investigated.
Table 2. Possible mechanisms of age-related expansion of Tregs.
The expansion of naturally occurring Tregs with advancing age could be due to a number of reasons listed here.
| Possible Mechanisms of Age-related Expansion of Tregs |
|---|
| Loss of naïve T cells opens “space” |
| Changes in Treg homing patterns |
| Increased conversion of CD4 T effector cells into CD4 T suppressor cells |
| Antigen-induced expansion of CD4 Treg cells |
| Survival advantage of CD4 Tregs compared to CD4 T effector cells |
CD8+ Regulatory T cells
Just like CD4+ T cells, CD8+ T cells lose naïve precursor cells with progressive age. Contraction of the naïve CD8 T cell compartment occurs at an even greater extent than in the CD4 T cell population [11]. Also, aging CD8 T cells sustain a number of functional defects spanning their ability to proliferate, to become activated, the magnitude of response to antigens and their cytotoxic ability [28]. While a major role of CD8+ T cells lies in lysing virally infected cells, there are a number of smaller CD8+ subpopulations with regulatory capacity. Tregs belonging to the CD8+ T cell compartment, although less well-studied than their CD4+ counterpart, are equally important in regulating immune responses [15]. Because the CD8+ Treg pool comprises of a variety of subsets, each with a unique phenotype, it is difficult to construct a simple picture of how their numbers and function are altered with advanced age. However, some clear parallels to the CD4+ Tregs can be drawn.
Like CD4+ Tregs, the percentage of CD8+FOXP3+ Tregs has been shown to be significantly increased in the blood of older individuals [31], as well as in the spleen and lymph nodes of aged mice [20] (Figure 1, Table 1). A corresponding increase in the absolute number of CD8+ Tregs has been shown in murine spleen and lymph nodes [20], suggesting that this is a genuine increase in numbers and not just a relative increase due to declining numbers of other CD8+ T cell subsets.
In contrast, inducible CD8+CCR7+ Tregs, which maintain a naïve phenotype even after a 6-day in vitro induction, clearly decrease with age [32] (Figure 1, Table 1). More specifically, peripheral blood lymphocytes from elderly donors have significantly lower capacity of inducing CD8+CCR7+ Tregs than younger ones following stimulation with anti-CD3 mAb and IL-15 in vitro. While the mechanism responsible for the reduction of inducible CD8+ Tregs is not known, their reduction is independent of the reduced number of precursor CD8+CD45RA+ naïve T cells [32]. Moreover, the expression levels of both FOXP3 and CD45RA in CD8+CCR7+ Tregs from older individuals were also lower than from younger donors. This decreased expression of FOXP3 is suggestive of a reduced suppressive capacity and could possible contribute to the rising risk for autoimmune disease in the elderly host. In vivo treatment with IL-15, also induced a smaller population of CD8+CD122+ T cells in the liver and spleen of aged mice compared to younger mice [33], suggesting a potential role for altered IL-15 signaling. The cytokine IL-15 is important in establishing central memory CD8+ T cells, and especially CD8+CD122+ T cells, which contain a population of central memory T cells expressing CD44+CD62L+CCR7+ that display Treg function [34]. The Treg (CD44+CD62L+CCR7+) portion of the IL-15 induced CD8+CD122+ T cells is considerably decreased in older mice [34]. In contrast, CD8+CD122+ T cells in old mice had a high percentage of the effector memory population of CD44+CD62L−CCR7−, which were shown to have a weaker regulatory function [34]. These findings are similar to the observation that CD4+ iTregs cannot be efficiently induced in aged mice, suggesting a consistent pattern whereby naturally existing Tregs (whether CD4+ or CD8+) are increased with age, whereas inducible Tregs decline with age.
In terms of function, the ability of aged naturally occurring CD8+ Tregs to suppress the proliferation and cytokine production of effector CD4+ T cells remains comparable between younger and elder individuals [31]. Interestingly, a defined subset of CD8+FOXP3+ Tregs lacks CD28 expression [35]. End-differentiated CD8+CD28− T cells are considered as a hallmark of immune aging, since they have been known for a long time to expand with progressing age, while the frequency of naïve CD8+CD28+ T cells decreases with age [36]. This indicates that the increase in CD8+FOXP3+CD28− Tregs is consistent with the increase in overall numbers of CD8+CD28− T cells. Whether the functional spectrum of CD8+CCR7+CD45RA+ Tregs, which display a naïve phenotype, and CD8+CD28− Tregs, which resemble end-differentiated effector T cells, are comparable or distinct is currently not known. The expression of CCR7 enables CD8+CCR7+ Tregs to traffic like a naïve cell and access storage places that are occupied by naïve cells, such as lymph nodes. In contrast, end-differentiated CD8+CD28− Tregs should display a distribution pattern like end-differentiated T cells, assigning them to CCL5-producing tissues sites, such as inflammatory lesions. In essence, the tissue distribution patterns and thus, the functional impact of these CD8 Treg populations, is predicted to be quite different.
In summary, naturally occurring CD8+ Tregs numbers appear to increase with age, while maintaining immunosuppressive function, whereas induction of CD8+ Tregs in the periphery declines with age (Figure 1, Table 1). Whether aged Tregs are fully functional or are biased to preferentially suppress specific CD4+ Th subsets and whether Bim also plays a role in maintaining higher numbers of CD8+ Tregs or another mechanism is responsible for the increase in numbers are questions that remain to be answered.
Immunosenescence of Tregs – Functional consequences
Since naturally occurring Tregs increase with age, but iTregs decline with age, it is difficult to delineate what the functional consequences are for the aging host. An increase in Tregs would be predicted to cause disease phenotypes that are consistent with aging (Table 3). Firstly, cancer is a disease mainly affecting the aging population [5]. Moreover, cancer incidence has been shown to correlate with an increase in Treg numbers. In gastric cancer tissue increased prevalence of CD8+FOXP3+ Tregs has been described, whose phenotype is that of effector memory cells lacking both CD45RA and CD27 [37]. CD4+ Tregs infiltrating cancer tissue and expressing an effector memory phenotype of CD44+CD62L−CCR7− have been shown, not only to be prevalent but also to be functionally able to suppress CD4+ T cell responses [38]. Percentages of CD4+ Tregs and FOXP3 expression correlate with lung cancer metastasis rates in elderly individuals [20]. While human studies of Tregs in cancer can only be correlative, Sharma et al were able to illustrate the link between cancer and Tregs in the murine system by demonstrating that increased numbers of Tregs in aged mice are indeed responsible for lack of anti-tumor responses. Recent data show that a tumor challenge results in a higher tumor load in aged mice compared to young mice and that tumor load correlates to an increase in CD4+ Tregs. Secondly, susceptibility to another major killer of the elderly population, infectious diseases, also goes hand in hand with Tregs numbers [39]. It is thought that an increase in Treg numbers leads to impaired anti-pathogen responses and may contribute to the high risk of disease reactivation typically encountered in individuals older than 60 years of age [39]. Indeed, Lages et al have demonstrated that presence of increased Treg numbers in aged mice is responsible for lack of an appropriate response against Leishmania major by suppressing IFN-γ production [21]. More recent studies from Williams-Bey et al have provided evidence on another, functionally important link between aging, Treg function and infectious susceptibility. Following influenza infection, aged mice, in addition to starting with higher Treg numbers, expand their Treg pool to a much greater extend than young mice, which results in decreased IFN-γ production by anti-viral CD8+ effector T cells [27]. Human data show that an increase of Tregs in the skin correlates with a reduced response to VZV challenge [40]. This mechanism could be of particular relevance in reactivation of herpes zoster infection, a typical complication in the aging population. Here, age-related defects in Treg biology could provide an explanation for the combination of poor vaccine responsiveness and increased susceptibility to viral reactivation [39]. Age-related reprogramming of CD4 T cells has been associated with a shift in intracellular kinases and phosphatases, majorly impacting the outcome of immune stimulation [41, 42]. Whether similar mechanisms hold for Treg cells is currently unknown. Lastly, causal links between increased Treg numbers and incidence of neurodegenerative disease have been suggested [43], since in a mouse model of optic nerve injury, neuron survival was higher in the absence of Tregs. This opens up the possibility that increased Treg numbers in older individuals may negatively impact neuron survival and the ability of the CNS to withstand and recover from injury.
Table 3. Predicted and observed consequences of increased and decreased Treg numbers.
Gain and loss of Treg function with progressive age has predictable consequences for the immune competence of the host. Trends for age-related morbidities in the general population are highlighted in orange; aging individuals become more susceptible to malignancy and infection (increased Treg function) but, at the same time, are more likely to develop autoimmunity (reduced Treg function).
| Age-related increase in Treg function |
Age-related loss of Treg function |
|
|---|---|---|
| Tumor cell development and growth |
Impaired anti-tumor responses |
More effective anti- tumor responses |
| Protection from infections |
Declining anti-microbial immune responses |
Improved anti-pathogen immunity |
| Self tolerance | Autoimmunity mostly in the young |
Autoimmunity mostly in the elderly |
| Tissue regeneration Wound healing |
Tissue degeneration | Effective tissue regeneration in the elderly |
 = Changes in aging hosts
= Changes in aging hosts
Another important aspect of the immune aging process is a syndrome of smoldering, low-grade inflammation [6] and the increasing risk to develop autoimmune disease in the elderly [44]. In light of insufficient data from aging humans it is not clear, whether this inflammatory syndrome results from enhanced immune responsiveness, from lack of physiologic immune suppression or both. Given the critical role of Treg in immune homeostasis, any decline in Treg competence would inevitably lead to a dysbalance of protective and pathogenic immunity and would favor chronic relentless and possibly tissue-damaging inflammation (Table 3). In view of increasing frequencies of Tregs with advancing age it is predictable that they are either less efficient in downregulating inflammation or fail to be recruited to the sites where such inflammation originates. Another possible mechanism involves functional skewing such that Treg in the elderly preferentially suppress selected T cell populations while leaving other relatively unaffected [29] and therefore allow certain proinflammatory cells to persist. Finally, Treg diversity must be considered. Evidence suggests that iTreg may be more susceptible to age-related insufficiency whereas nTreg appear to be rising with advancing age. Failure to oppose the inflammatory syndrome of the senescent immune system may reflect a decline in the inducibility and durability of iTreg. Appropriate studies are required to address the relevant issues. Is there a division of labor between nTreg and iTreg? Is the inflammatory syndrome of the elderly susceptible to Treg-mediated suppression? Do Tregs, either nTreg or iTreg, dampen life-saving anti-tumor responses? Is anti-pathogen immunity in the elderly subject to Treg-dependent regulation?
Given the important role that Treg dysbalance appears to play in the development of diseases afflicting primarily the elderly population, the possibility of harnessing Treg function has significant therapeutic potential [45]. Selective depletion or inhibition of Tregs is a possibility that has been explored in cancer models [46] and is a strategy that could be applied, not only to prevent and treat cancer, but also infections and could be used as an adjuvant to enhance responses to vaccination. By the same token, expanding Tregs could prove beneficial in treating the inflammation associated with aging. While there are still major technical obstacles to be overcome for both the targeted depletion and the ex vivo expansion of functional Tregs, the development of a successful biologic therapy based on Tregs will also depend on our ability to delineate the precise contribution of Tregs to the initiation and perpetuation of the diseases of the aging host.
References
- 1.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. 2012;24:488–493. doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011;2:524–537. [PMC free article] [PubMed] [Google Scholar]
- 3.Goronzy JJ, Li G, Yang Z, Weyand CM. The Janus head of T cell aging – autoimmunity and immunodeficiency. Front Immunol. 2013;4:131. doi: 10.3389/fimmu.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger B, Grubeck-Loebenstein B. Vaccines for the elderly. Clin Microbiol Infect. 2012;(suppl 5):100–108. doi: 10.1111/j.1469-0691.2012.03944.x. [DOI] [PubMed] [Google Scholar]
- 5.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Cevenini E, Monti D, Franceschi C. Inflammageing. Curr Opin Clin Nutr Metab Care. 2013;16:14–20. doi: 10.1097/MCO.0b013e32835ada13. [DOI] [PubMed] [Google Scholar]
- 7.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf J, Weinberger B, Arnold CR, Maier AB, Westendorp RG, Grubeck-Loebenstein B. The effect of chronological age on the inflammatory response of human fibroblasts. Exp Gerontol. 2012;47:749–753. doi: 10.1016/j.exger.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold CR, Wolf J, Brunner S, Herndler-Brandstetter D, Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age – age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;31:137–146. doi: 10.1007/s10875-010-9499-x. [DOI] [PubMed] [Google Scholar]
- 11.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu BC, Shang X, Frait KA, Hu JS, Komuniecki E, Miller RA, Chensue SW. Differential effects of ageing on cytokine and chemokine responses during type-1 (mycobacterial) and type-2 (schistosomal) pulmonary granulomatous inflammation in mice. Mech Ageing Dev. 2002;123:313–326. doi: 10.1016/s0047-6374(01)00372-4. [DOI] [PubMed] [Google Scholar]
- 13.Dejaco C, Duftner C, Schirmer M. Are regulatory T-cells linked with aging? Exp Gerontol. 2006;41:339–345. doi: 10.1016/j.exger.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Caccamo N, Todaro M, Sireci G, Meraviglia S, Stassi G, Dieli F. Mechanisms underlying lineage commitment and plasticity of human γδ T cells. Cell Mol Immunol. 2013;10:30–34. doi: 10.1038/cmi.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol Pathol. 2012;40:186–204. doi: 10.1177/0192623311430693. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 18.Raynor J, Lages CS, Shehata H, Hildeman DA, Chougnet CA. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. 2012;24:482–487. doi: 10.1016/j.coi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu BC, Stolberg VR, Zhang H, Chensue SW. Increased Foxp3(+) Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice. Mech Ageing Dev. 2007;128:618–627. doi: 10.1016/j.mad.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 21.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25 high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottenberg JE, Lavie F, Abbed K, Gasnault J, Le Nevot E, Delfraissy JF, Taoufik Y, Mariette X. CD4 CD25 high regulatory T cells are not impaired in patients with primary Sjogren’s syndrome. J Autoimmun. 2005;24:235–242. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu J, Moriizumi E. CD4+CD25− T cells in aged mice are hyporesponsive and exhibit suppressive activity. J Immunol. 2003;170:1675–1682. doi: 10.4049/jimmunol.170.4.1675. [DOI] [PubMed] [Google Scholar]
- 25.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 26.Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, Plas DR, Hildeman DA. A major role for Bim in regulatory T cell homeostasis. J Immunol. 2011;186:156–163. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams-Bey Y, Jiang J, Murasko DM. Expansion of regulatory T cells in aged mice following influenza infection. Mech Ageing Dev. 2011;132:163–170. doi: 10.1016/j.mad.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25−Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 29.Sun L, Hurez VJ, Thibodeaux SR, Kious MJ, Liu A, Lin P, Murthy K, Pandeswara S, Shin T, Curiel TJ. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging Cell. 2012;11:509–519. doi: 10.1111/j.1474-9726.2012.00812.x. [DOI] [PubMed] [Google Scholar]
- 30.Nikolich-Žugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin Immunol. 2012;24:356–364. doi: 10.1016/j.smim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simone R, Zicca A, Saverino D. The frequency of regulatory CD3+CD8+CD28− CD25+ T lymphocytes in human peripheral blood increases with age. J Leukoc Biol. 2008;84:1454–1461. doi: 10.1189/jlb.0907627. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Jagger AL, Konya C, Shimojima Y, Pryshchep S, Goronzy JJ, Weyand CM. CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells. J Immunol. 2012;189:2118–2130. doi: 10.4049/jimmunol.1200122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motegi A, Kinoshita M, Inatsu A, Habu Y, Saitoh D, Seki S. IL-15-induced CD8+CD122+ T cells increase antibacterial and anti-tumor immune responses: implications for immune function in aged mice. J Leukoc Biol. 2008;84:1047–1056. doi: 10.1189/jlb.0807530. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Shi Z, Okuno Y, Isobe K. Are CD8+CD122+ cells regulatory T cells or memory T cells? Hum Immunol. 2008;69:751–754. doi: 10.1016/j.humimm.2008.08.285. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–789. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28− T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng LS, Zhuang Y, Shi Y, Zhao YL, Wang TT, Chen N, Cheng P, Liu T, Liu XF, Zhang JY, Zuo QF, Mao XH, Guo G, Lu DS, Yu PW, Zou QM. Increased tumor-infiltrating CD8(+)Foxp3(+) T lymphocytes are associated with tumor progression in human gastric cancer. Cancer Immunol Immunother. 2012;61:2183–2192. doi: 10.1007/s00262-012-1277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanchot C, Terme M, Pere H, Tran T, Benhamouda N, Strioga M, Banissi C, Galluzzi L, Kroemer G, Tartour E. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron. 2013;6:147–157. doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 40.Vukmanovic-Stejic M, Sandhu D, Sobande TO, Agius E, Lacy KE, Riddell N, Montez S, Dintwe OB, Scriba TJ, Breuer J, Nikolich-Zugich J, Ogg G, Rustin MH, Akbar AN. Varicella zoster-specific CD4+Foxp3+ T cells accumulate after cutaneous antigen challenge in humans. J Immunol. 2013;190:977–986. doi: 10.4049/jimmunol.1201331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells – a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kipnis J, Avidan H, Caspi RR, Schwartz M. Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia. Proc Natl Acad Sci USA. 2004;101(suppl 2):14663–14669. doi: 10.1073/pnas.0404842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther. 2012;12:1383–1397. doi: 10.1517/14712598.2012.707184. [DOI] [PMC free article] [PubMed] [Google Scholar]