Abstract
Congenital hyperinsulinism of infancy (CHI) can be caused by inactivating mutations in the gene encoding short-chain 3-hydroxyacyl-CoA dehydrogenase (SCHAD), a ubiquitously expressed enzyme involved in fatty acid oxidation. The hypersecretion of insulin may be explained by a loss of interaction between SCHAD and glutamate dehydrogenase in the pancreatic β-cells. However, there is also a general accumulation of metabolites specific for the enzymatic defect in affected individuals. It remains to be explored whether hypoglycemia in SCHAD CHI can be uncoupled from the systemic effect on fatty acid oxidation. We therefore transplanted islets from global SCHAD knockout (SCHADKO) mice into mice with streptozotocin-induced diabetes. After transplantation, SCHADKO islet recipients exhibited significantly lower random and fasting blood glucose compared with mice transplanted with normal islets or nondiabetic, nontransplanted controls. Furthermore, intraperitoneal glucose tolerance was improved in animals receiving SCHADKO islets compared with those receiving normal islets. Graft β-cell proliferation and apoptosis rates were similar in the two transplantation groups. We conclude that hypoglycemia in SCHAD-CHI is islet cell–autonomous.
Introduction
Persistent hyperinsulinemic hypoglycemia in neonates, often termed congenital hyperinsulinism of infancy (CHI), comprises a group of inherited disorders in which the hallmarks are persistent hypoglycemia and inappropriately elevated insulin secretion (1). Both diagnosis and treatment of CHI are complicated. Medications tend to be ineffective, and partial or subtotal surgical resection of the pancreas may eventually be needed in many patients (1).
Mutations in nine different genes can cause CHI (2). These genes are expressed in the pancreatic β-cell, where they are involved in the regulation of insulin secretion. About half of the CHI cases are “channelopathies” being caused by inactivating mutations in the ABCC8 and KCNJ11 genes that encode subunits of the β-cells’ KATP channel (2,3). Most of the other genetic CHI etiologies can be classified as “metabolopathies,” as they directly involve dysregulated metabolic pathways due to mutated enzyme genes (2).
A classic example of the latter category is CHI caused by recessive mutations in HADH (4,5). This gene encodes short-chain 3-hydroxyacyl-CoA dehydrogenase (SCHAD), a mitochondrial enzyme that catalyzes the third of the four steps in fatty acid oxidation. More than 20 patients with SCHAD deficiency have now been reported. Intriguingly, they exhibit leucine-sensitive, diazoxide-responsive CHI rather than a phenotype typical for fatty acid oxidation disorders (6). The mechanism behind SCHAD CHI remained enigmatic for many years until Li et al. (7) investigated a global knockout mouse model (SCHADKO) and demonstrated that SCHAD serves to inhibit the enzyme glutamate dehydrogenase (GDH), another protein directly implicated in CHI (8).
However, HADH is ubiquitously expressed, suggesting that SCHAD deficiency is a metabolic disease with possible implications outside the context of the pancreatic β-cell. As such, in affected individuals there is abnormal accumulation of 3-hydroxybutyryl-carnitine in blood and 3-hydroxyglutaric acid in urine—metabolites that are both directly attributable to the enzymatic defect (4–6). To explore whether the impact of global knockout of the SCHAD enzyme on glucose homeostasis is secondary only to defects in the islets of Langerhans, we transplanted SCHADKO islets into mice with streptozotocin (STZ)-induced diabetes.
Research Design and Methods
Mouse Strains
Islet donor mice were of the SCHADKO strain (mixed B6/129 background) reported by Li et al. (7). Mice were bred as Hadh+/− heterozygotes and Hadh+/+ (wild type [WT]), and Hadh−/− (SCHADKO) offspring were used for the experiments. Genotyping was performed as previously described (7).
Recipient animals were male ICR-SCID mice purchased from Taconic (Hudson, NY). Diabetes was induced 1 week before transplantation by injection of 180 mg/kg i.p. STZ (Sigma-Aldrich, St. Louis, MO), freshly dissolved in sodium citrate buffer (pH 4.5). Morning blood glucose was determined in tail vein blood and measured by a OneTouch Ultra glucometer (LifeScan, Milpitas, CA). Recipient mice were considered diabetic when their random fed blood glucose level exceeded 350 mg/dL on at least two consecutive days.
The animals were kept on a 12-h light/dark cycle at 22°C and had ad libitum access to food and water. All animal experiments were approved by the Joslin Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
Isolation of Islets and Transplantation
Islets were isolated from 10- to 12-week-old male and female SCHADKO and WT mice as previously described (9). Four hundred islet equivalents were transplanted under the left kidney capsule of STZ-induced diabetic mice (10). The islets were gently centrifuged into a capillary plastic tubing shortly before transplantation, and graft volume was calculated. Random fed blood glucose and body weight were monitored on a weekly basis until nephrectomy of the islet graft–containing kidney in week 11. Recipient mice were considered cured of diabetes when the glucose level was <200 mg/dL for at least 2 consecutive weeks. The animals were killed after 3 consecutive days of hyperglycemia (>200 mg/dL) postnephrectomy.
Glucose Tolerance Testing
Donors
We used a similar approach to that reported by Li et al. (7) and performed glucose tolerance testing by oral administration of 20% dextrose at a dose of 2 g/kg body wt 3 weeks before transplantation. Male WT and SCHADKO mice were fasted overnight (16–18 h) with water available ad libitum. The next morning blood glucose was measured before (time point 0) and 15, 30, 60, and 120 min after glucose administration.
Recipients
To avoid the stress of oral gavage in recipients that underwent surgery and consistent with the routine approach in our laboratory, we performed intraperitoneal glucose tolerance testing (IPGTT) 10 weeks posttransplantation. The mice were fasted overnight (16–18 h), and blood glucose was measured before (time point 0) and 15, 30, 60, 90, and 120 min after injection of glucose at a dose of 2 g/kg body wt i.p. At 0, 30, and 90 min, 40–50 μL blood was collected for insulin measurement. Plasma insulin was measured by the Diabetes Research Center Specialized Assay Core at Joslin Diabetes Center using the rodent insulin ELISA kit (catalog no. 90010) from Crystal Chem (Downers Grove, IL).
Tissue Processing and Immunohistochemistry
Upon nephrectomy, the recipient kidneys were immediately fixed in 4% paraformaldehyde solution for 2 h and then stored in PBS at 4°C until processing, paraffin embedding, and sectioning at 5 μm thickness. Four WT grafts and three from the SCHADKO group were recovered after final sectioning and insulin staining. Primary antibody against SCHAD (catalog no. ab154088, rabbit polyclonal, Abcam, Cambridge, MA; dilution 1:1,000) was applied overnight at 4°C followed by secondary antibody (catalog no. 711-066-152, Jackson ImmunoResearch, West Grove, PA; dilution 1:400) and signal amplification by the TSA Fluorescein System (catalog no. NEL701A; PerkinElmer, Boston, MA). Primary antibody against insulin (catalog no. ab7842, guinea pig polyclonal, Abcam; dilution 1:400) was then applied at room temperature for 1 h, followed by secondary antibody (catalog no. 706-586-148, Jackson ImmunoResearch; dilution 1:400). For glucagon and Ki-67 staining, mouse monoclonal antibodies from, respectively, Sigma-Aldrich (catalog no. G2654, 1:8,000) and BD Biosciences, San Jose, CA (catalog no. 556003, 1:50), were used. Secondary antibody was from Jackson ImmunoResearch (catalog no. 715-546-150, 1:400). TUNEL staining was done with the ApopTag Fluorescein In Situ Apoptosis Detection kit (Merck Millipore, Darmstadt, Germany).
Statistics
Data are presented as mean ± SEM. Statistical significance between groups was tested with unpaired two-tailed Student t test.
Results
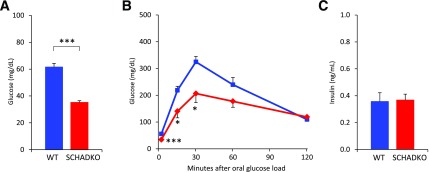
We confirmed that the donor (SCHADKO) mice exhibited the previously reported hypoglycemic phenotype (7) by measuring blood glucose levels after a 24-h fast and observing a significant difference between the WT and SCHADKO animals (62 ± 2.7 vs. 35 ± 1.3 mg/dL) (Fig. 1A). Oral glucose tolerance testing on overnight-fasted animals also showed significantly lower blood glucose levels in the SCHADKO mice at 15 and 30 min (Fig. 1B). The glycemic response evaluated as area under the curve, using basal glucose level as baseline, appeared lower in the SCHADKO animals but did not reach statistical significance (WT 18,400 ± 1,860 vs. SCHADKO 14,500 ± 2,230; P = 0.20). Again, this was similar to the previous observation (7). Upon fasting, circulating insulin levels were low and not significantly different between the groups (Fig. 1C). The insulin-to-glucose ratio, however, was different between WT and SCHADKO (0.006 ± 0.0011 vs. 0.011 ± 0.0019; P = 0.05).
Figure 1.
Glucose metabolism in male donor mice at age 7–8 weeks, i.e., 3 weeks before transplantation. A: Blood glucose in WT (n = 16) and SCHADKO (n = 16) animals fasted for 24 h. B: Oral glucose tolerance in WT (blue line, n = 7) and SCHADKO (red line, n = 6) animals. C: Serum insulin levels after an overnight fast in WT (n = 7) and SCHADKO (n = 6) animals. *Statistical significance at P = 0.05. ***Statistical significance at P = 0.001.
Next, we performed the transplantation experiment using 10- to 12-week-old SCHADKO animals or their WT littermates as islet donors and diabetic ICR-SCID mice as recipients. Mean transplanted islet volumes were 0.87 ± 0.12 mm3 for WT grafts vs. 0.98 ± 0.11 mm3 for SCHADKO grafts (P = 0.52). Among 12 recipients transplanted with WT islets, 6 (50%) had exhibited stable normoglycemia 3 weeks after transplantation. The corresponding cure rate among SCHADKO recipients was six of eight (75%) transplanted animals.
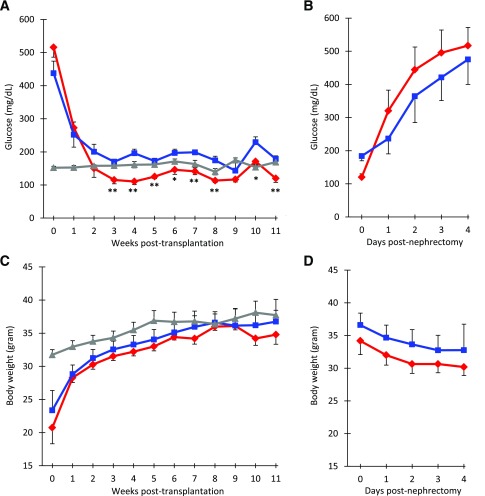
Both experimental groups showed a fall in blood glucose by 1 week after transplantation. Starting at 3 weeks, we observed a significant difference in random blood glucose levels between the groups at all but one time point, with the mice receiving SCHADKO islets always exhibiting lower levels (Fig. 2A). The average difference over the 3- to 11-week period between the two transplantation groups was 56 mg/dL. Random blood glucose values in the nontransplanted ICR-SCID control mice were generally between the values observed for the two transplanted groups. The small spike in blood glucose observed in week 10 in both transplanted groups likely reflects a stress response as a consequence of the IPGTT being performed the day prior to the weekly measurement of random blood glucose.
Figure 2.
Effect on random blood glucose (A and B) and body weight (C and D) of transplanting SCHADKO and WT islets into diabetic ICR-SCID mice. A: Measurements of random blood glucose over 11 weeks after transplantation. B: Measurements of random blood glucose after nephrectomy. C: Body weight changes over 11 weeks after transplantation. D: Measurements of body weight after nephrectomy. Time point 0 refers to the date of transplantation (A and C) or nephrectomy (B and D). The red and blue lines represent data from mice that received, respectively, SCHADKO (n = 6) or WT (n = 6) islets and returned to normoglycemia. In panels A and C, the gray line shows measurements in a control group of age-matched, nontransplanted, and euglycemic ICR-SCID mice (n = 8). *Statistical significance at P = 0.05. **Statistical significance at P = 0.01 comparing the SCHADKO and WT transplantation groups.
In the days after STZ injection, the mice lost a substantial part of their body weight due to the development of diabetes (not shown). However, after returning to normoglycemia upon islet transplantation, they rapidly regained weight and at week 8 had caught up to the body weights of nontransplanted control mice (Fig. 2C). The rate of weight gain was similar in the two transplanted groups.
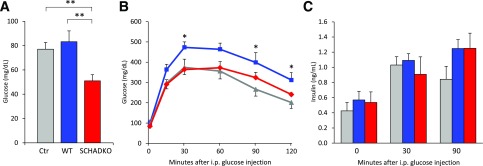
Five weeks after transplantation, blood glucose levels were measured in 24-h fasted mice. The animals receiving SCHADKO islets had significantly lower levels than both WT transplanted and nontransplanted mice (51 ± 5.0 vs. 83 ± 8.9 and 77 ± 5.5 mg/dL, respectively) (Fig. 3A). IPGTT performed at week 10 posttransplantation in overnight-fasted animals revealed that mice transplanted with WT islets exhibited significantly impaired glucose tolerance compared with both SCHADKO recipients and nontransplanted controls (area under the curve 35,600 ± 2,730 vs. 27,500 ± 2,520 [P = 0.05] and 24,100 ± 3,420 [P = 0.03], respectively) (Fig. 3B). The glucose tolerance was similar in the SCHADKO recipients and nontransplanted controls (P = 0.45), and the blood glucose levels were not significantly different at any of the time points (Fig. 3B). Circulating insulin levels were comparable at baseline (time 0) (Fig. 3C), and stimulation with glucose led to a similar secretory response at 30 and 90 mins in all three groups (Fig. 3C).
Figure 3.
Glucose metabolism in diabetic mice that received either normal (WT, n = 6; blue) or SCHADKO (n = 6; red) islets and in age-matched, nontransplanted, and euglycemic control ICR-SCID mice (Ctr) (n = 8; gray). A: Fasting blood glucose (24 h). Measurements were done 5 weeks after transplantation. **Statistical significance at P = 0.01. B: Intraperitoneal glucose tolerance at week 10 posttransplantation. *Statistical significance at P = 0.05 comparing the WT group with the controls. C: Serum insulin levels during IPGTT (0, 30, and 90 min after glucose injection).
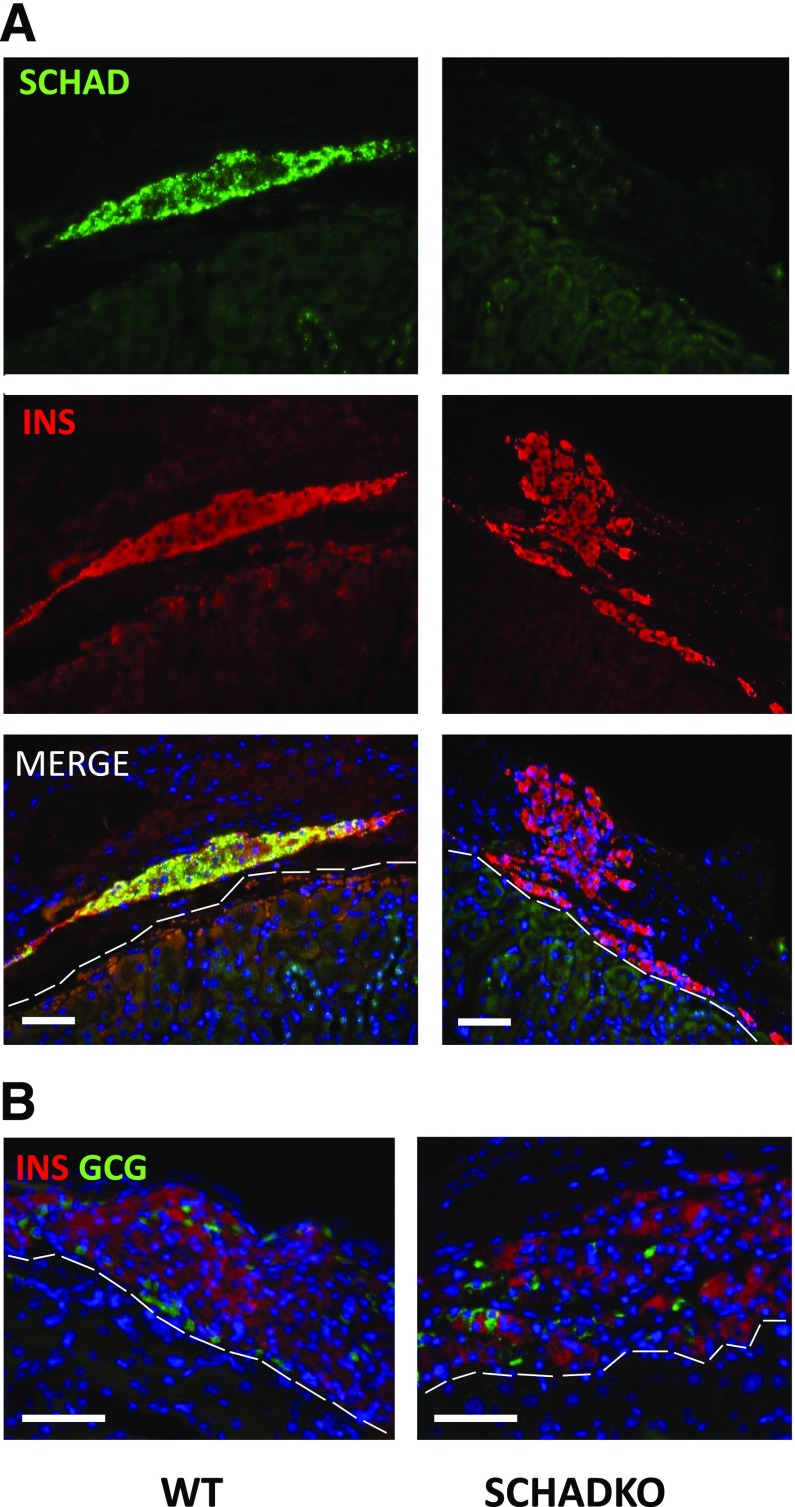
To confirm that the effects on normalization of the blood glucose were indeed secondary to transplanted islets, we removed the recipient kidney in both transplantation groups in week 11. Shortly thereafter, all mice became hyperglycemic and started to lose weight (Fig. 2B and D). Four days postnephrectomy, random blood glucose levels were generally >350 mg/dL and the animals were killed. The recipient kidneys were sectioned and immunostained for insulin and SCHAD, confirming that SCHAD protein was not expressed in the islet grafts derived from SCHADKO animals (Fig. 4A). We observed no systematic difference between groups with regard to the size of recovered grafts or β-cells counted per section. The β-cell proliferation rate, as evaluated by double staining for insulin and Ki-67, was between 0 and 0.5%, with no differences between groups. No apopotic β-cells were detected in any of the grafts by the TUNEL assay. Finally, there was no obvious difference in β-cell–to–α-cell ratio between WT and SCHADKO transplants (Fig. 4B). Percentages of glucagon-positive cells (fraction of all insulin- or glucagon-positive cells) were 6.3, 9.5, 13.0, and 14.3% for WT grafts and 13.7, 15.1, and 17.4% for SCHADKO grafts. Single cells positive for both markers were not seen in any section.
Figure 4.
Immunohistochemistry performed on islet grafts recovered after nephrectomy in the 11th week of the study. Representative sections from the WT and SCHADKO transplantation groups are shown. The dashed white lines indicate outline of the kidney parenchyma. Scale bars are 50 μm. A: Staining performed with fluorescent antibodies against SCHAD (green) and insulin (INS) (red). B: Staining performed with fluorescent antibodies against glucagon (GCG) (green) and insulin (red).
Discussion
The phenotype of CHI caused by SCHAD deficiency has been difficult to explain as a defect in fatty acid degradation. Initially, it was suggested that the protein acted as a coupling factor between fatty acid oxidation and glucose metabolism in the pancreatic β-cell and that the disease mechanism was KATP channel independent and involved accumulation of certain metabolites (4,5,11). However, more recent studies have reported that SCHAD interacts with and inhibits the enzyme GDH (7,12), a finding consistent with the protein-sensitive hyperinsulinemic hypoglycemia noted in some SCHAD-deficient patients (13).
However, it is well established that fatty acids and their metabolites can modulate insulin secretion (14–16), and newly discovered esters of hydroxy-fatty acids were described to have a surprising antidiabetes effect (17). Because some fatty acid–related metabolites are clearly increased both in humans with SCHAD deficiency (4–6) and in the corresponding animal model (7), it is possible that systemic metabolic changes play a role in the persistent hyperinsulinemic hypoglycemia characteristic of the disease. We therefore directly addressed this issue by transplanting pancreatic islets from either SCHADKO or WT animals into STZ-induced diabetic recipients.
The SCHADKO donor mice in our studies exhibited the phenotype reported previously (7), i.e., lower blood glucose levels in the fasted state and similar glycemic responses compared with WT mice (Fig. 1A and B). In contrast to Li et al. (7), we did not detect an increased basal insulin level in SCHADKO mice (Fig. 1C) but did observe an inappropriately elevated insulin-to-glucose ratio (7). Moreover, 3 weeks after transplantation the SCHADKO islet-transplanted mice presented a significantly lower random fed glucose level than mice transplanted with WT islets (Fig. 2A). Upon removal of the kidney that had received the graft, all animals in both transplantation groups rapidly lost weight and became overtly diabetic (Fig. 2B and D). Together, these results support the notion that the established euglycemia depended mostly on the functional islet graft.
We also considered the possibility that a quantitative effect may explain the lower blood glucose levels in the SCHADKO group. Thus, although the average graft volume pretransplantation was ∼10% smaller in the WT group compared with the SCHADKO group, the difference did not reach statistical significance (P = 0.52). Evaluation of the grafts recovered after nephrectomy did not reveal significant differences in β-cell number, rate of β-cell proliferation or apoptosis, or α-cell–to–β-cell ratio between groups. These data suggest that it is unlikely that graft size/composition or β-cell proliferation/survival directly contributes to the differences in blood glucose observed. Despite these observations, we cannot completely exclude the possibility that SCHADKO islets possess inherent biological properties, independent of the blood glucose–lowering impact of the genetic defect, that make them functionally superior to WT islets in our transplantation model.
We propose that SCHADKO islets alone are sufficient to recapitulate the hypoglycemic phenotype observed in the animal model of global SCHAD deficiency. Indeed, transplantation with SCHADKO islets was able to maintain glucose tolerance in the normal range in the recipients and was comparable with nontransplanted controls, in contrast to the attenuated glucose tolerance in animals receiving WT islets. Whether endogenous metabolites produced from the SCHAD-deficient islets contribute to the phenotype warrants further investigation.
Our study does not address a potential role of SCHAD in β-cells versus other cell types in the islet. In this regard, it is notable that insulin-independent glucagon suppression contributes to hypoglycemia in a mouse model of CHI caused by GDH mutation, suggesting that a signal generated via β-cell mitochondria and GDH specifically inhibits α-cells (18). As SCHAD inhibits GDH, impaired α-cell function may also be relevant for SCHAD deficiency, and of note, the first family described with the disease was regarded as glucagon deficient (19). The question of how SCHAD might affect glucagon secretion could be addressed in more detail by studying mice with β-cell–specific knockout of the Hadh gene.
Article Information
Acknowledgments. The SCHADKO mouse strain was kindly provided by Charles Stanley (Children’s Hospital of Philadelphia) and Arnold W. Strauss (University of Cincinnati College of Medicine). The authors are grateful to Brooke Sullivan (Joslin Diabetes Center) for assistance with islet transplantation.
Funding. The study was supported by a Fulbright fellowship to A.M. and by grants from the Novo Nordisk Foundation (NNF15OC0016330), Western Norway Regional Health Authority, KG Jebsen Foundation, and The Research Council of Norway (Norges Forskningsråd) (FRIMEDBIO 240788). P.R.N. was supported by a European Research Council Advanced Grant. C.W.L. was supported by a grant from the National Institutes of Health (K99-DK-090210). R.N.K. was supported by National Institutes of Health grants R01-DK-67536 and R01-DK-103215.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.M., J.H.-L., J.H., and R.M. researched data. A.M., P.R.N., C.W.L., G.W., and R.N.K. conceived the project. A.M. and R.N.K. directed and supervised the study. A.M. wrote the manuscript. All authors reviewed and edited the manuscript. A.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Kapoor RR, James C, Hussain K. Advances in the diagnosis and management of hyperinsulinemic hypoglycemia. Nat Clin Pract Endocrinol Metab 2009;5:101–112 [DOI] [PubMed] [Google Scholar]
- 2.Rahman SA, Nessa A, Hussain K. Molecular mechanisms of congenital hyperinsulinism. J Mol Endocrinol 2015;54:R119–R129 [DOI] [PubMed] [Google Scholar]
- 3.Sandal T, Laborie LB, Brusgaard K, et al. The spectrum of ABCC8 mutations in Norwegian patients with congenital hyperinsulinism of infancy. Clin Genet 2009;75:440–448 [DOI] [PubMed] [Google Scholar]
- 4.Clayton PT, Eaton S, Aynsley-Green A, et al. Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion. J Clin Invest 2001;108:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molven A, Matre GE, Duran M, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes 2004;53:221–227 [DOI] [PubMed] [Google Scholar]
- 6.Molven A, Helgeland G, Sandal T, Njølstad PR. The molecular genetics and pathophysiology of congenital hyperinsulinism caused by short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. In Monogenic Hyperinsulinemic Hypoglycemia Disorders. Frontiers in Diabetes. 21. Stanley CA, De León DD, Eds. Basel, Switzerland, Karger, 2012, p. 137–145 [Google Scholar]
- 7.Li C, Chen P, Palladino A, et al. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem 2010;285:31806–31818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palladino AA, Stanley CA. The hyperinsulinism/hyperammonemia syndrome. Rev Endocr Metab Disord 2010;11:171–178 [DOI] [PubMed] [Google Scholar]
- 9.Gotoh M, Ohzato H, Dono K, et al. Successful islet isolation from preserved rat pancreas following pancreatic ductal collagenase at the time of harvesting. Horm Metab Res Suppl 1990;25:1–4 [PubMed] [Google Scholar]
- 10.Davalli AM, Ogawa Y, Scaglia L, et al. Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice. Diabetes 1995;44:104–111 [DOI] [PubMed] [Google Scholar]
- 11.Martens GA, Vervoort A, Van de Casteele M, et al.. Specificity in beta cell expression of L-3-hydroxyacyl-CoA dehydrogenase, short chain, and potential role in down-regulating insulin release. J Biol Chem 2007;282:21134–21144 [DOI] [PubMed] [Google Scholar]
- 12.Filling C, Keller B, Hirschberg D, et al. Role of short-chain hydroxyacyl CoA dehydrogenases in SCHAD deficiency. Biochem Biophys Res Commun 2008;368:6–11 [DOI] [PubMed] [Google Scholar]
- 13.Heslegrave AJ, Kapoor RR, Eaton S, et al. Leucine-sensitive hyperinsulinaemic hypoglycaemia in patients with loss of function mutations in 3-Hydroxyacyl-CoA Dehydrogenase. Orphanet J Rare Dis 2012;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graciano MF, Valle MM, Kowluru A, Curi R, Carpinelli AR. Regulation of insulin secretion and reactive oxygen species production by free fatty acids in pancreatic islets. Islets 2011;3:213–223 [DOI] [PubMed] [Google Scholar]
- 15.Las G, Mayorek N, Dickstein K, Bar-Tana J. Modulation of insulin secretion by fatty acyl analogs. Diabetes 2006;55:3478–3485 [DOI] [PubMed] [Google Scholar]
- 16.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 2006;55(Suppl. 2):S16–S23 [DOI] [PubMed] [Google Scholar]
- 17.Yore MM, Syed I, Moraes-Vieira PM, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014;159:318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kibbey RG, Choi CS, Lee HY, et al. Mitochondrial GTP insensitivity contributes to hypoglycemia in hyperinsulinemia hyperammonemia by inhibiting glucagon release. Diabetes 2014;63:4218–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidnes J, Øyasaeter S. Glucagon deficiency causing severe neonatal hypoglycemia in a patient with normal insulin secretion. Pediatr Res 1977;11:943–949 [DOI] [PubMed] [Google Scholar]