Cardiovascular disease (CVD) associated with enhanced atherothrombosis is the leading cause of morbidity and mortality in patients with type 2 diabetes mellitus (T2DM) (1). Platelet hyperactivity in conjunction with endothelial dysfunction contributes to accelerated atherothrombosis in T2DM (1–3). The enhanced platelet reactivity in T2DM is caused by several factors including insulin resistance and chronic hyperglycemia (1–3). Although more intensive glycemic control has been shown to reduce the long-term risk of CVD, this treatment is often associated with weight gain and hypoglycemia (4,5). Thus, the development of novel antihyperglycemic agents without these negative side effects is needed. In this regard, incretin-based therapies have emerged as important agents in the treatment of T2DM (6). One such incretin therapy is glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1RAs) such as exenatide that provide supraphysiological concentrations of GLP-1 that stimulate GLP-1Rs (7–9). Another is dipeptidyl peptidase 4 (DPP-4) inhibitors, which prevent the proteolytic breakdown and inactivation of GLP-1 (6). Both GLP-1RAs and DDP-4 inhibitors improve glycemic control, reduce weight, and limit the risk of hypoglycemia, and thus they are regarded as therapeutic options for glycemic therapy in T2DM.
The clinical trials in T2DM patients with DPP-4 inhibitors and GLP-1RAs suggest that incretin-based therapies generally have neutral CVD outcome profiles in patients with T2DM except for adverse outcomes for a few DPP-4 inhibitors and perhaps an increased propensity for heart failure (6–9). The conventional beneficial effect of GLP-1RAs and DDP-4 inhibitors is that GLP-1 restores the glucose sensitivity of pancreatic β-cells by upregulation of glucose transporter 2 and glucokinase (6). GLP-1 also inhibits pancreatic β-cell apoptosis and stimulates the proliferation and differentiation of insulin-secreting β-cells (6). In addition, GLP-1RAs inhibit gastric secretion and motility and may reduce appetite (6,9). Recent studies have found cytoprotective actions of GLP-1 in different cell types beyond their effect on glucose metabolism. Importantly, emerging evidence suggests that augmentation of GLP-1 using GLP-1 analogs or DPP-4 inhibitors may potentially improve cardiovascular outcomes by controlling the cardiac autonomic nervous system, baroreceptor sensitivity, and the release of atrial natriuretic peptide; reduce systemic vascular resistance; and improve endothelial nitric oxide (NO) signaling in the vasculature (6). We and others have shown that DPP-4 inhibitors improve vascular function in an NO-dependent manner and enhance phosphorylation of Ser1177 in endothelial NO synthase (eNOS) (6,10,11). Impaired endothelial insulin metabolic signaling leading to decreased bioavailable NO is a potential factor contributing to increased platelet hyperactivity (3), suggesting an important role of interaction between DPP-4 inhibition/GLP-1R activation and NO production.
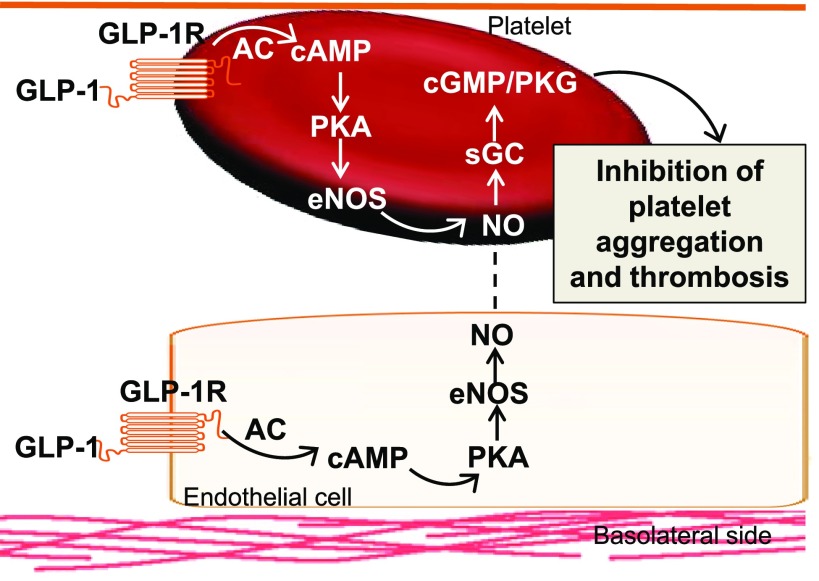
In this issue of Diabetes, Cameron-Vendrig et al. (12) explore the therapeutic effect of a GLP-1RA in the prevention of platelet aggregation and thrombosis by examining the effects of GLP-1R activation on platelet NO–mediated reduction in platelet aggregation. The GLP-1RA exenatide increased the release of cAMP and further inhibited platelet aggregation induced by thrombin, adenosine diphosphate, and collagen in a cultured human megakaryocyte cell line (12). Exenatide also inhibited thrombus formation under flow conditions ex vivo and in normoglycemic and hyperglycemic mice in vivo (10). However, the effects of exenatide on preventing platelet aggregation and thrombosis were abrogated in both GLP-1R and eNOS knockout mice in vivo (12). Although endothelial insulin resistance leading to decreased bioavailable NO is a factor in increased platelet hyperactivity, recent studies have shown that the presence of the NO signaling cascade within the platelet also modulates platelet aggregation (13–15) (Fig. 1). In this regard, adenylyl cyclase activation increases the levels of cAMP, which activate protein kinase A and phosphorylation/activation of eNOS (1). The increased NO in platelets stimulates cyclic guanosine monophosphate and protein kinase G by activating soluble guanylyl cyclase, which plays a crucial role in preventing platelet activation/aggregation (1) (Fig. 1). Therefore, an increase in bioavailable NO, either through endothelial or platelet production, is a critical determinant of platelet function, and this is perturbed in T2DM.
Figure 1.
Proposed mechanisms of GLP-1R activation in the prevention of platelet aggregation and thrombosis. AC, adenylyl cyclase; cGMP, cyclic guanosine monophosphate; PKG, protein kinase G; sGC, soluble guanylyl cyclase.
The current study provides new insight regarding the ability of GLP-1RAs to attenuate platelet aggregation and thrombosis by the activation of eNOS and NO production. This is translationally relevant, as GLP-1RA therapy has a potential application for the prevention of thrombotic events such as stroke, myocardial infarction, and deep vein thrombosis, which are common causes of morbidity and mortality in T2DM patients. Although these data highlight a central role of GLP-1RAs in the regulation of eNOS activity and platelet function, several caveats need to be considered. For one, this study did not investigate the direct effects of exenatide on eNOS activity and NO production in vitro and in vivo, which is important in understanding the interaction of GLP-1R and eNOS activation in the prevention of platelet activation. A specific platelet eNOS knockout model would be helpful to provide further evidence for a therapeutic role of GLP-1R–mediated eNOS activation in the reduction of the risk for atherothrombosis in T2DM patients. In this regard, the intrinsic expression of eNOS in platelets remains controversial, as some studies have reported the lack of eNOS activity in platelets (15–17). Further, the current study data does not exclude the role of exenatide in the reduction of platelet NO resistance, and it has been shown that platelet NO resistance induced by oxidative stress also plays an important role in thrombus formation in T2DM (18).
Overall, the data in the current study identify an important role of GLP-1RAs in the regulation of platelet activation/aggregation and provide evidence that the interaction of GLP-1R signaling and eNOS activation plays a role in exenatide inhibition of arterial thrombosis, thus providing a potential novel therapeutic strategy for the reduction in CVD in T2DM patients. Although GLP-1R expression has been shown by gene expression, GLP-1R protein could not be demonstrated in platelets in this study. As commercially available antibodies for GLP-1R have been shown to be nonspecific (19), the use of GLP-1R knockout mice in future studies is needed (12). Further studies are warranted to help more definitively understand the incretin-based therapies in the prevention of metabolic disorders and the associated cardiovascular complications in T2DM.
Article Information
Acknowledgments. The authors would like to thank Brenda Hunter (Diabetes and Cardiovascular Research Center, University of Missouri School of Medicine, Columbia, MO) for editorial assistance.
Funding. J.R.S. has received funding from the National Institutes of Health (R01-HL-73101-01A and R01-HL-107910-01) and the U.S. Department of Veterans Affairs Merit Review Award Program (0018).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1714.
References
- 1.Santilli F, Simeone P, Liani R, Davì G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat 2015;120:28–39 [DOI] [PubMed] [Google Scholar]
- 2.Suslova TE, Sitozhevskii AV, Ogurkova ON, et al. Platelet hemostasis in patients with metabolic syndrome and type 2 diabetes mellitus: cGMP- and NO-dependent mechanisms in the insulin-mediated platelet aggregation. Front Physiol 2015;5:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 2001;37:1053–1059 [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Genuth S, et al.; ACCORD Study Group . Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 6.Aroor AR, Sowers JR, Jia G, DeMarco VG. Pleiotropic effects of the dipeptidylpeptidase-4 inhibitors on the cardiovascular system. Am J Physiol Heart Circ Physiol 2014;307:H477–H492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White WB, Baker WL. Cardiovascular effects of incretin-based therapies. Annu Rev Med 2016;67:245–260 [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Ahrén B, Neubacher D, Patel S, Woerle HJ, Johansen OE. Efficacy and cardiovascular safety of linagliptin as an add-on to insulin in type 2 diabetes: a pooled comprehensive post hoc analysis. Can J Diabetes 2016;40:50–57 [DOI] [PubMed] [Google Scholar]
- 9.Ravassa S, Zudaire A, Díez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res 2012;94:316–323 [DOI] [PubMed] [Google Scholar]
- 10.Aroor AR, Sowers JR, Bender SB, et al. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology 2013;154:2501–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii M, Shibata R, Kondo K, et al. Vildagliptin stimulates endothelial cell network formation and ischemia-induced revascularization via an endothelial nitric-oxide synthase-dependent mechanism. J Biol Chem 2014;289:27235–27245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron-Vendrig A, Reheman A, Siraj MA, et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes 2016;65:1714–1723 [DOI] [PubMed] [Google Scholar]
- 13.Modrego J, Azcona L, Martín-Palacios N, et al. Platelet content of nitric oxide synthase 3 phosphorylated at Serine 1177 is associated with the functional response of platelets to aspirin. PLoS One 2013;8:e82574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 2004;84:903–934 [DOI] [PubMed] [Google Scholar]
- 15.Gambaryan S, Tsikas D. A review and discussion of platelet nitric oxide and nitric oxide synthase: do blood platelets produce nitric oxide from L-arginine or nitrite? Amino Acids 2015;47:1779–1793 [DOI] [PubMed] [Google Scholar]
- 16.Gambaryan S, Kobsar A, Hartmann S, et al. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost 2008;6:1376–1384 [DOI] [PubMed] [Google Scholar]
- 17.Apostoli GL, Solomon A, Smallwood MJ, Winyard PG, Emerson M. Role of inorganic nitrate and nitrite in driving nitric oxide-cGMP-mediated inhibition of platelet aggregation in vitro and in vivo. J Thromb Haemost 2014;12:1880–1889 [DOI] [PubMed] [Google Scholar]
- 18.Manrique C, Lastra G, Palmer J, Gardner M, Sowers JR. Aspirin and diabetes mellitus: revisiting an old player. Ther Adv Cardiovasc Dis 2008;2:37–42 [DOI] [PubMed] [Google Scholar]
- 19.Aroor A, Nistala R. Tissue-specific expression of GLP1R in mice: is the problem of antibody nonspecificity solved? Diabetes 2014;63:1182–1184 [DOI] [PubMed] [Google Scholar]