Abstract
Rationale
Impaired goal-directed motivation represents a debilitating class of symptoms common to psychological disorders including schizophrenia and some affective disorders. Despite the known negative impact of impaired motivation, there are currently no effective pharmacological interventions to treat these symptoms.
Objectives
Here, we evaluate the effectiveness of the serotonin 2C (5-HT2C) receptor selective ligand, SB242084, as a potential pharmacological intervention for enhancing goal-directed motivation in mice. The studies were designed to identify not only efficacy but also the specific motivational processes that were affected by the drug treatment.
Methods
We tested subjects following treatment with SB242084 (0.75 mg/kg) in several operant lever pressing assays including the following: a progressive ratio (PR) schedule of reinforcement, an effort-based choice task, a progressive hold down task (PHD), and various food intake tests.
Results
Acute SB242084 treatment leads to an increase in instrumental behavior. Using a battery of behavioral tasks, we demonstrate that the major effect of SB242084 is an increase in the amount of responses and duration of effort that subjects will make for food rewards. This enhancement of behavior is not the result of non-specific hyperactivity or arousal nor is it due to changes in food consumption.
Conclusions
Because of this specificity of action, we suggest that the 5-HT2C receptor warrants further attention as a novel therapeutic target for treating pathological impairments in goal-directed motivation.
Keywords: Serotonin receptor, Motivation, Locomotor activity, Feeding, SB242084, Operant
Introduction
Many patients with schizophrenia (50 %) and affective disorders (20–80 %) experience a pervasive inability to activate their behavior in pursuit of positive goals, reflecting a deficit in goal-directed motivation (Kiang et al. 2003; Roth 2004; Faerden 2009; Feil et al. 2003; Marin et al. 2003). Clinically, a paucity of goal-directed behavior is referred to as apathy (Markou et al. 2013), and the impact of this debilitating symptom has become increasingly recognized in recent years (Chase 2011; Treadway and Zald 2011, 2013). Impaired goal-directed motivation decreases a patient’s quality of life and functional outcomes (Kiang et al. 2003), which presents a challenge, as there are not approved pharmacological treatments with demonstrated effectiveness for alleviating these deficits (Chase 2011; Levy and Czernecki 2006). Some medications used to treat schizophrenia and affective disorders have even been shown to exacerbate these symptoms in preclinical models (Sanders et al. 2007; Simpson et al. 2011; Aberman et al. 1998; Salamone 2009) and in patients (Wongpakaran et al. 2007).
In healthy individuals, intact goal-directed motivation requires a normal hedonic response to an outcome, as well as the willingness to expend energy in pursuit of a goal. These two processes are known to be controlled by distinct neurobiological substrates (Berridge and Robinson 2003; Salamone et al. 2007). Substantial research indicates that hedonic reaction (the capacity to experience pleasure) is mostly intact in schizophrenia (Cohen and Minor 2010; Oorschot et al. 2011), whereas the willingness to expend effort is impaired (Treadway and Zald 2013). This has now been observed for some patients with depression (Treadway et al. 2009, 2012) and schizophrenia (Barch and Dowd 2010; Strauss et al. 2011) and represents a deficit for which the development of new pharmacological treatment strategies would be highly beneficial.
A new potential target for modulating goal-directed motivation is the serotonin 2C (5-HT2C) receptor. This receptor is expressed in several brain regions involved in various aspects of motivated behaviors, including the following: regions of the cortex (pyriform, cingulate, prefrontal), limbic areas (nucleus accumbens (NAcc), dorsal striatum, amygdala, hippocampus), and dopaminergic midbrain nuclei (ventral tegmental area (VTA), substantia nigra (SN))—observations made through studies of mRNA, radioactive ligand binding, and immunohistochemical analyses (reviewed in Fletcher and Higgins 2011).
Numerous recent studies have demonstrated that 5HT2C receptor activity can modulate the neuronal activity of dopamine (DA) neurons and DA release at the terminal sites of the mesolimbic, mesocortical, and niagro-striatal DA pathways (Alex and Pehek 2007; Di Matteo et al. 2008a, b). Importantly, DA neurotransmission is well known to be involved in motivated behavior and the willingness to exert effort toward a goal (Salamone et al. 2007). Specifically, low doses of DA antagonists and NAcc DA depletions lower subjects’ willingness to work, impairing the selection of high-effort/high-reward options while increasing the selections of low-effort options (Salamone et al. 1994, 2007; Nowend et al. 2001). Generally, the 5-HT2C receptor exerts an inhibitory influence on DA neurotransmission, as serotonin and 5-HT2C receptor agonists decrease the firing rates of DA neurons in the VTA and SN, leading to reductions in NAcc and striatal DA efflux, respectively. In contrast, antagonists and inverse agonists increase the firing rates of DA neurons and enhance DA efflux in the terminal targets of these neurons (reviewed in Alex and Pehek 2007; Di Matteo et al. 2008a, b).
Pharmacological modulation of the 5-HT2C receptor with the highly selective 5-HT2C receptor ligand SB242084 was previously reported to increase behavioral output in an effortful appetitive task in mice (Simpson et al. 2011). Although often referred to as an antagonist, SB242084 is one of several compounds displaying functional selectivity at the 5-HT2C receptor, meaning that its effects differ on the multiple downstream signaling pathways associated with the receptor. Specifically, SB242084 acts as an inverse agonist on phospholipase A2 (PLA2) and the inhibitory G protein, Gαi, but has agonist effects on phospholipase C (PLC) (De Deurwaerdère et al. 2004).
Here, we characterize the effects of SB242084 in several assays of motivated behavior (progressive ratio, an effort-based choice task, and a progressive hold down task) and then rule out possible alternative explanations for the observed increases in motivation (changes in non-goal-directed hyperactivity and feeding behavior). We also test the time course of the drug’s effect on behavior. Our data demonstrate that acute treatment with SB242084 specifically enhances goal-directed motivation, indicating that the 5-HT2C receptor should be considered as a target for the development of treatments for pathological deficits in motivation.
Methods
Subjects
Experiments used C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) which were 10 weeks old at the start of the experiments. The sex and the number of mice used are provided below. All experiments and animal care protocols were in accordance with the Columbia University and NYSPI Institutional Animal Care and Use Committees and Animal Welfare regulations.
Drug treatment
The selective 5-HT2C receptor ligand SB242084 (Tocris Bioscience, Ellisville, Missouri, USA) was dissolved in 0.9 % saline and injected intraperitoneally (IP) 20 min before the start of behavioral testing (doses used for each described below).
Behavioral procedures
Progressive ratio
Twenty-four male C57BL/6J mice were divided into two groups: vehicle control group (n=12) and SB242084-treated group (n=12). Regular home cage chow (Isopro RMH 3000 complete mouse diet; Prolab, Syracuse, NY) was provided in a restricted manner to maintain subjects at 85 % of ad lib baseline body weight. Unless otherwise noted, the apparatus used was identical to that used by Drew et al. (2007).
The protocol for operant lever press training was carried out as described in Drew et al. (2007), with some differences. Once all subjects lever pressed for evaporated milk reinforcers at a high rate, subjects were then tested on a PR (3) × 2 schedule of reinforcement. The press requirement started at 3 and was multiplied by 2 thereafter, following the function 3 × 2(n−1); where n is the trial number (i.e., 3, 6, 12, 24, 48, 96, 192, and so on). Sessions ended after 3 min elapsed without a subject making a single press or after 2 h, whichever came first. Subjects were tested on the PR (3) × 2 for five consecutive days receiving an IP injection of either saline or 0.75 mg/kg SB242084. The dose of 0.75 mg/kg was chosen based on previous work showing a behavioral effect (Simpson et al. 2011).
Effort-based choice task
Twenty male C57BL/6J mice were divided into two groups: saline control group (n=10) and SB242084-treated group (n= 10). Standard lever press training was carried out as described in Drew et al. (2007). In the effort-based choice task (EBCT), subjects were exposed to a random ratio (RR) schedule of reinforcement (subjects were reinforced after a variable number of responses), and there was a small petri dish of freely available chow present in the chamber. Sessions lasted 1 h, and the testing occurred 5 days per week for 3 weeks. In week 1, both groups were treated with vehicle and tested in a RR10; in week 2, while earning rewards on the RR10, controls received saline and the drug group received 0.75 mg/kg SB242084; in week 3, an RR20 was in effect and subjects received the same drug treatments as the previous phase.
Food intake
2-h chow intake
Twenty male and 20 female C57BL/6J mice were randomly assigned to a restricted feeding (RF) condition in which food was only available during the test or an ad lib feeding condition (n=10 per sex per condition) in which food was also freely available in the home cage. The feeding test consisted of 2-h access to home cage chow and water in a separate clean cage identical to the home cages of the subjects. Subjects were acclimated to the 2-h daily feeding session for 5 days, receiving vehicle injections each day. In the drug phase, subjects from both the RF and ad lib feeding conditions were randomly assigned to one of two treatment orders each lasting for 4 days: saline followed by 0.75 mg/kg SB242084 or the reverse order (n=5 subjects per feeding condition, drug treatment, order, and sex).
1-h milk intake
Twenty male C57BL/6J mice were randomly assigned to a saline (n=10)- or SB242084 (n = 10)-treated group for three consecutive days and given 1-h free access to the evaporated milk. Total milk consumed in the hour was determined by weighing the amount of milk consumed in the session.
Progressive hold down
Sixteen male C57BL/6J mice were used for the progressive hold down (PHD) experiment. Standard lever press training was carried out as described above. Next, mice were trained to make lever presses of extended durations (i.e., holding the lever in the depressed position until a required criterion time) as previously described (Bailey et al. 2015). Briefly, mice were first trained with a variable interval hold (VIH) schedule in which the required hold time at the start of each trial was randomly determined from an exponential distribution of times of a given mean. Subjects were successively trained on VIH schedules with means of 0.5, 1, 2, 3, 4, 5, 8, and 10 s. Sessions lasted 1 h or until 40 reinforcers were earned, whichever came first. When all subjects reached the criterion of 40 reinforcers for three consecutive days on a schedule, they were advanced to VIH schedules with a higher mean. After all subjects earned 40 reinforcers for four consecutive days on a VIH of 10 s, PHD testing began.
Subjects were then tested on a PHD (2.0 s) × 1.13 schedule—the first hold duration was 2 s and was multiplied by 1.13 for each trial thereafter, following the function 2× 1.13(n−1); (i.e., 3.26 s on the 5th trial, 6.0 s on the 10th trial, 20.39 s on the 20th trial, etc.). Subjects were then tested on a more difficult PHD (4 s) × 1.18 schedule—the first hold duration was 4 s and was multiplied by 1.18 thereafter, following the function 4 × 1.18(n−1); (i.e., 7.75 s on the fifth trial, 17.7 s on the tenth trial, etc.). Following 4 days of testing on PHD (4 s) × 1.18 in which all subjects received saline prior to testing, subjects were tested during the drug phase of the experiment. Two doses (0.75 and 1.5 mg/kg SB242084) were used. Subjects were randomly divided into two treatment orders and were tested on the PHD schedule for 3 days at one dose, followed by a 4-day vehicle washout period before being tested for 3 days at the second dose.
Progressive ratio: pretreatment
Twenty male C57BL/6J mice were divided into a saline control group (n=10) and a 0.75 mg/kg SB242084 group (n=10). All aspects of the PR experiment were identical to the previously described PR experiment with one exception. After learning to press at high rates, Subjects were treated for five consecutive days with either saline or 0.75 mg/kg SB242084 without any behavioral testing. In the next 5 days, subjects were tested on the PR without receiving any injections.
Progressive ratio: repeated exposure
Twenty male C57BL/6J mice were tested on the PR for four consecutive weeks. In the first baseline week, all subjects received daily vehicle injections prior to PR testing. Subjects were then divided into a control group (n=10) and a treatment group (n=10). Over the next 3 weeks, the control group received vehicle injections in every week, whereas the treatment group received 0.75 mg/kg SB242084 in week 1 (treatment 1), vehicle in week 2 (Washout), and 0.75 mg/kg SB242084 in week 3 (treatment 2).
Data analysis
All data were analyzed using two-tailed Student t tests or, where appropriate, repeated measure analysis of variance (ANOVA). In all experiments, data were averaged across all days of a specific treatment type (e.g., vehicle or SB242084 treatment) with the number of days provided in the figure legend. Planned comparisons are reported in the main text, and significant post hoc analyses are reported in the figure legends.
Results
SB242084 increases responding for food rewards in a progressive ratio schedule of reinforcement
It was previously reported that SB242084 increased responding in a PR schedule of reinforcement (Simpson et al. 2011). In a replication of this work, treatment with SB242084 led to a significant increase in lever presses (t(22)=3.971, p=0.0006; Fig. 1a) and significantly longer session durations (t(22)=3.355, p=0.0029). Figure 1b shows cumulative survival, the percentage of mice still working for rewards as a function of session time (sessions were terminated after 3 min without a response or after 2 h has elapsed). A Mantel-Cox log rank test revealed that SB242084-treated mice worked significantly longer than vehicle-treated subjects. As a result of this, treatment with SB242084 also led to a significant increase in the number of reinforcers earned (t(22)=3.710, p=0.0012; Fig. 1c).
Fig. 1.
SB242084 at 0.75 mg/kg increases responding in a progressive ratio. a Mean (SEM) number of lever presses made during the PR session. b Cumulative survival curves in the PR. c Mean (SEM) number of rewards earned in PR session. d Mean (SEM) response rate (presses/minute) as a function of trial number/reward number in the PR session. e Group average response rate from the peak of responding through the end of session fit with the negative exponential function y=a^(−b×n). f Mean (SEM) peak response rate (a) in PR session. g Mean (SEM) decay rate (b) in the PR session. Average of five consecutive days of testing in PR (3) × 2 for vehicle (n=12) and SB (n=12)-treated mice. **p<0.01
We further characterized SB242084’s effect on behavior in the PR by examining the rate of lever pressing during the session and observed that the rate increases to a peak rate by about the third trial and then slowly declines over the session (Fig. 1d). To evaluate the peak press rate and the rate of decline in responding over trials, we fit the response rate data starting on the third trial for each individual subject with negative exponential functions of the form y=(a×exp(−b×n)), where a is the y intercept of the function (reflecting the maximum response rate reached) b is the rate of decay (how fast the decline in the rate of responding occurred), and n is the trial number (Fig. 1e). The drug did not affect the maximum response rate, as the a parameter was not significantly different (t(22)=1.03, p=0.310; Fig. 1f). It did significantly change the decay rate, as the decline in response rate (b parameter) was slower in SB242084-treated mice (t(22) =2.835, p=0.009; Fig. 1g). Finally, both groups showed equal interest in consuming reinforcers, as the number of unconsumed rewards was the same in each group (vehicle: mean=1.08, SEM= 0.676; SB: mean=1.125, SEM=0.688; t(22)=0.064, p= 0.949).
SB242084 increases responding for a preferred reward in an effort-based choice task
To determine if the effects of SB242084 generalized across different assays of motivation, we tested mice in an EBCT to evaluate willingness to choose to work for a preferred reward. Figure 2a shows that subjects treated with 0.75 mg/kg SB242084 made more presses as there was a significant main effect of schedule (F(1,56)=108.7, p<0.001), a significant main effect of the drug (F(1,56)=7.28, p<0.01), and a significant drug by schedule interaction (F(1,56)=6.02, p=0.017). Post hoc comparisons revealed that the groups differed significantly only for the RR20 schedule. Subjects consumed more freely available chow as the effort requirement increased (F(1,56)=8.654, p=0.004) and SB242084 reduced chow consumption (F(1,56)=4.345, p=0.041). There was no drug by schedule interaction (F(1,56)=2.749, p=0.102). Again, there was no difference in the number of missed rewards between groups.
Fig. 2.
SB242084 at 0.75 mg/kg increases responding for a preferred reward in an effort-based choice task. a Mean (SEM) number of lever presses made during 1 h of an effort-based choice task under different ratio schedules. b Mean (SEM) total intake (g) of freely available chow during the 1 h effort-based choice task for the different ratio schedules. Average of 5 days of testing in RR 10 (Veh/Veh), 5 days of testing in RR 10 (Veh/SB), and 5 days of testing in RR 20 (Veh/SB) for vehicle (n=12) and SB (n=12)-treated mice. *p<0.05, with Bonferoni correction for multiple comparisons
SB242084 enhances goal-directed action in a progressive hold down task
To assess if SB242084 would increase responding across different types of work, we tested subjects in the PHD task, in which the increasing work requirement is the time that subjects must hold a lever in the depressed position. Subjects were first tested on a low-difficulty PHD (3 s) × 1.13 schedule, but because this was too easy, the median session duration was the maximum 120 min as most subjects were at a ceiling level of performance. We then tested all subjects on a more demanding PHD (4 s) × 1.18 schedule in order to be able to measure potential increases in performance when subjects were given SB242084.
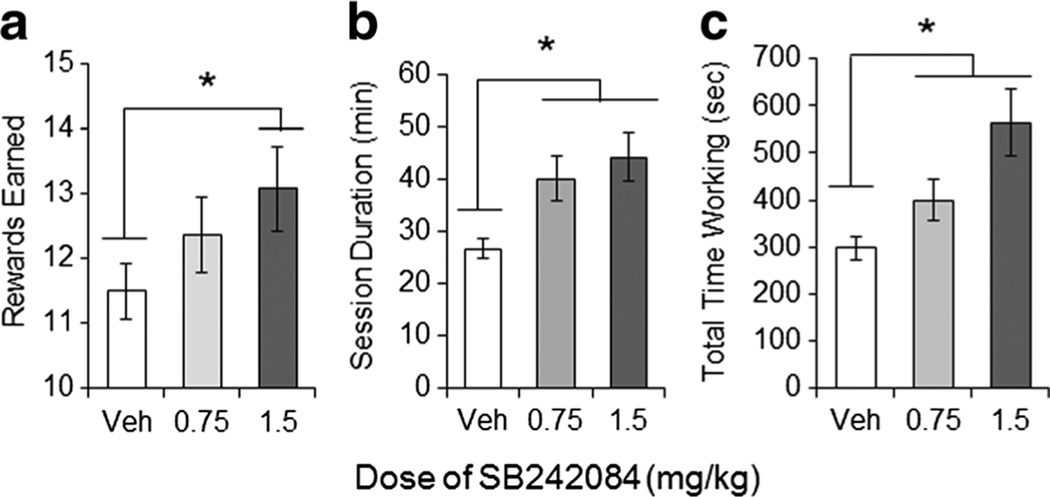
In the harder PHD schedule, SB242084 led to a dose-dependent increase in the number of reinforcers earned (F(2, 38)=3.329, p=0.041; Fig. 3a), as well the session durations (F(2,38)=7.846, p=0.0008; Fig. 3b), and the total time subjects spent successfully holding the lever down (F(2,38)=11.17, p<0.0001; Fig. 3c), showing that the drug increases responding for rewards in a manner similar to that which we observed in both the PR and the effort-based choice task.
Fig. 3.
SB242084 increases goal-directed action in a progressive hold down task. a Mean (SEM) number of rewards earned in the PHD session. b Mean (SEM) session duration (min) in the PHD task. c Mean (SEM) time spent successfully working in the PHD task. Average of 3 days of testing in each condition. *p<0.05, with Bonferoni correction for multiple comparisons
SB242084 effects on increased operant responding are not due to increases in non-goal-directed hyperactivity/arousal
We also assessed whether SB242084 enhanced non-goal-specific hyperactivity, as this could possibly occur independently of the drug’s effect of increasing goal-directed action. SB242084 treatment significantly increased the total number of lever presses in the PHD session (F(2,38) =14.63, p<0.0001; Fig. 4a). A contribution of hyperactivity would be expected to manifest itself in unsuccessful presses throughout a PHD session (see Bailey et al. 2015), but the within-session response profiles of subjects suggested that the increased responses were occurring mostly at the end of the session. Figure 4b shows the mean number of failed or unsuccessful presses per trial, where trial number 0 reflects the last trial attempted for every subject (i.e., subjects did not complete the requirement). Unsuccessful presses increase as the hold requirement becomes more difficult and the drug appeared to increase unsuccessful attempts in the final trial. We analyzed the first and last five trials of subjects to see if there was a group difference in the number of failed presses at the beginning or end of the session. Across the first five trials (Fig. 4c), there was no significant effect of trial (F(4,210)= 1.852; p=0.120), no significant effect of dose (F(2,210) = 1.115; p=0.330), and no dose × trial interaction (F(8,210)= 0.772; p=0.628). In the last five trials (Fig. 4d), there was a significant main effect of trial number (F(4,210)=5.237; p= 0.0004), but no significant main effect of dose (F(2,210)= 0.650; p=0.522), and despite the mean differences, there was no dose × trial interaction (F(8,210)=1.039; p=0.407), likely due to the high variability in the last trial.
Fig. 4.
SB242084 does not enhance non-goal-directed hyperactivity. a Mean (SEM) number of lever presses in the PHD session. b Mean (SEM) number of failed (unsuccessful) press attempts in the PHD session as a function of trial number from each subject’s last attempted trial (i.e., for each subject, 0 corresponds to their last trial and −1 corresponds to their penultimate trial, and so forth). c Mean (SEM) number of unsuccessful presses in each subject’s first five trials. d Mean (SEM) number of unsuccessful presses in each subject’s last five trials. Average of 3 days of testing in each condition. **p<0.01, with Bonferoni correction for multiple comparisons
SB242084 does not alter feeding behavior in either a hungry or sated state
We examined SB242084’s effects on feeding behavior to see if this was altered by the drug. In a 2-h food intake test (the same timescale used in the operant experiments), SB242084 had no effect on food intake in hungry or sated mice, in either males or females (Fig. S1a–b in Supplement). In a 1-h feeding intake test using evaporated milk (the same reward used in the operant experiments), SB242084 had no effect on the amount of milk consumed (Fig. S1c in Supplement).
Prior treatment with SB242084 has no effect on progressive ratio performance
Systemic treatment with SB242084 for five consecutive days has been recently shown to induce molecular changes in the brain and has antidepressant-like behavioral effects (Opal et al. 2013), so we tested this treatment regimen and examined behavior in the PR. When tested drug-free, pretreatment with SB242084 for 5 days had no effect on any dependent variables in the PR: number of presses, session duration, or reinforcers earned (Fig. S2a–c in Supplement).
The acute effect of SB242084 on goal-directed motivation can be reinstated repeatedly
We next tested whether there are post-administration carryover effects of the drug and whether or not the effectiveness of the drug is changed with repeated administration. All subjects were given vehicle baseline week of PR testing and assigned to two groups so that no baseline differences existed in any parameters. Control subjects then received 3 weeks of vehicle injections, and drug-treated subjects received treatment with SB242084 (Treatment 1), followed by a week of vehicle (Washout), followed by a week of SB242084 (Treatment 2) while being tested in the PR (Fig. 5a).
Fig. 5.
The acute effects of SB242084 (0.75 mg/kg) can be reinstated repeatedly. a Schematic of the experimental design used for the repeated administration of SB242084 experiment. b Mean (SEM) number of rewards earned in PR session for each treatment condition. c Mean (SEM) number of lever presses made in PR session for each treatment condition. d Mean (SEM) session duration (min) for each treatment condition. Average of 5 days of testing *p<0.05; **p<0.01, with Bonferoni correction for multiple comparisons
Pooled across the 2 weeks of drug treatments, the SB242084 group earned more rewards (t(36) =2.71, p= 0.010), pressed more (t(36)=2.437, p=0.020), and continued responding longer (t(36)=2.883, p=0.0068) than vehicle controls (Fig. 3a – c in Supplement), replicating our earlier findings (Fig. 1) and those of Simpson et al. (2011).
Planned comparisons were made to assess carryover effects and whether drug efficacy changes with repeated administration. Figure 5b – d shows data for each treatment week in the experiment. In week 3 (when both groups received vehicle), there were no differences in any aspect of PR performance [number of rewards earned (t(16)=1.352; p=0.195; Fig. 5a), number of lever presses (t(16)=0.463; p=0.649; Fig. 5b), and session duration (t(16)=0.551; p=0.588; Fig. 5c], again showing that the drug acts acutely but that it does not have carryover effects. Finally, in the drug group, Treatment 1 and Treatment 2 were compared and there were no significant differences between the two exposures in any of the measures [number of rewards earned (t(9) =0.001; p=1.000), the number of lever presses (t(9)=0.588; p=0.570), and session duration (t(9)=1.801; p=0.105)], showing that enhanced goal-directed responding can be reinstated repeatedly in the same subjects.
Discussion
Prolonged impairments in goal-directed motivation are a problematic class of symptoms for which no approved pharmacological treatments currently exist (Chase 2011; Levy and Czernecki 2006). Systemic treatment with the 5-HT2C receptor ligand SB242084 increases the firing rate of VTA DA neurons and enhances DA efflux at the NAcc in a manner that would be predictive of the drug-enhancing motivated behavior. It was previously shown that SB242084 increased lever press responding in a PR schedule of reinforcement (Simpson et al. 2011). Here, we investigate the effects of this compound on goal-directed motivation using a comprehensive series of assays and determine the conditions under which the drug produces behavioral effects.
SB242084 enhances behavioral output across several measures of motivated behavior
In the PR, SB242084 treatment increased lever pressing and also how long subjects pressed before quitting, which resulted in more rewards being earned—consistent with increased motivation (Hodos 1961; Aberman et al. 1998). We replicated this in an additional experiment with alternating weeks of SB242084 treatment. SB242084 increased all dependent measures in both exposures to the drug, replicating our first experiment and the observations in Simpson et al. (2011).
We next tested the effects of SB242084 in an EBCT, which gave subjects a choice between a high-effort option for a preferred reward (lever pressing for milk) and a low-effort option for a less preferred reward (consuming freely available home cage chow). SB242084 increased lever pressing for the preferred reward and decreased chow consumption, a pattern of results frequently interpreted as an increased willingness to work for a preferred reward (Salamone et al. 2007).
The results of both the PR and EBCT experiments demonstrate that SB242084 increases motivation for food rewards, but both of these tasks are response rate dependent (i.e., higher response rates benefit the subject and lead to more rewards). Because SB242084 has been shown to increase overall locomotor activity in an open field test (Fletcher et al. 2009), we wanted to test whether an increase in hyper-activity or general arousal could have contributed to the PR result. To assess this, we used the PHD task which requires subjects to make sustained responses of increasing durations, making increased willingness to work for a goal and increased response rates incompatible with one another. SB242084-treated subjects earned more rewards by continuing to hold the lever down for longer durations, clearly showing that the drug increases behavioral output in pursuit of a goal across different modalities of work requirements (i.e., making multiple lever presses or holding the lever down for longer durations).
In the PHD task, SB242084 also increased the number of total lever presses made, but the extra presses mainly occurred in the last trial when subjects were not able to meet the next hold requirement. This pattern may reflect continued persistence in light of repeated failure. The observation that the number of short duration unsuccessful presses did not differ throughout the entire session also suggests that the drug does not induce non-goal-specific hyperactivity as seen with meth-amphetamine (Bailey et al. 2015). Taken together, the results of the PR, EBCT, and PHD demonstrate that SB242084 increases subject’s persistence in responding across different operant tasks, independent of the type of work requirement demanded by the task.
Because an increase in hunger or feeding behavior could enhance operant responding for food, we conducted several experiments to examine this alternative explanation, which is plausible as several 5-HT2C receptor agonists have been shown to reduce food intake and bodyweight (Clifton et al. 2000; Dalton et al. 2006; reviewed in Fletcher et al. 2010). The experiments reported here found no effect of SB242084 on food intake in either males or females under various conditions, which is consistent with several previous reports (Vickers et al. 2000; Hewitt et al. 2002; Dalton et al. 2006; Fletcher et al. 2009).
The 5-HT2C receptor and reward-related behaviors
After observing that SB242084 can enhance the firing rates of DA VTA neurons and DA efflux in the NAcc, many studies have since examined 5-HT2C receptor’s involvement in reward-related behaviors. Several studies have looked at the effect of 5-HT2C ligands on a number of psychostimulant-induced behaviors, including the following: drug-induced locomotion, self-administration, and reinstatement. A generalized finding has been that 5-HT2C receptor agonists attenuate psychostimulant-induced behaviors, whereas antagonists and inverse agonists tend to enhance such behaviors (reviewed in Fletcher and Higgins 2011).
More specifically related to the present study, the effects of 5-HT2C ligands have been tested on operant responding for food. 5-HT2C receptor agonists have consistently been shown to reduce motivated responding for food rewards (Grottick et al. 2000; Ward et al. 2008; Cunningham et al. 2011; Higgins et al. 2013).
There have been two previous reports on the effects of SB242084 on motivation for food by Fletcher et al. 2010 and Bezzina et al. 2015 which may appear contradictory to the present findings because they do not report a significant increase in incentive motivation. There are a number of differences between these studies and the ones reported here, but in both studies, the overall level of effort/output required of subjects was significantly lower compared to most of the procedures used here. Like these studies, we found that when the effort requirement was low (RR10) in the EBCT, drug and control groups did not differ, but when the effort requirement increased (RR20), a significant drug effect emerged. If a task is too easy (or too hard), it may be difficult to study motivation-enhancing compounds.
Treatment conditions in which SB242084 can enhance goal-directed behavior
Given the apparent specificity of SB242084 on goal-directed motivation, determining the effective treatment conditions will be important for the development of future pharmacological treatments for impaired motivation. One recent study has shown that SB242084 given to mice for five consecutive days induces fast-acting antidepressant-like effects at both the behavioral and molecular levels (Opal et al. 2013). Using this same treatment regimen, we found that the drug was only effective when present during testing: 5 days of prior treatment with SB242084 had no effect on subsequent performance in the PR task in the absence of the drug. We also found that the acute effects of the drug can be reinstated repeatedly.
Potential mechanisms of action of SB242084 in enhancing motivation
One possible mechanism by which SB242084 may act to increase motivation in the present studies is through phasic modulation of mesolimbic DA neurotransmission. This is a plausible mechanism for the present results, as systemically administered 5-HT2C receptor ligands have been shown to alter the firing rate of DA VTA neurons (Di Giovanni et al. 2000; Di Matteo et al. 1999, 2000), altering DA release to the NAcc (Di Giovanni et al. 2001). Many studies have demonstrated DA’s involvement in motivation and the willingness to exert effort (Salamone et al. 2007), such that drugs which reduce and enhance DA neurotransmission lead to decreased and increased willingness to work, respectively (Salamone et al. 1994, 1991, 2007; Nowend et al. 2001). Future studies will be needed to determine the locations and mechanism through which SB242084 acts to increase goal-directed behavior.
5-HT2C receptor as a target for novel therapeutic interventions of impaired motivation
There have been previous suggestions that 5-HT2C receptor ligands may be useful in treating depression and schizophrenia (Simpson et al. 2011; Millan 2005). The present work provides support for the possibility that such drugs can help to acutely ameliorate the impaired motivation commonly seen in these two disorders.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health (NIMH) Grant 1R21MH104718 (E.H.S.) and 5R01MH068073 (P.D.B.) and National Institute of Health (NIH) Grant NS37919 (R.S.)
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-015-4135-3) contains supplementary material, which is available to authorized users.
Contributor Information
Matthew R. Bailey, Email: mrb2225@columbia.edu.
Eleanor H. Simpson, Email: es534@columbia.edu.
References
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113(2):296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MR, Jensen G, Taylor K, Mezias C, Williamson C, Silver R, Simpson EH, Balsam PD. A novel strategy for dissecting goal-directed action and arousal components of motivated behavior with a progressive hold-down task. Behav Neurosci. 2015;129:269–280. doi: 10.1037/bne0000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bezzina G, Body S, Cheung THC, Hampson CL, Bradshaw CM, Glennon JC, Szabadi E. Evidence for a role of 5-HT2C receptors in the motor aspects of performance, but not the efficacy of food reinforcers, in a progressive ratio schedule. Psychopharmacology. 2015;232:699–711. doi: 10.1007/s00213-014-3700-5. [DOI] [PubMed] [Google Scholar]
- Chase T. Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment. Neurotox Res. 2011;19:266–278. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Lee MD, Dourish CT. Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist, and d-fenfluramine on feeding patterns in the rat. Psychopharmacology. 2000;152:256–267. doi: 10.1007/s002130000504. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. Selective serotonin 5-HT2C receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology. 2011;61:513–523. doi: 10.1016/j.neuropharm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Lee MD, Kennett GA, Dourish CT, Clifton PG. Serotonin 1B and 2C receptor interactions in the modulation of feeding behaviour in the mouse. Psychopharmacology. 2006;185:45–57. doi: 10.1007/s00213-005-0212-3. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the Serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24:3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E. Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin2C/2B receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse. 2000;35:53–61. doi: 10.1002/(SICI)1098-2396(200001)35:1<53::AID-SYN7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, La Grutta V, Esposito E. m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience. 2001;103:111–116. doi: 10.1016/s0306-4522(00)00561-3. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242 084, a selective serotonin(2C) receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Di Matteo M, Di Giovanni G, Di Mascio M, Esposito E. Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res. 2000;865:85–90. doi: 10.1016/s0006-8993(00)02246-0. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. Di Giovanni G, Di Matteo V, Esposito E, editors. Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Serotonin-dopamine interaction: experimental evidence and therapeutic relevance. 2008;172:7–44. doi: 10.1016/S0079-6123(08)00902-3. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, Kandel ER, Malapani C, Balsam PD. Transient overexpression of striatal D-2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faerden A. Apathy is associated with executive functioning in first episode psychosis. BMC Psychiatr. 2009;9:1. doi: 10.1186/1471-244X-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil D, Razani J, Boone K, Lesser I. Apathy and cognitive performance in older adults with depression. Int J Geriatric Psychiatr. 2003;18:479–485. doi: 10.1002/gps.869. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Higgins G. Serotonin and reward-related behavior: focus on 5-HT2C receptors. In: Di Giovanni G, Esposito E, Di Matteo V, editors. 5-HT2C receptors in the pathophysiology of CNS disease. Vol. 22. Humana Press; 2011. pp. 293–324. [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT2C receptor ligands on motor activity and feeding behaviour in 5-HT2C receptor knockout mice. Neuropharmacology. 2009;57:259–267. doi: 10.1016/j.neuropharm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Sinyard J, Higgins GA. Genetic and pharmacological evidence that 5-HT2C receptor activation, but not inhibition, affects motivation to feed under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2010;97:170–178. doi: 10.1016/j.pbb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT2C receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Hewitt KN, Lee MD, Dourish CT, Clifton PG. Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol Biochem Behav. 2002;71:691–700. doi: 10.1016/s0091-3057(01)00709-2. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Lau W, de Lannoy IAM, Lee DKH, Izhakova J, Coen K, Le AD, Fletcher PJ. Evaluation of chemically diverse 5-HT2C receptor agonists on behaviours motivated by food and nicotine and on side effect profiles. Psychopharmacology. 2013;226:475–490. doi: 10.1007/s00213-012-2919-2. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Kiang M, Christensen BK, Remington G, Kapur S. Apathy in schizophrenia: clinical correlates and association with functional outcome. Schizophr Res. 2003;63:79–88. doi: 10.1016/s0920-9964(02)00433-4. [DOI] [PubMed] [Google Scholar]
- Levy R, Czernecki V. Apathy and the basal ganglia. J Neurol. 2006;253:vii54–vii61. doi: 10.1007/s00415-006-7012-5. [DOI] [PubMed] [Google Scholar]
- Marin RS, Butters MA, Mulsant BH, Pollock BG, Reynolds CF. Apathy and executive function in depressed elderly. J Geriatr Psychiatry Neurol. 2003;16:112–116. doi: 10.1177/0891988703016002009. [DOI] [PubMed] [Google Scholar]
- Markou A, Salamone JD, Bussey TJ, Mar AC, Brunner D, Gilmour G, Balsam P. Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. Neurosci Biobehav Rev. 2013;37:2149–2165. doi: 10.1016/j.neubiorev.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Oorschot M, Lataster T, Thewissen V, Lardinois M, Wichers M, van Os J, Delespaul P, Myin-Germeys I. Emotional experience in negative symptoms of schizophrenia—no evidence for a generalized hedonic deficit. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal MD, Klenotich SC, Morais M, Bessa J, Winkle J, Doukas D, Kay LJ, Sousa N, Dulawa SM. Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.144. [DOI] [PubMed] [Google Scholar]
- Roth RM. Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. Am J Psychiatry. 2004;161:157–159. doi: 10.1176/appi.ajp.161.1.157. [DOI] [PubMed] [Google Scholar]
- Salamone JDJD. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Sanders AC, Hussain AJ, Hen R, Zhuang X. Chronic blockade or constitutive deletion of the serotonin transporter reduces operant responding for food reward. Neuropsychopharmacology. 2007;32:2321–2329. doi: 10.1038/sj.npp.1301368. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, Kandel ER, Balsam PD. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry. 2011;69:928–935. doi: 10.1016/j.biopsych.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 2011;187:36–41. doi: 10.1016/j.psychres.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr Dir Psychol Sci. 2013;22:244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Benwell KR, Porter RH, Bickerdike MJ, Kennett GA, Dourish CT. Comparative effects of continuous infusion of mCPP, Ro 60-0175 and d-fenfluramine on food intake, water intake, body weight and locomotor activity in rats. Br J Pharmacol. 2000;130:1305–1314. doi: 10.1038/sj.bjp.0703443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Lefever TW, Jackson C, Tallarida RJ, Walker EA. Effects of a Cannabinoid1 receptor antagonist and Serotonin2C receptor agonist alone and in combination on motivation for palatable food: a dose-addition analysis study in mice. J Pharmacol Exp Ther. 2008;325:567–576. doi: 10.1124/jpet.107.131771. [DOI] [PubMed] [Google Scholar]
- Wongpakaran N, van Reekum R, Wongpakaran T, Clarke D. Selective serotonin reuptake inhibitor use associates with apathy among depressed elderly: a case-control study. Annals Gen Psychiatr. 2007;6:1–6. doi: 10.1186/1744-859X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.