Abstract
The major function of the uterus is to accept and provide a suitable environment for an embryo, ultimately leading the birth of offspring and successful propagation of the species. For this occur, there must be precise coordination of hormonal signalling within both the endometrial and myometrial components of this organ. Non-coding RNAs, specifically, microRNAs (miRNAs) have been shown to be essential for normal uterine development and function. Within this organ, miRNAs are proposed to fine-tune the actions of the female steroid hormones estradiol and progesterone. Not surprising, mis-expression of miRNAs has been documented in diseases of the endometrium and myometrium such as endometriosis and leiomyomas, respectively. In this chapter, I will review the current understanding on the role, regulation and function of non-coding RNAs focusing on miRNAs in both the normal physiology of the endometrium and myometrium as well as in pathologies of these tissues, namely endometriosis and leiomyomas.
Keywords: Uterus, Endometrium, Myometrium, Endometriosis, Leiomyoma, miRNA
9.1 Introduction
The uterus, often referred to as the womb, is an essential female organ necessary for successful reproduction. The proper development and function of the uterus is dependent on the proper interactions of a complex system involving gene transcription, post-transcriptional regulation and protein translation. This review will focus on the role of non-coding RNAs (ncRNAs) in the development and function of the uterus, with particular emphasis on the role of short RNAs such as microRNAs (miRNAs).
Non-coding RNAs (ncRNAs) are functional RNA molecules that are not translated into proteins and can be classified into long ncRNAs and short ncRNAs. While ncRNAs do not code for proteins, many of these molecules none the less have an important role in modulation of gene and protein expression. By far the examination of small RNAs, such as miRNAs, has gained the most attention of all of the ncRNAs (Taft et al. 2010). Not surprisingly, the majority of research conducted to understand the expression and function of small RNAs within the female reproductive tract has focused primarily on miRNAs. The objective of this chapter is to highlight the expression, regulation and functional role of miRNAs in uterine development and diseases.
9.2 Role of miRNAs/Small RNAs in Uterine Development and Function
The uterus is composed of three tissue layers, the endometrium, the myometrium and the perimetrium. The endometrium is the inner most layer or the lining of the uterine cavity. The endometrium is hormone-responsive and, in response to successive action of oestrogen and progesterone, provides the necessary environment for embryo attachment and establishment of pregnancy. The myometrium is the muscular layer, which separates the endometrium from the outer most layer, the perimetrium. While the myometrium remains relatively quiescent during the course of the reproductive cycle, the major function of the myometrium is to provide the contractile force necessary for expulsion of the foetus at the time of parturition.
Using animal models, which are genetically depleted of specific components of the small RNA/miRNA biogenesis pathway, it has been clearly established that this class of ncRNAs is essential for normal uterine development and function. DICER (DICER1 in mice) is an RNAse III enzyme that cleaves pre-miRNAs into transient RNA duplexes, which results in the generation of two strands of RNA. One of the strands is degraded while the remaining strand is the mature miRNA (Bartel 2004). Nagaraja and colleagues (2008) demonstrated that female mice in which Dicer1 was inactivated in Müllerian duct mesenchyme-derived tissues of the reproductive tract were sterile and displayed decreased ovulation rates, altered oocyte/embryo integrity, oviductual cysts and shorter uterine horns. Similarly, Hong and coworkers (2008) observed similar oviduct, ovulatory and uterine defects, but no significant impact of Dicer1 deletion on embryo development in vitro. However, embryos collected from day 3 of pregnancy in vivo were developmentally delayed in Dicer1-deficient mice compared to wild-type counterparts. Gonzalez and Behringer (2009) also observed similar defects within the oviduct, degenerated/unfertilized oocytes within the oviductal cysts and an inability to establish pregnancy. Further histological analysis demonstrated that Dicer1-deficient uteri contained less glandular tissue and exhibited what appeared to be early stage adenomyosis or growth of endometrial glands within the myometrial tissue layer. Taken together, the infertility and reproductive tract abnormalities characteristic of Dicer1-deficient female mice strongly suggest that DICER1 function and miRNA mediated post-transcriptional gene regulation are essential for normal female reproductive tract development and function.
With respect to the role of miRNAs in uterine tissue, although the first reports were in the areas of endometriosis (Pan et al. 2007) and uterine fibroids/leiomyomas (Wang et al. 2007), the majority of research has focused on the role of miRNAs in endometrial cancer. As it is beyond the scope of this review to cover in detail the role of these small RNAs in endometrial cancer, the reader is referred to reviews on this topic (Banno et al. 2013; Gilabert-Estelles et al. 2012; Lee et al. 2011). In this chapter, we will focus on the expression of the small non-coding RNAs, miRNAs, and their function within the uterus, with emphasis in endometrium and myometrium under normal and pathological states.
9.3 miRNAs in Endometrial Physiology and Pathology
9.3.1 Expression and Function of miRNAs in the Endometrium
The endometrium is the innermost layer of the uterus and functions to provide a suitable environment for establishment of pregnancy (embryo implantation) in response to the changing sex steroid levels that occur during the course of the menstrual/reproductive cycle. The first assessment of endometrial miRNA expression was conducted by Pan and colleagues (2007) using endometrial tissue from “normal” women compared to endometrium from women with endometriosis obtained during the early to mid-secretory stage of the menstrual cycle. Sixty-five miRNAs were detected above a predetermined threshold level. In endometrium from “normal” women (women without endometriosis in this study), miR-125b, miR-21, miR-145, miR-26a, miR-23b, miR-29a, and miR-99a were among the most abundantly expressed miRNAs. In ectopic (implant) and eutopic endometrium from women with endometriosis, there was a significant reduction in the expression of all of these miRNAs as well as several other miRNAs, with miR-451 being one of the most reduced miRNAs in the tissues from women with endometriosis. MicroRNA expression in normal endometrium was further characterized into miRNA expressed by endometrial stromal cells and glandular epithelial cells. Thirty-two miRNAs were shown to be differentially expressed between cell types. Of these, miR-20a, miR-21, and miR-26a were further assessed for steroidal regulation (Table 9.1).
Table 9.1.
Endometrial miRNAs whose expression is regulated by estradiol and/or progesterone
| miRNA | Cell/tissue type | Steroidal regulation | References |
|---|---|---|---|
| miR-20a | Human stromal cellsa | E2b ↓/MPAc ↓ | Pan et al. (2007) |
| miR-21 | E2 ↓/MPA ↓ | ||
| miR-26a | E2 ↓/MPA ↓ | ||
| miR-20a | Human glandular epithelial cells | E2 ↓/MPA ↑ | Pan et al. (2007) |
| miR-21 | E2 ↓/MPA ↓ | ||
| miR-26a | E2 ↑/MPA ↑ | ||
| miR-17-5p | Human stromal cells | E2 ↑/MPA ↑ | Toloubeydokhti et al. (2008) |
| miR-542-3p | E2 ↑/MPA ↑ | ||
| miR-23a | E2 ↓/MPA ↓ | ||
| miR-23b | E2 ↓/MPA no affect | ||
| miR-17-5p | Human glandular epithelial cells | E2 ↑/MPA ↑ | Toloubeydokhti et al. (2008) |
| miR-542-3p | E2 ↓/MPA ↓ | ||
| miR-23a | E2 ↓/MPA ↑ | ||
| miR-23b | E2 ↑/MPA ↑ | ||
| miR-155 | Mouse uterusd | E2 ↑ | Nothnick and Healy (2010) |
| miR-429 | |||
| miR-451 | |||
| miR-181b | Mouse uterus | E2 ↓ | Nothnick and Healy (2010) |
| miR-204 |
All studies using human endometrial stromal or epithelial cells were conducted in vitro
E2 = estradiol 17beta
MPA = medroxyprogesterone acetate
All studies using mouse uterine tissue were conducted in vivo
This same group (Toloubeydokhti et al. 2008) conducted a follow-up study in which miR-17-5p, miR-23a, miR-23b, and miR-542-3p expression was assessed (Table 9.1). Findings from this study revealed that miR-23b and miR-542-3p were expressed in lower levels and that miR-17-5p was expressed at higher levels in paired eutopic and ectopic endometrial tissue compared to “normal” eutopic endometrium and that oestradiol and MPA could regulate expression of all three of these miRNAs (Table 9.1). In this and the previously cited study (Pan et al. 2007), steroidal regulation could be blocked for some of the miRNAs using the oestrogen receptor antagonist ICI-182780, as well as the progesterone receptor antagonist RU-486. These observations suggest that steroidal regulation of miRNAs in endometrial stromal and glandular epithelial cells appears to occur via complex mechanisms.
A similar experimental design was implemented to compare miRNA expression between early secretory endometrium from women with and without endometriosis (Burney et al. 2009). Significantly lower levels of miR-34c-3p, miR-34c-5p, miR-9, miR-9*, miR-34b*, and the unannotated miRPlus_42 780 were expressed in the early secretory endometrium from women with endometriosis. Although additional miRNA profile information was not provided in this report, one may conclude that these miRNAs are expressed in early secretory endometrium from women without endometriosis and may play a role within the endometrium during the menstrual cycle.
The first report to examine hormonal regulation of miRNAs in the human endometrium to gain insight into the mechanisms of the opposing action of progesterone on that of oestradiol during the period of embryo implantation was conducted by Kuokkanen and colleagues (2010). Using isolated endometrial epithelial cells from women in the late proliferative versus the mid-secretory stages of the menstrual cycle, it was demonstrated that 12 miRNAs were expressed at significantly higher levels in the late proliferative stage (miR-29b, miR-29c, miR-30b, miR-30d, miR-31, miR-193a-3p, miR-203, miR-204, miR-200c, miR-210, miR-582-5p, and miR-345) compared to the mid-secretory stage. Based upon the up-regulation of these miRNAs coupled with the fact that these miRNAs are proposed to target cell cycle genes, it is tempting to speculate that these miRNAs may suppress cell proliferation during the secretory phase of the menstrual cycle.
In subsequent studies, miR-30b and miR-30d were further proposed to regulate human endometrial receptivity. First, Sha and colleagues (2011) performed a genome-wide analysis of small RNAs/miRNAs associated with endometrial receptivity in a population of women undergoing in vitro fertilization treatment. Comparing 7 days post LH surge to 2 days post LH surge (with 7 days post LH surge being representative of the “receptive” state of the endometrium), 20 miRNAs were determined to be differentially expressed. Of these, miR-30d, miR30b*, miR-31, and miR-30b showed the most significant up-regulation (6.92, 3.97, 3.32, and 2.99-fold, respectively).
Secondly, Altmäe and coworkers (2013) also compared 7 days post LH surge to 2 days post LH surge and again found that miR-30b and miR-30d were significantly up-regulated in the former group compared to the latter. Further, miR-494 and miR-923 were both down-regulated and predicted to target leukemia inhibitory factor (LIF), which has been proposed as a major factor necessary for embryo implantation in mammals (reviewed in Stewart 1994).
It should be noted that in both of these studies, the up-regulation of miR-30b and miR-30d were in agreement with the work of Kuokkanen et al. (2010). Collectively, both studies suggest that miR-30b and miR-30d up-regulation during the window of implantation may function to dampen expression of potential protein targets associated with cell cycle regulation such as CCNB1, RASSF2, and MMP7. A limitation to these studies as a whole was that data were restricted to miRNA profiling and prediction of their target genes by in silico algorithms. Functional studies demonstrating that these miRNAs target these proposed genes within the endometrium remain to be conducted.
Additional information with respect to miRNA mis-expression and altered embryo implantation was reported in 2011 by Petracco and colleagues. Homeobox A10 (HOXA10) is essential for normal uterine function/embryo implantation (Taylor et al. 1998) and is mis-expressed in the endometrium from women with endometriosis (Taylor et al. 1999). As HOXA10 is a proposed target of miR-135a/b, Petracco and colleagues (2011) examined miR-135a/b expression in endometrium from women with and without endometriosis and found that miR-135a/b was significantly increased in eutopic endometrial tissue from women with endometriosis and this elevated expression was associated with reduced levels of HOXA10 transcript expression (Fig. 9.1). Additional studies demonstrated that miR-135a/b bound to the 3′ UTR of HOXA10 as well as modulated HOXA10 transcript and protein expression in cultured endometrial stromal cells. These studies suggest that the over-expression of miR-135a/b in endometrium of women with endometriosis, a disease associated with impaired embryo implantation, may lead to reduced levels of HOXA10 expression which is necessary for embryo implantation and is one of the few studies demonstrating expression and function for a given miRNA in human endometrial tissue (Fig. 9.1).
Fig. 9.1.
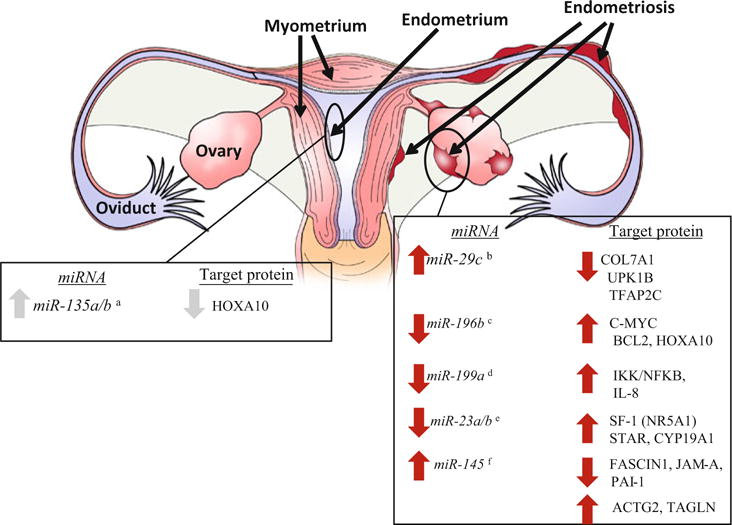
miRNAs and their validated targets in endometrial and endometriotic tissue. Left table summarizes miR-NAs and their predicted transcript/protein in endometrial tissue (indicated as the gray lining of the uterus in the figure) derived from studies incorporating human endometrial cells which have been validated. Right table summarizes miRNAs and their predicted transcript/protein in endometriotic tissue (indicated as red ectopic tissue in the figure) derived from studies incorporating human cells which have been validated. Superscripts refer to references with a = Petracco et al. 2011; b = Hawkins et al. 2011; c = Abe et al. 2013; d = Dai et al. 2012; e = Shen et al. 2013; f = Adammek et al. 2013. Validation refers to either assessing cellular events and/or mRNA and protein levels by modulation of endogenous miRNA levels
In addition to information gleaned from studies that incorporated human endometrial tissue, there is also limited information on miRNA profiles in mouse uterine tissue, with the bulk focusing on identifying miRNAs that are associated with and may play a role in embryo implantation. Initial characterization was performed by Chakrabarty and colleagues (2007) in which expression profiling was performed with oligonucleotide microarrays and compared the expression patterns of 380 miRNAs common to humans, mice, and rats, in day 1 (pre-receptive; oestrogen dominance) and day 4 (receptive; progesterone dominance) pregnant mouse uteri, with day 1 representing the presence of a vaginal plug. Thirty-two miRNAs were up-regulated during the receptive phase (progesterone dominance) and five were down-regulated compared to the pre-receptive (oestrogen dominance) phase. Of those up-regulated, miR-101a and miR-199a* were further examined and verified to regulate cyclo-oxygenase-2 (Cox2) expression, encoding an enzyme which is essential for embryo implantation. Additional studies have suggested that miR-21 (Hu et al. 2008), let-7a (Xia et al. 2010a) and miR-320 (Xia et al. 2010b) may be important for embryo implantation in rodents.
My laboratory (Nothnick and Healy 2010) recently utilized miRNA arrays followed by qRT-PCR validation to demonstrate that oestradiol upregulates miR-155, miR-429, and miR-451 expression in mouse uterine tissue, but decreases the expression of miR-181b and miR-204 (Table 9.1). Use of ICI 182,780 validated that this regulation was mediated via the classical oestrogen receptor pathway. Additional unpublished observations from our research group demonstrate that within the mouse uterus from ovariectomized mice, miR-709 is the most abundant miRNA followed by the let-7 family members and miR-26a. Mis-expression of miRNAs such as miR-709 has been proposed to contribute to the discordant pattern of expression between MMP9 protein and transcript expression detected in oestradiol-primed mouse uterine tissue (Nothnick 2008).
Oestradiol is a known proliferative agent within the endometrium and its actions thought to be fine-tuned through miRNAs (Lessey 2010). Oestradiol increased oestrogen receptor positive endometrial adenocarcinoma cell proliferation which was associated with an up-regulation of BCL2 and concurrent down-regulation of Bax (Zhang et al. 2012). As Bax repression was determined to occur post-transcriptionally, miRNA assessment revealed that members of the let-7 family (let7a–g) and miR-27a targeted Bax transcript. Surprisingly, as discussed above, none of these miRNAs were up-regulated in human endometrial cells or mouse uterus in response to oestrogen administration.
From our current knowledge, it appears that endometrial miRNAs are regulated by both oestradiol and progesterone. Some of these miRNAs appear to be modulated through the classical nuclear steroid receptor pathway while the regulation of others appears to be more complex. Future assessment will need to focus on the specific mechanisms by which oestradiol and progesterone modulate endometrial miRNA expression and how steroid modulation of these miRNAs in turn “fine-tune” steroid action downstream within this tissue.
To deepen our understanding on the role of miRNAs in uterine physiology and/or pathology, the function of any given miRNA needs to be understood. Functional data on the role of miRNAs within the uterus are beginning to accumulate with the majority of these studies using endometrial carcinoma cell lines. Pan and colleagues (2007) were perhaps the first investigators to postulate the possible function of miRNAs in endometrial/uterine tissue based upon algorithms for the identification of miRNA targets. Based upon this assessment, endometrial miRNAs were proposed to modulate diverse pathways including cellular proliferation, apoptosis, cell differentiation, and inflammation all of which are essential to normal endometrial biology. Further, it was proposed that dysregulation of these miRNA-modulated pathways could lead to the pathogenesis of endometrial pathologies such as endometriosis and endometrial cancer. Our current understanding on the functional roles of specific miRNAs in endometrial cells is derived primarily from studies incorporating human endometrial adenocarcinoma cell lines and as such, should be interpreted with caution as it is well established that mis-expression of miRNAs are associated with the malignant phenotype in a variety of cancers (Fabbri 2013). Nonetheless, our current understanding on the function of endometrial miRNAs from studies utilizing these models is outlined below and summarized in Table 9.2.
Table 9.2.
Endometrial carcinoma miRNAs, their expression level, proposed function and transcriptional targets
| miRNA | Cell/tissue type | ↑/↓ | Function | Proposed target | Validationa | References |
|---|---|---|---|---|---|---|
| miR-200a/b/c | HEC-1A, Ishikawa cell lines | ↑ | Proliferation | NAb | Yes | Lee et al. (2011) |
|
miR-141, miR-429 |
||||||
| miR-200c | HEC-1A, Ishikawa cell lines | ↑ | Proliferation | BRD7 | Yes | Park et al. (2012) |
|
miR-9, miR-27, |
Endometrial carcinoma tissue, Ishikawa cells | ↑ | NDc | FOXO1 (decreased) | ND | Myatt et al. (2010) |
|
miR-96, miR-153, |
||||||
|
miR-182, miR-183, |
||||||
| miR-186 | HEC-1B cells | Proliferation | FOXO1 | Yes | Myatt et al. (2010) | |
|
miR-7, miR-149, |
Endometrial carcinoma tissue | ↑ | ND | NA | ND | Myatt and Lam (2007) |
|
miR-449b, miR-204 |
||||||
| miR-204 | HEC-1A cell line | Migration, invasion | FOXC1 | Yes | Myatt and Lam (2007) | |
| miR-125b | Type II endometrial carcinoma cells | ↑ | Proliferation, invasion (in vitro and in vivo) | TP53INP1 | Yes | Jiang et al. (2011) |
Target validation was performed by 3″ UTR reporter construct assays and/or, miRNA transfection and Western analysis
NA = function or proposed target was not assessed in the cited study
ND = not determined
Cellular proliferation is one of the most-well-studied miRNA-mediated cellular events within the endometrium. Of the miRNAs to date, the miR-200 family is the most studied. miR-200a/b/c, miR-141, and miR-429 are up-regulated in both endometrioid endometrial adenocarcinoma and complex atypical hyperplasia compared to normal control endometrium (Snowdon et al. 2011). Since this report, subsequent studies (Lee et al. 2011; Park et al. 2012) further documented a functional role for these miRNAs in cancerous tissues as well as endometrial adenocarcinoma cell lines. Incorporation of anti-miR technology revealed that each of these miRNAs suppressed HEC-1A cell proliferation, but only anti-miR-141, -200c, and -429 inhibitors reduced growth of Ishikawa cells (Lee et al. 2011). Park and co-workers (2012) further demonstrated that miR-200c modulated HEC-1A and Ishikawa cell proliferation, survival and apoptosis and that this was associated, at least in part, via down-regulation of transcript for the tumour suppressor protein BRD7.
Additional investigations examined the potential role of miRNAs in modulation of tumour suppressor genes and their role in proliferation. FOXO1 expression is reduced in endometrial carcinoma (Goto et al. 2008; Myatt et al. 2010). Using miRNA target prediction programs, Myatt and colleagues (2010) identified a panel of highly conserved miRNAs, which could potentially target the 3′ UTR of FOXO1 transcript. Of these, miR-9, miR-27, miR-96, miR-128, miR-153, miR-182, miR-183, and miR-186 up-regulation correlated with loss of FOXO1 expression in both endometrial carcinoma tissue and Ishikawa cells. Individual over-expression of all of these miRNAs in HEC-1B cells, except miR-128, reduced FOXO1 protein expression. When inhibitors of miR-9, miR-27, miR-96, miR-153, miR-183, and miR-186 were transfected as a pool into Ishikawa cells, cell cycle arrest and apoptosis were induced.
FOXC1 belongs to the same family of forkhead box transcription factors as FOXO1 and its mis-expression has also been associated with carcinogenesis (Myatt and Lam 2007). Through analysis of differential expression, endometrial cancer expressed a set of dysregulated miRNAs, which included miR-7, miR-149, miR-449b, and miR-204. Of these, miR-204 was shown to target FOXC1 and functionally regulate endometrial adenocarcinoma (HEC-1A) cell migration and invasion (Chung et al. 2012). Similarly, the tumour suppressor, TP53INP1 is also modulated by miRNAs and its miRNA-induced suppression is associated with enhanced cell proliferation (Jiang et al. 2011). More specifically, miR-125b was found to up-regulated in type II endometrial carcinoma cells. Over-expression and inhibition studies using adenocarcinoma cell lines revealed that miR-125b modulated in vitro and in vivo cell proliferation and invasion and that these events were mediated via TP53INP1.
Within the field of endometrial biology, miRNA profiles have been generated for both human and rodent uterine tissue. Steroidal regulation of uterine/endometrial miRNAs has been investigated using both in vivo (rodent) and in vitro (human) models. Unfortunately, very few miRNAs and their targets have been validated in endometrial biology and/or disease with miR-135a/b being one of the few (Fig. 9.1). Currently, the majority of our functional understanding on the role of miRNAs within the uterus/endometrium has been formulated from studies that employed human endometrial adenocarcinoma cell lines. Collectively, this body of knowledge supports the concept that endometrial miRNAs regulate cellular proliferation, migration and/or invasion, and that members of the miR-200 family appear to be major players in these regulatory pathways. However, care must be taken when extrapolating miRNA regulation and/or functional data from cancerous cell types to that of “normal” endometrial cells that reside within the endometrium as there is strong existing data which demonstrates alterations between miRNA profiles in “normal” endometrium and endometrial adenocarcinoma tissue (reviewed in Fabbri 2013).
9.3.2 miRNAs Are Mis-expressed in Endometriosis
As discussed previously, it appears that within the endometrium, miRNAs function to modulate downstream gene expression of steroid-regulated genes important for normal reproductive cycles and fertility/embryo implantation. Endometriosis is a significant disease in which endometrial tissue grows ectopically within the pelvic cavity. This disease is associated with pelvic pain and infertility with the latter thought to be associated with embryo implantation insufficiencies among other pathological mechanisms. Over the last several years, miRNA expression profiles have been generated comparing miRNA expression within endometriotic implant tissue (ectopic endometrium) compared to that of the eutopic endometrium. The first study to profile endometriotic implant miRNA expression was conducted by Pan and colleagues (2007). Endometrial biopsy and endometriotic tissue was obtained from women of reproductive age during the early to mid-secretory stage (rising progesterone levels) of the menstrual cycle. Using miRNA array analysis, 48 miRNAs were identified as differentially expressed between ectopic and eutopic endometrium with the majority exhibiting approximately 40–60 % lower levels of expression in the ectopic endometrial (endometriosis) tissue. The reduced expression of these miRNAs in the endometriotic tissue was proposed to play a role in allowing target over-expression and subsequent enhancement of cellular events conducive to endometriotic implant survival and growth.
Filigheddu and co-workers (2010) conducted a similar miRNA profiling experiment comparing miRNA expression between ectopic and eutopic endometrium using tissue obtained during the proliferative stage of the menstrual cycle (cycle days 6–12, associated with rising estradiol levels). Fifty miRNAs were considered differentially expressed between tissue types. Again, the mis-expression of these miRNAs was postulated to allow for mis-expression of various cytokines, enzymes, growth factors, receptors and transcription regulators, all of which are proposed to play a role in the pathogenesis of endometriosis. In comparing the data presented in the study by Pan and colleagues to those of Filigheddu and coworkers, an interesting observation may be noted. Of the miRNAs profiled in the two studies, eight miRNAs were common to both reports; miR-100, miR-126, miR-143, miR-145, miR-17-5p, miR-29c, miR-30e-5p, and miR-99a. Interestingly, in the first study, which collected samples during rising endogenous progesterone levels, all of these miRNAs showed lower expression in the endometriotic tissue compared to the corresponding (same patient) eutopic endometrium, while in the latter study in which samples were collected during rising endogenous oestradiol levels, the expression of all eight miRNAs was markedly higher in the endometriotic implant tissue compared to corresponding eutopic endometrium. This may suggest altered steroidal regulation of these miRNAs in the ectopic tissue between the proliferative and secretory stages of the menstrual cycle.
Using endometriotic and corresponding eutopic endometrial tissue from patients in both the proliferative and secretory stages of the menstrual cycle, Ohlsson-Teague and colleagues (2009) identified 14 differentially expressed miRNAs that were up-regulated in the endometriotic implant tissue and 8 that were down-regulated in the endometriotic tissue. ANOVA analysis of the microarray data according to stage of menstrual cycle revealed no significant differences in miRNA profiles based upon stage of menstrual cycle. Of the 14 up-regulated miRNAs in this study, 6 miRNAs (miR-145, miR-143, miR-99a, miR-126, miR-100, and miR-29c) were common to the studies of Pan et al. (2007) and Filigheddu et al. (2010). These six miRNAs were all up-regulated in the study by Filigheddu and colleagues, but down-regulated in the Pan and co-workers study. The finding that the menstrual cycle stage did not appear to influence the level of these six miRNAs (unlike the earlier suggestion) may be due to the small sample sizes in the study by Ohlsson-Teague et al. (2009) in which only four and three subjects from the proliferative and secretory stages of the menstrual cycle were enrolled.
The first transcriptome-miRNA analysis of endometriotic endometriomas (endometriotic cysts of the ovary) was conducted in 2011 (Hawkins et al. 2011). The top 30 miRNAs expressed in endometriomas and the corresponding abundance of transcript in non-endometriosis control endometrium was determined. Many of the miRNAs identified in the previous three reports (Pan et al. 2007; Ohlsson Teague et al. 2009; Filigheddu et al. 2010) were again identified in the report by Hawkins and colleagues (2011). Further, in the study by Hawkins et al. the potential function of miR-29c, which exhibited the highest expression differential between tissue types, was evaluated. An in vitro cell culture system using primary human endometrial stromal cells was employed in which levels of miR-29c were either inhibited or up-regulated. P utative extracellular matrix (ECM) protein gene targets of miR-29c, COL7A1, UPK1B and TFAP2C, were down-regulated in cells over-expressing miR-29c and the direct effect on the 3′ UTR of the genes was confirmed (Fig. 9.1). Thus, mis-expression of miR-29c in endometriomas appears to functionally contribute to the mis-expression of ECM proteins associated with the disease.
Most recently, Abe and colleagues (2013) examined the miRNA profiles in endometriotic versus eutopic endometrial stromal cells. Stromal cells were isolated from ovarian endometriomas, while endometrial stromal cells were obtained from eutopic endometrium of women with leiomyomas but no visible signs of endometriosis. Twelve miRNAs were demonstrated to be differentially expressed between endometrioma and endometrial stromal cells, eight down-regulated in endometrioma stromal cells (miR-199b-5p, miR-503, miR-424, miR-196b, miR-199a-3p, miR-214, miR-29b, and miR-455-3p) and four up-regulated (miR-210, miR-100, miR-132*, and miR-181a). Of these, miR-196b that showed approximate 70 % reduction in expression was selected for further evaluation. Transfection of endometrioma stromal cells with mir-196b precursor, which leads to subsequent expression of mature miR-196b, resulted in rounded/polygonal cells that poorly adhered to culture vesicles as opposed to the normal dendritic/stellate shape that adhere to the cell culture plate. Increased expression of miR-196b was also associated with reduced endometrioma stromal cell viability and cell proliferation as well as increased apoptosis, which was associated with elevated caspase-3 and -7 activity. To evaluate the miR-196b-mediated pathways, cMYC, BCL2, and HOXA10 expression were examined. First, cMYC expression was higher in endometrioma stromal cells compared to cells from eutopic endometrium. Transfection of endometrioma stromal cells with mir-196b precursor revealed that the antiproliferative and pro-apoptotic activities are likely mediated through cMYC and BCL2, respectively, as miR-196b suppressed transcript levels of these factors. HOXA10 expression was lower in endometrioma stromal cells compared to cells from eutopic endometrium, which was correlated with lower levels of miR-196b in these cells. Lastly, it was determined that the suppressed expression of miR-196b in endometrioma stromal cells is a result of DNA hypermethylation. Collectively, these studies suggest that aberrant miR-196b expression plays a role in the events conducive to the cellular processes associated with the pathogenesis of endometriosis, and that this mis-expression may be epigenetic in nature and results in increased expression of cMYC, BCL2 and HOXA10 protein (Fig. 9.1).
Ramón and colleagues (2011) assessed the expression of miR-15b, miR-16, miR-17-5p, miR-20a, miR-21, miR-125a, miR-221, and miR-222 and correlated the levels of expression for these miRNAs with the angiogenic factors vascular endothelial growth factor A (VEGFA) and thrombospondin I (THBS1). When analysing paired specimens, ovarian endometriomas exhibited significantly lower levels of pro-angiogenic VEGFA mRNA and protein and higher levels of miR-125a and miR-222 compared to corresponding eutopic endometrium. In contrast, levels of the angiogenesis inhibitor THBS1 were significantly higher in endometriomas and this was associated with reduced levels of miR-17-5p. Significant inverse correlations were noted between miR-222 and VEGFA protein expression and miR-17-5p and THBS1 protein levels suggesting that the mis-expression of these miRNAs may at least in part contribute to the observed altered expression of these angiogenic factors and in doing so contribute to the pathogenesis of the disease.
Additional miRNAs, which have been shown to be mis-expressed in endometriotic tissue include miR-199a (Dai et al. 2012), miR-126 (Liu et al. 2012), miR-23a/23b (Shen et al. 2013) and miR-145 (Adammek et al. 2013). It is well established that endometriosis is an invasive disease associated with increased angiogenesis, which often draws comparison to the malignant process. Of these microRNAs miR-199a is down-regulated in several types of cancer (Shen et al. 2010; Cheung et al. 2011). Based upon these observations, Dai and coworkers (2012) evaluated miR-199a expression in matched ovarian endometriomas and eutopic endometrium as well as endometrium from women free of endometriosis. Compared to eutopic endometrium from women without endometriosis, miR-199a expression was lower in both the eutopic endometrium from women with endometriosis and ovarian endometriomas. Forced expression of miR-199a in endometrial stromal cells resulted in dampened cell adhesion, invasion and migration, which was associated with suppression of the IKK/NFκB pathway and reduced interleukin 8 expression suggesting a possible functional role for the mis-expressed levels of miR-199a in the pathogenesis of endometriosis (Fig. 9.1).
miR-126 is a proposed regulator of cell growth, adhesion, invasion and angiogenesis (Guo et al. 2008; Feng et al. 2010), all processes essential for endometriosis development and progression. miR-126 expression is significantly reduced in ovarian endometriomas and eutopic endometrium from women with endometriosis compared to eutopic endometrium from control subjects. Associated with this reduction in miR-126 was a significant increase in CRK mRNA and protein. Furthermore, a highly significant association was found between miR-126 expression in endometriomas and eutopic endometrium from endometriosis subjects and stage/score of endometriosis suggesting that the worse the disease, the lower the expression of miR-126. Unfortunately, the ability of miR-126 to modulate the events conducive to endometriosis pathogenesis such as proliferation, adhesion, invasion, and angiogenesis, and if these events are associated with altered CRK expression in endometriosis were not evaluated.
There is considerable evidence that steroidogenic factor 1 (SF1 or NR5A1) expression is increased in stromal cells from endometriotic tissue, but the mechanisms for this mis-expression are largely unknown. miR-23a/b expression is reduced in endometriotic and eutopic endometrium from women with endometriosis. As miR-23a/b is proposed to target NR5A1, the potential mechanistic link between miR-23a/b and NR5A1 expression in the pathogenesis of endometriosis was examined (Shen et al. 2013). Reduced levels of miR-23a/b expression was confirmed in ectopic endometriotic tissue and eutopic endometrium from women with endometriosis compared to eutopic endometrium from women free of endometriosis and this reduction was associated with elevated transcript levels of NR5A1 (SF1), STAR and CYP19A1 (Fig. 9.1). To confirm that miR-23a/b directly regulated NR5A1 (SF1) expression, luciferase reporter assays were conducted. Despite the fact that transfection with mir-23a/b precursor suppressed NR5A1 (SF1) transcript, miR-23a/b did not bind to the 3′ UTR of NR5A1 (SF1) suggesting that miR-23a/b mediation of NR5A1 expression is via an indirect mechanism.
miR-145 has been shown to be mis-expressed in endometriotic tissue compared to eutopic endometrium (Pan et al. 2007; Ohlsson-Teague et al. 2009; Filigheddu et al. 2010). Coupled with the fact that miR-145 putatively targets factors involved in cellular events (Götte et al. 2010) conducive to endometriosis survival and progression, Adammek and colleagues (2013) examined the expression and potential function of miR-145 in the pathogenesis of endometriosis. Transfection of the human immortalized epithelial endometriotic cell line 12Z with miR-145 resulted in reduced cell proliferation and invasion while in primary ectopic and eutopic endometrial stromal cells from endometriosis patients, miR-145 over-expression resulted in inhibition of cell proliferation. Further analysis revealed that miR-145 induced post-transcriptional down-regulation of proposed targets FASCIN1, PAI1, and JAMA at the transcript and protein level in 12Z cells. Direct binding of miR-145 to the 3′ UTR of JAMA was confirmed by luciferase reporter construct assays. In contrast, only FASCIN1 was shown to be modulated by miR-145 in primary ectopic and eutopic endometrial stromal cells from endometriosis patients. Cytoskeleton proteins ACTG2, TAGLN, and MYL9 were shown to be differentially regulated by miR-145 with over-expression of miR-145 leading to decreased ACTG2 transcript expression but increased TAGLN transcript expression while MYL9 expression was unaffected. Similarly, miR-145 over-expression in 12Z and stromal cells from both eutopic and ectopic endometrial cells from women with endometriosis resulted in decreased transcript expression of pluripotency and stemness-related markers. Modulation of protein expression or verification of direct binding via 3′ UTR reporter assays to further validate these targets were not performed. Although initial results on miR-145 expression in different endometriosis samples are conflicting (Pan et al. 2007; Ohlsson-Teague et al. 2009; Filigheddu et al. 2010), these data suggest that miR-145 appears to inhibit endometriotic cell proliferation and invasion as well as regulation of stem cell properties.
As discussed earlier, miRNAs are proposed to regulate steroid action within the endometrium to control the cellular events necessary for “normal” endometrial function. Mis-expression of miRNAs is in turn thought to contribute to the pathogenesis of diseases of endometrial tissue origin such as endometriosis. Within the field of endometriosis research, several miRNAs have emerged as potential players in modulating the events conducive to the establishment, survival and progression of the ectopic implant. Of these, functional data exists for only a few (Fig. 9.1). To truly understand which miRNAs are important in the pathogenesis of endometriosis and how they contribute to the disease, greater effort must be put forth to standardize what constitutes true “control” groups and what constitutes “endometriotic tissue”. Reported and unpublished observations strongly suggest that not only does miRNA expression vary based upon the “type” of implant, i.e. ovarian endometrioma versus peritoneal implant, among study subjects but that this expression also varies by type of implant within study subject.
9.4 miRNAs in Myometrial Physiology and Pathology
9.4.1 Expression and Function of miRNAs in the Myometrium
The role of miRNAs in normal myometrial physiology and function is just beginning to be explored. The primary function of the myometrium is to aid in the expulsion of the foetus during the birthing process via contraction. During the period of pregnancy, the myometrium is quiescent due to the high progesterone levels associated with pregnancy.
Renthal and colleagues (2010) first examined the potential role and regulation of miRNAs in myometrial function. In this study, microarray analysis was utilized to examine myometrial miRNA expression in term labour and non-labour human and mouse uterine tissue (pre-term and term). miR-200a, miR-200b, miR-200c, miR-141 and miR-429 (collectively referred to as the miR-200 family) were up-regulated in mouse myometrium from the day 18.5 group compared to the day 15.5 group, while in human tissue a similar pattern of expression was detected in labouring human myometrium compared to myometrium from term, non-labouring subjects. Expression of zinc finger E-box-binding homeobox 1 (ZEB1) and ZEB2, which are targets of the miR-200 family, was decreased demonstrating an inverse pattern of expression between miRNA and target protein in both mouse and human tissues. Further, an increase in expression of connexion 43 (CXN43) and oxytocin receptor (OXTR) was also detected in both mouse and human myometrium. Additional mouse models of preterm labour were used and revealed that there was an up-regulation of the miR-200 family and down-regulation of ZEB1/ZEB2. It was further revealed that ZEB1 is directly up-regulated by progesterone and that ZEB1 and ZEB2 inhibit the expression of contractile proteins CXN43 and OXTR. Collectively, these findings suggest that progesterone regulates the expression of the miR-200 family of microRNAs. In turn, the miR-200 family regulates ZEB1 and ZEB2 to modulate uterine contractility during pregnancy and labour (Fig. 9.2).
Fig. 9.2.
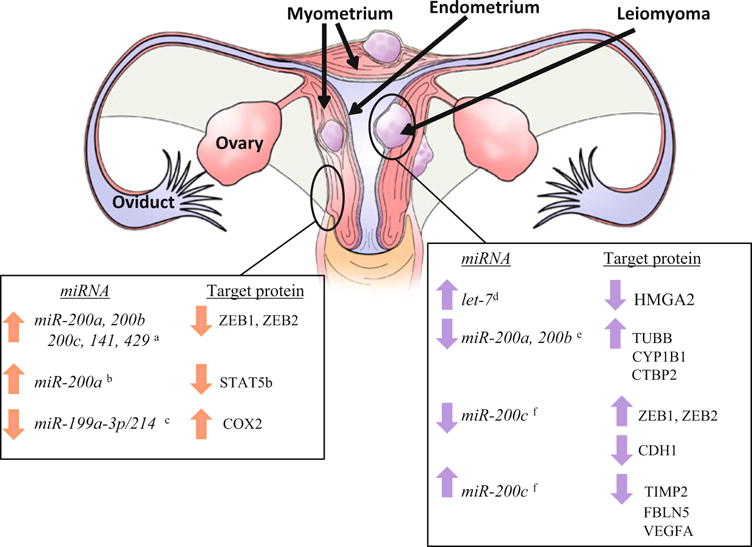
miRNAs and their validated targets in myometrial and leiomyoma tissue. Left table summarizes miR-NAs and their predicted transcript/protein in myometrial tissue (indicated as the pink layer of tissue in the figure) derived from studies incorporating human myometrial cells which have been validated. Right table summarizes miRNAs and their predicted transcript/protein in leiomyoma tissue (indicated as the lavender tissue within the myometrium) derived from studies incorporating human cells which have been validated. Superscripts refer to references with a = Renthel et al. 2010; b = Williams et al. 2012a; c = Williams et al. 2012b; d = Wang et al. 2007; e = Zavadil et al. 2010; f = Chuang et al. 2012. Validation refers to either assessing cellular events and/or mRNA and protein levels by modulation of endogenous miRNA levels
Subsequent studies by this research group (Williams et al. 2012a) further demonstrated that miR-200a plays a key role in initiating uterine contractility by enhancing the metabolism of progesterone in the myometrium in turn leading to reduced progesterone receptor function. Specifically, miR-200a represses STAT5b, which in turn leads to an increase in the expression of 20α-hydroxysteroid dehydrogenase (20α-HSD), a key progesterone-metabolizing enzyme. From these findings it was concluded that miR-200a plays a key role in the decline in progesterone action, which is necessary for initiation of labour through down-regulation of STAT5b (Fig. 9.2).
More recently, the same research group (Williams et al. 2012b) determined that while an increase in miR-200a plays a necessary role in labour induction, a decline in the expression of the miR-199a/214 cluster might also be important in the labour process. Both miR-199a-3p and miR-214 decline during labour in both mouse and human models employed in this study and this decrease was associated with an up-regulation of COX2 protein in both species during labour. It was further determined that oestrogens levels increase near term and are capable of stimulatory pro-inflammatory cascades, decrease miR-199a/214 expression and increase COX2 levels. These events could be blocked by progesterone, which often antagonizes many of the effects of oestrogens. Over-expression of miR-199a-3p and miR-214 inhibited COX2 protein and blocked tumour necrosis factor α-induced myometrial cell contractility. Together, these data could be interpreted to suggest that miR-199a-3p and miR-214 regulate myometrial contractility through the modulation of COX2 expression (Fig. 9.2).
Collectively, the studies from this research group provide strong insight into the role of the miR-200 and miR-199a/214 families in the process of uterine contractility. From these studies it appears that these miRNA families regulate the expression of ZEB1, ZEB2, STAT5B, and PTGS2 and of these, ZEB1 appears to be a central mediator of these opposing actions of progesterone and oestradiol on myometrial contractility.
9.4.2 miRNAs Are Mis-expressed in Uterine Leiomyomas and Contribute to Disease Pathogenesis
As discussed in the preceding paragraphs, we are just beginning to dissect the regulation of miRNAs in the myometrium and their role in the process of parturition. To date, there is no information on the potential role of miRNAs in abnormal labour/labour dystocia. With respect to miRNA function in abnormal myometrium, our current understanding is derived primarily from studies focusing on the role of miRNAs in the pathogenesis of uterine fibroids or leiomyomas. Uterine leiomyomas are benign smooth muscle (myometrium) neoplasms, which develop in women of reproductive age via uncertain mechanisms. As altered gene expression and/or function are predicted to play a role in the pathogenesis of leiomyomas, it is not surprising that miRNA expression has been examined in this tissue. The first study to assess myometrial miRNA expression was conducted by Wang and colleagues in 2007, in which they generated a miRNA signature associated with race, tumour size and target gene activity. Two hundred and six (206) miRNAs were examined and 45 were found to be dysregulated in uterine leiomyomas. Of these, the let-7 family, miR-21, miR-23b, miR-27a, and miR-30a were the most significantly up-regulated leiomyoma miRNAs. Let-7 expression was significantly higher in small (<3 cm) compared to large (>10 cm) leiomyomas and exhibited an inverse correlation with one of its proposed targets, HMGA2. The putative regulation of HMGA2 by let-7 was confirmed by transfection of leiomyoma cells with let-7 miRNA. In this same study, miR-29b, miR-32, miR-144, miR-197 and miR-212 were shown to be significantly down-regulated in leiomyomas. In contrast to the functional studies performed to validate let-7 regulation of HMGA2, only the predicted targets for these miRNAs were reported by Wang and coworkers (2007) without further validation.
Marsh and colleagues (2008) identified 46 miRNAs, which were differentially expressed in leiomyomas compared to myometrial tissue. Of the 46 detected, 19 were up-regulated and 27 were down-regulated. miR-542-3p was the most significant up-regulated microRNA with approximately 12-fold increase, while miR-498 was the most significantly down-regulated (approximately 2.5-fold). The authors further confirmed expression of miR-21, miR-34a, miR-125b, miR-139 and miR-323 by qRT-PCR, but did not examine either the regulation or function of these miRNAs in their study. The observation that miR-542-3p expression was the most significantly up-regulated miRNA in leiomyoma tissue was interesting in that this miRNAs has been proposed and validated to target survivin which suppresses cell growth (Yoon et al. 2010). If true, one may speculate that elevated miR-542-3p and subsequent reduction of survivin would lead to increased cell proliferation in fibroid tissue. miR-498 has been proposed to target ZEB2 (based on upon TargetScan analysis), which has been linked to cell proliferation and tumour growth (Qi et al. 2012). Thus, one may speculate that reduced levels of miR-498 in leiomyomas may be associated with increased levels of ZEB2 and enhancement of cellular proliferation.
Pan and colleagues (2008) used a multifaceted approach incorporating paired myometrial and leiomyoma tissue, myometrial (MSMC) and leiomyoma (LSMC) isolated cells, as well as leiomyoma cell lines T-LSMC and SK-LMS-1 to examine myometrial/leiomyoma miRNA expression. Ninety-one (91) miRNAs were identified which were expressed above myometrium thresholds with miR-20a, miR-21, miR26a, mir-18a, miR-206, miR-181a, and miR-142-5p expression confirmed by qRT-PCR. Further, steroidal regulation of these specific miRNAs was assessed in MSMC, LSMC, T-LSMC, and SK-LMS-1 cells. Compared to myometrium, leiomyomas expressed higher levels of miR-20a, miR-21, miR-26a, and miR-206, but expressed lower levels of miR-142-5p from Caucasians, but not in African Americans. African Americans also expressed lower levels of miR-181a in leiomyomas compared to matched myometrium while Caucasians expressed higher levels of miR-181a in leiomyoma tissue versus myometrium.
Comparison of primary cells LSMC and MSMC to T-LSMC and SKLM cell lines demonstrated that both cell lines expressed significantly higher levels of expression of miR-20a and miR-26a compared to both MSMC and LSMC. However, expression of these miRNAs was lower in the LSMC cells compared to that in MSMC. In contrast, miR-21 expression was lower in LSMC, tLSMC, and SKLM compared to MSMC but the cell lines exhibited significantly greater levels of miR-21 expression compared to MSMC. The potential impact of oestradiol and progesterone on the regulation of miR-20a, miR-26a, and miR-21 was evaluated. Oestradiol decreased miR-21 expression in MSMC but not in LSMC. The progesterone analogue, MPA, increased miR-21 expression in LSMC but not in MSMC. When combined, oestradiol and MPA increased miR-26a expression in MSMC but reduced its expression in LSMC. miR-20a expression was influenced by neither oestrogen nor MPA in either MSMC or LSMC cells.
Using uterine leiomyomas, Zavadil and coworkers (2010) profiled and analysed miRNA expression and evaluated the predicted target products of miR-21, miR-23b, miR-27a, miR-30a, and let-7s, which were the most significantly up-regulated, and miR-29b, miR-32, miR-144, miR-197, and miR-212, which were the most significantly down-regulated. Two hundred and forty-nine (249) down-regulated putative mRNA targets were identified which corresponded to the 5 up-regulated miRNAs and 97 up-regulated putative mRNA targets were identified which corresponded with the 5 down-regulated miRNAs. Protein localization and relative level of expression in leiomyoma versus myometrium were assessed for EGFR, ERα, GRIP1, Hamartin, HMGA1, HMGA2, IGF1, IGF2, Ki67, PDECGF, PI3K, PRA, RARα, RXRα, TGFα, and TSC2. Of these proteins, RXR, TFGα, TSC2, and PRA showed higher expression in myometrial tissue with RXR exhibiting the highest expression, while the remaining proteins exhibited greater expression in leiomyoma tissue with HMGA2 exhibiting the highest level of expression. Further, there was an inverse correlation between Ki67 expression and that of let-7. Moderate negative correlations were found between EGFR and miR-194-1 as well as between TGFα and miR-199a-2. Overall, most gene products and their corresponding miRNAs exhibited negative correlations.
In addition to the miRNAs listed above, miR-200a and miR-200b, the mir-15/mir-16 cluster and the let-7 family were also found to be significantly down-regulated in leiomyomas (Zavadil et al. 2010). Evaluation of putative targets of these miRNAs, which are over-expressed in leiomyoma tissue, revealed a number of over-expressed targets that may potentially contribute to the pathogenesis of the disease. Of these, the authors assessed TUBB, CYP1B1, CTBP2, TNPO1, and ATXN1, which are all putative targets of miR-200a, for validation and functionality. miR-200a suppressed transcript expression of TUBB, CYP1B1, and CTBP2 but not that of TNPO1 or ATXN1, which was also associated with reduced cell proliferation of leiomyoma cells and reversed their phenotype from fibroblast-like to that of a more pronounced epithelial phenotype. Further, let-7c was found to repress transcript expression of PPP1R12B, STARD13, TRIB1, BTG2, HMGA2, and ITGB3, but the impact on cell function or phenotype was not assessed.
Of the miRNAs studied in leiomyomas, miR-21 is one of the more studied yet its role in the pathogenesis of the disease is still largely unknown. Fitzgerald and colleagues (2012) recently examined the association between miR-21 and one of its targets, programmed cell death 4 (PDCD4) in leiomyomas and myometrial tissue. Consistent with the previously reported observations, leiomyoma tissue exhibited significantly higher levels of miR-21 expression compared to normal myometrium. However, the increased expression of miR-21 was associated with elevated (not reduced) levels of PDCD4 protein expression, which is in contrast to previous observations for miR-21 and PDCD4 in HeLa cells (Yao et al. 2009). Transfection of either immortalized myometrial or leiomyoma cells with blocking oligonucleotides to miR-21 resulted in increased PDCD4 protein expression. The authors suggested that these contrasting results indicate that PDCD4 may be regulated by a complex mechanism in which miR-21 may play a minimal role.
In addition to miR-21, the miR-200 family has also gained considerable interest in the pathogenesis of leiomyomas. miR-200c was recently demonstrated to be down-regulated in leiomyomas compared to myometrial tissue (Chuang et al. 2012). Using isolated cells from leiomyoma (LYO) and myometrial tissue (MYO) as well as the leiomyomasacrcoma cell line, SKLM-S1, it was demonstrated that miR-200c transfection (gain-of-function) repressed protein expression of ZEB1 and ZEB2 mRNA and protein, but increased E-cadherin (CDH1) transcript and protein expression (Fig. 9.2). Also associated with miR-200c gain-of-function was a change in cell morphology from elongated to round shape in both MYO and LYO (less so in SKLM-S1) and a reduction in cell viability and proliferation. Using luciferase reporter constructs, Chuang and colleagues (2012) also determined that miR-200c is capable of binding to the 3′ UTR seed sequence of tissue inhibitor of metalloproteinase 2 (TIMP2), fibulin 5 (FBLN5), and vascular endothelial growth factor A (VEGFA). Further, gain of miR-200c function studies indicated that miR-200c repressed TIMP2, FBLN5, and VEGFA mRNA and protein levels (with only SKLM-S1 cells exhibiting repressed VEGFA mRNA; Fig. 9.2). The authors concluded from this series of studies that miR-200c, via regulation of ZEBs, VEGFA, FBLN5, and TIMP2 expression, may contribute to leiomyoma growth and maintenance of their cellular characteristics (Fig. 9.2).
Myometrial miRNAs (miR-200 family) appear to be necessary for normal pregnancy and labour where they regulate uterine contractility (Fig. 9.2). Outside of this report, the bulk of our information on myometrial miRNAs is derived from studies that have focused on differential profiles between myometrium and leiomyomas (fibroids). Along these lines, current information is primarily limited to assessment of cell growth and proliferation as well as steroidal regulation of myometrial/leiomyoma miRNAs. As myometrial function is essential to parturition, additional studies are required to enhance our understanding on the regulation and function of miRNAs within this muscle layer. This analysis should also expand into myometrial dysfunction not only associated with the pathogenesis of uterine leiomyomas but also abnormal myometrial function/contraction during abnormal labour/birth.
9.5 Summary and Conclusions
In summary, the majority of research that has evaluated the role of non-coding RNAs in uterine development and function has been concentrated in the area of short non-coding RNAs, predominately miRNAs. Within the context of the uterus, miRNA profiles have been generated for human and rodent myometrial and uterine/endometrial tissue. Steroidal regulation of some of these miRNAs has been examined in isolated stromal and glandular epithelial systems. Emerging data from studies which have incorporated human endometrial adenocarcinoma cell lines has shed some insight into the potential function of these miRNAs within the cells of the endometrium and the pathways by which they may do so. The majority of the current information suggests that myometrial miRNAs appear to mediate myometrial contractility during pregnancy and labour, while uterine/endometrial miRNAs appear to regulate cellular proliferation, migration and/or invasion especially in the context of the case of endometriosis. The members of the miR-200 family appear to be major players in these processes in both myometrium and uterus/endometrium. Research on miRNA regulation and function within the endometrium and myometrium is occurring at a rapid rate. One can fully anticipate that future study will increase focus on dissecting the mechanisms which contribute to the expression/mis-expression of these miRNAs in endometrial and myometrial tissue/cells as well as further defining their specific functions and the mediators by which they modulate these cellular events. Forthcoming information and application of novel approaches will allow for an expansion of our knowledge on which specific miRNAs play a role in the normal and abnormal events within these tissues.
Acknowledgments
Gratitude is expressed to Mr Stanton Fernald for graphic design. A portion of the original work performed by the author and cited in this chapter was funded by grants HD069043 and HD056387 from the Eunice Kennedy Shriver Institute of Child Health and Development (NICHD/NIH) to WBN.
References
- Abe W, Nasu K, Nakada C, Kawano Y, Moriyama M, Narahara H. miR-196b targets c-myc and Bcl-2 expression, inhibits proliferation and induces apoptosis in endometriotic stromal cells. Hum Reprod. 2013;28:750–761. doi: 10.1093/humrep/des446. [DOI] [PubMed] [Google Scholar]
- Adammek M, Greve B, Kässens N, Schneider C, Brüggemann K, Schüring AN, Starzinski-Powitz A, Kiesel L, Götte M. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil Steril. 2013;99:1346–1355. doi: 10.1016/j.fertnstert.2012.11.055. [DOI] [PubMed] [Google Scholar]
- Altmäe S, Martinez-Conejero JA, Esteban FJ, Ruiz-Alonso M, Stavreus-Evers A, Horcajadas JA, Salumets A. MicroRNAs miR30b, miR-30d and miR-494 regulate human endometrial receptivity. Reprod Sci. 2013;20:308–317. doi: 10.1177/1933719112453507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno K, Yanokura M, Kisu I, Yamagami W, Susumu N, Aoki D. MicroRNAs in endometrial cancer. Int J Clin Oncol. 2013;18:186–192. doi: 10.1007/s10147-013-0526-9. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15:625–631. doi: 10.1093/molehr/gap068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 2007;104:15144–15149. doi: 10.1073/pnas.0705917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HH, Davis AJ, Lee TL, Pang AL, Nagrani S, Rennert OM, Chan WY. Methylation of an intronic region regulates miR-199a in testicular tumor malignancy. Oncogene. 2011;30:3404–3415. doi: 10.1038/onc.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TD, Panda H, Luo X, Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocrinol Relat Cancer. 2012;19:541–556. doi: 10.1530/ERC-12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung TK, Lau TS, Cheung TH, Yim SF, Lo KW, Siu NS, Chan LK, Yu MY, Kwong J, Doran G, Barroilhet LM, Ng AS, Wong RR, Wang VW, Mok SC, Smith DI, Berkowitz RS, Wong YF. Dysregulation of microRNAs-204 mediates migration and invasion of endometrial cancer by regulating FOXC1. Int J Cancer. 2012;130:1036–1045. doi: 10.1002/ijc.26060. [DOI] [PubMed] [Google Scholar]
- Dai L, Gu L, Di W. MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKβ/NF-κB pathway and reduced interleukin-8 expression. Mol Hum Reprod. 2012;18:136–145. doi: 10.1093/molehr/gar066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M. MicroRNAs and cancer: towards a personalized medicine. Curr Mol Med. 2013;13:751–756. doi: 10.2174/1566524011313050006. [DOI] [PubMed] [Google Scholar]
- Feng R, Chen X, Yu Y, Su L, Yu B, Li J, Cai Q, Yan M, Liu B, Zhu Z. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298:50–63. doi: 10.1016/j.canlet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol. 2010;2010:369549. doi: 10.1155/2010/369549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JB, Chennathukuzhi V, Koohestani F, Nowak RA, Christenson LK. Role of microRNA-21 and programmed cell death 4 in the pathogenesis of human uterine leiomyomas. Fertil Steril. 2012;98:726–734. doi: 10.1016/j.fertnstert.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Estelles J, Braza-Boils A, Ramon LA, Zorio E, Medina P, Espana F, Estelles A. Role of microR-NAs in gynecological pathology. Curr Med Chem. 2012;19:2406–2413. doi: 10.2174/092986712800269362. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Behringer RR. Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev. 2009;76:678–688. doi: 10.1002/mrd.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, Dina RE, Ishihara O, Takeda S, Lam EW, Bamberger AM, Ghaem-Maghami S, Brosens JJ. Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene. 2008;27:9–19. doi: 10.1038/sj.onc.1210626. [DOI] [PubMed] [Google Scholar]
- Götte M, Mohr C, Koo CY, Stock C, Vaske AK, Viola M, Ibrahim SA, Peddibhotla S, Teng YH, Low JY, Ebnet K, Kiesel L, Yip GW. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29:6569–6580. doi: 10.1038/onc.2010.386. [DOI] [PubMed] [Google Scholar]
- Guo C, Sah JF, Beard L, Wilson JK, Markowitz SD, Guda K. The non-coding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Gene Chromosome Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25:821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology. 2008;149:6207–6212. doi: 10.1210/en.2008-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su RW, Ma XH, Ni H, Lei W, Yang ZM. MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem. 2008;283:23473–23484. doi: 10.1074/jbc.M800406200. [DOI] [PubMed] [Google Scholar]
- Jiang F, Liu T, He Y, Yan Q, Chen X, Wang H, Wan X. MiR-125b promotes proliferation and migration of type II endometrial carcinoma cells through targeting TP53INP1 tumor suppressor in vitro and in vivo. BMC Cancer. 2011;11:425. doi: 10.1186/1471-2407-11-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod. 2010;82:791–801. doi: 10.1095/biolreprod.109.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Park YA, Choi JJ, Lee YY, Kim CJ, Choi C, Kim TJ, Lee NW, Kim BG, Bae DS. The expression of the miRNA-200 family in endometrial endometrioid carcinoma. Gynecol Oncol. 2011;120:56–62. doi: 10.1016/j.ygyno.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Lessey BA. Fine tuning of endometrial function by estrogen and progesterone through microRNAs. Biol Reprod. 2010;82:653–655. doi: 10.1095/biolreprod.110.083667. [DOI] [PubMed] [Google Scholar]
- Liu S, Gao S, Wang XY, Wang DB. Expression of miR-126 and Crk in endometriosis: miR-126 may affect the progression of endometriosis by regulating Crk expression. Arch Gynecol Obstet. 2012;285:1065–1072. doi: 10.1007/s00404-011-2112-6. [DOI] [PubMed] [Google Scholar]
- Marsh EE, Lin Z, Yin P, Milad M, Chakravanti D, Bulun SE. Differential expression of microRNAs species in human uterine leiomyoma versus normal myometrium. Fertil Steril. 2008;89:1771–1776. doi: 10.1016/j.fertnstert.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- Myatt SS, Wang J, Monteiro LJ, Chrisitan M, Ho KK, Fusi L, Dinea RE, Brosens JJ, Ghaem-Maghami S, Lam EW. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res. 2010;70:367–377. doi: 10.1158/0008-5472.CAN-09-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen RH, Han DY, Zhu HF, Agno JE, Gunaratne PH, DeMayo FJ, Matzuk MM. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–2352. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnick WB. Regulation of uterine matrix metalloproteinase-9 and the role of microRNAs. Semin Reprod Med. 2008;26:494–499. doi: 10.1055/s-0028-1096129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnick WB, Healy C. Estrogen induces distinct patterns of microRNA expression within the mouse uterus. Reprod Sci. 2010;17:987–994. doi: 10.1177/1933719110377472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23:265–275. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- Pan Q, Luo X, Chegini N. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med. 2008;12:227–240. doi: 10.1111/j.1582-4934.2007.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Park YA, Lee JW, Choi JJ, Jeon HK, Cho Y, Choi C, Kim TJ, Lee NW, Kim BG, Bae DS. The interactions between MicroRNA-200c and BRD7 in endometrial carcinoma. Gynecol Oncol. 2012;124:125–133. doi: 10.1016/j.ygyno.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Petracco R, Grechukhina O, Popkhadze S, Massasa E, Zhou Y, Taylor HS. MicroRNA 135 regulates HOXA10 expression in endometriosis. J Clin Endocrinol Metab. 2011;96:E1925–E1933. doi: 10.1210/jc.2011-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Song Y, Peng Y, Wang H, Long H, Yu X, Li Z, Fang L, Wu A, Luo W, Zhen Y, Zhou Y, Chen Y, Mai C, Liu Z, Fang W. ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion and apoptosis in glioma. PLoS ONE. 2012;7:e38842. doi: 10.1371/journal.pone.0038842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón LA, Braza-Boïls A, Gilabert-Estellés J, Gilabert J, España F, Chirivella M, Estellés A. microRNA expression in endometriosis and their relationship to angiogenic factors. Hum Reprod. 2011;26:1082–1090. doi: 10.1093/humrep/der025. [DOI] [PubMed] [Google Scholar]
- Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci U S A. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha A-G, Liu J-L, Jiang X-M, Ren J-Z, Ma C-H, Lei W, Su R-W, Yang Z-M. Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertil Steril. 2011;96:150–155. doi: 10.1016/j.fertnstert.2011.04.072. [DOI] [PubMed] [Google Scholar]
- Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G, Beckebaum S. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Yang S, Huang W, Xu W, Wang Q, Song Y, Liu Y. MicroRNA23a and microRNA23b deregulation derepresses SF-1 and upregulates estrogen signaling in ovarian endometriosis. J Clin Endocrinol Metab. 2013;98:1575–1582. doi: 10.1210/jc.2012-3010. [DOI] [PubMed] [Google Scholar]
- Snowdon J, Zhang X, Childs T, Tron VA, Feilotter H. The microRNAs-200 family is upregulated in endometrial carcinoma. PLoS ONE. 2011;6:e22828. doi: 10.1371/journal.pone.0022828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CL. The role of leukemia inhibitory factor (LIF) and other cytokines in regulating implantation in mammals. Ann N Y Acad Sci. 1994;734:157–165. doi: 10.1111/j.1749-6632.1994.tb21743.x. [DOI] [PubMed] [Google Scholar]
- Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101:1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–1331. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- Toloubeydokhti T, Pan Q, Luo X, Bukulmez O, Chegini N. The expression and ovarian steroid regulation of endometrial micro-RNAs. Reprod Sci. 2008;15:993–1001. doi: 10.1177/1933719108324132. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei J-J. A micro-RNA signature associated with race, tumor size and target gene activity in human uterine leiomyomas. Gene Chromosome Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci U S A. 2012a;109:7529–7534. doi: 10.1073/pnas.1200650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Renthal NE, Gerard RD, Mendelson CR. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol. 2012b;26:1857–1867. doi: 10.1210/me.2012-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia HF, Jin XH, Song PP, Cui Y, Liu CM, Ma X. Temporal and spatial regulation of let-7a in the uterus during embryo implantation in the rat. J Reprod Dev. 2010a;56:73–78. doi: 10.1262/jrd.09-088k. [DOI] [PubMed] [Google Scholar]
- Xia HF, Jin XH, Song PP, Cui Y, Liu CM, Ma X. Temporal and spatial regulation of miR-320 in the uterus during embryo implantation in the rat. Int J Mol Sci. 2010b;11:719–730. doi: 10.3390/ijms11020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun. 2009;388:539–542. doi: 10.1016/j.bbrc.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Yoon S, Choi YC, Lee S, Jeong Y, Yoon J, Baek K. Induction of growth arrest by miR-542-3p that targets survivin. FEBS Lett. 2010;584:4048–4052. doi: 10.1016/j.febslet.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Ye H, Liu Z, Wu J, Lee P, Hernando E, Soteropoulos P, Toruner GA, Wei JJ. Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PLoS ONE. 2010;5:e12362. doi: 10.1371/journal.pone.0012362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, He Y, Zhang X, Xing B, Sheng Y, Lu H, Wei Z. Estrogen receptor-regulated microRNAs contribute to the BCL2/BAX imbalance in endometrial adenocarcinoma and precancerous lesions. Cancer Lett. 2012;314:155–165. doi: 10.1016/j.canlet.2011.09.027. [DOI] [PubMed] [Google Scholar]


