Abstract
This study demonstrates body mass in middle and late adulthood as a consequence of the complex interplay among individuals’ genes, lifetime socioeconomic experiences, and the historical context in which they live. Drawing on approximately 9,000 genetic samples from the Health and Retirement Study, we first investigate how socioeconomic status (SES) over the life course moderates the impact of 32 established obesity-related genetic variants on body mass index (BMI) in middle and late adulthood. Further, we consider differences across birth cohorts in the genetic influence on BMI and cohort variations in the moderating effects of life-course SES on the genetic influence. Our analyses suggest that persistently low SES over the life course or downward mobility (e.g., high SES in childhood but low SES in adulthood) amplified the genetic influence on BMI, while persistently high SES or upward mobility (e.g., low SES in childhood but high SES in adulthood) compensated for such influence. For more recent birth cohorts, while the genetic influence on BMI became stronger, the moderating effects of lifetime SES on the genetic influence were weaker compared to earlier cohorts. We discuss these findings in light of social changes during the obesity epidemic in the United States.
Keywords: obesity, lifetime socioeconomic status, cohort, gene-environment interaction
INTRODUCTION
Currently in the United States, more than two-thirds of adults are overweight or obese (Flegal et al. 2012). This figure is alarming given that obesity is associated with numerous health problems such as diabetes, asthma, and high blood pressure (Mokdad et al. 2003). Obesity is a complex trait affected by genetic factors, socioeconomic status (SES), and historical context. In recent years, one important breakthrough in genomics is the discovery of specific genetic variants associated with obesity-related traits (Frayling et al. 2007; Loos et al. 2008; Meyre et al. 2009; Monda et al. 2013; Okada et al. 2012; Speliotes et al. 2010; Wen et al. 2012). These genetic variants, involved in various biological pathways such as energy balance and metabolism, play important roles in the development of obesity. At the societal level, socioeconomic factors have long been attributed as “fundamental causes” of health and mortality (Link and Phelan 1995). Research has consistently shown a relationship between low SES and poor health outcomes (Braveman et al. 2010; Kanjilal et al. 2006; Kennedy et al. 1998; Minkler et al. 2006; Thurston et al. 2005). In developed countries such as the United States, low SES is well documented to be associated with overweight and obesity (McLaren 2007; Sobal and Stunkard 1989). Moreover, recent decades have witnessed advances in food manufacturing and marketing practices, and growing cultural and technological adaption. These changes also contribute to increasing obesity in the United States (Keith et al. 2006; Reither et al. 2009).
This study seeks to tie up the three lines of inquiry, namely, genetic inheritance, SES, and socio-historical contexts, to advance our understanding of obesity. As shown by gene-environment interaction (G × E) studies (Boardman et al. 2014; Demerath et al. 2013; Rokholm et al. 2011), genetic, socioeconomic, and historical factors do not act independently, but interactively, to affect obesity-related traits. Extant G×E studies, however, have typically focused on socioeconomic factors measured at one time point, and paid less attention to transitions and trajectories of one’s socioeconomic status (SES) and changes in the historical context in which one lives. These life-course dynamics, which often provide opportunities for behavioral change (Elder 1985; Elder et al. 2003; Ryder 1965), can be critical in shaping the relationship between genotypes and phenotypes. This calls for an integration of genetic research and life-course sociology in the investigation of obesity.
There are three specific aims of this study. First, we examine how SES over the life course moderates the genetic influence on body mass index (BMI) in middle and late adulthood. Second, we consider differences across birth cohorts in the genetic influence based on the proposition that cohort differences reflect changes in the socio-historical context in which individual lives unfold. Third, we investigate cohort variations in the moderating effects of life-course SES on the genetic influence. To achieve these aims, we take advantage of the accelerated multi-cohort longitudinal design of the Health and Retirement Study (HRS), the large-scale genetic sample in HRS (N = 8816), and the recently established 32 obesity-related genetic variants in genomic studies.
CONCEPTUAL FRAMEWORK AND RESEARCH HYPOTHESES
Gene-Environment Interaction Models
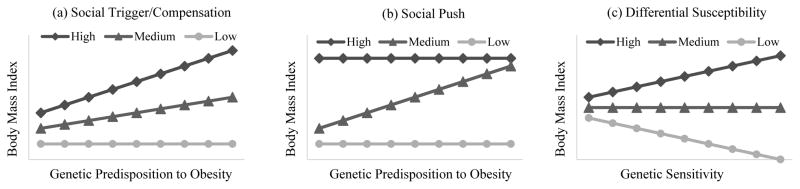
Genetic factors are influential in determining obesity-related traits, but their impact is, to a great extent, conditioned by an individual’s health behavior and the social context. Within the G×E paradigm, three different conceptual models have provided important explanations of how behavioral and contextual factors moderate genetic effects: social trigger/compensation, social push, and differential susceptibility.
The social trigger/compensation model includes two components and is graphically illustrated in Panel (a) of Figure 1. First, the social trigger component, also referred to as the diathesis stress model, emphasizes the harmful influences of adverse conditions (Ellis et al. 2011). Accordingly, unhealthy behaviors or environments may trigger or magnify effects of the risk alleles (Ravussin and Bouchard 2000). An example can be found in the study of Sonestedt et al. (2009), where the relationship between fat mass and the FTO (i.e., fat mass and obesity-associated protein) gene was observed to be stronger among those who reported a high-fat diet than those who reported a low-fat diet. Second, the compensation component underscores the protection of favorable conditions against genetic risk. As demonstrated by Kilpeläinen et al. (2011), higher degrees of physical activity were linked to a significant reduction in genetic risk of obesity. The compensation could be stronger such that genetic effects expressing under “normal” conditions do not manifest themselves under enriched behavioral or environmental conditions (i.e., strong compensation) (Shanahan and Boardman 2009). Rampersaud et al. (2008) found risk alleles of two polymorphisms on the FTO gene were strongly associated with increased BMI. Such associations were, nonetheless, completely attenuated among physically active individuals.
Figure 1.
Conceptual Gene-Environment Interaction Models
Note: Lines with circles represent genetic association with BMI in lowest level of environmental risk compared to medium (triangles) and high (diamonds) risk. The magnitude of the genetic association is indicated by the slope. A steeper slope means greater genetic association.
In contrast to the social trigger/compensation model, the social push model does not emphasize direct environmental effects on the genetic contribution. Rather, this model proposes that the genetic contribution can be camouflaged under strong environmental influences (Raine 2002). For example, individuals who keep a healthy diet and do regular physical exercises are less likely to become obese in spite of their genetic propensities. Also, if people live in an obesogenic environment and often eat unhealthy foods such as fatty fast foods or sugary desserts, they may gain weight regardless of whether they possess obesity-related genotypes or not. Under both circumstances, the environmental influences are the dominant causes and there is little room for genes to act. The genetic influences, therefore, are expected to be most salient among individuals exposed to medium levels of environmental risk. In a G×E study using twin data, the heritability of BMI was shown to be the highest for students attending schools with moderate body-size norms, whereas the genetic contribution is markedly less prominent for those in schools with stronger or weaker body-size norms (Boardman et al. 2012).
The differential susceptibility model has increasingly drawn attention in recent years (Belsky et al. 2007; Belsky and Pluess 2009). Accordingly, individuals with certain genotypes are more sensitive to environmental conditions. Compared to others, genetically sensitive individuals may find it easier to gain weight in obesogenic environments, whereas the same individuals are more likely to lose weight if they stay on a diet and keep physically active. As shown in Panel (c) of Figure 1, the direction of the genetic influence varies under differential conditions. Consequently, a genetic association with the outcome may be undetectable if environmental variations are ignored. There is empirical evidence for this differential susceptibility model that variants in the dopamine D4 receptor (DRD4) and the serotonin transporter gene (5HTTLPR) played protective roles under least-risky conditions, while the same variants produced genetic risks under most adverse social conditions (Daw et al. 2013; Simons et al. 2011; Mitchell et al. 2011).
Socioeconomic Status, Genes, and Obesity
In the present study, we use SES as our indicator of social environment. In contrast to previous G×E studies that mainly focused on socioeconomic factors measured at one time point (Boardman et al. 2014; Boardman et al. 2012; Guo et al. 2007), we consider the dynamics of individuals’ socioeconomic experiences over the life course. In the following we describe three distinct, but related, life-course perspectives: sensitive period, social accumulation, and social mobility. We then link the three perspectives and the G×E models to develop hypotheses on how timing, accumulation (i.e., duration), and stability of socioeconomic exposures moderate the genetic influence on obesity-related traits in middle and late adulthood.
The sensitive period perspective predicts that certain periods over the life course have stronger effects on later outcomes than other periods. A large number of studies have shown that one’s SES during childhood and adolescence is associated with obesity-related traits in adulthood (Pollitt et al. 2005; Senese et al. 2009). There are three major explanations for such an association. The first explanation focuses on affordability and availability of resources. Compared to energy-dense, less nutritious foods, healthy and nutrient-dense foods (e.g., fruits and vegetables) are typically more expensive, thus less affordable for low-SES families (Darmon and Drewnowski 2008; Neumark-Sztainer et al. 2003). Moreover, low-SES families are more likely than high-SES families to be located in poor communities with limited access to public exercise facilities. Also, because these communities are often viewed as unsafe, children’s physical activities outdoors are restricted by their parents (Lumeng et al. 2006). Lack of nutritious food and physical activity during early life stages could put individuals at higher risk of overweight or obesity throughout the life course. Secondly, children from low-SES families typically suffer more from family risks (e.g., marital instability and conflict), and consequently have greater difficulties with emotion regulation and social competence (Repetti et al. 2002; Troxel and Matthews 2004). Poor emotion regulation during childhood may result in higher degrees of anxiety, depression, eating disorders, and an inability to form and maintain strong relationships and to secure social support—all of which could raise the risk of obesity in later life (Alvarez et al. 2007; Anderson et al. 2006; Herzer et al. 2011). Thirdly, the relationship between childhood SES and adult obesity is also influenced by social norms on body weight and attitudes toward obesity (Power and Parsons 2000). Researchers have found that a desire to lose weight, which starts in adolescence or even earlier, is more common among women with high SES than those with low SES (Dornbusch et al. 2001; Jeffery and French 1996; Jeffery et al. 1991).
In contrast to the sensitive period perspective that stresses the importance of the timing of exposure, the social accumulation perspective emphasizes the overall amount or duration of SES-related exposures. It states that socioeconomic (dis)advantages over the life course accumulate to affect health outcomes. There is growing research considering cumulative (dis)advantage (i.e., duration) as a mechanism that produces health problems in adulthood. Based on multiple indicators of SES in childhood and early adulthood, a study of a British postwar cohort reports that the mortality for individuals with persistently low SES from childhood to early adulthood was three to five times higher than for those with persistently high SES (Kuh et al. 2002). Similar cumulative effects of low SES are found in a study of adults from Alameda County in the United States (Lynch et al. 1997). Padyab and Norberg (2014) examined SES disadvantage in three phases of life. They showed that higher levels of cumulative SES disadvantage were associated with higher BMI among women in late adulthood.
The social mobility perspective proposes that the direction of SES mobility over the life course has important implications for health outcomes at later stages. Upward mobility, an increase in SES after childhood, may lead to better health in later life (Cohen et al. 2010). In other words, the adverse effects of low SES earlier in life could be partially or fully remedied by higher SES at a later time. In contrast, downward mobility, a decline from higher to lower SES, may lead to poorer health, even for people with high SES in childhood. Heraclides and Brunner (2010) observed that participants experiencing downward mobility had a higher risk of overweight and obesity than those with stable and high SES throughout the life course. A later study based on a sample of Southeast Asians provided evidence that women experiencing upward social mobility had lower odds of obesity relative to those experiencing low SES throughout the life course (Malhotra et al. 2013).
Given the relationship between SES and various proximate factors (e.g., diet, physical activity, etc.) that modify the genetic contribution to obesity, we expect that genetic influences on obesity-related traits are socioeconomically moderated (H1). According to the social trigger/compensation perspective, genetic risk is likely to be triggered by exposure to low SES, but to be compensated for by exposure to high SES. The genetic influence on body mass in middle and late adulthood therefore is hypothesized to be greater for individuals who experienced lower levels of childhood SES, less cumulative socioeconomic advantage, or downward mobility over the life course than for those who experienced higher levels of childhood SES, more cumulative socioeconomic advantage, or upward mobility. From the social push perspective, the genetic contribution may be masked by environmental influences among most socioeconomically (dis)advantaged individuals. Accordingly, the genetic influence on body mass in middle and late adulthood is predicted to be more salient among individuals experiencing medium levels of childhood SES, cumulative socioeconomic (dis)advantage, or SES mobility than those experiencing highest/lowest levels of childhood SES, most/least cumulative socioeconomic advantage, or persistently high/low SES over the life course. Finally, from the differential susceptibility perspective, some individuals are genetically more sensitive than others to either high or low SES. These genetically sensitive individuals might have higher levels of body mass (i.e., higher risk of overweight or obesity) in middle or late adulthood than others if they experienced low SES over the life course, whereas such individuals are likely to have lower levels of body mass (i.e., lower risk of overweight or obesity) if they experienced high SES over the life course.
Cohort Effects as an Indicator of Historical Change
In this study, we use cohort effects as an indicator of historical change to examine whether the genetic influence on body mass in middle and late adulthood and the moderating effects of SES on the genetic influence are contingent upon historical context. A cohort is a group of individuals who experience same historical and social events at the same ages (Ryder 1965). Different cohorts may have diverse life experiences inasmuch as they encounter historical and social changes at various stages of their lives. In his classic work on the children of the Great Depression, Elder ([1974] 1999) demonstrated that the impact of early-life socioeconomic hardship on physical and emotional health varied by social class for the Oakland cohort (born in the early 1920s), namely that the most disadvantaged suffered the most adverse health consequences during adulthood. Importantly, when compared with a younger cohort (born in the late 1920s) who were more afflicted by the Great Depression, the Oakland males were better off in later life, possibly due to benefits of earlier entry into military service (Elder 1986). There is a growing interest in cohort effects on the genetic contribution to health outcomes. The genetic influence on smoking, for example, has been found to differ before and after the implementation of legislation on smoking behaviors (Boardman et al. 2010; Boardman et al. 2011; Kendler et al. 2000).
The cohort consideration has been extended to the study of obesity-related traits. Using cross-sectional data from southwestern Ohio, Demerath et al. (2013) examined birth-year variation in the associations between a genetic risk score based on obesity-related genetic variants and phenotypes such as BMI, waist circumference and skinfold thickness. They showed that the genetic association with BMI for males born in 1970, compared to those born in 1930, was three times as great. Such an increase in the genetic influence on BMI was attributed to the obesity epidemic. According to national surveys, the prevalence of obesity in the United States has risen 2.5-fold since the late 1970s (Flegal et al. 2012; Flegal et al. 1998; Flegal et al. 2010). Various factors have been claimed to be responsible for this obesity epidemic, such as advances in specific food manufacturing and marketing practices (e.g., the increasing availability of fast food and vending machines in public places), increased cultural and technological adaption, reduced physical activity, etc.(Keith et al. 2006). In this study, we use a nationally representative, longitudinal sample to revisit the cohort-effect hypothesis, namely that the genetic influence on body mass is greater among recent cohorts than earlier ones (H2).
Whereas the prevalence of obesity has increased in all SES groups since the 1970s, SES disparities of obesity have decreased for both male and female adults in the United States (Wang and Beydoun 2007). Specifically, the low-to-high SES ratio (i.e., prevalence in low SES group/prevalence in high-SES group) has reduced from 1.6 in the early 1970s to 1.1 in late 1990s for males, and from 3.4 to 1.3 for females. The high-SES group experienced the highest rate of increase in the prevalence of obesity (Zhang and Wang 2004). Therefore, we hypothesize that the cohort differences in the genetic influence on body mass are greater for more socioeconomically advantaged groups. In other words, the moderating effects of SES on the genetic influence are weaker in more recent cohorts than in earlier cohorts (H3). We test this hypothesis from each of the three life-course perspectives (i.e., sensitive period, social accumulation and social mobility).
It should be noted that the relationships among genes, SES, and body mass can be complicated by race or ethnicity. Importantly, the 32 obesity-related genetic variants used in this study were discovered in genome-wide association studies (GWAS) using white samples (Speliotes et al. 2010). It is uncertain whether these discoveries are replicable in other racial/ethnic groups (Belsky et al. 2013; Domingue et al. 2014). We did sensitivity analysis by combining the white and black samples. The main results are similar to those based on only white samples. This is likely due to the fact that whites comprise the majority of the sample. The small size of minority samples does not afford sufficient statistical power for separate G×E analysis. Moreover, our preliminary analysis shows higher SES at different life stages is associated with lower BMI among whites, whereas such a pattern does not exist among blacks. This is consistent with findings in previous studies of SES disparities in obesity-related traits (Braveman et al. 2010; Wang and Beydoun 2007; Zhang and Wang 2004). For these reasons, we focus on non-Hispanic whites in this study.
DATA AND MEASUREMENT
Data
Data for this study come from the Health and Retirement Study (HRS). HRS is a longitudinal study of Americans over age 50 conducted every two years from 1992 to 2012; it collects information on economic, health, social, and other factors relevant to aging and retirement. DNA samples were collected in 2006 and 2008. Of the collected samples, 13,129 were put into genotyping production using the Illumina Human Omni-2.5 Quad beadchip, and 12,507 passed the University of Washington Genetics Coordinating Center’s (GCC) standardized quality control processes. Among these samples, 8,816 are non-Hispanic whites and they compose our analytic sample.
Variable Measurement
Outcome Variable
The outcome variable in this study is BMI (weight [kg]/height[m]2). Respondents were asked to report their height at least one time (e.g., at entry into the study) and to report their weight at each wave. Based on height and weight information, we calculated BMI for all respondents at each wave.
Cohort Measure
Cohort is based on respondents’ birth year. HRS includes six birth cohorts with different entry years: the Study of Assets and Health Dynamics Among the Oldest Old (AHEAD) cohort (born before 1924) surveyed in 1993, 1995, and 1998–2012; Children of Depression (CODA) cohort (born 1924–1930) surveyed in 1998–2012; HRS cohort (born 1931–1941) surveyed from 1992–2012; War Baby (WB) cohort (born 1942–1947) surveyed in 1998–2012; Early Baby Boomers (EBB) cohort (born 1948–1953) surveyed in 2004–2012; and Mid Baby Boomers (MBB) cohort (born 1954–60) surveyed in 2010 and 2012. When the present study is conducted, genetic data are available for AHEAD, CODA, HRS, WB, and EBB cohorts. Importantly, unlike a cross-sectional study in which all measurements are taken at the same time, respondents of each cohort in HRS were repeatedly examined at different ages, allowing for cohort and age effects to be disentangled.
SES Measures
SES is multidimensional (i.e., it can be measured by educational attainment, occupational status, income, wealth, etc.), and the roles of different dimensions of SES vary over the life course. Specifically, one’s SES in childhood primarily depends on his/her parents’ SES. The transition from parental SES to one’s own SES is mainly achieved through education. In middle/late adulthood, the role of wealth becomes increasingly important. Accordingly, we used three life-course SES measures respectively for SES in childhood, young adulthood, and middle/late adulthood. Childhood SES is measured by father’s occupation (“What was your father’s occupation when you were 16?”). Young-adulthood SES is based on years of education (“What is the highest grade of school or year of college you completed?”). Middle/late-adulthood SES is based on household wealth (sum of all types of assets, pensions, etc.).1 Imputed income and wealth are both available in HRS. Wealth was chosen over income as research shows the former is a more accurate measure of SES among older adults (Allin et al. 2009).
The same SES measure may have somewhat different meaning for different cohorts. For example, it is likely that a high school degree indicated high SES for individuals in earlier cohorts, but medium/low SES for those in later cohorts.2 This issue was addressed by within-cohort standardization. Specifically, we recoded the SES measures into relative indicators based on a baseline sample. The baseline sample includes onset measures for all respondents (either provided DNA or did not).3 These measures were taken in 1992 for HRS, 1993 for AHEAD, 1998 for CODA and WB, and 2004 for EBB. We trichotomized respondents into low, medium, and high SES categories on the basis of the first and second tertiles of each of the three SES measures4 within each birth cohort in the baseline sample.
To test the moderating effects of cumulative socioeconomic (dis)advantage on the genetic influence on BMI, we constructed a cumulative socioeconomic advantage score (CAS) (Hallqvist et al. 2004; Heraclides and Brunner 2010; Loucks et al. 2009; Loucks et al. 2010; Luo and Waite 2005; Otero-Rodríguez et al. 2011). Each of the three life-course SES indicators was assigned a value of “1” for high SES and “0” for medium or low SES and then summed to form a total score, with possible values of 0, 1, 2, and 3. A higher value on this score indicates greater cumulative socioeconomic advantage.
To test the moderating effects of SES trajectories on the genetic influence, we defined eight mutually exclusive and exhaustive SES mobility trajectories based on respondents’ SES at three time points (childhood to early adulthood to middle/late adulthood): (1) low/medium childhood SES, low/medium young-adulthood SES, and low/medium middle/late -adulthood SES (LLL); (2) low/medium childhood SES, low/medium young-adulthood SES, and high middle/late-adulthood SES (LLH); (3) low/medium childhood SES, high young-adulthood SES, and low/medium middle/late-adulthood SES (LHL); (4) low/medium childhood SES, high young-adulthood SES, and high middle/late-adulthood SES (LHH); (5) high childhood SES, low/medium young-adulthood SES, and low/medium middle/late-adulthood SES (HLL); (6) high childhood SES, low/medium young-adulthood SES, and high middle/late-adulthood SES (HLH); (7) high childhood SES, high young-adulthood SES, and low/medium middle/late-adulthood SES (HHL); (8) high childhood SES, high young-adulthood SES, and high middle/late-adulthood SES (HHH) (Beckett 2000; Hallqvist et al. 2004; Heraclides and Brunner 2010; James et al. 2006; Loucks et al. 2010; Luo and Waite 2005; Otero-Rodríguez et al. 2011). In preliminary analyses, we tested for different specifications of the SES trajectories and conducted sensitivity tests. The results are not sensitive to the specification of young adulthood SES. To simplify the interpretation, we combined LLL and LHL into “stable and low,” HHH and HLH into “stable and high,” LLH and LHH into “upwardly mobile,” and HLL and HHL into “downwardly mobile.”
Genetic Measures
In this study, we focused on 32 BMI-related single-nucleotide polymorphisms (SNPs). The association between each of the SNPs and BMI has been repeatedly validated using independent samples of European ancestry (Speliotes et al. 2010). Sixteen of the 32 SNPs were not included on the HRS-used Illumina Human Omni-2.5 Quad beadchip, and so we instead used genotypes imputed utilizing the phase 1 reference panel from the 1000 Gnomes Project (Howie et al. 2011; Li et al. 2009). Genotype imputation was conducted using the IMPUTE2 software (Howie et al. 2009). The imputation quality R2 values range from .99 to 1.0 (see Appendix A for more details).
Appendix A.
Detailed Information about 32 Established SNPs for BMI
| Chromosome | Nearest Gene | GWAS SNPa | HRS SNP | Imputation Quality R2 | R2 with GWAS SNP | Allele |
GWAS Betac | HRS Beta | Effect Allele Frequencyd | |
|---|---|---|---|---|---|---|---|---|---|---|
| Effectc | Other | |||||||||
| 1 | SEC16B | rs543874 | rs543874 | 1.00 | 1.00 | G | A | .22 | .08 | .20 |
| 1 | TNNI3K | rs1514175 | rs1514175 | 1.00 | 1.00 | A | G | .07 | .09 | .47 |
| 1 | PTBP2 | rs1555543 | rs10489741b | 1.00 | 1.00 | C | A | .06 | .09 | .57 |
| 1 | NEGR1 | rs2815752 | rs2815752 | 1.00 | 1.00 | A | G | .13 | .09 | .62 |
| 2 | LRP1B | rs2890652 | rs2890652b | 1.00 | 1.00 | C | T | .09 | .05 | .19 |
| 2 | FANCL | rs887912 | rs887912b | 1.00 | 1.00 | T | C | .10 | .06 | .24 |
| 2 | RBJ | rs713586 | rs713586b | 1.00 | 1.00 | C | T | .14 | .20 | .52 |
| 2 | TMEM18 | rs2867125 | rs2867125 | 1.00 | 1.00 | C | T | .31 | .27 | .84 |
| 3 | CADM2 | rs13078807 | rs13078807 | 1.00 | 1.00 | G | A | .10 | −.04 | .17 |
| 3 | ETV5 | rs9816226 | rs9816226b | .99 | 1.00 | T | A | .14 | .21 | .82 |
| 4 | SLC39A8 | rs13107325 | rs13107325 | 1.00 | 1.00 | T | C | .19 | .36 | .06 |
| 4 | GNPDA2 | rs10938397 | rs10938397 | 1.00 | 1.00 | G | A | .18 | .10 | .40 |
| 5 | ZNF608 | rs4836133 | rs4836133b | 1.00 | 1.00 | A | C | .07 | .12 | .58 |
| 5 | FLJ35779 | rs2112347 | rs2112347b | 1.00 | 1.00 | T | G | .10 | .16 | .62 |
| 6 | TFAP2B | rs987237 | rs987237 | 1.00 | 1.00 | G | A | .13 | .04 | .18 |
| 6 | NUDT3 | rs206936 | rs206936 | 1.00 | 1.00 | G | A | .06 | .03 | .27 |
| 9 | LRRN6C | rs10968576 | rs10968576 | 1.00 | 1.00 | G | A | .11 | .26 | .28 |
| 11 | MTCH2 | rs3817334 | rs3817334b | 1.00 | 1.00 | T | C | .06 | .05 | .39 |
| 11 | RPL27A | rs4929949 | rs4929949b | 1.00 | 1.00 | C | T | .06 | −.03 | .49 |
| 11 | BDNF | rs10767664 | rs10767664b | 1.00 | 1.00 | A | T | .19 | .15 | .80 |
| 12 | FAIM2 | rs7138803 | rs7138803 | 1.00 | 1.00 | A | G | .12 | .14 | .34 |
| 13 | MTIF3 | rs4771122 | rs9512699b | 1.00 | .87 | G | A | .09 | .22 | .18 |
| 14 | PRKD1 | rs11847697 | rs10134820b | 1.00 | .74 | T | C | .17 | −.03 | .06 |
| 14 | NRXN3 | rs10150332 | rs10150332b | 1.00 | 1.00 | C | T | .13 | .03 | .23 |
| 15 | MAP2K5 | rs2241423 | rs2241423 | 1.00 | 1.00 | G | A | .13 | .21 | .72 |
| 16 | SH2B1 | rs7359397 | rs7359397b | 1.00 | 1.00 | T | C | .15 | .18 | .35 |
| 16 | FTO | rs1558902 | rs1558902b | 1.00 | 1.00 | A | T | .39 | .30 | .35 |
| 16 | GPRC5B | rs12444979 | rs12444979 | 1.00 | 1.00 | C | T | .17 | .25 | .87 |
| 18 | MC4R | rs571312 | rs571312 | 1.00 | 1.00 | A | C | .23 | .29 | .24 |
| 19 | KCTD15 | rs29941 | rs29941b | 1.00 | 1.00 | G | A | .06 | .09 | .70 |
| 19 | TMEM160 | rs3810291 | rs3810291 | 1.00 | 1.00 | A | G | .09 | .13 | .59 |
| 19 | QPCTL | rs2287019 | rs2287019 | 1.00 | 1.00 | C | T | .15 | .22 | .89 |
Note:
rs ids of BMI-related SNPs in Speliotes et al. (2010).
Imputed SNPs
Effect size in kg/m2 of BMI obtained from Speliotes et al. (2010).
Effect allele frequency in HRS.
Control Variables
The relationship between SES and health is complicated and could be bidirectional (Case et al. 2005; Conley and Bennett 2000; Smith 2009). Individuals in poor health may be trapped in low SES. To address reverse causality, we used a baseline health measure based on a survey question “Consider your health while you were growing up, from birth to age 16. Would you say that your health during that time was excellent, very good, good, fair, or poor?” In our preliminary analysis, poor health in childhood significantly predicted low SES in young and middle/late adulthood. We controlled childhood health as a covariate in all regression models including SES as a predictor of BMI. We also conducted a sensitivity analysis by removing individuals who reported that they were in poor health in early life. This is consistent with previous studies in which researchers restricted their sample to individuals who were not in poor health at the baseline (Qi et al. 2012; Willson et al. 2007). The alternative approach produced very similar results.
Age is measured at the time of each survey. In our analytical models, we centered age at the grand median to obtain more interpretable results. Other control variables include gender, region (i.e., in which census area the respondent was born), and rural (i.e., whether the respondent lived in a rural area at about age 10). As we previously mentioned, low SES is typically associated with unhealthy behaviors (e.g., eating an unhealthy diet, less physical exercise, etc.) that raise the risk of overweight or obesity, but smoking is an exception. Research has shown that lower SES is linked to a greater prevalence of smoking (Hiscock et al. 2012), which is known to be associated with lower BMI. Also, our preliminary analysis suggests that low-SES respondents were less likely to drink alcoholic beverages, and a lower frequency of drinking was associated with lower BMI. Therefore, we controlled for smoking (smoker or not), and drinking (ever drank alcoholic beverages or not) as two suppressors when assessing the relationship between SES and BMI. In addition, although we focused on non-Hispanic whites, population stratification within racial groups may confound the G×E results. To account for population stratification, we controlled for largest 10 principle components (PCs) in our G×E analysis (Price et al. 2006). These PCs were constructed on the basis of genome-wide SNPs with pair-wise squared correlation (R2) smaller than 0.2.
ANALYTIC STRATEGY
Genetic Predisposition Score
We first constructed a genetic predisposition score (GPS) by summing up the number of risk alleles across the 32 obesity-related SNPs (Belsky et al. 2013; Domingue et al. 2014). Before summation, each SNP was weighted according to its relative effect size (βcoefficient) on BMI. To have a more precise effect size of the SNPs, β coefficients were obtained from a meta-analysis of GWAS involving about 250,000 individuals that provides robust replication for the 32 SNPs (Speliotes et al. 2010).
Multivariate Analysis
We examined the interaction effects of obesity genes, SES, and cohort on BMI using multilevel models. Our sample consists of spousal pairs measured repeatedly over HRS waves. These measures are not independent from each other. Multilevel models have been developed to address the correlation structure among observations (Raudenbush and Bryk 2002). The following equations describe our model:
- Level 1:
- Level 2:
-
Level 3:
where BMIjit is the BMI measure for respondent i in household j at time t, for i = 1, ..., I, j = 1, ..., J, and t = 1, ..., Ti; Ti is the number of measurements ranging from 1 to 11; GPSji, SESji, and Cohortji respectively represent grand-median-centered GPS5, SES (i.e., childhood SES, cumulative advantage score, or SES mobility trajectory) and birth cohort for respondent i in household j; Cpji represents time-invariant covariates such as region, rural, PCs, and childhood health status for p = 1, ...P, with P being the maximum number of such covariates; Cqjit represents time-varying covariates such as age, age squared, smoking, and drinking, for q = 1, ...Q, with Q being the maximum number of such covariates; εjit is the level 1 residual term with εjit ~ N(0, σ2), and μj is the level 3 random effect at the household level with . The correlation between repeated measures (i.e., the level 2 random effect) is modeled using the SP(POW) structure in SAS 9.3 which provides a generalization of the autoregressive 1 structure (see Appendix B for more details).
To examine average GPS × SES interaction effects on BMI over birth cohorts (H1), we first estimated reduced models without GPS × Cohort, SES × Cohort, and GPS × SES × Cohort interactions (Models 1a–c). We then estimated an average GPS × Cohort interaction effect over SES groups (H2) by fitting a reduced model without SES, GPS × SES, SES × Cohort, and GPS × SES × Cohort terms (Model 2). Finally, to assess cohort differences in the moderating effects of SES on the genetic association with BMI (H3), we estimated the full model with all two- and three-way interaction terms among GPS, SES, and cohort (Models 3a–c). We compared models in which cohort is coded as a set of dummy variables with models where it is treated as a continuous variable with greater values indicating more recent cohorts (i.e., AHEAD = 1; CODA = 2; HRS = 3; WB = 4; EBB = 5). Results suggested linear cohort patterns, thus we report multivariate results based on the continuous cohort measure. As such, positive GPS × Cohort interaction indicates increased genetic association with BMI in more recent cohorts, and negative GPS × SES × Cohort interaction indicates decreased moderating effects of SES on the genetic association with BMI in more recent cohorts.
Appendix B.
To illustrate, suppose there are three respondents: A, B, and C. A and B were from household 1, and C was from household 2. A was measured in 1992 and 1994, B only in 1992, and C in 1993 and 1998. Then the covariance matrix for the 5 BMI measures will be:
|
|
Cohort Analysis Adjusting for Age
Although the accelerated multi-cohort longitudinal design of HRS allows us to disentangle age and cohort effects, it also has a limitation, namely that some ages were not observed for all cohorts. The AHEAD cohort was not observed before 70 years of age and the WB and EBB cohorts had not reached 70 when the most recent wave (2012) of data was collected. The differential age distribution by cohort may affect the accuracy of the cohort-difference estimates (Yang and Land 2013). Particularly, studies have shown age-related decline in lean body mass (Han et al. 2011; Villareal et al. 2005; Zamboni et al. 2005). Consequently cohort differences in genetic association with BMI might be driven by weight loss as the older cohorts are more likely to experience weight loss than the younger ones. To address this potential issue, we formed an age-comparable sample from overlapping age groups in AHEAD, CODA, and HRS cohorts. WB and EBB were not included because of their limited age overlap with other cohorts. For each cohort in the selected subsample, age ranges from 70 to 81 with a mean age of 73.4. Using the age-comparable sample, we replicated the cohort analysis and compared the results with those from the entire analytic sample.
RESULTS
Bivariate Relationships between Key Variables
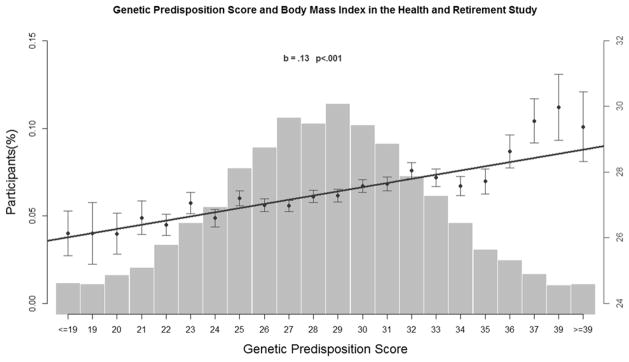
Before testing the hypotheses, we examined bivariate associations of GPS, SES, and Cohort with BMI. In HRS, GPS values range from 16 to 43 (i.e., number of risk alleles), with higher scores indicating greater genetic propensities to obesity. GPS is positively associated with BMI (see Figure 2). An increment of 10 risk alleles is associated with a 1.3 kg/m2 increase in BMI (i.e., about 10 lbs. for a 6-foot person). The first row in Table 2a shows the bivariate correlation between GPS and BMI for each of the five cohorts. As can be seen, the GPS-BMI correlation is stronger for more recent cohorts than earlier ones.
Figure 2.
Genetic Predisposition Score and Body Mass Index in the Health and Retirement Study
Note: GPS is calculated as the number of weighted risk alleles (ranging from 16 to 43) for each participant. The X axis represents GPS and the Y axis on right represents mean BMI (± S.E.) of participants in corresponding GPS category, with the line showing the regression of the mean BMI values on GPS. The histogram (Y axis on left) shows the percentage of participants in each GPS category.
Table 2a.
Genetic and Socioeconomic Associations with BMI (Standard Error) in Five Cohorts
|
|
AHEAD (Born before 1924) | CODA (Born 1924–1930) | HRS (Born 1931–1941) | WB (Born 1942–1947) | EBB (Born 1948–1953) | Overall |
|---|---|---|---|---|---|---|
| Mean Age = 74.0 | Mean Age = 69.1 | Mean Age = 56.1 | Mean Age = 52.4 | Mean Age = 52.7 | Mean Age = 58.9 | |
| (1) |
(2) |
(3) |
(4) |
(5) |
(6) |
|
| Genetic Associationa | .01(.03) | .12(.03)*** | .13(.02)*** | .17(.03)*** | .16(.04)*** | .13(.01)*** |
| (N = 909) | (N = 1480) | (N = 3483) | (N = 1521) | (N = 1423) | (N = 8816) | |
| Childhood SES | ||||||
| Low | 26.48(.32)* | 26.84(.29) | 27.12(.18)*** | 27.82(.28)* | 28.48(.34)** | 27.36(.12)*** |
| (N = 163) | (N = 268) | (N = 727) | (N = 361) | (N = 254) | (N = 1773) | |
| Medium | 25.58(.21)* | 26.64(.15) | 26.71(.11)*** | 27.24(.22)* | 28.41(.24)** | 26.94(.08)*** |
| (N = 344) | (N = 833) | (N = 1775) | (N = 685) | (N = 629) | (N = 4266) | |
| High | 25.51(.22)* | 26.68(.32) | 26.17(.19)*** | 26.77(.30)* | 27.11(.27)** | 26.41(.11)*** |
| (N = 302) | (N = 196) | (N = 540) | (N = 269) | (N = 363) | (N = 1670) | |
| Young Adulthood SES | ||||||
| Low | 26.22(.42)** | 27.44(.26)*** | 27.11(.20)*** | 28.03(.42)** | 30.00(.78)*** | 27.47(.15)*** |
| (N = 94) | (N = 275) | (N = 583) | (N = 182) | (N = 94) | (N = 1228) | |
| Medium | 26.54(.43)** | 27.35(.26)*** | 27.51(.16)*** | 27.50(.22)** | 28.68(.23)*** | 27.77(.11)*** |
| (N = 109) | (N = 229) | (N = 640) | (N = 668) | (N = 637) | (N = 2283) | |
| High | 25.45(.14)** | 26.35(.15)*** | 26.36(.10)*** | 26.88(.20)** | 27.20(.21)*** | 26.41(.06)*** |
| (N = 705) | (N = 975) | (N = 2255) | (N = 663) | (N = 677) | (N = 5275) | |
| Middle/Late Adulthood SES | ||||||
| Low | 26.38(.47)* | 27.61(.34)*** | 27.30(.21)*** | 28.37(.32)*** | 29.46(.40)*** | 27.91(.14)*** |
| (N = 107) | (N = 270) | (N = 683) | (N = 356) | (N = 315) | (N = 1731) | |
| Medium | 25.67(.23)* | 27.08(.19)*** | 27.01(.13)*** | 27.44(.24)*** | 28.63(.26)*** | 27.24(.09)*** |
| (N = 303) | (N = 509) | (N = 1258) | (N = 546) | (N = 525) | (N = 3141) | |
| High | 25.50(.17)* | 26.09(.15)*** | 26.16(.11)*** | 26.51(.20)*** | 26.82(.20)*** | 26.21(.07)*** |
| (N = 499) | (N = 701) | (N = 1542) | (N = 619) | (N = 583) | (N = 3944) | |
| Cumulative Advantage in SES (CAS) | ||||||
| CAS(=0) | 26.59(.50)** | 27.56(.25)*** | 27.53(.18)*** | 28.25(.30)*** | 29.58(.33)*** | 28.09(.13)*** |
| (N = 85) | (N = 261) | (N = 649) | (N = 441) | (N = 378) | (N = 1814) | |
| CAS(=1) | 25.99(.26)** | 26.84(.21)*** | 27.08(.14)*** | 27.04(.25)*** | 27.95(.27)*** | 27.05(.09)*** |
| (N = 241) | (N = 481) | (N = 1138) | (N = 443) | (N = 380) | (N = 2683) | |
| CAS(=2) | 25.54(.22)** | 26.17(.19)*** | 26.11(.14)*** | 26.65(.27)*** | 27.20(.29)*** | 26.27(.09)*** |
| (N = 316) | (N = 460) | (N = 982) | (N = 322) | (N = 330) | (N = 2410) | |
| CAS(=3) | 25.30(.28)** | 25.96(.46)*** | 25.46(.24)*** | 26.46(.48)*** | 26.06(.36)*** | 25.73(.15)*** |
| (N = 166) | (N = 94) | (N = 268) | (N = 104) | (N = 144) | (N = 776) | |
| SES Trajectory | ||||||
| Stable and Low | 26.09(.27)* | 27.17(.19)*** | 27.24(.13)*** | 27.98(.24)*** | 29.12(.26)*** | 27.59(.09)*** |
| (N = 245) | (N = 586) | (N = 1429) | (N = 644) | (N = 554) | (N = 3458) | |
| Downwardly Mobile | 25.69(.37)* | 27.52(.48)*** | 26.76(.31)*** | 27.05(.44)*** | 28.07(.45)*** | 27.03(.18)*** |
| (N = 120) | (N = 87) | (N = 228) | (N = 128) | (N = 171) | (N = 734) | |
| Upwardly Mobile | 25.66(.24)* | 26.14(.17)*** | 26.28(.13)*** | 26.59(.25)*** | 27.27(.28)*** | 26.36(.09)*** |
| (N = 262) | (N = 515) | (N = 1073) | (N = 402) | (N = 329) | (N = 2581) | |
| Stable and High | 25.39(.27)* | 26.02(.41)*** | 25.73(.23)*** | 26.51(.40)*** | 26.26(.30)*** | 25.93(.14)*** |
| (N = 182) | (N = 109) | (N = 312) | (N = 141) | (N = 192) | (N = 936) | |
Note: The first row includes estimated genetic association with BMI (β) for each cohort and analyses use t-test to test whether β equals to 0. Values in other rows are means of BMI calculated using onset measures in the analytic sample. In columns (1), (2), (3), (4), and (5), analyses use ANOVA to test for mean socioeconomic differences in BMI. In column (6), analyses use ANOVA to test for mean cohort differences in BMI.
p< .05;
p< .01;
p< .001.
Socioeconomic gaps in BMI (before age adjustment) are illustrated by the differences between rows in Table 2a, and inter-cohort variations by the differences between columns. In all five cohorts, BMI is typically higher among respondents with lower SES at each of the three life stages (two exceptions are young-adulthood-SES-BMI associations in AHEAD and HRS). Considering the cumulative socioeconomic advantage, BMI is higher for those who experienced SES advantages at fewer phases over the life course. With regard to the four SES mobility trajectories, BMI is lowest for individuals who experienced stable and high SES, higher for those who were upwardly mobile, higher for those who were downwardly mobile, and highest for those with stable and low SES throughout the life course. In almost all SES categories, BMI is higher in more recent cohorts than earlier ones.
The cohort patterns in BMI are likely to be confounded by differential age distribution across cohorts. Nevertheless, as demonstrated by Table 2b, such patterns still largely hold in the age-comparable sample where age is comparable across AHEAD, CODA, and HRS cohorts. This implies that the cohort patterns are not due to different age distributions across cohorts.
Table 2b.
Genetic and Socioeconomic Associations with BMI (Standard Error) in the Age-comparable Sample
|
|
AHEAD (Born before 1924) | CODA (Born 1924–1930) | HRS (Born 1931–1941) | Overall |
|---|---|---|---|---|
| Mean Age =73.4 | Mean Age =73.4 | Mean Age =73.4 | Mean Age ==73.4 | |
| (1) |
(2) |
(3) |
(4) |
|
| Genetic Associationa | .02(.04) | .12(.03)*** | .13(.02)*** | .12(.02)*** |
| (N = 844) | (N = 1449) | (N = 3302) | (N = 5595) | |
| Childhood SES | ||||
| Low | 26.58(.33)** | 27.03(.29) | 28.04(.21)** | 27.59(.15)*** |
| (N = 155) | (N = 263) | (N = 683) | (N = 1101) | |
| Medium | 25.66(.22)** | 26.71(.16) | 27.56(.13)** | 27.10(.09)*** |
| (N = 325) | (N = 816) | (N = 1690) | (N = 2831) | |
| High | 25.47(.23)** | 26.96(.32) | 27.06(.22)** | 26.59(.15)*** |
| (N = 276) | (N = 192) | (N = 510) | (N = 978) | |
| Young Adulthood SES | ||||
| Low | 26.17(.44)* | 27.50(.29)*** | 27.99(.24)*** | 27.67(.18)** |
| (N = 88) | (N = 265) | (N = 537) | (N = 890) | |
| Medium | 26.80(.46)* | 27.44(.29)*** | 28.34(.19)*** | 27.95(.15)*** |
| (N = 99) | (N = 227) | (N = 616) | (N = 942) | |
| High | 25.48(.15)* | 26.52(.15)*** | 27.31(.11)*** | 26.79(.08)*** |
| (N = 656) | (N = 956) | (N = 2146) | (N = 3758) | |
| Middle/Late Adulthood SES | ||||
| Low | 26.47(.49)* | 27.82(.38)*** | 28.45(.25)*** | 28.08(.20)*** |
| (N = 98) | (N = 263) | (N = 626) | (N = 987) | |
| Medium | 25.79(.24)* | 27.24(.19)*** | 27.82(.15)*** | 27.39(.11)*** |
| (N = 279) | (N = 499) | (N = 1192) | (N = 1970) | |
| High | 25.49(.17)* | 26.19(.15)*** | 27.08(.12)*** | 26.57(.09)*** |
| (N = 467) | (N = 687) | (N = 1484) | (N = 2638) | |
| Cumulative Advantage in SES (CAS) | ||||
| CAS(=0) | 26.76(.54)** | 27.55(.30)*** | 28.22(.22)*** | 27.92(.17)*** |
| (N = 77) | (N = 251) | (N = 609) | (N = 937) | |
| CAS(=1) | 26.13(.27)** | 27.10(.22)*** | 28.08(.17)*** | 27.57(.12)*** |
| (N = 229) | (N = 475) | (N = 1077) | (N = 1781) | |
| CAS(=2) | 25.56(.23)** | 26.22(.19)*** | 26.87(.16)*** | 26.47(.11)*** |
| (N = 294) | (N = 453) | (N = 937) | (N = 1684) | |
| CAS(=3) | 25.20(.29)** | 26.24(.47)*** | 26.64(.29)*** | 26.12(.11)*** |
| (N = 155) | (N = 91) | (N = 257) | (N = 503) | |
| SES Trajectory | ||||
| Stable and Low | 26.23(.28)* | 27.32(.21)*** | 28.11(.16)*** | 27.70(.12)*** |
| (N = 230) | (N = 572) | (N = 1345) | (N = 2147) | |
| Downwardly Mobile | 25.75(.40)* | 27.81(.48)*** | 27.47(.35)*** | 27.09(.24)** |
| (N = 107) | (N = 86) | (N = 210) | (N = 403) | |
| Upwardly Mobile | 25.71(.25)* | 26.19(.18)*** | 27.16(.15)*** | 26.68(.11)*** |
| (N = 250) | (N = 507) | (N = 1028) | (N = 1785) | |
| Stable and High | 25.30(.28)* | 26.28(.41)*** | 26.78(.27)*** | 26.25(.18)*** |
| (N = 169) | (N = 106) | (N = 300) | (N = 575) | |
Note: The first row includes estimated genetic association with BMI (β) for each cohort and analyses use t-test to test whether β equals to 0. Values in other rows are means of BMI calculated using onset measures in the analytic sample. In columns (1), (2), and (3), analyses use ANOVA to test for mean socioeconomic differences in BMI. In column (4), analyses use ANOVA to test for mean cohort differences in BMI.
p< .05;
p< .01;
p< .001.
Moderating Effects of SES on the Genetic Association with BMI (H1)
Table 3 displays the results of Models 1a–c testing for interactions of SES and GPS on BMI. As shown in the first column in Table 3, the interaction term in Model 1a (i.e., GPS × childhood SES) is not significant at the .05 level, suggesting that there is no difference in the genetic association with BMI among individuals with different levels of childhood SES.
Table 3.
Coefficients (Standard Error) of Multilevel Models Assessing Moderating Effects of SES on the Genetic Association with BMI (H1)
|
|
Sensitive Period (Model 1a) |
Social Accumulation (Model 1b) |
Social Mobility (Model 1c) |
|---|---|---|---|
| Genetic Predisposition Score (GPS)a | .12(.03)*** | .07(.04)† | .07(.04)† |
| Childhood SES | |||
| Low | .57(.16)*** | ||
| Medium | .31(.13)* | ||
| High | |||
| Cumulative Advantage in SES (CAS) | |||
| CAS(=0) | 1.74(.20)*** | ||
| CAS(=1) | 1.20(.19)*** | ||
| CAS(=2) | .46(.18)* | ||
| CAS(=3) | |||
| SES Trajectory | |||
| Stable and Low | 1.53(.17)*** | ||
| Downwardly Mobile | 1.01(.23)*** | ||
| Upwardly Mobile | .32(.17)† | ||
| Stable and High | |||
| GPS × Childhood SES | |||
| GPS × Low SES | .04(.04) | ||
| GPS × Medium SES | −.00(.03) | . | |
| GPS × High SES | |||
| GPS × Cumulative Advantage in SES (CAS) | |||
| GPS × CAS(=0) | .10(.05)* | ||
| GPS × CAS(=1) | .07(.05) | ||
| GPS × CAS(=2) | .03(.05) | ||
| GPS × CAS(=3) | |||
| GPS × SES Trajectory | |||
| GPS × Stable and Low | .09(.04)* | ||
| GPS × Downwardly Mobile | .11(.06)* | ||
| GPS × Upwardly Mobile | .01(.04) | ||
| GPS × Stable and High | |||
| Covariates | |||
| Ageb | .16(.01)*** | .16(.01)*** | .16(.01)*** |
| Age2 | −.01(.00)*** | −.01(.00)*** | −.01(.00)*** |
| Cohort | .87(.07)*** | .86(.07)*** | .91(.07)*** |
| Age × Cohort | −.01(.00)** | −.01(.00)** | −.01(.00)** |
| Female | −.82(.10)*** | −.74(.09)*** | −.90(.09)*** |
| Male | |||
| Childhood Health | −.02(.06) | −.01(.06) | −.00(.06) |
| Smoking (Yes) | −.67(.05)*** | −.66(.05)*** | −.66(.05)*** |
| Smoking (No) | |||
| Drinking (Yes) | .09(.02)*** | .09(.02)*** | .09(.02)*** |
| Drinking (No) | |||
| Midwest | .48(.19)** | .47(.19)* | .48(.19)** |
| Northeast | .13(.20) | .17(.20) | .15(.20) |
| South | −.02(.19) | −.01(.19) | −.01(.19) |
| Other | −.10(.38) | −.13(.38) | −.11(.38) |
| West | |||
| Rural | .20(.11) | .14(.11) | .21(.11)* |
| Urban | |||
| Random-Effect Variance | |||
| σ2 | 15.22(.35)*** | 15.29(.35)*** | 15.11(.34)*** |
| ρ | .94(.00)*** | .94(.00)*** | .94(.00)*** |
| 10.04(.46)*** | 10.04(.46)*** | 10.23(.45)*** | |
| Sample Size | 7,670 | 7,670 | 7,695 |
Note: All models control for the largest 10 principal components for adjusting population stratification. Model 1 also controls for young adulthood and middle/late adulthood SES;
GPS is grand median centered;
Age is grand median centered.
p< .05;
p< .01;
p< .001 (one-tailed tests)
p< .05;
p< .01;
p< .001 (two-tailed tests).
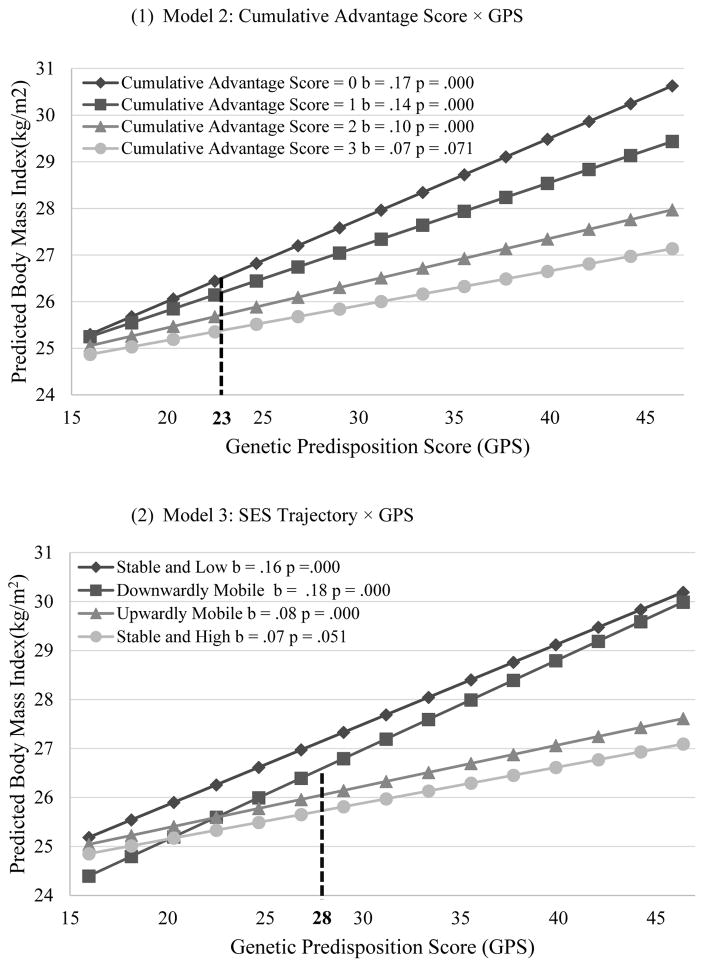
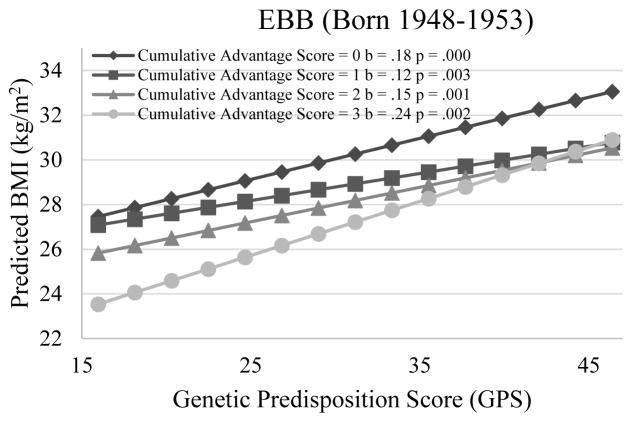
Results from Model 1b are consistent with the prediction of the social trigger/compensation model: that is, the genetic influence on BMI is greater for individuals who experienced less socioeconomic advantage than for those who experienced more socioeconomic advantage over the life course. Panel (1) of Figure 3 shows the genetic association with BMI for respondents with different CAS. Specifically, for respondents who experienced high SES at all three phases (i.e., CAS = 3), an increment of 10 risk alleles is associated with .7 increases in BMI (P = .07). The genetic association with BMI increased to 1.0, 1.4, and 1.7, respectively for those who experienced high SES at two, one and zero phases (P<.05 for interaction). Viewed differently, the socioeconomic differences in BMI may depend on genetic propensity. As can be seen, cumulative socioeconomic advantage significantly decreases an individual’s BMI when his/her GPS is greater than 23. This implies that the socioeconomic gap in BMI manifests only for individuals with higher genetic propensities to overweight or obesity, but not for those with lower genetic propensities.
Figure 3.
Genetic Association with BMI for Individuals with Differential Life-Course SES
Notes: (1) Genetic association with BMI is weaker for individuals experiencing more socioeconomic advantage than those experiencing less socioeconomic advantage over the life course. (2) Genetic association with BMI is weaker for those experiencing stable and high SES or upward mobility than those experiencing stable and low SES or downward mobility. (3) Cumulative socioeconomic advantage significantly decreases an individual’s BMI only when his/her GPS is greater than 23 (i.e., possessing more than 23 risk alleles). (4) The average BMI in the stable and high trajectory is significantly lower than that in the downward mobile trajectory only for those with a GPS greater than 28.
Results from Model 1c support the social trigger/compensation model, which predicts that the genetic influence on BMI is greater among individuals who experienced stable and low SES or downward mobility, compared to those who experienced stable and high SES or upward mobility. As Panel (2) of Figure 3 displays, the increases in BMI per increment of 10 risk alleles are .7 for stable and high, .8 for upwardly mobile, 1.8 for downwardly mobile, and 1.6 for stable and low (P<.01 for interaction). Again, these results also suggest that the socioeconomic gaps in BMI are conditioned on genetic propensity. BMI in the downward mobility trajectory is significantly higher than that in the stable and high trajectory only for respondents with a GPS greater than 28.
Cohort Differences in the Genetic Association with BMI (H2)
The first two columns in Table 4 demonstrate results of testing cohort differences in the genetic association with BMI based on the whole analytic sample and the age-comparable sample. In line with H2, Model 2 shows positive GPS × Cohort interactions, net of age, gender, and other covariates, suggesting increased genetic influence on BMI in more recent cohorts. On average, the genetic association with BMI increased by .03 in one cohort over a prior cohort (P<.01 for interaction).
Table 4.
Coefficients (Standard Error) of Multilevel Models Assessing Cohort Differences in the Genetic Association with BMI (H2) and Cohort Differences in the Moderating Effects of SES Based on the Age-comparable Sample and the Whole Analytic Sample (H3)
| Cohort Difference (Model 2)
|
Sensitive Period (Model 3a)
|
Social Accumulation (Model
3b) |
Social Mobility (Model 3c)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Whole Sample | Age- comparable Sample | Whole Sample | Age- comparable Sample | Whole Sample | Age- comparable Sample | Whole Sample | Age- comparable Sample | |
|
|
|
|
|
|
||||
| Genetic Predisposition Score (GPS) × Cohort | .03(.01)** | .05(.02)* | .04(.02)† | .09(.05)† | .08(.03)** | .11(.06)† | .08(.03)** | .12(.06)* |
| GPS × Childhood SES | ||||||||
| GPS × Low SES | .11(.08) | .20(.12)† | ||||||
| GPS × Medium SES | .02(.07) | .04(.09) | ||||||
| GPS × High SES | ||||||||
| GPS × Cumulative Advantage in SES (CAS) | ||||||||
| GPS × CAS(=0) | .22(.10)* | .30(.18)† | ||||||
| GPS × CAS(=1) | .22(.09)* | .19(.12) | ||||||
| GPS × CAS(=2) | .11(.08) | .23(.11)* | ||||||
| GPS × CAS(=3) | ||||||||
| GPS × SES Trajectory | ||||||||
| GPS × Stable and Low | .22(.08)** | .24(.12)* | ||||||
| GPS × Downwardly Mobile | .31(.11)** | .37(.15)* | ||||||
| GPS × Upwardly Mobile | .13(.08)† | .20(.11)† | ||||||
| GPS × Stable and High | ||||||||
| GPS × Childhood SES × Cohort | ||||||||
| GPS × Low × Cohort | −.04(.03) | −.11(.07) | ||||||
| GPS × Medium × Cohort | −.01(.03) | −.02(.06) | ||||||
| GPS × High × Cohort | ||||||||
| GPS × Cumulative Advantage in SES (CAS) × Cohort | ||||||||
| GPS × CAS(=0) × Cohort | −.07(.04)† | −.11(.11) | ||||||
| GPS × CAS(=1) × Cohort | −.09(.04)* | −.04(.08) | ||||||
| GPS × CAS(=2) × Cohort | −.05(.04) | −.11(.07) | ||||||
| GPS × CAS(=3) × Cohort | ||||||||
| GPS × SES Trajectory × Cohort | ||||||||
| GPS × Stable and Low × Cohort | −.06(.03)* | −.07(.07) | ||||||
| GPS × Downwardly Mobile × Cohort | −.09(.04)* | −.13(.10) | ||||||
| GPS × Upwardly Mobile × Cohort | −.06(.04)† | −.12(.07)† | ||||||
| GPS × Stable and High × Cohort | ||||||||
| Sample Size | 7,670 | 4,562 | 7,670 | 4,562 | 7,670 | 4,562 | 7,695 | 4,567 |
Note: The age-comparable sample is selected from overlapping age groups in AHEAD, CODA, and HRS cohorts. For each cohort in the age-comparable sample, age ranges from 70 to 81 with a mean age of 73.4. All models control for grand-median-centered age, age squared, sex, childhood health, region, rural, smoking, drinking, and the largest 10 principal components for adjusting population stratification. Sensitive-period models also control for young adulthood and middle/late adulthood SES.
p< .05;
p< .01;
p< .001 (one-tailed tests)
p< .05;
p< .01;
p< .001 (two-tailed tests).
Cohort Differences in the Moderating Effects of SES (H3)
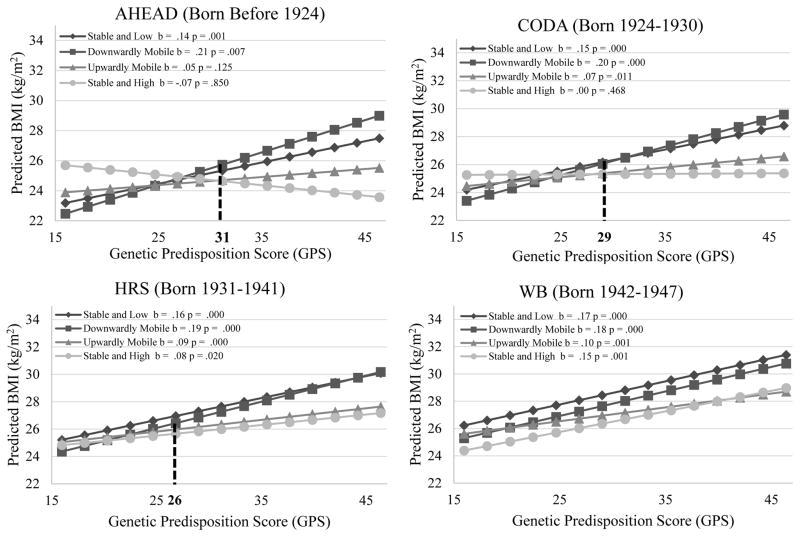
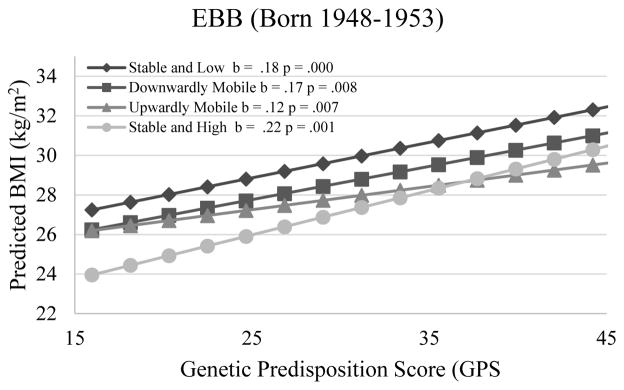
Columns 3–8 in Table 4 show cohort differences in the moderating effects of SES on the genetic influence. Models 3b and 3c show negative three-way GPS × cumulative advantage/mobility × Cohort interactions (P<.05 for interaction). Figures 4 and 5 show that the GPS × cumulative advantage/mobility interactions for BMI are weaker in more recent cohorts. Consistent with H3, cohort differences in the genetic association with BMI are greater in socioeconomically advantaged groups than in disadvantaged groups. Whereas in AHEAD, CODA, and HRS the genetic association is much weaker among respondents experiencing upward mobility or stable and high SES than those experiencing downward mobility or stable and low SES, in WB and EBB there is no significant difference in the genetic association across SES groups.
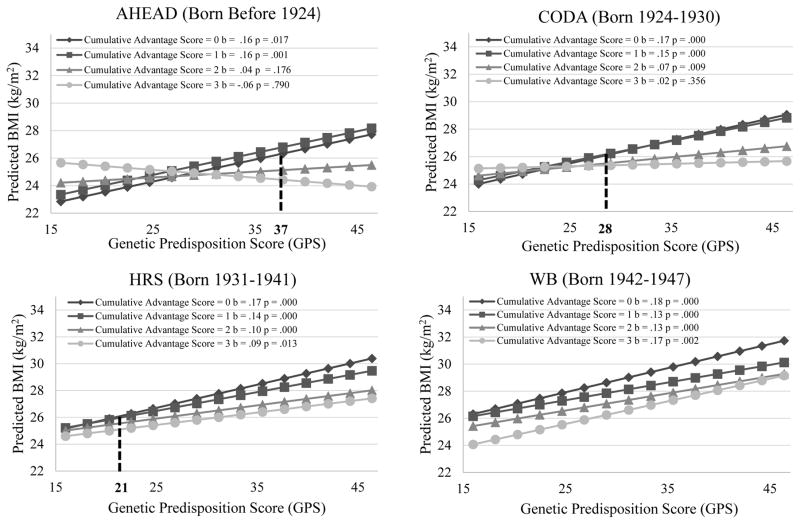
Figure 4.
Genetic Association with BMI for Individuals with Different Levels of Cumulative Socioeconomic Advantages by Birth Cohort Net of Age and Other Covariates (Estimated Using the Whole Analytic Sample)
Note: (1) In AHEAD, CODA, and HRS cohorts, the genetic association with BMI is much weaker among those experiencing high SES in at least two of the three life stages (i.e., CAS = 2 or 3) than others (i.e., CAS = 0, 1). In WB and EBB, however, there is no significant difference in the genetic association with BMI across SES groups. (2) The mean BMI of individuals with CAS = 3 is significantly lower that of those with CAS = 0 when the GPS is greater than 37 in AHEAD, 28 in CODA, 21 in HRS. Such interaction patterns are less obvious or nonexistent in WB and EBB.
Figure 5.
Genetic Association with BMI for Individuals in Different SES Mobility Trajectories by Birth Cohort Net of Age and Other Covariates (Estimated Using the Whole Analytic Sample)
Note: (1) In AHEAD, CODA, and HRS cohorts, the genetic association with BMI is much weaker among those experiencing stable and high SES or upward mobility than those experiencing stable and low SES or downward mobility. In WB and EBB, however, there are no significant differences in the genetic association with BMI across SES groups. (2) The mean BMI in the stable and high trajectory is significantly lower than that in the downward mobile trajectory when the GPS is greater than 31 in AHEAD, 29 in CODA, 26 in HRS. Such interaction patterns are less obvious or nonexistent in WB and EBB.
In spite of reduced statistical power, the direction and magnitude of two- and three-way interactions estimated from the age-comparable sample are mostly consistent with that based on the whole sample. Again, this suggests that our cohort-difference results are robust to differences in the age distribution across cohorts.
Assessing the Effect of Selection Bias
While our analysis shows significant interactions of SES, obesity-related genes, and birth cohort on BMI in middle and late adulthood, the story is, in fact, more complicated. Selection processes could have played a role. The final step of our analysis is to assess the effect of selection bias.
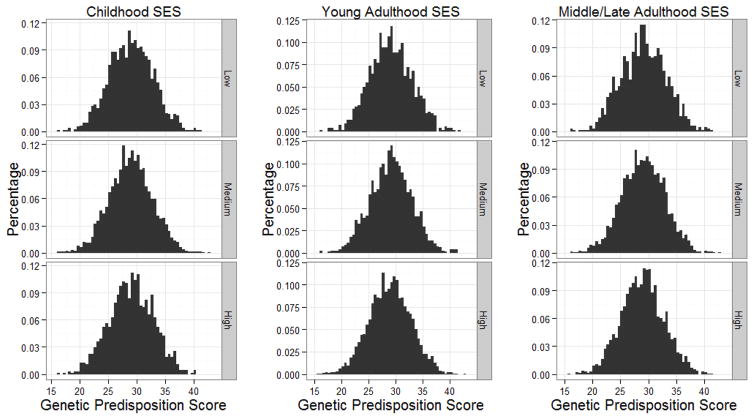
Two types of selection can confound our results. First, one’s exposure to SES-related environments may depend upon his or her genotype (rGE). Individuals with certain genotypes might be more likely to develop obesity-related health problems, and these problems in childhood may adversely affect their socioeconomic opportunities in later life (Haas 2006; Palloni et al. 2009). The existence of such rGE can bias our GPS × SES analysis (Jaffee and Price 2007; Wagner et al. 2013). To detect rGE, we examined correlations between GPS and SES measures. Differential distribution of the GPS by SES would indicate the existence of rGE. Figure 6 demonstrates that the mean and variance of the GPS are similar across different SES categories in childhood, young adulthood, and middle/late adulthood. Table 5 shows the comparison of the mean GPS across different SES categories within each cohort. Overall, there are no clear SES patterns in the distribution of GPS.
Figure 6.
Distribution of the Genetic Predisposition Score by SES
Note: The distribution of the genetic predisposition score is similar across levels of SES in childhood, young adulthood, and Middle/late adulthood.
Table 5.
Mean Genetic Predisposition Score (Standard Error) by SES in Five Birth Cohorts
| AHEAD (Born before 1924) | CODA (Born 1924–1930) | HRS (Born 1931–1941) | WB (Born 1942–1947 | EBB (Born 1948–1953) | Overall | |
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
|
|
|
|
|
|
|
|
| Childhood SES | ||||||
| Low | 29.10(.29) | 28.96(.22) | 29.10(.14) | 28.89(.21) | 29.04(.25) | 29.02(.09) |
| Medium | 28.74(.20) | 29.11(.13) | 29.06(.09) | 28.82(.15) | 29.38(.15) | 29.05(.06) |
| High | 28.67(.22) | 28.99(.25) | 29.13(.17) | 29.35(.24) | 29.10(.20) | 29.06(.09) |
| Young Adulthood SES | ||||||
| Low | 28.42(.43) | 29.06(.23) | 29.16(.16) | 29.08(.31) | 29.65(.42) | 29.11(.11) |
| Medium | 29.40(.32) | 29.14(.24) | 29.28(.15) | 28.97(.16) | 29.22(.15) | 29.16(.08) |
| High | 28.66(.14) | 29.05(.12) | 28.99(.08) | 29.04(.15) | 29.13(.15) | 28.98(.05) |
| Middle/Late Adulthood SES | ||||||
| Low | 28.40(.39) | 28.70(.23) | 29.27(.15) | 29.38(.21)* | 29.42(.23) | 29.18(.10)** |
| Medium | 28.77(.20) | 29.22(.17) | 29.02(.11) | 29.08(.17)* | 29.26(.17) | 29.08(.07) |
| High | 28.75(.17) | 29.10(.14) | 29.03(.10) | 28.73(.15)* | 29.04(.15) | 28.96(.06) |
| Cumulative Advantage in SES (CAS) | ||||||
| CAS(=0) | 29.78.40) | 28.96(.22) | 29.44(.15) | 28.81(.20) | 29.43(.20) | 29.23(.09) |
| CAS(=1) | 28.45(.23) | 29.31(.17) | 29.01(.11) | 29.26(.18) | 29.38(.21) | 29.11(.07)* |
| CAS(=2) | 28.72(.22) | 28.80(.17) | 28.81(.12) | 28.61(.20) | 28.98(.22) | 28.79(.08) |
| CAS(=3) | 28.92(.31) | 29.36(.37) | 29.54(.23) | 29.30(.40) | 28.91(.27) | 29.23(.13) |
| SES Trajectory | ||||||
| Stable and Low | 28.94(.23) | 29.12(.15) | 29.17(.10) | 29.01(.16) | 29.33(.17) | 29.14(.07) |
| Downwardly Mobile | 28.46(.35) | 28.55(.38) | 28.83(.28) | 29.43(.33) | 29.44(.33) | 28.98(.15)* |
| Upwardly Mobile | 28.77(.24) | 29.02(.16) | 28.95(.11) | 28.58(.19) | 29.18(.21) | 28.92(.07) |
| Stable and High | 28.81(.29) | 29.34(.34) | 29.35(.22) | 29.28(.34) | 28.79(.25) | 29.12(.12) |
Note: In columns (1), (2), (3), (4), and (5), analyses use ANOVA to test for mean SES differences in GPS. In column (6), analyses use ANOVA to test for mean cohort differences in GPS.
p< .05;
p< .01;
p< .001.
Second, DNA samples were taken in 2006 and 2008, when ages of AHEAD range from 83–101, CODA from 76–82, HRS from 65–75, WB from 65–70, and EBB from 53–60. The retention of healthier, more affluent respondents in older cohorts can lead to confounded cohort-difference results that reflect differential characteristics between robust survivors and others in the population, rather than “real” effects of socio-historical changes. Specifically, AHEAD and CODA have a higher proportion of robust survivors, who may be less genetically predisposed to obesity than participants in other cohorts. Yet results in Table 5 show that the distribution of GPS does not significantly differ across birth cohorts in most SES groups. Furthermore, we replicated the cohort-difference analyses using a more homogeneous subsample that includes all “survival elites,” who were observed at age 80 or older. As Table 6 demonstrates, despite reduced statistical power, results estimated using the Survival Elites subsample are consistent with those based on the whole sample, indicating that our cohort-difference findings are robust against potential confounding from differential surviving across cohorts.
Table 6.
Assessing Selection Bias: Coefficients (Standard Error) of Multilevel Models Based on the Survival Elites Subsample and the Whole Analytic Sample
|
|
Social Accumulation
|
Social Mobility |
||||
|---|---|---|---|---|---|---|
| Whole Sample | Survival Elites Subsample | Whole Sample | Survival Elites Subsample | Whole Sample | Survival Elites Subsample | |
|
|
|
|
|
|||
| Genetic Predisposition Score (GPS) × Cohort | .03(.01)** | .04(.03) | .08(.03)** | .12(.08) | .08(.03)** | .16(.08)* |
| GPS × Cumulative Advantage in SES (CAS) | ||||||
| GPS × CAS(=0) | .22(.10)* | .29(.19) | ||||
| GPS × CAS(=1) | .22(.09)* | .26(.11)* | ||||
| GPS × CAS(=2) | .11(.08) | .23(.10)* | ||||
| GPS × CAS(=3) | ||||||
| GPS × SES Trajectories | ||||||
| GPS × Stable and Low | .22(.08)** | .24(.11)* | ||||
| GPS × Downwardly Mobile | .31(.11)** | .41(.14)** | ||||
| GPS × Upwardly Mobile | .13(.08)† | .24(.10)* | ||||
| GPS × Stable and High | ||||||
| GPS × Cumulative Advantage in SES (CAS) × Cohort | ||||||
| GPS × CAS(=0) × Cohort | −.07(.04) | −.10(.15) | ||||
| GPS × CAS(=1) × Cohort | −.09(.04)* | −.13(.11) | ||||
| GPS × CAS(=2) × Cohort | −.05(.04) | −.11(.10) | ||||
| GPS × CAS(=3) × Cohort | ||||||
| GPS × SES Trajectory × Cohort | ||||||
| GPS × Stable and Low × Cohort | −.06(.03)* | −.11(.10) | ||||
| GPS × Downwardly Mobile × Cohort | −.09(.04)* | −.31(.15)* | ||||
| GPS × Upwardly Mobile × Cohort | −.06(.04)† | −.21(.10)* | ||||
| GPS × Stable and High × Cohort | ||||||
| Sample Size | 7,670 | 2,066 | 7,670 | 2,066 | 7,695 | 2,068 |
Note: The Survival Elites subsample includes respondents who lived to at least age 80. All models control for grand-median-centered age, age squared, sex, childhood health, region, rural, smoking, drinking, and the largest 10 principal components for adjusting population stratification.
p< .05;
p< .01;
p< .001 (one-tailed tests)
p< .05;
p< .01;
p< .001 (two-tailed tests).
DISCUSSSION AND CONCLUSIONS
This study makes important contributions to the G×E literature and the life-course paradigm. First of all, one of the biggest challenges in G×E research is to provide convincing evidence that social context interacts with genetic makeup to influence health outcomes. Over the decades, sociologists have developed a sophisticated understanding of social context, which is “multilevel, multidomain, longitudinal.” (Boardman et al. 2013: S64) Yet incorporating all these complexities in social context has been proved difficult and most empirical studies have considered only one environmental factor at a time (Boardman et al. 2014; Guo et al. 2008; Pescosolido et al. 2008; Shanahan et al. 2008; Simons et al. 2011). In this paper, we take on the challenge to examine how socioeconomic experiences over the life course and historical context moderate the genetic influence on BMI in middle and late adulthood. We developed hypotheses combining distinct G×E conceptual models (i.e., social trigger/compensation, social push, and differential susceptibility) and life-course perspectives addressing the SES-obesity relationship (i.e., sensitive period, social accumulation, and social mobility). Our empirical findings based on the HRS genetic sample support the social trigger/compensation model, which predicts that stable and low SES or downward mobility amplify the genetic influence on BMI, whereas stable and high SES or upward mobility compensate for such influence. However, there is no evidence that the genetic influence on BMI differs by levels of SES at a single life stage (e.g., childhood). This highlights the importance of considering timing, duration, and stability of socio-environmental exposures in the investigation of G×E interactions.
Secondly, viewed from a different perspective, our G×E findings also suggest that socioeconomic influences may depend on individuals’ genetic make-up. In our sample, higher SES was associated with lower BMI, but mainly among respondents with relatively high GPS. Without genetic information, the socioeconomic gaps in BMI would be unobservable. This underscores the significance of incorporating genetic factors into empirical models and theoretical discussions regarding social inequality in health outcomes.
Thirdly, we provided evidence for the increased genetic influence on BMI in more recent birth cohorts. This finding is consistent with previous research based on regional data (Demerath et al. 2013). Importantly, we assessed the cohort differences using nationally representative data. Also, the accelerated multi-cohort longitudinal design of HRS offers us opportunity to disentangle cohort effects and age effects. We demonstrated that the cohort-difference results were, for the most part, maintained when using a subsample with an equal age distribution across birth cohorts. These results provide additional evidence that the cohort-difference findings may be somewhat affected, but not predominantly driven by age effects (e.g., age-related weight loss).
Fourthly, we found that the cohort differences in the genetic association with BMI was primarily among the most affluent individuals (see Figure 7). This might be attributable to social changes during the obesity epidemic such as increased cultural and technological adaptation (Ryder 1965). It is likely that affluent individuals in more recent cohorts had longer “screen time” (e.g., time spent in front of a computer) and engaged less in physical activity compared to their counterparts in earlier cohorts. Such social changes might lead to epigenetic modifications that regulate gene expression, thereby exacerbating genetic risk of obesity. Currently large-scale human studies of epigenetic mechanisms are still rare, and the relationship between obesity-related traits and epigenetic modifications is not well understood. Extant studies have shown an association of FTO gene variants and methylation variation (Almén et al. 2012; Bell et al. 2010). These studies, however, are typically small and lack statistical power to assess complex interactions among the environment, genotype, and epigenetic modifications. We hope advances in epigenetic technology in conjunction with increasingly available large-scale data will facilitate our understanding of specific mechanisms through which changes in social context shape the genetic influences on obesity-related traits in the future.
Figure 7.
Genetic Association with BMI for Individuals in Stable and Low and Stable and High Trajectories by Birth Cohort
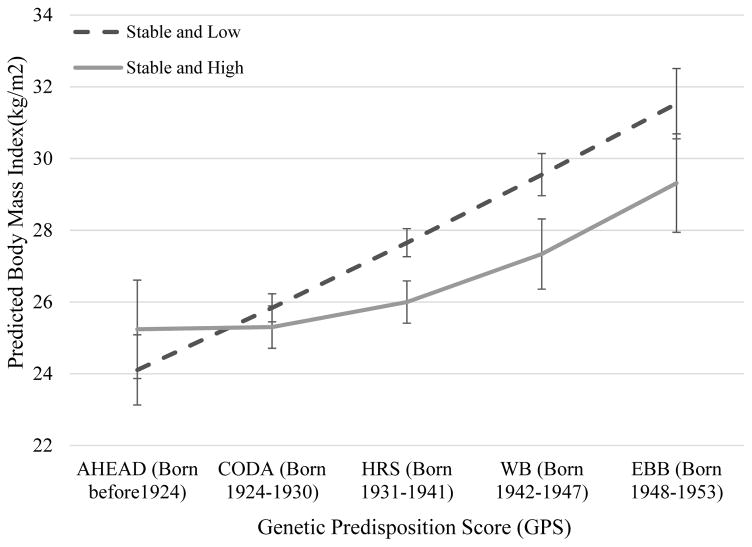
Note: Cohort differences in the genetic association with BMI are indicated by changes in the slope. I bars indicate 95% confidence intervals. The Slope becomes steeper in more recent cohorts for the stable and high group but not for the stable and low group, suggesting that the genetic association with BMI is greater in more recent cohorts for individuals experiencing stable and high SES, while there is little cohort difference in the genetic association with BMI for those experiencing stable and low SES.
Finally, we conducted G×E analysis using a genetic predisposition score based on 32 established genetic variants related to BMI. This polygenic score approach helps to minimize the multiple testing problem in single-variant analysis and maximize statistical power. Identified in GWAS using stringent criteria (e.g., each variant-BMI association with p-value < 5 × 10−8) and replicated in independent samples (Speliotes et al. 2010), the 32 SNPs provide credibility for assessment of genetic association with BMI.
It is important to mention that although our empirical findings are consistent with the social trigger/compensation model, they do not necessarily disprove the other G×E models. The social push model predicts that genetic influences are most significant in benign environments, but may be masked under extreme environments. A critical challenge in assessing this model is that some extreme environmental conditions may not be captured in the survey data. For instance, individuals suffering from prolonged starvation during a famine were unlikely to become obese in spite of their genetic makeup. Such individuals, however, might not be included in the sample. Moreover, as previously mentioned, the 32 genetic variants used to construct the GPS are based on GWAS meta-analysis assuming homogenous effects of risk alleles across samples. This assumption is, to some extent, at odds with the differential susceptibility model, which predicts that genetic effects may have opposite directions under differential environmental conditions (e.g., positive effects under adverse conditions but negative effects under favorable conditions). Such genetic variants might exist, but they do not stand out when their average effects on the whole population are assessed ignoring environmental variations. In other words, the current GWAS methods select against genetic variants that satisfy the differential susceptibility model. Discovery of such genetic variants may require a genome-wide gene-environment interaction (GWGEI) approach that allows heterogeneous genetic effects under differential environmental conditions (Boardman et al. 2014).
We acknowledge some limitations in the current study. We used three different indicators in assessing SES at childhood, young adulthood, and middle/late adulthood. Shifts in these indicators may affect the interpretability of the SES effects. A more refined lifetime SES indictor might be a composite measure with varying weights on different dimensions of SES (e.g., parental SES has more weight in measuring childhood SES, educational attainment has more weight in measuring young adulthood SES, and household wealth has more weight in measuring middle/late adulthood SES). In HRS, however, income and wealth information is only available after age 50, and educational attainment is the only indicator of young adulthood SES. Moreover, we did not include minority samples in our analysis. Although genetic information is available for some minorities (e.g., blacks and Asians), their sample size is insufficient to achieve adequate statistical power for separate G×E analysis. Future research can use more refined measures and extend the analysis in this study to other racial populations when data become available.
Despite these limitations, this study demonstrates body mass in middle and late adulthood as a consequence of the complex interplay among individuals’ genes, dynamic socioeconomic experiences, and historical context in which they live. Nowadays molecular genetic data are increasingly available in large-scale datasets (e.g., the Fragile Families Study, the Framingham Heart Study, the National Longitudinal Study of Adolescent to Adult Health, the Wisconsin Longitudinal Study), thereby providing researchers unprecedented opportunities to study interactive influences of socio-environmental factors and genetic factors on health and social behaviors. The theoretical framework and methods in this paper could be expanded to study other complex traits of interest to social scientists.
Table 1.
Analytic Sample Summary Statistics by Cohort: Health and Retirement Study
| AHEAD (Born before 1924) |
CODA (Born 1924–1930) |
HRS (Born 1931–1941) |
WB (Born 1942–1947) |
EBB (Born 1948–1953) |
|
|---|---|---|---|---|---|
| Mean/% (SE) | Mean/% (SE) | Mean/% (SE) | Mean/% (SE) | Mean/% (SE) | |
|
|
|
|
|
|
|
| Dependent Variable | |||||
| BMIa | 25.66(.13) | 26.71(.12) | 26.69(.08) | 27.28(.14) | 28.07(.15) |
| Independent Variables | |||||
| Genetic Predisposition Score (GPS) | 28.71(.13) | 29.07(.10) | 29.07(.07) | 29.01(.10) | 29.21(.10) |
| Childhood SES (Father’s Occupation) | |||||
| Low | .18 | .18 | .21 | .24 | .18 |
| Medium | .38 | .56 | .51 | .45 | .44 |
| High | .33 | .14 | .16 | .18 | .26 |
| Missing | .11 | .12 | .12 | .13 | .12 |
| Young Adulthood SES (Years of Education) | |||||
| Low | .10 | .19 | .17 | .12 | .07 |
| Medium | .12 | .15 | .18 | .44 | .45 |
| High | .78 | .66 | .65 | .44 | .48 |
| Middle/Late Adulthood SES (Wealth) | |||||
| Low | .12 | .18 | .20 | .23 | .22 |
| Medium | .33 | .35 | .36 | .36 | .37 |
| High | .55 | .47 | .44 | .41 | .41 |
| Cumulative Advantage in SES (CAS) | |||||
| CAS(=0) | .09 | .18 | .19 | .29 | .27 |
| CAS(=1) | .27 | .33 | .33 | .29 | .27 |
| CAS(=2) | .35 | .31 | .28 | .21 | .23 |
| CAS(=3) | .18 | .06 | .08 | .07 | .10 |
| CAS Missing | .11 | .12 | .13 | .14 | .13 |
| SES Trajectory | |||||
| Stable and Low | .27 | .39 | .41 | .42 | .39 |
| Downwardly Mobile | .13 | .06 | .06 | .08 | .13 |
| Upwardly Mobile | .29 | .35 | .31 | .27 | .23 |
| Stable and High | .20 | .08 | .09 | .09 | .13 |
| SES Trajectory Missing | .11 | .12 | .13 | .14 | .12 |
| Covariates | |||||
| Age | 73.98(.12) | 69.12(.09) | 56.09(.06) | 52.43(.06) | 52.70(.05) |
| Female | .62 | .56 | .54 | .61 | .56 |
| Region | |||||
| Midwest | .31 | .35 | .34 | .31 | .38 |
| Northeast | .19 | .23 | .22 | .22 | .20 |
| South | .30 | .24 | .30 | .28 | .21 |
| West | .19 | .10 | .10 | .10 | .13 |
| Other | .00 | .01 | .04 | .02 | .00 |
| Region Missing | .01 | .07 | .00 | .07 | .08 |
| Rural | |||||
| Rural | .35 | .44 | .48 | .47 | .42 |
| Urban | .52 | .53 | .49 | .51 | .57 |
| Rural Missing | .13 | .03 | .03 | .02 | .01 |
| Smokinga | |||||
| Smoker | .05 | .09 | .21 | .24 | .21 |
| Nonsmoker | .95 | .91 | .79 | .76 | .79 |
| Drinkinga | |||||
| Ever Drank | .62 | .62 | .66 | .66 | .71 |
| Never Drank | .38 | .38 | .34 | .34 | .29 |
| N | 909 | 1480 | 3483 | 1521 | 1423 |
Note:
For time-varying variables, summary statistics are based on the onset measure in the analytic sample.
Appendix C.
Coefficients (Standard Error) of Multilevel Models Assessing Moderating Effects of SES on the Genetic Association with BMI for Females and Males in HRS
| Sensitive Period |
Social Accumulation
|
Social Mobility |
||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
|
|
|
|
|
|||
| Genetic Predisposition Score (GPS)a | .13(.05)** | .15(.04)*** | .06(.07) | .07(.06) | .06(.07) | .09(.05)† |
| GPS × Childhood SES | ||||||
| GPS × Low SES | .02(.06) | .01(.06) | ||||
| GPS × Medium SES | −.02(.05) | −.01(.05) | ||||
| GPS × High SES | ||||||
| GPS × Cumulative Advantage in SES (CAS) | ||||||
| GPS × CAS(=0) | .08(.08) | .09(.07) | ||||
| GPS × CAS(=1) | .08(.08) | .09(.07) | ||||
| GPS × CAS(=2) | .05(.08) | .08(.07) | ||||
| GPS × CAS(=3) | ||||||
| GPS × SES Trajectory | ||||||
| GPS × Stable and Low | .09(.07) | .08(.06) | ||||
| GPS × Downwardly Mobile | .13(.09) | .15(.08)† | ||||
| GPS × Upwardly Mobile | .00(.07) | .04(.06) | ||||
| GPS × Stable and High | ||||||
| Sample Size | 4,325 | 3,345 | 4,325 | 3,345 | 4,340 | 3,355 |
Note: To test if the main findings differ substantially between genders, we estimated G×E models separately for males and females. Results show similar patterns in the major associations of interest between males and females. All models control for grand-median-centered age, age squared, sex, childhood health, region, rural, smoking, drinking, and the largest 10 principal components for adjusting population stratification. Sensitive-period models also control for young adulthood and middle/late adulthood SES.
GPS is grand median centered;
p< .05;
p< .01;
p< .001 (one-tailed tests)
p< .05;
p< .01;
p< .001 (two-tailed tests).
Appendix D.
Bivariate Genome-wide Covariance Estimates For BMI and SES Measures
| Childhood SES | Young Adulthood SES | Late Adulthood SES | Cumulative Advantage in SES | SES Trajectory | |
|---|---|---|---|---|---|
| Genetic variance | |||||
| BMI | 5.49(1.86) | 5.80(1.84) | 5.89(1.84) | 5.86(1.84) | 5.92(1.84) |
| SES | .08(.03) | .19(.04) | .20(.04) | .34(.08) | .32(.11) |
| Cov(BMI, SES) | −.26(.18) | −.31(.19) | −.08(.21) | −.32(.27) | −.22(.32) |
| Residual varaince | |||||
| BMI | 20.54(1.32) | 20.33(1.31) | 20.27(1.31) | 20.29(1.30) | 20.25(1.30) |
| SES | .39(.02) | .39(.03) | .44(.03) | .64(.05) | 1.03(.08) |
| Cov(BMI, SES) | −.03(.13) | −.16(.14) | −.44(.14) | −.51(.19) | −.57(.23) |
| Phenotypic variance | |||||
| BMI | 26.03(.71) | 26.13(.71) | 26.16(.71) | 26.15(.71) | 26.17(.71) |
| SES | .47(.01) | .58(.02) | .64(.02) | .98(.03) | 1.35(.04) |
| Heritability | |||||
| BMI | .21(.07) | .22(.07) | .23(.07) | .22(.07) | .23(.07) |
| SES | .17(.07) | .32(.06) | .32(.06) | .35(.07) | .23(.08) |
| rGE Test | |||||
| rG | −.39(.30) | −.30(.18) | −.07(.18) | −.22(.19) | −.16(.23) |
| df | 1 | 1 | 1 | 1 | 1 |
| p-value | .14 | .10 | .70 | .22 | .50 |
Note: To detect potential correlation between SES variables and genetic variants other than the 32 GWAS SNPs used to construct the GPS, we employed bivariate genome-wide-complex-trait-analysis (GCTA) model to assess rGE based on the whole genome (Lee et al. 2012). As a result, there is no significant evidence that SES and BMI are influenced by common variants from the whole genome.
Acknowledgments
Many thanks go to Michael Shanahan, Yang Claire Yang, Tiantian Yang, Yi Li, the ASR editors, and anonymous reviewers for their helpful comments on the manuscript. An earlier version of this manuscript was presented at the 2014 Annual Meeting of the Population Association of America in Boston and 2014 Demography Daze at Duke University. We are grateful to the Carolina Population Center (R24 HD050924) for general support.
Footnotes
To minimize reverse causality, we chose wealth measured at each respondent’s entry into the study.
We conducted sensitivity analyses based on absolute SES measures (e.g., below high school, high school, above high school). The major findings remain, suggesting our findings are robust to different coding strategies.
When constructing the SES indicators, we used the baseline sample instead of the analytic sample to minimize potential biases due to sample selection.
Father’s occupation is transformed into occupation prestige score (NORC scale) before being recoded into a relative childhood SES indicator. Years of education was trichotomized for males and females separately.
Grand-median-centered GPS allows us to interpret the intercept as the average level of BMI for individuals with medium genetic propensities to overweight or obesity.
References
- Allin Sara, Masseria Cristina, Mossialos Elias. Measuring Socioeconomic Differences in Use of Health Care Services by Wealth Versus by Income. American Journal of Public Health. 2009;99:1849–1855. doi: 10.2105/AJPH.2008.141499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almén Markus Sällman, Jacobsson Josefin A, Moschonis George, Benedict Christian, Chrousos George P, et al. Genome Wide Analysis Reveals Association of a FTO Gene Variant with Epigenetic Changes. Genomics. 2012;99:132–137. doi: 10.1016/j.ygeno.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Alvarez Jennifer, Pavao Joanne, Baumrind Nikki, Kimerling Rachel. The Relationship between Child Abuse and Adult Obesity among California Women. American Journal of Preventive Medicine. 2007;33:28–33. doi: 10.1016/j.amepre.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Anderson Sarah E, Cohen Patricia, Naumova Elena N, Must Aviva. Association of Depression and Anxiety Disorders with Weight Change in a Prospective Community-Based Study of Children Followed up into Adulthood. Archives of Pediatrics and Adolescent Medicine. 2006;160:285–291. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- Beckett Megan. Converging Health Inequalities in Later Life--an Artifact of Mortality Selection? Journal of Health and Social Behavior. 2000;41:106–119. [PubMed] [Google Scholar]
- Bell Christopher G, Prokopenko Inga, Morison Ian M, Mill Jonathan, Pidsley Ruth, et al. Integrated Genetic and Epigenetic Analysis Identifies Haplotype-Specific Methylation in the FTO Type 2 Diabetes and Obesity Susceptibility Locus. PloS One. 2010;5:e14040. doi: 10.1371/journal.pone.0014040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky Daniel W, Moffitt Terrie E, Sugden Karen, Williams Benjamin, Houts Renate, et al. Development and Evaluation of a Genetic Risk Score for Obesity. Biodemography and Social Biology. 2013;59:85–100. doi: 10.1080/19485565.2013.774628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky Jay, Bakermans-Kranenburg Marian J, van Ijzendoorn Marinus H. For Better and for Worse: Differential Susceptibility to Environmental Influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky Jay, Pluess Michael. Beyond Diathesis Stress: Differential Susceptibility to Environmental Influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Boardman Jason D, Domingue Benjamin W, Blalock Casey L, Haberstick Brett C, Harris Kathleen M, et al. Is the Gene-Environment Interaction Paradigm Relevant to Genome-Wide Studies? The Case of Education and Body Mass Index. Demography. 2014;51:119–139. doi: 10.1007/s13524-013-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman Jason D, Roettger Michael E, Domingue Benjamin W, McQueen Matthew B, Haberstick Brett C, et al. Gene–Environment Interactions Related to Body Mass: School Policies and Social Context as Environmental Moderators. Journal of Theoretical Politics. 2012;24:370–388. doi: 10.1177/0951629812437751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman Jason D, Daw Jonathan, Freese Jeremy. Defing the Environment in Gene-Environment Research: Lessons From Social Epidemiology. American Journal of Public Health. 2013;1:S64–72. doi: 10.2105/AJPH.2013.301355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman Jason D, Blalock Casey L, Pampel Fred C. Trends in the Genetic Influences on Smoking. Journal of Health and Social Behavior. 2010;51:108–123. doi: 10.1177/0022146509361195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman Jason D, Blalock Casey L, Pampel Fred C, Hatemi Peter K, Heath Andrew C, et al. Population Composition, Public Policy, and the Genetics of Smoking. Demography. 2011;48:1517–1533. doi: 10.1007/s13524-011-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic Disparities in Health in the United States: What the Patterns Tell Us. American Journal of Public Health. 2010;100(Suppl 1):S186–96. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case Anne, Fertig Angela, Paxson Christina. The Lasting Impact of Childhood Health and Circumstance. Journal of Health Economics. 2005;24:365–389. doi: 10.1016/j.jhealeco.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Cohen Sheldon, Janicki-Deverts Denise, Chen Edith, Matthews Karen A. Childhood Socioeconomic Status and Adult Health. Annals of the New York Academy of Sciences. 2010;1186:37–37. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Conley Dalton, Bennett Neil G. Is Biology Destiny? Birth Weight and Life Chances. American Sociological Review. 2000;65:458–467. [Google Scholar]
- Darmon Nicole, Drewnowski Adam. Does Social Class Predict Diet Quality? American Journal of Clinical Nutrition. 2008;87:1107. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- Daw Jonathan, Shanahan Michael, Harris Kathleen Mullan, Smolen Andrew, Haberstick Brett, et al. Genetic Sensitivity to Peer Behaviors: 5HTTLPR, Smoking, and Alcohol Consumption. Journal of Health and Social Behavior. 2013;54:92–108. doi: 10.1177/0022146512468591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath Ellen W, Towne Bradford, Choh Audrey C, Johnson William, Curran Joanne E, et al. The Positive Association of Obesity Variants with Adulthood Adiposity Strengthens over an 80-Year Period: A Gene-by-Birth Year Interaction. Human Heredity. 2013;75:175–185. doi: 10.1159/000351742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingue Benjamin W, Belsky Daniel W, Harris Kathleen Mullan, Smolen Andrew, McQueen Matthew B, et al. Polygenic Risk Predicts Obesity in Both White and Black Young Adults. PloS one. 2014;9:e101596. doi: 10.1371/journal.pone.0101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch Sanford M, Erickson Kristan Glasgow, Laird Jennifer, Wong Carol A. The Relation of Family and School Attachment to Adolescent Deviance in Diverse Groups and Communities. Journal of Adolescent Research. 2001;16:396–422. [Google Scholar]
- Elder GH. C. Social Science Research, editor. In: Life Course Dynamics : Trajectories and Transitions, 1968–1980. Elder GH Jr, editor. Ithaca: Cornell University Press; 1985. [Google Scholar]
- Elder GH. Military Times and Turning Points in Men’s Lives. Developmental Psychology. 1986;22:233–245. [Google Scholar]
- Elder GH. Children of the Great Depression: Social Change in Life Experience. Boulder, CO: Westview Press; [1974] 1999. 25th Anniversary Ed. [Google Scholar]
- Elder GH, Johnson MK, Crosnoe R. Emergence and Development of Life Course Theory. In: Mortimer JT, Shanahan MJ, editors. Handbook of the Life Course. New York, NY: Kluwer; 2003. pp. 3–22. [Google Scholar]
- Ellis Bruce J, Thomas Boyce W, Belsky Jay, Bakermans-Kranenburg Marian J, van Ijzendoorn Marinus H. Differential Susceptibility to the Environment: An Evolutionary–Neurodevelopmental Theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of Obesity and Trends in the Distribution of Body Mass Index among Us Adults, 1999–2010. Journal of the American Medical Association. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and Obesity in the United States: Prevalence and Trends, 1960–1994. International Journal of Obesity and Related Metabolic Disorders : Journal of the International Association for the Study of Obesity. 1998;22:39. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- Flegal Katherine M, Carroll Margaret D, Ogden Cynthia L, Curtin Lester R. Prevalence and Trends in Obesity among Us Adults, 1999–2008. Journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Frayling Timothy M, Rayner Nigel W, Shields Beverley, Harries Lorna W, Barrett Jeffrey C, et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Guang, North Kari E, Gorden-Larsen Penny, Bulik Cynthia M, Choi Seulki. Body Mass, DRD4, Physical Activity, Sedentary Behavior, and Family Socioeconomic Status: The Add Health Study. Obesity (Silver Spring, Md) 2007;15:1199–1206. doi: 10.1038/oby.2007.640. [DOI] [PubMed] [Google Scholar]
- Guo Guang, Roettger Michael E, Cai Tianji. The Integration of Genetic Propensities into Social-Control Models of Delinquency and Violence among Male Youths. American Sociological Review. 2008;73:543–568. [Google Scholar]
- Haas Steven A. Health Selection and the Process of Social Stratification: The Effect of Childhood Health on Socioeconomic Attainment. Journal of Health and Social Behavior. 2006;47:339–354. doi: 10.1177/002214650604700403. [DOI] [PubMed] [Google Scholar]
- Hallqvist Johan, Lynch John, Bartley Mel, Lang Thierry, Blane David, et al. Can We Disentangle Life Course Processes of Accumulation, Critical Period and Social Mobility? An Analysis of Disadvantaged Socio-Economic Positions and Myocardial Infarction in the Stockholm Heart Epidemiology Program. Social Science and Medicine. 2004;58:1555–1562. doi: 10.1016/S0277-9536(03)00344-7. [DOI] [PubMed] [Google Scholar]
- Han TS, Tajar Abdelouahid, Lean MEJ. Obesity and Weight Management in the Elderly. British Medical Bulletin. 2011;97:169–196. doi: 10.1093/bmb/ldr002. [DOI] [PubMed] [Google Scholar]
- Heraclides A, Brunner E. Social Mobility and Social Accumulation across the Life Course in Relation to Adult Overweight and Obesity: The Whitehall II Study. Journal of Epidemiology and Community Health. 2010;64:714–719. doi: 10.1136/jech.2009.087692. [DOI] [PubMed] [Google Scholar]
- Herzer Michele, Zeller Meg H, Rausch Joseph R, Modi Avani C. Perceived Social Support and Its Association with Obesity-Specific Health-Related Quality of Life. Journal of Developmental and Behavioral Pediatrics. 2011;32:188–195. doi: 10.1097/DBP.0b013e318208f576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes CL. Obesity, Disease, and Functional Limitation in Later Life. Demography. 2000;37:73–82. [PubMed] [Google Scholar]
- Hiscock Rosemary, Bauld Linda, Amos Amanda, Fidler Jennifer A, Munafò Marcus. Socioeconomic Status and Smoking: A Review. Annals of the New York Academy of Sciences. 2012;1248:107–123. doi: 10.1111/j.1749-6632.2011.06202.x. [DOI] [PubMed] [Google Scholar]
- Howie Bryan, Marchini Jonathan, Stephens Matthew. Genotype Imputation with Thousands of Genomes. G3: Genes, Genomics, Genetics. 2011;1:457–469. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie Bryan N, Donnelly Peter, Marchini Jonathan. A Flexible and Accurate Genotype Imputation Method for the Next Generation of Genome-Wide Association Studies. PLoS Genetics. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-Environment Correlations: A Review of the Evidence and Implications for Prevention of Mental Illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James Sherman A, Fowler-Brown Angela, Raghunathan Trevillore E, Van Hoewyk John. Life-Course Socioeconomic Position and Obesity in African American Women: The Pitt County Study. American Journal of Public Health. 2006;96:554–560. doi: 10.2105/AJPH.2004.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, French SA. Socioeconomic Status and Weight Control Practices among 20- to 45-Year-Old Women. American Journal of Public Health. 1996;86:1005–1010. doi: 10.2105/ajph.86.7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, French SA, Forster JL, Spry VM. Socioeconomic Status Differences in Health Behaviors Related to Obesity: The Healthy Worker Project. International Journal of Obesity. 1991;15:689–696. [PubMed] [Google Scholar]
- Kanjilal S, Gregg EW, Cheng YJ, Zhang P, Nelson DE, et al. Socioeconomic Status and Trends in Disparities in 4 Major Risk Factors for Cardiovascular Disease among Us Adults, 1971–2002. Archives of Internal Medicine. 2006;166:2348–55. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- Keith SW, Fontaine KR, Wang C, Aronne LJ, Wright SM, et al. Putative Contributors to the Secular Increase in Obesity: Exploring the Roads Less Traveled. International Journal of Obesity. 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- Kendler Kenneth S, Thornton Laura M, Pedersen Nancy L. Tobacco Consumption in Swedish Twins Reared Apart and Reared Together. Archives of General Psychiatry. 2000;57:886–892. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- Kennedy BP, Kawachi I, Glass R, Prothrow-Stith D. Income Distribution, Socioeconomic Status, and Self-Rated Health in the United States: Multilevel Analysis. British Medical Journal. 1998;317:917–21. doi: 10.1136/bmj.317.7163.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpeläinen Tuomas O, Sandholt Camilla Helene, Marmot Michael, Holzapfel Christina, Autenrieth Christine S, et al. Physical Activity Attenuates the Influence of FTO Variants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children. PLoS Medicine. 2011;8:e1001116. doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Hardy R, Langenberg C, Richards M, Wadsworth ME. Mortality in Adults Aged 26–54 Years Related to Socioeconomic Conditions in Childhood and Adulthood: Post War Birth Cohort Study. British Medical Journal. 2002;325:1076–1080. doi: 10.1136/bmj.325.7372.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Yang J, Goddard ME, Visscher PM, Wray NR. Estimation of Pleiotropy between Complex Diseases Using Single-Nucleotide Polymorphism-Derived Genomic Relationships and Restricted Maximum Likelihood. Bioinformatics. 2012;28:2540–2542. doi: 10.1093/bioinformatics/bts474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yun, Willer Cristen, Sanna Serena, Abecasis Gonçalo. Genotype Imputation. Annual Review of Genomics and Human Genetics. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link Bruce G, Phelan Jo. Social Conditions as Fundamental Causes of Disease. Journal of Health and Social Behavior. 1995;35:80–94. [PubMed] [Google Scholar]
- Loos Ruth JF, Beckmann Jacques S, Caulfield Mark J, Berndt Sonja I, Jacobs Kevin B, et al. Common Variants near Mc4r Are Associated with Fat Mass, Weight and Risk of Obesity. Nature Genetics. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks Eric B, Lynch John W, Pilote Louise, Fuhrer Rebecca, Almeida Nisha D, et al. Life-Course Socioeconomic Position and Incidence of Coronary Heart Disease: The Framingham Offspring Study. American Journal of Epidemiology. 2009;169:829–836. doi: 10.1093/aje/kwn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks Eric B, Pilote Louise, Lynch John W, Richard Hugues, Almeida Nisha D, et al. Life Course Socioeconomic Position Is Associated with Inflammatory Markers: The Framingham Offspring Study. Social Science and Medicine. 2010;71:187–195. doi: 10.1016/j.socscimed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng Julie C, Appugliese Danielle, Cabral Howard J, Bradley Robert H, Zuckerman Barry. Neighborhood Safety and Overweight Status in Children. Archives of Pediatrics and Adolescent Medicine. 2006;160:25–31. doi: 10.1001/archpedi.160.1.25. [DOI] [PubMed] [Google Scholar]
- Luo Ye, Waite Linda J. The Impact of Childhood and Adult SES on Physical, Mental, and Cognitive Well-Being in Later Life. Journals of Gerontology Series B Psychological Sciences Social Sciences. 2005;60:S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Shema SJ. Cumulative Impact of Sustained Economic Hardship on Physical, Cognitive, Psychological and Social Functioning. New England Journal of Medicine. 1997;337:1889–95. doi: 10.1056/NEJM199712253372606. [DOI] [PubMed] [Google Scholar]
- Malhotra Rahul, Malhotra Chetna, Chan Angelique, Østbye Truls. Life-Course Socioeconomic Status and Obesity among Older Singaporean Chinese Men and Women. Gerontology Series B: Psychological Sciences Social Sciences. 2013;68:117–127. doi: 10.1093/geronb/gbs102. [DOI] [PubMed] [Google Scholar]
- McLaren L. Socioeconomic Status and Obesity. Epidemiologic Reviews. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- Meyre David, Proença Christine, Gaget Stefan, Körner Antje, Kovacs Peter, et al. Genome-Wide Association Study for Early-Onset and Morbid Adult Obesity Identifies Three New Risk Loci in European Populations. Nature Genetics. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- Minkler M, Fuller-Thomson E, Guralnik JM. Gradient of Disability across the Socioeconomic Spectrum in the United States. New England Journal of Medicine. 2006;355:695–703. doi: 10.1056/NEJMsa044316. [DOI] [PubMed] [Google Scholar]
- Mitchell Colter, Notterman Daniel, Brooks-Gunn Jeanne, Hobcraft John, Garfinkel Irwin, et al. Role of Mother’s Genes and Environment in Postpartum Depression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8189–8193. doi: 10.1073/pnas.1014129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, et al. Prevalence of Obesity, Diabetes, and Obesity-Related Health Risk Factor, 2001 . Journal of the American Medical Association. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Monda Keri L, Bielak Lawrence F, Schadt Eric E, Chen Guanjie, Graff Mariaelisa, et al. A Meta-Analysis Identifies New Loci Associated with Body Mass Index in Individuals of African Ancestry. Nature Genetics. 2013;45:690–696. doi: 10.1038/ng.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumark-Sztainer Dianne, Wall Melanie, Perry Cheryl, Story Mary. Correlates of Fruit and Vegetable Intake among Adolescents. Findings from Project Eat. Preventive Medicine. 2003;37:198–208. doi: 10.1016/s0091-7435(03)00114-2. [DOI] [PubMed] [Google Scholar]
- Okada Y, Go MJ, Zheng W, Kato N, Wu JY, et al. Common Variants at CDKALL1 and KLF9 Are Associated with Body Mass Index in East Asian Populations. Nature Genetics. 2012;44:302–U105. doi: 10.1038/ng.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Rodríguez Andrea, León-Muñoz Luz María, Banegas José R, Guallar-Castillón Pilar, Rodríguez-Artalejo Fernando, et al. Lifecourse Socioeconomic Position and Change in Quality of Life among Older Adults: Evidence for the Role of a Critical Period, Accumulation of Exposure and Social Mobility. Journal of Epidemiology and Community Health. 2011;65:964–971. doi: 10.1136/jech.2010.113555. [DOI] [PubMed] [Google Scholar]
- Padyab Mojgan, Norberg Margareta. Socioeconomic Inequalities and Body Mass Index in Västerbotten County, Sweden: A Longitudinal Study of Life Course Influences over Two Decades. International Journal for Equity in Health. 2014;13:35. doi: 10.1186/1475-9276-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palloni Alberto, Milesi Carolina, White Robert G, Turner Alyn. Early Childhood Health, Reproduction of Economic Inequalities and the Persistence of Health and Mortality Differentials. Social Science and Medicine. 2009;68:1574–1582. doi: 10.1016/j.socscimed.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescosolido Bernice A, Perry Brea L, Scott Long J, Martin Jack K, Jr, Nurnberger John I, et al. Under the Influence of Genetics: How Transdisciplinarity Leads Us to Rethink Social Pathways to Illness. American Journal of Sociology. 2008;114:S171–S201. doi: 10.1086/592209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the Evidence for Models of Life Course Socioeconomic Factors and Cardiovascular Outcomes: A Systematic Review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Parsons T. Nutritional and Other Influences in Childhood as Predictors of Adult Obesity. The Proceedings of the Nutrition Society. 2000;59:267–272. doi: 10.1017/s002966510000029x. [DOI] [PubMed] [Google Scholar]
- Preacher Kristopher J, Curran Patrick, Bauer Daniel J. Computational Tools for Probing Interactions in Multiple Linear Regression, Multilevel Modeling, and Latent Curve Analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Price Alkes L, Patterson Nick J, Plenge Robert M, Weinblatt Michael E, Shadick Nancy A, et al. Principal Components Analysis Corrects for Stratification in Genome-Wide Association Studies. Nature Genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, et al. Sugar-Sweetened Beverages and Genetic Risk of Obesity. New England Journal of Medicine. 2012;367:1387–96. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine Adrian. Biosocial Studies of Antisocial and Violent Behavior in Children and Adults: A Review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Rampersaud E, Mitchell BD, Pollin TI, Fu M, Shen H, et al. Physical Activity and the Association of Common FTO Gene Variants with Body Mass Index and Obesity. Archives of Internal Medicine. 2008;168:1791–7. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Ravussin Eric, Bouchard Claude. Human Genomics and Obesity: Finding Appropriate Drug Targets. European Journal of Pharmacology. 2000;410:131–145. doi: 10.1016/s0014-2999(00)00811-6. [DOI] [PubMed] [Google Scholar]
- Reither Eric N, Hauser Robert M, Yang Yang. Do Birth Cohorts Matter? Age-Period-Cohort Analyses of the Obesity Epidemic in the United States. Social Science and Medicine. 2009;69:1439–1448. doi: 10.1016/j.socscimed.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti Rena L, Taylor Shelley E, Seeman Teresa E. Risky Families: Family Social Environments and the Mental and Physical Health of Offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Rokholm Benjamin, Silventoinen Karri, Tynelius Per, Gamborg Michael, Sørensen Thorkild IA, et al. Increasing Genetic Variance of Body Mass Index During the Swedish Obesity Epidemic. PloS One. 2011;6:e27135. doi: 10.1371/journal.pone.0027135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder NB. The Cohort as a Concept in the Study of Social Change. American Sociological Review. 1965;30:843–861. [PubMed] [Google Scholar]
- Senese LC, Almeida ND, Fath AK, Smith BT, Loucks EB. Associations between Childhood Socioeconomic Position and Adulthood Obesity. Epidemiologic Reviews. 2009;31:21–51. doi: 10.1093/epirev/mxp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan Michael J, Boardman Jason D. Genetics and Behavior in the Life Course: A Promising Frontier. In: Giele GHEaJZ., editor. The Craft of Life Course Research. New York: Guilford; 2009. pp. 215–35. [Google Scholar]
- Shanahan Michael J, Vaisey Stephen, Erickson Lance D, Smolen Andrew. Environmental Contingencies and Genetic Propensities: Social Capital, Educational Continuation, and Dopamine Receptor Gene DRD2. American Journal of Sociology. 2008;114(Suppl):S260–S286. doi: 10.1086/592204. [DOI] [PubMed] [Google Scholar]
- Simons Ronald L, Lei Man Kit, Beach Steven RH, Brody Gene H, Philibert Robert A, et al. Social Environment, Genes, and Aggression: Evidence Supporting the Differential Susceptibility Perspective. American Sociological Review. 2011;76:883–912. doi: 10.1177/0003122411427580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith James P. The Impact of Childhood Health on Adult Labor Market Outcomes. The Review of Economics and Statistics. 2009;91:478–489. doi: 10.1162/rest.91.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobal Jeffery, Stunkard Albert J. Socioeconomic Status and Obesity: A Review of the Literature. Psychological Bulletin. 1989;105:260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfalt E, et al. Fat and Carbohydrate Intake Modify the Association between Genetic Variation in the FTO Genotype and Obesity. American Journal of Clinical Nutrition. 2009;90:1418–25. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- Speliotes Elizabeth K, Mägi Reedik, Buchanan Thomas A, Randall Joshua C, Vedantam Sailaja, et al. Association Analyses of 249,796 Individuals Reveal 18 New Loci Associated with Body Mass Index. Nature Genetics. 2010;42:937–U53. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston Rebecca C, Kubzansky Laura D, Kawachi Ichiro, Berkman Lisa F. Is the Association between Socioeconomic Position and Coronary Heart Disease Stronger in Women Than in Men? American Journal of Epidemiology. 2005;162:57–65. doi: 10.1093/aje/kwi159. [DOI] [PubMed] [Google Scholar]
- Troxel Wendy M, Matthews Karen A. What Are the Costs of Marital Conflict and Dissolution to Children’s Physical Health? Clinical Child and Family Psychology Review. 2004;7:29–57. doi: 10.1023/b:ccfp.0000020191.73542.b0. [DOI] [PubMed] [Google Scholar]
- Villareal Dennis T, Apovian Caroline M, Kushner Robert F, Klein Samuel, et al. Obesity in Older Adults: Technical Review and Position Statement of the American Society for Nutrition and NAASO, the Obesity Society. American Journal of Clinical Nutrition. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- Wagner Brandon, Li Jiang, Liu Hexuan, Guo Guang. Gene-Environment Correlation: Difficulties and a Natural Experiment-Based Strategy. American Journal of Public Health. 2013;103(Suppl 1):S167–73. doi: 10.2105/AJPH.2013.301415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Youfa, Beydoun May A. The Obesity Epidemic in the United States--Gender, Age, Socioeconomic, Racial/Ethnic, and Geographic Characteristics: A Systematic Review and Meta-Regression Analysis. Epidemiologic Reviews. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- Wen WQ, Tabara Y, Gu DF, Zhu DL, Haiman CA, et al. Meta-Analysis Identifies Common Variants Associated with Body Mass Index in East Asians. Nature Genetics. 2012;44:307–U112. doi: 10.1038/ng.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson Andrea E, Shuey Kim M, Elder Glen H. Cumulative Advantage Processes as Mechanisms of Inequality in Life Course Health. American Journal of Sociology. 2007;112:1886–1924. [Google Scholar]
- Yang Y, Land Kenneth. Age-Period-Cohort Analysis_: New Models, Methods, and Empirical Applications. Boca Raton, FL: CRC Press; 2013. [Google Scholar]
- Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, et al. Health Consequences of Obesity in the Elderly: A Review of Four Unresolved Questions. International Journal of Obesity. 2005;29:1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- Zhang Qi, Wang Youfa. Trends in the Association between Obesity and Socioeconomic Status in U.S. Adults: 1971 to 2000. Obesity Research. 2004;12:1622–1632. doi: 10.1038/oby.2004.202. [DOI] [PubMed] [Google Scholar]