Abstract
Laser capture microdissection (LCM) is a powerful method to isolate specific populations of cells for subsequent analysis such as gene expression profiling, for example, microarrays or ribonucleic (RNA)-Seq. This technique has been applied to frozen as well as formalin-fixed, paraffin-embedded (FFPE) specimens with variable outcomes regarding quality and quantity of extracted RNA. The goal of the study was to develop the methods to isolate high-quality RNA from islets of Langerhans and pancreatic duct glands (PDG) isolated by LCM. We report an optimized protocol for frozen sections to minimize RNA degradation and maximize recovery of expected transcripts from the samples using quantitative real-time polymerase chain reaction (RT-PCR) by adding RNase inhibitors at multiple steps during the experiment. This technique reproducibly delivered intact RNA (RIN values 6–7). Using quantitative RT-PCR, the expected profiles of insulin, glucagon, mucin6 (Muc6), and cytokeratin-19 (CK-19) mRNA in PDGs and pancreatic islets were detected. The described experimental protocol for frozen pancreas tissue might also be useful for other tissues with moderate to high levels of intrinsic ribonuclease (RNase) activity.
Keywords: Islets, Laser capture microdissection – LCM, Pancreas, PDGs, RNase inhibitors
Introduction
Powerful new tools are available to characterize the molecular signatures of tissues of interest in health and disease based on ribonucleic acid (RNA) and/or protein profiling. Laser capture microdissection (LCM) is a technique that theoretically enables isolation of RNA and/or protein from a compartment of interest within an organ. After identification of the compartment by microscopy, laser dissection is used to procure a sample of the cells of interest to permit subsequent gene expression and/or proteome profiling.1,2
LCM has been performed on formalin-fixed paraffin embedded (FFPE) as well as frozen tissues, with advantages and disadvantages for either procurement method.3 Sections from FFPE tissue typically have well-preserved morphology at the expense of poor RNA integrity, due to the cross linking of proteins and nucleic acids caused by formalin fixation. Although LCM has been widely applied to various types of frozen tissues, isolating high-quality RNA often still represents a challenge and requires stringent protocols to be followed, which is crucial for the performance of down-stream experiments.4 Ribonuclease (RNase) activity, either endogenous or due to external contamination, represents the most important barrier to extraction of high-quality RNA from tissues. Endogenous RNase levels vary widely between tissue types, being most abundant in pancreas, gall bladder, and skin.5 The focus here was to establish a method to reproducibly isolate high-quality RNA from LCM-derived tissue samples from pancreas.
The use of standard protocols employed in tissues with much lower RNase levels present than in pancreas was not sufficient for use in pancreatic tissue.6,7 Improved protocols have more recently been developed for LCM of pancreatic islets.8 However, due to the intrinsic autofluorescence of beta cells, islets are more easily identified in pancreatic sections than are pancreatic duct glands (PDG). Additionally, pancreatic islets have lower endogenous RNase activity than exocrine pancreas. We therefore set out to establish a protocol to permit reproducible recovery of high-quality RNA following LCM of the tissue compartments of interest in the pancreas.9-11 For the purposes of this work, we focused on the PDG compartment,12 recently proposed as a potential pancreatic stem cell niche, as well as pancreatic islets.
Materials and Methods
Tissue procurement
Humans
Pancreas was procured from a brain-dead non-diabetic organ donor by the Juvenile Diabetes Research Association network for pancreatic organ donors with diabetes (nPOD), coordinated through the University of Florida in Gainesville, Florida (Case ID 6104 and 6165). All procedures were in accordance with federal guidelines for organ donation and the University of Florida Institutional Review Board. The nPOD employs a standardized preparation procedure for pancreata recovered from cadaveric organ donors. The pancreas is divided into three main regions (head, body, and tail) followed by serial transverse sections throughout the medial to lateral axis, allowing sampling of the entire pancreas organ while maintaining anatomical orientation. As preparation is completed within 2 h, tissue integrity is maintained. Tissues intended for frozen blocks are trimmed to no larger than 1.5 × 1.5 cm, placed in molds with optimal cutting temperature compound (OCT) and snap-frozen in an isopentane-dry ice bath. For the presented experiments, 10 cryosections (8 μm) were cut from the body of the pancreas.
Rats
Studies were approved by the University of California Los Angeles Institutional Animal Care and Use Committee. Sprague Dawley wild-type rats were bred and housed with a standard of 12 h light/dark cycle at the University of California Los Angeles (UCLA) animal housing facility and fed ad libitum with regular chow diet. Following euthanasia, rat pancreas was rapidly dissected and then divided into two portions (head and body of pancreas, and tail of pancreas). The pancreata were processed as described. Ten to fifteen sections were cut from the head of the pancreas for each LCM experiment. Each individual experiment presented represents 1–2 rats. LCM and RNA isolation from both human and rat tissue was performed at UCLA.
Optimized LCM protocol
Rodent pancreas isolation and specimen freezing (Day 1)
An icy chamber for freezing of pancreatic tissue was prepared-by placing dry ice in a suitable container (e.g. styrofoam box, ~6″ × 5″ × 5″) and slowly filling it with isopentane until the level was just above the dry ice. Bubbling of isopentane occurred upon addition to dry ice, but once this had subsided, the mixture was ready for use.
Rodent pancreas was harvested, placed into a culture dish with cold phosphate saline, and any non-parenchymal tissue such as adipose or connective tissue was removed.
The bottom of a cryomold was covered with a thin layer of cold OCT compound and the specimen was placed into the mold after blotting on clean paper towel. Forceps were used to gently press the organ to the bottom of the cryomold; an embedding ring was placed on top and more OCT was carefully added until the specimen was completely covered and the ring was filled about half way. The cryomold was transferred into cooled isopentane using a perforated spoon; care was taken to not disturb the tissue orientation and to wait for the OCT to completely solidify (about 2 min). The processed specimen was placed on dry ice and transferred to a −80 °C freezer and stored in the −80 °C freezer wrapped in aluminum foil until needed. Human pancreata blocks received from nPod were processed as described below (Day 2).
Slide preparation and sectioning (Day 2)
The cryostat was pre-cooled to −20 °C (CM1900, Leica Microsystems; Wetzlar, Germany) and cryoblocks were transferred on dry ice and allowed to equilibrate for at least 30 min prior to cutting (but not exceeding 1 h, to avoid unnecessary thawing). Polyethylene naphthalate (PEN) membrane slides were ultraviolet irradiated at 260 mJ/cm2 for 30 min in order to sterilize them. Sections of 8 um thickness were cut, rotating the wheel slowly and holding down the glass shield lightly. Each section was mounted on the center of a labeled, room temperature PEN slide by moving the slide toward the specimen, thereby allowing the mounting media to melt and the tissue to be attached to the slide.
Each slide was placed in a pre-cooled slide box on dry ice immediately, limiting contact with air and moisture and stored at −80 °C when the desired number of slides were cut. Frequent cycling of the tissue block from −80 to −20 °C for cryosectioning may accelerate RNA degradation so, for best results, a sufficient number of sections for multiple LCM sessions (if possible) should be cut and mounted.
Preparation of reagents for LCM (Day 2)
RNase-free glass coplin jars were prepared for staining by rinsing with 100% Ethanol (EtOH), followed by distilled water, Ambion® RNase Zap® (Ambion®; Carlsbad, CA), followed by RNase-free water. The jars were allowed to dry before sealing with a glass lid and tape.
Staining solutions were prepared: 50-ml falcon tubes were filled with RNase-free 75% EtOH, 95% EtOH, 100% EtOH, RNase-free water (Arcturus® HistoGene Frozen Section Staining Kit, KIT0401) and cooled down to −20 °C (alcoholic solutions) or 4 °C (water). A 15-ml Falcon tube was filled with hematoxylin (3 ml; Gill No.1, Richard-Allan Scientific, Fisher Scientific).
Staining and LCM (Day 3)
RNase contamination was avoided by cleaning surfaces and tools with RNase Zap® solution. Under a chemical hood, 100-μl RNase inhibitor (ProtectRNA™ 500×, R7397; Sigma, St. Louis, MO) was added to each 50-ml falcon tube with staining solutions, mixed, and poured into labeled coplin jars. A volume of 6-μl RNase inhibitor was added to the hematoxylin solution. The surfaces of the microscope, slide, and tube holder were pre-cleaned with RNase Zap®.
The slide box with cryosections was kept on dry ice during the LCM procedure and one slide removed at a time for staining and subsequent laser microdissection. Each slide was thawed briefly (after removing it from dry ice) and subsequently stained for hematoxylin and eosin (H&E) in Coplin Jars immediately before proceeding to laser microdissection. Solutions had to be ice-cold and were prepared the day before use in Falcon tubes and stored at 2-8 °C. The RNase inhibitor was added shortly before (+/− 5 min) the staining procedure. The solutions were used for up to 20 slides and then replaced.
The staining protocol for cryosections was followed:
| Solution | Time (sec) | |
|---|---|---|
| 1 | 75% EtOH | 30 |
| 2 | RNase-free water | 30 |
| 3 | Hematoxylin | 40 |
| 4 | RNase-free water | 30 |
| 5 | 75% EtOH | 30 |
| 6 | 95% EtOH | 30 |
| 7 | 100% EtOH | 30 |
| 8 | Airdry | 120 |
LCM experiment were performed using an LMD7000 Laser Microdissection system (Leica; Wetzlar, Germany) cutting into a 0.5-ml tube cap (Axygen Scientific Inc, Union City, CA) filled with 10 μl of extraction buffer (PicoPure® RNA Isolation Kit, KIT0204) and 0.5 μl of RNase inhibitor (1 U/μl) (SUPERase·IN™, AM2694; Ambion® Carlsbad, CA). To avoid RNA degradation, staining and dissection was finished within 20 min and the tube placed with cap down on dry ice immediately.
RNA isolation (Day 3)
RNA isolation was performed on the day of the LCM experiment. Samples were thawed on ice (cap down) and spun for a few seconds once thawed. RNA was isolated following the protocol from Arcturus® PicoPure® RNA Isolation Kit (Applied Biosystems™; Foster City, CA) incorporating a DNase step and an extra spin at 16,000 rcf for membrane drying (see RNA Isolation Protocol for frozen LCM samples).
A volume of 0.75 μl SUPERase·IN™ RNase inhibitor was added to isolated RNA (for 15 μl sample), 1 μl was ali-quoted for Agilent testing and stored at −80 °C until further processing. The procedures for Day 2 (slide preparation and sectioning) and Day 3 (preparation of reagents for LCM, Staining and LCM, and RNA isolation) may all be combined into Day 2.
Quality control
RNA quality was checked with an Agilent Bioanalyzer 2100 using an RNA 6000 Pico LabChip kit (Agilent Technologies, Santa Clara, CA) (http://www.genomics.agilent.com/article.jsp?crumbAction=push&pageId=1648). An RNA Integrity Number (RIN) was computed by the instrument, using an algorithm as previously described.13 Briefly, RNA quality is estimated in each sample based on the fragment size distribution, including two peaks corresponding to 18S and 28S ribosomal RNAs, as well as a signal in the small molecular weight area, corresponding to small RNAs. This distribution is then compared to a large database of RNA samples and an RIN is computed.13,14
cDNA preparation and quantitative real time PCR
An amount of 0.5 μg of total RNA from each sample was denatured at 65 °C and then reverse transcribed using SuperscriptIII reverse transcriptase (Invitrogen) at 50 °C for 1 h. Real-time quantitative polymerase chain reaction (qPCR) was performed using ABI7900HT (Applied Biosystems™; Foster City, CA) with initial denaturation at 95 °C for 20 s, followed by 40 cycles of 94 °C for 1 s and 60 °C for 20 s, then continued with a dissociation stage. Each qPCR reaction contained 1 × Fast SYBR® Green Master Mix (Applied Biosystems™; Foster City, CA), 1 μM of each primer, and 400 ng cDNA. Relative mRNA expression of a certain target gene was determined using the comparative cycle threshold (Ct) method, where the amount of target cDNA was normalized to the internal control, cyclophilin cDNA. Gene expression is always depicted relative to the levels in pancreatic islets with a hypothetical value of 1. Values are means ± standard error of the mean of three independent experiments. Primer sequences are as follows: β-actin F:CCAACCGTGAAAAGATGACC, R: CCAGAGGCATACAGGGACAG; Insulin (human) F:AGAAGAGGCCATCAAGCAGA, R:CAGGTGTTGGTTCACAAAGG; Glucagon (human) F:GTACAAGGCAGCTGGCAAC, R:TGGGAAGCTGAGAATGATCTG; Mucin6 (human) F:CCAATCCCCAAGCTCATTTA, R:TGGTGCCTGCCTGTACTGGTGTG; CK-19 (human) F:GCCACTACACGACCATCC, R:CAAACTTGGTTCGGAAGTCAT; Insulin-1 (rat) F:TCATAGACCATCAGCAAGCAG, R:CTTGGGCTCCCAGAGGAC; Mucin6 (rat) F: GGCCCACAGTGACAGAGGCAA, R: TCCCCCTTCACCTTGGCTGGTA; CK-19 (rat) F:CTGGGTGGCAATGAGAAGAT, R: TCAAACTTGGTCCGGAAGTC.
The cDNA after reverse transcription was tested with an Agilent Bioanalyzer 2100 using a High Sensitivity D NA Analysis Kit (http://www.genomics.agilent.com/en/product.jsp?cid=AG-PT-105&tabId=AG-PR-1069).
Results and Discussion
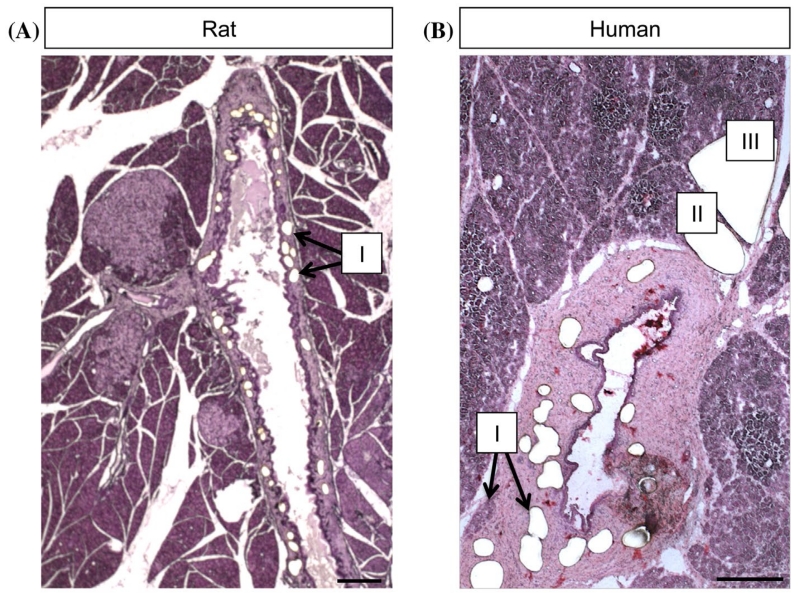
For the experiments described, PDGs were dissected first, followed by pancreatic islets (Fig. 1). The duration of staining and dissection of both compartments from a single slide were completed within 20 min. Samples were placed on dry ice (cap down) and RNA isolation was performed the same day.
Figure 1. Tissue sections from rodent and human pancreas. Shown are frozentissue sections (8 μm) of rat (A) and human pancreas (B), stained with hematoxylin, after LCM of PDGs (I), islets (II) and acinar tissue (III). Note that PDGs are embedded in mesenchyme surrounding large ducts and therefore are easy to identify. Scale bar = 200 μm.
The first consideration in procuring high-quality RNA from LCM aliquots of pancreatic tissue is the preservation of the pancreas. Since pancreas undergoes rapid autolysis following death, pancreas must be rapidly removed and preserved. Tissue preservation is typically achieved by either formalin fixation and paraffin embedding (FFPE) or cryopreservation. FFPE tissue has advantages over that of frozen tissue as it permits approaches that more readily facilitate identification of the tissue compartment of interest and more human pathological tissue archives of FFPE than frozen tissue are available. However, formalin’s excellent characteristics for tissue preservation, creating cross links between proteins, chemical alterations of RNA bases, and fragmentation, makes it almost impossible to isolate intact RNA from FFPE tissue. Optimized RNA extraction techniques (deparaffinization of samples, prolonged lysis, and proteinase K digestion) have enabled better recovery of RNA (up to 30%) compared to fresh tissue; however, quality remains poor as RNA is highly fragmented.15
The quality of RNA integrity (RIN) is measured by an algorithm applied to electrophoretic RNA analysis and is reported as a scale of 1 (totally degraded) to 10 (intact). Values of 7 or more are considered necessary for valid gene expression profiles. The RIN value has superseded the prior reliance on the 28S to 18S rRNA ratio (optimally 2:1) as the latter was unreliable. Multiple factors can influence RNA quality, such as time from organ isolation to freezing, storage, and staining.16,17
The process of degradation of RNA by RNase activation begins immediately upon excision of the tissue. RNases may also then be reactivated in an aqueous environment once the tissue is defrosted, a necessary step for LCM sample preparation (e.g. before cryosectioning) and during any staining procedures.18 We and others have found that the published protocols for LCM developed for use in organs with low-RNase expression are not suitable for pancreatic tissue. For example, LCM was used to isolate pancreatic islets9-11 with resulting RIN values of 2–4 and no detectable rRNA ratio. A more recent improved protocol for LCM of pancreatic islets did significantly improve the mean R/N value to 5.8.8
Treatment with RNase inhibitors
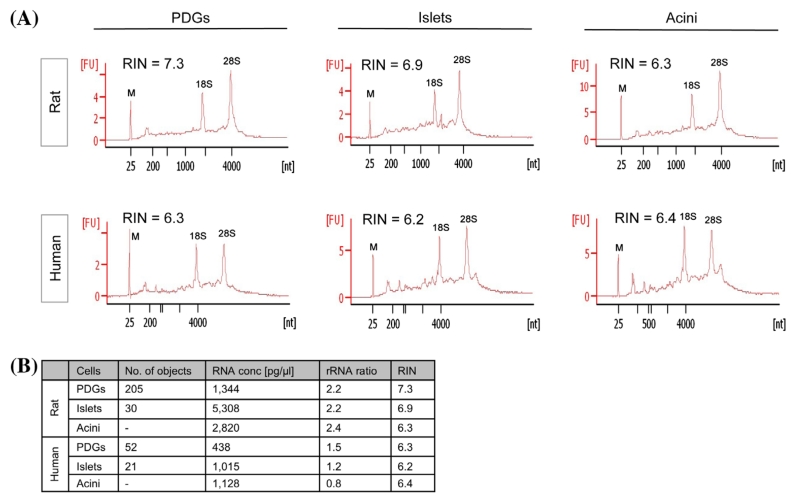
We investigated the potential of adjusting RNase inhibition to obtain intact RNA from LCM-derived samples from frozen rat as well as human pancreas. The use of RNase inhibitors at different steps of LCM protocols has been investigated by others, but with inconsistent results. Kube and colleagues reported improved RIN values from prostate cancer samples by adding RNase inhibitors to each staining solution,19 whereas RNase inhibition in colorectal tumors decreased overall RNA quality.6 Preservation of RNA integrity was furthermore reported for endometrial cancer tissue by adding RNase inhibitor immediately after RNA extraction.20 As RNase activity is particularly high in the pancreas, we evaluated the effect of application of RNase inhibition during both the staining procedure preceding LCM and during LCM of PDGs, islets and acinar tissue from rodent and human pancreas (Fig. 2A). ProtectRNA™ RNase inhibitor (1 × final concentration; Sigma Aldrich; St.Louis, MO) was added to all staining solutions. In addition, 5 U of SUPERase·IN™ RNase inhibitor (Ambion®; Carlsbad, CA) was added to the extraction buffer and again after RNA isolation. The detailed protocol is described in Materials and Methods (Optimized protocol). One hundred and twelve PDGs and 30 islets from rat pancreatic sections were dissected; for human samples, 52 PDGs and 21 islets were collected. By this approach, excellent yields of high-quality RNA were consistently obtained from LCM-derived pancreatic cryosections, never previously reported for pancreatic tissue using 10–15 sections (8 μm). For rat pancreatic tissue, RNA quality was slightly higher, with RIN values measured between 6 and 7 for islets and PDGs and rRNA ratios ranging from 2.2 to 2.4. Human samples had RIN values around 6 (6.2–6.4) for both islets and PDGs and rRIN ratios between 0.8 and 1.5 (Fig. 2B). RNA yield for rodent PDG samples was 168 pg/PDG and islets 2,477 pg/islet. The corresponding values for human tissue samples were 118 pg/PDG and 677 pg/islet. The optimized protocol was repeated multiple times with different rodent (n = 5) and human (n = 4) specimens resulting in RNA yields of 130 ± 14 and 100 ± 34 pg/PDG, rRNA ratios of 1.9 ± 0.3 and 1.4 ± 0.1, and RIN values of 6.2 ± 0.3 and 6.0 ± 0.3.
Figure 2. RNA inhibition results in excellent RNA quality and quantity of laser microdissected rat and human pancreatic samples. (A) The presence of RNA inhibitors in staining solutions, as well as during and after the LCM experiment, resulted in isolation of RNA of excellent quality with RIN values ranging from 6.2 to 7.3 in rat and human tissue. Positions of 18S and 28S ribosomal RNA are indicated and serve as an additional quality control. M indicates marker peak. (B) Summary of parameters represented in the electropherograms in (A).
Gene expression determined in dissected samples by real-time PCR
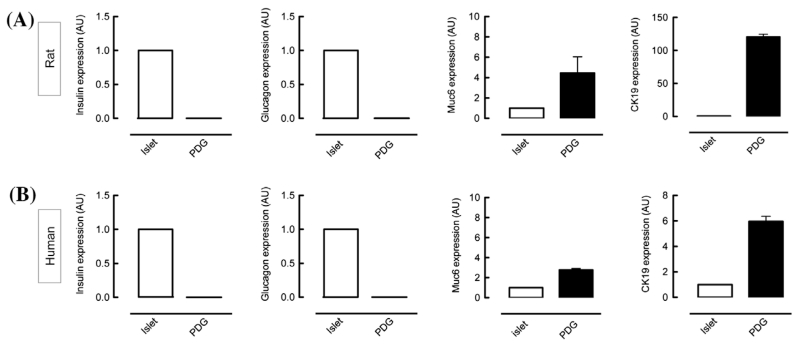
In order to perform down-stream experiments such as RNA-Seq, it is essential to test not only the quality of the isolated RNA, but also the expression of genes expected to be present in the isolated cell population. Therefore, mRNA expression of characteristic islet cell genes (insulin and glucagon) as well as a PDG/ductal cell marker mucin6 (muc6) and cytokeratin-19 (CK-19) were analyzed (Fig.3). As expected, high levels of Muc6 and CK-19 were expressed in PDGs compared to islets. Although at very low levels, transcripts of insulin and glucagon were found to be present in the PDGs (approximately 1000× less compared to islet levels).
Figure 3. Additional validation of RNA quality by mRNA transcript identification. The mRNA expression levels in dissected samples in rat (A) and human (B) PDGs were compared to those of pancreatic islets (set as 1). As expected, islets express high levels of insulin-1 and glucagon. Although at very low levels, these transcripts were also detected in PDGs. The characteristic marker Mucin6 and CK-19 were found in PDGs, with lower levels present in islets.
The primary goal was to establish a protocol that permits recovery of intact RNA from pancreas after LCM. The prior efforts used to optimize RNA integrity after LCM in other tissues by adjusting conditions of section collection and fixation were not sufficient in pancreas. Instead, because of the very high endogenous RNase activity in pancreas, a protocol was developed with an emphasis on RNase inhibition.
The critical modification required to obtain usable quality RNA from pancreas after LCM was the use of RNase inhibition at multiple steps during the experimental procedure. To maximize the RNA quality, RNase inhibitors were used during the entire staining procedure, as well as during laser microdissection and they were also added to samples after RNA isolation. By this modification, we began with well-cryopreserved pancreas and were able to reproducibly procure good recovery of high-quality RNA after LCM of pancreas.
Modern amplification techniques have made it possible to work with extremely low amounts of RNA (1 pg) and perform gene expression analysis subsequently. However, in order to achieve more robust results and minimize the loss of rare transcripts, it is desirable to recover at least 1 ng of material. With the described optimized protocol, we were able to demonstrate for the first time high-quality RNA with minimal degradation as reflected by presence of 18S and 28S ribosomal RNA and RIN values ~7 in laser microdissected pancreatic tissue. Combined with the amount of recovered RNA (5–45 pg depending on the cell type collected), these results provide a good basis for down-stream experiments such as RNA-Seq.
Acknowledgments
This research was performed with the support of the nPOD, a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International. Organ Procurement Organizations partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/our-partners.php.
Funding This work was supported by NIH/NIDDK [DK077967], Larry L. Hillblom Foundation, and the Juvenile Diabetes Research Foundation. We appreciate the editorial assistance of Bonnie Lui from the Hillblom Islet Research Center at UCLA.
List of Abbreviations
- CK-19
cytokeratin-19
- EtOH
ethanol
- FFPE
formalin-fixed paraffin embedded
- LCM
laser capture microdissection
- Muc6
mucin6
- PDGs
pancreatic duct glands
- PEN
polyethylene naphtalate
- RIN
RNA integrity number
- qPCR
quantitative RT-PCR
- RNase
ribonuclease
Footnotes
Disclaimer Statements
Contributors Alexandra E. Butler performed the studies, assisted in study design and interpretation and writing the manuscript. Aleksey Matveyenko assisted in performing studies and study interpretation. David Kirakossian and Johanna Park assisted in performing the studies. Tatyana Gurlo assisted in performing the studies and manuscript preparation. Peter Butler contributed to the study design, study interpretation and preparation of the manuscript.
Conflict of interest The authors have no conflicts of interest.
References
- 1.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, et al. Laser capture microdissection. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 2.Suarez-Quian CA, Goldstein SR, Pohida T, Smith PD, Peterson JI, Wellner E, et al. Laser capture microdissection of single cells from complex tissues. Biotechniques. 1999;26:328–35. doi: 10.2144/99262rr03. [DOI] [PubMed] [Google Scholar]
- 3.Farragher SM, Tanney A, Kennedy RD, Paul Harkin D. RNA expression analysis from formalin fixed paraffin embedded tissues. Histochem Cell Biol. 2008;130:435–45. doi: 10.1007/s00418-008-0479-7. [DOI] [PubMed] [Google Scholar]
- 4.Copois V, Bibeau F, Bascoul-Mollevi C, Salvetat N, Chalbos P, Bareil C, et al. Impact of RNA degradation on gene expression profiles: Assessment of different methods to reliably determine RNA quality. J Biotechnol. 2007;127:549–59. doi: 10.1016/j.jbiotec.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Sorrentino S. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell Mol Life Sci. 1998;54:785–94. doi: 10.1007/s000180050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Wang L, Zhu T, Gao X, Li J, Wu Y, et al. Improvement of tissue preparation for laser capture microdissection: application for cell type-specific miRNA expression profiling in colorectal tumors. BMC Genomics. 2010;11:163–175. doi: 10.1186/1471-2164-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang WZ, Oeschger FM, Lee S, Molnár Z. High quality RNA from multiple brain regions simultaneously acquired by laser capture microdissection. BMC Mol Biol. 2009;10:69–78. doi: 10.1186/1471-2199-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturm D, Marselli L, Ehehalt F, Richter D, Distler M, Kersting S, et al. Improved protocol for laser microdissection of human pancreatic islets from surgical specimens. J Visualized Exp.: JoVE. 2013;71:1–6. doi: 10.3791/50231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marselli L, Thorne J, Ahn YB, Omer A, Sgroi DC, Libermann T, et al. Gene expression of purified β-Cell tissue obtained from human pancreas with laser capture microdissection. J Clin Endocrinol Metab. 2008;93:1046–53. doi: 10.1210/jc.2007-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marselli L, Sgroi DC, Bonner-Weir S, Weir GC. Laser capture microdissection of human pancreatic beta-cells and RNA preparation for gene expression profiling. Methods Mol Biol. 2009;560:87–98. doi: 10.1007/978-1-59745-448-3_8. [DOI] [PubMed] [Google Scholar]
- 11.Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS ONE. 2012;7:e30415. doi: 10.1371/journal.pone.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate shh-induced metaplasia. Gastroenterology. 2010;138:1166–77. doi: 10.1053/j.gastro.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3–16. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller O, Lightfoot S, Schroeder A. Notes. 2004. RNA integrity number (RIN) – standardization of RNA quality control. Publication Number 5989-1165EN Contract No.: Publication Number 5989-1165EN. [Google Scholar]
- 15.Chung JY, Braunschweig T, Hewitt SM. Optimization of recovery of RNA from formalin-fixed, paraffin-embedded Tissue. Diagn Mol Pathol. 2006;15:229–36. doi: 10.1097/01.pdm.0000213468.91139.2d. [DOI] [PubMed] [Google Scholar]
- 16.Medeiros F, Rigl CT, Anderson GG, Becker SH, Halling KC. Tissue handling for genome-wide expression analysis: a review of the issues, evidence, and opportunities. Arch Pathol Lab Med. 2007;131:1805–16. doi: 10.5858/2007-131-1805-THFGEA. [DOI] [PubMed] [Google Scholar]
- 17.Jewell SD, Srinivasan M, McCart LM, Williams N, Grizzle WH, LiVolsi V, et al. Analysis of the Molecular Quality of Human Tissues. Am J Clin Pathol. 2002;118:733–41. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- 18.Fend F, Emmert-Buck MR, Chuaqui R, Cole K, Lee J, Liotta LA, et al. Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis. Am J Pathol. 1999;154:61–6. doi: 10.1016/S0002-9440(10)65251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kube DM, Savci-Heijink CD, Lamblin AF, Kosari F, Vasmatzis G, Cheville JC, et al. Optimization of laser capture microdissection and RNA amplification for gene expression profiling of prostate cancer. BMC Mol Biol. 2007;8:25–38. doi: 10.1186/1471-2199-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings M, McGinley CV, Wilkinson N, Field SL, Duffy SR, Orsi NM. A robust RNA integrity-preserving staining protocol for laser capture microdissection of endometrial cancer tissue. Anal Biochem. 2011;416:123–5. doi: 10.1016/j.ab.2011.05.009. [DOI] [PubMed] [Google Scholar]