Significance
By laser surgery, genetics, and pharmacology, we demonstrate that neurons of the nematode Caenorhabditis elegans undergo a novel form of regeneration that is largely independent of defined regeneration pathways, including DLK, which underlies axon regeneration from C. elegans to mammals. Our results indicate genetic and molecular connections between DLK-independent regeneration and a previously studied activity-dependent ectopic axon outgrowth in C. elegans. We also note numerous similarities with lesion-conditioned regeneration, in which reduction of sensory activity triggers robust axon regeneration in the mammalian CNS. Our study unites disparate forms of neurite outgrowth to uncover the molecular mechanisms that modulate regeneration in the adult CNS and suggests that ectopic outgrowth might represent a powerful gene discovery platform for regeneration.
Keywords: lesion conditioning, axon regeneration, femtosecond laser ablation, DLK-1, activity-dependent ectopic axon outgrowth
Abstract
During development, a neuron transitions from a state of rapid growth to a stable morphology, and neurons within the adult mammalian CNS lose their ability to effectively regenerate in response to injury. Here, we identify a novel form of neuronal regeneration, which is remarkably independent of DLK-1/DLK, KGB-1/JNK, and other MAPK signaling factors known to mediate regeneration in Caenorhabditis elegans, Drosophila, and mammals. This DLK-independent regeneration in C. elegans has direct genetic and molecular links to a well-studied form of endogenous activity-dependent ectopic axon outgrowth in the same neuron type. Both neuron outgrowth types are triggered by physical lesion of the sensory dendrite or mutations disrupting sensory activity, calcium signaling, or genes that restrict outgrowth during neuronal maturation, such as SAX-1/NDR kinase or UNC-43/CaMKII. These connections suggest that ectopic outgrowth represents a powerful platform for gene discovery in neuronal regeneration. Moreover, we note numerous similarities between C. elegans DLK-independent regeneration and lesion conditioning, a phenomenon producing robust regeneration in the mammalian CNS. Both regeneration types are triggered by lesion of a sensory neurite via reduction of neuronal activity and enhanced by disrupting L-type calcium channels or elevating cAMP. Taken as a whole, our study unites disparate forms of neuronal outgrowth to uncover fresh molecular insights into activity-dependent control of the adult nervous system’s intrinsic regenerative capacity.
One of the principal goals of modern neuroscience is the comprehensive understanding and therapeutic application of neuronal regeneration (1). This goal is particularly relevant in the case of the mammalian central nervous system (CNS), which regenerates poorly. Efforts to enhance axon regeneration generally fall into two broad categories: eliminating or blocking nonpermissive extrinsic inhibitors or promoting a neuron’s intrinsic regenerative capacity (2). Although much research in previous decades focused on the extrinsic angle, recent encouraging developments, particularly in invertebrate models, increasingly examine the cell intrinsic control of regeneration. Studies demonstrate that axon regeneration recruits or recapitulates mechanisms involved in a diverse range of biological processes, including synapse formation, stress response, apoptosis, and development.
During development, neuronal electrical activity acts as a common intracellular feedback mechanism to establish appropriate connections and modulate outgrowth (3). Subsequently, a neuron transitions from a state of rapid growth to a stable morphology, and neurons within the adult mammalian CNS lose their ability to effectively regenerate in response to injury. A striking exception to this paradigm is lesion conditioning, a phenomenon exemplified by the dorsal root ganglion (DRG), where peripheral axon damage enables robust central axon regeneration (4). The peripheral lesion is thought to revert the neuron to a growth-permissive state in an activity-dependent manner (5). These observations suggest that activity-dependent inhibitors of neurite outgrowth may represent a potent therapeutic target for enhancing neuronal regeneration in the face of injury or disease.
The nematode Caenorhabditis elegans is a well-established and powerful model system (6) for the genetic and molecular study of both activity-dependent neuronal development and regeneration. Specifically, several studies (7–9) have observed spontaneous ectopic axon outgrowths from four bipolar sensory neurons of mutant animals raised under stressed conditions (high temperature). An extensive genetic study indicated that the mutations disrupt sensory activity, which triggers cell-specific ectopic outgrowth after the neurons establish their initial pattern of innervations (10). More recently, the application of advanced subcellular laser ablation techniques (11) to C. elegans has permitted quantitative in vivo study of single-neuron regeneration in this genetically tractable system. Several studies have revealed the role of conserved mitogen-activated protein kinase (MAPK) pathways, including the dual leucine zipper kinase (DLK) and the parallel but coordinated c-Jun N-terminal kinase (JNK) pathways (12, 13, 14). Other studies have clarified the beneficial role of caspase activity (15) and the role of calcium signaling (16, 17). Thus, C. elegans, by virtue of its facile genetics and amenability to precise laser surgery, is an excellent system for studying axon outgrowth and regeneration.
In a previous study, we sought to dissect the subcellular components of the C. elegans ASJ sensory neurons by femtosecond laser surgery to establish their role in development and behavior (18). ASJ axons rapidly regenerate to the vicinity of their original synaptic targets, but regeneration is prevented by mutation of dlk-1, which plays a crucial role in neuronal regeneration in C. elegans (12), Drosophila (19), and mammals (20, 21). Remarkably, although dlk-1(ju476) completely prevents regeneration after axotomy, we discovered that strong DLK-independent regeneration returns under two conditions, which we explore in this study. First, regeneration occurs after severing both the axon and sensory dendrite, indicating that sensory dendrite cuts trigger robust DLK-independent regeneration. Second, even without a dendrite cut, regeneration occurs under mutations that also trigger ectopic axon outgrowth. Here, we demonstrate that this novel type of regeneration is largely independent of both the DLK-1/DLK and the KGB-1/JNK pathways. We find numerous direct links between this DLK-independent regeneration and ectopic outgrowth in the same neurons. Finally, we note similarities between DLK-independent regeneration in C. elegans and regeneration after lesion conditioning in mammals, including shared phenotypes, role of L-type voltage-gated calcium channel (L-VGCC), and mediation by cyclic adenosine monophosphate (cAMP). As such, our study establishes a novel regeneration pathway in C. elegans, where physical, genetic, or pharmacological lesion of a sensory neurite or sensory signaling removes activity-dependent developmental inhibitors to permit robust regeneration.
Results
Transection of the Axon and Sensory Dendrite Triggers DLK-Independent Regeneration in the ASJ Neuron.
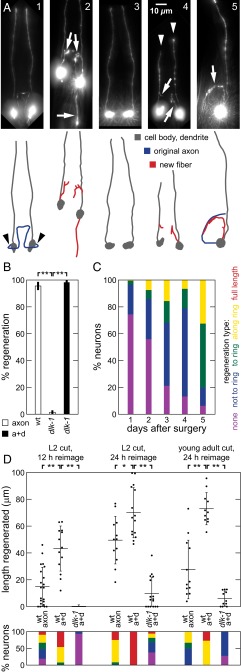
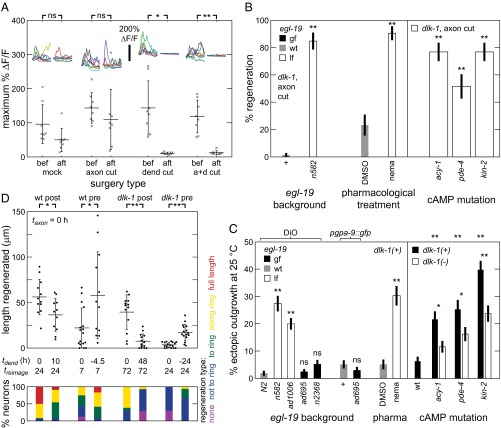
The ASJ neuron in C. elegans is one of the amphids, a set of 12 stereotyped bilateral bipolar sensory neurons, each composed of a cell body, a dendrite terminating in sensory cilia in the nose, and an axon mediating the synaptic connections in a central neuropil called the nerve ring (Fig. 1 A1 and A5) (22). In wild-type (WT) C. elegans, in vivo laser severing of ASJ axons results in robust regeneration, with >95% of neurons displaying regenerative outgrowth within several days (Fig. 1 A2 and B), whereas dendrite transection results in little or no growth, even after 4 days (18, 23). Consistent with previous studies of several neuron types in C. elegans (12, 13, 18), the dlk-1(ju476) mutation eliminates conventional DLK-1–mediated axon regeneration after axotomy alone, with <5% of postsurgery neurons regenerating (Fig. 1 A3 and B). Remarkably, we find that concomitantly (Δt < 1 min) severing both the ASJ axon and the sensory dendrite (axon+dendrite, a+d) restores regeneration rates in dlk-1 animals (Fig. 1 A4 and B), suggesting that severing the sensory dendrite triggers a nonconventional (i.e., DLK-independent) regeneration pathway that is not activated by axotomy alone. To confirm results in a different neuron, we performed the same surgeries on the ASH amphid neuron (Fig. S1B). Results are consistent with findings in the ASJ neuron, showing a reduction in regenerated length from dlk-1 and an increase in regenerated length from an additional dendrite cut.
Fig. 1.
Sensory dendrite cuts enhance ASJ regeneration in C. elegans WT and dlk-1. (A) Postsurgery GFP fluorescence images and line drawings of ASJ neuron with soma and dendrite (dark gray), normal axon path (blue), and regenerated fiber (red) in (A1–A4) dorsal-ventral and (A5) lateral views. White arrows indicate regenerative growth, white arrowheads indicate cut dendrites, and black arrowheads indicate typical axotomy location. (A1) Mock surgery. (A2) WT regeneration. (A3) Lack of regeneration in dlk-1 after axon surgery. (A4) Regeneration in dlk-1 after axon+dendrite surgery. (A5) Regeneration directed along ring in dlk-1; tax-4 after axon surgery. (B) dlk-1 eliminates and dendrite cut restores regeneration. Percentage of neurons regenerating after surgery (45 ≤ n ≤ 68). (C) Regenerated fibers aimed at the nerve ring, where original synaptic targets are located. Percentage of neurons with indicated regeneration extent defined by fiber with farthest outgrowth. Full-length regeneration included in regeneration “growth along ring” category. Data are aggregated across multiple genetic backgrounds, all containing dlk-1 (80 ≤ n ≤ 337). Fig. S1C shows single-background data. (D) Dendrite cuts enhance axon regeneration in the WT. (Upper) Total regeneration lengths for various surgeries and backgrounds. Each point indicates a single animal measurement. Only 1 of 15 dlk-1 neurons regenerated for L2 surgery (12-h reimaging; at L3); points are not shown for simplicity in this case. (Lower) Percentage of neurons with indicated regeneration extent defined by farthest outgrowth as in C. Data are represented as average ± SD. a+d, axon+dendrite. *P < 0.05; **P < 0.001.
Fig. S1.
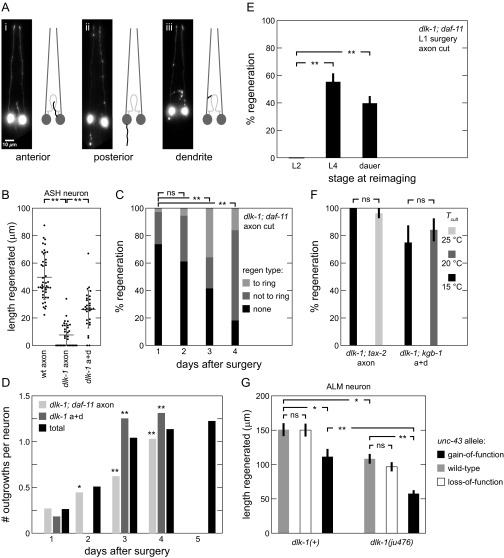
Extended characterization of DLK-independent regeneration. (A) Fundamental regeneration types. Postsurgery GFP fluorescence images and line drawings of ASJ neuron, with soma and dendrite (dark gray), normal axon path (light gray), and regenerated fiber (black) shown in dorsal-ventral view. (B) ASH total regeneration lengths for various surgeries and backgrounds. Each point indicates a single animal measurement. (C) Regenerations are aimed at nerve ring. Percentage of neurons with indicated regeneration extent defined by fiber with farthest outgrowth. P values are calculated comparing percentage of neurons with regeneration reaching ring (53 ≤ n ≤ 141). Additional data in Fig. 1C. (D) Number of regenerated outgrowths increases after surgery. Average number of outgrowths per neuron after surgery. Total data aggregated across multiple genetic backgrounds (51 ≤ n ≤ 337, except n = 11 for dlk-1 axon+dendrite on day 1). (E) DLK-independent regeneration occurs late in development. Percentage of neurons regenerating at indicated times after L1 surgery (n = 41 for L2, 67 for L4, and 88 for dauer). (F) DLK-independent regeneration is not temperature-sensitive. Percentage regeneration of various backgrounds under different temperatures (n = 27, 27, 12, and 19 from left to right). (G) Multiple pathways mediate regeneration in ALM neuron; dlk-1 and unc-43(gf) independently reduce length of ALM axon regeneration in dlk-1(+) and dlk-1 backgrounds (20 ≤ n ≤ 29). Data are represented as average ± SD. a+d, axon+dendrite; ns, not significant. *P < 0.05; **P < 0.001.
Both conventional regeneration after axotomy alone in WT animals and DLK-independent regeneration generate similar outgrowths of three basic morphologies, although the dynamics of regeneration are distinct (see below). The ASJ axon normally projects ventrally from the cell body and then extends along the nerve ring, where it makes numerous synaptic connections with other neurons (blue lines in Fig. 1 A1 and A5). As shown in Fig. 1A and Fig. S1A, regeneration projects from the cell body anteriorly to the ring and follows the ring posteroventrally (Fig. 1 A2, A4, and A5), projects posteriorly from the cell body (Fig. 1A2), or occasionally projects off the dendrite and follows the ring posteroventrally (Fig. 1 A2 and A4). The outgrowth morphologies also match ectopic axon outgrowths from the same neurons triggered by genetic defects in sensory activity (see below) (10). As shown in Fig. 1C, we quantified the regeneration extent and found that >80% of DLK-independent regeneration successfully grows into the nerve ring by 5 d postsurgery (e.g., Fig. 1 A2, A4, and A5). These data include regeneration from dlk-1 axon+dendrite cuts as well as axon cuts in double mutants, with dlk-1 and mutations triggering regeneration listed in Fig. 2B (see below). SI Results (Fig. S1 C and D) details additional observations of this regeneration, illustrating and quantitatively comparing regeneration in single backgrounds. Thus, DLK-independent regeneration is morphologically similar to conventional regeneration, and both are clearly directed toward the original axon targets in the nerve ring.
Fig. 2.
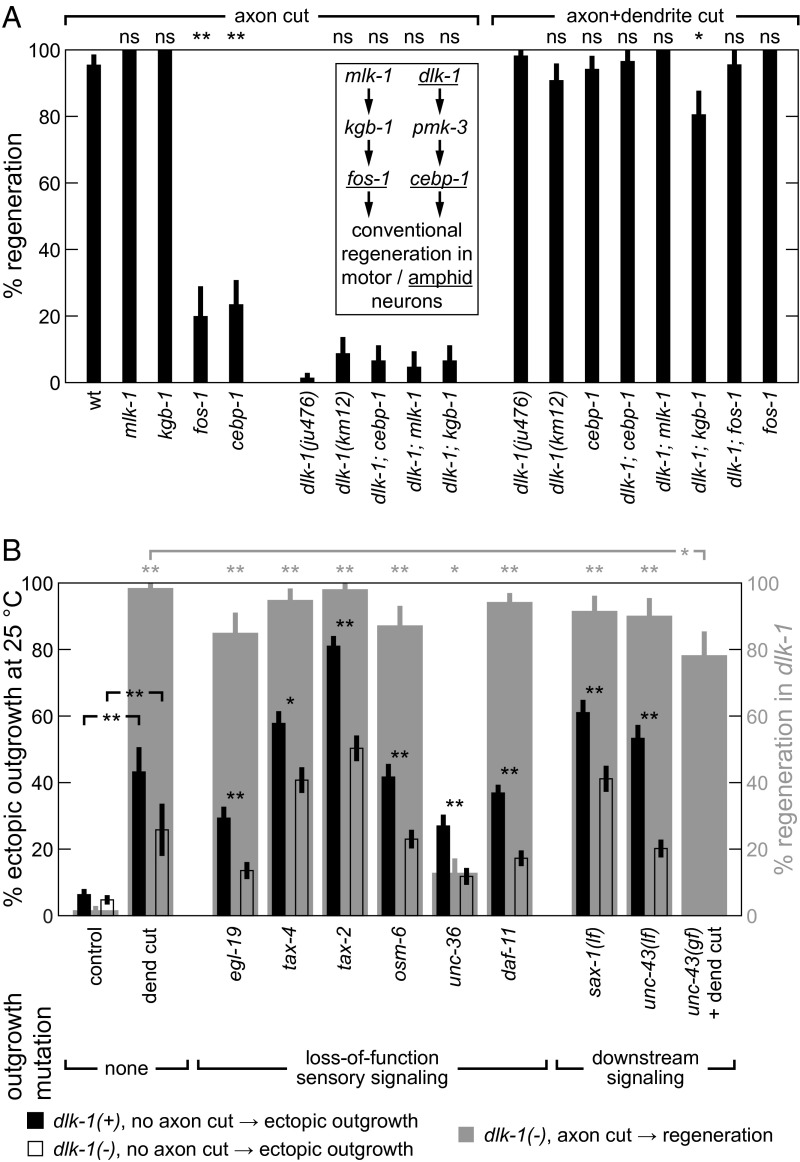
(A) MAPK genes are not critical for DLK-independent regeneration. Percentage of neurons regenerating after indicated surgery (30 ≤ n ≤ 68, except for n ≥ 20 for strains with fos-1 or mlk-1). WT and dlk-1 data are replicated from Fig. 1B. P values are calculated comparing data point with control value (leftmost data point in each group). (B) Comparison of DLK-independent regeneration and ectopic outgrowth. Gray-shaded results: Outgrowth-inducing mutations and dendrite cuts trigger DLK-independent regeneration. Percentages of neurons regenerating after axon lesion are shown (30 ≤ n ≤ 68). P values (indicated above plot) are calculated comparing data point with control (i.e., dlk-1 with axon cut), except for unc-43(gf). Leftmost two columns are replicated from Fig. 1B. Black and clear bars: Dendrite cuts trigger and dlk-1 partly mediates ectopic outgrowth. Percentages of neurons with ectopic outgrowth (n ≥ 150, except n ≥ 31 for dendrite cut data). P values (indicated directly above data) are calculated comparing measurements from different dlk-1 alleles or as indicated. Data are represented as average ± SD. ns, Not significant. *P < 0.05; **P < 0.001.
Given that a dendrite cut enhances regeneration in dlk-1, we next sought to determine if this enhancement exists in the WT or arises by disruption of dlk-1. We, therefore, examined the impact of dendrite cuts on regeneration in WT animals. After surgery, most ASJ neurons in WT animals substantially regenerate; therefore, to quantify regeneration after different surgeries, we measured regenerated outgrowth length and penetrance into the nerve ring (Materials and Methods and Fig. S2). As shown in Fig. 1D, in WT ASJ neurons the addition of a dendrite cut generates significantly longer regeneration with greater success penetrating into the nerve ring compared to axon lesion alone. Enhancement occurs both at relatively short regeneration times of 12 and 24 h after surgery during the L2 larval stage and after surgery during the later young adult stage. In contrast, DLK-independent regeneration resulting from surgery in L1 or L2 typically begins during the L4 larval stage: reimaging before L4 (about 24 h after L2) reveals little regeneration in dlk-1 mutants (Fig. 1D and Fig. S1E). Although DLK-mediated regeneration appears to initiate within about 12 h after surgery or less if enhanced by dendrite cut, DLK-independent regeneration initiates from 24 h to several days after surgery (Fig. 1C). Axon+dendrite surgeries have a similar effect in young adult animals, resulting in relatively modest regeneration in dlk-1 compared to WT after 24 h. Despite the limited DLK-independent regeneration at earlier reimaging times shown in Fig. 1D, reimaging at later times confirms continued regeneration that, although slower than DLK-mediated regeneration, eventually reaches and extends along the nerve ring (Figs. 1 C and D and 3D for length measurement). These observations suggest that, in WT animals, both DLK-mediated regeneration and a novel DLK-independent regeneration are active in a single neuron and enhanced by dendrite surgery. In this context, mutation of dlk-1 eliminates DLK-mediated regeneration, revealing the weaker but distinct DLK-independent regeneration.
Fig. S2.
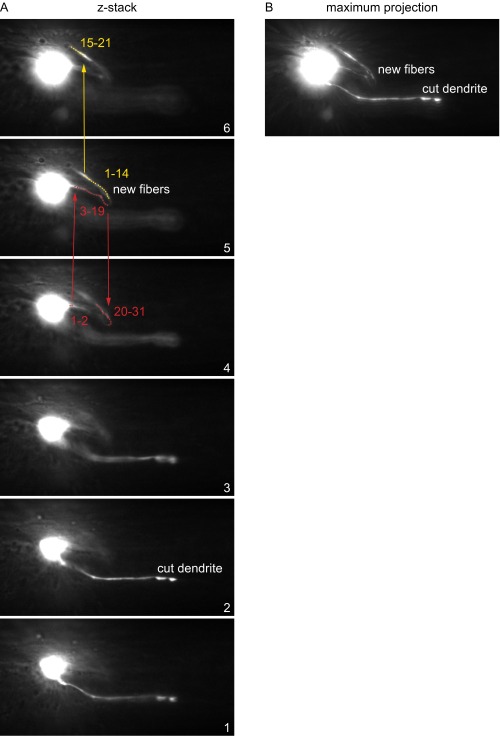
Procedure to measure regenerated axon lengths. (A) Slices of a z stack showing regenerated fiber. In the ImageJ plugin 3D Distance Tool, we selected points (indicated by numbers) unidirectionally along the entire length of the regenerated fiber. Red points track a new outgrowth from the cell body, and yellow points indicate a second branch. (B) Maximum projection of the z stack in A.
Fig. 3.
(A) Dendrite lesion abolishes sensory-evoked activity in ASJ neuron. Intracellular calcium dynamics before (bef) and after (aft) ASJ surgery. Each x indicates a single animal measurement. Insets show 30-s calcium traces. (B and C) Reduction of EGL-19 function and elevation of cAMP signaling trigger DLK-independent regeneration and ectopic outgrowth. Modulation of function by genetic lesion of egl-19 and pharmacology. (B) Percentage of neurons regenerating (n ≥ 30). P values are calculated comparing data point with dlk-1, except for that nemadipine-treated was compared with DMSO. Leftmost two columns are replicated from Fig. 2B. (C) Percentage of neurons with ectopic outgrowth (n ≥ 150). ASJ visualized by DiO or fluorescent protein. Significance indicators positioned directly above data are calculated comparing data point with control value (leftmost data point in each group). Significance indicators positioned between data points compare data from different dlk-1 alleles. WT data in Right are replicated from Fig. 2B. (D) Dendrite cuts enhance axon regeneration in a time-dependent manner. (Upper) Total regeneration lengths for various surgeries and backgrounds. Each sequential surgery set is processed concurrently with a concomitant surgery set. All axons were cut at taxon = 0 h. Dendrite cut (tdend) and reimaging (treimage) were at indicated times. Each point indicates a single animal measurement. (Lower) Percentage of neurons with indicated regeneration extent defined by farthest outgrowth. Data are represented as average ± SD. a+d, axon+dendrite; ns, not significant. *P < 0.05; **P < 0.001.
DLK-Independent Regeneration after ASJ Axon+Dendrite Lesion Is Independent of Defined MAPK Signaling Pathways.
To further define the mechanisms underlying ASJ regeneration, we tested additional known genetic modulators by noting the frequency of regeneration after the axon or axon+dendrite surgery types (Fig. 2A). First, we tested dlk-1(km12), which produces regeneration rates not statistically different from dlk-1(ju476) after either type of surgery and confirms that the dlk-1 regeneration phenotypes are not limited to one dlk-1 allele. Second, we tested MAPK genes that mediate conventional motor neuron regeneration in C. elegans (24) for a role in conventional ASJ regeneration. As shown in Fig. 2A, Inset, conventional axon regeneration in motor neurons requires a coordinated activation of the DLK-1/PMK-3/CEBP-1 p38 and the parallel but coregulated MLK-1/KGB-1/FOS-1 JNK MAPK pathways (14, 25). Although mutation of cebp-1 and fos-1, which encode C/EBP and Fos basic region-leucine zipper transcription factors, significantly reduces conventional regeneration, mutation of mlk-1 and kgb-1 does not (Fig. 2A, Left). Thus, unlike motor neuron regeneration, ASJ conventional regeneration (Fig. 2A, Inset) does not require mlk-1 or kgb-1. Third, we tested MAPK genes for roles in DLK-independent regeneration. Double mutants of dlk-1 and these genes (Fig. 2A) do not exhibit significantly different regeneration rates from dlk-1 after either type of surgery (only kgb-1 shows a mild 20% reduction after a+d). These results suggest that DLK-independent regeneration involves a novel mechanism largely independent of both the dlk-1 and kgb-1 MAPK pathways shown to mediate most C. elegans regeneration.
DLK-Independent Regeneration Shares Phenotypic and Genetic Links with Ectopic Axon Outgrowth.
Although DLK-independent regeneration is distinct from previously defined axon regeneration, we observe striking similarities to another type of amphid growth called activity-dependent ectopic axon outgrowth, which occurs primarily at 25 °C cultivation. Both mechanisms produce similar morphologies, with projections running either posteriorly (7) or into the nerve ring (Fig. 1A). In addition, both ectopic outgrowth (10) and ASJ DLK-independent regeneration appear in the later stages of the animal’s lifecycle, well after the amphid sensory neurons have developed their final morphology: regeneration in animals with dlk-1 usually does not emerge until the L4 larval stage or later (Fig. 1D and Fig. S1E). SI Results lists more details on ectopic outgrowth characteristics.
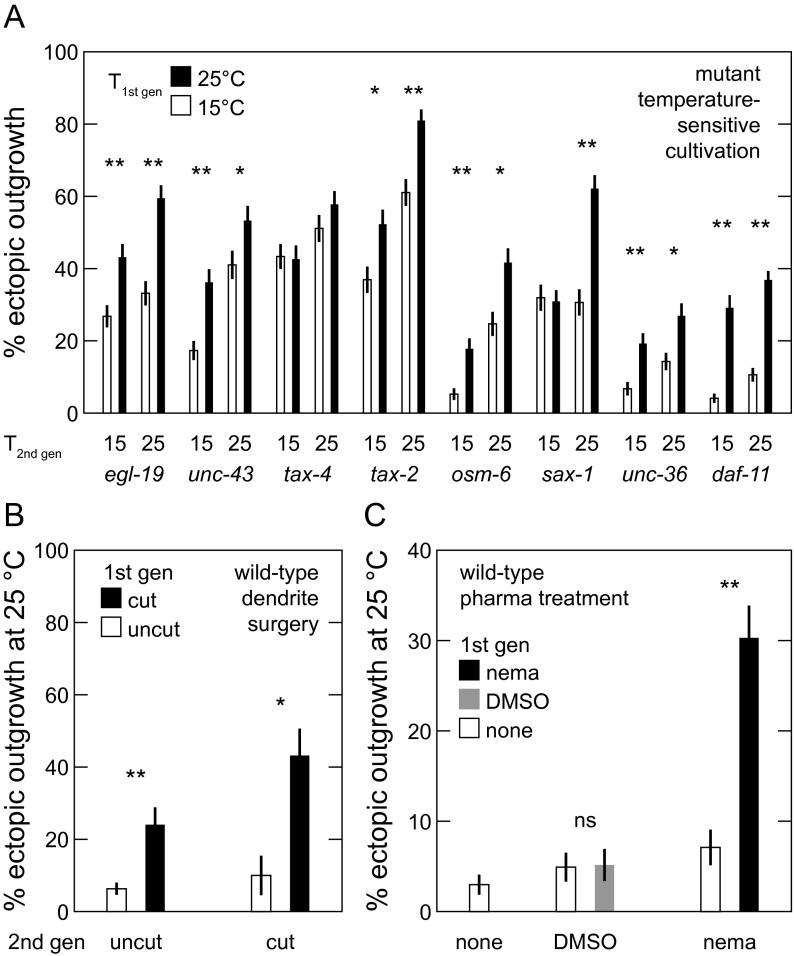
Based on these phenotypic similarities, we asked if the same physical or genetic lesions might trigger both ectopic outgrowth and DLK-independent regeneration. First, dendrite cuts trigger DLK-independent axon regeneration, and we find that dendrite lesions alone can trigger ectopic axon outgrowth (Fig. 2B, left gray bars) under conditions required for ectopic outgrowth (i.e., 25 °C cultivation and surgery on two successive generations—see SI Results for discussion of maternal effect). Second, we tested an array of mutations that reduce sensory activity and trigger ectopic outgrowth to assess their role in triggering DLK-independent regeneration. These “outgrowth” mutations disrupt genes encoding the L-VGCC α1 subunit Cav1.2 (egl-19/CACNA1C) and α2/δ-1 subunit (unc-36/CACNA2D1), cyclic nucleotide-gated channel subunits α and β (tax-4/CNGA1 and tax-2/CNGB1, respectively), a transmembrane guanylate cyclase involved in signal transduction (daf-11), and intraflagellar transport protein 52 (osm-6, which fails to develop functional sensory dendrites) (10). As shown in Fig. 2B, gray bars, most outgrowth mutations also successfully trigger DLK-independent regeneration after axotomy without a dendrite lesion at lower temperatures that generate less ectopic outgrowth (additional details are in SI Results). The rates of ectopic outgrowth and regeneration are strongly correlated (Fig. S3B). The results above strongly suggest that the same activity-dependent mechanism that triggers ectopic outgrowth also triggers DLK-independent regeneration after axon damage.
Fig. S3.
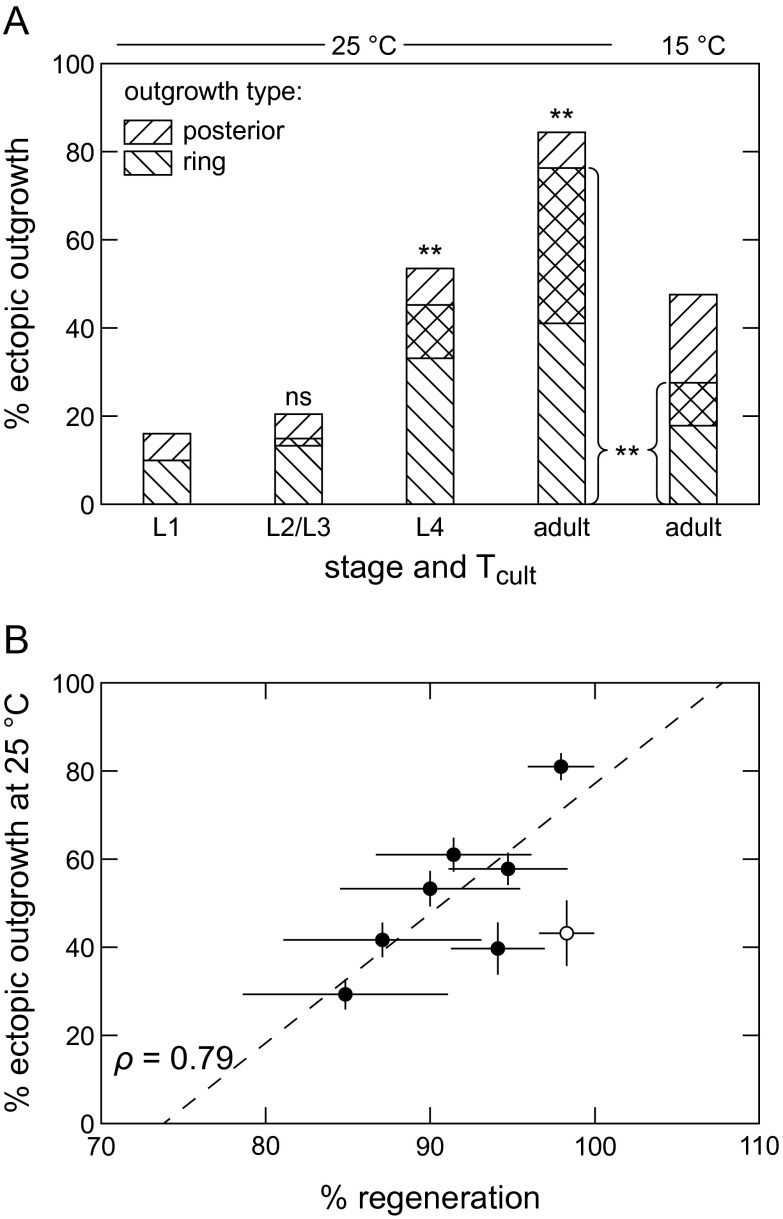
(A) Extra axons in ring is a novel form of ectopic outgrowth. Percentage of neurons with type of ectopic outgrowth in tax-2; trx-1. Extra axons in ring appear in later stages and are temperature-sensitive. P values are calculated comparing percentage of neurons with ring outgrowth at a given time with L1 percentage (157 ≤ n ≤ 185). (B) Ectopic outgrowth and DLK-independent regeneration rates are correlated. Data are adapted from Fig. 2 B and C. Black circles are mutants with axon cuts. The white circle is dlk-1 with axon+dendrite cuts. The line is best fit for mutant data. Data are represented as average ± SD. ns, Not significant. **P < 0.001.
Dendrite cuts enhance regeneration under DLK-mediated and DLK-independent processes (Fig. 1D), and, therefore, we next asked if ectopic outgrowth can occur independent of dlk-1. Comparing ectopic outgrowth in WT dlk-1(+) and dlk-1(ju476) backgrounds (Figs. 2B, black and clear bars, and 3C, Right), we find a significant but incomplete reduction of ectopic outgrowth under dlk-1. These data suggest that both DLK-mediated and DLK-independent processes underlie ectopic outgrowth, similar to regeneration.
Finally, we asked if ectopic outgrowth and DLK-independent regeneration might share the same underlying regulation. Ectopic outgrowth is regulated by proteins involved in the control of neurite outgrowth in development and cell maintenance (9). For example, SAX-1 is a conserved nuclear dbf2-related (NDR) family kinase that stabilizes neurite morphology cell autonomously (26), and UNC-43 is a homolog of Ca2+/calmodulin-dependent protein kinase II (CaMKII) that modulates neurite outgrowth during development and mediates activity-dependent neural plasticity (27–29). These two proteins also restrict ectopic outgrowth in C. elegans (9). We found that the sax-1(ky211) and unc-43(n1186) loss-of-function (lf) mutations generate DLK-independent regeneration without a dendrite cut (Fig. 2B, gray bars). In a complementary manner, the gain-of-function (gf) mutation unc-43(n498) successfully inhibits regeneration after axon+dendrite surgery, decreasing it by ∼20%. To confirm these findings in another neuron, we performed surgery on the ALM axon in dlk-1 and unc-43 mutant backgrounds. As further described in SI Results, results are consistent with ASJ findings indicating two parallel regenerative pathways that are at least partly redundant: one that is DLK-mediated, and a second one that is DLK-independent and negatively regulated by unc-43. In summary, the results above demonstrate many connections between DLK-independent regeneration and ectopic outgrowth, suggesting that they share a common activity-dependent mechanism for triggering and control of neuronal growth.
Reduction of Sensory and Calcium Activity and Elevation of cAMP Signaling Trigger DLK-Independent Regeneration and Ectopic Outgrowth.
Recent publications have directly demonstrated in multiple amphid neurons that ectopic outgrowth mutations reduce neuronal activity (summarized in SI Results). Given its role in triggering activity-dependent ectopic outgrowth, we directly tested if sensory dendrite lesion might reduce ASJ activity. Bright light stimulates ASJ neuron activity (30), which we measured by monitoring cytoplasmic calcium levels using the genetically encoded calcium-sensitive fluorophore GCaMP3 (31). Before surgery, the ASJ neurons generate a robust response to 30 s of 10 mW/mm2 blue light (“bef” conditions in Fig. 3A). After mock ablations or axotomy, the light response (“aft”) to a subsequent 30 s of 10 mW/mm2 blue light is not statistically different from the initial response. However, severing sensory dendrites (either alone or with axotomy) completely eliminates the light response. Thus, sensory dendrite lesion but not axon lesion abolishes ASJ sensory-evoked activity. Interestingly, the DRG neurons of the mammalian CNS display the same phenotype (5), where lesion of the peripheral sensory but not central axon abolishes DRG sensory-evoked activity. Although endogenous (i.e., resting) activity of mammalian DRG neurons is not significantly changed by neurite lesions, the loss of sensory-evoked activity appears to be an important signal to trigger central axon regeneration by lesion conditioning.
The L-VGCC and, specifically, its pore-forming subunit Cav1.2 are important components in neuronal calcium signaling and play a central role in lesion conditioning (5). We performed experiments to further define the channel's role in DLK-independent regeneration and ectopic outgrowth. As noted above, genetic disruption of the CACNA1C homolog egl-19 triggers DLK-independent regeneration without dendrite cut (Fig. 3B). Likewise, the EGL-19 antagonist nemadipine-A (32) also triggers robust DLK-independent regeneration without dendrite cut (Fig. 3B): we find significantly more neurons regenerated under nemadipine-A than its solvent vehicle dimethyl sulfoxide (DMSO). By contrast, the original ectopic outgrowth studies indicate that gain-of-function mutation egl-19(ad695) triggers ectopic outgrowth (10). To more fully understand this possible discrepancy, we examined the impact of both egl-19(lf) and egl-19(gf) mutations on ectopic outgrowth. Contrary to the original study, our results, including the sequencing of strains from the original study (SI Results), demonstrate that egl-19(lf) but not egl-19(gf) triggers ectopic outgrowth (Fig. 3C). Moreover, blocking EGL-19 with nemadipine-A also triggers ectopic outgrowth (Fig. 3C). These results demonstrate that reductions of EGL-19 function trigger ectopic outgrowth and DLK-independent regeneration. Because calcium influx through voltage-gated calcium channels plays a critical role in neuronal activation and sensory signaling, these findings are consistent with an activity-inhibited regenerative pathway. In mammals, reduced L-VGCC calcium current contributes to axon regeneration of DRG neurons (5), suggesting a possible mechanistic link to the outgrowth mechanisms that we observe here.
Increased cAMP signaling enhances regeneration in C. elegans as well as the mammalian CNS (16, 33–36). Therefore, we tested if elevated cAMP signaling can induce DLK-independent regeneration in the ASJ neuron. Adenylate cyclase catalyzes the production of cAMP, which is degraded by cAMP phosphodiesterase. Gain-of-function adenylate cyclase acy-1(md1756gf) (37) and loss-of-function phosphodiesterase PDE4 pde-4(ce268) (38) both increase neuronal cAMP signaling and enable robust regeneration of the ASJ after axotomy alone in a dlk-1 mutant background (Fig. 3B). Likewise, constitutive elevation of the cAMP signaling effector protein kinase A by mutation of its regulatory subunit kin-2 (37) also enables DLK-independent regeneration after axotomy alone. Consistent with our other findings, these mutations also trigger ectopic outgrowth (Fig. 3C). These results indicate that, similar to lesion conditioning, elevated cAMP signaling enhances DLK-independent regeneration and ectopic outgrowth.
In a classic lesion conditioning experiment, peripheral sensory axotomy before central axotomy, known as preconditioning, more strongly improves central axon regeneration than concomitant axotomies and vice versa, suggesting a cellular intrinsic growth capacity that is enhanced by peripheral axotomy (39). To assess if the ASJ exhibits a similar phenotype, we separated sensory dendrite and axon surgeries by 5–48 h. Azide anesthesia likely reduces regeneration, and therefore, we processed each sequential surgery set concurrently with a concomitant surgery set, treating these animals alike except for laser surgery. Comparing the regenerated axon length in both WT and dlk-1 animals, we find that dendrite surgery after axon surgery (tdend > 0 h; “post” comparison) produces less regeneration than concomitant surgeries (tdend = 0 h), whereas dendrite surgery before axon surgery (tdend < 0 h; “pre” comparison) produces more (Fig. 3D). These results underscore the dendrite lesion’s crucial role in enhancing axon regeneration and provide another phenotypic similarity between DLK-independent regeneration and mammalian lesion-conditioned regeneration.
SI Results
Additional Characterization of DLK-Independent Regeneration.
We conducted extensive sets of experiments to characterize cell-autonomous, DLK-independent regeneration. In total, we processed over 3,500 neurons by severing the axon and/or dendrite.
As noted in previous studies (56), we confirmed that axon transections near the cell body lead to regenerative outgrowth from the cell body but that transections farther from the cell body lead more often to regeneration from the severed axon stump. The total regeneration length is similar, regardless of the position of damage or regeneration. In dlk-1, dendrite cuts together with axon cuts in the nerve ring lead to clear regeneration from the ring in 4 of 11 neurons. In dlk-1; daf-11, 3 of 14 neurons cut in the commissure regenerate from the axon, whereas 8 of 8 neurons cut in the ring regenerate from the axon. This regeneration roughly follows the original axon path, making discrimination between normal and regenerated neurites difficult. Also, laser ablation in the nerve ring may lead to collateral damage given the close proximity of adjacent axons. For these reasons, despite the possibility of mechanistic differences between regeneration after complete and distal axotomies, we chose to sever axons close to the cell body.
As mentioned in the text, ASJ regeneration generally consisted of three morphologies, which are shown in Fig. S1A. Regenerated neurites projected anteriorly to the ring and then followed it posteroventrally (Fig. S1A, i), projected posteriorly (Fig. S1A, ii), or projected off the dendrite and followed the ring posteroventrally (Fig. S1A, iii). As shown in Fig. 1C, regenerated neurites across all of the backgrounds generally grew toward the ring and on reaching the ring, continued growing along the ring. The percentage of regenerated neurites in dlk-1; daf-11 reaching the ring increased significantly between days 1 and 3–4 (Fig. S1C). Postsurgery neurons extended an increasing number of outgrowths during the days after surgery (Fig. S1D).
Similar to ectopic outgrowth (10), we also noted a late time of DLK-independent regeneration after L1 life stage surgery as shown in Fig. S1E. By L2, none of the neurons had regenerated, but about one-half of the neurons regenerated by L4 or dauer (a facultative post-L2 life stage). In contrast to ectopic outgrowth, we observe no temperature dependence in DLK-independent regeneration rates at 15 °C, 20 °C, or 25 °C (Fig. S1F).
To confirm our findings in another neuron type, we performed the same surgeries on the ASH neuron in the L2 larval stage with reimaging after 3 d (Fig. S1B). The ASH is an amphid sensory neuron type with similar morphology to ASJ comprising an axon extending into the nerve ring and a distinct sensory dendrite projecting anteriorly to the animal’s nose. As expected, neurons in WT animals strongly regenerated, generally by projecting from the dendrite portion near the ring. In contrast to the ASJ, where only 1 of 68 neurons regenerated in dlk-1, about one-half the ASH neurons in dlk-1 animals regenerated, although the regeneration was significantly shorter than in the WT. Adding a sensory dendrite lesion significantly increases the regenerated length in dlk-1. Thus, the ASH results confirm ASJ findings that dlk-1 reduces conventional regeneration and dendrite lesion enhances DLK-independent regeneration.
Regenerating Backgrounds.
We tested mutations shown to block sensory signaling in the ASJ neurons for a role in triggering DLK-independent regeneration. As shown in Fig. 2B, excepting unc-36, each of the mutations that trigger ectopic outgrowth also triggers DLK-independent regeneration without dendrite lesion. Regeneration rates of dlk-1; unc-36, although statistically higher than dlk-1 regeneration rates, belie an abortive regeneration phenotype. Regenerated neurites are unusually thin, the average regenerated length is 5 μm, and none of the regenerated neurites reach the ring (20–25 μm from the cell body). Thus, we consider unc-36 a nonregenerating background.
As summarized in Fig. S3B, we note a correlation between ectopic outgrowth and DLK-independent regeneration rates. In Fig. S3B, we have included only mutants that regenerate (see above). The ρ correlation factor for all of the mutant data is 0.79, represented by the dotted line in Fig. S3B, and most of the noncorrelation is caused by the daf-11 data at the point (x, y) = (95%, 40%), which were acquired using temperature shifting and partially in dauer. Without the daf-11 data point, the correlation increases to 0.95.
ALM Results Confirm unc-43 Regeneration Phenotype.
We performed regeneration assays in the ALM mechanosensory neuron to test the existence of parallel conventional and DLK-independent pathways in another neuron type. The ALM is a well-established model neuron for axon regeneration in Caenorhabditis elegans (15). The ALM axon is the only major process extending from the cell body and mediates both axonal connections within the nerve ring and touch sensation along its length. We performed ALM axotomy in unc-43(lf) and unc-43(gf) 20 μm from the cell body in young adult animals and measured outgrowth after 24 h (Fig. S1G). We find that, although dlk-1 mutation completely eliminates conventional regeneration in some other neuron types, such as motor neurons (12) and the ASJ, dlk-1 reduces ALM regeneration by only ∼30% (Fig. S1G), confirming results of our previous study (15). In addition, unc-43(gf) but not unc-43(lf) significantly decreases regenerative outgrowth in both the WT and dlk-1. Thus, as with the ASJ neuron, the ALM neuron displays two parallel regenerative pathways that are at least partly redundant: a conventional pathway that is DLK-mediated, and a second pathway that is DLK-independent and negatively regulated by unc-43.
Role of trx-1 in Neurite Growth Mechanisms.
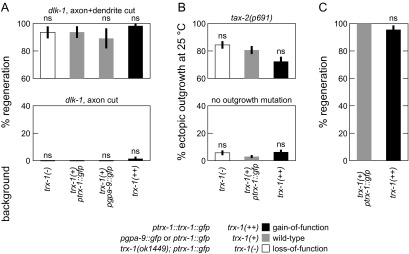
In many experiments, we visualized the ASJ by ptrx-1::trx-1::gfp, an extrachromosomal translational fusion GFP, because it was the clearest and brightest cell-specific GFP. Because this transgene overexpresses functional TRX-1 thioredoxin, we examined the role of trx-1 in the relevant neurite growth mechanisms (Fig. S4). After axon+dendrite cuts and after only axotomy, regeneration rates are not statistically different between trx-1(+) WT animals and either mutants with null allele trx-1(ok1449) (57) or animals expressing the translational fusion GFP (Fig. S4A). Likewise, ectopic outgrowth rates with and without a tax-2 mutation are not statistically different between the above backgrounds (Fig. S4B). Finally, conventional regeneration rates between trx-1(+) and trx-1(++) are not statistically different (Fig. S4C). By eye, we also did not notice any gross differences in morphology or extent of neurite growths. These data indicate that trx-1 is not crucially involved in DLK-independent regeneration, ectopic outgrowth, or conventional regeneration, permitting the use of ptrx-1::trx-1::gfp for ASJ visualization.
Fig. S4.
trx-1 is not crucially involved in DLK-independent regeneration, ectopic outgrowth, and conventional regeneration. No significant difference in regeneration rates between various trx-1 alleles and trx-1(+) for (A) DLK-independent regeneration, (B) ectopic outgrowth, and (C) conventional regeneration (32 ≤ n ≤ 68 for regeneration data, except n ≥ 18 for pgpa-9::gfp; 170 ≤ n ≤ 235 for ectopic outgrowth data). Regeneration trx-1(++) data are replicated from Fig. 1B. Data are represented as average ± SD. ns, Not significant.
Neuronal Activity in Ectopic Outgrowth Mutants and Processed Animals.
Although we demonstrate that dendrite cuts abolish ASJ sensory-evoked activity (Fig. 3A), recent studies have also assessed the impact of loss-of-function ectopic outgrowth mutations, dendrite surgery, and nemadipine treatment on neuronal sensory-evoked activity. In cultured mechanosensory neurons, egl-19(ad1006) and unc-36(e251) significantly reduce the calcium influx resulting from depolarization in response to 100 mM K+ (58), and egl-19(n582) reduces ASH sensory calcium response to high osmolarity (1 M glycerol) (59). As observed in acutely dissected animals by perforated voltage clamp (−70 mV), tax-2(p671) and tax-2(p691) eliminate light-induced ASJ current (30). Similarly, tax-2(p671), tax-4(p678), daf-11(ks67), and daf-11(m47) eliminate GTPγS-stimulated ASJ current (60). ASJ (61) and AFD (62) calcium concentration changes responding to temperature shifts were not observed in tax-4(p678). AWCON response to benzaldehyde was eliminated by osm-6(p811) and restored by cell-specific osm-6(+) rescue in AWC but not ASEL (63). In addition, dendrite cuts reduce AFD activity elicited by temperature stimuli in a cell-specific manner (64), and nemadipine-A strongly inhibited AWA responses to diacetyl (65). Taken together, the above results strongly indicate that our interventions (mutations, dendrite surgery, and nemadipine), which trigger ectopic outgrowth and DLK-independent regeneration, also decrease sensory-evoked activity in the ASJ. It is important to note that, although the effects on ASJ sensory-evoked activity are well-documented, little is known about the effects that these perturbations might have on any endogenous ASJ activity.
EGL-19 Ectopic Outgrowth Characterization.
Peckol et al. (10) examined the role of weak gain-of-function allele egl-19(ad695) in triggering ectopic outgrowth in the amphid neurons ASI, AWB, ASE, and ASJ in separate strains. Figure 4 in ref. 10 indicates that egl-19(ad695) triggers ectopic outgrowth in ASI and ASJ but not in ASE or AWB. Because the original ectopic outgrowth studies were completed before the advent of inexpensive, rapid, and accessible genetic sequencing, we directly verified the alleles. The ad695 allele is a missense mutation A906V (66). Sequencing by primers CTTCTCGATATCCTCGTCGT (left) and GTCTTCGAATCCTCTCGAAC (right) confirmed that outgrowing strains (fluorescent in ASI or ASJ) do not carry ad695 and that strains carrying ad695 (fluorescent in AWB) do not outgrow. Together with results from Fig. 3D, these data indicate that the gain-of-function allele ad695 does not trigger ectopic outgrowth, consistent with our findings that reductions of egl-19 function trigger DLK-independent regeneration.
Ectopic Outgrowth Characteristics.
Four original papers from the Bargmann laboratory identified four distinguishing phenotypic characteristics of ectopic outgrowth (7–10). First, outgrowth extends ectopically: only 5% of WT background animals outgrow ectopic axons (10). Second, ectopic outgrowth is temperature-sensitive: neurons outgrow significantly more often at higher temperatures (8). Third, ectopic outgrowth occurs late in the life cycle of the animal, well after completion of the affected neurons’ normal development (8). Fourth, the primary morphology observed was an axon extending posteriorly from the cell body, although ectopic outgrowth was typically observed with dyes and noncell-specific GFPs, which could complicate the observation of some morphologies (7).
Regeneration Vs. Ectopic Outgrowth.
Some regenerative outgrowth can be attributed to ectopic outgrowth. However, the regeneration rate in all outgrowing strains is significantly greater than the ectopic outgrowth rate, and therefore, there is significant regeneration triggered by outgrowth mutations. For some mutant strains exhibiting strong ectopic outgrowth, such as tax-2, we cultivated and performed surgery at 15 °C to reduce ectopic outgrowth. We did not note any significant changes in regeneration at different temperatures (Fig. S1F), although ectopic outgrowth rates can change by over 50% (Fig. S5). Dendrite cut and nemadipine-triggered regenerative outgrowth is unlikely to arise from ectopic outgrowth processes, because one generation of surgeries or nemadipine is not sufficient to trigger ectopic outgrowth. A discussion of the ectopic outgrowth maternal effect is below and in Fig. S5 B and C.
Fig. S5.
Ectopic outgrowth exhibits a maternal effect. Ectopic outgrowth depends on (A) temperature of parent cultivation in mutant backgrounds (152 ≤ n ≤ 223), (B) cutting of parent dendrites (30 ≤ n ≤ 206), and (C) pharmacological treatment of parents (155 ≤ n ≤ 235). Some data are replicated from Figs. 2 and 3. Data are represented as average ± SD. ns, Not significant. *P < 0.05; **P < 0.001.
Supernumerary Axons in the Nerve Ring Are a Novel Form of Ectopic Outgrowth.
Our study demonstrates that DLK-independent regeneration and ectopic outgrowth share many characteristics. Our experiments confirming that axon transections farther from the cell body lead to new projection at the axon tip and growth along the ring (see above), thus, raise the possibility that there exists a corresponding morphology of ectopic outgrowth. Other studies have also noted supernumerary axons in the ring (67). Indeed, using cell-specific GFP in a tax-2; trx-1 background, we observed ASJ axons that sprout from a section of the original axon within the ring and grow alongside the original axon. We ascertained that these aberrant axons were, in fact, ectopic outgrowths by confirming that they appear late in development and are temperature-sensitive (Fig. S3A). In our study, we include this novel morphology in scoring ectopic outgrowth; however, supernumerary axons were not noted in the original ectopic outgrowth studies, which primarily used noncell-specific dyes and fluorescent proteins. In some cases, this distinction leads to significant differences with published results. For example, we observe unc-43 animals outgrowing 53% of neurons rather than 8% (9).
Ectopic Outgrowth Exhibits a Maternal Effect.
Surprisingly, we note that ectopic outgrowth exhibits a maternal effect, regardless of the type of triggering lesion. In a maternal effect, the phenotype of an individual is fully or partially determined by the phenotype or environment of its parent. We note a maternal effect caused by cultivation temperature (Fig. S5A) by varying the first generation cultivation temperature while holding fixed the second generation cultivation temperature and then measuring second generation ectopic outgrowth. All tested strains, except tax-4, indicate some significant difference in second generation outgrowth percentage depending on first generation cultivation temperature. We also note a maternal effect caused by triggering by dendrite surgery (Fig. S5B). The presence of a dendrite surgery in the first generation significantly increased ectopic outgrowth in the second generation, regardless of the presence of a second generation dendrite surgery or the side severed. Finally, we note a maternal effect in nemadipine triggering of ectopic outgrowth, where application of nemadipine but not DMSO significantly affects ectopic outgrowth in the second generation. Importantly, DLK-independent regeneration in C. elegans does not seem to exhibit the same maternal effect. DLK-independent regeneration is not temperature-sensitive (Fig. S1F), and nearly all neurons regenerate after surgery in the first generation.
Note that all ectopic outgrowth data, except for those in Fig. S5, are taken with the intervention (temperature, surgery, and pharmacology) applied to at least two successive generations to eliminate the maternal effect.
Complexity in Neuronal Regeneration.
Fig. 4 presents a generalized picture of the DLK-independent regeneration that we define in this study. However, it is important to note that our results also suggest additional complexities in neuronal outgrowth and regeneration. First, the data in Fig. 1D indicate that dendrite lesions in WT animals enhance regeneration before the L4 stage, when DLK-independent regeneration from dendrite lesion is first observed. Moreover, even after surgery in young adults, when DLK-independent regeneration is active, dendrite lesions increase WT-regenerated length beyond what might be predicted from strictly adding DLK-independent regenerated length, suggesting that dendrite injury enhances both DLK-independent and DLK-mediated regeneration pathways. Thus, we observe several forms of regeneration, including a conventional DLK-mediated regeneration triggered by axon cut only, a DLK-independent regeneration triggered by axon+dendrite cut, and an enhanced DLK-mediated regeneration also triggered by axon+dendrite cut. Second, overlapping but distinct sets of genes and molecules mediate various forms of regeneration in different neuron types. For instance, the JNK pathway in C. elegans critically underlies conventional motor axon regeneration (14) but does not underlie conventional ASJ regeneration or DLK-independent regeneration (Fig. 2A). Third, at least one gene underlying ectopic outgrowth in the ASJ (daf-11) is not expressed in other outgrowing neurons, suggesting that some of the molecular mechanisms mediating a common activity-dependent signal may be cell type-specific. Fourth, although L-VGCCs mediate sensory signaling that inhibits DLK-independent regeneration, in other contexts, they mediate beneficial calcium signals during the damage response: blocking L-VGCCs with nemadipine-A results in decreased calcium response and reduced conventional regeneration after PLM axotomy (16). Thus, our observations not only demonstrate a novel form of regeneration but also begin to illuminate a greater underlying complexity in regeneration processes.
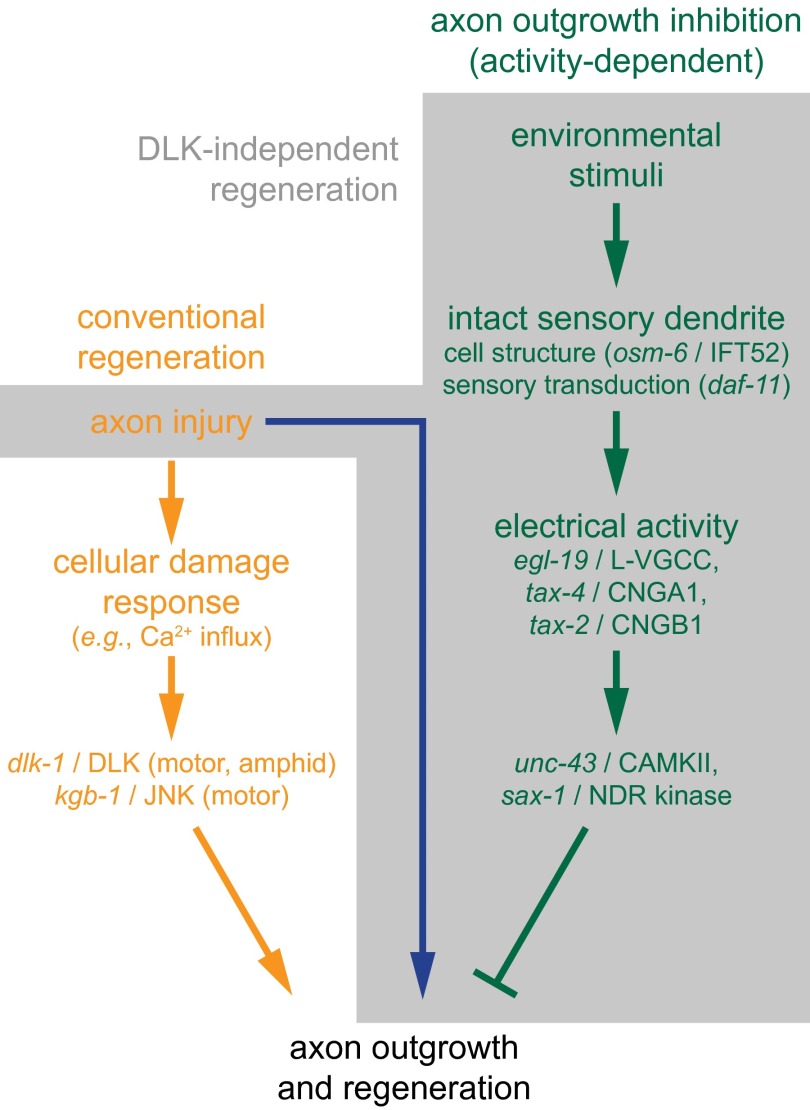
Fig. 4.
Neurite growth mechanisms. Simplified working model of neurite growth mechanisms in C. elegans. The orange and green pathways represent conventional regeneration and ectopic outgrowth, respectively. The shaded pathways comprise a DLK-independent regeneration, which shares characteristics with mammalian lesion conditioning.
Discussion
Our work describes regeneration in C. elegans amphid neurons that is triggered by lesion of both the axon and sensory dendrite. This novel regeneration is largely independent of DLK-1/DLK, KGB-1/JNK, and other known MAPK signaling factors underlying neuronal regeneration in C. elegans and other organisms. Although a recent study showed that dendrite regeneration in Drosophila dendritic arborization neurons is also independent of both DLK and JNK (40), an analogous mechanism initiated by axon surgery has not been defined.
Our results indicate a direct link between DLK-independent regeneration and activity-dependent ectopic axon outgrowth, with comparable morphologies, late time of outgrowth, overlapping set of inducing mutations and surgeries, and at least partial DLK independence. Our work suggests that examining nematode ectopic outgrowth could provide a fruitful source of insight into other vertebrate and invertebrate regeneration mechanisms and vice versa. Similar ectopic outgrowths occur in mammals and are often associated with disease states. For instance, defects in the tax-2 homolog CNGB1 and the L-VGCC gene CACNA1F trigger ectopic outgrowth in mouse models of retinitis pigmentosa (41) and congenital stationary night blindness (42). Information gained from these models may be combined with results in neuronal regeneration to inform multiple areas of study.
DLK-independent regeneration and ectopic outgrowth in C. elegans also share numerous similarities with lesion-conditioned regeneration observed in the mammalian CNS. They are all triggered by genetic or pharmacological inhibition of L-VGCCs or by damage to sensory neurites causing a reduction of sensory activity. Furthermore, all are mediated by cAMP activity. Importantly, DLK-independent and lesion-conditioned regeneration also exhibit a similar regeneration phenotype dependent on the sequence and timing of neurite lesions, including a preconditioning effect. In mammals, DLK underlies preconditioning of peripheral axon regeneration (21), a conditioning effect on DRG neurons in vitro (43), and retinal ganglion cell regeneration after optic nerve crush (44). Nonetheless, the similarities with the C. elegans DLK-independent regeneration observed here suggest the possibility of modeling aspects of CNS lesion conditioning in genetically accessible systems.
Although regenerative processes vary with cell type and context (SI Results), our findings suggest a simple working model for regeneration and outgrowth in C. elegans (Fig. 4). Physical damage of the axon leads to conventional regeneration through specific damage-induced calcium signaling that initiates dlk-1– and kgb-1–mediated regeneration (orange pathway in Fig. 4). Independently, sensory activity of the intact neuron normally suppresses ectopic outgrowth (green pathway in Fig. 4) in C. elegans. Our results and previous studies (SI Results) indicate that the dendritic cilia transduce external stimuli to generate amphid sensory activity. Activity propagates by ion channels, resulting in calcium influx through EGL-19/L-VGCC and activation of SAX-1/NDR kinase and UNC-43/CaMKII, which inhibit outgrowth. Disruption of this pathway at any point by dendrite lesion, genetic mutation, or pharmacology removes the suppression, enabling neurite growth within two contexts. Ectopic outgrowth occurs without axotomy when animals are grown under stressed conditions. Alternatively, axon injury (blue line in Fig. 4) can activate DLK-independent regeneration. Thus, this novel DLK-independent regeneration (gray area in Fig. 4) requires both a central axon injury signal and the elimination of activity-dependent developmental blocks mediated by the peripheral sensory neurite.
Our study identifies key negative regulators of regeneration, sax-1 and unc-43, which also restrict outgrowth during neuronal maturation. SAX-1 and its Drosophila homolog Tricornered negatively regulate dendritic growth during development to establish correct sensory neurite tiling in worms and flies (45, 46). In mammals, NDR kinases suppress formation of supernumerary axons during polarization of developing hippocampal neurons in vitro and are activated by calcium (47, 48), suggesting a possible regulatory link with neuronal activity and the underlying calcium signal (9). Likewise, increased CaMKII activity causes maturation of dendritic morphology, slowing outgrowth and stabilizing dendritic structures in developing neurons of the Xenopus optic tectum (29). A recent study revealed a specific developmental pathway, whereby calcium entry through L-VGCCs in response to sensory activity controls dendrite complexity in the Xenopus visual system as well as cultured rat cortical neurons. These results suggest that CaMKII activation in response to elevated calcium up-regulates transcription of the GTPase Rem2 to restrict dendrite outgrowth and branching (49). Taken together, our findings suggest that the same activity-dependent pathways restricting neurite outgrowth during development through activation of SAX-1/NDR kinase and UNC-43/CaMKII can also constitutively block regeneration after axonal injury in the adult.
Electrical activity is a key modulator of neurite outgrowth during development and after injury. Within this context, our work establishes the ASJ neuron in C. elegans as a powerful experimental model for studying DLK-independent regeneration and suggests ectopic outgrowth as a tractable gene discovery platform. Employing this model, we demonstrate that, after axonal injury, neuronal sensory activity restricts outgrowth by known developmental regulators of neurite outgrowth. Removal of this inhibition triggers a novel regeneration pathway largely independent of DLK and JNK signaling. The striking similarities between DLK-independent regeneration in C. elegans and lesion-conditioned regeneration in the mammalian CNS suggest a conserved mechanism. As such, our study establishes a framework for defining the mechanisms that modulate a neuron’s intrinsic growth capacity and ultimately enable robust regeneration within the adult nervous system.
Materials and Methods
Cultivation and Strains.
Following standard methods (6), we cultured strains on nematode growth medium (NGM) plates inoculated with OP50 bacteria (i.e., seeded plates), the primary food source for laboratory C. elegans, and maintained animals at 20 °C unless otherwise stated. We cultivated strains at 25 °C for ectopic outgrowth experiments, except for daf-11, which we temperature-shifted from 15 °C to 25 °C after the dauer entry decision (8). Experiments in liquid solution used NGM buffer, which includes the same inorganic salts as NGM agar. We confirmed the genotype of all mutant strains [except egl-19(ad1006), which is not defined] by polymerase chain reaction and gel electrophoresis (larger deletions) or sequencing (small-scale mutations) as well as defined phenotypes. Primer sequences are available on request. SI Materials and Methods lists the fluorescent and mutant parent strains used to generate this study’s strains. Unless otherwise stated, dlk-1 refers to dlk-1(ju476).
Imaging and Neurite Surgery.
We followed established procedures for immobilization, imaging, surgery by femtosecond laser ablation, and postsurgery reimaging (23, 50). We immobilized L4 and adult animals with 5 mM sodium azide and younger animals with 2 mM sodium azide, except for in calcium imaging experiments (see below). We used cell-specific green fluorescent protein (GFP) (51), GCaMP (calcium-sensitive fluorophore) (31, 52), and DiOC18(3), also known as DiO (53) to image neurons. We primarily visualized the ASJ by ptrx-1::trx-1::gfp, an extrachromosomal translational fusion GFP, because it was the clearest and brightest cell-specific GFP. Overexpression of this transgene has no effect on outgrowth rates (Fig. S4). We scored individual neurons for ectopic outgrowth of greater than 2 μm, similar to established procedures (10). In the course of this study, we found that ectopic outgrowth displays a strong maternal effect (i.e., treatment of one generation modulates ectopic outgrowth rates in the subsequent generation). These results are detailed in SI Results and Fig. S5. Thus, to avoid confounding effects, all strains were subject to the same conditions or surgery for multiple generations in the ectopic outgrowth experiments, except as noted.
Femtosecond laser surgery has submicrometer precision in the sample bulk (23) and permits cell-specific surgery of the ASJ dendrite within the amphid bundle and axon proximal to the amphid commissure (18). Unless otherwise noted, we performed laser surgery in L2 or L3 life stage with 3-nJ pulses at 10-kHz repetition rate, severing axons within several micrometers of the cell body and dendrites near their midpoint. Under these conditions, severed dendrites can persist for days, but severed axons typically decay within 1 d. Although a report noted AWB regeneration after dendrite surgery (54), we do not observe regeneration after ASJ dendrite surgery (23). Unless otherwise stated, we reimaged animals 3–5 d after surgery, and we only counted animals with successful surgery, noted by the absence of a neurite with normal morphology. For most experiments, we scored individual neurons for the presence of regeneration (greater than 2 μm) rather than measuring outgrowth length. The reasons underlying the above methodology are listed in SI Materials and Methods. We measured regenerated fiber lengths by using the ImageJ plugin 3D Distance Tool available at the NIH ImageJ website. The plugin measures the distance between successive selected points in a z stack. As depicted in Fig. S2A, we selected points unidirectionally along the entire length of the regenerated fiber (slices 4–6) and measured distinct branches separately (red and yellow points in Fig. S2A). Summing the distances, we obtained the total regenerated length of new fibers (maximum projection shown in Fig. S2B).
Calcium Imaging.
We measured intracellular calcium levels as an indicator of neuronal activity using animals expressing ASJ-specific GCaMP3. Following standard procedures (15), we immobilized C. elegans using 2% (wt/wt) agar pads with 0.05% tetramisole. The ASJ neuron displays a robust response to higher intensities of the same 480-nm blue light (30) used for GCaMP3 imaging, and therefore, we located the ASJ cell body using low light illumination (<1 mW/mm2) that does not elicit a response and stimulated neurons with higher intensities. In agreement with earlier studies, we first measured response to a range of illumination stimuli and found robust response to intensities of 10 mW/mm2 and above but lower response at lower intensities. We used 10 mW/mm2 for all subsequent experiments. Animals were left in the dark for >30 s before exposure to 30 s of intense light while recording 1 frame per second. As described previously (55), we quantified GCaMP3 fluorescent intensities as the average intensity across the ASJ cell body normalized to the initial intensity (i.e., in the first frame). As shown in Fig. 3A, responses are temporally variable, and therefore, we quantified the response size from each trial as the maximum amplitude change in fluorescence across the 30-s recording. For experiments combining Ca2+ imaging with laser surgery, we measured the neurons response, performed the surgery as noted above, allowed animals a 30-min recovery, and measured the light response of the neuron again.
Nemadipine-A Treatment.
We made a stock solution of 10 mg/mL nemadipine in dimethyl sulfoxide (DMSO) and diluted 4.2 μL stock solution (or DMSO) in 76 μL NGM buffer. We made nemadipine (or DMSO) plates by spreading this mixture on the surface of a 6-cm seeded plate. We allowed plates to dry overnight, introduced gravid animals on the plates the next day, and picked L2 or L3 animals for surgery after two more days.
Statistics and Interpretation of Results.
Most of the ASJ regeneration and ectopic outgrowth data are binary: we score whether or not neurons regrow or outgrow. We calculated P values for these data by Fisher’s exact test. For regenerated length measurements, we calculated P values by the unpaired, unequal variance, two-tailed t test. For calcium imaging data, we calculated P values by the paired, two-tailed t test. For ALM regeneration length data, we conducted a one-way analysis of variance (ANOVA) to compare the effect of genetic background on regenerated length in WT and mutant dlk-1 and unc-43 conditions. There is a highly significant effect of genetic background on regenerated length for the six conditions [F(5,131) = 14.7; P < 0.0001]. We performed posthoc comparisons using the Tukey test. Data are represented as average ± standard deviation (SD). We indicate values that differ at P < 0.05 (*) and P < 0.001 (**) levels.
SI Materials and Methods
Strains.
The parent strains that we crossed to generate the animals used in our experiments are listed below.
Fluorescent strains:
ASJ: KJ560 dpy-20; jhEx560[ptrx-1::trx-1::gfp; dpy-20(+)] (from Joohong Ahnn, Hanyang University, Seoul, Korea) (57, 68),
ASJ: OE3265 lin-15ab; ofIs1[lin-15ab+; ptrx-1::gfp] (from Peter Swoboda, Karolinska Institute, Huddinge, Sweden),
ASJ: NL1606 dpy-20(e1282)IV; pkIs586[pgpa-9::gfp; dpy-20(+)],
ASJ: CX3797 lin-15(n765)X; kyIs150[tax-2Δ::gfp; lin-15(+)]IV (from Cornelia Bargmann, The Rockefeller University, New York),
ASJ: PS6388 pha-1(e2123ts)III; him-5(e1490)V; syEx1248[gpa-9::GCaMP3; pha-1(+)] (from Paul Sternberg, California Institute of Technology, Pasadena, CA) (69), and
ALM: ZB154 zdIs5[mec-4::GFP; lin-15(+)] I (from Monica Driscoll, Rutgers, The State University of New Jersey, Piscataway, NJ).
Mutant strains:
CZ5730 dlk-1(ju476)I (70),
KB3 kgb-1(um3)IV (from Monica Driscoll) (14),
VZ1 trx-1(ok1449)II,
MT6129 egl-19(n2368)IV,
DA695 egl-19(ad695)IV,
DA1006 egl-19(ad1006)IV,
MT1212 egl-19(n582)IV,
MT1092 unc-43(n498)IV (from Cornelia Bargmann),
MT2605 unc-43(n1186n498)IV,
CX2948 tax-4(p678)III (from Cornelia Bargmann),
CX2217 tax-2(p691)I (from Cornelia Bargmann),
PR811 osm-6(p811)V (from Cornelia Bargmann),
CX3492 sax-1(ky211)X (from Cornelia Bargmann),
CB251 unc-36(e251)III,
JT195 daf-11(sa195)V (from Piali Sengupta, Brandeis University, Waltham, MA),
KU12 dlk-1(km12)I,
RB1908 mlk-1(ok2471)V,
KG522 acy-1(md1756)III,
KG744 pde-4(ce268)II,
KG532 kin-2(ce179)X,
MJB1296 fos-1(km30)V / nT1[qIs51]IV;V (from Michael Bastiani, University of Utah, Salt Lake City), and
MJB1148 cebp-1(u819)X; ovIs268[punc-47::gfp]I (from Michael Bastiani).
Strains listed without a source originate from the Caenorhabditis Genetics Center.
Scoring Neuronal Growth.
We primarily scored individual neurons for the presence of regeneration rather than measuring length for several reasons. First, regenerated length depends on the genetic background and the time between surgery and reimaging, which must be long because of the slow rate of DLK-independent regeneration (Fig. 1D). Several backgrounds exhibit slow development, delayed regeneration, or generally poor health, which complicates quantitative comparisons of regenerated length. Second, simple binary scoring of regeneration is sufficient in most cases to demonstrate triggering of DLK-independent regeneration, and practically, it is more feasible and reliable over the large number of experiments performed here. Data are ambiguous only for unc-36 (see above) and in the WT background, where ASJ regeneration is highly robust (Fig. 1D). In both cases, we rely on length measurements to discern differences in regeneration and interpret results. Third, binary scoring increases our ability to compare regeneration with published and current ectopic outgrowth measurements.
Acknowledgments
We thank Cornelia Bargmann for strains and extensive help with ectopic outgrowth experiments. We also thank Peter Swoboda, Antonio Miranda-Vizuete, Piali Sengupta, Joohong Ahnn, Paul Sternberg, Monica Driscoll, and Michael Bastiani for strains and/or guidance. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. The authors were supported, in part, by the Massachusetts Life Sciences Center and NIH Grant R21NS078580. M.M.M. was supported, in part, by National Institute of General Medical Sciences Predoctoral Training Grant GM008541.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5465.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600564113/-/DCSupplemental.
References
- 1.Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr Biol. 2005;15(18):R749–R753. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Liu K, Tedeschi A, Park KK, He ZG. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34(2011):131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444(7120):707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PM, Issa VMK. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309(5971):791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- 5.Enes J, et al. Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr Biol. 2010;20(13):1154–1164. doi: 10.1016/j.cub.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 6.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17(4):695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 8.Coburn CM, Mori I, Ohshima Y, Bargmann CI. A cyclic nucleotide-gated channel inhibits sensory axon outgrowth in larval and adult Caenorhabditis elegans: A distinct pathway for maintenance of sensory axon structure. Development. 1998;125(2):249–258. doi: 10.1242/dev.125.2.249. [DOI] [PubMed] [Google Scholar]
- 9.Zallen JA, Peckol EL, Tobin DM, Bargmann CI. Neuronal cell shape and neurite initiation are regulated by the Ndr kinase SAX-1, a member of the Orb6/COT-1/warts serine/threonine kinase family. Mol Biol Cell. 2000;11(9):3177–3190. doi: 10.1091/mbc.11.9.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peckol EL, Zallen JA, Yarrow JC, Bargmann CI. Sensory activity affects sensory axon development in C. elegans. Development. 1999;126(9):1891–1902. doi: 10.1242/dev.126.9.1891. [DOI] [PubMed] [Google Scholar]
- 11.Chung SH, Mazur E. Surgical applications of femtosecond lasers. J Biophotonics. 2009;2(10):557–572. doi: 10.1002/jbio.200910053. [DOI] [PubMed] [Google Scholar]
- 12.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323(5915):802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138(5):1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nix P, Hisamoto N, Matsumoto K, Bastiani M. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc Natl Acad Sci USA. 2011;108(26):10738–10743. doi: 10.1073/pnas.1104830108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinan-Lucarre B, et al. The core apoptotic executioner proteins CED-3 and CED-4 promote initiation of neuronal regeneration in Caenorhabditis elegans. PLoS Biol. 2012;10(5):e1001331. doi: 10.1371/journal.pbio.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 2010;30(9):3175–3183. doi: 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, et al. Neuronal regeneration in C. elegans requires subcellular calcium release by ryanodine receptor channels and can be enhanced by optogenetic stimulation. J Neurosci. 2014;34(48):15947–15956. doi: 10.1523/JNEUROSCI.4238-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung SH, Schmalz A, Ruiz RCH, Gabel CV, Mazur E. Femtosecond laser ablation reveals antagonistic sensory and neuroendocrine signaling that underlie C. elegans behavior and development. Cell Reports. 2013;4(2):316–326. doi: 10.1016/j.celrep.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong X, et al. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191(1):211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh A, Horiuchi M, Bannerman P, Pleasure D, Itoh T. Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem Biophys Res Commun. 2009;383(2):258–262. doi: 10.1016/j.bbrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Shin JE, et al. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74(6):1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 23.Chung SH, Clark DA, Gabel CV, Mazur E, Samuel AD. The role of the AFD neuron in C. elegans thermotaxis analyzed using femtosecond laser ablation. BMC Neurosci. 2006;7:30. doi: 10.1186/1471-2202-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nix P, et al. Axon regeneration genes identified by RNAi screening in C. elegans. J Neurosci. 2014;34(2):629–645. doi: 10.1523/JNEUROSCI.3859-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raivich G, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43(1):57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Zallen JA, Kirch SA, Bargmann CI. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development. 1999;126(16):3679–3692. doi: 10.1242/dev.126.16.3679. [DOI] [PubMed] [Google Scholar]
- 27.Hell JW. CaMKII: Claiming center stage in postsynaptic function and organization. Neuron. 2014;81(2):249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greer PL, Greenberg ME. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59(6):846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279(5348):222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- 30.Ward A, Liu J, Feng Z, Xu XZS. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat Neurosci. 2008;11(8):916–922. doi: 10.1038/nn.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6(12):875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwok TCY, et al. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441(7089):91–95. doi: 10.1038/nature04657. [DOI] [PubMed] [Google Scholar]
- 33.Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34(6):885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 34.Qiu J, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34(6):895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Hisamoto N, Matsumoto K. Axon regeneration is regulated by Ets-C/EBP transcription complexes generated by activation of the cAMP/Ca2+ signaling pathways. PLoS Genet. 2015;11(10):e1005603. doi: 10.1371/journal.pgen.1005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blesch A, et al. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: Superiority to camp-mediated effects. Exp Neurol. 2012;235(1):162–173. doi: 10.1016/j.expneurol.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schade MA, Reynolds NK, Dollins CM, Miller KG. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the G alpha(s) pathway and define a third major branch of the synaptic signaling network. Genetics. 2005;169(2):631–649. doi: 10.1534/genetics.104.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlie NK, Thomure AM, Schade MA, Miller KG. The Dunce cAMP phosphodiesterase PDE-4 negatively regulates G alpha(s)-dependent and G alpha(s)-independent cAMP pools in the Caenorhabditis elegans synaptic signaling network. Genetics. 2006;173(1):111–130. doi: 10.1534/genetics.105.054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23(1):83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 40.Stone MC, Albertson RM, Chen L, Rolls MM. Dendrite injury triggers DLK-independent regeneration. Cell Reports. 2014;6(2):247–253. doi: 10.1016/j.celrep.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michalakis S, et al. Characterization of neurite outgrowth and ectopic synaptogenesis in response to photoreceptor dysfunction. Cell Mol Life Sci. 2013;70(10):1831–1847. doi: 10.1007/s00018-012-1230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansergh F, et al. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14(20):3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- 43.Valakh V, Frey E, Babetto E, Walker LJ, DiAntonio A. Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol Dis. 2015;77:13–25. doi: 10.1016/j.nbd.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watkins TA, et al. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci USA. 2013;110(10):4039–4044. doi: 10.1073/pnas.1211074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallegos ME, Bargmann CI. Mechanosensory neurite termination and tiling depend on SAX-2 and the SAX-1 kinase. Neuron. 2004;44(2):239–249. doi: 10.1016/j.neuron.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Emoto K, et al. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119(2):245–256. doi: 10.1016/j.cell.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 47.Yang R, Kong E, Jin J, Hergovich A, Püschel AW. Rassf5 and Ndr kinases regulate neuronal polarity through Par3 phosphorylation in a novel pathway. J Cell Sci. 2014;127(Pt 16):3463–3476. doi: 10.1242/jcs.146696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millward TA, Heizmann CW, Schäfer BW, Hemmings BA. Calcium regulation of Ndr protein kinase mediated by S100 calcium-binding proteins. EMBO J. 1998;17(20):5913–5922. doi: 10.1093/emboj/17.20.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghiretti AE, et al. Rem2 is an activity-dependent negative regulator of dendritic complexity in vivo. J Neurosci. 2014;34(2):392–407. doi: 10.1523/JNEUROSCI.1328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 1995;48:225–250. doi: 10.1016/s0091-679x(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalfie M, Kain S. Green Fluorescent Protein: Properties, Applications, and Protocols. Wiley Interscience; Hoboken, NJ: 2006. [Google Scholar]
- 52.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19(2):137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 53.Herman RK, Hedgecock EM. Limitation of the size of the vulval primordium of Caenorhabditis elegans by lin-15 expression in surrounding hypodermis. Nature. 1990;348(6297):169–171. doi: 10.1038/348169a0. [DOI] [PubMed] [Google Scholar]
- 54.Wu Z, et al. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci USA. 2007;104(38):15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung SH, Sun L, Gabel CV. In vivo neuronal calcium imaging in C. elegans. J Vis Exp. 2013;74(2013):e50357. doi: 10.3791/50357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabel CV, Antoine F, Chuang CF, Samuel AD, Chang C. Distinct cellular and molecular mechanisms mediate initial axon development and adult-stage axon regeneration in C. elegans. Development. 2008;135(6):1129–1136. doi: 10.1242/dev.013995. [DOI] [PubMed] [Google Scholar]
- 57.Miranda-Vizuete A, et al. Lifespan decrease in a Caenorhabditis elegans mutant lacking TRX-1, a thioredoxin expressed in ASJ sensory neurons. FEBS Lett. 2006;580(2):484–490. doi: 10.1016/j.febslet.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 58.Frøkjaer-Jensen C, et al. Effects of voltage-gated calcium channel subunit genes on calcium influx in cultured C. elegans mechanosensory neurons. J Neurobiol. 2006;66(10):1125–1139. doi: 10.1002/neu.20261. [DOI] [PubMed] [Google Scholar]
- 59.Kato S, Xu Y, Cho CE, Abbott LF, Bargmann CI. Temporal responses of C. elegans chemosensory neurons are preserved in behavioral dynamics. Neuron. 2014;81(3):616–628. doi: 10.1016/j.neuron.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, et al. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat Neurosci. 2010;13(6):715–722. doi: 10.1038/nn.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohta A, Ujisawa T, Sonoda S, Kuhara A. Light and pheromone-sensing neurons regulates cold habituation through insulin signalling in Caenorhabditis elegans. Nat Commun. 2014;5:4412. doi: 10.1038/ncomms5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura KD, Miyawaki A, Matsumoto K, Mori I. The C. elegans thermosensory neuron AFD responds to warming. Curr Biol. 2004;14(14):1291–1295. doi: 10.1016/j.cub.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 63.Leinwand SG, Chalasani SH. Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegans. Nat Neurosci. 2013;16(10):1461–1467. doi: 10.1038/nn.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark DA, Biron D, Sengupta P, Samuel AD. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci. 2006;26(28):7444–7451. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larsch J, Ventimiglia D, Bargmann CI, Albrecht DR. High-throughput imaging of neuronal activity in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2013;110(45):E4266–E4273. doi: 10.1073/pnas.1318325110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee RYN, Lobel L, Hengartner M, Horvitz HR, Avery L. Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 1997;16(20):6066–6076. doi: 10.1093/emboj/16.20.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crump JG, Zhen M, Jin Y, Bargmann CI. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron. 2001;29(1):115–129. doi: 10.1016/s0896-6273(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 68.Jee C, Vanoaica L, Lee J, Park BJ, Ahnn J. Thioredoxin is related to life span regulation and oxidative stress response in Caenorhabditis elegans. Genes Cells. 2005;10(12):1203–1210. doi: 10.1111/j.1365-2443.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 69.Zaslaver A, et al. Hierarchical sparse coding in the sensory system of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2015;112(4):1185–1189. doi: 10.1073/pnas.1423656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakata K, et al. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120(3):407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]