Significance
Data from a longitudinal neuroimaging study beginning in the preschool period and including three brain scans through school age and early adolescence were used to investigate the effects of maternal support on the development of the hippocampus. Consistent with animal findings showing that early support enhances hippocampal development and later adaptive coping, findings demonstrated that early childhood maternal support predicted a steeper hippocampal growth trajectory. The data also suggested that early childhood was a sensitive period when the effects of support had a more powerful effect on hippocampal growth. The hippocampal growth trajectory was associated with better emotion regulation in early adolescence. Findings suggest that enhancing early childhood maternal support fosters healthy childhood brain development and emotion functioning.
Keywords: maternal support, hippocampus, sensitive period, preschool, emotions
Abstract
Building on well-established animal data demonstrating the effects of early maternal support on hippocampal development and adaptive coping, a few longitudinal studies suggest that early caregiver support also impacts human hippocampal development. How caregiving contributes to human hippocampal developmental trajectories, whether there are sensitive periods for these effects, as well as whether related variation in hippocampal development predicts later childhood emotion functioning are of major public health importance. The current study investigated these questions in a longitudinal study of preschoolers assessed annually for behavioral and emotional development, including observed caregiver support. One hundred and twenty-seven children participated in three waves of magnetic resonance brain imaging through school age and early adolescence. Multilevel modeling of the effects of preschool and school-age maternal support on hippocampal volumes across the three waves was conducted. Hippocampal volume increased faster for those with higher levels of preschool maternal support. Subjects with support 1 SD above the mean had a 2.06 times greater increase in total hippocampus volume across the three scans than those with 1 SD below the mean (2.70% vs. 1.31%). No effect of school-age support was found. Individual slopes of hippocampus volume were significantly associated with emotion regulation at scan 3. The findings demonstrate a significant effect of early childhood maternal support on hippocampal volume growth across school age and early adolescence and suggest an early childhood sensitive period for these effects. They also show that this growth trajectory is associated with later emotion functioning.
A large body of developmental data from studies of rodents has clearly established that the early experience of a highly nurturing caregiver has a powerful effect on hippocampal development in the rat pup, through an epigenetic mechanism (1–4). Building on these findings in animals, an increasing body of data in humans has emerged suggesting that early experiences of support, or conversely of abuse, neglect, or adversity, similarly impact human hippocampal development (5–7). The hippocampus, a region dense with glucocorticoid receptors, plays an integral role in the hypothalamic pituitary axis stress response (8, 9). Related to this role, reductions in hippocampal volume have been implicated in maladaptive stress reactivity and coping, as well as in affective psychopathology (10, 11). Therefore, a greater understanding of the environmental factors, particularly early caregiving experiences, that contribute to healthy hippocampal development in humans is of significant public health importance. Further, it is critical to examine whether variation in hippocampal development related to early caregiving predicts later childhood emotion functioning, as would be expected based on the animal data but not yet established in humans (12).
Although retrospective studies establish a link between childhood trauma and abuse and decreases in hippocampal volume in adults, these findings are limited by the known bias and possible inaccuracy of retrospective accounts of early childhood experiences by adult reporters (13, 14). More recently, some prospective data have become available to inform this issue. Using one wave of scan data from the study sample presented here, we have previously reported a link between higher early childhood maternal support and larger hippocampal volumes measured at school age in nondepressed subjects (15). Another prospective study that followed a small sample of cocaine-exposed infants from birth through adolescence reported decreased hippocampal volumes in adolescents who experienced higher maternal nurturance at age 4 (16). Although this effect was opposite what would be expected from the animal literature, the use of a relatively small sample exposed to drugs in utero may represent a unique developmental trajectory. Alterations in patterns of connectivity between the medial prefrontal cortex and amygdala have been reported in children who experienced early maternal deprivation (17, 18). Notably, another unique prospective study of a small group of children exposed to chronically depressed and less nurturing mothers displayed increases in amygdala volumes but no changes in hippocampus when scanned at age 10 (19). These conflicting findings underscore the need for further investigation of these relationships in larger samples with broader early risk exposures. In addition, there is a need to examine brain outcomes across the trajectory of brain development using multiple scan waves longitudinally. It is possible that disparate findings may reflect the unique risk trajectories of the various study samples, small sample sizes, as well as the limitations of cross-sectional imaging outcomes. As such, the question of whether the effect of early caregiver support impacts hippocampal development across its growth trajectory is of key interest. The current study aimed to address this question using a longitudinal neuroimaging design across childhood to investigate whether there are effects of support on brain development from school age through early adolescence.
Another key question that has been of interest in the study of the effects of caregiving quality on emotional, cognitive, and related brain development has been whether there are sensitive periods during which these environmental factors may have a particularly powerful effect on developmental outcomes. Such sensitive periods in development, when environmental exposures have a uniquely large and formative effect on neural structure and function, are well-established in visual and sensory-motor systems (20, 21). There has been some emerging evidence for sensitive periods in emotional development as well. This includes data from the Bucharest Early Intervention Project, where institutionalized children randomized to foster care before the age of 2 had superior cognitive and socioemotional outcomes to those randomized at later ages (22). Similarly, Rao et al. (16) report evidence for a sensitive period for the effects of maternal support (although in the opposite direction of that expected from animal studies), with childhood support at age 4 predicting adolescent brain outcomes whereas later childhood support at age 8 did not. If sensitive periods for the effects of supportive caregiving on brain development could be identified, it would have very important early intervention and prevention implications.
To address these questions, we sought to investigate whether experiences of maternal support predicted the trajectory of hippocampal development through school age and early adolescence using a longitudinal neuroimaging study with three waves of structural imaging data. We also tested whether caregiver support during the preschool versus school-age periods had unique effects on hippocampal outcomes. Last, to better interpret the functional significance of any effects found on hippocampal growth trajectories, we examined whether these trajectories predicted emotion reactivity and/or regulation at late school age.
Results
Subjects were 127 children participating in a longitudinal study of preschool depression who met all inclusion/exclusion criteria for magnetic resonance brain imaging at the first of three scan waves at ages 7–13. These children had usable left and/or right hippocampus T1-weighted MRI volume data at one or more scan waves and maternal support data, as well as other data of interest in the trajectory of hippocampal development, including IQ and income-to-needs ratio. Demographic characteristics of the study sample are detailed in Table 1. Multilevel linear models (MLMs) were used to examine the effect of maternal support on hippocampus volume over three scan waves. Two measures of maternal support, one assessed during the preschool period (centered at mean 11.65) and one assessed at school age before the first scan (centered at mean 30.60), were included as independent variables in the models. At the wave where preschool maternal support was assessed, 66% of subjects lived with both maternal and paternal figures in the home. The models included random intercept and slope components with an unstructured covariance matrix between the two. Time was coded as scan number, and covariates were age at scan 1 (centered at mean 10.54 y), quadratic age at scan 1, gender (1 male, 0 female), income-to-needs ratio at scan 1 (centered at mean 1.72), IQ (centered at mean 103.68), and total gray matter volume. Degrees-of-freedom calculations used the method of Kenward and Roger (23), and analyses were conducted using SAS version 9.3.
Table 1.
Characteristics of the sample (n = 127)
| Mean | SD | |
| Age at scan 1, y | 10.54 | 1.06 |
| Income-to-needs ratio at scan 1 | 1.72 | 0.99 |
| Preschool maternal support | 11.65 | 8.30 |
| School-age maternal support | 30.60 | 9.07 |
| IQ score | 103.68 | 15.65 |
| % | N | |
| Male gender | 51.18 | 65 |
| Internalizing diagnosis through scan 1* | 68.50 | 87 |
| Externalizing diagnosis through scan 1† | 52.76 | 67 |
| Number of scans completed | ||
| One scan | 11.81 | 15 |
| Two scans | 29.92 | 38 |
| Three scans | 58.27 | 74 |
Internalizing diagnoses included MDD, generalized anxiety disorder, separation anxiety disorder, and posttraumatic stress disorder.
Externalizing diagnoses included attention-deficit/hyperactivity disorder, conduct disorder, and oppositional defiant disorder.
Maternal Support During Preschool and Developmental Trajectories of Hippocampus Volume During School Age and Early Adolescence.
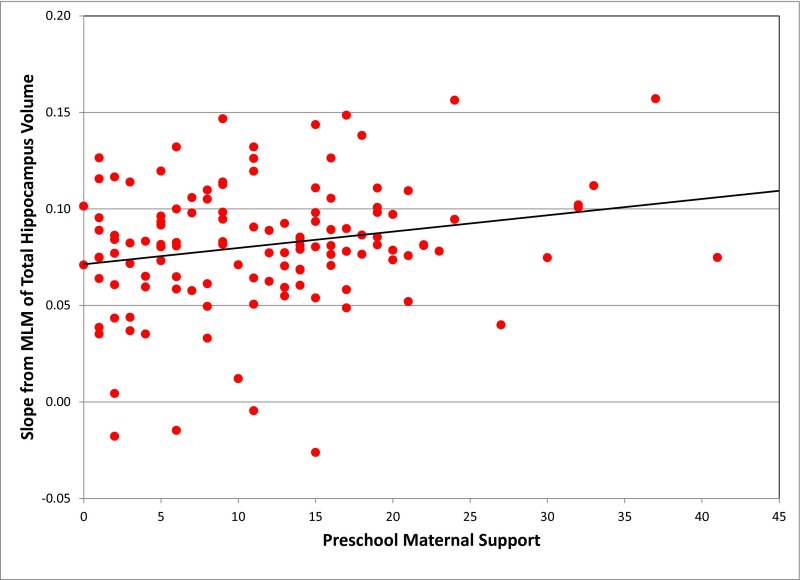
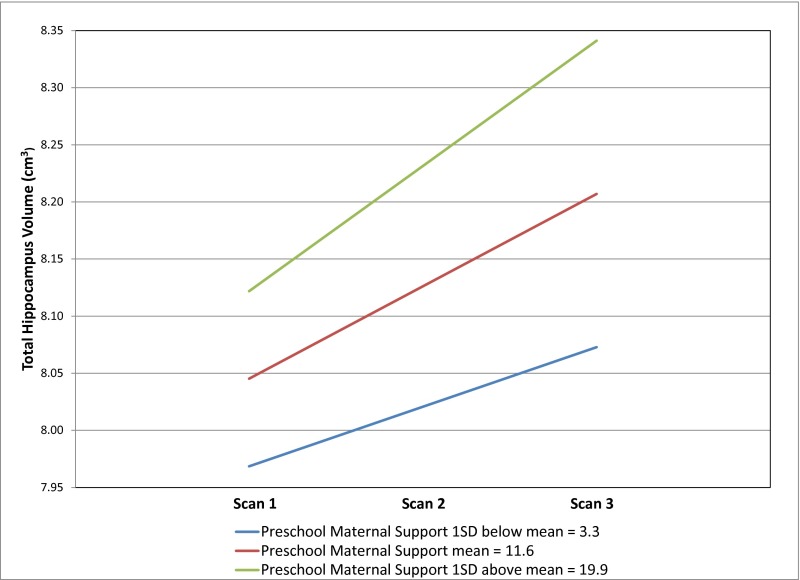
The results of the MLM of total hippocampus volume are shown in Table 2. There was a significant increase in total hippocampus volume over time (P < 0.0001). In addition, the main effect of total gray matter volume was significantly positively associated with hippocampus volume (B = 0.0053, SE = 0.0007, t = 7.61, P < 0.0001). IQ score was not significantly associated with hippocampus volume. Neither preschool (P = 0.4629) nor school-age (P = 0.4846) maternal support was significant as a main effect; however, the interaction between preschool maternal support and time was significantly associated with hippocampus volume in a positive direction. That is, total hippocampus volume increased faster for those with higher levels of preschool maternal support. Specifically, subjects with preschool maternal support 1 SD above the mean (19.9) compared with subjects with preschool maternal support 1 SD below the mean (3.3) had a 2.06 times greater increase in total hippocampus volume from scan 1 to 3 (2.70% vs. 1.31%). Fig. 1 shows individually estimated total hippocampus volume slopes as a function of preschool maternal support. Effect size was calculated by taking the correlation of preschool maternal support with subjects’ individual slope estimates, and the effect size for this model was 0.22. Fig. S1 shows estimated trajectories of total hippocampus volume by preschool maternal support.
Table 2.
Multilevel linear model of total hippocampus volume by preschool and school-age maternal support (n = 127)
| Estimate, cm3 | SE | t | P | |
| Intercept | 7.9360 | 0.0957 | 82.93 | <0.0001 |
| Age | 0.1024 | 0.0528 | 1.94 | 0.0546 |
| Age-squared | 0.0202 | 0.0469 | 0.43 | 0.6676 |
| Gender, male 1, female 0 | 0.0568 | 0.1159 | 0.49 | 0.6250 |
| Income-to-needs ratio | 0.0285 | 0.0740 | 0.39 | 0.7007 |
| IQ score | 0.0070 | 0.0043 | 1.63 | 0.1059 |
| Total gray matter volume* | 0.0053 | 0.0007 | 7.61 | <0.0001 |
| Time† | 0.0809 | 0.0106 | 7.65 | <0.0001 |
| Preschool maternal support | 0.0058 | 0.0078 | 0.74 | 0.4629 |
| School-age maternal support | 0.0059 | 0.0084 | 0.70 | 0.4846 |
| Preschool maternal support × time | 0.0035 | 0.0012 | 2.83 | 0.0059 |
| School-age maternal support × time | −0.0001 | 0.0011 | −0.10 | 0.9228 |
Total hippocampus volume was subtracted from total gray matter volume.
Time indicates scan number.
Fig. 1.
Individually estimated slopes over time for total hippocampus volume as a function of preschool maternal support (n = 127).
Fig. S1.
Estimated trajectories of total hippocampus volume by preschool maternal support in n = 127 subjects.
In contrast to preschool support, there was no significant change in total hippocampus volume over time as a function of school-age maternal support (B = −0.0001, SE = 0.0011, t = −0.10, P = 0.9228). To further evaluate any effect of school-age maternal support, an MLM of total hippocampus volume with school-age, but not preschool, maternal support as an independent variable was conducted. The school-age maternal support by time interaction in that model was still not significant (B = 0.0012, SE = 0.0011, t = 1.12, P = 0.2647). Importantly, although preschool and school-age support are correlated at 0.40, there was ample variance in the study sample of children who had high preschool support and low school-age support and vice versa to allow us to test the differential effects of these two variables (SI Materials and Methods).
Of note, the significant preschool maternal support by time interaction remained (B = 0.0034, SE = 0.0012, t = 2.79, P = 0.0067) when internalizing diagnosis through scan 1 [major depressive disorder (MDD), generalized anxiety disorder, separation anxiety disorder, and/or posttraumatic stress disorder] and externalizing diagnosis through scan 1 (attention-deficit/hyperactivity disorder, conduct disorder, and/or oppositional defiant disorder), and their interactions with time were included as covariates in the MLM.
To further investigate the relationship between maternal support and hippocampus volume, separate MLMs were run for left and right hippocampus volume. Table 3 details the results of the models by hemisphere. As in the model of total hippocampus volume, the main effects of time and total gray matter volume were significantly associated with left and right hippocampus volume (P < 0.0001). Additionally, higher IQ scores were significantly associated with greater right hippocampus volume (B = 0.0046, SE = 0.0023, t = 2.02, P = 0.0460). To determine whether verbal or nonverbal IQ had a greater effect on hippocampus volume, additional MLMs were run replacing IQ score with verbal IQ and, separately, nonverbal IQ. Results indicated that verbal IQ was driving the effect, as seen in Tables S1 and S2.
Table 3.
Multilevel linear models of left and right hippocampus volume by preschool and school-age maternal support (n = 127)
| Estimate, cm3 | SE | t | P | |
| Left hippocampus volume | ||||
| Intercept | 3.8856 | 0.0512 | 75.85 | <0.0001 |
| Age | 0.0525 | 0.0278 | 1.89 | 0.0619 |
| Age-squared | 0.0020 | 0.0248 | 0.08 | 0.9358 |
| Gender, male 1, female 0 | 0.0142 | 0.0619 | 0.23 | 0.8195 |
| Income-to-needs ratio | −0.0270 | 0.0392 | −0.69 | 0.4921 |
| IQ score | 0.0022 | 0.0023 | 0.97 | 0.3361 |
| Total gray matter volume* | 0.0031 | 0.0004 | 7.50 | <0.0001 |
| Time† | 0.0414 | 0.0069 | 6.02 | <0.0001 |
| Preschool maternal support | 0.0022 | 0.0043 | 0.51 | 0.6119 |
| School-age maternal support | 0.0079 | 0.0045 | 1.73 | 0.0854 |
| Preschool maternal support × time | 0.0019 | 0.0008 | 2.37 | 0.0203 |
| School-age maternal support × time | −0.0009 | 0.0007 | −1.22 | 0.2257 |
| Right hippocampus volume | ||||
| Intercept | 4.0539 | 0.0505 | 80.31 | <0.0001 |
| Age | 0.0485 | 0.0277 | 1.75 | 0.0829 |
| Age-squared | 0.0173 | 0.0247 | 0.70 | 0.4842 |
| Gender (male 1, female 0) | 0.0272 | 0.0612 | 0.44 | 0.6578 |
| Income-to-needs ratio | 0.0472 | 0.0390 | 1.21 | 0.2278 |
| IQ score | 0.0046 | 0.0023 | 2.02 | 0.0460 |
| Total gray matter volume* | 0.0025 | 0.0004 | 6.55 | <0.0001 |
| Time† | 0.0414 | 0.0059 | 7.05 | <0.0001 |
| Preschool maternal support | 0.0034 | 0.0041 | 0.83 | 0.4102 |
| School-age maternal support | −0.0017 | 0.0044 | −0.37 | 0.7089 |
| Preschool maternal support × time | 0.0014 | 0.0007 | 2.10 | 0.0389 |
| School-age maternal support × time | 0.0007 | 0.0006 | 1.21 | 0.2308 |
Left/right hippocampus volume was subtracted from total gray matter volume.
Time indicates scan number.
Table S1.
Total, left, and right hippocampus volume by preschool and school-age maternal support with verbal IQ score as covariate (n = 127)
| Estimate, cm3 | SE | t | P | |
| Total hippocampus volume | ||||
| Intercept | 21.7366 | 6.9605 | 3.12 | 0.0023 |
| Age | 0.0988 | 0.0524 | 1.89 | 0.0619 |
| Age-squared | 0.0205 | 0.0466 | 0.44 | 0.6602 |
| Gender, male 1, female 0 | 0.0549 | 0.1153 | 0.48 | 0.6347 |
| Income-to-needs ratio | 0.0128 | 0.0747 | 0.17 | 0.8638 |
| Verbal IQ score | 0.1359 | 0.0686 | 1.98 | 0.0499 |
| Total gray volume* | 0.0053 | 0.0007 | 7.64 | <0.0001 |
| Time† | 0.0809 | 0.0106 | 7.65 | <0.0001 |
| Preschool maternal support | 0.0056 | 0.0078 | 0.71 | 0.4770 |
| School-age maternal support | 0.0046 | 0.0084 | 0.54 | 0.5883 |
| Preschool maternal support × time | 0.0035 | 0.0012 | 2.83 | 0.0059 |
| School-age maternal support × time | −0.0001 | 0.0011 | −0.10 | 0.9202 |
| Left hippocampus volume | ||||
| Intercept | 8.6509 | 3.6870 | 2.35 | 0.0206 |
| Age | 0.0514 | 0.0277 | 1.85 | 0.0661 |
| Age-squared | 0.0023 | 0.0246 | 0.09 | 0.9249 |
| Gender, male 1, female 0 | 0.0138 | 0.0618 | 0.22 | 0.8235 |
| Income-to-needs ratio | −0.0330 | 0.0396 | −0.83 | 0.4073 |
| Verbal IQ score | 0.0469 | 0.0363 | 1.29 | 0.1989 |
| Total gray volume* | 0.0031 | 0.0004 | 7.51 | <0.0001 |
| Time† | 0.0413 | 0.0069 | 6.02 | <0.0001 |
| Preschool maternal support | 0.0020 | 0.0043 | 0.48 | 0.6310 |
| School-age maternal support | 0.0073 | 0.0046 | 1.60 | 0.1131 |
| Preschool maternal support × time | 0.0019 | 0.0008 | 2.37 | 0.0204 |
| School-age maternal support × time | −0.0009 | 0.0007 | −1.22 | 0.2259 |
| Right hippocampus volume | ||||
| Intercept | 12.7820 | 3.6548 | 3.50 | 0.0007 |
| Age | 0.0462 | 0.0275 | 1.68 | 0.0957 |
| Age-squared | 0.0174 | 0.0244 | 0.71 | 0.4777 |
| Gender, male 1, female 0 | 0.0255 | 0.0608 | 0.42 | 0.6764 |
| Income-to-needs ratio | 0.0378 | 0.0392 | 0.96 | 0.3369 |
| Verbal IQ score | 0.0859 | 0.0360 | 2.39 | 0.0187 |
| Total gray volume* | 0.0025 | 0.0004 | 6.61 | <0.0001 |
| Time† | 0.0414 | 0.0059 | 7.06 | <0.0001 |
| Preschool maternal support | 0.0033 | 0.0041 | 0.80 | 0.4225 |
| School-age maternal support | −0.0024 | 0.0044 | −0.54 | 0.5885 |
| Preschool maternal support × time | 0.0014 | 0.0007 | 2.10 | 0.0393 |
| School-age maternal support × time | 0.0007 | 0.0006 | 1.20 | 0.2338 |
Total/left/right hippocampus volume was subtracted from total gray matter volume.
Time indicates scan number.
Table S2.
Total, left, and right hippocampus volume by preschool and school-age maternal support with nonverbal IQ score as covariate (n = 127)
| Estimate, cm3 | SE | t | P | |
| Total hippocampus volume | ||||
| Intercept | 12.7056 | 5.9581 | 2.13 | 0.0351 |
| Age | 0.1009 | 0.0532 | 1.90 | 0.0604 |
| Age-squared | 0.0156 | 0.0472 | 0.33 | 0.7419 |
| Gender, male 1, female 0 | 0.0550 | 0.1167 | 0.47 | 0.6384 |
| Income-to-needs ratio | 0.0457 | 0.0736 | 0.62 | 0.5358 |
| Nonverbal IQ score | 0.0461 | 0.0577 | 0.80 | 0.4257 |
| Total gray volume* | 0.0053 | 0.0007 | 7.67 | <0.0001 |
| Time† | 0.0813 | 0.0106 | 7.68 | <0.0001 |
| Preschool maternal support | 0.0068 | 0.0079 | 0.87 | 0.3883 |
| School-age maternal support | 0.0087 | 0.0082 | 1.06 | 0.2935 |
| Preschool maternal support × time | 0.0035 | 0.0012 | 2.83 | 0.0059 |
| School-age maternal support × time | −0.0001 | 0.0011 | −0.09 | 0.9262 |
| Left hippocampus volume | ||||
| Intercept | 5.0653 | 3.1336 | 1.62 | 0.1087 |
| Age | 0.0518 | 0.0280 | 1.85 | 0.0665 |
| Age-squared | 0.0003 | 0.0248 | 0.01 | 0.9891 |
| Gender, male 1, female 0 | 0.0131 | 0.0621 | 0.21 | 0.8338 |
| Income-to-needs ratio | −0.0216 | 0.0388 | −0.56 | 0.5797 |
| Nonverbal IQ score | 0.0114 | 0.0304 | 0.38 | 0.7078 |
| Total gray volume* | 0.0031 | 0.0004 | 7.58 | <0.0001 |
| Time† | 0.0416 | 0.0069 | 6.06 | <0.0001 |
| Preschool maternal support | 0.0025 | 0.0043 | 0.59 | 0.5552 |
| School-age maternal support | 0.0088 | 0.0044 | 1.99 | 0.0485 |
| Preschool maternal support × time | 0.0019 | 0.0008 | 2.37 | 0.0203 |
| School-age maternal support × time | −0.0009 | 0.0007 | −1.22 | 0.2254 |
| Right hippocampus volume | ||||
| Intercept | 7.3264 | 3.1452 | 2.33 | 0.0216 |
| Age | 0.0476 | 0.0281 | 1.70 | 0.0928 |
| Age-squared | 0.0144 | 0.0249 | 0.58 | 0.5643 |
| Gender, male 1, female 0 | 0.0262 | 0.0619 | 0.42 | 0.6732 |
| Income-to-needs ratio | 0.0581 | 0.0389 | 1.49 | 0.1381 |
| Nonverbal IQ score | 0.0317 | 0.0305 | 1.04 | 0.3010 |
| Total gray volume* | 0.0026 | 0.0004 | 6.62 | <0.0001 |
| Time† | 0.0417 | 0.0059 | 7.08 | <0.0001 |
| Preschool maternal support | 0.0041 | 0.0042 | 0.98 | 0.3302 |
| School-age maternal support | 0.0001 | 0.0044 | 0.03 | 0.9789 |
| Preschool maternal support × time | 0.0014 | 0.0007 | 2.10 | 0.0393 |
| School-age maternal support × time | 0.0007 | 0.0006 | 1.21 | 0.2290 |
Total/left/right hippocampus volume was subtracted from total gray matter volume.
Time indicates scan number.
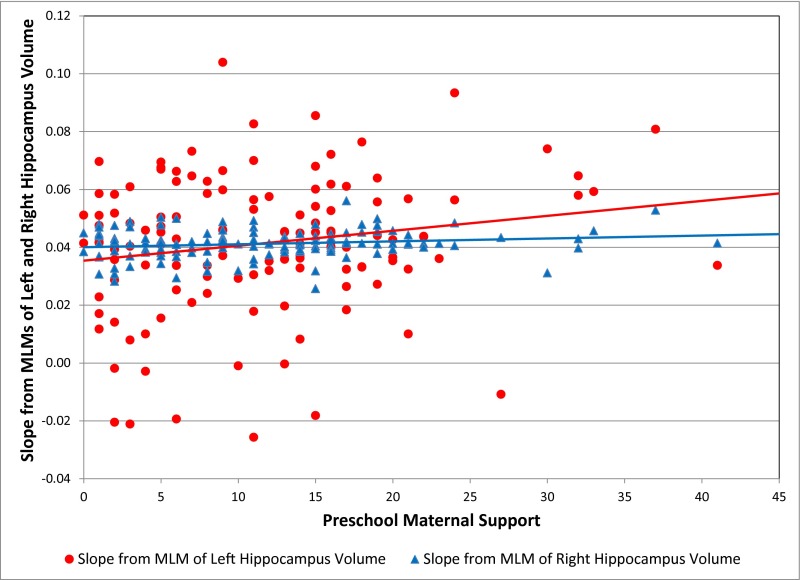
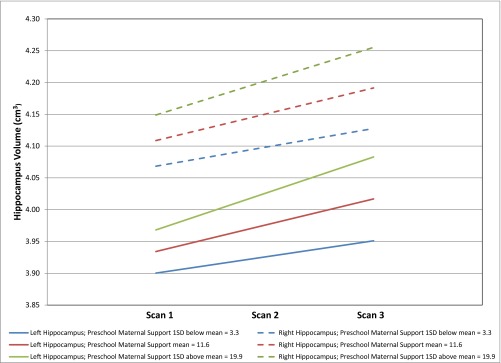
Similar to the total hippocampus volume model, the main effects of preschool and school-age maternal support were nonsignificant, but the interaction of preschool maternal support and time was significantly associated with both left (B = 0.0019, SE = 0.0008, t = 2.37, P = 0.0203) and right hippocampus volume (B = 0.0014, SE = 0.0007, t = 2.10, P = 0.0389). Specifically, subjects with preschool maternal support 1 SD above the mean (19.9) compared with subjects with preschool maternal support 1 SD below the mean (3.3) had a 2.22 times greater increase in left hippocampus volume from scan 1 to 3 (2.89% vs. 1.30%) and a 1.76 times greater increase in right hippocampus volume from scan 1 to 3 (2.56% vs. 1.46%). Fig. 2 shows individually estimated left and right hippocampus volume slopes as a function of preschool maternal support. Effect sizes were 0.18 for left and 0.17 for right hippocampus. Fig. S2 shows estimated trajectories of left and right hippocampus volume by preschool maternal support.
Fig. 2.
Individually estimated slopes over time for left and right hippocampus volume as a function of preschool maternal support (n = 127).
Fig. S2.
Estimated trajectories of left and right hippocampus volume by preschool maternal support in n = 127 subjects.
As with the MLM of total hippocampus volume, the preschool maternal support by time interaction remained significant when internalizing diagnosis through scan 1 and externalizing diagnosis through scan 1, and their interactions with time were included as covariates in the models of left (B = 0.0019, SE = 0.0008, t = 2.37, P = 0.0206) and right hippocampus volume (B = 0.0014, SE = 0.0007, t = 2.09, P = 0.0404).
Because school-age maternal support was not significantly associated with hippocampus volume trajectory, the MLMs were rerun without this independent variable. Removing school-age maternal support from the model resulted in an increased sample size of n = 143 subjects with the same effects found (Tables S3 and S4).
Table S3.
Multilevel linear model of total hippocampus volume by preschool maternal support (n = 143)
| Estimate, cm3 | SE | t | P | |
| Intercept | 7.9519 | 0.0862 | 92.26 | <0.0001 |
| Age | 0.0974 | 0.0422 | 2.31 | 0.0227 |
| Age-squared | 0.0123 | 0.0269 | 0.46 | 0.6490 |
| Gender, male 1, female 0 | 0.0590 | 0.1086 | 0.54 | 0.5877 |
| Income-to-needs ratio | 0.0615 | 0.0594 | 1.04 | 0.3023 |
| IQ score | 0.0069 | 0.0038 | 1.80 | 0.0738 |
| Total gray matter volume* | 0.0051 | 0.0006 | 7.81 | <0.0001 |
| Time† | 0.0838 | 0.0097 | 8.60 | <0.0001 |
| Preschool maternal support | 0.0079 | 0.0069 | 1.15 | 0.2534 |
| Preschool maternal support × time | 0.0030 | 0.0010 | 2.94 | 0.0041 |
Total hippocampus volume was subtracted from total gray matter volume.
Time indicates scan number.
Table S4.
Multilevel linear models of left and right hippocampus volume by preschool maternal support (n = 143)
| Estimate, cm3 | SE | t | P | |
| Left hippocampus volume | ||||
| Intercept | 3.8948 | 0.0467 | 83.39 | <0.0001 |
| Age | 0.0464 | 0.0225 | 2.06 | 0.0410 |
| Age-squared | 0.0025 | 0.0143 | 0.18 | 0.8608 |
| Gender, male 1, female 0 | 0.0067 | 0.0586 | 0.11 | 0.9090 |
| Income-to-needs ratio | 0.0088 | 0.0317 | 0.28 | 0.7820 |
| IQ score | 0.0028 | 0.0021 | 1.38 | 0.1708 |
| Total gray matter volume* | 0.0030 | 0.0004 | 7.84 | <0.0001 |
| Time† | 0.0436 | 0.0063 | 6.93 | <0.0001 |
| Preschool maternal support | 0.0042 | 0.0038 | 1.10 | 0.2726 |
| Preschool maternal support × time | 0.0015 | 0.0007 | 2.30 | 0.0237 |
| Right hippocampus volume | ||||
| Intercept | 4.0598 | 0.0452 | 89.82 | <0.0001 |
| Age | 0.0479 | 0.0221 | 2.17 | 0.0318 |
| Age-squared | 0.0094 | 0.0141 | 0.67 | 0.5065 |
| Gender, male 1, female 0 | 0.0337 | 0.0571 | 0.59 | 0.5568 |
| Income-to-needs ratio | 0.0429 | 0.0311 | 1.38 | 0.1701 |
| IQ score | 0.0038 | 0.0020 | 1.91 | 0.0590 |
| Total gray matter volume* | 0.0025 | 0.0004 | 6.83 | <0.0001 |
| Time† | 0.0430 | 0.0056 | 7.75 | <0.0001 |
| Preschool maternal support | 0.0035 | 0.0036 | 0.96 | 0.3408 |
| Preschool maternal support × time | 0.0014 | 0.0006 | 2.33 | 0.0219 |
Left/right hippocampus volume was subtracted from total gray matter volume.
Time indicates scan number.
SI Materials and Methods present additional analyses replicating our previous findings (15) at scan 1, only with the larger sample used in the current report (see Tables S5 and S6).
Table S5.
Total hippocampus volume at scan 1 by preschool MDD severity and preschool maternal support (n = 111)
| Estimate, cm3 | SE | t | P | |
| Intercept | 7.5258 | 0.2164 | 34.79 | <0.0001 |
| Male gender | 0.3019 | 0.1417 | 2.13 | 0.0354 |
| Preschool MDD severity | 0.0519 | 0.0596 | 0.87 | 0.3862 |
| Preschool maternal support | 0.0456 | 0.0130 | 3.50 | 0.0007 |
| Preschool MDD severity × maternal support | −0.0071 | 0.0039 | −1.82 | 0.0716 |
Table S6.
Total hippocampus volume at scan 1 by preschool MDD severity and preschool maternal support, with total gray volume (n = 111)
| Estimate, cm3 | SE | t | P | |
| Intercept | 7.9171 | 0.1891 | 41.87 | <0.0001 |
| Male gender | −0.0342 | 0.1278 | −0.27 | 0.7895 |
| Total gray volume | 0.0076 | 0.0011 | 6.89 | <0.0001 |
| Preschool MDD severity | 0.0321 | 0.0498 | 0.64 | 0.5205 |
| Preschool maternal support | 0.0248 | 0.0113 | 2.20 | 0.0303 |
| Preschool MDD severity × maternal support | −0.0046 | 0.0033 | −1.39 | 0.1668 |
Does the Trajectory of Hippocampal Development Predict Emotion Functioning?
Individual-subject intercepts and slopes were generated from the MLM of total hippocampus volume by preschool maternal support and were investigated as potential predictors of the Children’s Emotion Management Scale–Sadness (CEMS-S) (24) dysregulation and coping subscales. Individual-subject slopes were significantly associated with CEMS-S dysregulation and coping in a general linear model with individual intercepts and slopes as independent variables (B = −26.41, SE = 12.53, t = −2.11, P = 0.0374).
SI Materials and Methods
Stability of Supportive Behaviors Used with Preschool vs. School-Age Children.
Using a tertiary split, caregivers were classified as being low-, mid-, and high-support based on the total number of observed supportive behaviors used during a structured parent–child interaction task when children were in the preschool period of development. An additional three-way split was conducted based on observed parent–child interactions that were conducted when the same children were school-age. Descriptive analyses revealed that ∼60% of mothers in the low-support group when their children were preschoolers were also classified as low-support when their children were school-age. The remaining 40% showed increased support (i.e., classified as mid- or high-support) behaviors when their children were school-age. Approximately 30% of caregivers in the mid-support group when their children were in preschool remained in the mid-support group when their children were school-age. Of the remaining 70% of the preschool mid-support group, ∼37% were categorized as low-support at school age and 33% were in the high-support group at school age. Last, ∼60% of caregivers in the high-support group when their children were preschoolers were also classified as being high-support when their children were school-age. Thus, the results indicated that caregiving support of preschool children varies from caregiving support observed when the same children reach school age. That is, being highly supportive during interactions with preschool children did not mean the same was true when the same children were school-age. However, the total number of supportive behaviors exhibited with preschool children was significantly associated with the total number of supportive behaviors observed when the same children were school-age (r = 0.40, P < 0.0001).
Replication of Findings from Luby et al. (15).
The MLM presented in the main text examined the effects of preschool maternal support on both the intercept and slope of hippocampal volume over time. The model showed significant effects of preschool maternal support on the slope or trajectory of hippocampal volume over time, with lower maternal support associated with a shallower slope of hippocampal volume increase over time. This effect held with a number of important covariates in the model, including whole-brain gray matter volume. However, it was intriguing that there was no significant effect of preschool maternal support on the intercept of hippocampal volume (i.e., average volume). Our previous report, Luby et al. (15), did find a significant effect of preschool maternal support on hippocampal volume at the first scan, which could be thought of as analogous to an intercept effect. The current sample used a different methodology for quantifying hippocampal volume and is also larger than the one used in ref. 15, as data acquisition and processing were still ongoing for the first time point. Thus, we sought to determine whether we could replicate our prior findings in this larger sample. We ran essentially the same model 1 as in ref. 15 with the current sample for scan 1. Importantly, model 1 from ref. 15 included sex, preschool MDD severity, and preschool maternal support, but did not include whole-brain gray matter volume. As shown in Table S5, we clearly replicated the finding of a main effect of preschool maternal support on hippocampal volume for scan 1 with this larger sample. In the current manuscript, we included total gray volume as a covariate to specifically address the degree to which any effect of maternal support on hippocampal volume was over and above effects on the whole brain. Thus, we reran this model including total gray volume. As shown in Table S6, the effect of preschool maternal support was still significant at scan 1 with total gray volume in the model, although the effect size was reduced.
Discussion
These study findings in a group of children observed in interaction with their caregivers during the preschool period and then scanned three times across school age and early adolescence are consistent with the body of animal literature documenting the powerful effects of maternal support on hippocampal development. Study findings confirm and extend earlier cross-sectional findings in the same sample and suggest that failure to find similar effects in smaller samples may be related to type II error, and findings of opposite effects in a cocaine-exposed population may be unique to those exposures. Findings from this longitudinal neuroimaging study extend the literature in three important and previously unidentified ways. First, these results demonstrate that the effect of maternal support extends beyond one outcome measure of hippocampal volume at early school age and has an influence on the trajectory of hippocampal volume growth into later school age and early adolescence. Second, the findings demonstrate that maternal support measured during the preschool period has a stronger effect on hippocampal volume trajectories compared with support measured at school age, consistent with the hypothesis of a sensitive period for the effects of support on hippocampal development. Third, alterations in the trajectory of hippocampal growth had functional significance for adaptive outcomes, as evidenced by the association between hippocampal volume trajectories and a later measure of emotion regulation.
Consistent with the animal literature, alterations in hippocampal growth trajectories related to early maternal support were associated with more adaptive later emotion regulation (12). The hippocampus is a key brain region known to impact several areas of cognitive and emotion functioning including emotion regulation (10). Therefore, the identification of modifiable environmental exposures that can impact the development of the hippocampus and in turn later emotion functioning is a finding of key public health importance. These findings should inform prevention and early intervention strategies, a number of which have been designed and shown to effectively enhance maternal support (25–27).
These findings are limited by the fact that the study sample was enriched for preschoolers with emotional and behavioral problems. Although the findings held when the effects of these variables were accounted for, this group could have a unique risk trajectory from healthy developing children or children under other risk conditions. Based on this, future investigations of these risk relationships should now be explored in general-population samples. In addition, future studies should further investigate whether the effects found had regional anterior versus posterior subfield specificity in the hippocampus, an issue the current study was unable to address due to the current limitations of FreeSurfer methodology. Although study findings suggest that there is a sensitive period for preschool versus school-age maternal support on the trajectory of hippocampal volume growth, future studies that are specifically designed to more definitively address this question are now needed.
Conclusions
These study findings provide, to our knowledge, the first evidence for the long-term effects of early maternal support on the development of the hippocampus through school age and early adolescence. They also suggest that maternal support during the preschool period may represent a sensitive period when these experiences have a uniquely powerful effect on brain development. The finding that maternal support experienced during the preschool period has a tangible impact on the trajectory of hippocampal development through school age and early adolescence further underscores the importance of public health efforts to enhance maternal support during early childhood, a goal that has proven to be both feasible and cost-effective (28).
Materials and Methods
All study procedures were approved in advance by the Washington University Institutional Review Board and informed consent/assent was obtained from all study subjects (as age-appropriate) and their legal guardians. Subjects were participants in an ongoing 11-y longitudinal neuroimaging study. Behavioral/developmental assessments began when subjects were 3.0- to 5.11-y-old and continued annually over six waves with scanning starting at age 6.11–12.11 and repeated approximately every 18 mo for three waves of neuroimaging; n = 127 subjects who completed at least one scan (out of three), had maternal support data available at both preschool and school age (before the first scan), and had a valid IQ score were included in the primary analysis.
Maternal Preschool and School-Age Support Defined.
Maternal support in the present study was conceptualized as the degree to which mothers view and approach their children with positive regard overall as well as their efforts to be emotionally and developmentally aware of their children’s emotional well-being. Furthermore, caregiver support includes their ability to facilitate their children’s sense of autonomy by supporting and validating their child’s intent to lead and strategize to solve problems. More specifically, support was coded during an interval when parents expressed predominantly positive emotions during the mildly stressful task, when they remained calm, and when they were reassuring when reacting to the child’s emotional expressions elicited by the parent–child interaction task. Supportive behaviors included acknowledging the child’s accomplishments on the task or behaviors related to the parent–child interaction. Support includes verbally encouraging the child with positive emotional regard (e.g., “You’re really good at this” or “You got another one right”). Another example of support occurs when a child becomes visibly upset/anxious and the parent changes their proximity to the child so that they are closer and the caregiver may rub the child’s back to help him/her regulate.
Preschool Maternal Support.
Preschool maternal support was measured at the second annual study wave when subjects aged 3.11–6.11 and their caregivers engaged in a mildly stressful task in the laboratory. This task, known as “the waiting task,” required the child to wait for 8 min before opening a brightly wrapped gift sitting within arm’s reach while the parent completed questionnaires. This paradigm was designed to evoke mild stress for both parent and child. Staff trained to acceptable interrater reliability coded the interaction for supportive caregiving strategies the parent used to help regulate the child’s impulse and desire to open the gift before the appropriate amount of time had elapsed. Each instance of specific types of supportive caregiving strategies used by the parent was counted as 1 unit and summed to give an overall preschool maternal support score. Thus, each parent’s supportive caregiving score represents the total number of supportive behaviors they were observed using over the course of the 8-min task. Prior findings suggest that caregiving support behaviors coded during this task have good psychometric properties (29, 30). Specifically, Cronbach’s alpha for the combined supportive behaviors coded was alpha = 0.86.
School-Age Maternal Support.
Subjects and their caregivers completed a different developmentally appropriate mildly stressful task, “the puzzle task,” at the third annual study wave (child age 4.11–7.11) and again at their fifth annual assessment wave ∼4–5 y later (child age 8.3–12.6). In this task, a puzzle of the United States is placed between the parent and child under a cardboard box. The child is asked to attempt to put together a puzzle within an enclosed box so that they cannot see the pieces but are instructed only by the parent, who can see. Children are told that they cannot look and puzzle pieces are given to the parent, who can clearly see the puzzle board and where the child’s hands are. Parents are told to give the child instructions on how to place each piece, and both are told to finish the puzzle in 5 min to earn a prize. The examiner then sets a timer and tells the pair to begin. The 5-min task was separated into 30-s segments during which coders trained to adequate interrater reliability provided ratings for the frequency of parents’ supportive caregiving behaviors observed during the task. Maternal support data coded from the puzzle task were only included in these analyses if the child completed the task after age 6.0 and before their first scan. In instances where the subject had two ratings of maternal support from the puzzle task, school-age maternal support was calculated as the mean of the two values. Out of the n = 127 subjects included in this analysis, n = 120 (94%) had the same caregiver at preschool and school-age assessments of support.
Income-to-Needs Ratio.
An income-to-needs ratio was calculated as the total family income at the first scan divided by the federal poverty level based on family size for the year of data collection.
IQ Score.
Subjects were assessed with either the Kaufman Brief Intelligence Test (KBIT) (31) or the Wechsler Abbreviated Scale of Intelligence (WASI) (32) (depending upon age at study wave) to determine verbal and nonverbal intelligence.
Children’s Emotion Management Scale–Sadness.
The CEM-S measures is a parent report of the child’s sadness. The 11 items are on a Likert scale of hardly ever (1), sometimes (2), and often (3) and are summed to create inhibition, dysregulation, and coping subscales. The coping subscale was reverse-scored and added to the dysregulation subscale to create an overall measure of dysregulation and coping.
Imaging and Hippocampal Quantification.
Two 3D T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) scans were acquired sagittally on a Siemens 3.0T Tim Trio using a 12-channel head coil [repetition time (TR) 2,300 ms, echo time (TE) 3.16 ms, inversion time (TI) 1,200 ms, flip angle 8°, 160 slices, 256 × 256 matrix, field of view 256 mm, 1.0-mm3 voxels, 6:18 min per scan]. The two MPRAGE scans were assessed visually, and the best one was selected for further processing by blinded raters. The selected MPRAGE for each wave was processed using the longitudinal stream in the FreeSurfer software package, version 5.3 (surfer.nmr.mgh.harvard.edu) (33). Several processing steps, such as skull stripping, Talairach transformations, and atlas registration, as well as spherical surface maps and parcellations were initialized with common information from an unbiased within-patient template. This longitudinal stream reduces the bias that would otherwise be present in selecting a single scan result as baseline, and significantly increases reliability and statistical power (34). The white and pial surfaces generated by FreeSurfer were visually inspected and, when necessary, appropriate edits were performed and the surfaces were regenerated. For ∼10% of scans, poor scan quality required discarding them from further analysis (n = 15, 11, and 7 at the three waves, respectively). In those cases, all data were generated from within-subject templates using the remaining scans. Hippocampal and total gray matter volumes (sum of cortical, subcortical, and cerebellar gray matter) for the left and right hemispheres were obtained from the “Hippocampus” and “TotalGrayVol” measures, respectively, in Freesurfer’s “aseg.stats” report.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.T.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601443113/-/DCSupplemental.
References
- 1.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 2.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 3.Fish EW, et al. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 4.Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26(3-4):139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Bremner JD, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27(4):951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 7.Driessen M, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 8.De Kloet E. Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol. 1991;12(2):95–164. [Google Scholar]
- 9.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3(8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapolsky RM. Mothering style and methylation. Nat Neurosci. 2004;7(8):791–792. doi: 10.1038/nn0804-791. [DOI] [PubMed] [Google Scholar]
- 13.Vythilingam M, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159(12):2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teicher MH, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1-2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 15.Luby JL, et al. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci USA. 2012;109(8):2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao H, et al. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. Neuroimage. 2010;49(1):1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gee DG, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gee DG, et al. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA. 2011;108(34):14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox SE, Levitt P, Nelson CA., III How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sininger YS, Doyle KJ, Moore JK. The case for early identification of hearing loss in children. Auditory system development, experimental auditory deprivation, and development of speech perception and hearing. Pediatr Clin North Am. 1999;46(1):1–14. doi: 10.1016/s0031-3955(05)70077-8. [DOI] [PubMed] [Google Scholar]
- 22.Nelson CA, III, et al. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 23.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–997. [PubMed] [Google Scholar]
- 24.Zeman J, Shipman K, Penza-Clyve S. Development and initial validation of the Children’s Sadness Management Scale. J Nonverbal Behav. 2001;25(3):187–205. [Google Scholar]
- 25.Lieberman AF. Child-parent psychotherapy: A relationship-based approach to the treatment of mental health disorders in infancy and early childhood. In: Sameroff AJ, McDonough SC, Rosenblum KL, editors. Treating Parent-Infant Relationship Problems: Strategies for Intervention. Guilford; New York: 2004. pp. 97–122. [Google Scholar]
- 26.Marvin R, Cooper G, Hoffman K, Powell B. The Circle of Security project: Attachment-based intervention with caregiver-pre-school child dyads. Attach Hum Dev. 2002;4(1):107–124. doi: 10.1080/14616730252982491. [DOI] [PubMed] [Google Scholar]
- 27.Luby J, Lenze S, Tillman R. A novel early intervention for preschool depression: Findings from a pilot randomized controlled trial. J Child Psychol Psychiatry. 2012;53(3):313–322. doi: 10.1111/j.1469-7610.2011.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olds DL. The nurse-family partnership: An evidence-based preventive intervention. Infant Ment Health J. 2006;27(1):5–25. doi: 10.1002/imhj.20077. [DOI] [PubMed] [Google Scholar]
- 29.Belden AC, Luby JL. Preschoolers’ depression severity and behaviors during dyadic interactions: The mediating role of parental support. J Am Acad Child Adolesc Psychiatry. 2006;45(2):213–222. doi: 10.1097/01.chi.0000189133.59318.5e. [DOI] [PubMed] [Google Scholar]
- 30.Belden AC, Sullivan JP, Luby JL. Depressed and healthy preschoolers’ internal representations of their mothers’ caregiving: Associations with observed caregiving behaviors one year later. Attach Hum Dev. 2007;9(3):239–254. doi: 10.1080/14616730701455395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2nd Ed Pearson; Bloomington, MN: 2004. [Google Scholar]
- 32.The Psychological Corporation . Wechsler Abbreviated Scale of Intelligence (WASI) PsychCorp; San Antonio: 1999. [Google Scholar]
- 33.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: A robust approach. Neuroimage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]