Significance
Research initiatives in Huntington’s disease (HD) have traditionally focused on defining the biological processes mediating neuronal dysfunction and death during adult life. In contrast to these classical approaches, we have shown that selective exposure to the pathogenic protein during development recapitulates characteristic features of HD. These findings support the hypothesis that putative HD-associated developmental impairments in neurogenesis contribute to regional cellular vulnerabilities to late-life stressors leading to dysfunction culminating in cell death. An implication of our findings is that the spectrum of developmental alterations can be held in check for many decades after the birth of these vulnerable neuronal subtypes, thus defining a previously unidentified window for therapeutic interventions at a time when irreversible neural degeneration has not yet occurred.
Keywords: neurodegeneration, prodromal, plasticity
Abstract
Recent studies have identified impairments in neural induction and in striatal and cortical neurogenesis in Huntington’s disease (HD) knock-in mouse models and associated embryonic stem cell lines. However, the potential role of these developmental alterations for HD pathogenesis and progression is currently unknown. To address this issue, we used BACHD:CAG-CreERT2 mice, which carry mutant huntingtin (mHtt) modified to harbor a floxed exon 1 containing the pathogenic polyglutamine expansion (Q97). Upon tamoxifen administration at postnatal day 21, the floxed mHtt-exon1 was removed and mHtt expression was terminated (Q97CRE). These conditional mice displayed similar profiles of impairments to those mice expressing mHtt throughout life: (i) striatal neurodegeneration, (ii) early vulnerability to NMDA-mediated excitotoxicity, (iii) impairments in motor coordination, (iv) temporally distinct abnormalities in striatal electrophysiological activity, and (v) altered corticostriatal functional connectivity and plasticity. These findings strongly suggest that developmental aberrations may play important roles in HD pathogenesis and progression.
Huntington’s disease (HD) is an inherited neurodegenerative disorder caused by abnormal expansion of a polyglutamine tract in the amino terminal end of the protein, huntingtin (Htt). HD is characterized by the onset and progression of motor alterations frequently commencing during the fourth decade of life and associated with degeneration of the striatum, cortex, and other brain areas. Research initiatives in the field have traditionally focused on defining the biological processes mediating neuronal dysfunction and death during adult life. More recently, our studies and others have shown that the HD pathogenic mutation impairs the specification and maturation of striatal medium spiny neurons (MSNs), the primary target of HD neurodegeneration, and alters cortical progenitor cell divisions and neurogenesis by causing deregulation of mitotic spindle orientation (1, 2). Although the role of these diverse developmental defects for HD pathogenesis remains undefined, they have the potential to impair the integrity of striatal and cortical neuronal homeostasis and function through multiple inductive interactions. Indeed, these changes may provide an explanation for studies, including the Neurobiological Predictors of Huntington’s Disease (PREDICT-HD) and TRACK-HD studies, showing electrophysiological abnormalities, discrete brain structural as well as volumetric changes, and cognitive and motor deficits occurring long before disease onset (3–8). We therefore propose that selective exposure to mutant Htt (mHtt) during neural development can recapitulate characteristic features of HD. To examine this possibility, we used a conditional mHtt-ablation model allowing selective developmental expression of mHtt.
Results
The Generation of BACHD Mice Selectively Expressing mHtt During Development.
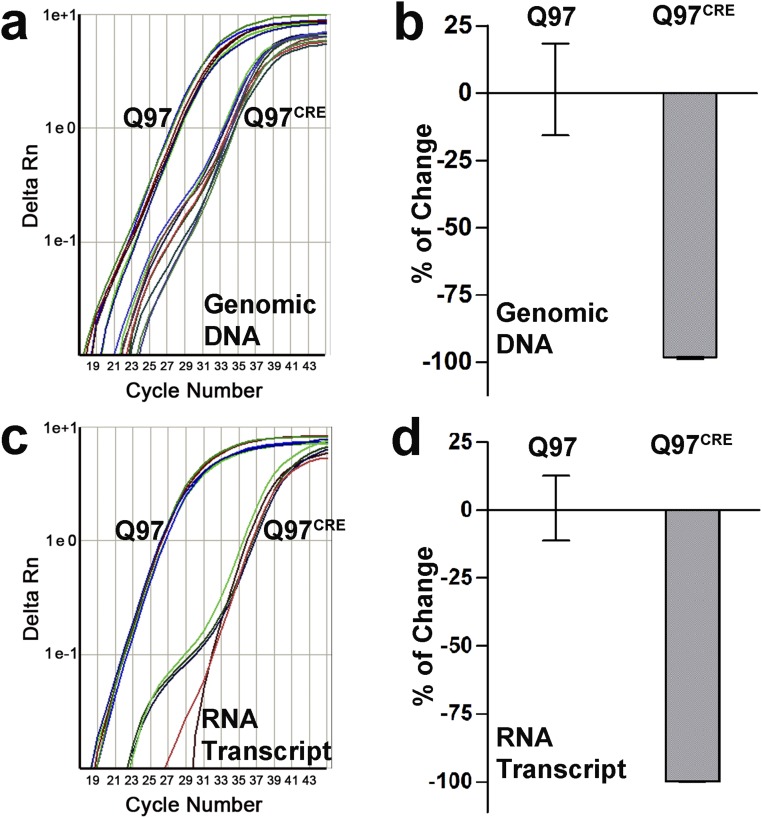
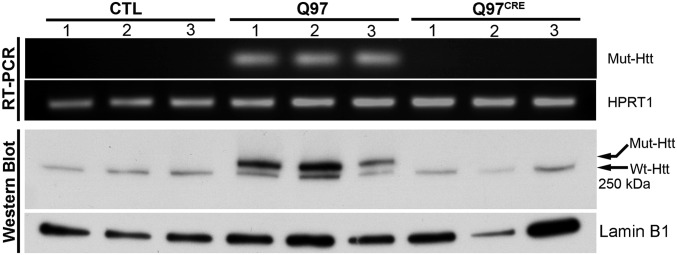
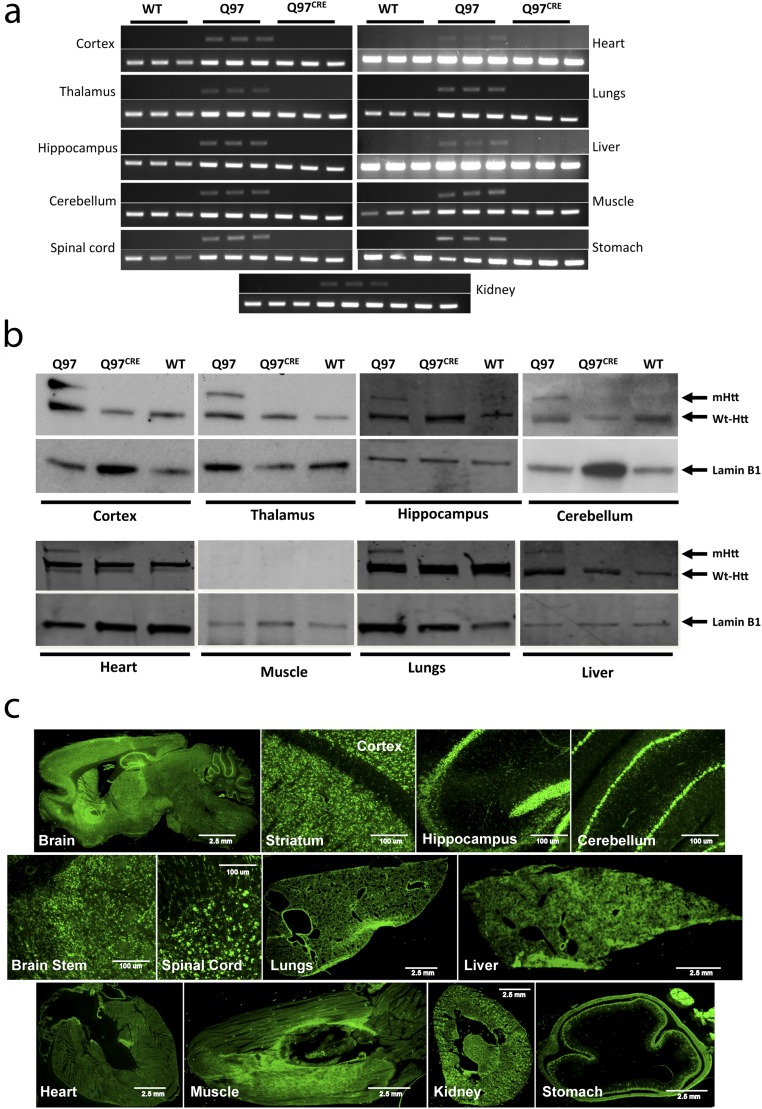
We interbred BACHD mice (9) carrying the full-length human mHtt gene modified to harbor a floxed exon 1 coding for 97 polyglutamine repeats (HD juvenile repeat range) with mice carrying the tamoxifen-inducible Cre recombinase (CRE) driver system (CAG-Cre/Esr1) (10). Their offspring were subsequently treated with tamoxifen at postnatal day (PND) 21 to generate mice expressing mHtt: (i) only during development (Q97CRE), (ii) during development and adult life (Q97), and (iii) in WT controls not expressing mHtt (WT). In 3-mo-old mice, the efficiency of mHtt tamoxifen-induced brain gene ablation was examined at genomic (Fig. S1 A and B), transcript, and protein levels (Fig. 1 and Fig. S1 C and D). Quantitative real-time PCR (QRT-PCR) analysis using primers targeting the floxed region of mHtt revealed a 98.2% reduction of the genomic amplicon in Q97CRE compared with Q97 mice (Fig. S1 A and B). Further analysis of mHtt RNA expression showed a 99.8% reduction of transcript expression in Q97CRE compared with WT mice (Fig. 1, Upper and and Fig. S1 C and D). Accordingly, no mHtt protein was detected by Western blot analysis (Fig. 1, Lower). Consistent with previous studies (10), we also verified efficient levels of recombination throughout the neuraxis and in nonneural tissues (Fig. S2). These observations validate the use of the BACHD mouse model to study the selective effects of mHtt expression during development.
Fig. S1.
CREER yields highly efficient levels of mHtt excisional recombination in Q97CRE mice. CRE-mediated excisional recombination after tamoxifen administration at PND21 was examined in 3-mo-old striatal specimens. High Q97CRE QRT-PCR cycle thresholds of DNA (nper strain = 8, A and B) and RNA (nper strain = 4, C and D) demonstrate very efficient levels of CREER-mediated excisional recombination of mHtt [relative quantification (RQ) = 0.018, CI95% = 0.015–0.021 and RQ = 0.002, CI95% = 0.001–0. 002, fold change of Q97CRE relative to Q97 for DNA and RNA, respectively]. Rn is the fluorescence of the reporter dye divided by the fluorescence of a passive reference dye.
Fig. 1.
CREER yields highly efficient levels of mHtt excisional recombination in Q97CRE mice. In contrast to striatal Q97 specimens, RT-PCR amplicons using primers that selectively detect the presence of mutant exon 1 of the Htt cDNA (Top) and Western blot analysis probed with the mAb 2166 anti-Htt antibody (∼350 kDa, Bottom) show no discernible bands in control and Q97CRE striatal specimens (nper strain = 3).
Fig. S2.
CREERT2 yields highly efficient levels of mHtt excisional recombination in Q97CRE mice throughout the nervous system and in nonneural tissues. BACHD:CAG-CreERT2 mice received an oral dose (1.125 mg⋅d−1) of tamoxifen given via feeding tube for five consecutive days at PND21 to generate mice expressing mHtt (i) only during development (Q97CRE) or (ii) during development and adult life (Q97) and (iii) WT controls not expressing mHtt (WT). The efficiency of mHtt tamoxifen-induced brain and body gene ablation was examined at genomic (A) and protein (B) levels, respectively, in a cohort of 3-mo-old mice. In contrast to striatal Q97 specimens, PCR amplicons using primers that selectively detect the presence of mutant exon 1 (A) and Western blot (B) analysis probed with the mAb2166 anti-Htt antibody (∼350 kDa, Bottom) show no discernible bands in control and Q97CRE specimens. Tissue-specific expression of CreERT2 was also verified in CAG-CreERT2 mice carrying a floxed inducible reporter (zsGreen1). (C) After tamoxifen recombination at PND21, 3-mo-old specimens widely expressed the fluorescence protein throughout the brain and body.
Q97CRE Mice Display HD-Like Profiles of Striatal Vulnerability to Cell Death.
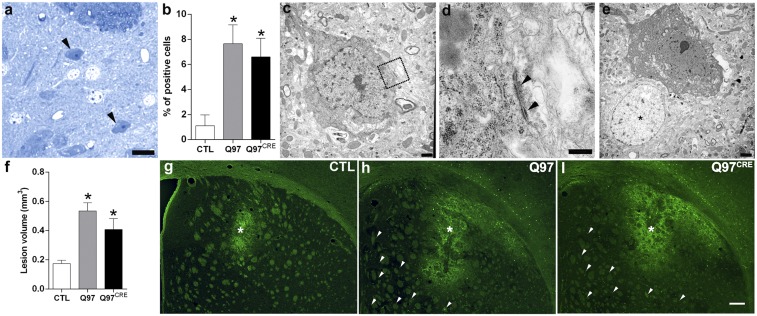
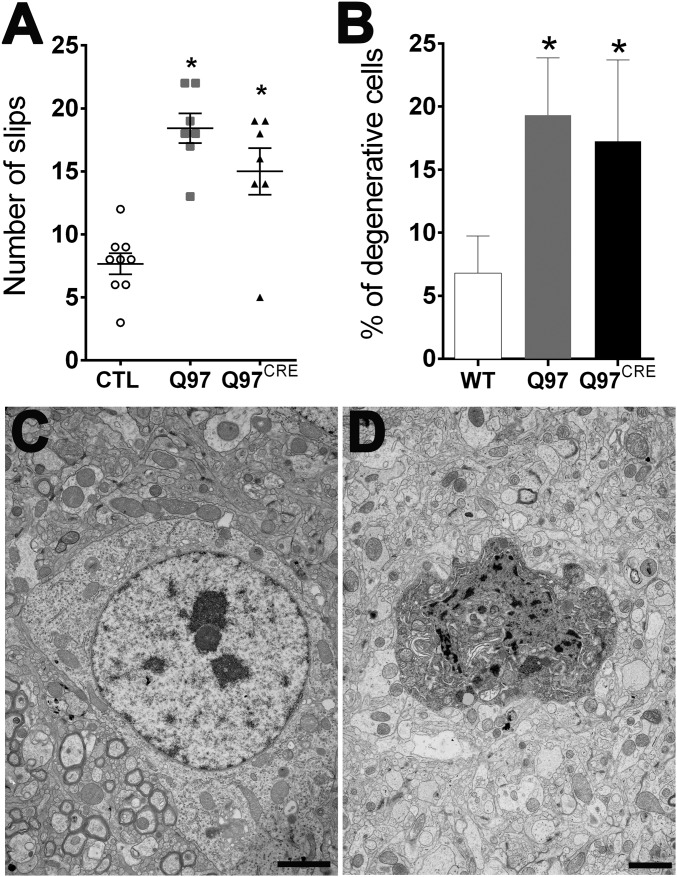
Striatal vulnerability to neurodegeneration is a seminal hallmark of HD. To investigate the presence of HD striatal pathology in Q97CRE mice, we studied gross morphological changes in brain specimens of 9-mo-old mice. We found that striatal volumes [F(2,12) = 1.28, P = 0.311], as well as the numbers of striatal NeuN+ neuronal species [F(2,21) = 1.86, P = 0.18], were not different between the three experimental groups. To identify neurodegenerative changes more thoroughly, we used toluidine blue staining of semithin striatal sections from the lateral aspect of the striatum (Fig. 2 A and B), a region previously shown to display the largest number of degenerating neurons in Q97 mice (9). Degenerating cells in the striatum appear dark when stained with toluidine blue. We found that compared with WT specimens [1.1%, 0.6–1.9 coefficient interval (CI95%)], both Q97CRE (6.6%, 5.3–8.1 CI95%) and Q97 (7.6%, 6.3–9.1 CI95%) specimens showed larger numbers of darkly stained degenerating neurons (Fig. 2A), many of which exhibited atrophic morphologies (Fig. 2A, arrowheads). Electron microscopic techniques further confirmed the presence of darkly stained atrophic neurons displaying morphological features of neurodegeneration, not only in the Q97 striatum but also in the Q97CRE striatum (Fig. 2 C–E). The neuronal identity of degenerating cells was confirmed by the detection of axosomatic synapses in these species (Fig. 2 C, outlined box and D). These results suggest that selective exposure to mHtt during neural development creates a state of striatal cell vulnerable to cell death.
Fig. 2.
Q97CRE mice display late-life appearance of striatal cellular degeneration and enhanced vulnerability to QA. Semithin sections (1 μm) of 9-mo-old striatum of female mice show that the number of darkly stained degenerating toluidine blue-positive cells was significantly higher in both Q97CRE (A, arrowheads) and Q97 mice compared with CTL mice (B, percentage ± 95% confidence interval). (C–E) Electron micrographic images showing representative degenerating cells in the striatum of Q97CRE mouse specimens. D corresponds to the boxed area in C, illustrating the presence of an axosomatic synapse (arrowheads). The asterisk in E identifies the nucleus of a normal striatal cell. (G–I) Representative striatal specimens that received a single intrastriatal injection of QA and were thereafter treated with Fluoro-Jade C to label dying cells (an asterisk depicts the site of the QA lesion). Arrowheads in H and I depict scattered Fluoro-Jade C–positive cells throughout the striatum. (Scale bars: A, 100 μm; D, 0.33 μm; C and E, 1 μm; I, 220 μm.) *P < 0.05 for statistical comparisons between CTL and either Q97CRE or Q97 specimens.
One provocative additional possibility is that this vulnerability might even be present early in life. In support of this possibility, previous studies have shown that early in life, HD mouse models exhibit an increased striatal vulnerability to NMDA-mediated excitotoxicity both in vivo and in vitro (11, 12). However, these studies do not clarify whether the cellular vulnerability is a consequence of developmental abnormalities or the continuing effects of the mutant protein during adult life. To investigate this seminal issue, we injected either the NMDA agonist quinolinic acid (QA) or PBS unilaterally into the corresponding dorsal aspect of the striatum of 3-mo-old Q97CRE mice. Seven days postinjection, striatal lesions were quantified using Fluoro-Jade C (Millipore), a cell death marker. PBS-mediated lesions were similar across all experimental groups [F(2,11) = 0.245, P = 0.786], and were smaller than those lesions resulting from QA [t(8) = 4.5, t(6) = 8.2, and t(8) = 4.6; P < 0.01 for Q97CRE, Q97, and control (CTL) mice, respectively]. Moreover, both Q97CRE and Q97 striata displayed approximately twofold larger QA lesions compared with those lesions seen in WT specimens [F(2,11) = 10.64, P = 0.002; Tukey’s honest significant difference (HSD) post hoc test, P = 0.031 and P = 0.002, respectively; Fig. 2 F–I, asterisks]. These results reveal that mHtt displays an active role in sculpting this cellular vulnerability to neurodegeneration during neural development.
Q97CRE Mice Partially Recapitulate Motor Deficits of Q97 Mice.
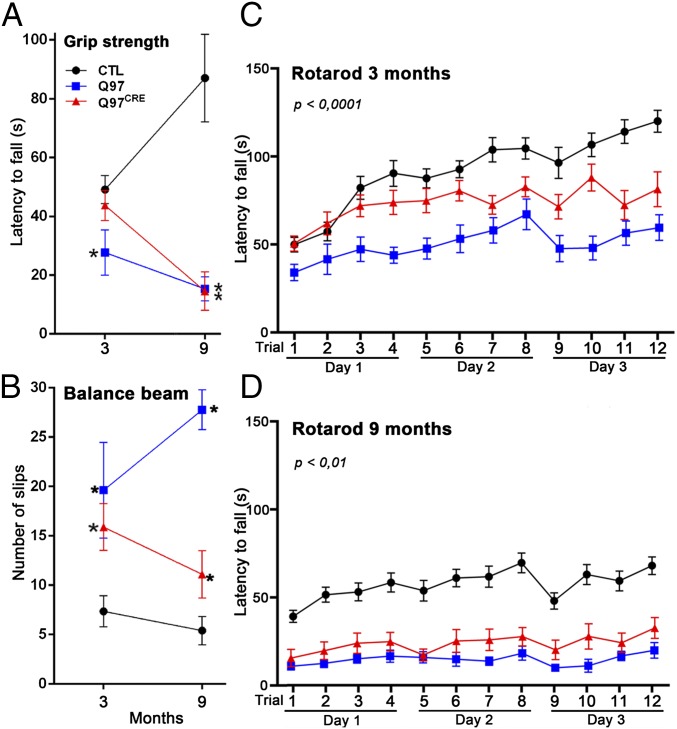
Progressive motor alterations are a clinical hallmark of HD, and they appear years before pathological changes are observed in patients with HD. However, whether selective developmental expression of mHtt is sufficient to produce progressive behavioral impairments remains unknown. We performed comparative behavioral analysis in a cohort of Q97CRE, Q97, and CTL mice at 3 and 9 mo of age (Fig. 3). To control for the confounding effects of weight in our behavioral examinations, all analyses were adjusted for this variable (13). We studied muscle strength with the Grip test. At 3 mo of age, Q97 but not Q97CRE mice displayed shorter latencies to fall in comparison to CTL mice [F(2,27) = 2.86, P = 0.073; Tukey’s HSD post hoc test, P = 0.045; Fig. 3A]. However, at 9 mo of age, both Q97 and Q97CRE mice exhibited shorter latencies to fall compared with CTL mice [F(2,27) = 20.68, P < 0.0001; Fig. 3A]. These observations indicate the presence of progressive reductions in muscle strength for both Q97CRE and Q97 mice. To examine motor coordination, we measured the number of slips of the mice using the balance beam test. Compared with CTL mice, both 3- and 9-mo-old Q97CRE and Q97 mice displayed higher numbers of slips [F(2,26) = 3.92, P = 0.032 and F(2,28) = 39.4, P < 0.0001, respectively; Fig. 3C]. In addition, the number of slips at 9 mo, but not at 3 mo, were significantly higher in Q97CRE compared with Q97 mice [t(16) = 4.99, P = 0.0001 and t(15) = 0.59, P = 0.6, respectively]. To examine motor coordination further, we used the rotarod test (Rotamex-5; Columbus Instruments). We assessed differences among groups using a mixed linear ANOVA model with repeated measurements. This model revealed differences in the latencies of mice at 3 mo (Fig. 3B) and 9 mo (Fig. 3D) of age to fall across all experimental models examined. Compared with CTL mice, the individual latencies to fall of Q97CRE and Q97 mice were shorter at both 3 mo [F(1,22) = 8.40, P = 0.0122 and F(1,21) = 34.4, P < 0.01 for genotype effects, respectively; Fig. 3B] and 9 mo [F(1,24) = 5.75, P = 0.02 and F(1,23) = 8.98, P < 0.01 for genotype effects, respectively; Fig. 3D] of age. Moreover, although comparisons between mutant strains (Q97CRE and Q97) revealed that Q97CRE mice displayed longer latencies to fall than Q97 mice at 3 mo of age [F(1,17) = 7.73, P = 0.013 for genotype effect; Fig. 3B], but not at 9 mo of age [F(1,17) = 0.08, P = 0.77 for genotype effect; Fig. 3D], indicating the preferential influences of mHtt during early but not later life. Overall, these data demonstrate that Q97CRE mice display deficits in motor coordination, suggesting that selective expression of mHtt during the developmental period partially recapitulates the motor deficits of HD.
Fig. 3.
Q97CRE mice have analogous, although less severe, motor deficits compared with Q97 mice. Behavioral examinations were performed at 3 and 9 mo of age in a single cohort of female mice from each experimental group (n = 10, n = 9, and n = 12 for Q97CRE, Q97, and CTL mice, respectively). Compared with CTL mice, both Q97CRE and Q97 mice displayed deficits in limb grip strength (paw grip endurance hanging wire test) at 9 mo of age (A) and a higher number of slips in the balance beam test at 3 and 9 mo of age (B). (C and D) Consistently, repeated rotarod tests at 3 and 9 mo of age also revealed significant deficits in motor coordination in Q97CRE mice. Individual values represent the mean ± SEM. P values (*) depicted at the top or next to each experimental group in A and B represent the statistical comparison with corresponding age-matched controls.
Q97CRE and Q97 Mice Display Age-Dependent Alterations in Striatal Spiking Activity.
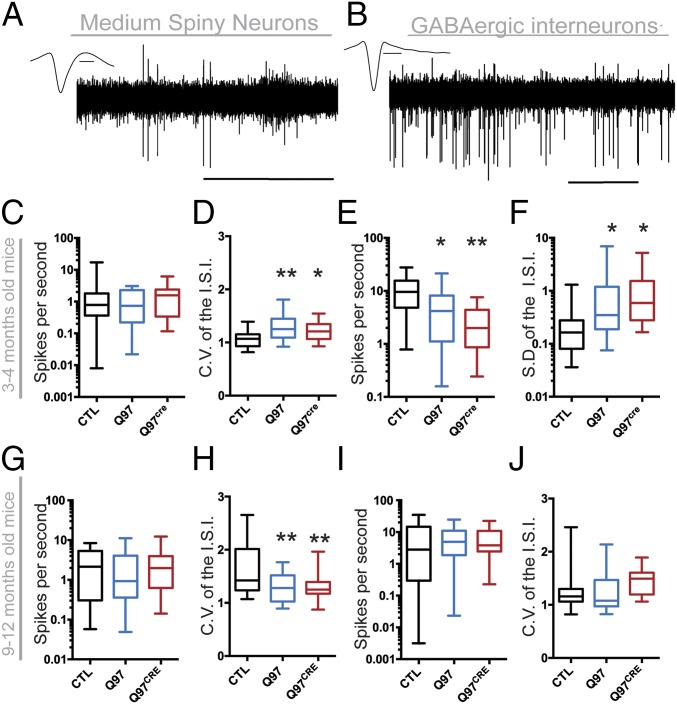
A number of studies have demonstrated defects in in vivo striatal spiking activity (14, 15) and in vitro age-dependent alterations in different striatal cell types (14, 16, 17) in various HD mouse models. We sought to determine whether mHtt-associated developmental effects contribute to these electrophysiological alterations. We implanted a drivable microarray to record single-unit activities within the dorsolateral striatum of behaving 3- to 4-mo-old and 9- to 12-mo-old Q97CRE mice (Fig. 4). The recorded units were classified according to their waveform (18) as either putative MSNs (Fig. 4A) or GABAergic interneurons (GINs; Fig. 4B). The proportions of units classified as MSNs and GINs were not different between the three genotypes in 3- to 4-mo-old and 9- to 12-mo-old mice [∼50% for MSN and GINs at both age ranges; χ2(2) = 0.566, P = 0.753 and χ2(2) = 1.877, P = 0.391, respectively]. At 3 to 4 mo of age, the firing rate of MSNs was similar among the experimental groups [H(2,66) = 0.557, P = 0.756; Fig. 4C), although the coefficient of variation (CV) of the interspike interval (ISI) of MSNs was increased in both Q97CRE and Q97 mice [F(2,66) = 8.22, P < 0.001, P = 0.032; Fig. 4D]. In contrast, the firing rate of striatal GINs was decreased [H(2,60) = 16.31, P < 0.001; Fig. 4E] in both Q97CRE and Q97 mice. Because the firing rate of GINs was different among groups, we used the SD of the ISI as a more appropriate measure of spiking variability than the CV of the ISI. We observed that the SD of the ISI for GINs was increased for both Q97 and Q97CRE mice [F(2,60) = 3.19, P = 0.048; Fig. 4F]. These observations during the 3- to 4-mo age range suggest generalized alterations in the striatal spiking activity as a result of the presence of mHtt during development. At 9 to 12 mo of age, the MSN firing rate was similar among all genotypes [H(2,75) = 1.50, P < 0.472; Fig. 4G]. However, the CV of the ISI of MSNs was decreased [F(2,74) = 7.09, P < 0.001; Fig. 4H]. For GINs, both the firing rate [H(2,68) = 0.56, P < 0.752; Fig. 4I] and the CV of the ISI [F(2,68) = 1.69, P < 0.192; Fig. 4J] were similar across all experimental groups. Therefore, by contrast, during the 9- to 12-mo age range, we observed that alterations in striatal spiking activity were selective for MSNs rather than GINs. These overall observations demonstrate that both MSNs and GINs display age-dependent alterations in their spiking activity. Moreover, our results reveal that mHtt-associated developmental defects contribute to alterations in both age- and neuronal subtype-dependent striatal spiking activity.
Fig. 4.
Both Q97CRE and Q97 mice exhibit abnormal striatal electrophysiological activity. (A and B) Drivable microarrays were implanted within the dorsolateral striatum to record the single-unit activity of awake, behaving 3- to 4-mo-old and 9- to 12-mo-old mice. (C) In 3- to 4-mo-old mice, MSN firing rates from Q97 (n = 22) and Q97CRE (n = 20) mice were similar to CTL (n = 27) mice. (D) ISI CV was increased in Q97 and Q97CRE MSNs compared with CTL MSNs. (E) GIN firing rates from Q97 (n = 21) and Q97CRE (n = 15) mice were significantly lower than in CTL (n = 27) mice. (F) In comparison to CTL units, the SD of the ISI of GINs was higher for both Q97 and Q97CRE units. In 9- to 12-mo-old mice, MSN firing rates from Q97 (n = 23) and Q97CRE (n = 27) mice were similar to CTL (n = 26) mice (G); however, the CV of the ISI was decreased in both Q97 (n = 23) and Q97CRE (n = 27) mice in comparison to CTL (n = 26) units (H). No differences were detected in the firing rate (I) or the CV of the ISI of GINs (J). Data are shown as whisker box plots representing the median (at the center), interquartile range (outer box), and minimum and maximum values (whiskers). *P < 0.05; **P < 0.001.
Q97CRE and Q97 Mice Display Alterations in Corticostriatal Communications and Plasticity.
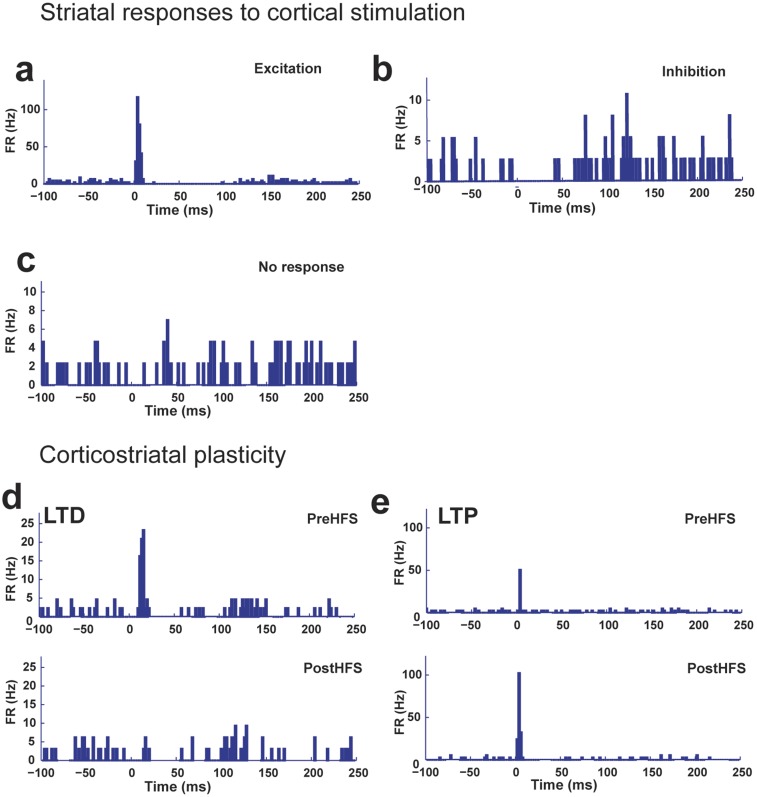
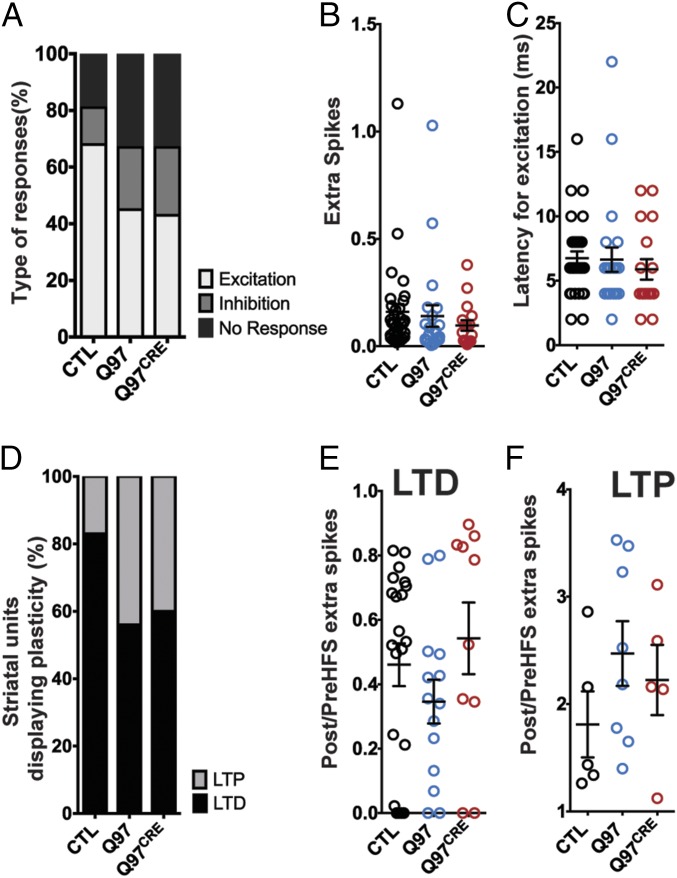
Several electrophysiological alterations in the corticostriatal pathway have been implicated in HD. They include late-onset loss of cortical innervation of the striatum and defects in corticostriatal plasticity in acute brain slices from HD mouse models (19–21). However, whether selective expression of the mHtt during the developmental period contributes to corticostriatal dysfunction is not known. We addressed this issue by analyzing corticostriatal pathway properties in 9- to 12-mo-old freely moving Q97CRE mice. We implanted an eight-wire microarray in the striatum and an electrical microstimulator within the motor cortex of experimental mice. We then assessed the effects of cortical stimulation on the responsivity of striatal unit activity. We found that cortical stimulation induced the following responses: (i) excitation, defined as the transient increase in the firing rate (Fig. S3A); (ii) inhibition, characterized by the transient suppression of striatal activity (Fig. S3B); and (iii) no response (Fig. S3C). Because the motor cortex provides the primary excitatory input to the dorsolateral striatum, we focused our analysis on units displaying excitation as a response. We found that there is an ∼20% decrease of striatal units displaying excitatory response in both Q97 and Q97CRE mice compared with CTL mice [χ2(2) = 15.53, P < 0.0003; Fig. 5A]. These findings suggest a decrease in functional corticostriatal connectivity. However, we observed that in those cells displaying excitation, the number of spikes elicited after cortical stimulation [H(2,70) = 3.923, P = 0.140; Fig. 5B] and the excitatory response latency [H(2,70) = 2.422, P = 0.297; Fig. 5C] were similar among all genotypes. This finding suggests that the remaining cortical inputs retain the ability to modulate the firing of striatal neurons in the HD mouse models. Our overall observations imply that the reduction of the cortical input to striatal cells is a consequence of selective developmental expression of mHtt.
Fig. S3.
Types of striatal response to cortical stimulation and effects of HFS on striatal units. A depiction of types of responses of striatal cells to cortical stimulation [excitation (A), inhibition (B), and no response (C)] is shown. (D) Peristimulus histogram (PSTH) for a striatal cell displaying LTD. (Upper) Striatal unit before HFS. (Lower) Same striatal unit displaying a reduction in the number of spikes elicited after HFS. (E) PSTH for a striatal unit displaying LTP. (Upper) Striatal unit before HFS. (Lower) Same striatal unit displaying an increase in the number of spikes elicited. FR, firing rate.
Fig. 5.
Q97CRE mice display impairments in corticostriatal connectivity and plasticity. (A) Proportion of cells exhibiting an excitatory response to cortical stimulation was reduced in both Q97 and Q97CRE mice in comparison to CTL mice (CTL = 47, Q97 = 49, and Q97CRE = 47 cells). (B) Correspondence to the summary data for the number of extra spikes elicited in striatal units displaying excitation by cortical stimulation (CTL = 32, Q97 = 22, and Q97CRE = 17 cells). The number of extra spikes was similar among all experimental groups. (C) Correspondence to the summary data for latency to elicited excitation in striatal units by cortical stimulation. The latency was similar among all experimental groups. (D) Proportion of striatal cells undergoing LTP is increased in both Q97 and Q97CRE mice in comparison to CTL mice (CTL = 27, Q97 = 22, and Q97CRE = 15 cells). (E and F) Summary data for striatal units undergoing LTD or LTP, respectively, for all experimental groups. (E) Ratio of spikes before and after HFS for cells undergoing LTD was not statistically different among genotypes. (F) Ratio of spikes before and after HFS for cells undergoing LTP among all experimental groups was not statistically different among genotypes. We used six to eight mice per genotype in all of the experimental paradigms. The mean and SEM are displayed in B, C, E, and F, and each individual point represents a striatal unit.
It is known that decreased inputs to the striatum are able to alter corticostriatal plasticity (22, 23). Therefore, we hypothesized that the relative corticostriatal functional disconnection is also able to impair corticostriatal plasticity in HD. In a previous work, we have shown that delivery of high-frequency stimulation (HFS) to the motor cortex of freely moving mice is able to induce bidirectional striatal plasticity (24). Using the same experimental paradigm, we observed striatal units that displayed decreased [long-term depression (LTD); Fig. S3D] or increased [long-term potentiation (LTP); Fig. S3E] evoked spikes post-HFS in comparison to pre-HFS in all experimental mice. We find that the proportion of units displaying LTP was increased ∼25% at the expense of units displaying LTD in both Q97 and Q97CRE mice in comparison to CTL mice [χ2(2) = 19.02, P < 0.0001; Fig. 5D]. For units that we categorized as undergoing LTD, the ratio of extra spikes post-HFS/pre-HFS was comparable among all experimental mice [H(2,45) = 3.382, P = 0.184; Fig. 5E]. Similarly, in both Q97 and Q97CRE mouse units undergoing LTP display, there were no differences in the ratio of extra spikes post-HFS over pre-HFS compared with CTL mice [H(2,18) = 2.464, P = 0.304; Fig. 5F]. These overall results suggest that the relative HD-mediated corticostriatal disconnection has the potential to alter bidirectional striatal plasticity and, additionally, that previously demonstrated mHtt-associated developmental alterations in both the striatum and cortex may furnish the regional substrates to mediate these dynamic processes.
Discussion
We have shown that selective exposure to mHtt during development recapitulates characteristic features of the HD phenotype. The findings in Q97CRE mice of striatal degeneration, early susceptibility to NMDA-mediated excitotoxicity, progressive motor coordination deficits, impairments in striatal spiking activity, and defects in corticostriatal plasticity and functional connectivity suggest that developmental abnormalities play an important role in HD pathogenesis.
We have postulated that mHtt-associated developmental dysfunction contributes to HD neurodegeneration (1). We and other groups have previously shown impairments in early embryonic development and stem cell-mediated striatal neurogenesis in HD mouse and cellular models (1, 25–27). These impairments may exert long-term disease-modifying effects (27), including selective neuronal vulnerability to degeneration. This concept is supported by the fact that Q97CRE mice display HD-like neurodegeneration.
Ablation of mHtt after PND21 in Q97CRE mice resulted in reductions in muscle strength and alterations in locomotor activity and motor coordination. However, these defects were not as severe as those defects observed in Q97 mice. These partial defects in the Q97CRE mice suggest that developmental mechanisms alone may not be sufficient to yield the entire spectrum of HD pathologies, thus potentially supporting a disease model encompassing two pathogenic components: one developmental and the other reflecting the ongoing toxic effects of mHtt. As a corollary, selective targeting of mHtt during postnatal life may not be sufficient to prevent or cause complete rescue of the HD phenotype. Consistently, although cumulative studies have reported that postnatal ablation or reduction of mHtt delays HD onset (28, 29), prolongs longevity of mice (28, 30, 31), stabilizes brain atrophy (28, 32), and improves motor functions (28, 33), targeting of the pathogenic protein does not lead to full reversal of the HD phenotype. It is also possible that these profiles of partial recovery reflected the fact that gene targeting was performed at a time when the deleterious effects of mHtt had already resulted in irreversible pathophysiological changes. However, Wang et al. (30) targeted mHtt well before the establishment of neuropathological disease hallmarks, thus making it unlikely that the absence of full recovery was a consequence of delayed therapeutic interventions. Overall, the evidence from postnatal mHtt targeting studies further suggests that additional pathogenic mechanisms are operating in response to the presence of mHtt during adult life.
Different studies have shown the involvement of multiple striatal cell types (MSNs, GINs, oligodendrocytes, and astrocytes) in addition to other cardinal brain regions and cell types in HD (34–37). All of these cellular subtypes are elaborated in a regional- and temporal-specific manner during neural development. Striatal cell types are, for example, derived from distinct regional ventral telencephalic progenitor pools, with MSNs derived from the lateral ganglionic eminence and GINs derived preferentially from the medial ganglionic eminence. These and other cellular components of the evolving striatum define an integrated functional microenvironment. In the context of mHtt, impairments of these and other striatal cellular subtypes likely contribute to progressive dysfunction of multiple regional progenitor domains, including the striatum. The presence of these developmental alterations would suggest a mechanism for generating both temporal and spatial cellular vulnerabilities that are known to occur in HD.
We have observed a change in the proportion of cells undergoing corticostriatal LTD and LTP after delivering HFS to the motor cortex in both Q97 and Q97CRE mice in comparison to CTL mice (Fig. 5). Although these alterations can be explained by loss in corticostriatal functional connectivity, other mechanisms must also contribute to these alterations. One possibility for the decreased proportion of cells undergoing LTD reflects impairments in different mechanisms of striatal LTD. As a proof of principle, we have previously demonstrated that using an antagonist (AM251) of cannabinoid receptor 1 (CBR1), we reduced the proportion of cells undergoing LTD in freely moving mice (24). These findings suggest that alterations in CBR1 or other components of the signaling pathways of striatal LTD could contribute to the corticostriatal plasticity defects in HD. Accordingly, patients with HD and mouse models display alterations in molecular components of striatal LTD, including cannabinoid receptors (38–40) and the mGluR5 signaling pathway (41–43). Previous studies have shown that using an agonist for CBR1 (WIN 55,212-2) (44) or mGluR5 (42, 45) modulators in HD mouse models ameliorates several alterations associated with corticostriatal dysfunction. Alterations in excitatory-inhibitory (E-I) balance can also deregulate corticostriatal plasticity (46). Changes in inhibitory (47, 48) and excitatory (14, 17) inputs to different striatal neurons (MSN-D1 and MSN-D2, fast-spiking interneurons, and persistent low-threshold interneurons) have been observed in BACHD (at both 2 and 12 mo of age) (47) and other mouse models of HD, thereby suggesting another mechanism to explain the alterations in corticostriatal plasticity. These defects in E-I balance may be established during development, given the fact that associated synaptic alterations are present very early in HD mouse models. These overall observations suggest that the alterations in corticostriatal plasticity may be mediated by multiple mechanisms occurring in parallel, including those mechanisms exhibiting a developmental origin.
Although the use of mice carrying a mixed C57BL-6J/FVB-NJ background might potentially act as a confounding factor in our analyses, this possibility seems unlikely because (i) the HD-like phenotype exhibited by our mixed C57BL-6J/FVB-NJ BACHD (Q97) strain was similar to the HD-like phenotype previously described in other studies using the FVB-NJ BACHD strain, thus indicating that the mixed background did not alter the pathogenic effects of mHtt in our mouse model (9); (ii) Q97 and Q97CRE HD-like phenotypes (e.g., neuronal vulnerability to excitotoxicity and neuronal degeneration, electrophysiological abnormalities) were consistently observed to be in the same direction throughout our studies, despite the fact that these phenotypes involved cohorts of mice obtained from different litter pools; (iii) our findings of neuronal degeneration and impairments of motor coordination were further confirmed in a separate cohort of experimental mice in which the FVB-NJ background was outbred following 10 consecutive matings with WT C57BL mice (Fig. S4); and (iv) our corticostriatal communications and plasticity experiments were performed using our purebred C57BL mouse models (Fig. 5), and both Q97 and Q97CRE mice display similar alterations compared with CTL mice. These convergent observations suggest that mHtt is sufficient to produce robust pathological impairments, NMDA-mediated excitotoxicity, behavioral abnormalities, and electrophysiological alterations under these breeding paradigms.
Fig. S4.
C57BL Q97CRE mice exhibit motoric and striatal neurodegenerative changes. The FVB-NJ genetic background was outbred for 10 successive matings using double-heterozygous BACHD+/CAG−CREER+ offspring with WT C57BL mice. (A) At 9 mo of age, the motoric performance of tamoxifen-treated CTL, Q97, and Q97CRE mice was examined with the balance beam test. Compared with controls, Q97CRE and Q97 mice have significantly higher numbers of slips (mean ± SEM; mean comparisons were performed with the Kruskal–Wallis test, whereas post hoc analyses used Dunn’s multiple comparisons test). (B) At 12 mo of age, the striata of Q97 and Q97CRE mice were examined with electron microscopy and exhibited enhanced neuronal degeneration (bars depict percentage ± 95% confidence interval; each comparison was made using the χ2 test). *P < 0.05. (C and D) Representative normal and degenerating neurons in CTL and Q97CRE specimens, respectively. (Scale bars: C and D represent 2 μm.)
These studies illuminate a developmental window associated with HD pathogenesis that has major implications for our understanding of the mechanisms governing the process of neurodegeneration. These observations have the potential to identify new classes of biomarkers and innovative therapeutic targets to delay, reverse, or prevent the onset and progression of HD, with potential relevance to other neurodegenerative diseases. In fact, the stages of premanifest HD likely represent the time interval during which therapeutic strategies “may provide the most positive possible outcome for patients and their families affected by this devastating disease” (5). Our findings also suggest that disease-specific profiles of propagation throughout the neuraxis are likely due to regional cellular vulnerabilities programmed during neural development rather than exclusively or preferentially due to mechanisms of prion-like propagation of pathogenic forms of neurodegenerative disease proteins (49).
Materials and Methods
Mouse Models.
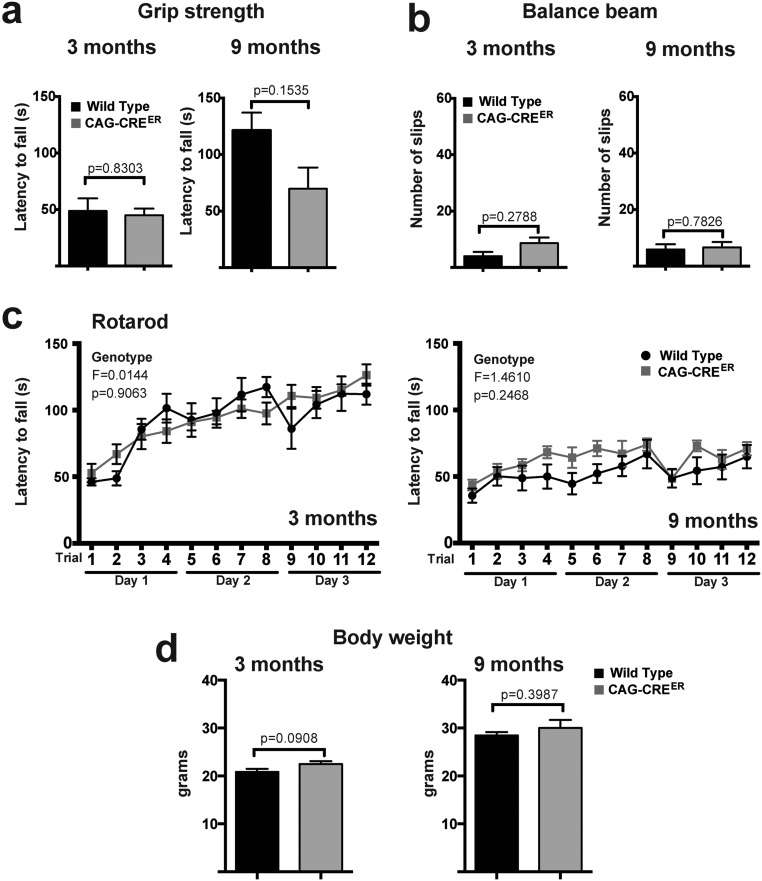
All studies were conducted with the approval of the Albert Einstein Animal Institute and were in compliance with all ethical guidelines and regulations (Institutional Review Board #00023382). CTL mice (Fig. 3) encompassed a combination of WT and CAG-CREER mice; we confirmed that there were no behavioral differences among them (Fig. S5). Detailed breeding paradigms are described in SI Materials and Methods.
Fig. S5.
WT and CAG-CREER mice display similar performance in motor coordination tests and exhibit comparable body weight. Female mice (n = 8 per genotype: WT and CAG-CREER) were examined at 3 and 9 mo of age for motor coordination. Mouse performance in the grip strength (A), balance beam (B), and rotarod (C) tests is depicted. (D) Mouse body weight of both genotypes. P values in A, B, and D were calculated using the Student t test. F and P values in C represent the genotype effects calculated using a general linear ANOVA model. All values depicted correspond to mean ± SEM.
Experimental Procedures.
Detailed descriptions of all of our experimental procedures, including mouse models, behavioral tests, in vivo electrophysiology, tissue processing and staining, immunoblots, cell quantification and striatal volumetric analyses, QA stress paradigms, QRT-PCR, and statistical analyses, can be found in SI Materials and Methods.
SI Materials and Methods
Mouse Models.
BACHD (9) mice (FVB-NJ, stock no. 008197; The Jackson Laboratory) were interbred with CAG-Cre/Esr1 mice (C57BL/6J, stock no. 004682; The Jackson Laboratory) carrying the tamoxifen-inducible Cre driver system. The resulting mice, BACHD/CAG-Cre/Esr1, were further mated with additional CAG-Cre/Esr1 mice, and at PND21, their offspring, BACHDhemizygous/CAG-Cre/Esr1 (both homozygous and hemizygous, and hereafter referred to as CRE+), BACHDhemizygous/CRE−, BACHD−/CRE+, and BACHD−/CRE−, received an oral dose (1.125 mg⋅d−1) of tamoxifen given via feeding tube for five consecutive days. The resulting experimental mice were grouped as Q97CRE [BACHDhemizygous/CRE, expressing human mutant huntingtin (hmHtt) only during development], Q97 (BACHDhemizygous/CRE−, expressing hmHtt throughout life), and littermate controls never expressing hmHtt (BACHD−/CRE+ and BACHD−/CRE). Both male and female mice were used unless otherwise specified. Excisional recombination of hmHtt exon 1 was mediated using the CAG-CRE/Esr1 tamoxifen approach. Prior studies have shown widespread expression of CRE/Esr1 from the CAG promoter, including the cortex, cerebellum, and hippocampus in the brain (10). Mouse genotyping was performed from tail biopsies before tamoxifen administration (PND21), and further confirmed postmortem from forebrain specimens. This approach allowed us to verify the presence of robust CRE-mediated excisional recombination in both neural and nonneural tissues for the entire cohort of mice examined in these studies. To accomplish this goal, the primers we used (5′-aggtcggtgcagaggctcctc-3′ and 5′-ccgctcaggttctgctttta-3′) generated a 651-bp amplicon from exon 1 of the human Htt gene in mice lacking CRE/Esr1. Because our preliminary studies, using a functional observation battery, motoric tests, and weight analysis, did not reveal significant differences between BACHD−/CRE+ and BACHD−/CRE− mice (Fig. S5), these genotypes were merged as a single experimental control group for our comparative analyses. All mice, irrespective of their genotype, were treated with tamoxifen.
Behavioral Tests.
Free locomotion, motor strength, and motor coordination were examined in female mice longitudinally at 3 and 9 mo of age with the open-field, grip strength, balance beam, and rotarod tests (Rotamex-5; Columbus Instruments). All tests were performed after 1 h of acclimation in the testing room during the light cycle. In the balance beam test, motor coordination deficits were measured as the number of missteps while traversing a round wooden beam, as previously described (50, 51). Before the test, all mice were pretrained on a wide plank (3 in) to encourage reliable crossing. The start side was brightly illuminated, and the other side had a small, darkened chamber that contained palatable food (cereal) to encourage the mice to cross. Immediately after pretraining, slips were assessed on a 1.5-cm-diameter beam, defined by when the paw slipped below the midline of the beam. In the accelerating rotarod test, each mouse was placed on the rotarod with increasing speed, from 0 to 10 cm⋅s−1 (increasing by 0.2 cm⋅s−1 every 6 s) for 300 s. Each mouse received five trials a day for three consecutive days. The latency to falling off the rotarod within this time period was recorded. In the grip strength test, we used the paw grip endurance hanging wire test, which consists of placing mice upside down on a wire grid and defining the latency to falling, recorded in seconds.
In Vivo Electrophysiology of Awake, Freely Moving Mice.
Single units were recorded using a custom-made, eight-wire, drivable microarray (52) consisting of 100 μm of Teflon insulated tungsten wires with a 50-μm core. Microarrays were implanted into the right dorsolateral striatum of anesthetized mice (1:4 male/female ratio, stereotactic coordinates: mediolateral, 2.5 mm; anterior-posterior, +0.55 mm; dorsoventral, −1.2 mm). Recordings started 2 wk after implantation. Mice freely moving on a 10-cm-diameter platform were recorded using a head stage (Trucker–Davis Technologies) connected to an amplifier (5,000× amplification, bandpass: 150 Hz–10 kHz), and the signal was digitalized at 20 kHz with a National Instruments card (PCI-MIO-16XE) using a custom-written software program in Labview (National Instruments). Wires were lowered 75 or 150 μm⋅d−1 as needed to a maximum of 1.5 mm to ensure that the units recorded were in the dorsal aspect of the striatum. Wire placement was confirmed postmortem using 50-μm brain slices stained with cresyl violet (Sigma). Recorded signals were wavelet-filtered (53) using MATLAB (MathWorks), and spike sorting was performed offline using principle components analysis in an Offline Sorter (Plexon). Waveform analyses were performed in MATLAB, with valley and peak referring to the negative and positive deflections, respectively, of the waveforms corresponding to the rising phase and the downstroke of the cell’s action potential. As described elsewhere (18), we classified the units using the peak FWHM. Units with a peak FWHM > 250 μs were labeled as MSNs, whereas units with peak FWHM < 200 μs were labeled as GINs. Similar mean values for the CV and for firing rates of MSNs were obtained using a more stringent classification criteria (peak FWHM > 300 μs), thus validating the peak FWHM classification threshold used.
Motor Cortex Stimulation and Corticostriatal Plasticity.
This procedure was used as previously described (24). Briefly, we positioned a metal tube at the bottom of the microarray and used it to stimulate the motor cortex. We delivered pulses of 200 μs at the maximum stimulus intensity that did not elicit movement in the mouse (the range was 6–22 μA). Finally, we recorded striatal units while stimulating the motor cortex every 7 s for at least 200 trials before and after the delivery of HFS. Recorded signals were processed as described in the previous section, and analysis was performed using custom-made MATLAB scripts.
Tissue Processing and Staining.
Cellular preparations were fixed and processed for immunocytochemistry, as described elsewhere (1). Briefly, under deep anesthesia, mice were perfused transcardially with ice-cold 0.25 mg/mL heparin in PBS (10 mL) followed by 4% (wt/vol) paraformaldehyde (PFA; 50 mL), and their brains were thereafter harvested. For histological techniques, the brains were then postfixed overnight in 4% (wt/vol) PFA and incubated in sucrose [20% (wt/vol)] until total submersion, and were subsequently flash-frozen in a cryomatrix resin and stored at −80 °C until use. For Fluoro-Jade C and immunostaining procedures, specimens were cryosectioned using 30-μm slices. NeuN (mAb377, 1:100 dilution; Millipore) primary antibody incubation was performed overnight at 4 °C. For electron microscopy techniques, perfused brains were sliced (1-mm-thick sections), fixed for an additional hour in a solution composed of 2% (wt/vol) PFA/2.5% (vol/vol) glutaraldehyde in 0.1 M cacodylate buffer, and then rinsed in this buffer. Samples were thereafter osmicated with 1% osmium tetroxide for 1 h, rinsed in buffer and then water, and incubated with 2% (wt/vol) uranyl acetate for 1 h. Specimens were subsequently dehydrated in alcohol mixtures and embedded in Epon LX112.
Immunoblot Analysis.
After tissue homogenization and further sonication, 30 μg of protein from 3-mo-old striata (1:2 female/male ratio) was denatured at 99 °C for 1 min in 1:1 Bio-Rad Laemmli buffer with 20:1 beta-mercaptoethanol and then loaded into 4–15% Bio-Rad precast Tris-Glycine-extended polyacrylamide gels with Tris/glycine/SDS buffer and transferred onto a PVDF membrane. After blocking, the membrane was then incubated overnight at 4 °C with primary antibody against Htt (mAb2166, 1:2,000 dilution; Millipore) and, later, with lamin B1 as a housekeeping protein (1:2,000 dilution; Abcam). Thereafter, membranes were washed, incubated for 1 h at room temperature with the respective HRP-conjugated secondary antibody (Sigma NA931V sheep anti-mouse and NA934V donkey anti-rabbit, 1:5,000 dilution), and washed again, and, finally, immunoreactive bands were detected with the Amersham ECL Plus chemiluminescent detection system (BioExpress).
Cell Quantification and Striatal Volumetric Analyses.
Cells were quantified by scoring the number of neuronal cell types reactive for NeuN in an area of 0.153 mm2 located in the external striatum corresponding to 0.74 mm per bregma (n = 8 per experimental group). The striatal volume was estimated according to Cavalieri’s principle (54). Accordingly, six coronal brain slices from female specimens separated by 360-μm intervals (from bregma, 1.54 mm) were immunostained with the MSN marker DARPP32 (sc-11365, 1:50 dilution; Santa Cruz Biotechnology) and photographed at 4× magnification with an Olympus BX51 microscope coupled with a Sensicam digital camera (Cooke Corporation), and the striatal area in each section was measured with ImageJ (NIH) software (55). Data were expressed as the striatal average of Cavalieri volume ± SEM (cubic millimeters, n = 4 per experimental group). Degenerating neurons were quantified by assessing the number of darkly stained toluidine blue-positive neurons from each replicate [12 fields (0.21 mm2); n = 4, n = 4, and n = 3 for Q97CRE, Q97, and control specimens, respectively] from semithin sections (1 μm) of the lateral striatum of 9- and 12-mo-old mice.
QA Stress Paradigms.
The procedure was performed with adaptations as described elsewhere (11). Briefly, under 2% isoflurane anesthesia, each mouse received 0.4 μL of QA, 1 nM in PBS, via intrastriatal injection, at a rate of 0.1 μL⋅min−1 into the right striatum. As an internal control, 0.4 μL of PBS at a similar rate was also injected into the left striatum. The site of the stereotactic injection was located 0.8 mm posterior to bregma, 1.8 mm distal to bregma, and 3.5 mm from the bregma. One week after the injections, mice were euthanized and their brains were harvested as described above. Cryosectioned brain slices were serially collected every 120 μm from bregma, 1.6 mm, and further stained with Fluoro-Jade C following the manufacturer’s instructions. Image capture was performed using an Olympus BX51 microscope coupled with a Sensicam digital camera. With the examiner blinded to the genotype, the area of the lesion of every slice was measured using ImageJ software and the volume of the lesion was calculated according to Cavalieri’s principle (54). We used only male specimens for this experimental paradigm. Data were expressed as the average volume ± SEM (cubic millimeters) of mice per group (n = 5, n = 4, and n = 5 for controls, Q97, and Q97CRE mice, respectively).
QRT-PCR Analysis.
These studies were performed with minor adaptations from techniques described elsewhere (1). For quantitative analyses of mHtt transcript expression, striatal RNA (n = 4 per time point per experimental group, 1:3 female/male ratio) obtained from 3- and 9-mo-old mice treated with tamoxifen was individually extracted using TRIzol Reagent (Invitrogen/Life Technologies), treated with Turbo-DNase (Invitrogen/Life Technologies), and subsequently processed for cDNA synthesis using standard techniques. QRT-PCR was performed with Power SYBR Green Master Mix (Invitrogen) per the manufacturer’s instructions on an ABI 7000 Sequence Detector System (Applied Biosystems). The primer set used to target mHtt selectively was 5′-gccccattcattgccccggt-3′ and 5′-agcttttccagggtcgccatgg-3′. For a housekeeping gene, we used primer sets targeting HPRT1: 5′-agcaggtgttctagtcctgtggc-3′ and 5′-gcgacaatctaccagagggtaggc-3′. For study of CRE-mediated excisional recombination efficiency at the genomic level, the DNA phase of the TRIzol Reagent was further purified, posttreated with RNase, and quantified using fluorometric techniques. Thereafter, 100 pg of DNA was mixed with the Power SYBR Green Master Mix and run in an ABI 7000 Real-Time PCR system (Applied Biosystems) using the primers described above for mHtt and the housekeeping gene, HPRT1. Data collection and quality assessment were performed using 1.1 RQ Software (Applied Biosystems).
Statistical Analyses.
Depending on the data distribution, mean comparisons were performed with either a one-way ANOVA/unpaired Student two-tailed t test or the Kruskall–Wallis/Mann–Whitney U test. The comparison between means of dependent samples was accomplished with the paired two-tailed Student t test, and the comparison of proportions was accomplished with the χ2 test. For rotarod test comparisons, we used a linear model of repeated measurements ANOVA. The n values reported correspond to biological replicates. The raw data from each parameter were prescreened for outlier values with Grubb’s extreme Studentized deviate test. Further, we examined the variance within each group with Bartlett’s test and the F test and determined whether the data exhibited a Gaussian distribution with the D’Agostino–Pearson omnibus normality test. Depending on the analysis, post hoc comparisons were performed with either Tukey’s HSD test or Dunn’s test, depending on whether the data displayed or did not display a Gaussian distribution/equal variance, respectively. For these tests, unless otherwise indicated, the statistical differences between samples were assigned using a probability of at least <0.05.
Acknowledgments
This work was supported by US NIH Grant NS073758 (to A.E.M.); Grant NS079750 (to K.K.); and Grants NS071571 (EUREKA), NS096144, and HD071593, as well as by grants from the F. M. Kirby, Alpern Family, Harold and Isabel Feld, and Roslyn and Leslie Goldstein Foundations (to M.F.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603871113/-/DCSupplemental.
References
- 1.Molero AE, et al. Impairment of developmental stem cell-mediated striatal neurogenesis and pluripotency genes in a knock-in model of Huntington’s disease. Proc Natl Acad Sci USA. 2009;106(51):21900–21905. doi: 10.1073/pnas.0912171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina-Calavita M, et al. Mutant huntingtin affects cortical progenitor cell division and development of the mouse neocortex. J Neurosci. 2014;34(30):10034–10040. doi: 10.1523/JNEUROSCI.0715-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings DM, et al. Aberrant cortical synaptic plasticity and dopaminergic dysfunction in a mouse model of Huntington’s disease. Hum Mol Genet. 2006;15(19):2856–2868. doi: 10.1093/hmg/ddl224. [DOI] [PubMed] [Google Scholar]
- 4.Nopoulos PC, et al. PREDICT-HD Investigators and Coordinators of the Huntington Study Group Smaller intracranial volume in prodromal Huntington’s disease: Evidence for abnormal neurodevelopment. Brain. 2011;134(Pt 1):137–142. doi: 10.1093/brain/awq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulsen JS, et al. PREDICT-HD Investigators and Coordinators of the Huntington Study Group Clinical and Biomarker Changes in Premanifest Huntington Disease Show Trial Feasibility: A Decade of the PREDICT-HD Study. Front Aging Neurosci. 2014;6:78. doi: 10.3389/fnagi.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi S, et al. Deficits of glutamate transmission in the striatum of toxic and genetic models of Huntington’s disease. Neurosci Lett. 2006;410(1):6–10. doi: 10.1016/j.neulet.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 7.Tabrizi SJ, et al. TRACK-HD Investigators Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: Analysis of 36-month observational data. Lancet Neurol. 2013;12(7):637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 8.Ross CA, Tabrizi SJ. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10(1):83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 9.Gray M, et al. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008;28(24):6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 11.Graham RK, et al. Differential susceptibility to excitotoxic stress in YAC128 mouse models of Huntington disease between initiation and progression of disease. J Neurosci. 2009;29(7):2193–2204. doi: 10.1523/JNEUROSCI.5473-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeron MM, et al. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron. 2002;33(6):849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 13.Kudwa AE, et al. Increased Body Weight of the BAC HD Transgenic Mouse Model of Huntington’s Disease Accounts for Some but Not All of the Observed HD-like Motor Deficits. PLoS Curr. 2013 doi: 10.1371/currents.hd.0ab4f3645aff523c56ecc8ccbe41a198. 5. Available at www.ncbi.nlm.nih.gov/pmc/articles/PMC3770835/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.André VM, et al. Differential electrophysiological changes in striatal output neurons in Huntington’s disease. J Neurosci. 2011;31(4):1170–1182. doi: 10.1523/JNEUROSCI.3539-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cayzac S, Delcasso S, Paz V, Jeantet Y, Cho YH. Changes in striatal procedural memory coding correlate with learning deficits in a mouse model of Huntington disease. Proc Natl Acad Sci USA. 2011;108(22):9280–9285. doi: 10.1073/pnas.1016190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cepeda C, et al. Transient and progressive electrophysiological alterations in the corticostriatal pathway in a mouse model of Huntington’s disease. J Neurosci. 2003;23(3):961–969. doi: 10.1523/JNEUROSCI.23-03-00961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi PR, et al. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J Neurosci. 2009;29(8):2414–2427. doi: 10.1523/JNEUROSCI.5687-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43(6):883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Deng YP, Wong T, Bricker-Anthony C, Deng B, Reiner A. Loss of corticostriatal and thalamostriatal synaptic terminals precedes striatal projection neuron pathology in heterozygous Q140 Huntington’s disease mice. Neurobiol Dis. 2013;60:89–107. doi: 10.1016/j.nbd.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinstry SU, et al. Huntingtin is required for normal excitatory synapse development in cortical and striatal circuits. J Neurosci. 2014;34(28):9455–9472. doi: 10.1523/JNEUROSCI.4699-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plotkin JL, et al. Impaired TrkB receptor signaling underlies corticostriatal dysfunction in Huntington’s disease. Neuron. 2014;83(1):178–188. doi: 10.1016/j.neuron.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picconi B, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6(5):501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 23.Thiele SL, et al. Selective loss of bi-directional synaptic plasticity in the direct and indirect striatal output pathways accompanies generation of parkinsonism and l-DOPA induced dyskinesia in mouse models. Neurobiol Dis. 2014;71:334–344. doi: 10.1016/j.nbd.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nat Neurosci. 2014;17(12):1767–1775. doi: 10.1038/nn.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorincz MT, Zawistowski VA. Expanded CAG repeats in the murine Huntington’s disease gene increases neuronal differentiation of embryonic and neural stem cells. Mol Cell Neurosci. 2009;40(1):1–13. doi: 10.1016/j.mcn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen GD, Gokhan S, Molero AE, Mehler MF. Selective roles of normal and mutant huntingtin in neural induction and early neurogenesis. PLoS One. 2013;8(5):e64368. doi: 10.1371/journal.pone.0064368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehler MF, Gokhan S. Developmental mechanisms in the pathogenesis of neurodegenerative diseases. Prog Neurobiol. 2001;63(3):337–363. doi: 10.1016/s0301-0082(00)00052-6. [DOI] [PubMed] [Google Scholar]
- 28.Kordasiewicz HB, et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74(6):1031–1044. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Lebron E, Denovan-Wright EM, Nash K, Lewin AS, Mandel RJ. Intrastriatal rAAV-mediated delivery of anti-huntingtin shRNAs induces partial reversal of disease progression in R6/1 Huntington’s disease transgenic mice. Mol Ther. 2005;12(4):618–633. doi: 10.1016/j.ymthe.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, et al. Suppression of polyglutamine-induced toxicity in cell and animal models of Huntington’s disease by ubiquilin. Hum Mol Genet. 2006;15(6):1025–1041. doi: 10.1093/hmg/ddl017. [DOI] [PubMed] [Google Scholar]
- 31.Boudreau RL, et al. Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington’s disease mice. Mol Ther. 2009;17(6):1053–1063. doi: 10.1038/mt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101(1):57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 33.Harper B. Huntington disease. J R Soc Med. 2005;98(12):550. doi: 10.1258/jrsm.98.12.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EH, et al. Cortical interneuron loss and symptom heterogeneity in Huntington disease. Ann Neurol. 2014;75(5):717–727. doi: 10.1002/ana.24162. [DOI] [PubMed] [Google Scholar]
- 35.Myers RH, et al. Decreased neuronal and increased oligodendroglial densities in Huntington’s disease caudate nucleus. J Neuropathol Exp Neurol. 1991;50(6):729–742. doi: 10.1097/00005072-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Reiner A, et al. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci USA. 1988;85(15):5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong X, et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci. 2014;17(5):694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington’s disease: A comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97(3):505–519. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 39.Lastres-Becker I, et al. Loss of mRNA levels, binding and activation of GTP-binding proteins for cannabinoid CB1 receptors in the basal ganglia of a transgenic model of Huntington’s disease. Brain Res. 2002;929(2):236–242. doi: 10.1016/s0006-8993(01)03403-5. [DOI] [PubMed] [Google Scholar]
- 40.Pouladi MA, et al. Marked differences in neurochemistry and aggregates despite similar behavioural and neuropathological features of Huntington disease in the full-length BACHD and YAC128 mice. Hum Mol Genet. 2012;21(10):2219–2232. doi: 10.1093/hmg/dds037. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro FM, Paquet M, Cregan SP, Ferguson SS. Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets. 2010;9(5):574–595. doi: 10.2174/187152710793361612. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro FM, et al. Metabotropic glutamate receptor-mediated cell signaling pathways are altered in a mouse model of Huntington’s disease. J Neurosci. 2010;30(1):316–324. doi: 10.1523/JNEUROSCI.4974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribeiro FM, Pires RG, Ferguson SS. Huntington’s disease and Group I metabotropic glutamate receptors. Mol Neurobiol. 2011;43(1):1–11. doi: 10.1007/s12035-010-8153-1. [DOI] [PubMed] [Google Scholar]
- 44.Chiodi V, et al. Unbalance of CB1 receptors expressed in GABAergic and glutamatergic neurons in a transgenic mouse model of Huntington’s disease. Neurobiol Dis. 2012;45(3):983–991. doi: 10.1016/j.nbd.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Doria JG, et al. Metabotropic glutamate receptor 5 positive allosteric modulators are neuroprotective in a mouse model of Huntington’s disease. Br J Pharmacol. 2013;169(4):909–921. doi: 10.1111/bph.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paille V, et al. GABAergic circuits control spike-timing-dependent plasticity. J Neurosci. 2013;33(22):9353–9363. doi: 10.1523/JNEUROSCI.5796-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cepeda C, et al. Multiple sources of striatal inhibition are differentially affected in Huntington’s disease mouse models. J Neurosci. 2013;33(17):7393–7406. doi: 10.1523/JNEUROSCI.2137-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dvorzhak A, Semtner M, Faber DS, Grantyn R. Tonic mGluR5/CB1-dependent suppression of inhibition as a pathophysiological hallmark in the striatum of mice carrying a mutant form of huntingtin. J Physiol. 2013;591(4):1145–1166. doi: 10.1113/jphysiol.2012.241018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11(3):155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulinello M, et al. Acquired infection with Toxoplasma gondii in adult mice results in sensorimotor deficits but normal cognitive behavior despite widespread brain pathology. Microbes Infect. 2010;12(7):528–537. doi: 10.1016/j.micinf.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanley JL, et al. The mouse beam walking assay offers improved sensitivity over the mouse rotarod in determining motor coordination deficits induced by benzodiazepines. J Psychopharmacol. 2005;19(3):221–227. doi: 10.1177/0269881105051524. [DOI] [PubMed] [Google Scholar]
- 52.du Hoffmann J, Kim JJ, Nicola SM. An inexpensive drivable cannulated microelectrode array for simultaneous unit recording and drug infusion in the same brain nucleus of behaving rats. J Neurophysiol. 2011;106(2):1054–1064. doi: 10.1152/jn.00349.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takekawa T, Isomura Y, Fukai T. Accurate spike sorting for multi-unit recordings. Eur J Neurosci. 2010;31(2):263–272. doi: 10.1111/j.1460-9568.2009.07068.x. [DOI] [PubMed] [Google Scholar]
- 54.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 55.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]