Significance
Immune parameters exhibit circadian periodicity. We show that disease progression and survival following intranasal infection with a neurotropic virus depends on the circadian time of infection. Infection occurring at the start of the rest period leads to higher mortality than infection at the start of the active period and is associated with increased numbers of inflammatory cells and higher levels of chemokine (C-C motif) ligand 2 (CCL2). The highest expression of REV-ERBα, a component of the molecular circadian clock, coincides with the start of the active cycle, when vesicular stomatitis virus infection leads to lower mortality. Inhibiting REV-ERBα–mediated suppression of CCL2 at the start of the active cycle abolishes protection in these animals, thus providing insight into circadian regulation of immune response.
Keywords: circadian, inflammation, immune response, monocytes, vesicular stomatitis virus
Abstract
Certain components and functions of the immune system, most notably cytokine production and immune cell migration, are under circadian regulation. Such regulation suggests that circadian rhythms may have an effect on disease onset, progression, and resolution. In the vesicular stomatitis virus (VSV)-induced encephalitis model, the replication, caudal penetration, and survivability of intranasally applied VSV depends on both innate and adaptive immune mechanisms. In the current study, we investigated the effect of circadian time of infection on the progression and outcome of VSV-induced encephalitis and demonstrated a significant decrease in the survival rate in mice infected at the start of the rest cycle, zeitgeber time 0 (ZT0). The lower survival rate in these mice was associated with higher levels of circulating chemokine (C-C motif) ligand 2 (CCL2), a greater number of peripherally derived immune cells accumulating in the olfactory bulb (OB), and increased production of proinflammatory cytokines, indicating an immune-mediated pathology. We also found that the acrophase of molecular circadian clock component REV-ERBα mRNA expression in the OB coincides with the start of the active cycle, ZT12, when VSV infection results in a more favorable outcome. This result led us to hypothesize that REV-ERBα may mediate the circadian effect on survival following VSV infection. Blocking REV-ERBα activity before VSV administration resulted in a significant increase in the expression of CCL2 and decreased survival in mice infected at the start of the active cycle. These data demonstrate that REV-ERBα–mediated inhibition of CCL2 expression during viral-induced encephalitis may have a protective effect.
Circadian rhythms are daily oscillations of physiological and behavioral parameters driven by the suprachiasmatic nucleus (SCN), the “master clock” of the organism, through humoral and neural outputs (1). At the molecular level, the clock is comprised of at least three interlocking transcription loops, which regulate circadian oscillations in gene expression within the cell (1). In the immune system, intrinsic clocks have been identified in various cell lineages, including macrophages, lymphocytes, and microglia (1–3). Direct control of immune gene expression, among them proinflammatory cytokines, by the clock transcription factors BMAL and REV-ERBα also has been demonstrated (4–7). Furthermore, some of the functional aspects of immune cells, such as the rate of phagocytosis, lymphocyte proliferation, and cytolytic activity, are under circadian control (8–10). The migration of hematopoietic progenitors and mature leukocytes also displays daily variation, which can be particularly consequential to disease outcomes (1–3, 11, 12).
Monocytes are bone marrow-derived cells characterized by Ly6chi/ CCR2hi expression, which are recruited to the sites of infection and injury, including those occurring in the CNS (2, 3, 13, 14). These cells have the capacity to differentiate into macrophages and dendritic cells (DC), perform effector functions such as antigen presentation and T-cell stimulation, and produce proinflammatory cytokines and chemokines, all directed to contain and clear infection (8, 9, 13, 15). However, poorly controlled migration of these cells may result in immune-mediated pathology and tissue damage, as has been shown for stroke, experimental autoimmune encephalomyelitis (EAE), and traumatic brain injury (16–18).
Intranasal (i.n.) vesicular stomatitis virus (VSV) infection is a well-established murine encephalitis model. A single application of VSV leads to infection and viral replication within sensory neurons in the neuroepithelium. Within 12–24 h the virus is transported to the olfactory bulb (OB) via the olfactory nerve, spreads through deeper layers, and reaches the olfactory ventricle 4 d postinfection (dpi). The virus spreads retrograde via olfactory circuits and along the ventricular system and reaches the hindbrain by 8 dpi (19). By 4 dpi VSV infection also leads to the accumulation of a mixed cellular infiltrate in the OB containing brain-resident microglia (MG) and dendritic cells (bDC) as well as CD45hi/CD11bhi and CD45hi/CD11bint cells of peripheral origin (20). In the current study, we tested the hypothesis that the immune response to VSV is under circadian control and examined the molecular and cellular immune mechanisms underlying such circadian regulation. We observed that the poor survival rate of mice infected at the beginning of the rest cycle [zeitgeber time 0 (ZT0)] (hereafter “ZT0 mice) was associated with higher levels of circulating chemokine (C-C motif) ligand 2 (CCL2), a proinflammatory chemokine, and a greater proportion of infiltrating CD45hi/CD11bhi and CD45hi/CD11bint cells, identified as proinflammatory monocytes, in the OB. Pretreatment of VSV-infected mice with a REV-ERBα antagonist (SR8278) resulted in a significant increase in CCL2 mRNA expression in mice infected at ZT12 (hereafter “ZT12 mice”) and a decrease in their survival. These data demonstrate that REV-ERBα–mediated inhibition of CCL2 expression during viral-induced encephalitis may have neuroprotective effects and hence improve survival.
Results
Circadian Time of Infection Affects Disease Progression and Survival.
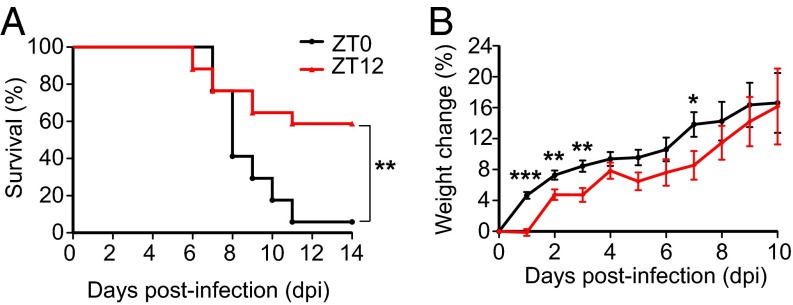
To test the hypothesis that the circadian time of infection impacts VSV-induced encephalitis, two groups of male mice, maintained on opposing light:dark cycles, were infected either at the start of the active period (ZT12) or at the end of the active period (ZT0). Disease progression (measured by weight loss) and the mortality rate of the animals were monitored daily for 2 wk following the infection. We found that viral infection at ZT12 resulted in a 40% mortality rate, as expected with an LD50 dose. The same dose of virus administered at ZT0 led to a 95% mortality rate (Fig. 1A). Consistent with the poor survival rate, mice infected at ZT0 lost a higher percentage of body weight than mice infected at ZT12, and the differences were most pronounced during the first 4 dpi (Fig. 1B). These data demonstrate that animals are more susceptible to VSV-induced encephalitis at the end of their active cycle (ZT0) and indicate circadian/diurnal regulation of the antiviral response.
Fig. 1.
Circadian time of infection affects survival following VSV i.n. administration. Two groups of mice were maintained on opposite light–dark cycles. For one group of mice VSV infection (LD50 dose applied i.n.) was done at the start of the light cycle (ZT0), and for the other group i.n. VSV application was done at the start of dark cycle (ZT12). (A) Survival curves are a composite of three independent experiments [total n = 17 per group, log-rank (Mantel–Cox) test, P = 0.004]. (B) Disease progression is expressed as the percent of weight change following VSV infection. Compiled data are expressed as mean ± SEM (total n = 17 per group; Student’s t test, *P < 0.05, **P < 0.01, ***P < 0.001).
Immune Response to i.n. VSV Infection Is Initiated in the Periphery.
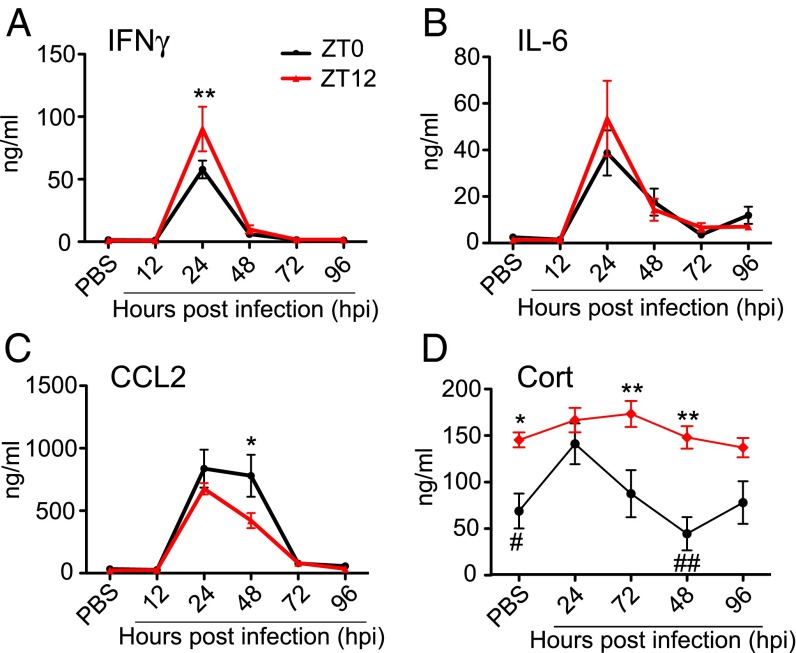
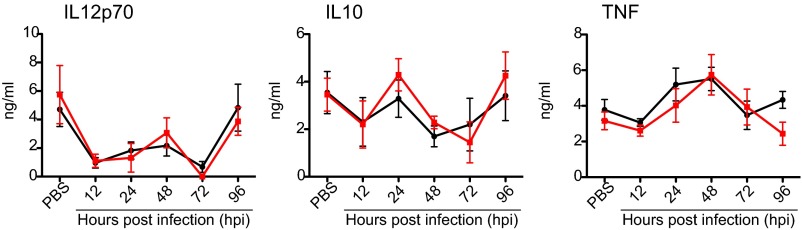
To examine the inflammatory response elicited by VSV i.n. administration at two circadian time points, we measured circulating cytokine levels after infection. Two groups of mice were infected at either ZT0 or ZT12, and blood samples were collected 12, 24, 48, 72, or 96 h postinfection (hpi). Control mice were treated with PBS solution (the vehicle for VSV), and blood samples were collected 96 h posttreatment. Levels of IFNγ, IL-6, and CCL2 in the blood of VSV-infected mice were elevated significantly at 24 hpi (Fig. 2 A–C and Table S1). IFNγ and IL-6 returned to baseline (the level in the PBS-treated cohort) at 48 hpi, but CCL2 levels were sustained and declined only at 72 hpi. Moreover, the magnitude of cytokine increase was different in the two circadian groups: ZT12 mice had higher levels of IFNγ at 24 hpi, and ZT0 mice had higher CCL2 at 48 hpi (Fig. 2 A–C and Table S1). Moderate but significant changes were detected in the levels of TNF and IL12p70 following VSV infection, but IL-10 levels were not affected (Fig. S1 and Table S1). Together these data demonstrate that the systemic immune response to i.n. VSV infection is very rapid and depends on the circadian time of infection.
Fig. 2.
Circadian time of infection affects cytokine and hormone levels following VSV i.n. administration. Blood samples were collected from ZT0 and ZT12 mice under the control (PBS-treated) condition and at the indicated time points following VSV infection. (A–C) Circulating cytokine levels were measured using cytometric bead array. Data compiled from two independent experiments are expressed as mean ± SEM (total n = 6 per time point, two-way ANOVA, Bonferroni's posttest; *P < 0.05, **P < 0.01, ZT12 vs. ZT0). (D) Blood CORT levels were measured by RIA. Data compiled from two independent experiments are expressed as mean ± SEM (total n = 6 per time point; two-way ANOVA, Bonferroni's posttest, *P < 0.05, **P < 0.01, ZT12 vs. ZT0; comparison within each circadian group was done using Student’s t test, #P < 0.05, ##P < 0.001 vs. ZT0 acrophase).
Table S1.
Statistical analysis of circulating cytokine and hormone levels in VSV-infected mice
| Cytokine/hormone | Circadian time of infection, ZT | Hours postinfection | Interaction | |||
| F(1,5) | P | F(1,5) | P | F(1,5) | P | |
| IFNγ | 2.798 | n.s. | 52.06 | <0.0001 | 2.571 | 0.039 |
| IL-6 | 0.137 | n.s. | 14.40 | <0.0001 | 0.665 | n.s. |
| CCL2 | 1.238 | n.s. | 28.42 | <0.0001 | 1.956 | n.s. |
| IL-12p70 | 0.000 | n.s. | 4.847 | 0.001 | 0.263 | n.s. |
| IL-10 | 0.242 | n.s. | 2.347 | n.s | 0.273 | n.s. |
| TNF | 1.725 | n.s. | 10.43 | 0.005 | 1.885 | n.s. |
| CORT | 39.71 | P < 0.0001 | 3.442 | 0.015 | 1.300 | n.s. |
Fig. S1.
Circulating cytokine levels following VSV i.n. administration. Circulating levels of IL-12p70, IL10, and TNF were measured in the blood samples collected from PBS-treated control mice and VSV-infected mice at the indicated time points. Cytokine levels were measure by cytometric bead array.
In addition to proinflammatory cytokines, we measured levels of circulating corticosterone (CORT), an antiinflammatory hormone that displays circadian oscillation and mediates synchronization of peripheral clocks. CORT levels were measured in blood samples collected from ZT0 and ZT12 mice under baseline (PBS-treated) conditions and at 24, 48, 72, or 96 h following VSV infection (Fig. 2D). As expected, CORT levels in PBS-treated control mice were higher at ZT12 than at ZT0. Following VSV i.n. administration, mice infected at ZT12 maintain CORT levels comparable to those in controls at 24, 48, 72, and 96 hpi. In contrast CORT levels in mice infected at ZT0 are significantly increased at 24 hpi and return to the basal (PBS-treated) level by 48–72 hpi (Fig. 2D and Table S1). These data indicate that VSV infection affects CORT secretion in mice infected at ZT0 and that this effect may contribute to neuroinflammation and the poor survival of these mice.
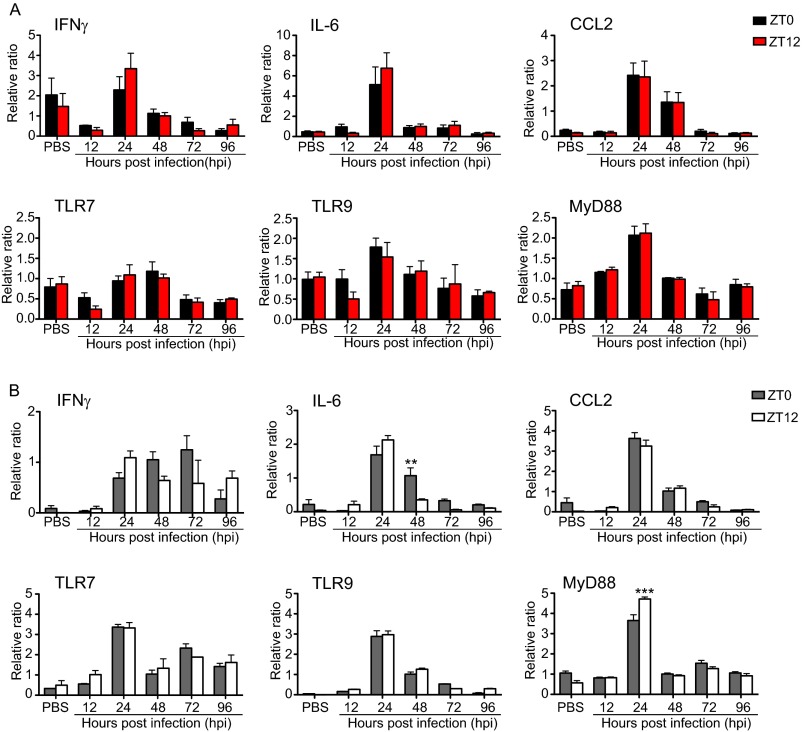
VSV administered i.n. travels not only to the brain but also to the lung, spleen, and cervical lymph nodes (21, 22). Thus, to characterize the whole-body immune response elicited by the virus, we examined the circadian regulation of the peripheral immune response mounted in the lung and spleen. We found that mRNA levels of the proinflammatory cytokines IL-6, IFNγ, and CCL2 are elevated by 24 hpi in the spleen and lung tissue of mice infected at ZT0 and ZT12 (Fig. S2A and B) and that this elevation coincides with the rise of inflammatory cytokines in blood (Fig. 2 A–C). Our data also showed that Toll-like receptor 7 (TLR7), TLR9, and MyD88 mRNA expression is uniquely induced in the lung but not in the spleen at the same time point (Fig. S2A and B).
Fig. S2.
Time course of gene expression in the spleen (A) and lung (B) of VSV-infected mice. Tissue was collected from PBS-treated and VSV-infected mice at the indicated time points. Total RNA was isolated, and transcript levels were measured by qPCR using Taqman gene-expression assays. Data are expressed as mean ± SEM (n = 6–8 per time point; two-way ANOVA followed by Bonferroni's multiple comparison test, **P < 0.01, ***P < 0.001, ZT0 vs. ZT12).
Proinflammatory Monocytes Accumulate in the OB of VSV-Infected Mice.
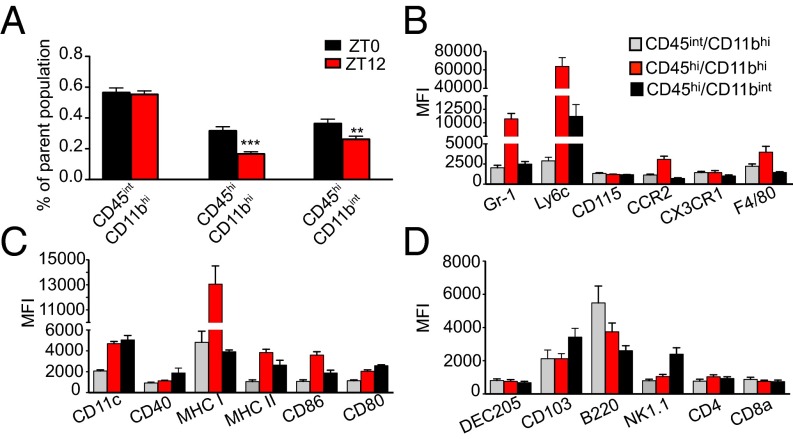
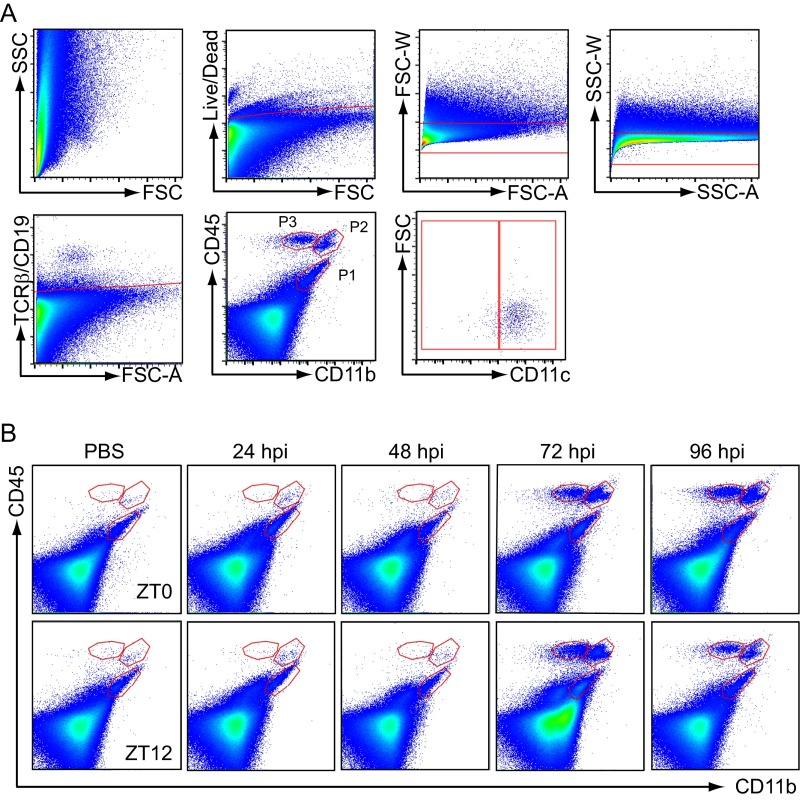
We next examined whether the circadian time of infection affects the cellular response to VSV in mice infected at ZT0 and ZT12. As described previously (20), cell populations isolated from VSV-infected OB contain brain-resident MG (CD45int/CD11bhi/CD11c−) and bDC (CD45int/CD11bhi/CD11c+) (Fig. 3A and Fig. S3B). A time-course analysis of VSV infection also revealed that by 72 hpi two additional cell populations, CD45hi/CD11bhi and CD45hi/CD11bint, accumulated in VSV-infected OB (Fig. S3B), and the proportion of these cells at 96 hpi was significantly higher in mice infected at ZT0 than in mice infected at ZT12 (Fig. 3A).
Fig. 3.
Circadian time of infection affects immune cell populations in the OB of VSV-infected mice. Mice were killed at 96 h following VSV, and OB tissue was processed for flow cytometry analysis. (A) Cells pregated on live/singlets/lin− were analyzed based on the expression of CD45 and CD11b. Three distinct populations were detected: CD45int/CD11bhi, CD45hi/CD11bhi, and CD45hi/CD11bint. Compiled data are expressed as mean ± SEM of the percent of parent population (n = 50 for each population; Student’s t test, **P < 0.01, ***P < 0.001, ZT12 vs. ZT0). (B–D) CD45/CD11b cell populations were phenotyped using flow cytometry. Only data for ZT0 group are shown because no phenotypic differences were found between the ZT0 and ZT12 groups. Data compiled from two or three independent experiments are expressed as mean ± SEM of geometric mean of fluorescence intensity (MFI) for each antibody (total n = 4–6 per group.)
Fig. S3.
Immune cell analysis in VSV-infected mouse OB tissue by flow cytometry. (A) Gating strategy used for FACS and flow cytometry experiments: Cells were gated on live/dead, forward-scatter (FSC) singlets, side-scatter (SSC) singlets, TCRβ/CD19-negative, CD45/CD11b cells. Three cell populations distinguished based on CD45 and CD11b expression (P1, CD45int/CD11bhi; P2, CD45hi/CD11bhi; and P3, CD45hi/CD11bint) were each gated further on CD11c cells. (B) Three cell populations (P1, CD45int/CD11bhi; P2, CD45hi/CD11bhi; and P3, CD45hi/CD11bint) were analyzed by flow cytometry 24–96 hpi in control (PBS-treated) and VSV-infected mouse OB.
To understand better the role of the CD45+/CD11b+ cell populations in VSV-induced pathology, we examined the phenotype of these cells using flow cytometry (Fig. 3 B–D). We found that CD45hi/CD11bhi cells [previously shown to be peripherally derived (20)] are Gr-1hi, Ly6chi, CCR2+, F4/80+, CD11c+, and MHC II+. These cells also express the costimulatory molecules CD86 and CD80 and markers for specialized DC subsets, CD103 and B220. These data indicate that CD45hi/CD11bhi cells contain proinflammatory monocytes as well as effector DC and macrophage subpopulations. Analysis of the CD45hi/CD11bint population also revealed the presence of CD11c+, NK1.1+, B220+ cells, which resemble IFN-producing killer DCs. Moreover, CD45hi/CD11bint cells contain a Ly6c+ subpopulation, indicating a monocytic lineage. Notably, no phenotypic differences were found among immune cell populations derived from ZT0 and ZT12 mice.
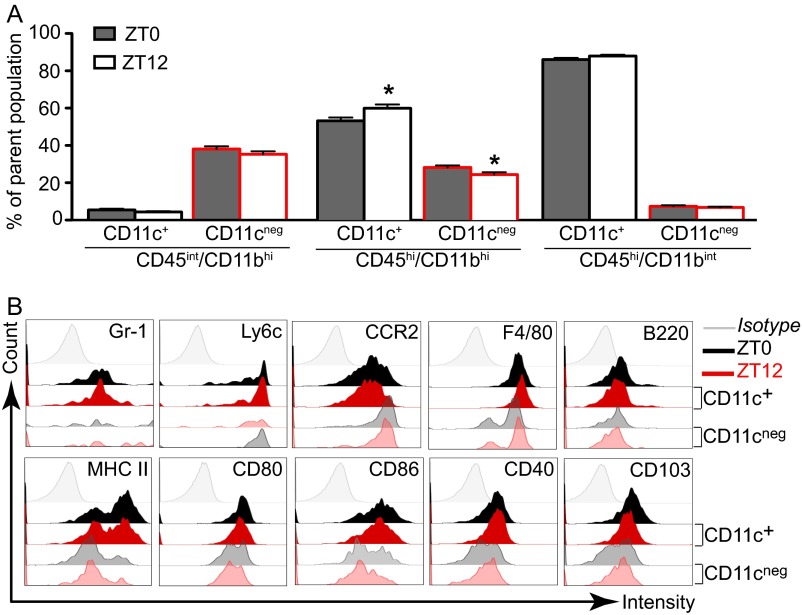
To examine the subpopulations of effector DCs and macrophages, which derive from proinflammatory monocytes, we next examined CD11c+ and CD11c− subsets within CD45/CD11b cells. We found that CD45hi/CD11bhi cells isolated from ZT12 VSV-infected mice contained a significantly higher proportion of CD11c+ cells and a significantly lower proportion of CD11c− cells compared with CD45hi/CD11bhi cells from ZT0 mice (Fig. S4A). No difference in the numbers of CD11c+ and CD11c− cells was found among other cell subsets in either ZT0 or ZT12 mice. Further phenotyping of CD45hi/CD11bhi/CD11c+ and CD45hi/CD11bhi/CD11c− cells also revealed that CD11c+ cells display a more activated phenotype and express higher levels of MHC II and the costimulatory molecules CD86 and CD40, as is consistent with the phenotype of monocyte-derived DCs (Fig. S4B). These data indicate that the disproportional accumulation of proinflammatory and effector cells in the OB of VSV-infected mice is influenced by the time of infection.
Fig. S4.
Analysis of CD11c+ and CD11c− subpopulations in the OB tissue. Mice were killed 96 h following VSV infection, and OB tissue was processed for flow cytometry analysis. Cells pregated on live/singlets/lin− were analyzed based on the expression of CD45/CD11b. (A) The proportion CD11c+ and CD11c− subsets was determined within three immune cell populations. Data are expressed as mean ± SEM of the percent of the parent population (total n = 50 for each population; Student’s t test, *P < 0.05, ZT0 vs. ZT12). (B) The phenotype of CD11c+ and CD11c− cells was characterized further using a variety of markers characteristic of monocytes and other immunological subsets. Representative histograms for cells isolated from ZT0 and ZT12 mice OB are shown.
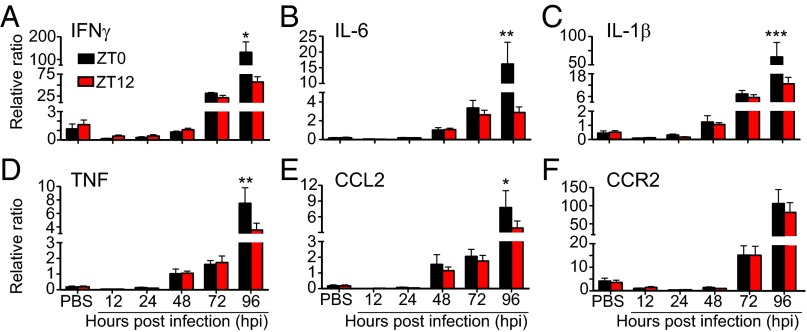
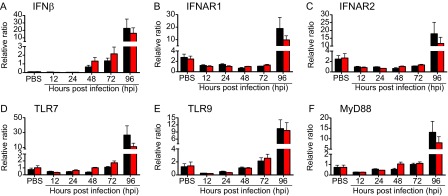
VSV Infection Leads to Up-Regulation of Cytokines in the OB 96 hpi.
We next examined whether the time of VSV infection affects the level of proinflammatory cytokine mRNA expression in the OB tissue at 12, 24, 48, 72, and 96 hpi. We found that i.n. VSV inoculation led to up-regulation of all genes examined by 48–72 hpi and reached significantly high levels at 96 hpi (Fig. 4 and Table S2). At the same time points, mice infected at ZT0 displayed significantly higher levels of IL1β, IL-6, TNF, IFNγ, and CCL2 mRNA expression than mice infected at ZT12 (Fig. 4 A–E). We also found that the expression of various antiviral factors and pattern-recognition receptors was elevated in the OB of VSV-infected mice at 96 hpi and that the expression of these factors and receptors was consistently higher in the OB of ZT0 mice than in the OB of ZT12 mice (Fig. S5 and Table S2). These data indicate that the circadian time of infection affects the changes in proinflammatory gene expression induced by VSV inoculation.
Fig. 4.
Circadian time of infection affects gene expression in the OB of VSV-infected mice. Total RNA was isolated from OB tissue of VSV-infected mice at the indicated time points. Transcript levels for proinflammatory cytokines (A–D); chemokine (E), and chemokine receptor (F) were measured by qPCR. Data compiled from two independent experiments are expressed as mean ± SEM (n = 6–8 per time point; two-way ANOVA, Bonferroni's posttest, *P < 0.05, **P < 0.01, ***P < 0.001, ZT0 vs. ZT12).
Table S2.
Statistical analysis of gene expression in the OB of VSV-infected mice
| Transcript | Circadian time of infection, ZT | Hours postinfection | Interaction | |||
| F(1,5) | P | F(1,5) | P | F(1,5) | P | |
| TLR7 | 0.700 | n.s. | 6.99 | <0.0001 | 0.955 | n.s. |
| TLR9 | 0.008 | n.s. | 12.06 | <0.0001 | 0.034 | n.s. |
| MyD88 | 0.234 | n.s. | 10.46 | <0.0001 | 0.353 | n.s. |
| IFNβ | 0.124 | n.s. | 6.79 | <0.0001 | 0.227 | n.s. |
| IFNAR1 | 0.823 | n.s. | 6.79 | <0.0001 | 0.823 | n.s. |
| IFNAR2 | 0.375 | n.s. | 8.98 | <0.0001 | 0.490 | n.s. |
| IL1β | 3.248 | n.s. | 7.34 | <0.0001 | 2.996 | 0.017 |
| IL6 | 2.781 | n.s. | 5.24 | <0.0001 | 2.400 | 0.047 |
| TNF | 1.930 | n.s. | 14.58 | <0.0001 | 2.201 | n.s. |
| IFNγ | 1.889 | n.s. | 13.00 | <0.0001 | 1.367 | n.s |
| CCL2 | 2.046 | n.s. | 11.61 | <0.0001 | 1.388 | n.s. |
| CCR2 | 0.058 | n.s. | 14.96 | <0.0001 | 0.053 | n.s. |
Fig. S5.
Expression of immune genes in the OB of VSV-infected mice. Total RNA was isolated from OB tissue of VSV-infected mice at the indicated time points. Transcript levels for antiviral cytokine and receptors (A–C) and pattern-recognition receptors (D–F) were measured by qPCR using Taqman gene-expression assays. Data are expressed as mean ± SEM (n = 6–8 per time point; two-way ANOVA followed by Bonferroni's multiple comparison test).
Expression of Circadian Clock Genes in the Steady-State OB.
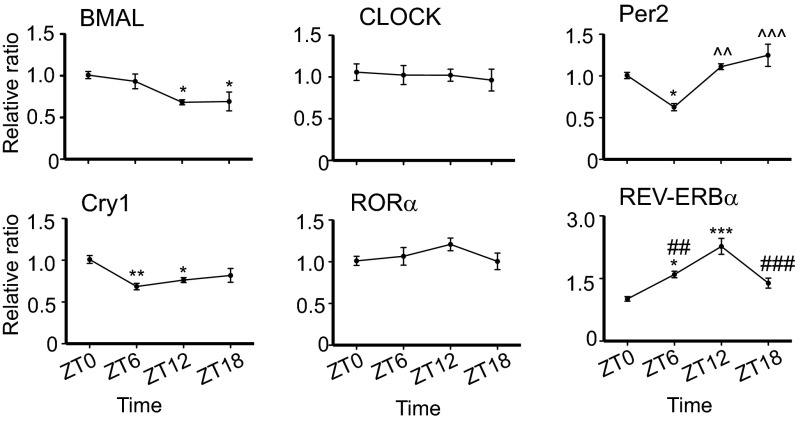
To identify the factors responsible for the circadian effect on VSV-induced encephalitis, we analyzed the expression of clock genes in the OB tissue of naive mice. Analysis of mRNA levels at four different time points—ZT0, ZT6, ZT12, and ZT18—revealed that BMAL, Per2, Cry1, and Rev-ERBα mRNA levels displayed rhythmic changes over the 24-h time period; however CLOCK and RORα mRNA did not (Fig. 5). Furthermore, significant differences between ZT0 and ZT12 time points were detected in the transcript levels of BMAL, Cry1, and Rev-ERBα, with BMAL and Cry1 mRNA displaying a lower expression and Rev-ERBα mRNA showing higher expression at ZT12 (Fig. 5). Notably, this expression pattern of REV-ERBα mRNA in the OB tissue appears to be antiphase with its expression in the SCN (23). However, this pattern is not surprising in light of evidence that the OB represents a functional circadian pacemaker that is capable of producing autonomous and entrainable rhythms independent of the SCN (24).
Fig. 5.
Expression of circadian clock genes in the OB tissue of naive mice. Total RNA was isolated from OB tissue at the indicated time points. Transcript levels for circadian clock genes were measured by qPCR. Data compiled from three independent experiments are expressed as mean ± SEM (total n = 6 per time point; one-way ANOVA, Bonferroni's posttest, *P < 0.05, **P < 0.01, ***P < 0.001 vs. ZT0; ^^P < 0.01, ^^^P < 0.001 vs. ZT6; ##P < 0.01, ###P < 0.001 vs. ZT12).
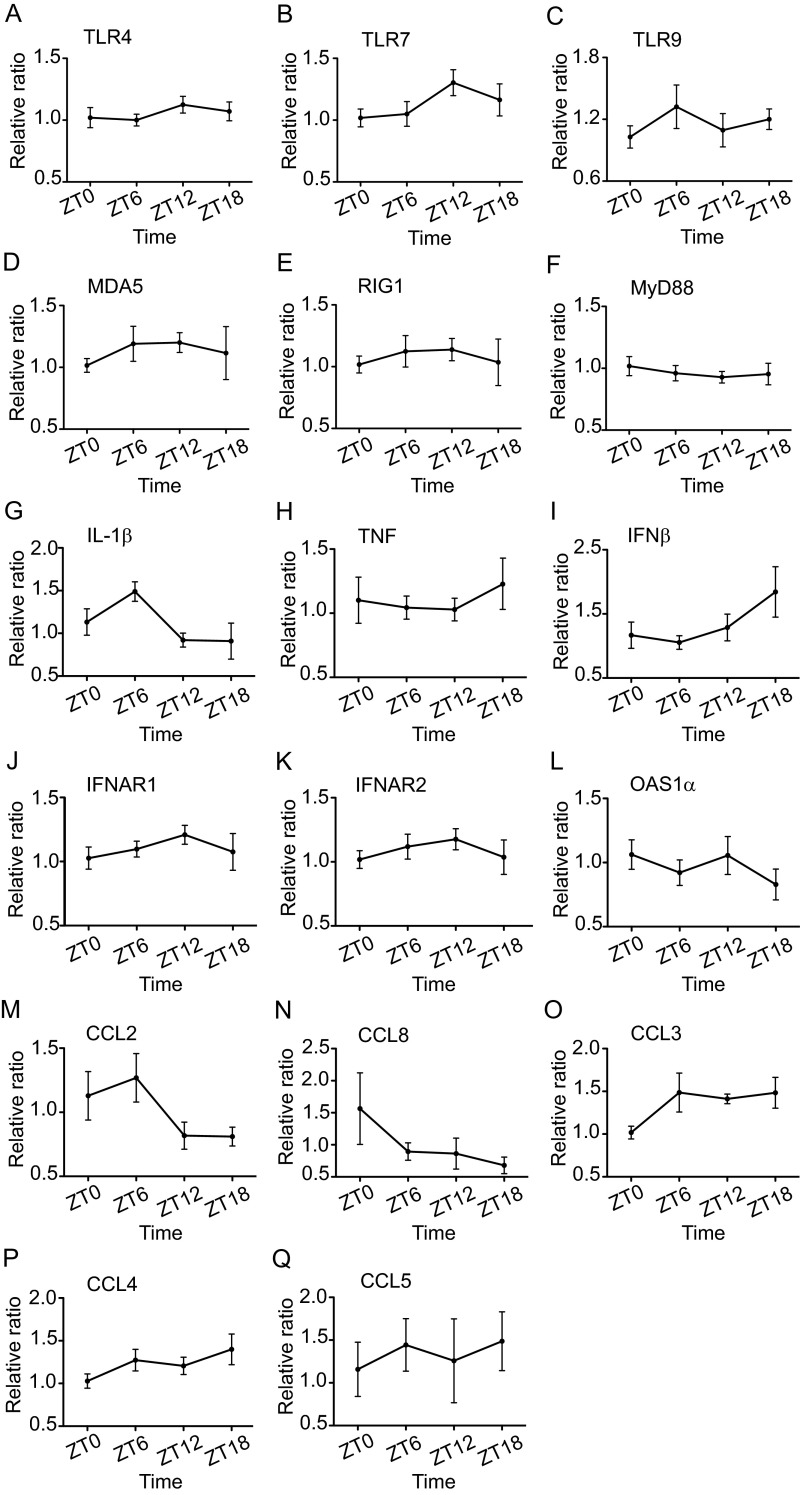
We also analyzed the daily variation in the expression of various immune genes involved in pattern recognition (Fig. S6 A–F), inflammation and antiviral response (Fig. S6 G–L), and cell migration (Fig. S6 M–Q). We found no significant differences in the mRNA levels of the examined genes across time points in the uninfected mice. However, slight time-of-day variations in the mRNA expression levels of TLR7, IL1β, IFNβ, IFNAR1 and IFNAR2, CCL2, and CCL3 were detected.
Fig. S6.
Expression of immune genes in the OB tissue of naive mice. Total RNA was isolated from OB tissue at the indicated time points. Transcript levels for pattern-recognition receptors (A–F), proinflammatory cytokines (G and H), antiviral cytokine and receptors (I–L), and chemokines (M–Q) were measured by qPCR using Taqman gene-expression assays. Data are expressed as mean ± SEM and represent a composite of three independent experiments (total n = 6 per time point).
REV-ERBα Mediates the Circadian Effect on Survival Following VSV i.n. Administration.
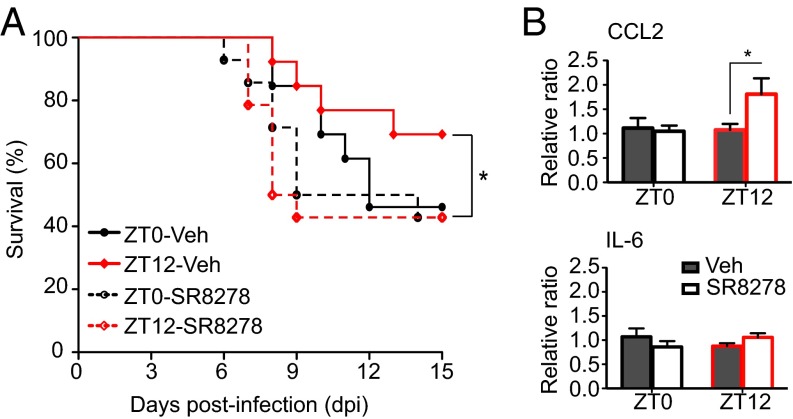
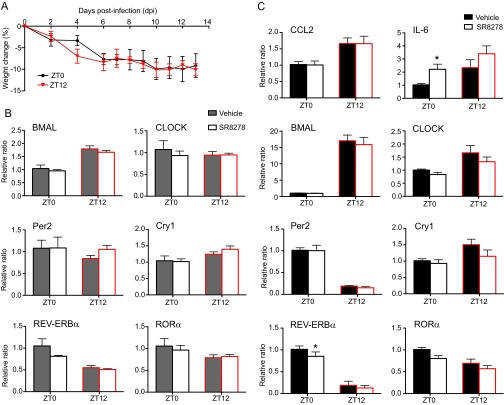
Differences detected in the expression of REV-ERBα in the OB tissue between the ZT0 and ZT12 time points led us to hypothesize that the circadian difference seen in VSV-induced encephalitis may be mediated by REV-ERBα. To test this hypothesis, we used a synthetic antagonist for REV-ERBα, SR8278, to block the activity of the receptor in the OB of VSV-infected mice. This compound has been shown to alter the mRNA levels of REV-ERBα target genes in vitro and in vivo and to produce behavioral changes in mice following microinfusion into the ventral midbrain (25, 26). We administered two doses of SR8278 i.n., the first 4 h before infection and the second concomitant with VSV i.n. administration. To account for the drug-only effect, we also treated groups of mice with the SR8278/PBS combination. We found that blocking REV-ERBα activity in mice infected with VSV at the start of their active phase (ZT12) significantly reduced the survival rate compared with vehicle-treated ZT12 mice (Fig. 6A). In contrast, SR8278 treatment had no effect on the survival of VSV-infected versus vehicle-treated ZT0 mice (Fig. 6A). SR8278 had no effect on the health and survival of PBS-treated mice (Fig. S7A).
Fig. 6.
Inactivation of REV-ERBα during VSV i.n. administration leads to poor survival. Two groups of mice were treated i.n. with either SR8278 (20 µg) or with vehicle solution administered twice: 4 h before and concomitant with VSV infection. (A) Survival curves are a composite of two independent experiments [total n = 13 per group; log-rank (Mantel–Cox) test, *P = 0.047 for ZT12-vehicle vs. ZT12-SR8278). (B) Total RNA was isolated from OB tissue of SR8278- or vehicle-treated mice 12 h after VSV i.n. administration. Transcript levels for CCL2 and IL-6 were measured by qPCR. Data compiled from two independent experiments are expressed as mean ± SEM (total n = 5 per time point; two-way ANOVA, Bonferroni's posttest, *P < 0.05, ZT12-vehicle vs. ZT12-SR8278).
Fig. S7.
Effects of SR8278 treatment. (A) Animals treated with SR8278/PBS were monitored for 14 d. Health score is expressed as the percent of weight change following treatment. Data are expressed as mean ± SEM (total n = 3 per group). (B and C) Total RNA was isolated from OB (B) and lung (C) tissue of SR8278- or vehicle-treated mice 12 h after VSV i.n. administration. Transcript levels for the indicated genes were measured by qPCR using Taqman gene-expression assays. Data compiled from two independent experiments are expressed as mean ± SEM (total n = 5 per time point; two-way ANOVA, Bonferroni's posttest, *P < 0.05, ZT0-vehicle vs. ZT0-SR8278).
To explore the molecular mechanism of the effect of REV-ERBα on VSV-induced mortality, we measured the expression of REV-ERBα target genes as well as clock genes following treatment with SR8278 or vehicle. It has been demonstrated that i.n. delivery of compounds could spread to lung tissue as well as the OB; therefore we selected these two sites for the analysis (27, 28). Blocking REV-ERBα activity with SR8278 led to a significant up-regulation of CCL2 mRNA 12 h posttreatment in the OB tissue of ZT12 mice, compared with vehicle-treated ZT12 mice (Fig. 6B). No effect on the mRNA expression of IL-6, BMAL, or any other clock genes was found in the OBs of either ZT0 or ZT12 mice (Fig. 6B and Fig. S7B). In contrast, changes in the expression levels of RORα (decreased) and IL-6 (increased) mRNA were found in the lung tissue of ZT0 mice (Fig. S7C). These changes did not affect the survival rate of the animals. These data suggest that REV-ERBα–mediated circadian effect on CCL2 expression in the OB affects the immune response to VSV-induced encephalitis and the survival of the animal.
CCL2 Is Expressed in Resident and Infiltrating Cells of the OB.
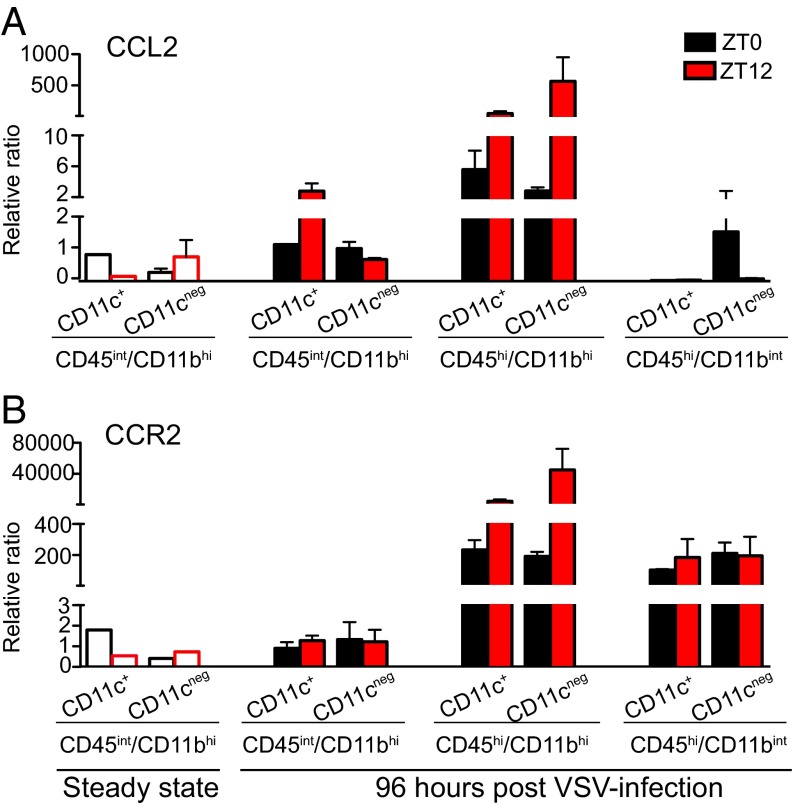
To identify the cellular source of CCL2 in the OB, we examined the expression levels of CCL2 mRNA in cell populations isolated from OB of naive and VSV-infected mice using FACS. We found that brain-resident MG and bDC both express low levels of CCL2 mRNA in steady state (Fig. 7A). VSV infection induces CCL2 mRNA expression at 96 hpi only in bDCs isolated from ZT12 mice. Interestingly, two cell populations that infiltrate the OB of VSV-infected mice at 96 hpi, CD45hi/CD11bhi and CD45hi/CD11bint, display drastically different levels of CCL2 mRNA expression: Both CD11c+ and CD11c− cells within the CD45hi/CD11bhi population express high levels of CCL2 mRNA, and the expression level is higher in mice infected at ZT12. In contrast, only the CD45hi/CD11bint/CD11c− cells isolated from ZT0 mice display CCL2 expression; no CCL2 mRNA was detected in cells isolated from ZT12 mice (Fig. 7A).
Fig. 7.
CCL2 and CCR2 expression in OB immune cells. (A and B) CD45int/CD11bhi, CD45hi/CD11bhi, and CD45hi/CD11bint cells isolated from the OB of VSV-infected mice at 96 hpi were FACS sorted based on CD11c expression. The only population present in the OB of untreated mice, CD45int/CD11bhi, was analyzed also (steady state, open bars). Total RNA was isolated, and transcript levels for CCL2 (A) and CCR2 (B) were measured by qPCR. Data compiled from five independent experiments are expressed as mean ± SEM (total n = 5).
The expression pattern of CCR2 mRNA in the sorted cell populations (Fig. 7B) was in agreement with our flow cytometry data (Fig. 3B): (i) the CD45int/CD11bhi population expresses low levels of CCR2 in steady state and following VSV challenge, and (ii) both CD11c+ and CD11c− subpopulations of CD45hi/CD11bhi and CD45hi/CD11bint cells express high levels of CCR2 96 h following VSV. Interestingly, differences were observed in the levels of CCR2 mRNA in the CD45hi/CD11bhi cell subpopulation isolated from ZT0 and ZT12 mice; however these differences were not reflected in the cell-surface expression of CCR2 as seen by flow cytometry (Fig. S4B).
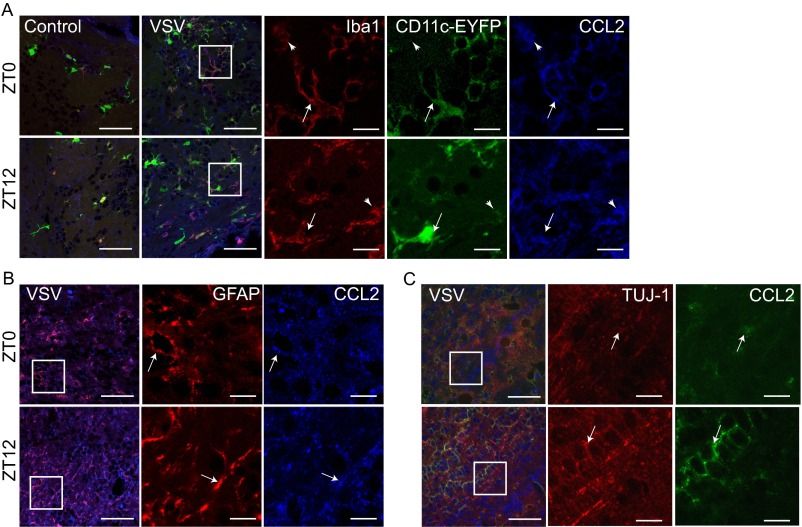
To confirm further the source of CCL2 in the VSV-infected OB, we examined the expression of CCL2 in OB tissue obtained from control and VSV-treated mice. Double immunolabeling for CCL2 and cell markers for MG (IbaI) (Fig. S8A), astroglia (GFAP) (Fig. S8B), and neurons (TUJ-1) (Fig. S8C) was performed. We found that both Iba1+ MG and GFAP+ astrocytes express CCL2 at 96 h following VSV i.n. administration. Moreover, in the CD11c-EYFP transgenic mouse OB we also detected colocalization of CCL2 with EYFP+ bDCs (Fig. S8A). Little or no CCL2 expression was detected in mitral cells of the OB, but diffuse labeling of CCL2 was evident in the granule cell layer of the OB (Fig. S8C).
Fig. S8.
CCL2 expression in the OB. Immunofluorescent labeling was performed on PFA-fixed control (PBS-treated) and VSV-infected OB tissue at 96 hpi. (A) CCL2 labeling of OB immune cells; CCL2 is shown in blue, Iba1 is shown in red, and CD11c-EYFP is shown in green. Arrows indicate cells coexpressing CCL2/IbaI and CD11c-EYFP; arrowheads indicate cells that coexpress only CCL2 and IbaI. (B) CCL2 labeling of astroglia cells; CCL2 is shown in blue, and FGAP is shown in red. Arrows indicate cells colabeled with CCL2 and GFAP. (C) CCL2 labeling of neurons; CCL2 is shown in green, and TUJ-1 is shown in red. Arrows indicate cells colabeled with CCL2 and TUJ-1. White squares denote areas in which a single label is shown. (Scale bars: 25 µm for overlaid panels, 10 µm for single-label panels.)
Discussion
Our results demonstrate that the circadian time of infection has a significant effect on the development and resolution of VSV-induced encephalitis. Specifically, we found that viral inoculation applied at the end of the active cycle (ZT0) leads to poor survival and is associated with a higher level of circulating CCL2 and a higher proportion of CD45hi/CD11bhi and CD45hi/CD11bint cells (identified as inflammatory monocytes) in the OB. We also have demonstrated that relieving REV-ERBα–mediated inhibition of CCL2 mRNA expression using receptor antagonist treatment during virus-induced encephalitis results in decreased survival.
The circadian effect on immune response has been demonstrated in number of infection models, including LPS-induced inflammation, pneumococcal infection, and cecal ligation puncture (CLP)-induced sepsis (1, 4, 12, 14). It is noteworthy that the acrophase of the circadian effect in each of these experimental models falls at different times in the daily cycle, suggesting differential underlying mechanisms. For example, clearance of Streptococcus pneumoniae from the lung depends on the circadian variation in the expression of CXCL5 and subsequent neutrophil recruitment (12), whereas circadian expression of TLR9 in mouse spleen determines the severity of response in CLP-induced sepsis (4).
Cytokines, chemokines, and hormones, which are critical regulators of immune function, also display circadian oscillation. For example, the induction of IL-6, IL-12 (p40), CCL2, and CCL5 in circulation and peritoneal exudate cells is far greater in mice challenged with LPS at ZT12 than at ZT0 (7). In our study, we also found that the VSV-induced increase in circulating inflammatory cytokines depends on the time of infection, with IFNγ and IL-6 levels being higher in mice infected at ZT12 and the level of CCL2 being higher and more sustained in mice infected at ZT0. It is noteworthy that we have not identified the specific source of the circulating cytokines following VSV challenge. However, we found the up-regulation of proinflammatory cytokine mRNA in the spleen and lung tissues of infected mice at the same time point (24 hpi) as increases in blood cytokine levels, suggesting that spleen and/or lung immune cells may be the source of circulating “alert” cytokines at this early stage of infection. These data are in agreement with a previous report that the infectious VSV is transiently present in lungs and spleens at 24 hpi (21) and may be able to initiate peripheral immune responses.
It must be noted that our experimental paradigm, which required having the measures for time-course experiments taken 12 h out of phase between groups, may have impacted data interpretation in cases where parameters showed circadian fluctuations. However, most measures taken (circulated cytokines, mRNA levels, cell numbers) displayed differences between two circadian groups only at specific time points (being comparable at all other time points), allowing us to conclude that circadian variation at the time of sample collection was not a significant factor for data interpretation.
Reciprocal regulation between cytokines and glucocorticoids has been described in various infection models: Pathogen-induced cytokines increase CORT production, which through a negative feedback loop down-regulates cytokine levels and attenuates inflammation (29, 30). We found that CORT levels are differentially affected by VSV, depending on the time of infection. In mice infected at the end of the active cycle (ZT0, the nadir of CORT), we observed an increase in the blood CORT levels coinciding with an increase in circulating cytokines at 24 hpi. In contrast, mice infected at the start of the active cycle (ZT12, the acrophase of CORT) maintained stable CORT levels for the following 96 hpi (measured daily at ZT12). Thus, CORT, acting through the glucocorticoid receptor and antagonizing NF-κB, may limit cytokine expression in ZT12 mice (31). It has been long recognized that acute stress and the associated surge in glucocorticoid levels result in a decrease in blood monocyte numbers (32) and have a protective effect during immune challenge (33). Our data showing a lower proportion of monocytes accumulating in the OB and better survival of mice infected at the peak of endogenous CORT at ZT12 are in accordance with these previous findings.
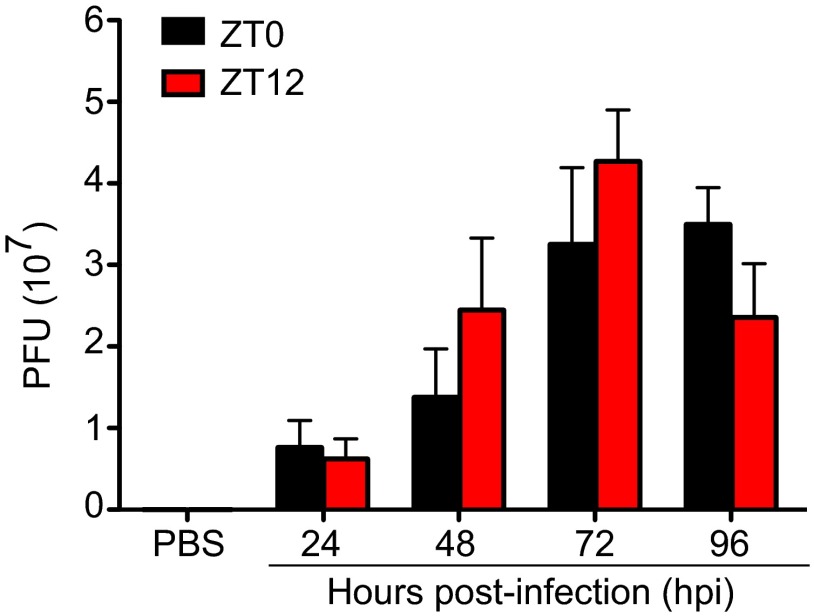
Monocyte infiltration is an important step in the defense against pathogens. However, uncontrolled accumulation of these cells at the site of infection, particularly in the CNS, may lead to immune-mediated pathology and irreparable tissue damage as seen in viral encephalitis models, stroke, traumatic brain injury, and EAE (13, 16–18, 34). In the current study we identified the CD45hi/CD11bhi/Ly6chi/CCR2+ cell population accumulating in the OB following VSV infection as proinflammatory monocytes. Notably, greater numbers of these cells and higher levels of proinflammatory cytokines, characteristics of an overactive immune response, were detected in the OB of mice infected at the end of the active cycle (ZT0), and these mice also were more susceptible to virus-induced mortality. At the same time, the amounts of virus present in the OB of mice infected with VSV at either ZT0 or ZT12 did not differ (Fig. S9). These data point to circadian regulation of monocyte trafficking into the brain and the critical role these cells may play in immune-mediated pathology.
Fig. S9.
Viral titer in the OB of VSV-infected mice. Total RNA was isolated from OB tissue at the indicated time points, and the VSV glycoprotein transcript was amplified using qPCR. The VSV titer (pfu) was calculated based on a logarithmic standard curve generated with known amounts of purified virus. Data are expressed as mean ± SEM and represent a composite of two independent experiments (total n = 4–5 per time point).
The chemokine ligand CCL2 and its receptor CCR2 are the critical signaling factors that mediate monocyte egress from bone marrow and recruitment to the site of infection (14, 35). Interfering with CCR2–CCL2 signaling by means of genetic modification or pharmacological agents has a protective effect in a number of inflammatory conditions in both the periphery and the CNS (13, 14, 16). Previously, BMAL was shown to regulate CCL2 expression in Ly6chi monocytes, and REV-ERBα was implicated in the regulation of CCL2 expression in peritoneal macrophages (5, 8, 11). We found that VSV-induced up-regulation of CCL2 is under circadian control, with mice infected at ZT0 displaying higher levels of CCL2 in circulation and in the OB tissue. To identify the factor(s) involved in the circadian regulation of CCL2, we focused on REV-ERBα for two reasons: (i) REV-ERBα has been shown to regulate the expression of two critical proinflammatory factors, CCL2 and IL-6 (5, 6), and (ii) we have found that the acrophase of REV-ERBα mRNA expression in the OB is at ZT12 and coincides with the acrophase of survival following VSV infection.
Using a REV-ERBα antagonist 4 h before and concomitantly with VSV i.n. administration led to decreased survival of ZT12 mice and was accompanied by the increased production of CCL2 mRNA in the OB. At present we cannot exclude the possibility that SR8278 also may have effects elsewhere in the brain that then could feed back or forward to the OB. Surprisingly, we did not find that the REV-ERBα antagonist had an effect on the survival or OB gene expression in ZT0 animals, although we did detect increased expression of IL-6 mRNA and decreased expression of RORα mRNA in the lung tissue of SR8278-treated ZT0 mice. We hypothesize that the efficiency of antagonist action may depend on the level of REV-ERBα expression at the time of treatment; hence the lack of response in the OB of ZT0 animals may be caused by a low level of REV-ERBα expression at the time of treatment.
Together these data suggest that (i) the effect of blocking REV-ERB activity depends on circadian/diurnal fluctuation in its expression level, and (ii) REV-ERBα–mediated regulation of CCL2 expression affects VSV-induced neuroinflammation, possibly through monocyte migration. Thus, this study reveals a previously unidentified physiological regulatory mechanism underlying disease progression and survival in virus-induced encephalitis and a potential target for therapeutic amelioration of similar viral CNS infections.
Materials and Methods
Mouse Models.
All experimental procedures were approved by The Rockefeller University Animal Care and Use Committee. Male, wild-type, 6- to 7-wk-old C57BL/6 or CD11c/eyfp transgenic mice (on the C57BL/6 background) were used interchangeably for all experiments. Animals were either bred in The Rockefeller University facilities or purchased from The Jackson Laboratory.
Viral Infection Model.
Wild-type VSV, Indiana Serotype (ATCC VR-158) was used in all experiments. Virus purification and the VSV LD50 (7,000 pfu) for i.n. administration were determined in 5- to 7-wk-old male mice as described previously (20). For intranasal infection model see SI Materials and Methods.
Cytokine Profiling and CORT RIA.
VSV-infected animals were killed by rapid decapitation at the indicated time points postinfection. PBS-treated control mice were killed 96 h posttreatment. Cytokines were measured in plasma samples using a BD Cytometric Bead Array Mouse Inflammatory Cytokine Kit (BD Bioscience) according to the manufacturer’s protocol and were analyzed immediately on a LSR-II flow cytometer. CORT levels were measured in the plasma of PBS- and VSV-treated animals using the Corticosterone Double Antibody RIA Kit (MP Biomedicals) according to the manufacturer’s instructions and were read on a Cobra II Auto-Gamma Counter (Perkin-Elmer).
Quantitative PCR.
TaqMan gene-expression assays were used to measure mRNA levels for genes of interest. Eukaryotic 18S rRNA or GAPDH (both from Life Technologies) was used as an internal control. The samples were run on a LightCycler480 real-time PCR thermal cycler (Roche), and the relative ratio of the expression of each gene was calculated using the 2ΔΔCt method (36). For tissue collection, RNA isolation, and cDNA synthesis, see SI Materials and Methods.
Flow Cytometry and FACS.
Virally challenged mice were killed 96 hpi by rapid decapitation, and single-cell suspensions were obtained by digestion with collagenase D (Roche), dispase II (Roche), and DNase I (Life Technologies) and repeated trituration using a GentleMACS Dissociator (Miltenyi Biotec). For antibodies and the staining procedure, see SI Materials and Methods.
Antagonist Treatment.
Mice adjusted to opposite light–dark cycles were treated with the REV-ERBα antagonist SR8278 (Tocris) or vehicle solution in conjunction with VSV. Animal health and survival were monitored daily for 15 d. For gene-expression analysis, mice were killed 12 h after VSV i.n. administration or the last SR8278 treatment. Tissue was extracted and processed for RNA isolation. For detailed treatment procedures and doses used, see SI Materials and Methods.
See SI Materials and Methods for additional details on the mouse models, antibodies, quantitative PCR (qPCR), immunofluorescence, and the statistics used in this article.
SI Materials and Methods
Mouse Models and Viral Infection Model.
Following weaning at 21 d, all experimental mice were split into two groups and maintained under a 12-h:12-h light:dark cycle with ad libitum access to chow and water. To allow manipulations to be undertaken at the same external clock time, one half of the mice were maintained on a reverse light:dark cycle. All animals were allowed at least 18–21 d to adjust to the respective light:dark cycle before experimental testing. For i.n. infection, mice were lightly anesthetized with 3–4% (vol/vol) isofluorane, and 5 μL VSV in PBS was applied per naris. Control mice were treated with only 5 μL PBS per naris. Virus was administered at one of two time points: at ZT0 (the start of the light/rest cycle) or at ZT12 (the start of the dark/active cycle).
Tissue Collection and RNA Isolation.
Naive, PBS-treated, or VSV-infected mice were killed by rapid decapitation at the indicated time points. Brains, spleens, and lungs were removed, dissected, and rapidly frozen on dry ice. Total RNA was isolated from the OB, spleen tissue, or lung tissue using the RNeasy Mini kit (Qiagen) and QIAcube according to the manufacturer’s instructions. The total RNA (2.5 µg) was converted into cDNA using the High-Capacity cDNA Reverse Transcription kit (Life Technologies), and the resultant cDNA was used as a template for qPCR.
Flow Cytometry and FACS.
OB cells from single-cell suspensions were washed and treated with anti-mouse CD16/32 Fc block (eBioscience). Live/Dead Fixable Aqua (Life Technologies) or DAPI stain (Sigma) was used for dead cell selection, and indicated antibodies were used for the staining of cell-surface markers. For flow cytometry analysis cells were fixed with the BD Fix/Perm kit (BD Bioscience) and were analyzed with a BD LSR-II flow cytometer (BD Bioscience) using FACS Diva software. Fixed cells postacquisition data analysis was performed using FlowJo software (TreeStar). See Table S3 for the complete antibody list. See Fig. S3A for a representative gating strategy.
Table S3.
Antibodies used in flow cytometry analysis
| Primary antibody | Clone | Isotype | Source |
| CD45 | 3D-F11 | Rat IgG2b κ | eBioscience |
| CD11b | M1/70 | Rat IgG2b κ | eBioscience |
| CD11c | N418 | Ar Ham IgG | eBioscience |
| F4/80 | BM8 | Rat IgG2a κ | eBioscience |
| CD103 | 2E 7 | Ar Ham IgG | eBioscience |
| CX3CR1 | Goat IgG | R&D Systems | |
| MHC I | 28-14-8 | Mouse IgG2a κ | eBioscience |
| MHC II (I-A/ I-E) | M5/114.15.2 | Rat IgG2b κ | eBioscience |
| TCR-β | H57-597 | Ar Ham IgG | eBioscience |
| CD19 | eBio1D3 | Rat IgG2a κ | eBioscience |
| CD86 | GL1 | Rat IgG2a κ | eBioscience |
| CD80 | 16-10A1 | Ar Ham IgG | eBioscience |
| CD40 | 1C10 | Rat IgG2a κ | eBioscience |
| CD4 | GK1.5 | Rat IgG2b κ | eBioscience |
| CD8 | 53-6.7 | Rat IgG2a κ | eBioscience |
| Dec205 | 205yekta | Rat IgG2a κ | eBioscience |
| NK1.1 | PK136 | Mouse IgG2a κ | eBioscience |
| B220 | RA3-6B2 | Rat IgG2a κ | eBioscience |
| Gr-1 | RB6-8C5 | Rat IgG2b κ | eBioscience |
| Ly6C | HK1.4 | Rat IgG2c κ | eBioscience |
| CD115 | AFS98 | Rat IgG2a κ | eBioscience |
| CCR2 | 475301 | Rat IgG2b | R&D Systems |
Ar Ham, Armenian hamster.
Antagonist Treatment.
The antagonist and vehicle solution was applied twice: 4 h before infection at ZT20 and ZT8, respectively, and concomitantly with infection at ZT0 and ZT12, respectively. Drug was administered i.n. by applying 5 µL of solution (2 µg/µL in 2.5% DMSO-saline, total treatment dose 20 µg) per naris under light isoflurane anesthesia. The other half of each circadian group was treated with vehicle solution (2.5% DMSO-saline) alone. Viral infection was done as described using a 5 × 104 pfu dose of wild-type VSV. The control group also was treated with SR8278 in conjunction with PBS to account for the drug-only effect.
Fluorescence Immunohistochemistry.
Tissue collected for fluorescent microscopy was harvested from mice perfused transcardially with a 4% paraformaldehyde solution under sodium pentobarbital anesthesia (150 mg/kg, i.p.) (Sigma Aldrich) as previously described (35). Brains were collected, postfixed, cryoprotected in 30% sucrose solution, and embedded into optimal cutting temperature (OCT) reagent (Sakura Finetek). Coronal sections (20 µm) of the OB were cut on a Leica CM3050S cryostat (Leica Biosystems) and were collected on glass slides. Sections were blocked in 0.5% BSA followed by incubation in a primary antiserum overnight at 4 °C. Primary antibodies used were rabbit polyclonal α-Iba1 (Wako) used at 1:1,000; goat polyclonal α–MCP-1(CCL2) (R-17; Santa Cruz Biotechnology) used at 1:100; rabbit polyclonal α-GFAP (ab7260; Abcam) used at 1:500; and mouse monoclonal α–TUJ-1 (BioLegend) used at 1:500. Sections then were incubated with the species-appropriate fluorescent-tagged Alexa-Fluor secondary antibodies (Life Technologies) for 1 h at room temperature. Fluorescence-labeled sections were imaged with a Nikon Eclipse 90i epifluorescent microscope (Nikon) or an inverted TCS SP5 laser-scanning confocal microscope (Leica Biosystems) at the Rockefeller Bioimaging Resource Center.
Calculation of VSV Load.
The viral load in OB samples isolated from VSV-infected mice was measured at the indicated time points. Total RNA was isolated, and random primers were used for cDNA synthesis from equal amounts of RNA. qPCR was performed using SYBR Green (Life Technologies) to amplify an 87-bp piece of VSV glycoprotein using the following primers: forward, 5′-CCCAAGAGTCACAAGGCTATT-3′; reverse, 5′-GTCCATACCAGCGGAAATCA-3′. To generate a standard curve, viral RNA was isolated from a known amount of purified VSV using TRIzol reagent (Life Technologies), and cDNA synthesis and qPCR were performed in parallel with OB samples.
Acknowledgments
We thank Dr. Paul D’Agostino and Zahrah Masheeb for contributions to the early stages of the study; Dr. Young Cheul Chung and Daniel Cole for technical assistance; and The Rockefeller University’s Comparative Bioscience Center, Flow Cytometry Resource Center, and the Bio-Imaging Resource Center for expert technical assistance. This research is supported by a gift from the Peter Deane Trust (to K.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520489113/-/DCSupplemental.
References
- 1.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LAJ. Circadian clock proteins and immunity. Immunity. 2014;40(2):178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Silver AC, Arjona A, Hughes ME, Nitabach MN, Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav Immun. 2012;26(3):407–413. doi: 10.1016/j.bbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonken LK, et al. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun. 2015;45:171–179. doi: 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36(2):251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato S, et al. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192(1):407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, et al. Direct and indirect suppression of interleukin-6 gene expression in murine macrophages by nuclear orphan receptor REV-ERBα. ScientificWorldJournal. 2014;2014(5):685854. doi: 10.1155/2014/685854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbs JE, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109(2):582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30(4):621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342(6159):727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arjona A, Boyadjieva N, Sarkar DK. Circadian rhythms of granzyme B, perforin, IFN-gamma, and NK cell cytolytic activity in the spleen: Effects of chronic ethanol. J Immunol. 2004;172(5):2811–2817. doi: 10.4049/jimmunol.172.5.2811. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen KD, et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341(6153):1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs J, et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20(8):919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terry RL, et al. Inflammatory monocytes and the pathogenesis of viral encephalitis. J Neuroinflammation. 2012;9(1):270. doi: 10.1186/1742-2094-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192(7):1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): Evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010;30(4):769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammond MD, et al. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34(11):3901–3909. doi: 10.1523/JNEUROSCI.4070-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiss CS, Plakhov IV, Komatsu T. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann N Y Acad Sci. 1998;855:751–761. doi: 10.1111/j.1749-6632.1998.tb10655.x. [DOI] [PubMed] [Google Scholar]
- 20.D'Agostino PM, et al. Viral-induced encephalitis initiates distinct and functional CD103+ CD11b+ brain dendritic cell populations within the olfactory bulb. Proc Natl Acad Sci USA. 2012;109(16):6175–6180. doi: 10.1073/pnas.1203941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trottier MD, Lyles DS, Reiss CS. Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. J Neurovirol. 2007;13(5):433–445. doi: 10.1080/13550280701460565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornish TE, Stallknecht DE, Brown CC, Seal BS, Howerth EW. Pathogenesis of experimental vesicular stomatitis virus (New Jersey serotype) infection in the deer mouse (Peromyscus maniculatus) Vet Pathol. 2001;38(4):396–406. doi: 10.1354/vp.38-4-396. [DOI] [PubMed] [Google Scholar]
- 23.Onishi H, et al. Rev-erbalpha gene expression in the mouse brain with special emphasis on its circadian profiles in the suprachiasmatic nucleus. J Neurosci Res. 2002;68(5):551–557. doi: 10.1002/jnr.10226. [DOI] [PubMed] [Google Scholar]
- 24.Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: Are circadian oscillators present throughout the mammalian brain? Eur J Neurosci. 2007;25(11):3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- 25.Kojetin D, Wang Y, Kamenecka TM, Burris TP. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem Biol. 2011;6(2):131–134. doi: 10.1021/cb1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung S, et al. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell. 2014;157(4):858–868. doi: 10.1016/j.cell.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan MB, Chauhan NB. Brain Uptake of Neurotherapeutics after Intranasal versus Intraperitoneal Delivery in Mice. J Neurol Neurosurg. 2015;2(1):009. [PMC free article] [PubMed] [Google Scholar]
- 28.Southam DS, Dolovich M, O’Byrne PM, Inman MD. Distribution of intranasal instillations in mice: Effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L833–L839. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 29.Ruzek MC, Miller AH, Opal SM, Pearce BD. Characterization of early cytokine responses and an interleukin (IL)-6–dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185(7):1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruzek MC, Pearce BD, Miller AH, Biron CA. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J Immunol. 1999;162(6):3527–3533. [PubMed] [Google Scholar]
- 31.Rao NAS, et al. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Res. 2011;21(9):1404–1416. doi: 10.1101/gr.118042.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: A tale of three hormones--Curt Richter Award winner. Psychoneuroendocrinology. 2012;37(9):1345–1368. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhabhar FS. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol Res. 2014;58(2-3):193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 34.Getts DR, et al. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205(10):2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsou C-L, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117(4):902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmittgen and Livak (2008) Analyzing real-time PCR data by the comperative CT method. Nature Protocols 3(6):1101–1108. [DOI] [PubMed]