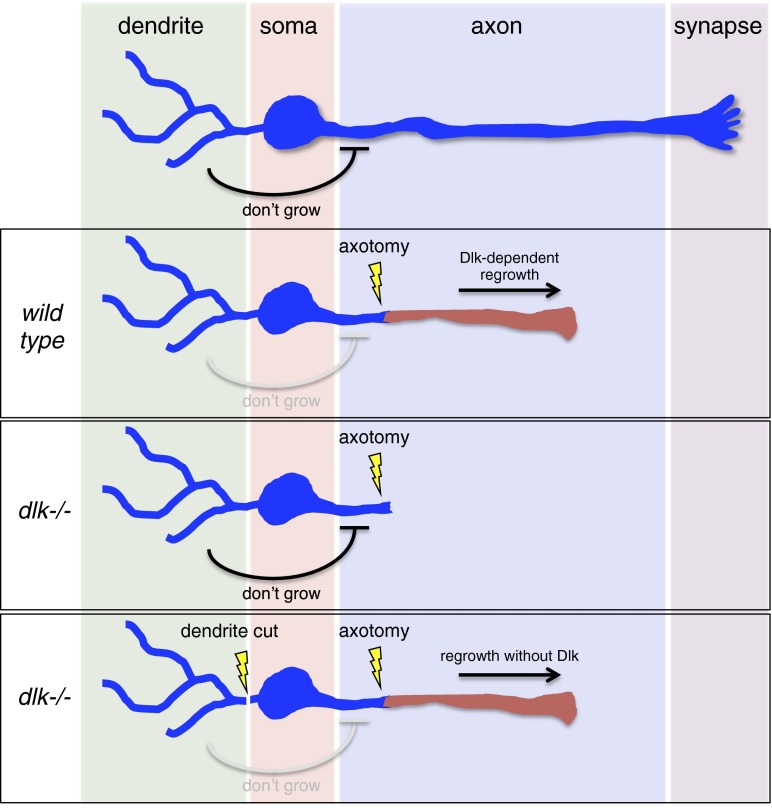
The development of approaches to regenerate neuronal connections that are lost after nervous system injury or during disease has proven enormously challenging. In the mammalian CNS the problem appears to be (at least) twofold. First, local extrinsic cues potently suppress axon outgrowth. However, efforts to blunt growth inhibitory effects of these molecules have not resulted in widespread regrowth of axons after lesion (1). The second problem is that functionally mature neurons, in contrast to developing neurons, appear to have a low intrinsic capacity for growth (2). Preconditioning lesions, where one axonal branch of a neuron is severed, can significantly enhance the outgrowth of other axonal branches in response to subsequent axotomy (3). The effect of the preconditioning lesion has been thought to result from the first lesion driving neuronal de-differentiation and resetting its fate to something more like a developing neuron (4), but the precise mechanisms involved have remained unclear. In PNAS, Chung et al. (5) use an elegant combination of genetics, laser ablations, and pharmacology to demonstrate that dendrites actively repress regenerative outgrowth in functionally mature neurons through a pathway that is independent of the well-conserved dual leucine zipper kinase (DLK)-regulated regeneration cascade (Fig. 1). Moreover, this pathway shares important cellular and molecular features with a previously described form of stress-induced ectopic axon outgrowth, suggesting common mechanisms may underlie these processes.
Fig. 1.
Dendritic control of axon growth and regrowth. Dendrites actively suppress ectopic sprouting, even in wild-type uninjured axons, through the Dlk-independent EGL-19/TAX2,4/UNC-43/SAX-1 pathway. In wild-type animals, Dlk activity is sufficient to drive axon regrowth after axotomy (red axon), even in the presence of this inhibitory pathway, but regeneration is completely suppressed in dlk−/− animals because of inhibitory signals from dendrites. Cutting dendrites in the dlk−/− background or eliminating components of the EGL-19/TAX2,4/UNC-43/SAX-1 pathway relieves this antigrowth signal and results in Dlk-independent axonal outgrowth.
Chung et al. (5) investigate sensory regulation of regeneration in Caenorhabditis elegans ASJ sensory neurons. These neurons are bilaterally symmetrical and bipolar: from each ASJ neuronal cell body projects a single dendrite with sensory cilia and an axonal connection to the nerve ring (central ganglion of the nematode nervous system), and each compartment of the cell can be easily resolved by microscopy. Chung et al. use femtosecond laser surgery to sever ASJ axons near the cell soma and demonstrate that regeneration is robust and specific to axons. Following laser axotomy in wild-type, >95% of neurons show significant regeneration. In contrast, dendrite transection results in little to no outgrowth. Prior work has shown that regeneration in a variety of experimental systems is strongly dependent on DLK-1 (6–9). Mutation of dlk-1 also eliminated regeneration after laser cutting of ASJ axons. Surprisingly, however, Chung et al. (5) found that simultaneous cutting of the ASJ axon and dendrite restores the regenerative capacity of ASJ axons even in dlk-1 mutants. Thus, dendrite lesion appears to activate a novel type of Dlk-independent axon regrowth. The regeneration of axons in this situation is substantial, with 80% of regenerated axons extending to the nerve ring neuropil within 5 d. How well these axons generate functional connections in the nerve ring remains an open question.
Unraveling the genetic basis of axonal regeneration has been challenging, so the discovery of a Dlk-independent regenerative event is an important step forward for modeling axonal responses to neuronal injury. Although it is a possibility that such regenerative effects following dendrite lesion are specific to dlk-1 mutants, Chung et al. (5) show that severing ASJ sensory dendrites in wild-type animals also significantly enhances the axonal regenerative response compared with axotomy-alone controls, arguing against this possibility. Importantly, the authors also identify cellular and molecular features that distinguish this form of regeneration from previously described regenerative events. For example, Dlk-independent regeneration proceeds more slowly compared with conventional regeneration, initiating around 24 h after surgery, compared with ∼12 h in control axons, and this is true in both young and old worms. Additionally, conventional MAPK genes downstream of Dlk are not required—including the PMK-3/CEBP-1 p38 pathway—nor was the parallel MLK-1/KGB-1/FOS-2 JNK MAPK cascade involved. It therefore appears that a novel regenerative pathway underlies Dlk-independent regeneration.
Previous studies had identified a collection of mutants that exhibited activity-dependent ectopic axon outgrowth under conditions of elevated culture temperature (i.e., stress). Could these outgrowth pathways be linked genetically? Consistent with this possibility, mutations that resulted in stress-induced ectopic outgrowth, including disruptions of the cyclic-nucleotide–gated channel subunits tax-4/CNGA1 and tax-2/CNGB1 or UNC-43/CaMKII, also promoted Dlk-independent axon regeneration in the absence of dendrite lesion. Reciprocally, dendrite lesion alone was sufficient to drive ectopic axon outgrowth in the absence of axotomy at elevated culture temperatures.
To further explore possible links with activity-regulated ectopic axon outgrowth, preconditioning lesions, and neural activity, Chung et al. (5) investigated the role of the L-type voltage-gated calcium channel (VGCC) α1 subunit egl-19/CACNA1C, which is also known to play a key role in lesion conditioning (4). Genetic disruption of egl-19 triggered Dlk-independent regeneration in axons in the absence of dendrite cut, as did the EGL-19 antagonist nemadipine-A. Finally, through gain-of-function and loss-of-function analyses, increases in cAMP signaling were shown to enhance the Dlk-independent regeneration response after axotomy. Modulation by the VGCC α1 subunit in particular is consistent with the notion that changes in membrane voltage, and potentially sensory neuron activity itself, are an important part of the regulatory mechanism controlling axonal outgrowth.
One of the additional intriguing aspects of the Chung et al. (5) study is the link the authors found between neuronal injury and maintenance of sensory function. Remarkably, neuronal injury affected ASJ activity in a cell compartment-specific manner. Using optogenetic stimulation of ASJ in combination with ASJ Ca2+ imaging, Chung et al. show that axotomy of ASJ neurons did not affect their responsiveness to photostimulation. In contrast, severing dendrites or axons and dendrites simultaneously completely eliminated the light-responsiveness of ASJ. This observation is strikingly similar to the response of mammalian DRG neurons, where lesion of peripheral but not central axon branches eliminates sensory-evoked neuronal activity (4). It is also consistent with the notion that dendrite lesion might promote Dlk-independent regeneration by inhibition of ASJ sensory activity.
However, the story is not quite that simple. Curiously, the initiation of Dlk-independent outgrowth after dendrite lesion depends critically on the order of the injury to each neuronal compartment. Severing the dendrite led to robust Dlk-independent outgrowth, as did simultaneous dendrite and axonal injury. However, severing the axon first, followed by the dendrite, did not activate Dlk-independent axonal outgrowth. Thus, dendrite injury can somehow prime the cell for enhanced outgrowth but this effect is eliminated by prior axonal injury. If simple deprivation of sensory activity in the dendrite is the key negative regulator of axonal outgrowth, one might anticipate the order of injury of axons versus dendrites would not be critical. In addition, whereas the genetic analysis is consistent with the notion that sensory activity in dendrites may actively suppress axonal outgrowth, these experiments do not definitively show that neural activity per se negatively regulates outgrowth. The mutations tested affect activity-dependent outgrowth but do not necessarily silence neuronal activity. Experiments using approaches that allow for acute silencing of the ASJ neurons or dendrites will allow for a deeper investigation into the potential role of sensory activity in modulating axonal growth. Nonetheless, the present work (5) makes a strong case that initial severing of dendrites somehow releases a constitutive inhibition of axonal growth.
The study by Chung et al. (5) offers an intriguing new look into the cellular and molecular basis of axon outgrowth and dendrite–axon interactions in mature neurons. Using this system, one should be able to provide deep molecular insight into the question of why neurons remain so morphologically stable after maturation and don’t spontaneously sprout new neurites. The identification of these Dlk-independent axonal outgrowth molecules provides an exciting new molecular foothold into a novel pathway that might be capable of promoting axonal outgrowth after injury. New screens to identify additional modulators of stress-induced axonal sprouting could prove a fruitful way to exploit the exceptional power of C. elegans genetics to delineate these important neuron regeneration pathways.
Acknowledgments
Work in the M.M.F. and M.R.F. laboratories is supported by the National Institutes of Health National Institutes of Neurological Disorders and Stroke, Grants R01 NS059991 (to M.R.F.) and R01 NS064263 (to M.M.F.).
Footnotes
The authors declare no conflict of interest.
See companion article on page E2852.
References
- 1.Geoffroy CG, Zheng B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol. 2014;27:31–38. doi: 10.1016/j.conb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309(5971):791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- 4.Enes J, et al. Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr Biol. 2010;20(13):1154–1164. doi: 10.1016/j.cub.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Chung SH, et al. Novel DLK-independent neuronal regeneration in Caenorhabditis elegans shares links with activity-dependent ectopic outgrowth. Proc Natl Acad Sci USA. 2016;113:E2852–E2860. doi: 10.1073/pnas.1600564113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323(5915):802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh A, Horiuchi M, Bannerman P, Pleasure D, Itoh T. Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem Biophys Res Commun. 2009;383(2):258–262. doi: 10.1016/j.bbrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Shin JE, et al. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74(6):1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong X, et al. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191(1):211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]