Abstract
The present study integrated microRNA (miRNA) and mRNA expression data obtained from atrial fibrillation (AF) tissues and healthy tissues, in order to identify miRNAs and target genes that may be important in the development of AF. The GSE28954 miRNA expression profile and GSE2240 mRNA gene expression profile were downloaded from the Gene Expression Omnibus. Differentially expressed miRNAs and genes (DEGs) in AF tissues, compared with in control samples, were identified and hierarchically clustered. Subsequently, differentially expressed miRNAs and DEGs were searched for in the miRecords database and TarBase, and were used to construct a regulatory network using Cytoscape. Finally, functional analysis of the miRNA-targeted genes was conducted. After data processing, 71 differentially expressed miRNAs and 390 DEGs were identified between AF and normal tissues. A total of 3,506 miRNA-mRNA pairs were selected, of which 372 were simultaneously predicted by both miRecords and TarBase, and were therefore used to construct the miRNA-mRNA regulatory network. Furthermore, 10 miRNAs and 12 targeted mRNAs were detected, which formed 14 interactive pairs. The miRNA-targeted genes were significantly enriched into 14 Gene Ontology (GO) categories, of which the most significant was gene expression regulation (GO 10468), which was associated with 7 miRNAs and 8 target genes. These results suggest that the screened miRNAs and target genes may be target molecules in AF development, and may be beneficial for the early diagnosis and future treatment of AF.
Keywords: atrial fibrillation, microRNA-mRNA interactions, microarray expression profiles, bioinformatics
Introduction
Atrial fibrillation (AF), which is the most common type of arrhythmia, is characterized by an irregular and rapid heartbeat. AF has strong associations with other cardiovascular diseases, including heart failure, coronary artery disease, valvular heart disease, diabetes mellitus, and hypertension (1). The prevalence of AF within a population increases with age, and 8% of people over 80 years old have AF (2). AF accounts for 1/3 of hospital admissions for cardiac rhythm disorders, and the rate of admissions for AF has risen in recent years (3).
The molecular mechanisms underlying AF-associated cardiovascular risk factors have gained attention in recent years, since a clear understanding of these mechanisms is essential for further AF management. Regulation of gene expression by microRNAs (miRNAs) has also garnered much attention. miRNAs belong to a class of endogenous small regulatory RNA molecules, which target mRNAs and trigger either translational suppression or mRNA degradation (4). Abnormal expression of several miRNAs has been reported to be associated with various types of disease, including AF (5). Luo et al (6) demonstrated that miR-26 family members were significantly downregulated in left atrial appendages from a canine model of AF (miR-26a), and in right atrial appendages from patients with AF (miR-26a and miR-26b). Gene expression profiling has also been used to investigate various types of disease, and a range of gene signatures have been identified using DNA chips (7). Chen et al (7) identified 31 differentially expressed genes, which were associated with transcriptional regulation, signal transduction, and structural components, in a porcine model of AF using a low-density cDNA array. This study indicated that four and a half LIM domains 1 and cardiac ankyrin repeat protein may have important regulatory roles in AF activation.
miRNAs, as important regulators of mRNAs, are excellent biomarkers for disease development. mRNA and miRNA markers always function through relationship networks. It has previously been reported that miRNA-1 levels are markedly reduced in human AF, which may contribute to upregulation of Kir2.1 subunits, leading to increased cardiac inward-rectifier potassium current (IK1) (8). Furthermore, miR-26 has been suggested as a potentially important regulator of potassium channel, inwardly rectifying subfamily J, member 2 gene expression and, via IK1, a determinant of AF susceptibility (6).
The present study aimed to screen the differentially expressed miRNAs and genes (DEGs) in AF tissues compared with control samples, using expression profile data downloaded from an online database (GSE28954 and GSE2240). In addition, a regulatory network was constructed using Cytoscape and a functional analysis of miRNA-targeted genes was performed. The results of the present study may help elucidate the mechanisms underlying AF pathogenesis and contribute to the improvement of AF treatment.
Materials and methods
Datasets
A dataset containing mRNA gene expression profiles for 10 patients with AF and 10 control samples (9,10) was downloaded from the Gene Expression Omnibus (GEO) deposited on the public National Center for Biotechnology Information database (accession number: GSE2240) (http://www.ncbi.nlm.nih.gov/geo/). U133A and U133B microarray chips (Affymetrix, Inc., Santa Clara, CA, USA), together representing 44,928 probe sets, were used to analyze each human heart sample. A dataset containing miRNA expression profiles for 10 patients with AF and 5 healthy control samples (11) was downloaded from the GEO (accession number: GSE28954). miRNA expression profiling was conducted using the 15 human samples (10 AF and 5 healthy controls) using microarrays covering whole miRNAs from Agilent Technologies (Human miRNA Microarrays: G4470B, cat. no. 019118 and V3, cat. no. 021827; Santa Clara, CA, USA). According to the original data, all studies were approved by the appropriate Human Ethics Committees and informed consent was obtained from all of the patients.
Data processing and significance analysis
The raw miRNA and mRNA microarray data of all AF and control samples were normalized simultaneously using the robust multiarray average (RMA) method (12), implemented in R/Bioconductor project (https://www.bioconductor.org/). DEGs between AF and control samples were calculated using the significance analysis of microarray (SAM) method (13). Furthermore, each miRNA expression comparison was tested between AF patients and healthy controls using Student's t-test (14), and raw P-values were adjusted using the Benjamini-Hochberg method (15). Fold changes between the two groups were also calculated. A false discovery rate (FDR)<0.05 from Student's t-test, and P<0.05 from SAM were used to identify differentially expressed miRNAs and DEGs.
Hierarchical cluster analysis of selected miRNAs and genes
The hierarchical cluster analysis of differentially expressed miRNAs was performed using CLUSTER3.0 (16), and the hierarchical clustering heat-map was visualized by TreeView (17).
Identification of potential target genes of miRNAs
The candidate target genes of the identified miRNAs were predicted using miRecords (18) (http://mirecords.biolead.org/; no longer accessible) and TarBase (19) (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=tarbase/index/). The miRNAs differentially expressed between AF patients and healthy controls were searched for in the miRecords database and TarBase, which contains validated miRNA-target interactions. In addition, the probability of a miRNA-target interaction existing between differentially expressed miRNAs and DEGs was predicted by miRecords (score ≥50, energy ≤−20). The untranslated region (UTR) location was also predicted.
Construction of a miRNA-target network
miRNAs regulate target gene expression via mRNA degradation or translational inhibition (20,21). The expression of miRNA-targeted genes that are regulated by mRNA degradation are negatively correlated with the expression of miRNA regulators. Therefore, a miRNA-mRNA regulatory network was constructed containing the paired miRNA-mRNA expression profiles, and the putative target genes of miRNAs were predicted. Predictive and validated miRNA-mRNA pairs were extracted from the miRecords database and TarBase, and only the miRNA-mRNA pairs predicted by both miRe-cords database and TarBase were retained. Subsequently, a miRNA-mRNA regulatory network was constructed via Cytoscape (22).
Functional analysis of targeted genes from the network
To obtain a better understanding of the biological function of miRNAs and their target genes in AF, a functional analysis of the miRNA-targeted genes was carried out using the Database for Annotation, Visualization and Integrated Discovery (23) (https://david.ncifcrf.gov/). FDR<0.05 was set as the cut-off criterion.
Results
Differentially expressed miRNAs and DEGs, and cluster analysis
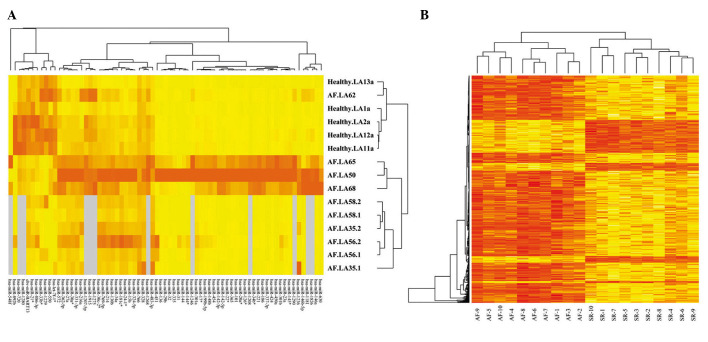
Following normalization using the RMA algorithm and SAM analysis of raw data, 71 significantly differentially expressed miRNAs and 390 DEGs were identified between the AF and normal tissues. In addition, the expression levels of these miRNAs and mRNAs were analyzed via heat-maps (Fig. 1).
Figure 1.
(A) Heat-map of selected microRNA expression and (B) mRNA expression in the atrial fibrillation (AF) and normal control samples. Red indicates high expression, yellow indicates low expression and gray indicates missing values. SR, sinus rhythm.
Construction of the miRNA-target network
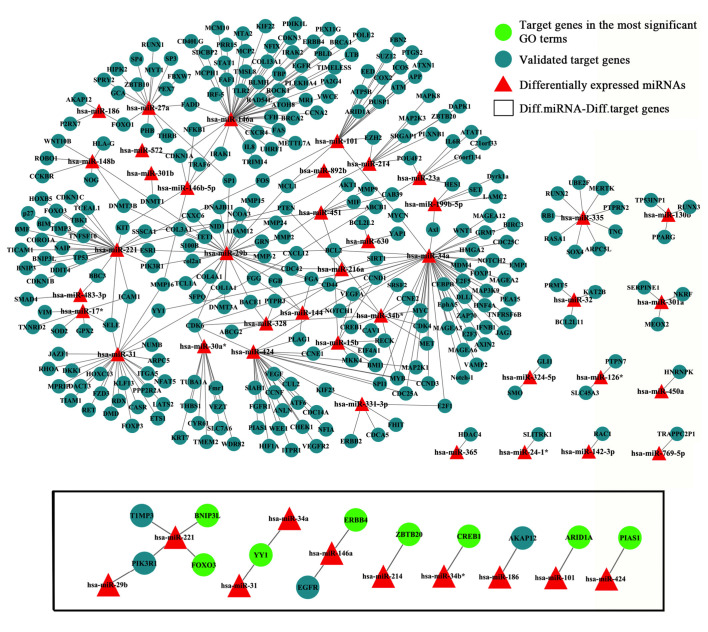
Using the criteria: Score ≥50 and energy ≤−20, 3,506 miRNA-mRNA pairs were predicted. A total of 372 validated miRNA-mRNA relationship pairs were simultaneously predicted by the miRecords database and TarBase, and were therefore used to construct the miRNA-mRNA regulatory network (Fig. 2, upper panel). Furthermore, 10 miRNAs and 12 targeted mRNAs were identified, which formed 14 interactive pairs (Fig. 2, lower panel). The selected miRNAs were upregulated in AF tissues, whereas the mRNAs were downregulated in the AF tissues. The expression levels of the 14 miRNA-mRNA pairs are presented in Table I, which indicated that the selected miRNAs were upregulated in AF tissues, whereas the mRNAs were downregulated.
Figure 2.
Candidate microRNA (miRNA)-mRNA target network was constructed using Cytoscape. Red triangles represent differentially expressed miRNAs; blue circles represent miRNA-targeted genes; the black box refers to the 14 interactive pairs of selected miRNAs and their targets that were predicted by miRe-cords and TarBase; and the green circles represent target genes involved in the most significantly enriched Gene Ontology terms. Diff., differentially expressed.
Table I.
Selected miRNA-mRNA pairs.
| Diff. miRNAs | Diff. & validated targets | miRNA-mRNA expression |
|---|---|---|
| hsa-miR-186 | AKAP12 | Up-down |
| hsa-miR-101 | ARID1A | Up-down |
| hsa-miR-221 | BNIP3L | Up-down |
| hsa-miR-34b* | CREB1 | Up-down |
| hsa-miR-146a | EGFR | Up-down |
| hsa-miR-146a | ERBB4 | Up-down |
| hsa-miR-221 | FOXO3 | Up-down |
| hsa-miR-424 | PIAS1 | Up-down |
| hsa-miR-221 | PIK3R1 | Up-down |
| hsa-miR-29b | PIK3R1 | Up-down |
| hsa-miR-221 | TIMP3 | Up-down |
| hsa-miR-31 | YY1 | Up-down |
| hsa-miR-34a | YY1 | Up-down |
| hsa-miR-214 | ZBTB20 | Up-down |
Diff., differentially expressed; miRNA/miR, microRNA; Up, upregulated; down, downregulated.
Functional analysis of miRNA-targeted genes
The miRNA-targeted genes were significantly enriched into 14 Gene Ontology (GO) categories (Table II). The GO term gene expression (GO10468) was the most significantly enriched, and was associated with 8 miRNAs (miR-146a, miR-31, miR-34a, miR-221, miR-424, miR34b*, miR-214, miR-101) and 8 target genes [zinc finger and BTB domain containing 20 (ZBTB20), Erb-B2 receptor tyrosine kinase 4 (ERBB4), YY1 transcription factor (YY1), cAMP response element-binding protein (CREB1), BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like (BNIP3L), AT rich interactive domain 1A (ARID1A), protein inhibitor of activated STAT, 1 (PIAS1), forkhead box O3 (FOXO3)]. No significantly enriched Kyoto Encyclopedia of Genes and Genomes pathway was detected.
Table II.
Enriched GO terms of target genes.
| GO term ID | FDR | Description | Target genes |
|---|---|---|---|
| 10468 | 0.00895 | Gene expression | ZBTB20, ERBB4, YY1, CREB1, BNIP3L, ARID1A, PIAS1, FOXO3 |
| 19219 | 0.00973 | Nucleobase, nucleoside, nucleotide and nucleic acid metabolic process | ZBTB20, ERBB4, YY1, CREB1, AKAP12, ARID1A, PIAS1, FOXO3 |
| 48522 | 0.00115 | Positive regulation of cellular process | EGFR, ERBB4, CREB1, BNIP3L, AKAP12, ARID1A, PIAS1, FOXO3, PIK3R1 |
| 48518 | 0.00115 | Positive regulation of biological process | EGFR, ERBB4, CREB1, BNIP3L, AKAP12, ARID1A, PIAS1, FOXO3, PIK3R1 |
| 31326 | 0.00352 | Cellular biosynthetic process | EGFR, ZBTB20, ERBB4, YY1, CREB1, AKAP12, ARID1A, PIAS1, FOXO3 |
| 51171 | 0.00352 | Nitrogen compound metabolic process | EGFR, ZBTB20, ERBB4, YY1, CREB1, AKAP12, ARID1A, PIAS1, FOXO3 |
| 9889 | 0.00352 | Biosynthetic process | EGFR, ZBTB20, ERBB4, YY1, CREB1, AKAP12, ARID1A, PIAS1, FOXO3 |
| 60255 | 0.00179 | Macromolecule metabolic process | EGFR, ZBTB20, ERBB4, YY1, CREB1, BNIP3L, ARID1A, PIAS1, FOXO3, TIMP3 |
| 80090 | 0.00254 | Primary metabolic process | EGFR, ZBTB20, ERBB4, YY1, CREB1, AKAP12, ARID1A, PIAS1, FOXO3, TIMP3 |
| 31323 | 0.00292 | Cellular metabolic process | EGFR, ZBTB20, ERBB4, YY1, CREB1, AKAP12, ARID1A, PIAS1, FOXO3, TIMP3 |
| 19222 | 0.00115 | Metabolic process | EGFR, ZBTB20, ERBB4, YY1, CREB1, BNIP3L, AKAP12, ARID1A, PIAS1, FOXO3, TIMP3 |
| 50794 | 0.00292 | Regulation of cellular process | EGFR, ZBTB20, ERBB4, YY1, CREB1, BNIP3L, AKAP12, ARID1A, PIAS1, FOXO3, TIMP3, PIK3R1 |
| 50789 | 0.00351 | Regulation of biological process | EGFR, ZBTB20, ERBB4, YY1, CREB1, BNIP3L, AKAP12, ARID1A, PIAS1, FOXO3, TIMP3, PIK3R1 |
| 65007 | 0.00463 | Biological regulation | EGFR, ZBTB20, ERBB4, YY1, CREB1, BNIP3L, AKAP12, ARID1A, PIAS1, FOXO3, TIMP3, PIK3R1 |
FDR, false discovery rate; GO, gene ontology.
Discussion
miRNAs are negative regulators of gene expression, which regulate various biological processes via inhibition of their target gene expression. It is important to identify miRNA targets and elucidate their complex regulatory networks, in order to contribute to a systematic understanding of pathological changes in medical and clinical studies. AF is associated with alterations to several proteins, molecules and pathways. As a result, these changes result in disease progression, which may develop into heart failure. In the present study, a very strict criteria was adopted to identify miRNA targets. Briefly, the target genes had to be significantly differentially expressed, and predicted by both miRecords and TarBase. Consequently, 14 miRNA-mRNA pairs were identified to have a high probability of association with AF.
Compared with previous studies, the miRNAs selected in the present study included genes that have been reported to be involved in heart disease, including miR-221 (7,24) and miR-101 (25,26). In the present study, ARID1A gene expression was shown to be predominantly regulated by miR-101, which binds with the 3′-UTR of ARID1A, thus inducing ARID1A instability or translational inhibition. miR-101 and its target gene ARID1A participated in the most significant GO term (GO: 10468, regulation of gene expression) and therefore may be considered a specific biosignature of AF. In addition, several other miRNAs, including miR-146a, miR-31, miR-34a, miR-221, miR-424, miR34b* and miR-214, were identified to be strongly associated with AF. The regulation of miRNA-mRNA pairs differs in various diseases, resulting in disease-specific expression profiles that interact via a complex regulatory network. Although numerous methods have been used to identify regulatory miRNA-mRNA pairs in disease, no method is perfect or able to determine all miRNA-mRNA pairs.
The present study identified 8 target genes: ZBTB20, ERBB4, YY1, CREB1, BNIP3L, ARID1A, PIAS1 and FOXO3. It has previously been demonstrated that ErbB receptors (ErbB2, ErbB3, ErbB4) are overexpressed in the atrium, compared with the ventricle, and are downregulated in AF (27). It has therefore been hypothesized that downregulation of ErbB may contribute to the propensity for structural remodeling in AF and congestive heart failure. Furthermore, ZBTB20 (28), YY1 (29), BNIP3L (30), PIAS1 (31) and FOXO3 (32) have been suggested to be associated with AF. Notably, no previous study has reported a relationship between CREB1 expression and AF.
There are several limitations to the present study. Firstly, the miRNA and mRNA data of AF and normal tissues were obtained from different studies, which may affect the results. Furthermore, the results are based on pure public data and further experimental validation is required, such as target miRNA and mRNA knockdown experiments.
In conclusion, the miRNA-mRNA interactions identified in the present study may act as biosignatures for AF, and may provide evidence for the identification of a novel agent for the diagnosis and gene-targeted therapy of AF.
References
- 1.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators: A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:1935–1944. doi: 10.1016/j.jacc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Gilligan DM, Joyner CA, Bundy GM. Multidisciplinary collaboration for the treatment of atrial fibrillation: Convergent procedure outcomes from a single center. Innovation in CRM. 2013;4:1396–1403. [Google Scholar]
- 4.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Zhang Y, Wang N, Pan Z, Gao X, Zhang F, Zhang Y, Shan H, Luo X, Bai Y, et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation. 2010;122:2378–2387. doi: 10.1161/CIRCULATIONAHA.110.958967. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, Lin H, Xiao L, Maguy A, Qi XY, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123:1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CL, Lin JL, Lai LP, Pan CH, Huang SK, Lin CS. Altered expression of FHL1, CARP, TSC-22 and P311 provide insights into complex transcriptional regulation in pacing-induced atrial fibrillation. Biochim Biophys Acta. 2007;1772:317–29. doi: 10.1016/j.bbadis.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Girmatsion Z, Biliczki P, Bonauer A, Wimmer-Greinecker G, Scherer M, Moritz A, Bukowska A, Goette A, Nattel S, Hohnloser SH, Ehrlich JR. Changes in microRNA-1 expression and IK1 upregulation in human atrial fibrillation. Heart Rhythm. 2009;6:1802–1809. doi: 10.1016/j.hrthm.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kääb S, Hinterseer M, et al. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: Expression of a ventricular-like genomic signature. Circ Res. 2005;96:1022–1029. doi: 10.1161/01.RES.0000165480.82737.33. [DOI] [PubMed] [Google Scholar]
- 10.Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kääb S, Pfeufer A, et al. Functional profiling of human atrial and ventricular gene expression. Pflugers Arch. 2005;450:201–208. doi: 10.1007/s00424-005-1404-8. [DOI] [PubMed] [Google Scholar]
- 11.Cooley N, Cowley MJ, Lin RC, Marasco S, Wong C, Kaye DM, Dart AM, Woodcock EA. Influence of atrial fibrillation on microRNA expression profiles in left and right atria from patients with valvular heart disease. Physiol Genomics. 2012;44:211–219. doi: 10.1152/physiolgenomics.00111.2011. [DOI] [PubMed] [Google Scholar]
- 12.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S. A comprehensive evaluation of SAM, the SAM R-package and a simple modification to improve its performance. BMC Bioinformatics. 2007;8:230. doi: 10.1186/1471-2105-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawilowsky SS (2005): Misconceptions leading to choosing the t test over the Wilcoxon Mann-Whitney U test for shift in location parameter. Journal of Modern Applied Statistical Methods. 2005;4(2):598–600. [Google Scholar]
- 15.Shaffer JP. Multiple hypothesis testing. Annual Review of Psychology. 1995;46:561–584. doi: 10.1146/annurev.ps.46.020195.003021. [DOI] [Google Scholar]
- 16.Yeung KY, Ruzzo WL. Principal component analysis for clustering gene expression data. Bioinformatics. 2001;17:763–774. doi: 10.1093/bioinformatics/17.9.763. [DOI] [PubMed] [Google Scholar]
- 17.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. MiRecords: An integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009;37(Database issue):D105–D110. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethupathy P, Corda B, Hatzigeorgiou AG. TarBase: A comprehensive database of experimentally supported animal microRNA targets. RNA. 2006;12:192–197. doi: 10.1261/rna.2239606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng X, Li Y, Walters KA, Rosenzweig ER, Lederer SL, Aicher LD, Proll S, Katze MG. Computational identification of hepatitis C virus associated microRNA-mRNA regulatory modules in human livers. BMC Genomics. 2009;10:373. doi: 10.1186/1471-2164-10-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Brannon AR, Reddy AR, Alexe G, Seiler MW, Arreola A, Oza JH, Yao M, Juan D, Liou LS, et al. Identifying mRNA targets of microRNA dysregulated in cancer: With application to clear cell renal cell carcinoma. BMC Syst Biol. 2010;4:51. doi: 10.1186/1752-0509-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 24.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 25.Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, Lin H, Xiao L, Maguy A, Qi XY, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123:1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao PK, Kumar RM, Farkhondeh M, Baskerville S, Lodish HF. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci USA. 2006;103:8721–8726. doi: 10.1073/pnas.0602831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kääb S, Hinterseer M, et al. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: Expression of a ventricular-like genomic signature. Circ Res. 2005;96:1022–1029. doi: 10.1161/01.RES.0000165480.82737.33. [DOI] [PubMed] [Google Scholar]
- 28.Mao S, Huang S. Toll-like receptors signaling in glomerular diseases. J Recept Signal Transduct Res. 2014;34:81–84. doi: 10.3109/10799893.2013.864676. [DOI] [PubMed] [Google Scholar]
- 29.Kharlap MS, Timofeeva AV, Goryunova LE, Khaspekov GL, Dzemeshkevich SL, Ruskin VV, Akchurin RS, Golitsyn SP, Beabealashvilli RSh. Atrial appendage transcriptional profile in patients with atrial fibrillation with structural heart diseases. Ann N Y Acad Sci. 2006;1091:205–217. doi: 10.1196/annals.1378.067. [DOI] [PubMed] [Google Scholar]
- 30.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Role for IGFBP7 in senescence induction by BRAF. Cell. 2010;141:746–747. doi: 10.1016/j.cell.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson MD, Li QJ, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, Iñiguez-Lluhí JA, Martens JR. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc Natl Acad Sci USA. 2007;104:1805–1810. doi: 10.1073/pnas.0606702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi S, Tsirigos A, Amoroso A, Mascellani N, Rigoutsos I, Calin GA, Volinia S. OMiR: Identification of associations between OMIM diseases and microRNAs. Genomics. 2011;97:71–76. doi: 10.1016/j.ygeno.2010.10.004. [DOI] [PubMed] [Google Scholar]