Abstract
Psoriasis is a common chronic inflammatory and T cell-meditated skin disease. Runt-related transcription factor 3 (RUNX3), one of the runt-domain family of transcription factors, has been reported to be a susceptibility gene for psoriasis. The present study was designed to delineate the role and underlying mechanism of RUNX3 involved in the differentiation of T helper (Th) 17 and Th22 cells in psoriasis. The results of the present study demonstrated that the expression of RUNX3 increased significantly in CD4-positive (CD4+) T cells from patients with psoriasis, compared with healthy controls. In addition, increased levels of interleukin (IL)-6, IL-20 and IL-22, and increased frequencies of Th17 and Th22 cells were found in the patients with psoriasis patients, compared with the healthy controls. It was also found that the overexpression of RUNX3 increased the levels of Th17- and Th22-associated cytokines in the CD4+ T cells from the healthy controls. However, the inhibition of RUNX3 reduced the levels of the associated cytokines and decreased the frequency of Th17 and Th22 cells in the CD4+ T cells from the patients with psoriasis. Taken together, the present study suggested that RUNX3 regulated the differentiation of Th17 and Th22 cells in psoriasis, providing a promising therapeutic strategy for the treatment of psoriasis.
Keywords: psoriasis, runt-related transcription factor 3, T helper 17, T helper 22
Introduction
Psoriasis is a common chronic inflammatory skin disease with a prevalence of 0.2-3%, depending on the population of origin (1). It is well known that psoriasis is a T-cell mediated disease, with a disturbed balance in the interplay of keratinocytes and immune cells (2). The pathogenesis of CD4-positive (CD4+) T helper (Th) lymphocytes is important in psoriasis progression. It has been reported that interleukin (IL-22), as well as IL-17, are involved in the pathogenesis of psoriasis (3,4). These two cytokines can be secreted by Th17 cells. In addition, a previous study identified T cells, which produced IL-22 alone, without IL-17 or interferon γ (IFNγ), and these were subsequently termed as Th22 cells (5). Th17 and Th22 cells are affected by the cytokine, IL-23, which is also involved in psoriasis (6). A further study demonstrated that the frequencies of Th17 and Th22 cells were significantly elevated in patients with psoriasis. These studies demonstrated that Th17 and Th22 cells are important in the pathogenesis of psoriasis (7).
An increasing number of studies have demonstrated the vital role of genetics in the development of psoriasis, and >30 loci have been identified as susceptibility genes in psoriasis (8,9). In a genome-wide association study, runt-related transcription factor 3 (RUNX3) was identified as a genetic risk factor for psoriasis (10). In addition, a previous study demonstrated that the expression of RUNX3 was increased in patients with psoriasis patients, confirming this result (11). RUNX3 is one of the runt-domain family of transcription factors, which has been reported to be a tumor suppressor gene, and regulates cell proliferation and apoptosis in several types of human tumor (12). RUNX3 is commonly expressed in the spleen, peripheral blood, spinal cord cells, bone marrow, B cells and T cells (13). The inactivation of RUNX3 is considered to be associated with the occurrence and development of various human diseases, including gastric and colon cancer (14,15) and other epithelial diseases (16). RUNX3 is important in the function of the immune system. Studies have reported that RUNX3-deficient mice spontaneously develop two immunological abnormalities: Airway hypersensitivity and colitis (17,18). Following RUNX3 knockdown in T cells, the target mice spontaneously developed asthma-like features, including elevated levels of IgA, IgE and IgG1, and the infiltration of eosinophils and lymphocytes in the lung (19). Furthermore, RUNX3 is important in the differentiation of T cells (20). However, its role and underlying mechanism in the differentiation of Th17 and Th22 cells in psoriasis have not been investigated in detail.
In the present study, the expression level of RUNX3 and the frequencies of Th17 and Th22 cells in psoriasis were determined. In particular, the role of RUNX3 in regulating the differentiation of CD4+ T cells and the underlying mechanism in psoriasis were investigated. By the forced overexpression and knockdown of RUNX3, the present study determined the importance of RUNX3 in psoriasis through examining its effect on regulating the levels of Th17 and Th22 cells. Taken together, the results of the present study may identify a novel therapeutic target and provide a foundation for gene therapy in psoriasis.
Materials and methods
Patients
The study cohort in the present study comprised 32 patients with psoriasis (20 males and 12 females), as well as 30 healthy control individuals (18 males and 12 females). The age of the patients with psoriasis was between 20 and 48 years the healthy controls, between 22 and 50 years old. The patients were recruited from the Dermatological department of the First Affiliated Hospital, Xinxiang Medical University (Weihui, China) from July 2013 to June 2014. All patients were diagnosed by clinical features and disease activity was assessed by Psoriasis Area and Severity Index (21), patients were excluded if they had any of the following conditions: Diabetes mellitus; renal failure; history of neoplasm; major cardiovascular and cerebrovascular disease. The healthy controls were recruited as volunteers at the First Affiliated Hospital and Xinxiang Medical University (Weihui, China. The study was approved by the ethics committee of Xinxiang Medical University (approval no. 2014055) and written informed consent was provided by all participants.
Specimen collection
Fasting venous peripheral blood samples (10 ml) were collected from the patients with psoriasis and the healthy control individuals. Following centrifugation for 15 min at 335.4 × g and 4°C, the serum samples were stored at −80°C until further use. CD4+ T cells were isolated from each blood sample using a human CD4+ positive selection magnetic column (Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany), according to the manufacturer's protocol. The absolute counts of the CD4+ T cells were measured using flow cytometry. The purity of the CD4+ T cells was typically >90, according to the protocols, and the cells were cultured in human T cell culture AIM V Medium CTS at a density of 105 at 5% CO2 and 37°C (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were grown to 80% confluence.
RNA extraction and reverse transcription-quantitative polymerase chain reaction analysis (RT-qPCR)
Total RNA was extracted from the cultured CD4+ T cells using TRIzol reagent and chloroform (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols and the following temperature protocol: 30°C for 10 min and 42°C for 30 min. The concentration and purity of the total RNA were detected using an ultraviolet (UV) spectrophotometer (BioSpectrometer; Eppendorf AG, Hamburg, Germany). cDNA was synthesized using M-MLV reverse transcriptase (Clontech Laboratories, Palo Alto, CA, USA). The mRNA was analyzed using an RT-qPCR mixture system containing cDNA templates, primers synthesized by Shanghai Sangon Biological Engineering and Technology & Services Co., Ltd. (Shanghai, China), and Absolute SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Inc.). The PCR primers (Shanghai Sangon Biological Engineering and Technology & Services Co., Ltd., Shanghai, China) used were as follows: RUNX3 F 5′-ACCTGTCACAACGGCCAGAAC-3′,R 5′-TTCCAGTGAGGACAGGCCAAG-3′; β-actin F 5′-GGCGGCACCACCATGTACCCT-3′, R 5′-AGGGGCCGGACTCGTCATACT-3′. PCR cycling conditions were as follows: Enzyme activation 95°C 15 min per cycle, denaturation 95°C at 15 sec per 40 cycles and annealing/extension at 60°C for 60 sec using a LightCycler sequence detection system (480; Roche Diagnostics, Indianapolis, IN, USA). The gene mRNA expression levels were normalized to those of β-actin. Data were analyzed using the 2−ΔΔCq method (22). This experiment was performed three times.
Western blot analysis
Total protein was extracted from the CD4+ T cells using a radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Inc.) and quantified using a UV spectrophotometer. A total of 25 μg protein per lane separated by 10% sodium dodecyl sulfate-polyacrylamide gel (Thermo Fisher Scientific, Inc.) electrophoresis. Following being transferred onto nitrocellulose membranes (Thermo Fisher Scientific, Inc.) and blocked in Tris-buffered saline-Tween buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) containing 5% nonfat dry milk for 30 min, the membranes were incubated for 16 h at 4°C with polyclonal rabbit anti-RUNX3 (cat. no. sc-30197; 1:1,000) or polyclonal rabbit anti-β-actin antibodies (cat. no. sc-7210; 1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by incubation with the corresponding horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (cat. no. sc-2004; 1:1,000; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature and washed twice with phosphate-buffered saline. A fluorescent Western blotting detection system (Thermo Fisher Scientific, Inc.) was used. Densitometry analysis was performed using MultiGauge 3.1 software (Thermo Fisher Scientific, Inc.). The protein expression levels were normalized to those of β-actin.
Flow cytometric analysis
The percentages of Th17 and Th22 in CD4+ T cells were detected using flow cytometric analysis, according to the manufacturer's protocols. Briefly, the cells at a density of 105 were incubated with phorbol ester, ionomycin and monensin (all Sigma-Aldrich, St. Louis, MO, USA) for 4 h at 37°C. The cells were then stained with fluorescein isothiocyanate (FITC)-labeled mouse anti-human CD4 (cat. no. 550628; 1:100; BD Biosciences, San Diego, CA, USA), phycoerythrin (PE)-labeled mouse monoclonal anti-IL-17 (cat. no. 12-7178; 1:100; eBioscience, Inc., San Diego, CA, USA), and PE-labeled monoclonal mouse anti-IL-22 (cat. no. 12-7229-41; 1:100; eBioscience, Inc.), respectively, for 30 min at room temperature. The results were analyzed using CellQuest Pro 5.1 software (BD Biosciences, Franklin Lakes, NJ, USA).
CD4+ T cell transfection
For the transfection of the CD4+ T cells, RUNX3 small interfering (si)RNA was purchased from GenePharma (Shanghai, China), and lentiviral vectors containing the RUNX3 gene were constructed (GeneChem Co., Ltd., Shanghai, China). The CD4+ T cells were seeded at 106 cells/well transfected with the RUNX3 lentiviral plasmid, empty lentiviral vector, RUNX3 siRNA and siRNA control, respectively, according to the instructions provided with the Lipofectamine RNAiMAX reagent (Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h of transfection at room temperature, the cells were collected for further analysis.
Enzyme-linked immunosorbent assay (ELISA)
The cytokine concentrations were measured using corresponding quantification ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer's protocols. Optical density values (OD values) were read at 450 nm using a microplate reader (SynergyH1; BioTek, Winooski, VT, USA) following the assay. The concentrations of cytokines were calculated, according to the corresponding OD value and concentration of the standard substance.
Statistical analysis
All data were processed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). The results are expressed as the mean ± standard deviation. Statistical analysis was calculated using one-way analysis of variance followed by a Bonferroni test. P≤0.05 were considered to indicate a statistically significant difference.
Results
Expression of RUNX3 is increased in CD4+ T cells from patients with psoriasis
To determine the expression level of RUNX3, CD4+ T cells were isolated from blood samples from each patient with psoriasis and control subject. The mRNA expression of RUNX3 was measured using RT-qPCR. As shown in Fig. 1A, the mRNA expression level of RUNX3 was significantly increased in the patients with psoriasis patients, compared with the healthy controls. Western blot analysis showed that the protein level of RUNX3 was also significantly elevated in the patients with psoriasis, compared with the healthy controls (Fig. 1B). These results revealed a notable upregulation of RUNX3 in CD4+ T cells from patients with psoriasis, which is relevant to psoriasis development.
Figure 1.
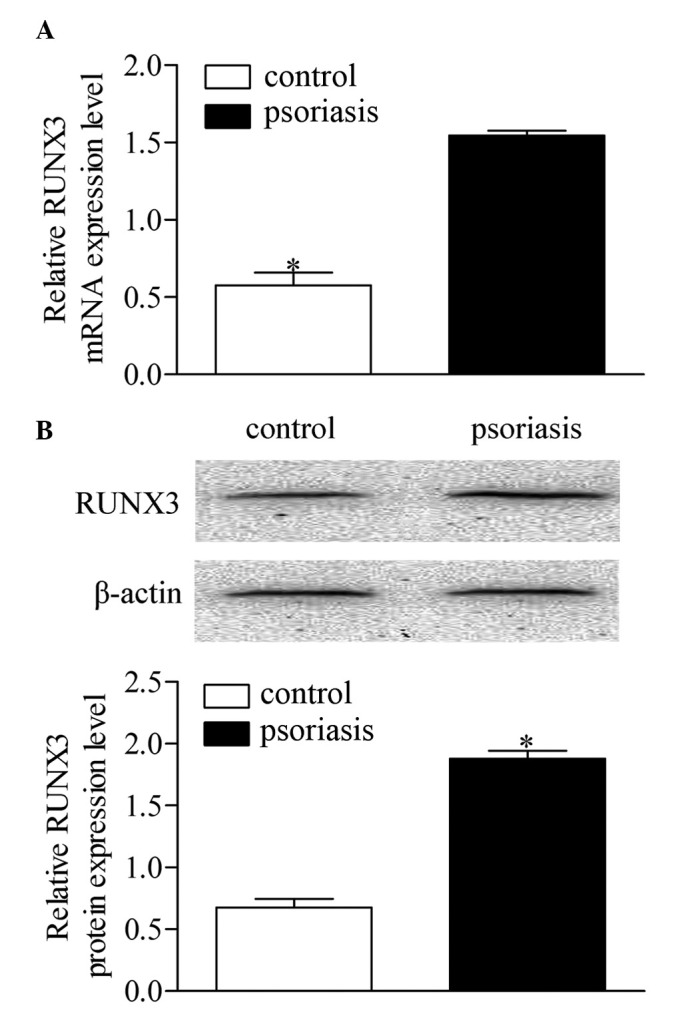
Expression of RUNX3 in CD4+ T cells from patients with psoriasis and healthy controls. (A) mRNA expression levels of RUNX3 were measured using reverse transcription-quantitative polymerase chain reaction analysis. (B) Protein expression levels of RUNX3 were measured using Western blot analysis. Data are presented as the mean ± standard deviation of three experiments. *P<0.05, vs. control group. RUNX3, runt-related transcription factor 3.
Th17 and Th22 cells are dysregulated in patients with psoriasis
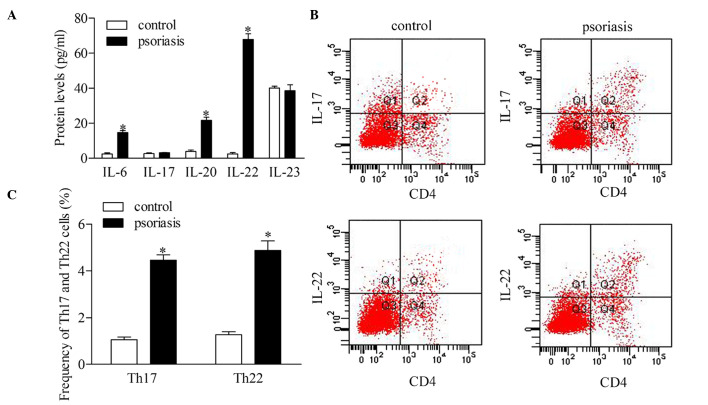
To investigate the roles of Th17 and Th22 cells, the present study detected the serum levels of IL-6, IL-17, IL-20, IL-22 and IL-23 in the supernatant of CD4 T cells of patients with psoriasis. The statistical analyses revealed that the protein expression levels of IL-6, IL-20 and IL-22 were significantly higher in the patients with psoriasis, compared with the healthy controls. However, no significant differences were found in the concentrations of IL-17 and IL-23 between the psoriatic patients and control group (Fig. 2A). To further confirm the dysregulation of Th17 and Th22 cells, the present study evaluated the frequency of Th17 and Th22 cells using flow cytometric analysis (Fig. 2B and C). The results showed that the frequencies of the Th17 and Th22 cells increased significantly in the patients with psoriasis, compared with the healthy controls.
Figure 2.
Comparison of Th17 and Th22 in CD4+ T cells from patients with psoriasis and healthy controls. (A) Expression levels of Th17 and Th22-associated cytokines were measured using an enzyme-linked immunosorbent assay. (B) Frequencies of Th17 and Th22 cells were detected using flow cytometry. (C) Percentages of Th17 and Th22 cells in patients with psoriasis and healthy controls. Data are presented as the mean ± standard deviation of three experiments. *P<0.05, vs. control group. Th, T helper; IL, interleukin.
RUNX3 overexpression increases concentrations of cytokines associated with Th17 and Th22 in normal CD4+ T cells
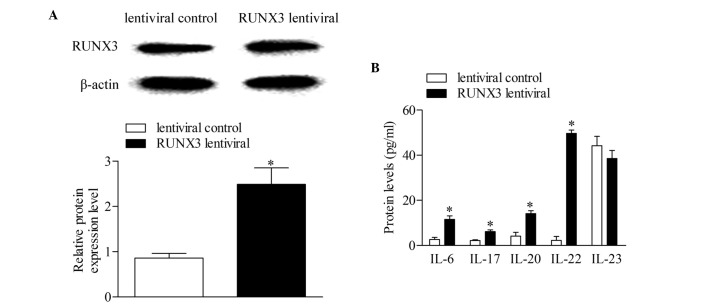
The RUNX3 lentiviral plasmid and empty lentiviral vector were transfected into CD4+ T cells from healthy controls to investigate the effect of RUNX3 overexpression in normal CD4+ T cells. Following 48 h of transfection, the expression level of RUNX3 was detected using Western blot analysis. The results showed that the expression of RUNX3 increased significantly in the CD4+ T cells transfected with the RUNX3 lentiviral plasmid, compared with the empty lentiviral vector-transfected control group (Fig. 3A). In addition, the concentrations of IL-6, IL-17, IL-20 and IL-22 increased significantly with RUNX3 overexpression, compared with the control group. No significant differences were observed in the concentrations of IL-23 between groups (Fig. 3B).
Figure 3.
Overexpression of RUNX3 regulates cytokines associated with Th17 and Th22 in CD4+ T cells from healthy controls. (A) Expression levels of RUNX3 were measured using Western blot analysis. (B) Expression levels of Th17 and Th22-associated cytokines was measured using an enzyme-linked immunosorbent assay. Data are presented as the mean ± standard deviation of three experiments. *P<0.05, vs. control group. RUNX3, runt-related transcription factor 3; Th, T helper; IL, interleukin.
RUNX3 knockdown inhibits the production of cytokines associated with Th17 and Th22 in CD4+ T cells from patients with psoriasis
The RUNX3 siRNA and siRNA control were transfected into CD4+ T cells from patients with psoriasis to detect whether the inhibition of RUNX3 can reverse immune dysfunction. The results of the Western blot analysis showed that the expression of RUNX3 decreased significantly in the CD4+ T cells transfected with RUNX3 siRNA, compared with the control group (Fig. 4A). Additionally, the concentrations of IL-6, IL-20 and IL-22 decreased significantly following RUNX3 siRNA transfection, compared with the control group. No significant differences were observed in the concentrations of IL-17 and IL-23 between groups (Fig. 4B).
Figure 4.
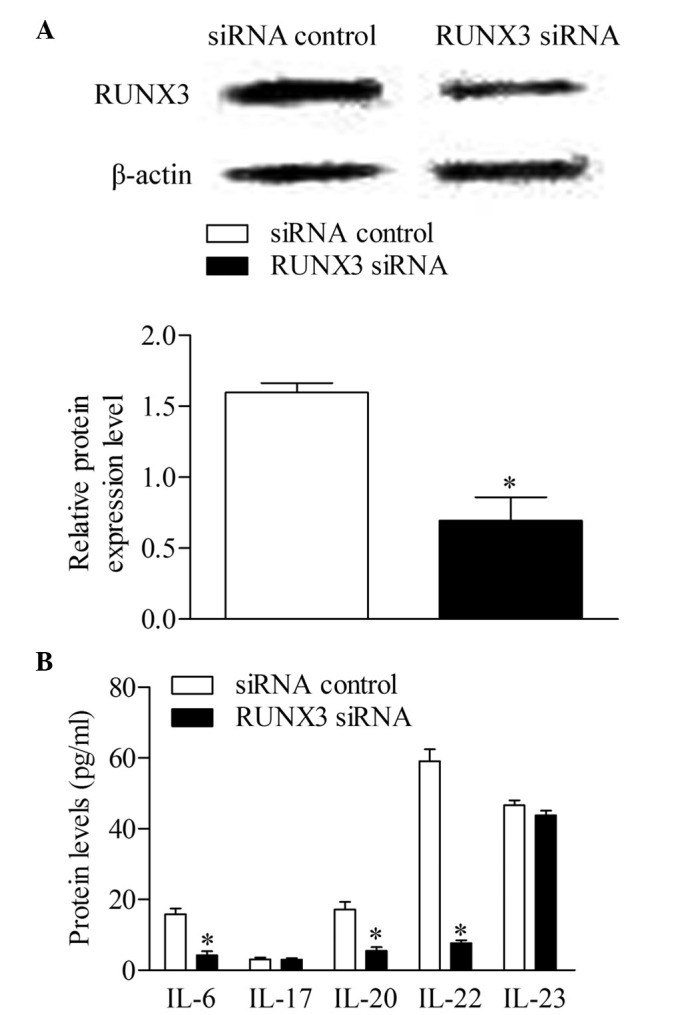
Inhibition of RUNX3 regulates cytokines associated with Th17 and Th22 in CD4+ T cells from patients with psoriasis. (A) Expression levels of RUNX3 were measured using Western blot analysis. (B) Expression levels of Th17 and Th22-associated cytokines were measured using an enzyme-linked immunosorbent assay, Data are presented as the mean ± standard deviation of three experiments. *P<0.05, vs. control group. RUNX3, runt-related transcription factor 3; Th, T helper; IL, interleukin; siRNA small interfering RNA.
RUNX3 knockdown represses the frequency of Th17 and Th22 cells in CD4+ T cells from patients with psoriasis
To further investigate the effect of RUNX3 inhibition on Th17 and Th22 cells in CD4+ T cells, the present study detected the frequencies of Th17 and Th22 cells from patients with psoriasis transfected with RUNX3 siRNA and the siRNA control using flow cytometry. The results showed that the frequencies of Th17 and Th22 cells in the RUXN3 siRNA transfection group were decreased significantly, compared with those in the control group (Fig. 5).
Figure 5.
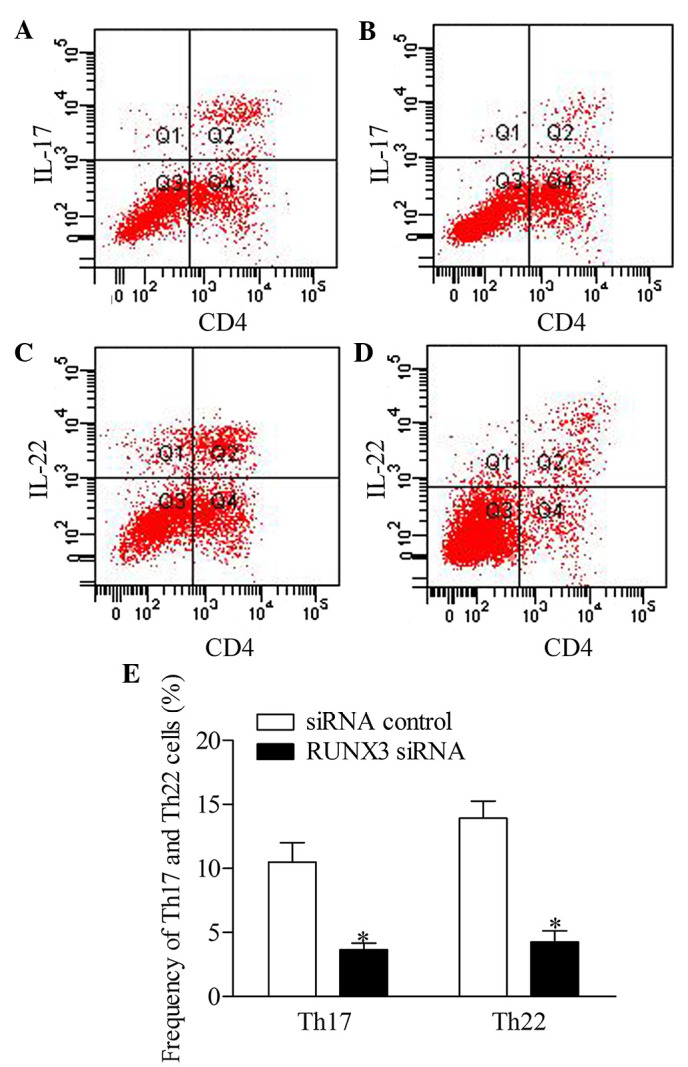
Inhibition of RUNX3 regulates the frequencies of Th17 and Th22 in CD4+ T cells from patients with psoriasis. The frequencies of Th17 and Th22 cells in CD4+ T cells from patients with psoriasis transfected with RUNX3 siRNA or an siRNA control was detected using flow cytometry. (A and B) Flow cytometric analysis of Th17 in CD4+ T cells from patients with psoriasis. The percentages of cells in the Q2 region represent the percentage of Th17 cells. (C and D) Flow cytometric analysis of Th22 in CD4+ T cells from patients with psoriasis. The percentages of cells in the Q2 region represent the percentage of Th22 cells. (E) Percentages of Th17 and Th22 cells in CD4+ T cells from patients with psoriasis transfected with RUNX3 siRNA or an siRNA control. Data are presented as the mean ± standard deviation of three experiments. *P<0.05, vs. control group. RUNX3, runt-related transcription factor 3; Th, T helper; IL, interleukin; siRNA, small interfering RNA.
Discussion
Psoriasis is well known as a T cell-dependent autoimmune disease of the joints and skin (23). Studies have suggested that there is a genetic contribution, and >30 risk loci for psoriasis have been identified (9). Genetic factors explain 68% of the variation in the susceptibility to psoriasis (24). Tsoi et al demonstrated that RUNX3 may be a susceptibility gene for psoriasis (10). In addition, Apel et al further confirmed that RUNX3 is a candidate for involvement in psoriasis and other T cell-mediated diseases (11). The present study showed that the expression level of RUNX3 increased significantly in CD4+ T cells from patients with psoriasis, compared with the healthy controls, which confirmed that RUNX3 is a susceptibility gene for psoriasis and was consistent with the previous reports.
CD4+ T cells are the most important element of adaptive systems and immune homeostasis. According to the local cytokine environment and the nature of the encountered stimulus itself, naïve CD4+ T cells are differentiated into various subsets of Th or regulatory T cells (25). One of the T cell subsets, Th17 cells, is known for producing IL-17, IL-6, IL-21, IL-22 and tumor necrosis factor (TNF) (26). Th17 cells are differentiated from naïve CD4+ T cells under the effect of the transforming growth factor β (TGF-β), IL-6 and IL-1, whereas their expansion and survival are controlled predominantly by IL-23 (27,28). Previous investigation has shown that Th17 cells can be generated by IL-6, IL-23 and IL-1β in the absence of TGF-β (29). It has been reported that cytokines produced by Th17 cells initiate parakeratosis and acanthosis hyperkeratosis (30). Th17 cells have been demonstrated to be involved in the development of T cell migration and activation, neutrophil and monocyte chemotaxis, and neovascularization (31).
Th17 cells were originally considered to be the predominant source in the production of the IL-22 cytokine, however studies have shown that certain CD4+ T cells secrete IL-22 alone, without IL-17, and these have subsequently been termed Th22 cells (32). Th22 cells also produce cytokines, including IL-26 and IL-13. Studies have demonstrated that TNF-α and IL-6 can promote the Th22 phenotype, with the assistance of plasmacytoid dendritic cells (33,34). The levels of Th17 and Th22 cells have been shown to be abnormal in patients with psoriasis (7). In the present study, it was found that the serum levels of IL-6, IL-20 and IL-22 were elevated in patients with psoriasis patients, compared with healthy control individuals. It is widely known that IL-6 contributes to the inflammatory and autoimmune processes of Th17 cells (31), and elevated serum levels of IL-6 have been observed in patients with psoriasis in numerous studies (35,36). IL-20 is produced by keratinocytes in the presence of IL-22, IL-17 and TNF-α, but not IFN-γ or IL-20 itself (37). The effect of IL-20 often appears in the later phases of psoriasis pathogenesis (38), and increased levels of IL-20 have been noted in lesional skin, as well as in the blood, in patients with psoriasis (39). In addition, the present study found that the frequencies of Th17 and Th22 cells were elevated in the patients with psoriasis, compared with the healthy controls. No significantly differences were found in the concentrations of IL-17 and IL-23 between the patients with psoriasis and control group. This confirmed the involvement of Th17 and Th22 cells in the pathogenesis of psoriasis, and the increased level of IL-22 without elevated IL-17 level suggested that Th22 cells are more important in the inflammatory process.
As has been previously reported, RUNX3 is important in the differentiation of T cells (20). Thus, the present study investigated the potential role and mechanism of RUNX3 in regulating Th17 and Th22 cells in psoriasis. The effect of RUNX3 overexpression on the expression levels of Th17 and Th22 cell-associated cytokines was compared with normal CD4+ T cells. The results showed that the levels of IL-6, IL-17, IL-20 and IL-22 increased significantly due to the overexpression of RUNX3, similar to the physiological features in psoriasis. Currently, the RUNX3 gene has become important in potential therapeutic targets for psoriasis. Liang et al (40) demonstrated that signaling transducer and activator of transcriptions 4, which is a central mediators in the generation of inflammation during protective immune responses and immune-mediated diseases, may be an effective therapeutic target for autoimmune diseases, including psoriasis (40). It has been reported that RUNX3 is a therapeutic target for gastric cancer and can be involved in a various human diseases, including psoriasis, according to its function in immune dysfunction (41). In the present study, the effect of RUNX3 knockdown was detected in CD4+ T cells from patients with psoriasis. The results showed that the inhibition of RUNX3 suppressed the production of IL-6, IL-17, IL-20 and IL-22. In addition, the frequencies of Th17 and Th22 cells decreased significantly following transfection with RUNX3 siRNA. These results suggested that the inhibition of RUNX3 regulated Th17 and Th22 cells, leading to an improvement in immune dysfunction in psoriasis.
In conclusion, the present study demonstrated that RUNX3 was upregulated in CD4+ T cells from patients with psoriasis. In addition, the importance of Th17 and Th22 cells in psoriasis was confirmed. Notably, the present study found that the inhibition of RUNX3 affected the differentiation of CD4+ T cells, which downregulated the frequencies of Th17 and Th22 cells in psoriasis. Consequently, targeting RUNX3 may support a promising therapeutic strategy for the treatment of psoriasis.
Acknowledgments
This study was supported by the Science and Technology Reasearch Key Project of The Education Department, Henan province (grant no. 13A320852) and the Science and Technology Research Plan in Xinxiang City (grant no. ZG13022).
Abbreviations
- Ps
psoriasis
- Th
T helper
- IL
interleukin
- IFNγ
interferon γ
- RUNX3
runt-related transcription factor 3
References
- 1.Sales R, Torres T. Psoriasis and metabolic syndrome. Acta Dermatovenerol Croat. 2014;22:169–174. [PubMed] [Google Scholar]
- 2.Diani M, Altomare G, Reali E. T cell responses in psoriasis and psoriatic arthritis. Autoimmun Rev. 2015;14:286–292. doi: 10.1016/j.autrev.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, Skapenko A. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 4.Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G, Yssel H, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E. IL-22-producing ῾T22᾽ T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252.e2. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauli A, Mathieu A. Psoriatic arthritis: Genetics and pathogenesis. Reumatismo. 2012;64:71–78. doi: 10.4081/reumatismo.2012.71. [DOI] [PubMed] [Google Scholar]
- 7.Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, Smith M, Thomas R, Gaston H. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther. 2013;15:R136. doi: 10.1186/ar4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang H, Jin X, Li Y, Jiang H, Tang X, Yang X, Cheng H, Qiu Y, Chen G, Mei J, et al. A large-scale screen for coding variants predisposing to psoriasis. Nat Genet. 2014;46:45–50. doi: 10.1038/ng.2827. [DOI] [PubMed] [Google Scholar]
- 9.Garber K. Genetics: Deep exploration. Nature. 2012;492(Suppl):S56–S57. doi: 10.1038/492S56a. [DOI] [PubMed] [Google Scholar]
- 10.Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apel M, Uebe S, Bowes J, Giardina E, Korendowych E, Juneblad K, Pasutto F, Ekici AB, McManus R, Ho P, et al. Variants in RUNX3 contribute to susceptibility to psoriatic arthritis, exhibiting further common ground with ankylosing spondylitis. Arthritis Rheum. 2013;65:1224–1231. doi: 10.1002/art.37885. [DOI] [PubMed] [Google Scholar]
- 12.Chen HX, Wang S, Wang Z, Zhang ZP, Shi SS. Overexpression of RUNX3 inhibits malignant behaviour of Eca109 cells in vitro and vivo. Asian Pac J Cancer Prev. 2014;15:1531–1537. doi: 10.7314/APJCP.2014.15.4.1531. [DOI] [PubMed] [Google Scholar]
- 13.Yagi R, Junttila IS, Wei G, Urban JF, Jr, Zhao K, Paul WE, Zhu J. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010;32:507–517. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blyth K, Cameron ER, Neil JC. The RUNX genes: Gain or loss of function in cancer. Nat Rev Cancer. 2005;5:376–387. doi: 10.1038/nrc1607. [DOI] [PubMed] [Google Scholar]
- 15.He SY, Jiang RF, Jiang J, Xiang YS, Wang L. Investigation of methylation and protein expression of the gene in colon carcinogenesis. Biomed Rep. 2015;3:687–690. doi: 10.3892/br.2015.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H, et al. RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell. 2008;14:226–237. doi: 10.1016/j.ccr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Brenner O, Levanon D, Negreanu V, Golubkov O, Fainaru O, Woolf E, Groner Y. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc Natl Acad Sci USA. 2004;101:16016–16021. doi: 10.1073/pnas.0407180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fainaru O, Woolf E, Lotem J, Yarmus M, Brenner O, Goldenberg D, Negreanu V, Bernstein Y, Levanon D, Jung S, Groner Y. Runx3 regulates mouse TGF-beta-mediated dendritic cell function and its absence results in airway inflammation. EMBO J. 2004;23:969–979. doi: 10.1038/sj.emboj.7600085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naoe Y, Setoguchi R, Akiyama K, Muroi S, Kuroda M, Hatam F, Littman DR, Taniuchi I. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djuretic IM, Cruz-Guilloty F, Rao A. Regulation of gene expression in peripheral T cells by Runx transcription factors. Adv Immunol. 2009;104:1–23. doi: 10.1016/S0065-2776(08)04001-7. [DOI] [PubMed] [Google Scholar]
- 21.Oji V, Luger TA. The skin in psoriasis: Assessment and challenges. Clin Exp Rheumatol. 2015;93(Suppl 5):S14–19. [PubMed] [Google Scholar]
- 22.Wang G, Wang L, Sun S, Wu J, Wang Q. Quantitative measurement of serum microRNA-21 expression in relation to breast cancer metastasis in Chinese females. Ann Lab Med. 2015;35:226–232. doi: 10.3343/alm.2015.35.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghoreschi K, Weigert C, Röcken M. Immunopathogenesis and role of T cells in psoriasis. Clin Dermatol. 2007;25:574–580. doi: 10.1016/j.clindermatol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Lonnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Heritability of psoriasis in a large twin sample. Br J Dermatol. 2013;169:412–416. doi: 10.1111/bjd.12375. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11:625–630. doi: 10.1016/j.micinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg WB, McInnes IB. Th17 cells and IL-17 a-focus on immunopathogenesis and immunotherapeutics. Semin Arthritis Rheum. 2013;43:158–170. doi: 10.1016/j.semarthrit.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peck A, Mellins ED. Breaking old paradigms: Th17 cells in autoimmune arthritis. Clin Immunol. 2009;132:295–304. doi: 10.1016/j.clim.2009.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpe E, Battistini L, Borsellino G. Advances in T helper 17 cell biology: Pathogenic role and potential therapy in multiple sclerosis. Mediators Inflamm. 2015;2015:475158. doi: 10.1155/2015/475158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asarch A, Barak O, Loo DS, Gottlieb AB. Th17 cells: A new paradigm for cutaneous inflammation. J Dermatolog Treat. 2008;19:259–266. doi: 10.1080/09546630802206686. [DOI] [PubMed] [Google Scholar]
- 32.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Yang B, Zhou M, Li L, Zhou H, Zhang J, Chen H, Wu C. Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans. Eur J Immunol. 2009;39:1472–1479. doi: 10.1002/eji.200838811. [DOI] [PubMed] [Google Scholar]
- 34.N, Pan HF, Ye DQ. Th22 in inflammatory and autoimmune disease: Prospects for therapeutic intervention. Mol Cell Biochem. 2011;353:41–46. doi: 10.1007/s11010-011-0772-y. [DOI] [PubMed] [Google Scholar]
- 35.Kaur S, Zilmer K, Leping V, Zilmer M. Comparative study of systemic inflammatory responses in psoriasis vulgaris and mild to moderate allergic contact dermatitis. Dermatology. 2012;225:54–61. doi: 10.1159/000339866. [DOI] [PubMed] [Google Scholar]
- 36.Chandran V. Soluble biomarkers may differentiate psoriasis from psoriatic arthritis. J Rheumatol Suppl. 2012;89:65–66. doi: 10.3899/jrheum.120247. [DOI] [PubMed] [Google Scholar]
- 37.Sabat R, Wolk K. Research in practice: IL-22 and IL-20: Significance for epithelial homeostasis and psoriasis pathogenesis. J Dtsch Dermatol Ges. 2011;9:518–523. doi: 10.1111/j.1610-0387.2011.07611.x. English, German. [DOI] [PubMed] [Google Scholar]
- 38.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 39.Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, Kunz S, Döcke WD, Asadullah K, Volk HD, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: A novel immunological cascade with potential relevance in psoriasis. Eur J Immunol. 2009;39:3570–3581. doi: 10.1002/eji.200939687. [DOI] [PubMed] [Google Scholar]
- 40.Liang Y, Pan HF, Ye DQ. Therapeutic potential of STAT4 in autoimmunity. Expert Opin Ther Targets. 2014;18:945–960. doi: 10.1517/14728222.2014.920325. [DOI] [PubMed] [Google Scholar]
- 41.Subramaniam MM, Chan JY, Yeoh KG, Quek T, Ito K, Salto-Tellez M. Molecular pathology of RUNX3 in human carcinogenesis. Biochim Biophys Acta. 2009;1796:315–331. doi: 10.1016/j.bbcan.2009.07.004. [DOI] [PubMed] [Google Scholar]