Abstract
Aldosterone (ALD) is a well-known hormone, which may initiate renal injury by inducing mesangial cell (MC) injury in chronic kidney disease (CKD); however, the molecular mechanism remains unknown. The aim of the present study was to investigate the effects of p53 on ALD-induced MC apoptosis and elucidate the underlying molecular mechanism. For the in vivo studies, rats were randomly assigned to receive normal saline or ALD for 4 weeks. The ratio of MC apoptosis was analysed by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay. In addition, the expression level and localisation of p53, a well-known cell apoptosis-associated key protein, were detected by immunofluorescence. For the in vitro studies, rat MCs were incubated in medium containing either buffer (control) or ALD (10−6 M) for 24 h. The cell apoptosis ratio was assessed by flow cytometry, and the expression level of p53 was assessed by reverse transcription quantitative polymerase chain reaction and western blotting. In order to confirm the role of p53 in ALD-regulated cell apoptosis, a rescue experiment was performed using targeted small interfering (si)RNA to downregulate the expression of p53. The ALD-treated rats exhibited greater numbers of TUNEL-positive MCs and higher expression levels of p53 when compared with the control group. Furthermore, the ratio of MC apoptosis and the p53 expression level were significantly increased following ALD exposure, compared with the control group. Additionally, in the rescue experiment, the effects of ALD on MC were blocked by downregulating the expression level of p53 in MCs. The present study hypothesized that ALD may directly contribute to the occurrence of MC apoptosis via p53, which may participate in ALD-induced renal injury.
Keywords: aldosterone, mesangial cell, apoptosis, p53, injury
Introduction
Chronic kidney disease (CKD) has become a worldwide public health issue due to increased morbidity, premature mortality and the associated health care costs (1). Globally, the mortality rate induced by kidney disease has risen by 83% since 1990 (2), exerting a great pressure on the health care system. Recently, clinical and experimental reports confirmed that aldosterone (ALD) may initiate the development and progression of chronic renal injury, in addition to the classical effects on sodium and potassium transport in the renal tubules (3–6). Furthermore, this mineralocorticoid receptor has been localized to preglomerular vasculature, mesangial cells (MCs) and fibroblasts, as well as distal tubular cells of the nephron in the rat kidney (7), suggesting a possible direct role of this mineralocorticoid hormone in kidney damage. For example, infusion of ALD induces glomerulosclerosis and tubulointerstitial fibrosis in rats (8,9). Similarly, in hypertensive remnant kidney rats, ALD infusion reverses the renoprotective effects of angiotensin-converting enzyme inhibitors (4). ALD also shows a direct deleterious influence on kidney cells, such as MCs, podocytes, proximal tubular epithelial cells and fibroblasts (10–13). Particularly, spironolactone, an ALD receptor antagonist, inhibits ALD-induced MC apoptosis (14).
The underlying mechanism of ALD-induced cell apoptosis has been increasingly investigated. Nagase and Fujita (15) reported that ALD evoked glomerular podocyte injury via oxidative stress and serum/glucocorticoid regulated kinase 1 upregulation. Additionally, evidence from animal studies indicated that renal injury in ALD-infused rats is associated with increases in NADPH expression and reactive oxygen species levels (8). There are also other signalling pathways, such as the Wnt/wingless signalling pathway (16), and the p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase (PI3K)/Akt signalling pathways (17). However, the precise mechanism for ALD-induced renal injury is not well understood.
MCs are crucial in maintaining the structural integrity of the glomerular microvascular bed, providing mesangial matrix homeostasis and modulating glomerular filtration (18). They are considered to be one of the main target cells of various pathogenic factors, including high glucose levels, high pressure and high serum levels of transforming growth factor-β and platelet-derived growth factor (19). Previous studies show that apoptosis occurs during various types of chronic kidney disease, including diabetic nephropathy, immunoglobulin (Ig)A nephropathy and lupus nephritis (20–22). Previously, it was demonstrated that MC apoptosis induces an increased severity of albuminuria in mice (23) and MC apoptosis is directly involved in the pathogenesis of progressive glomerulosclerosis (24), which may eventually progresses to end-stage renal disease. Furthermore, MC loss is also observed in the late stage of diabetic nephropathy and apoptosis is hypothesized to be involved (20). These findings indicate that MC apoptosis is crucial for the development of renal injury.
Cell apoptosis is regulated by multiple genes, among which p53 has been the most widely investigated and has been shown to have an important role in regulating apoptosis. As a tumour suppressor gene, p53 acts as an apoptosis regulator by modulating cell apoptosis via control of the G1 arrest checkpoint of the cell cycle (25). Accumulating evidence has indicated that p53 is critical for the process of cell apoptosis, and p53 may be an upstream regulator of the PI3K/Akt signalling pathway.
To the best of our knowledge, there are few studies regarding the association between ALD and the upstream regulator of the PI3K/Akt signalling pathway, and the underlying mechanism for ALD-induced kidney injury has not been fully elucidated. Thus, the aim of the present study was to evaluate the potential association between ALD and p53. Furthermore, the current study may provide a basis for clinical investigations into the underlying mechanism of ALD-induced renal injury.
Materials and methods
Materials
Rat MCs were obtained from The Chinese Center for Type Culture Collection (Wuhan, China) and ALD was obtained from Sigma-Aldrich (St. Louis, MO, USA). Gibco fetal bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM) and SYBR® Green Mix were supplied by (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Penicillin, streptomycin, TRIzol reagent and Lipofectamine® 2000 were obtained from Invitrogen (Thermo Fisher Scientific, Inc.). Rabbit anti-p53 polyclonal antibody (cat. no. AF0879) was supplied by Affinity BIO (Burwood, Victoria, AU) and rabbit anti-β-actin polyclonal antibody (cat. no. AP0060) was purchased from Bioworld Technology, Inc. (St. Louis Park, MN, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG polyclonal antibody (cat. no. DZP-03) was purchased from Dizhao Biotech (Nanjing, China).
Cell culture
The cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in an atmosphere containing 5% CO2. Glomerular mesangial cells (GMCs) between passages 3 and 6 were used in the present study. To determine the effects of ALD on MCs, equal numbers of growth-arrested MCs (preincubated in serum-free DMEM for 24 h at 37°C in 5% CO2) were incubated in medium (with 10% FBS) containing either buffer (control) or ALD (10−6 M) for 24 h at 37°C. Cells were analysed at the end of the incubation period.
RNA interference
MCs transfected with either an empty expression vector (pGPU6-NC-shRNA) or a p53-silenced vector (pGPU6/GFP/Neo-shRNA; GenePharma, Co., Ltd., Shanghai, China) were established in our laboratory, the sequences of the two cDNA fragments were as follows: Sense, 5′-GGAGGATTCACAGTCGGATAT-3′ for p53 (siRNA); and sense, 5′-GTTCTCCGAACGTGTCACGT-3′ for the negative control (NC). siRNA targeting p53 or its NC were transfected using Lipofectamine® 2000. MCs in growth medium without antibiotics were transfected in serum-free DMEM containing 10 µl Lipofectamine® 2000 with 4.0 ug siRNA per well (6-well plate) for 4–6 h, and the medium was then replaced with growth medium for 48 h. The MCs were serum-starved for 24 h and stimulated with or without 10−6 M ALD for 24 h. Cells were analysed at the end of the incubation period.
Reverse transcription-quantitative polymerase chain reaction (qPCR)
Total RNA, obtained from rat MCs by TRIzol extraction, was purified by processing with an RNeasy Mini kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer's protocol. Quantification and quality checks were performed using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA), respectively. RNA (1 µg) from each sample was reverse transcribed to cDNA using a random hexamer primer and the Thermo Scientific™ RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). Primers for each long non-coding RNA were designed according to Primer 3 (http://sourceforge.net/projects/primer3/) and verified against the National Center for Biotechnology Information Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to ensure a unique amplification product. The following primers were used for qPCR to measure p53 mRNA expression: Sense, 5′-TCTCCCCAGCAAAAGAAAAA-3′ and antisense, 5′-CTTCGGGTAGCTGGAGTGAG-3′ (expected product of ~168 bp) by. The control rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were as follows: Sense, 5′-CAAGTTCAACGGCACAGTCAA-3′ and antisense, 5′-TGGTGAAGACGCCAGTAGACTC-3′ (expected product of ~149 bp). PCR was performed on a ViiA™ 7 Dx Real-Time PCR Instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.) with 20 µl reaction mixtures consisting of 1 µl cDNA, 1 µl each of the forward and reverse primers, 10 µl SYBR® Green Mix (containing Taq DNA polymerase and dNTPs) and 7 µl dH2O. The cycling conditions were as follows: 1 cycle at 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec, 56°C for 20 sec and 72°C for 1 min. The relative expression levels were calculated using the 2−ΔΔCq method (26), following normalization to GAPDH.
Western blotting
p53 protein expression was assessed by western blotting. The cells were lysed in radioimmunoprecipitation assay buffer containing 150 mM NaCl, 50 mM Tris-Cl (pH 7.6), 5 mM EDTA, 0.5% NP-40, 0.5% Triton X-100 containing 10 µg/ml each of leupeptin, aprotinin, and antipain, 1 mM sodium orthovanadate and 0.5 mM phenylmethanesulfonyl fluoride (all Beyotime Institute of Biotechnology, Shanghai, China). The protein concentration was determined using the bicinchoninic acid assay (Thermo Fisher Scientific, Inc.). Total protein (50 µg) was loaded onto each lane, separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). After being blocked with 5% skimmed milk in Tris-buffered saline (pH 7.6; Beyotime Institute of Biotechnology) at room temperature, the membrane was incubated at 4°C overnight with rabbit anti-p53 (1:1,000) and anti-β-actin (1:1,000) primary antibodies. Following incubation with HRP-conjugated goat anti-rabbit secondary antibody (1:5,000), the immune complexes were detected by enhanced chemiluminescence western blotting reagents (Beyotime Institute of Biotechnology). Data were normalized to β-actin protein expression levels and relative protein expression levels were determined using AlphaView Stand Alone Analysis Software 3.0 (ProteinSimple, San Jose, CA, USA).
Detection of apoptosis
Apoptosis was detected by annexin V/propidium iodide (PI) staining using an Annexin V-FITC Apoptosis Detection kit (Vazyme, Piscataway, NJ, USA). Briefly, cells (1×106 per sample) were treated with 0.25% trypsin (Invitrogen; Thermo Fisher Scientific, Inc.), washed twice with cold phosphate-buffered saline (PBS) and resuspended in 100 µl 1X annexin V-binding buffer. The cells were subsequently stained with 5 µl annexin V-fluorescein isothiocyanate, and 5 µl PI at 4°C for 10 min in the dark, then supplemented with 400 µl 1X binding buffer and analysed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA). The data were processed using BD Accuri™ C6 software (BD Biosciences).
Animal treatment
All experimental procedures were performed according to the guidelines for the care and use of animals established by Fudan University (Shanghai, China). The present study was approved by the Medical Ethics Committee of Nanjing Medical University (Nanjing, China). Male Sprague-Dawley rats (n=16; age, 5–6 weeks; weight, ~190 g), obtained from the Animal Center of Fudan University, were housed in individual cages in a temperature-controlled room under a 12-h light/dark cycle and with ad libitum access to food and water. After 2 weeks of acclimatization, the rats underwent a right uninephrectomy or sham surgical procedure following intraperitoneal injection with 1% pentobarbital (40 mg/kg; Beyotime Institute of Biotechnology). After 2 weeks of recovery from surgery or sham surgery, a mini-osmotic pump (model 2004; Alzet, Cupertino, CA, USA) was implanted subcutaneously (sc) to administer the vehicle (control) or ALD (at 0 weeks), and the rats (weight, 260–290 g) were randomly divided into two groups for 4 weeks: Group 1, vehicle [normal saline (sc), n=8]; group 2, ALD [0.75 µg/h (sc), n=8]. The rats in the two groups received 1% NaCl in drinking water throughout the experimental period.
Systolic blood pressure (SBP) was measured in the conscious state by tail-cuff plethysmography (BP-98A; Softron Co., Tokyo, Japan) at weeks 0 and 4 during the treatment period. 24-h urine samples were collected following a 24-h acclimatization period in metabolic cages. Urinary protein excretion was determined using ELISA kits from Exocell, Inc. (Philadelphia, PA, USA; cat. no. NR002). Urine and plasma creatinine levels were analysed using an assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China; cat. no. C011-1).
Immunohistochemistry
The rats were sacrificed by tail vein air embolism following anesthetization with 1% pentobarbital (40 mg/kg), after which the renal cortices of the rats were dissected, fixed with 4% buffered formaldehyde, embedded in paraffin (both Beyotime Institute of Biotechnology), deparaffinized and rehydrated. Sections (thickness, 4 µm) were stained with periodic acid-Schiff, and haematoxylin and eosin (both Beyotime Institute of Biotechnology), according to standard protocol, and visualized using a light microscope (CX32; Olympus Corporation, Tokyo, Japan). Subsequently, the sections were pretreated with 0.3% H2O2 in methanol (both Beyotime Institute of Biotechnology) for 15 min to quench the endogenous peroxide activity and boiled at 100°C for 10 min in a 10% citrate buffer (Beyotime Institute of Biotechnology) for antigen retrieval. Sections were then incubated overnight at 4°C with rabbit anti-p53 polyclonal antibody (1:200; cat. no. sc-1311-R; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), followed by incubation with HRP-conjugated goat anti-rabbit IgG antibody (1:100; cat. no. BA1054; Boster Systems, Inc., Pleasanton, CA, USA) for 30 min at 37°C. Following washing three times with PBS, the sections were incubated with 3,3′-diaminobenzidine substrate for visualization under a microscope (CX32). NCs were prepared by replacing the primary antibodies with PBS.
Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) studies
Apoptosis in renal tissue samples was analysed by TUNEL assay using a kit from Roche Diagnostics (Indianapolis, IN, USA; cat. no. 11684795910). Briefly, 4-µm deparaffinised renal sections were exposed to terminal transferase in the presence of a nucleotide mixture containing fluorescein-2′-deoxyuridine 5′-triphosphate and were examined by fluorescence microscopy (Axio Observer. A1; Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
Data are presented as the mean ± standard deviation of at least three independent experiments. Statistical analysis was assessed using one-way analysis of variance followed by Student-Newman-Keuls q test or Dunnett's multiple comparison tests, with the SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics and renal function
The kidney/body weight ratio, BP, urine volume, final urinary ALD, and urinary albumin/creatinine were all significantly increased in the ALD group when compared with control rats. However, no significant differences in body weight and creatinine clearance were observed between the two groups (Table I).
Table I.
Biological parameters in control and ALD-infused rats at 4 weeks.
| Parameter | Group
|
|
|---|---|---|
| Control | ALD | |
| Body weight (g) | 460.00±10.00 | 452.00±11.00 |
| Kidney weight/body weight ratio (mg/g) | 5.70±0.30 | 9.10±0.50a |
| Blood pressure (mmHg) | 142.00±3.00 | 18.01±5.00a |
| Creatinine clearance (ml/min) | 3.20±0.30 | 3.00±0.30 |
| Urine volume (ml) | 13.00±3.00 | 4.01±9.00a |
| Final urinary ALD (mg/24 h) | 0.04±0.02 | 0.16±0.04a |
| Albumin/creatinine (mg/mg) | 1.50±0.70 | 9.00±1.20a |
Data are presented as the mean ± standard deviation.
P<0.05 vs. the control. ALD, aldosterone.
Effects of ALD on MC apoptosis in vivo
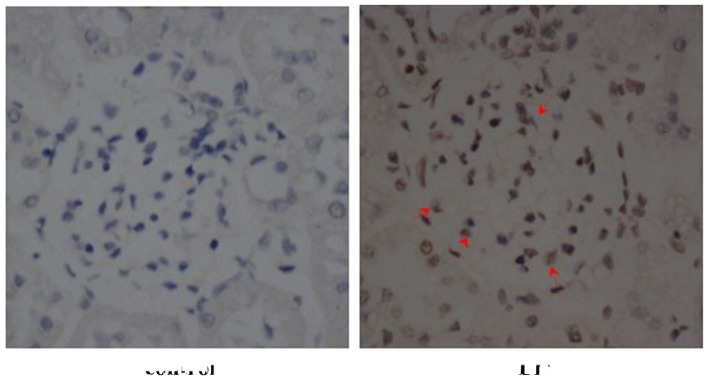
To investigate the effects of ALD on MC apoptosis, rats were randomly assigned to receive normal saline or ALD for 4 weeks and the ratio of MC apoptosis was analysed using a TUNEL assay. As shown in Fig. 1, renal cortical sections of ALD-treated rats demonstrated a greater number of TUNEL-positive MCs (red arrows) than that in the control rat sections. The data indicated that treatment with ALD induced MC apoptosis in vivo. The present study was unable to differentiate the MCs from other types of cell that may be simultaneously undergoing apoptosis. However, as the apoptotic cells were also located in the mesangium it was hypothesized that ALD induced MC apoptosis.
Figure 1.
Effects of ALD on MC apoptosis in in vivo studies (magnification, ×400; staining, periodic acid-Schiff and hematoxylin and eosin). Renal cortical sections of ALD-treated rats demonstrated greater numbers of terminal deoxynucleotidyl transferase dUTP nick end labelling-positive MCs (red arrow) when compared with sections from control rats. The control rats exhibited higher numbers of normal cells than the ALD-treated group. ALD, aldosterone; MC, mesangial cell.
Effects of ALD on p53 expression levels
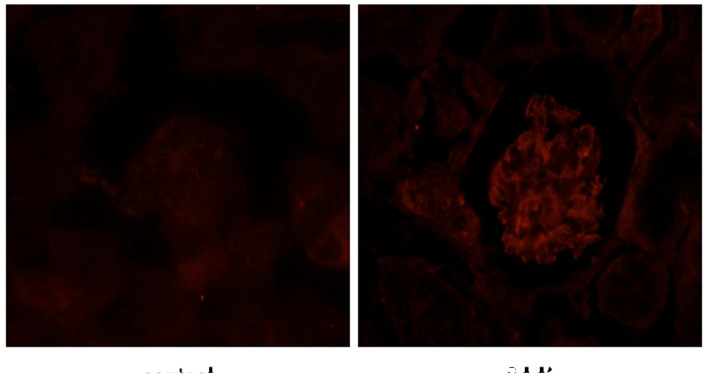
To determine the effects of ALD on the expression level and localization of p53, rats were randomly assigned to receive normal saline or ALD for 4 weeks and the expression levels of p53 were detected by immunohistochemistry. The level of p53 expression was markedly higher in the renal cortical sections from ALD-infused rats, as compared with those from the control group, and p53 protein was predominantly expressed in the renal cortex (Fig. 2). Conversely, there was little p53 protein expressed in the normal saline tissue samples. These results suggested that ALD induced the expression of p53 that was observed in the renal cortical sections.
Figure 2.
Effects of ALD on the distribution of p53 (magnification, ×400). The level of p53 expression was markedly higher in renal cortical sections from ALD-infused rats, as compared with those from the control group rats, as determined by immunohistochemical analyses. Furthermore, the p53 protein was predominantly expressed in the renal cortex, whereas negligible p53 protein expression was observed in the normal tissue samples. ALD, aldosterone.
Effects of ALD on MC apoptosis in vitro
To determine the effects of ALD on MC apoptosis, standard flow cytometry was performed. MCs were transfected with either an empty expression vector (pGPU6-NC-shRNA) or a p53-silenced vector (pGPU6/GFP/Neo-shRNA) for 48 h separately, and co-cultured with or without 10−6 M ALD for 24 h. MC apoptosis was measured by flow cytometry (Fig. 3) and the ALD group demonstrated a significantly higher level of apoptotic cells than the control group (29.11±1.33% vs. 66.55±2.55%; P<0.05). However, this effect of ALD was significantly inhibited by knockdown of p53 with siRNA (50.30±3.60%; P<0.05). The results indicated that ALD increased the MC apoptosis ratio by upregulating p53 expression (Fig. 3).
Figure 3.
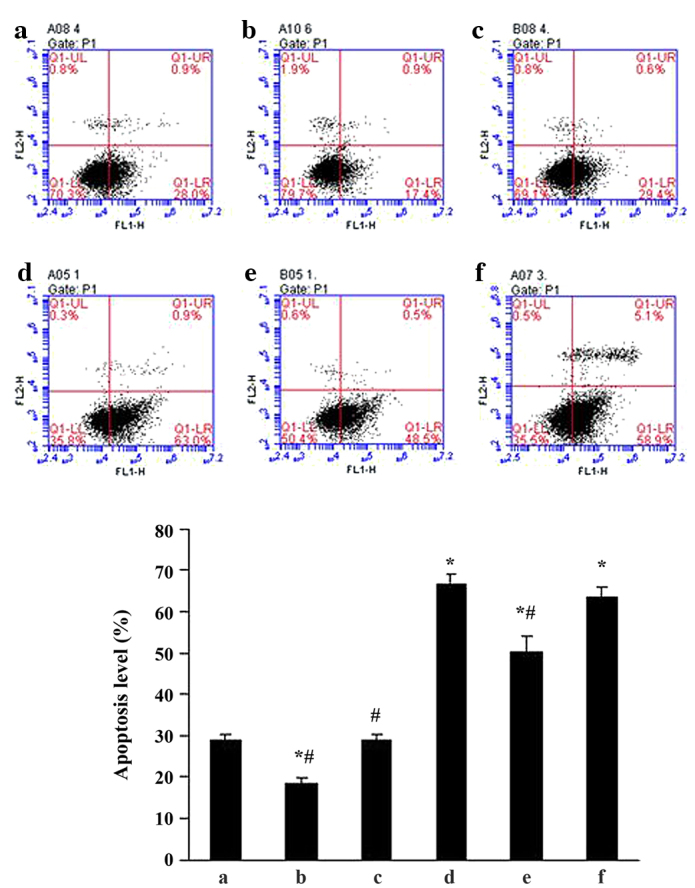
Effects of ALD and p53 siRNA on rat mesangial cell apoptosis. (a) Con; (b) siRNA-p53; (c) siRNA-con; (d) ALD; (e) ALD + siRNA-p53; (f) ALD + siRNA-con. The ALD group demonstrated a higher level of apoptotic cells than the con group (29.11±1.33 vs. 66.55±2.55%; P<0.05). However, this effect of ALD was markedly inhibited by knockdown of p53 with siRNA (50.30±3.60%; P<0.05). Values are presented as means ± standard deviation from three independent experiments. *P<0.05 vs. con; #P<0.05 vs. ALD. ALD, aldosterone; siRNA, small interfering RNA; con, control.
Effects of ALD on p53 mRNA expression in rat MCs
RT-qPCR was performed to evaluate the effects of ALD on p53 mRNA expression. MCs were transfected with either an empty expression vector (pGPU6-NC-shRNA) or a p53-silenced vector (pGPU6/GFP/Neo-shRNA) for 48 h separately. The MCs were then co-cultured with or without 10−6 M ALD for 24 h and the p53 mRNA expression level was measured by RT-qPCR (Fig. 4). Taking the mRNA level in the control as 1, the p53 mRNA level was significantly increased by ALD (1.49±0.23; P<0.05); however, these changes were significantly suppressed by knockdown of p53 with siRNA (0.82±0.03; P<0.05).
Figure 4.
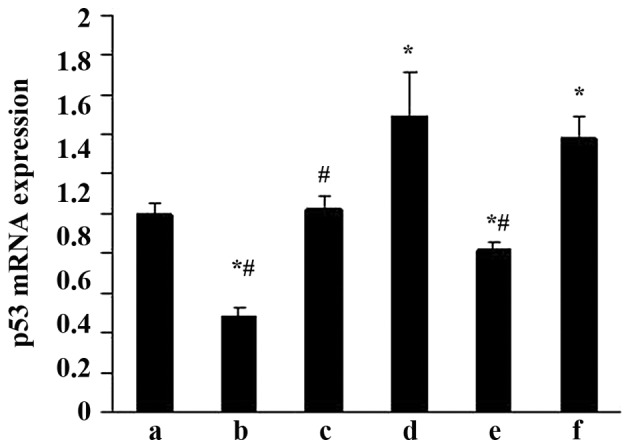
Effects of ALD and p53 siRNA on p53 mRNA expression in rat mesangial cells. (a) Con; (b) siRNA-p53; (c) siRNA-con; (d) ALD; (e) ALD + siRNA-p53; (f) ALD + siRNA-con. Taking the mRNA level in the con as 1, the p53 mRNA level was significantly increased by ALD (1.49±0.23; P<0.05); however, these changes were markedly suppressed by knockdown of p53 with siRNA (0.82±0.03; P<0.05). Values are presented as means ± standard deviation from three independent experiments. *P<0.05 vs. con; #P<0.05 vs. ALD. ALD, aldosterone; siRNA, small interfering RNA; con, control.
Effects of ALD on p53 protein expression in rat MCs
The effects of ALD on p53 protein expression were examined by western blotting. MCs were transfected with either an empty expression vector (pGPU6-NC-shRNA) or a p53-silenced vector (pGPU6/GFP/Neo-shRNA) for 48 h separately, and co-cultured with or without 10−6 M ALD for 24 h. The p53 protein expression was measured by western blotting (Fig. 5). Compared with the control group, the p53 protein level was significantly upregulated in the ALD group at the end of the incubation period (1.00±0.05 vs. 1.83±0.24; P<0.05); whereas the expression level of the p53 protein was significantly decreased following knockdown of p53 with siRNA (0.89±0.03; P<0.05).
Figure 5.
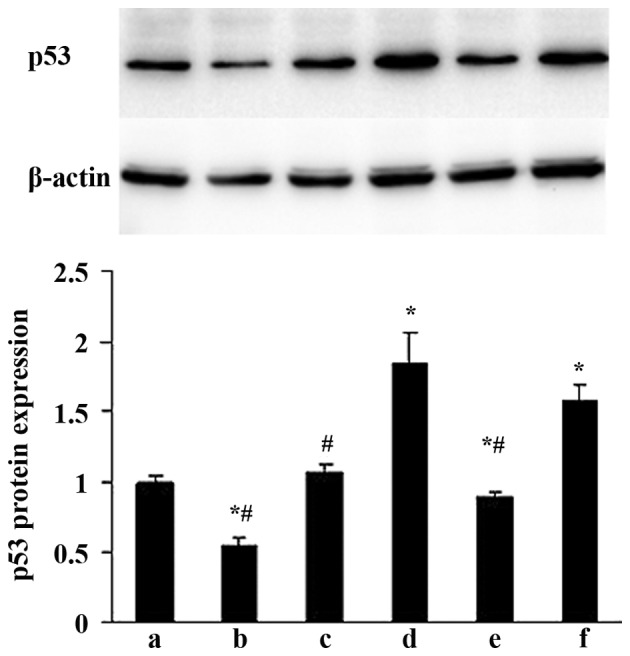
Effects of ALD and p53 siRNA on p53 protein expression levels in rat mesangial cells. (a) Con; (b) siRNA-p53; (c) siRNA-con; (d) ALD; (e) ALD + siRNA-p53; (f) ALD + siRNA-con. At the end of the incubation period, the p53 protein level was upregulated in the ALD group when compared with the con group (1.00±0.05 vs. 1.83±0.24; P<0.05); whereas the p53 protein levels were significantly decreased by knockdown of p53 with siRNA (0.89±0.03; P<0.05). Values are presented as means ± standard deviation from three independent experiments. *P<0.05 vs. con; #P<0.05 vs. ALD. ALD, aldosterone; siRNA, small interfering RNA; con, control.
Discussion
In the present study, the role of p53 in ALD-induced MC injury was investigated. In vivo studies demonstrated that ALD infusion significantly increased SBP, the kidney/body weight ratio, and the urinary protein/creatinine ratio. Additionally, renal cortical sections from ALD-infused rats showed a greater numbers of TUNEL-positive MCs and higher levels of p53 expression. Furthermore, ALD promoted cell apoptosis in cultured MCs, and increased the expression levels of p53 mRNA and protein. The pro-apoptotic effect of ALD was attenuated by knockdown of p53 with siRNA. The results of the present study indicate that the pro-apoptotic effect of ALD on MCs is mediated through p53.
ALD, a key component of the circulating renin-angiotensin-ALD system, has been increasingly recognized as an important factor in the development and progression of CKD, independent of its haemodynamic effects (4,5,27,28). Previous studies show that ALD is associated with hypertension (29), albuminuria (30) and glomerulosclerosis (31) as an independent agent in renal failure and diabetic nephropathy models. In the present study, the effects of ALD on MC apoptosis were investigated. It was found that ALD-treated rats showed a greater number of TUNEL-positive MCs, which is indicative of the pro-apoptotic effect of ALD on MCs in vivo. Furthermore, the ALD group showed higher levels of apoptotic cells than the control group in cultured MCs, which demonstrates that ALD promotes MC apoptosis in vivo and in vitro, indicating the possible involvement of ALD in the progression of renal injury. Although there have been numerous studies on the role of ALD-induced renal injury, the exact mechanisms responsible for the pro-apoptotic effects of ALD on renal cells remain unclear.
Apoptosis is essential in the developmental processes and tissue homeostasis of the majority of multicellular organisms; deregulation of apoptosis has been implicated in the pathogenesis and progression of many disease states (32). Cell apoptosis is an active response to an altered microenvironment and is modulated by families of proteins with a multitude of positive and negative regulators acting at serial steps along a programmed pathway (33). The tumour suppressor, p53 is crucial in the regulation of the cell cycle and apoptosis. In response to a variety of stresses, such as DNA damage, the wild-type p53 protein induces growth arrest at the G1/S phase of the cell cycle until damage is repaired or until cell suicide is triggered via apoptosis depending on the severity of the damage. While muted p53 lost this function and cells with damaged DNA were able to proliferate (34). p53 triggers apoptosis via transcription-dependent and -independent signalling pathways. As a transcriptional regulator, the activation of p53 may downregulate the anti-apoptotic gene product, B-cell lymphoma 2 and upregulate the pro-apoptotic gene product, BCL2-associated X protein to trigger myocyte apoptosis (35–37). The cyclin dependent kinase inhibitor, p21Waf1/Cip1 is also a direct p53-dependent target gene that induces the cell cycle arrest response to p53 (38). p53 is capable of initiating apoptosis via transcription-independent pathways. Recent studies demonstrated that WW domain-containing oxidoreductase is involved in binding and stabilizing p53 in mitochondria and deletion of this gene significantly abolishes p53 apoptotic function (39).
Since p53 is an important molecule that regulates cell apoptosis, the expression of p53 was evaluated in renal cortical tissue samples and in cultured MCs. The results of the current study indicated that ALD-treated rats and ALD-cultured MCs exhibited higher levels of p53 expression when compared with normal controls, which indicated that ALD promotes p53 expression in vivo and in vitro. Apoptosis is induced in MCs exposed to ALD, while knockdown of p53 with siRNA significantly inhibited ALD-induced apoptosis, which suggests the involvement of p53 in the pro-apoptotic effect of ALD. Han et al (32) reported that cyclosporine A, also induced MC apoptosis by upregulating the pro-apoptotic factor, p53. In addition to MCs, ALD exerts a direct pro-apoptotic influence on other kidney cells, including podocytes and proximal tubular epithelial cells (10,11).
In conclusion, the present study indicates that ALD induced MC apoptosis via p53 in vitro and in vivo. These findings partly elucidate the potential mechanism by which ALD induces kidney injury; however, additional studies involving a mineralocorticoid receptor antagonist are required. Furthermore, inhibiting the ALD system may represent a novel therapeutic strategy to prevent MC injury in the progression of CKD.
Acknowledgments
The present study was supported by the Scientific Research Program from Nanjing Medical University (grant nos. 2011NJMU251 and 2014NJMUZD068) and the Health department of Nanjing, China (grant no. QYK11226).
References
- 1.White SL, Cass A, Atkins RC, Chadban SJ. Chronic kidney disease in the general population. Adv Chronic Kidney Dis. 2005;12:5–13. doi: 10.1053/j.ackd.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:CD007004. doi: 10.1002/14651858.CD007004.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai L, Chen J, Hao CM, Lin S, Gu Y. Aldosterone promotes fibronectin production through a Smad2-dependent TGF-beta1 pathway in mesangial cells. Biochem Biophys Res Commun. 2006;348:70–75. doi: 10.1016/j.bbrc.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 6.Liang W, Chen C, Shi J, Ren Z, Hu F, van Goor H, Singhal PC, Ding G. Disparate effects of eplerenone, amlodipine and telmisartan on podocyte injury in aldosterone-infused rats. Nephrol Dial Transplant. 2011;26:789–799. doi: 10.1093/ndt/gfq514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Vecchio L, Procaccio M, Vigano S, Cusi D. Mechanisms of disease: The role of aldosterone in kidney damage and clinical benefits of its blockade. Nat Clin Pract Nephrol. 2007;3:42–49. doi: 10.1038/ncpneph0362. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–848. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 9.Fan YY, Baba R, Nagai Y, Miyatake A, Hosomi N, Kimura S, Sun GP, Kohno M, Fujita M, Abe Y, Nishiyama A. Augmentation of intrarenal angiotensin II levels in uninephrectomized aldosterone/salt-treated hypertensive rats; renoprotective effects of an ultrahigh dose of olmesartan. Hypertens Res. 2006;29:169–178. doi: 10.1291/hypres.29.169. [DOI] [PubMed] [Google Scholar]
- 10.Kiyomoto H, Rafiq K, Mostofa M, Nishiyama A. Possible underlying mechanisms responsible for aldosterone and mineralocorticoid receptor-dependent renal injury. J Pharmacol Sci. 2008;108:399–405. doi: 10.1254/jphs.08R02CR. [DOI] [PubMed] [Google Scholar]
- 11.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: Roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 12.Nagase M, Fujita T. Endocrinological aspects of proteinuria and podocytopathy in diabetes: Role of the aldosterone/mineralocorticoid receptor system. Curr Diabetes Rev. 2011;7:8–16. doi: 10.2174/157339911794273919. [DOI] [PubMed] [Google Scholar]
- 13.Epstein M. Aldosterone blockade: An emerging strategy for abrogating progressive renal disease. Am J Med. 2006;119:912–919. doi: 10.1016/j.amjmed.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Zhu D, Yu H, He H, Ding J, Tang J, Cao D, Hao L. Spironolactone inhibits apoptosis in rat mesangial cells under hyperglycaemic conditions via the Wnt signalling pathway. Mol Cell Biochem. 2013;380:185–193. doi: 10.1007/s11010-013-1672-0. [DOI] [PubMed] [Google Scholar]
- 15.Nagase M, Fujita T. Aldosterone and glomerular podocyte injury. Clin Exp Nephrol. 2008;12:233–242. doi: 10.1007/s10157-008-0034-9. [DOI] [PubMed] [Google Scholar]
- 16.Merkel CE, Karner CM, Carroll TJ. Molecular regulation of kidney development: Is the answer blowing in the Wnt? Pediatr Nephrol. 2007;22:1825–1838. doi: 10.1007/s00467-007-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Liang W, Jia J, van Goor H, Singhal PC, Ding G. Aldosterone induces apoptosis in rat podocytes: Role of PI3-K/Akt and p38MAPK signaling pathways. Nephron Exp Nephrol. 2009;113:e26–e34. doi: 10.1159/000228080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abboud HE. Mesangial cell biology. Exp Cell Res. 2012;318:979–985. doi: 10.1016/j.yexcr.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Brunskill EW, Potter SS. Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. BMC Nephrol. 2012;13:70. doi: 10.1186/1471-2369-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalla Vestra M, Saller A, Mauer M, Fioretto P. Role of mesangial expansion in the pathogenesis of diabetic nephropathy. J Nephrol. 2001;14(Suppl 4):S51–S57. [PubMed] [Google Scholar]
- 21.Kitamura H, Shimizu A, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N. Apoptosis in glomerular endothelial cells during the development of glomerulosclerosis in the remnant-kidney model. Exp Nephrol. 1998;6:328–336. doi: 10.1159/000020540. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N. Apoptosis in progressive crescentic glomerulonephritis. Lab Invest. 1996;74:941–951. [PubMed] [Google Scholar]
- 23.Mishra R, Emancipator SN, Kern T, Simonson MS. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int. 2005;67:82–93. doi: 10.1111/j.1523-1755.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama H, Kashihara N, Makino H, Yamasaki Y, Ota A. Apoptosis in glomerular sclerosis. Kidney Int. 1996;49:103–111. doi: 10.1038/ki.1996.14. [DOI] [PubMed] [Google Scholar]
- 25.Ford HL, Sclafani RA, DeGregori J. Cell cycle and growth control: Biomolecular regulation and cancer. In: Stein GSaP AB, editor. Cell cycle regulatory cascades. Hoboken, New Jersey: Wiley-Liss; 2004. pp. 95–128. [Google Scholar]
- 26.Livak, Schmittgen Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Chrysostomou A, Becker G. Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N Engl J Med. 2001;345:925–926. doi: 10.1056/NEJM200109203451215. [DOI] [PubMed] [Google Scholar]
- 28.Rocha R, Chander PN, Zuckerman A, Stier CT., Jr Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension. 1999;33:232–237. doi: 10.1161/01.HYP.33.1.232. [DOI] [PubMed] [Google Scholar]
- 29.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 30.Miric G, Dallemagne C, Endre Z, Margolin S, Taylor SM, Brown L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br J Pharmacol. 2001;133:687–694. doi: 10.1038/sj.bjp.0704131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB. Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol. 2005;16:3306–3314. doi: 10.1681/ASN.2004090804. [DOI] [PubMed] [Google Scholar]
- 32.Han SY, Chang EJ, Choi HJ, Kwak CS, Park SB, Kim HC, Mun KC. Apoptosis by cyclosporine in mesangial cells. Transplant Proc. 2006;38:2244–2246. doi: 10.1016/j.transproceed.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Oltvani ZN, Korsmeyer SJ. Checkpoints of dueling dimers foli death wishes. Cell. 1994;79:189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 34.Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 35.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 36.Pierzchalski P, Reiss K, Cheng W, Cirielli C, Kajstura J, Nitahara JA, Rizk M, Capogrossi MC, Anversa P. p53 induces myocyte apoptosis via the activation of the renin-angiotensin system. Exp Cell Res. 1997;234:57–65. doi: 10.1006/excr.1997.3604. [DOI] [PubMed] [Google Scholar]
- 37.Leri A, Fiordaliso F, Setoguchi M, Limana F, Bishopric NH, Kajstura J, Webster K, Anversa P. Inhibition of p53 function prevents renin-angiotensin system activation and stretch-mediated myocyte apoptosis. Am J Pathol. 2000;157:843–857. doi: 10.1016/S0002-9440(10)64598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.el-Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol. 1998;8:345–357. doi: 10.1006/scbi.1998.0097. [DOI] [PubMed] [Google Scholar]
- 39.Chang NS. A potential role of p53 and WOX1 in mitochondrial apoptosis (review) Int J Mol Med. 2002;9:19–24. [PubMed] [Google Scholar]