Abstract
The versican family is important in the modulation of inflammation, however, the role of versican V1 (V1) in lipo-polysaccharide (LPS)-induced acute lung injury (ALI) and the underlying mechanisms remain to be elucidated. To investigate this, the present study performed experiments in male C57BL/6J mice, which were randomly divided into a normal control group (control; n=6), an LPS-stimulated ALI group (LPS; n=6), a scramble small interfering (si)RNA group (scramble; n=6), a V1-siRNA group (V1-siRNA; n=6), a scramble siRNA and LPS-stimulated group (scramble+LPS; n=6) and a V1-siRNA and LPS-stimulated group (V1-siRNA+LPS; n=6). On day 1, the mice were anesthetized, and 5 nmol scramble siRNA or V1-siRNA were administered intratracheally. On day 3, LPS (1 mg/kg) or phosphate-buffered saline (50 µl per mouse) were injected intratracheally. All the mice were anesthetized and sacrificed on day 4, and samples were collected and analyzed. The mRNA and protein expression levels were examined using reverse transcription-quantitative polymerase chain reaction analysis, immunohistochemical staining and western blot analysis. ALI was evaluated based on lung injury scores, cell counts and total protein concentrations in the bronchoalveolar lavage fluid (BALF). Inflammatory mediators were detected using an enzyme-linked immunosorbend assay. V1 was increased by LPS in the mouse ALI model, whereas specific V1 knockdown induced higher lung injury scores, and higher total cell counts and protein concentrations in the BALF. Tumor necrosis factor-α (TNF)-α was upregulated, and interleukin-6 exhibited an increasing trend. The expression of toll-like receptor 2 (TLR2), but not TLR4, increased, and the nuclear factor (NF)-κB pathway subunit, P65, was phosphorylated. Taken together, the expression of V1 was upregulated by LPS, and V1 inhibition resulted in the aggravation of LPS-induced ALI via the activation of TLR2-NF-κB and release of TNF-α.
Keywords: versican, lipopolysaccharide, acute lung injury, toll-like receptor 2, nuclear factor-κB, tumor necrosis factor-α
Introduction
Extracellular matrix (ECM) contributes to the overall mechanical properties of tissues. ECM is also considered to modulate disease progression, and current understanding of the modulatory function of ECM suggests that the stiffness of the ECM affects tumor cell progression (1). Tumor-associated ECM remodeling is characterized by increased ECM deposition and the stiffness of the matrix, which leads to persistent migration and tissue invasion of cancer cells (2). It is known that ECM is involved in cell movement, attachment, proliferation and differentiation, and in orchestrating inflammation (3–7). Versican is a large ECM proteoglycan, encoded on human chromosome 5 and spanning >90kb. There are globular structures at the N-terminal (G1 domain) and C-terminal (G3 domain) of the protein core, similar to the other members of the lectican family (8,9). The G1 domain is characterized by a hyaluronan binding region. The G3 domain consists of epidermal growth factor-like domains, a carbohydrate recognition domain and a complement binding domain. Between G1 and G3 in versican, core proteins are present, where chondroitin sulfate glycosaminoglycan (GAG) side chains attach (8). There are at least five splice variants of versican, termed V0, V1, V2, V3 and V4, which differ predominantly in the sizes of their core proteins, generated by alternative splicing of the mRNA encoding the two GAG chain binding domains. V0 (is silenced by the same siRNA as V1, as it contains the full length protein encoded by all the exons) and V1 are distributed widely in adult tissues; V1 is the principal proteoglycan found in pulmonary ECM (10). V2 appears to be expressed only in the central nervous system (11–14). The V3 variant is generally expressed at low levels in adult tissues (14). V4 has been identified as an additional isoform in breast cancer (15). The G3 domain promotes cell adhesion through its interaction with β1 integrin and activation of focal adhesion kinase (16). In addition, versican binds with CD44 through its GAG chains or G1 hyaluronan binding domain to promote cell proliferation, migration and adhesion, and enhance the spread of tumors and inflammation (17–19). Versican binds to hyaluronan to affect T lymphocyte phenotypes and cytokine secretion (19). Versican also acts as an endogenous ligand of toll-like receptors (TLRs), generating a rapid inflammatory response (20). Several reports have shown that, particularly in tumors, V1 is a natural ligand of TLR2. By activating TLR2:TLR6 complexes and inducing tumor necrosis factor (TNF)-α secretion, V1 markedly enhances tumor metastatic growth (21,22).
Acute lung injury (ALI) is a type of severe inflammatory disease, which is life-threatening and is characterized by inflammatory damage of the alveolar capillary membrane, increased proteinaceous edema and the production of inflammatory cytokines. LPS, a major constituent of the outer membranes of Gram-negative bacteria, has been identified as a pivotal risk factor and prominent stimulus in the pathogenesis of ALI (23,24). LPS challenge induces neutrophil infiltrations, triggers acute inflammatory responses and generates early pathological changes in the lungs. The intratracheal administration of LPS in experimental animals is a suitable and reproducible method for investigations involving ALI. The predominant LPS sensor in the host is the TLR superfamily, of which TLR2 and TLR4 are the primary sensors of LPS, acting as transmembrane receptors and signal transduction molecules (25). On binding with LPS, TLRs activate the nuclear factor (NF)-κB pathway through myeloid differentiation factor 88, resulting in the production of pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, recruiting neutrophils in the lung. As the most widely investigated member of the TNF super family, TNF-α is important in the homeostasis and pathophysiology of ALI. LPS administration induces high levels of TNF-α, which exacerbates ALI (26). TNF-α also downregulates the gene expression of surfactant protein A in lung epithelial cells via the p38 mitogen-activated protein kinase signal transduction pathway, resulting in the loss of lung surfactant (27).
As versican has complex roles in inflammation, and as V1 is the predominant isoform present in the lungs, the present study hypothesized that V1 is also involved in the modulation of lung inflammation. The present study aimed to investigate the effects of V1 knockdown in ALI. As our previous in vitro study demonstrated that high levels of V1 are expressed in the lungs, and increase further following insult by LPS, it is possible to silence V1 and examine its function in ALI. This may demonstrate that V1 is one of the pivotal regulators in the inflammation of ALI and, therefore establish a novel target for the treatment of ALI.
Materials and methods
Animals
Male specific pathogen-free C57BL/6J mice (age, 6–8 weeks) were obtained from Shanghai Laboratory Animal Center (SLAC Laboratory Animal Co., Ltd., Shanghai, China). The mice were housed under a 12 h light/dark cycle and at a constant temperature of 22±2°C with food and water available ad libitum. The mice were allowed to acclimate to these conditions for 7 days prior to initiation of the experiment. All experimental procedures in the present study were approved by the Institutional Ethics Committee of Fudan University (Shanghai, China) and were performed, according to the guidelines for experimental animals developed by Fudan University.
Animal model establishment and sample collection
The male C57BL/6J mice were randomly divided into a normal control group (control; n=6), an LPS-stimulated ALI group (LPS; n=6), a scramble small interfering (si)RNA group (scramble; n=6), a V1-siRNA group (V1-siRNA; n=6), a scramble siRNA and LPS-stimulated group (scramble+LPS; n= 6) and a V1-siRNA and LPS-stimulated group (V1-siRNA+LPS; n=6). On day 1, the mice were anesthetized with tribromoethanol (1.2%; 20 ml/kg; Sigma-Aldrich, St. Louis, MO, USA), and then suspended vertically and intubated with a section of 22G intravenous catheter. The scramble siRNA (5 nmol) and the V1-siRNA modified with cholesterol and methyl (5′-GCAATTACCACCTCACCTA-3′) dissolved in phosphate-buffered saline (PBS), obtained from RiboBio Co., Ltd. (Guangzhou, China), were administered separately in the different treatment groups. On day 3, LPS (1 mg/kg; from Pseudomonas aeruginosa 10; Sigma-Aldrich) or PBS (50 µl per mouse) were administered intratracheally, as described above. All the mice were anesthetized and sacrificed by cervical dislocation on day 4, bronchoalveolar lavage fluid (BALF) was collected using 1.5 ml PBS divided into two volumes, each of which was injected and aspirated repeatedly five times in the trachea through the catheter. Blood samples were also collected, and the lungs were removed. The lung tissues were fixed, using 4% paraformaldehyde (1.0×0.8×0.5 cm; Sangon Biotech, Co., Ltd., Shanghai, China), for hematoxylin and eosin (H&E; Jiancheng Bioengineering Institute, Nanjing, China) and immunohistochemical staining, the rest of the lung tissues were frozen at −80°C.
Assessments of protein concentration and cell count in the BALF
The recovery of BALF was >85%. The total protein concentration of the BALF was determined using a bicinchoninic acid (BCA) protein assay, according to the manufacturer's protocol (Beyotime Institute of Biotechnology, Haimen, China). For counting of the cells in the BALF, the BALF was centrifuged at 300 × g for 5 min at 4°C, and the sediment was treated with 200 µl erythrocyte lysate (Yeasen Biotechnology Co., Ltd., Shanghai, China) for 10 min, centrifuged at 300 × g for 10 min at 4°C, and washed with PBS. Subsequently, 200 µl PBS was added to resuspend the cells, which were then counted under an inverted microscope (Eclipse TS100-F; Nikon Corporation, Tokyo, Japan).
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the lung tissues using an Ultrapure RNA kit (CW Biotech Co., Ltd., Peking, China), according to the manufacturer's protocol. Total RNA (500 ng) was transcribed and cDNA was synthesized according to the manufacturer's protocol (Shanghai Yeasen Biotechnology Co., Ltd., Shanghai, China). Hieff™ qPCR SYBR® Green Master mix (Shanghai Yeasen Biotechnology Co., Ltd; 10 µl Master Mix, 0.4 µl Forward Primer (10 mM), 0.4 µl Reverse Primer (10 mM), 1 µl cDNA and 8.2 µl ddH2O) was used, and qPCR was performed, according to the manufacturer's protocol, using the Master Cycler ep realplex PCR system (Eppendorf, Hamburg, Germany). The cycling conditions were as follows: 95°C for 5 min (pre-denaturation), 95°C for 10 sec, 60°C for 30 sec (40 cycles), 95°C for 15 sec, 60°C for 60 sec and 95° for 15 sec (melting). The expression levels of target mRNAs were normalized to that of GAPDH using the 2−ΔΔCq method (28). The primer pairs (Sangon Biotech, Co., Ltd.) used were as follows: V1, forward 5′-GCTGTAAACGTCGATTGAGTG-3′ and reverse 5′-TCTTCACTGCAAGGTTCCTC-3′; GAPDH, forward 5′-CATGGCCTTCCGTGTTCCTA-3′ and reverse 5′-GCGGCACGTCAGATCCA-3′; TNF-α, forward 5′-CCTGTAGCCCACGTCGTAG-3′ and reverse 5′-GGGAGTAGACAAGGTACAACCC-3′; IL-6, forward 5′-TCCAGTTGCCTTCTTGGGAC-3′ and reverse 5′-GTGTAATTAAGCCTCCGACTTG-3′; TLR2, forward 5′-GCGGACTGTTTCCTTCTG AC-3′ and reverse 5′-CCAAAGAGCTCGTAGCATCC-3′; and TLR4, forward 5′-CAGCAAAGTCCCTGATGACA-3′ and reverse 5′-AGAGGTGGTGTAAGCCATGC-3′.
Immunohistochemistry
Immunohistochemistry was performed using the paraffin (Beyotime Institute of Biotechnology)-embedded tissue sections [size, 4 µm; sliced using a Microtome (5,000 smz) from Campden Instruments Ltd., Loughborough, England] mounted on glass slides. The slides were deparaffinized and rehydrated. Antigens were retrieved using 10 mM citrate solution (Beyotime Institute of Biotechnology) and a microwave oven on high heat for 3 min, and a medium heat for 12 min, and then blocked using 10% bovine serum albumin (Beyotime Institute of Biotechnology) for 30 min at room temperature. The slides were then immunostained using the following primary antibodies at 4°C overnight: Monoclonal mouse anti-human versican (#MABT161; 1:100; EMD Millipore, Billerica, MA, USA), polyclonal rabbit anti-human phosphorylated (p)-P65 [(Ser536); #11014; 1:150; Signalway Antibody LLC, College Park, MD, USA], polyclonal rabbit anti-human TLR2 (#BA1716) and TLR4 (#BA1717) (1:100; Wuhan Boster Biological Technology Co., Ltd., Wuhan, China). This was followed by incubation with horseradish peroxidase(HRP)-conjugated goat anti-mouse and rabbit secondary antibody (#CK500605A; GeneTech, Shanghai, China) for 1 h at room temperature. Diaminobenzidine (GeneTech) reactions were performed, and the slides were reacted with hematoxylin for 30 sec, washed with running water, dehydrated in a graded series of ethanol and xylene (Sangon Biotech, Co., Ltd.), then mounted with a coverslip. The immunoreactivity was observed and images were captured under an inverted microscope (Eclipse TS100-F).
Western blot analysis
Total protein was extracted from the lung tissues using cold lysis buffer (Beyotime Institute of Biotechnology), and concentrations were measured using a BCA protein assay. The samples (30 µg/sample) were separated by 10% SDS-PAGE (Beyotime Institute of Biotechnology) and transferred onto polyvinylidene fluoride membranes (EMD Millipore). The membranes were blocked with skimmed milk, and incubated overnight at 4°C with the following primary antibodies: Anti-TLR2, TLR4 and GAPDH (monoclonal; 1:1,000; Wuhan Boster Biological Technology Co., Ltd.). The membranes were incubated with HRP-conjugated secondary antibodies (1:1,000; Beyotime Institute of Biotechnology). The immunoreactive signals were visualized using an enhanced chemiluminescence detection system (Beyotime Institute of Biotechnology).
Enzyme-linked immunosorbent assay (ELISA)
The lung tissues were homogenized with saline and centrifuged at 12,000 × g for 20 min at 4°C, and the resulting supernatants were used as homogenates, with the other samples being BALF and plasma. For the ELISA analysis, IL-6 and TNF-α kits (BioLegend, San Diego, CA, USA) were used. The levels of TNF-α and IL-6 were determined according to the manufacturer's protocol.
Statistical analysis
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was used for data analysis. Data are expressed as the mean ± standard division. Comparisons among three or more experimental groups were performed using one-way analysis of variance. Significant differences between groups were analyzed using Student's t-test or a Mann-Whitney U test, when appropriate. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of V1 increases in the ALI mouse model
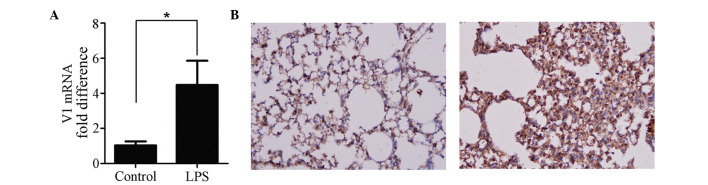
The expression of V1 was increased in the ALI mouse model. The mRNA (Fig. 1A) and protein (Fig. 1B) expression levels of V1 were altered by LPS. The mRNA expression of V1 was increased >4-fold, and the immunohistochemical analyses of the lung tissues confirmed that a higher level of V1 was expressed following ALI (Fig. 1B).
Figure 1.
Expression of V1 in the LPS-induced mouse ALI model. (A) Reverse transcription-quantitative polymerase chain reaction analysis was used to detect the mRNA expression of V1. The level of gene transcription increased >4-fold in ALI. (B) Representative images of immunohistochemistry staining (magnification, ×200). Compared with the control (left), the intensity of the expression of V1 was more marked in the ALI mice (right). Data are expressed as the mean ± standard division (*P<0.05). ALI, acute lung injury; LPS, lipopolysaccharide; V1, versican V1.
Lung injury is aggravated by V1-siRNA
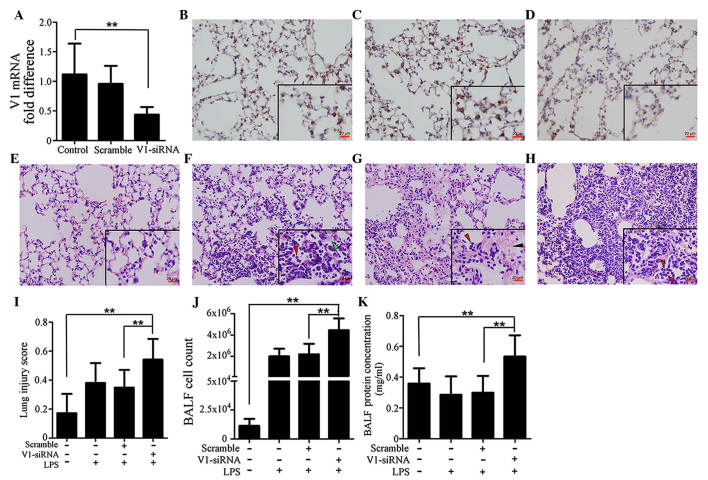
In order to investigate the role of V1 in ALI, V1-siRNA was administered in the lungs to inhibit V1. The levels of transcription decreased in the V1-siRNA group by >50%, compared with the control group, whereas no significant differences were observed between the control and scramble siRNA groups (Fig. 2A). The changes in the protein expression levels of V1 were in accordance with those of mRNA, which were confirmed using immunohistochemical staining (Fig. 2B–D). Injury to the mouse lung tissues was also investigated, as described previously (29) in the H&E-stained sections. The lung injury score in the V1-siRNA+LPS group was significantly higher, compared with those in the control, LPS, scramble+LPS groups (Fig. 2E–H). Fig. 2I shows histograms of the scores. Cell count and total protein concentration in the BALF were also detected. Consistent with the lung injury scores, the cell count and protein concentration were higher in the V1+LPS group (Fig. 2J and K).
Figure 2.
Specific knockdown of V1 aggravates immune reactions in the mouse lung. (A) mRNA expression of V1 was decreased by siRNA. No significant differences were found between the normal and scramble siRNA-treated groups. Immunohistochemical assays (magnification, ×200; scale bar in vignettes=20 µm) showed that the V1 expression intensity was consistent in the (B) control and (C) scramble siRNA groups, but weaker in the (D) V1-siRNA group. Representative images of H&E-stained sections (magnification, ×200; scale bars in vignettes=20 µm) in the (E) lungs of the control group were normal, with clear bronchial and alveolar structures. (F) At 24 h post-LPS administration (1 mg/kg; intratracheally), typical pathological changes were observed, with patchy neutrophil infiltration (red arrows) and liquid entering the alveolar cavity (green arrows). (G) In the scramble siRNA pre-treated mouse, the LPS-stimulated pathological changes were almost the same, with neutrophil infiltration (red arrows) and deposition of fibrin strands (black arrows). (H) In the V1-siRNA+LPS group, there was increased neutrophil infiltration in alveolar cavities (red arrows), and reduced clarity of bronchial and alveolar structures, compared with the control ALI group. (I) Lung injury scores were assessed, according to the H&E-stained sections. Higher scores were observed in the V1 knockdown group, which confirmed the existence of an aggravated inflammation in the V1 knockdown group. (J) Cell count and (K) total protein concentrations in the BALF also confirmed that V1 inhibition in the mouse led to a more severe reaction. Data are presented as the mean ± standard deviation (**P<0.01). siRNA, small interfering RNA; ALI, acute lung injury; LPS, lipopolysaccharide; V1, versican V1; BALF, bronchoalveolar lavage fluid.
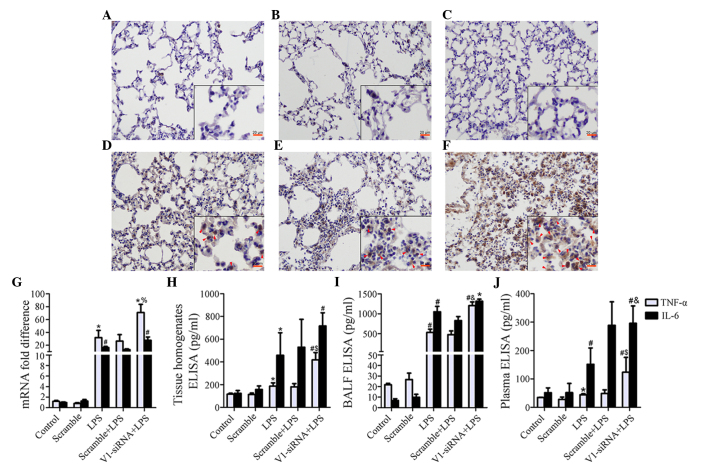
V1 knockdown-associated inflammatory aggravation is associated with activation of NF-κB and deregulated release of TNF-α and IL-6
Although levels remained stable prior to LPS stimulation in the control, scramble and V1-siRNA groups (Fig. 3A–C), marked NF-κB signaling pathway activation was induced by LPS in the V1-siRNA group, compared with the control and scramble siRNA groups (Fig. 3D–F). Several cytokines were detected in the present study (data not shown), TNF-α was found to be negatively correlated with V1. The mRNA expression of TNF-α was increased by LPS, however, V1 knockdown exhibited more marked transcriptional activity, compared with the other treatment groups (Fig. 3G). The protein levels of TNF-α also increased significantly in the lung tissue homogenates, BALF and plasma (Fig. 3H–J). The expression of IL-6 increased, but the significant difference between the LPS and V1-siRNA+LPS groups was only observed in the plasma samples (Fig. 3G–J).
Figure 3.
Increased severity of inflammatory reaction is mediated by NF-κB and TNF-α. (A–C) The immunostaining of NF-κB subunit P65 is demonstrated in the control, scramble and V1-siRNA groups (magnification, ×200; scale bar in vignettes=20 mm). P65 remained stable prior to LPS administration. (D–F) The immunostaining of P65 was demonstrated in the LPS, scramble+LPS and V1-siRNA+LPS groups (magnification, ×200; scale bar in vignette=20 mm). P65 was phosphorylated following LPS insult, immunostaining was more marked in the (F) V1-siRNA+LPS group, compared with the (D) LPS and (E) scramble+LPS groups (red arrows show positive staining). (G) mRNA levels of TNF-α were increased in all ALI groups, but were higher in the V1-siRNA+LPS group. (H–J) ELISA indicated TNF-α as a mediator of the severe inflammatory reaction, as TNF-α was the only molecule to show a consistent increasing trend in the (H) tissue homogenates, (I) BALF and (J) plasma. TNF-α was higher in the V1-siRNA+LPS group in all samples, IL-6 increased without statistical significance. Data are presented as the mean ± standard deviation. *P<0.05, vs. control group; #P<0.01, vs. control group; %P<0.05, vs. LPS group; $P=0.01, vs. LPS group; &P<0.01, vs. LPS group. NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α, IL-6, interleukin-6; siRNA, small interfering RNA; ALI, acute lung injury; LPS, lipopolysaccharide; V1, versican V1; BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay.
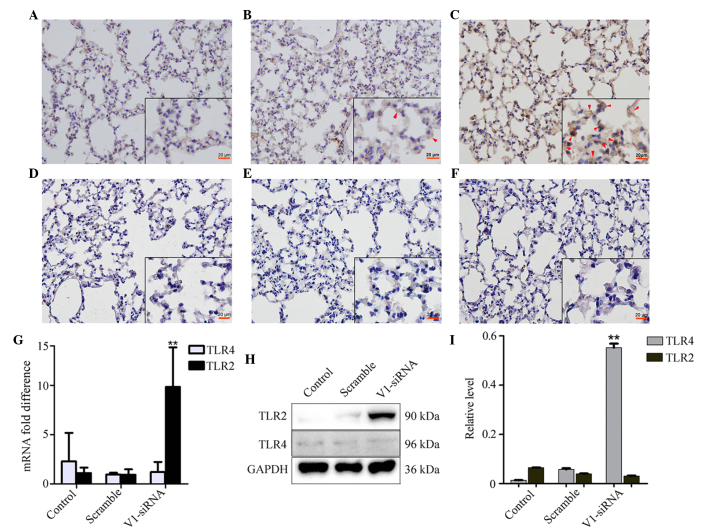
V1 knockdown induces the expression of TLR2, but not TLR4
The expression of TLR2 was increased in the V1 knockdown group, however, no notable changes were observed in the control and scramble siRNA groups (Fig. 4A–C). No significant differences were found in the expression levels of TLR4 among the groups (Fig. 4D–F). The mRNA expression of TLR2, but not TLR4, increased in the V1 knockdown group, which was consistent with the changes observed in the mRNA and protein expression levels (Fig. 4G and I).
Figure 4.
Changes in the expression levels of TLR2 and TLR4 in the different groups. (A–C) Levels of TLR2 remained stable in the (A) control and (B) scramble groups, but were altered in the V1-siRNA group. Red arrows show positive staining. (Magnification, ×200; scale bar in vignettes=20 µm). The levels of TLR4 altered marginally in the (D) control, (E) scramble and (F) V1-siRNA groups. The (G) mRNA and (H) protein levels of TLR2 and TLR4 showed the same changes as those observed in the immunostaining. (I) Relative protein level calculated as the target grey value/GAPDH grey value from the western blot. **P<0.01, vs. control. NF-κB, nuclear factor-κB; TLR, toll-like receptor; siRNA, small interfering RNA; ALI, acute lung injury; LPS, lipopolysaccharide; V1, versican V1.
Discussion
Previous reports have indicated that secreted proteoglycans act as signaling molecules; in various inflammatory diseases and in response to LPS, activated macrophages synthesize and secrete versican (30–33) and, according to the results of the present study, LPS induces V1 secretion in the lungs. It appeared that V1 increase was not only confined to macrophages, but spreads all over the lungs. The present study found that LPS also induced the expression of V1 in lung fibroblasts and A549 cells in vitro (data not shown), which supports the ubiquitous LPS-associated expression of V1. The TGF-β1 signaling pathway is associated with the synthesis of versican in lung fibroblasts (34). The versican promoter contains a typical TATA box, together with other 5′-flanking elements, allowing for the regulation of versican in different situations, such as cancer, inflammation, and growth and development. V1 is also modulated by micro (mi)RNAs, and myocardin represses V1 through the induction of miR-143 in smooth muscle cells (35).
Activation of the NF-κB pathway was more marked following V1 knockdown, which may be the predominant reason for the deregulated TNF-α and IL-6 release, and aggravated immune reactions. NF-κB signaling-induced TNF-α synthesis is now widely accepted, in which the κB subunit binds to the 5′-untranslated region of TNF-α to promote gene transcription (36). The present study suggested two possible reasons for alterations in TNF-α levels, one of which is the direct release from local affected cells, as V1-siRNA and LPS can be taken up by epithelial cells, macrophages and fibroblasts, which are all sources of TNF-α. The second is that, in LPS-insulted lung fibroblasts, V1 knockdown increases the production of IL-6 and IL-8 (data not shown), and these cytokines in vivo may stimulate macrophages to release TNF-α in a paracine manner. The present study examined TNF-α, IL-6, IL-8 and myeloperoxidase (data not shown), however, only TNF-α increased significantly in all samples.
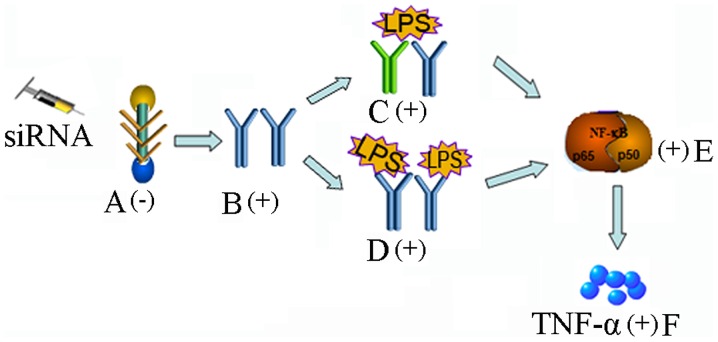
The activation of NF-κB occurs via signaling through the TLR superfamily and its adaptors, and the pro-inflammatory function of proteoglycans is always associated with TLR2 and TLR4 (20). Therefore, the present study examined the levels of TLR2 and TLR4 in the lung sections. Of note, the level of TLR2 increased markedly, unlike TLR4. Versican derived from Lewis lung carcinoma cells has been shown to interact with TLR2 and its co-receptors, TLR6 and CD14, on macrophages, inducing the secretion of TNF-α and IL-6, indicating the association between the tumor and its pro-metastatic microenvironment (20–22,37). Versican also binds with TLR2 on ovarian tumor cells to stimulate tumor cell proliferation (38). Although the present study is the first, to the best of our knowledge, to show that V1 knockdown can induce the expression of TLR2, it is understandable as, being a natural ligand of TLR2, a decreases in versican may lead to an increase in TLR2 in response. However, the exact mechanism requires further investigation. The TLR2 promoter contains binding motifs of several transcription factors; a series of 5′-truncated TLR2 promoter-luciferase constructs are required for further investigation of the reasons for TLR2 synthesis. The canonical LPS sensor is TLR4, however, the present study found minimal changes in TLR4 in the lungs, thus not providing an explanation for the severe reaction in the V1-knockdown mice. The upregulation of TLR2 may, at least partially, provide an explanation. The present study hypothesized that, as proteoglycan binds with TLR2 on cells, as a type of comparative competitor of other pathogen-derived ligands, a decrease in proteoglycan may increase the release of receptors for LPS on the cell surface and result in a more severe reaction. However, as far as we know, certain types of proteoglycans interact with TLR2/4 and a second TLR, which is not involved in pathogen sensing, thereby exacerbating the host response to microbial invasion (20). Prior to LPS insult, this complex may exist on the cell surface, and increase further following TLR2 increase, acting as a latent threat to cell homeostasis. Following the synchronous upregulation of TLR2 and LPS stimulation, a marked inflammatory reaction occurs, however, further investigations are required to elucidate the consequences of LPS/versican and TLR interactions on TLR signaling (Fig. 5).
Figure 5.
Possible mechanisms involved. (A) Expression of V1 is suppressed by siRNA; (B) TLR2 increases; (C) TLR2 and a second TLR (not involved in pathogen sensing) form a complex on the cell surface, inducing a severe reaction; (D) increased TLR2 located on the cell surface; (E) activation of the downstream NF-κB pathway; (F) increased release of TNF-α results in a more severe reaction. NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α, siRNA. small interfering RNA; ALI, acute lung injury; LPS, lipopolysaccharide; V1, versican V1.
Inhibiting the expression of versican in cancer cells inhibits the inflammation associated with these tumors (21,22,39). However, the mouse ALI model is a non-cancerous model, and the mechanisms of ALI are complicated. Due to activated NF-κB and increased TNF-α, the total immune reaction is more marked. The present study demonstrated that the same condition existed in the V1 knockdown fibroblasts in vitro (data not shown). The anti-inflammatory effect of proteoglycan has also been confirmed in human uterine cervical fibroblasts, in which primary cervical fibroblasts were treated with proteoglycan and LPS concomitantly, and significant decreases in IL-6 and IL-8 were observed (40).
The present study provided novel evidence of V1 modulating inflammation in the lungs and indicated its potential regulatory role in V1-TLR2 interactions. Further investigations are required to investigate the mechanisms involved in the upregulation of TLR2, and the effects of LPS/versican and TLR interactions on TLR signaling.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (grant no. 81470231).
References
- 1.Seewaldt V. ECM stiffness paves the way for tumor cells. Nat Med. 2014;20:332–333. doi: 10.1038/nm.3523. [DOI] [PubMed] [Google Scholar]
- 2.Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–48. doi: 10.1016/j.copbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 4.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanekar S, Borg TK, Terracio L, Carver W. Modulation of heart fibroblast migration and collagen gel contraction by IGF-I. Cell Adhes Commun. 2000;7:513–523. doi: 10.3109/15419060009040308. [DOI] [PubMed] [Google Scholar]
- 6.Valiente-Alandi I, Schafer AE, Blaxall BC. Extracellular matrix-mediated cellular communication in the heart. J Mol Cell Cardiol. 2016;91:228–237. doi: 10.1016/j.yjmcc.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P. The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol. 2015;6:150. doi: 10.3389/fimmu.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson-Sjöland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, Rydell-Törmänen K, Bjermer L, Malmström A, Karlsson JC, Westergren-Thorsson G. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology. 2015;25:243–251. doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampson PM, Boyd RB, Pietra GG, Fishman AP. Glycosaminoglycan biosynthesis in the isolated perfused rat lung. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:1648–1654. doi: 10.1152/jappl.1984.57.6.1648. [DOI] [PubMed] [Google Scholar]
- 11.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 12.Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fässler R, Zimmermann DR. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J Neurosci. 2009;29:7731–7742. doi: 10.1523/JNEUROSCI.4158-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZW, Zhang JP, Zhou TT, Feng WH, Jiao BH. Does the expression of versican isoforms contribute to the pathogenesis of neurodegenerative diseases? Arch Med Res. 2011;42:258–260. doi: 10.1016/j.arcmed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Cattaruzza S, Schiappacassi M, Ljungberg-Rose A, Spessotto P, Perissinotto D, Mörgelin M, Mucignat MT, Colombatti A, Perris R. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J Biol Chem. 2002;277:47626–47635. doi: 10.1074/jbc.M206521200. [DOI] [PubMed] [Google Scholar]
- 15.Kischel P, Waltregny D, Dumont B, Turtoi A, Greffe Y, Kirsch S, De Pauw E, Castronovo V. Versican overexpression in human breast cancer lesions: Known and new isoforms for stromal tumor targeting. Int J Cancer. 2010;126:640–650. doi: 10.1002/ijc.24812. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Chen L, Zheng PS, Yang BB. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002;277:12294–12301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- 17.Yang BL, Zhang Y, Cao L, Yang BB. Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem. 1999;72:210–220. doi: 10.1002/(SICI)1097-4644(19990201)72:2<210::AID-JCB5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Ang LC, Zhang Y, Cao L, Yang BL, Young B, Kiani C, Lee V, Allan K, Yang BB. Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J Neuropathol Exp Neurol. 1999;58:597–605. doi: 10.1097/00005072-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–161. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013;280:2165–2179. doi: 10.1111/febs.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Xu GL, Jia WD, Ma JL, Li JS, Ge YS, Ren WH, Yu JH, Liu WB. Ligation of TLR2 by versican: A link between inflammation and metastasis. Arch Med Res. 2009;40:321–323. doi: 10.1016/j.arcmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Wang HM, Bodenstein M, Markstaller K. Overview of the pathology of three widely used animal models of acute lung injury. Eur Surg Res. 2008;40:305–316. doi: 10.1159/000121471. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induce acute lung injury model in respiratory medicine. Expert Rev Respir Med. 2010;4:773–783. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- 25.Netea MG, van Deuren M, Kullberg BJ, Cavaillon JM, Van der Meer JW. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002;23:135–139. doi: 10.1016/S1471-4906(01)02169-X. [DOI] [PubMed] [Google Scholar]
- 26.Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, Secher T, Gasse P, Lima C, Coelho FR, et al. Toll-like receptor and tumor necrosis factor dependent endotoxin-induced acute lung injury. Int J Exp Pathol. 2007;88:387–391. doi: 10.1111/j.1365-2613.2007.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNF alpha in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fink L, Seeger W, Ermert L, Hänze J, Stahl U, Grimminger F, Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med. 1998;4:1329–1333. doi: 10.1038/3327. [DOI] [PubMed] [Google Scholar]
- 29.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM, Acute Lung Injury in Animals Study Group An official American thoracic society workshop report: Features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toeda K, Nakamura K, Hirohata S, Hatipoqlu OF, Demircan K, Yamawaki H, Oqawa H, Kusachi S, Shiratori Y, Ninomiya Y. Versican is induced in infiltrating monocytes in myocardial infarction. Mol Cell Biochem. 2005;280:47–56. doi: 10.1007/s11010-005-8051-4. [DOI] [PubMed] [Google Scholar]
- 31.Asplund A, Fridén V, Stillemark-Billton P, Camejo G, Bondjers G. Macrophages exposed to hypoxia secrete proteoglycans for which LDL has higher affinity. Atherosclerosis. 2011;215:77–81. doi: 10.1016/j.atherosclerosis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Gill S, Wight TN, Frevert CW. Proteoglycans: Key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec (Hoboken) 2010;293:968–981. doi: 10.1002/ar.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, Wight TN, Frevert CW. Reprint of: A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol. 2014;35:162–173. doi: 10.1016/j.matbio.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westergren-Thorsson G, Antonsson P, Malmström A, Heinegård D, Oldberg A. The synthesis of a family of structurally related proteoglycans in fibroblasts is differently regulated by TFG-beta. Matrix. 1991;11:177–183. doi: 10.1016/S0934-8832(11)80156-3. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Hu G, Zhou J. Repression of versican expression by microRNA-143. J Biol Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raabe T, Bukrinsky M, Currie RA. Relative contribution of transcription and translation to the induction of tumor necrosis factor-alpha by lipopolysaccharide. J Biol Chem. 1998;273:974–980. doi: 10.1074/jbc.273.2.974. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Miao L, Wang L. Inflammation amplification by versican: The first mediator. Int J Mol Sci. 2012;13:6873–6882. doi: 10.3390/ijms13066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang F, Wu KY, Wan HY. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS One. 2013;8:e56616. doi: 10.1371/journal.pone.0056616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said N, Sanchez-Carbayo M, Smith SC, Theodorescu D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Invest. 2012;122:1503–1518. doi: 10.1172/JCI61392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuyama A, Tanaka K, Kakizaki I, Kasai K, Chiba M, Nakamura T, Mizunuma H. Anti-inflammatory effect of proteoglycan and progesterone on human uterine cervical fibroblasts. Life Sci. 2012;90:484–488. doi: 10.1016/j.lfs.2011.12.024. [DOI] [PubMed] [Google Scholar]