Abstract
Cuscutae semen has been shown to have beneficial effects in the treatment of vitiligo, recorded in the Chinese Pharmacopoeia, whereas the effects of its constituent compounds remains to be elucidated. Using a tetrazolium bromide assay, the present study found that hyperoside (0.5–200 µg/ml) significantly increased the viability of human melanocytes in a time- and dose-dependent manner. The present study used a cell model of hydrogen peroxide (H2O2)-induced oxidative damage to examine the effect of hyperoside on human primary melanocytes. The results demonstrated that hyperoside pretreatment for 2 h decreased cell apoptosis from 54.03±9.11 to 17.46±3.10% in the H2O2-injured melanocytes. The levels of oxidative stress in the mitochondrial membrane potential of the melanocytes increased following hyperoside pretreatment. The mRNA and protein levels of B-cell lymphoma-2/Bcl-2-associated X protein and caspase 3 were regulated by hyperoside, and phosphoinositide 3-kinase/AKT and mitogen-activated protein kinase signaling were also mediated by hyperoside. In conclusion, the results of the present study demonstrated that hyperoside protected the human primary melanocytes against oxidative damage.
Keywords: hyperoside, apoptosis, oxidative stress
Introduction
Vitiligo is a common type of dermatosis, characterized by depigmentation of the skin and mucosae due to the loss of melanocytes, most likely as a result of autoimmune effects (1). It is predominantly caused by the destruction of melanocytes in the skin, mucous membranes and the retina, which results in white patches of skin on different parts of the body (1,2). The hair growing in these vitiligo-affected areas usually turns white. At present, leucoderma treatment is predominantly focused on drug therapy, surgical treatment and physical therapy (3). The transplantation of cultured autologous pure melanocytes contributes significantly in leucoderma therapy. Melanocytes are considered to be more vulnerable to the damaging effects of oxidative stress, compared with keratinocytes and fibroblasts (4–6). Oxidative stress is also one of the inducing factors causing vitiligo (7). Therefore, the repair of injured melanocytes and the renewal of melanocytes are key for the treatment of vitiligo.
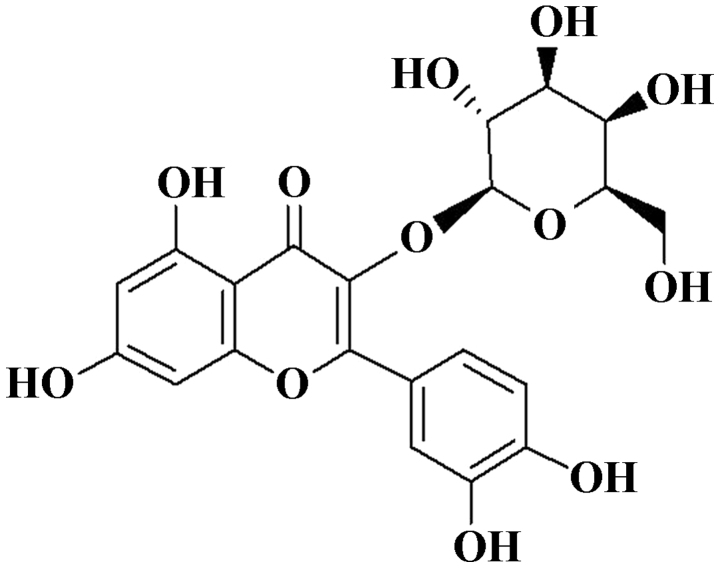
Traditional Chinese medicine has been used for various disease therapies in China for thousands of years (8). Cuscutae semen is the dry root of Cuscuta australis and Cuscuta chinensis, which has been used for treating various kidney conditions in China (9,10). It has also long been used for drinking (11). According to the Chinese Pharmacopoeia (2005, 2010) (12,13), Cuscutae semen has favorable effects on vitiligo treatment, and it is Component of the Chinese herbal compound prescription, Chi Tu Ting, which is used extensively in the treatment of leucoderma (14). Bioactive compounds, including alkaloids, anthraquinones, hyperoside, flavonoids, glycosides, sterols, tannic acid and saccharides are secondary metabolites found in Cuscutae semen (15,16). However, there has been little screening of the specific compounds closely associated with the effect of Cuscutae semen on vitiligo. In our previous study, six compounds obtained from Cuscutae semen, including quercetin, astragalin, quercetin-3-O-β-D-galactoside-7-O-β-glucoside, β-carotene, lutein and hyperoside [2-(3,4-dihydro xyphenyl)-3-(B-D-gala-ctopyranosyloxy)-5,7-dihydroxy] and hyperoside (Fig. 1) exhibited significant effects in melanogenesis.
Figure 1.
Chemical structure of hyperoside.
In the present study, the protective ability of hyperoside against hydrogen peroxide (H2O2)-induced damage in melanocytes was investigated, and the possible mechanisms involved was examined. The results of these investigations aimed to provide novel direction and understanding for the treatment of vitiligo.
Materials and methods
Melanocyte culture
Human primary epidermal melanoyctes (cat. no. PCS-200-012) were purchased from American Type Culture Collection (ATCC; Rockville, MD, USA) and cultured in dermal cell basal medium supplemented with a melanocyte growth kit and antimicrobials/antimycotics (0.5 ml gentamicin-amphotericin B and 0.5 ml penicillin-streptomycin-amphotericin B) (ATCC). All cultures were incubated in a humidified incubator with 5% CO2 at 37°C.
Hyperoside
Hyperoside, with a purity of 98.78%, was obtained as a canary yellow needle-shaped crystal (Nanjing Zelang Medical Technological Co., Ltd., Nanjing, China). The hyperoside was dissolved in an appropriate volume of dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) and diluted to the desired concentrations prior to use, with the final concentration of DMSO maintained <0.5%.
Cell viability
A standard tetrazolium bromide (MTT) assay was used to assess cell viability. Briefly, the melanocytes (5×103 cells/well) were seeded into 96-well plates. The cells were treated with hyperoside (0, 2, 10 and 50 µg/ml) for 2 h at 37°C, following which the melanocytes were exposed to H2O2 (200 µM) for 24 h at 37°C. Subsequently, 50 ml MTT (Sigma-Aldrich, St. Louis, MO, USA) solution (2 mg/ml) in phosphate-buffered saline (PBS) was added to each well and incubated for an additional 4 h at 37°C. The medium was then removed, and the cells were incubated with 200 µl DMSO in the dark for 30 min to dissolve the violet crystals. The absorbance was read at 570 nm on an automatic microplate reader (550; Bio-Rad Laboratories, Inc., Hercules, CA, USA), with DMSO as a blank control. All assays were performed in multiples of give and repeated at least three times.
Cell apoptosis assessment
Following treatment with hyperoside (0, 2, 10 and 50 µg/ml) for 2 h, the H2O2 (200 µM)-treated melanocytes were stained with Annexin V-propium iodide (Beyotime Institute of Biotechnology, Shanghai, China), and apoptosis rates were analyzed using a flow cytometer (FACS-Calibur; BD Biosciences, Hercules, CA, USA).
Mitochondria membrane potential (MMP)
Rhodamine-123 (Rho-123) dye (Sigma-Aldrich) was used to detect the changes in MMP in the cells. The cells (5×104 cells/well) were cultured in a 24-well plate. Following hyperoside (0, 2, 10 and 50 µg/ml) pretreatment for 2 h, and H2O2 exposure for 24 h, the cells were washed with PBS, incubated with Rho-123 (10 mg/ml) at 37°C for 20 min and subsequently subjected to flow cytometry.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The reverse transcription reactions were performed using M-MuLV reverse transcriptase (Promega Corporation, Madison, WI, USA), following the same protocol as Baek et al (17). Samples were pretreated with DNase (Promega Corporation) for 1 h at 37°C in order to avoid contamination. The qPCR reaction was performed on an ABI 7500 (Applied Biosystems; Thermo Fisher Scientific, Inc.) thermal cycler using 5 µl cDNA template, 1 µl primer pairs (10 µM) and 10 µl of a standard SYBR Green PCR kit (Thermo Fisher Scientific, Inc.) to a final reaction volume of 20 µl. The following cycling parameters were used: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 45 sec and a final extension step at 72°C for 5 min. The relative mRNA expression levels of target genes were compared with GAPDH, which were calculated using the 2−ΔΔCq method (18). The primers used for each gene (synthesized by Generay Biotech Co., Ltd., Shanghai, China) were as follows: B cell lymphoma-2 (Bcl-2), forward 5′-AGACCGAAGTCCGCAGAACC-3′ and reverse 5′-GAGACCACACTGCCCTGTTG-3′ (product 113 bp); Bcl-2-associated X protein (Bax), forward 5′-GCGACTGATGTCCCTGTCTC-3′ and reverse 5′-GGCCTCAGCCCATCTTCTTC-3′ (product 132 bp); caspase 3, forward 5′-AACTGGACTGTGGCATTGAG-3′ and reverse 5′-ACAAAGCGACTGGATGAACC-3′ (product 161 bp); and GAPDH, forward 5′-ATCACTGCCACCCAGAAG-3′ and reverse 5′-TCCACGACGGACACATTG-3′ (product 191 bp). The experiment was repeated three times.
Western blot analysis
The treated and untreated melanocytes were harvested and washed twice with PBS, followed by lysis in ice-cold radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) with freshly added 0.01% protease inhibitor cocktail (Sigma-Aldrich) and incubation on ice for 30 min. The cell lysate was centrifuged at 12,000 × g for 10 min at 4°C. The supernatant (20–30 µg protein) was run on a 10% SDS-PAGE gel and transferred electrophoretically onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membranes were then blocked with 5% skim milk, followed by incubation with primary antibodies at 4°C. Antibodies against rabbit polyclonal Bcl-2 (cat. no. Sc-492; 1:400), and rabbit polyclonal Bax (cat. no. Sc-493; 1:400) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), rabbit polyclonal caspase 3 (cat. no. ab44976; 1:500) was purchased from Abcam (Cambridge, UK). Antibodies against rabbit monoclonal phosphorylated (p)-AKT (cat. no. 4060; 1:1,000), rabbit polyclonal AKT (cat. no. 9272; 1:1,000), rabbit monoclonal p-p38 (cat. no. 4511; 1:1,000), rabbit monoclonal p38 (cat. no. 8690; 1:1,000) and rabbit monoclonal GAPDH (cat. no. 5471; 1:1,500) were purchased from Cell Signaling Technology (Danvers, MA, USA). The blots were then incubated for 1 h at room temperature with goat anti-mouse secondary antibody (cat no. A0216; 1:1,000; Beyotime Institute of Biotechnology) or polyclonal goat anti-rabbit secondary antibody (cat no. A0208; 1:1,000; Beyotime Institute of Biotechnology) and visualized using enhanced chemiluminescence (EMD Millipore).
Statistical analysis
The GraphPad Prism 5.0 software system (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis. Data are expressed as the mean ± standard deviation. Student's t-test was used to compared the differences between two groups, and one-way analysis of variance was used for comparing more than two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
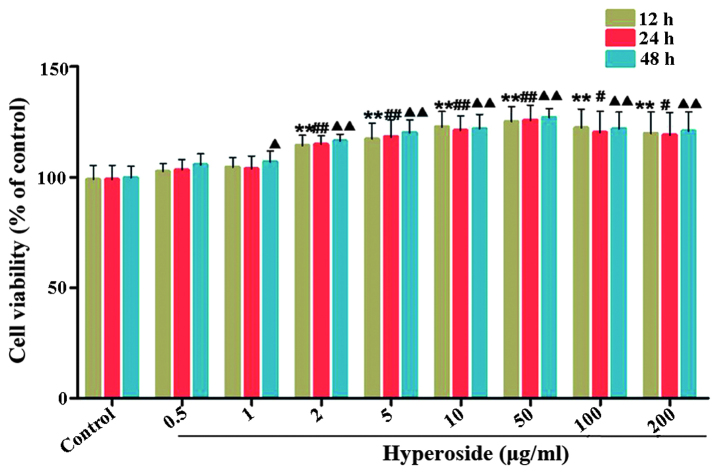
Hyperoside stimulates melanocyte proliferation
For the purpose of evaluating the proliferation promoting ability of hyperoside on melanocytes, cell viability was detected following treatment with different concentrations of hyperoside. As shown in Fig. 2, hyperoside significantly increased the proliferation of the melanocytes in a time- and dose-dependent manner, compared with the control group. However, treatment with high concentrations of hyperoside (100 and 200 µg/ml) had no significant effects on cell viability, compared with the 50 µg/ml hyperoside treatment group. As a result, doses of 5, 10 and 50 µg/ml were selected for treatment in the subsequent investigations.
Figure 2.
Effects of hyperoside on the proliferation of melanocytes. (A) Following exposure of melanocytes to various concentrations of hyperoside (0, 0.5, 1, 2, 5, 10, 50, 100 and 200 µg/ml) for 12, 24 and 48 h, cell viability was determined using a tetrazolium bromide assay. Data are expressed as the mean ± standard deviation (n=6), #, ▲P<0.05 and **, ##, ▲▲P<0.01, vs. control.
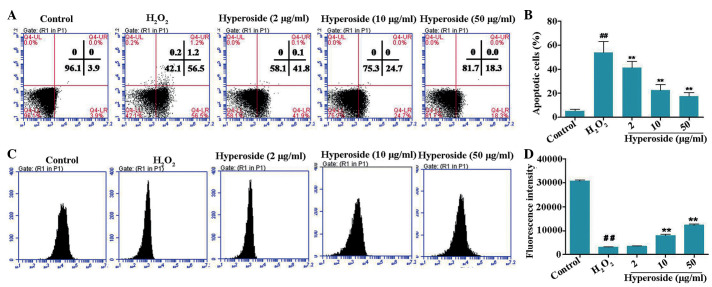
Hyperoside protects melanocytes against H2O2-induced apoptosis
Oxidative damage to human melanocytes is one of the inducing factors causing vitiligo (19). The results of the Annexin V/propidium iodide staining in the present study showed that treatment of the melanocytes with H2O2 (200 µM) resulted in a significant increase in apoptotic rates, compared with the control group (Fig. 3). Pretreatment with hyperoside (2, 10 and 50 µg/ml) for 2 h led to a notable decrease in the apoptotic rates of the melanocytes, compared with the H2O2-treated group. These results indicated the protective effects of hyperoside against H2O2-induced apoptosis in melanocytes.
Figure 3.
Effects of hyperoside on H2O2-induced apoptosis and MMP of human primary melanocytes. (A and B) Melanocytes were treated with different concentrations of hyperoside (0, 2, 10 and 50 µg/ml) for 2 h, and then exposed to H2O2 (200 µM) for 24 h. An Annexin V assay was used for apoptosis detection. (C and D) Melanocytes were treated with different concentrations of hyperoside (0, 2, 10 and 50 µg/ml) for 2 h, anf then exposed to H2O2 (200 µM) for 6 h. A Rhodamine-123 assay was used for MMP detection. Data are presented as the mean ± standard deviation (n=3). ##P<0.01, vs. control; **P<0.01, vs. H2O2-treated melanocytes. H2O2, hydrogen peroxide; MMP, mitochondrial membrane potential.
The breakdown in MMP is an early stage of the apoptotic process. In the present study, the effects of hyperoside on the MMP were evaluated using a Rho-123 assay. As shown in Fig. 3B, a notable reduction in the MMP was observed in the H2O2-treated human primary melanocytes. Compared with the H2O2-treated control cells, 1.91-fold and 3.45-fold increases in MMP were detected in the 10 and 50 µg/ml hyperoside pretreatment groups, respectively.
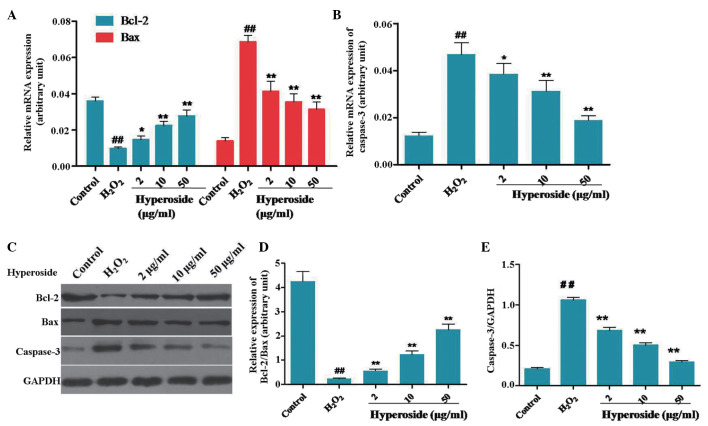
Hyperoside mediates the expression levels of Bcl-2, Bax and caspase 3 in H2O2-induced melanocytes
The relative expression levels of Bcl-2/Bax and caspase 3 are crucial in the process of cell apoptosis (20,21). In the present study, the mRNA and protein expression levels of Bcl-2/Bax and caspase 3 were measured using RT-qPCR and Western blot analyses, respectively. As shown in Fig. 4A, the expression of Bcl-2 was downregulated, and the expression of Bax was upregulated, in the melanocytes exposed to H2O2. Hyperoside effectively increased the relative mRNA expression levels of Bcl-2 and decreased those of Bax. As shown in Fig. 4B, the mRNA expression levels of caspase 3 were enhanced in the melanocytes exposed to H2O2, whereas hyperoside (2, 10 and 50 µg/ml) led to a dose-dependent reduction in the mRNA expression of caspase 3, by 17.23, 64.78 and 79.23%, respectively. As shown in Fig. 4C and D, the relative protein expression levels of Bcl-2/Bax were decreased by H2O2 treatment, whereas hyperoside treatment effectively upregulated the levels of Bcl-2/Bax, in a dose-dependent manner. On examination of the protein levels of caspase 3 by Western blotting, the protein expression levels increased significantly following exposure to H2O2, and decreased significantly following hyperoside treatment (Fig. 4B).
Figure 4.
Effect of hyperoside on the expression levels of Bcl-2, Bax and caspase 3 in H2O2-treated melanocytes. (A and B) Melanocytes were treated with different concentrations of hyperoside (0, 2, 10 and 50 µg/ml) for 2 h, and then exposed to H2O2 (200 µM) for 3 h. Reverse transcription-quantitative polymerase chain reaction analysis was used to determine the mRNA expression levels of Bcl-2, Bax and caspase 3. (C–E) Following the treatment with H2O2 for 6 h, the protein levels of Bcl-2, Bax and caspase 3 were detected using Western blotting. Data are presented as the mean ± standard deviation (n=6), ##P<0.01, vs. control; *P<0.05 and **P<0.01 vs. H2O2-treated melanocytes. Bcl-2, B cell lymphoma-2; Bax, Bcl-2-associated X protein; H2O2, hydrogen peroxide.
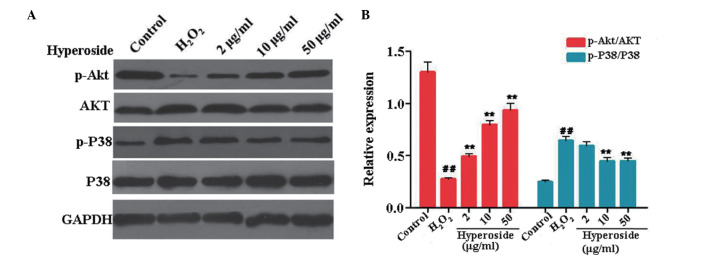
Hyperoside regulates PI3K/AKT and MAPK signaling H2O2-treated melanocytes
PI3K/AKT and MAPK signaling are crucial in cell apoptosis, proliferation, differentiation and various cellular functions (22,23). The phosphorylation of AKT exerts a protective effect in cell apoptosis, whereas the phosphorylation of p38 MAPK stimulates the process of apoptosis (24,25). In the present study, Western blot analysis was performed to evaluate the phosphorylation of AKT and p38. As shown in Fig. 5A and B, the levels of p-AKT/AKT in the melanocytes treated with H2O2 were markedly lower, compared with those in the control, and the expression levels of p-AKT/AKT in the groups pretreated with different doses of hyperoside (2, 10 and 50 µg/ml) were increased by 83.3, 103.1 and 236.5%, respectively, compared with those of the H2O2-treated group. By contrast, the expression of p-p38/p38 was increased on exposure to H2O2, and reduced following hyperoside treatment (Fig. 5A and B).
Figure 5.
Effect of hyperoside on the expression levels of p-AKT and p-p38 in H2O2-treated melanocytes. (A and B) Melanocytes were treated with different concentrations of hyperoside (0, 2, 10 and 50 µg/ml) for 2 h, and then exposed to H2O2 (200 µM) for 6 h. Western blot analysis was performed to identify the protein levels of p-AKT, AKT, p-p38 and p38 in the menlanocytes, and GAPDH was detected as a sample loading control. Data are presented as the mean ± standard deviation (n=3). ##P<0.01, vs. control; **P<0.01, vs. H2O2-treated melanocytes. p-, phosphorlyated; H2O2, hydrogen peroxide.
Discussion
The repair of injured melanocytes is one of the most important driving forces in the treatment of vitiligo. As recorded in the Chinese Pharmacopoeia (2005, 2010), Cuscutae semen shows beneficial effect in vitiligo treatment, which is also contained within the prescribed Chinese herbal compound, Chi Tu Ding, which is used extensively for the treatment of leucoderma (14). Wang et al reported that an ethanol fraction from Cuscutae semen significantly affected melanogenesis by regulating the enzymatic activity of tyrosinase in zebrafish (26). Ma et al (27) demonstrated that the ethanol extract of Cuscutae semen was effective in inducing the adhesion and migration of melanocytes, and offered potential in the treatment of vitiligo treatment. However, the pharmacodyamic material basis of Cuscutae semen in the vitiligo treatment remains to be elucidated. In the present study, six compounds from Cuscuta australis were obtained, and hyperoside was found to exhibit marked effects on the induction of melanogenesis in human primary melanocytes.
Melanocytes are considered to be more vulnerable to the damaging effects of oxidative stress, compared with keratinocytes and fibroblasts (4–6), and oxidative stress is one of inducing factors causing vitiligo. In the present study, it was shown that hyperoside significantly reduced the apoptosis of cultured human melanocytes treated with H2O2. PI3K/AKT and MAPK signaling are reported to be important regulators of cell apoptosis. The phosphorylation of AKT exerts protective effects in cell apoptosis, whereas the phosphorylation of p38 MAPK stimulates the process of apoptosis (28,29). H2O2-treatment significantly decreased the phosphorylation of AKT, but increased the phosphorylation of p38. Pretreament with hyperoside partially reversed these effects of on the phosphorylation of AKT and p38. Taken together, these data demonstrated that hyperoside protected the human primary melanocytes against H2O2-induced apoptosis via the regulation of PI3K/AKT and p38 signaling.
Mitochondrial dysfunction caused by oxidative stress can result in a decease in MMP levels (30). In the present study, the MMP levels of the H2O2-treated melanocytes pretreated with hyperoside were notably increased, comparison with those of the H2O2-only treated melanocytes. The loss of MMP causes an increase in the permeability of the MMP, followed by the release of pro-apoptotic molecules, including cytochrome c. The release of cytochrome c from the mitochondria interacts with ATP, Apaf-1 and caspase 9, and subsequently activates caspase 3, which consequently elicits caspase-dependent apoptotic cell death (31). In the present study, the mRNA and protein expression levels of casepase 3 in the H2O2-treated melanocytes with hyperoside pretreatment were significantly decreased, compared with those in the H2O2-treated melanocytes without pretreatment. These results indicated that hyperoside showed protective effects towards the human primary melanocytes from oxidative damage by inhibiting the mitochondrial apoptotic pathway.
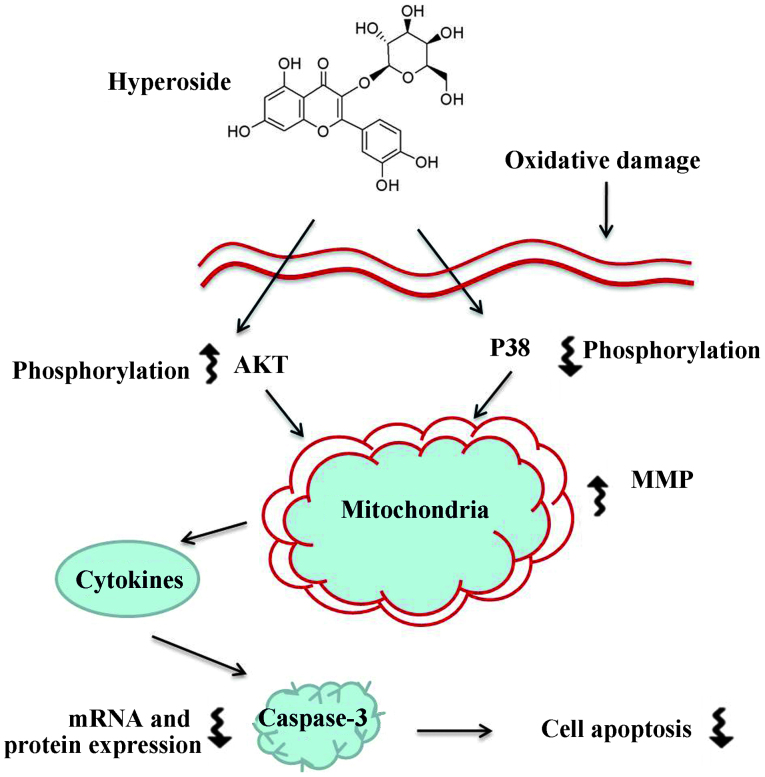
Taken together, the results of the present study lead to the hypothesis that hyperoside protects melanocytes against oxidative damage by activating AKT, inhibiting p38 phosphorylation and suppressing mitochondrial apoptosis signaling,. These findings may provide further insight into vitiligo therapy (Fig. 6), and hyperoside may be a useful therapeutic agent in the treatment of vitiligo.
Figure 6.
Mechanisms of hyperoside-induced enhancement of melanogenesis, and the protection of human primary melanocytes against oxidative stress. MMP, mitochondrial membrane potential.
Acknowledgments
This study was supported by the China Postdoctoral Science Foundation (grant no. 2014M562671) and the National Natural Science Foundation (grant no. 81201243).
References
- 1.Ruiz-Argüelles A, Brito GJ, Reyes-Izquierdo P, Pérez-Romano B, Sánchez-Sosa S. Apoptosis of melanocytes in vitiligo results from antibody penetration. J Autoimmun. 2007;29:281–286. doi: 10.1016/j.jaut.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg NB. Recent advances in childhood vitiligo. Clin Dermatol. 2014;32:524–530. doi: 10.1016/j.clindermatol.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Guerra L, Dellambra E, Brescia S, Raskovic D. Vitiligo: Pathogenetic hypotheses and targets for current therapies. Curr Drug Metab. 2010;11:451–467. doi: 10.2174/138920010791526105. [DOI] [PubMed] [Google Scholar]
- 4.Liu CF, Hu CL, Chiang SS, Tseng KC, Yu RC, Pan TM. Beneficial preventive effects of gastric mucosal lesion for soy-skim milk fermented by lactic acid bacteria. J Agric Food Chem. 2009;57:4433–4438. doi: 10.1021/jf900465c. [DOI] [PubMed] [Google Scholar]
- 5.Hoogduijn MJ, Cemeli E, Ross K, Anderson D, Thody AJ, Wood JM. Melanin protects melanocytes and keratinocytes against H2O2-induced DNA strand breaks through its ability to bind Ca2+ Exp Cell Res. 2004;294:60–67. doi: 10.1016/j.yexcr.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Valverde P, Manning P, Todd C, McNeil CJ, Thody AJ. Tyrosinase may protect human melanocytes from the cytotoxic effects of the superoxide anion. Exp Dermatol. 1996;5:247–253. doi: 10.1111/j.1600-0625.1996.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 7.Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, Ramachand AV, Dalai S, Begum R. Vitiligo: Interplay between oxidative stress and immune system. Exp Dermatol. 2013;22:245–250. doi: 10.1111/exd.12103. [DOI] [PubMed] [Google Scholar]
- 8.He X, Yang W, Ye M, Wang Q, Guo D. Differentiation of Cuscuta chinensis and Cuscuta australis by HPLC-DAD-MS analysis and HPLC-UV quantitation. Planta Med. 2011;77:1950–1957. doi: 10.1055/s-0030-1271186. [DOI] [PubMed] [Google Scholar]
- 9.Tang JL, Liu BY, Ma KW. Traditional chinese medicine. The Lancet. 2008;372:1938–1940. doi: 10.1016/S0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- 10.Yang HM, Shin HK, Kang YH, Kim JK. Cuscuta chinensis extract promotes osteoblast differentiation and mineralization in human osteoblast-like MG-63 cells. J Med Food. 2009;12:85–92. doi: 10.1089/jmf.2007.0665. [DOI] [PubMed] [Google Scholar]
- 11.Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats. Food Chem Toxicol. 2008;46:1771–1777. doi: 10.1016/j.fct.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 12.China Pharmacopoeia Committee . Chinese pharmacopoeia. Chinese Medicine Science and Technology Press; Beijing, China: 2005. p. 740. [Google Scholar]
- 13.State Pharmacopoeia Committee . Chinese pharmacopoeia. Chinese Medicine Science and Technology Press; Beijing: 2010. pp. 70–71. [Google Scholar]
- 14.Jang JY, Kim HN, Kim YR, Choi YH, Kim BW, Shin HK, Choi BT. Aqueous fraction from Cuscuta japonica seed suppresses melanin synthesis through inhibition of the p38 mitogen-activated protein kinase signaling pathway in B16F10 cells. J Ethnopharmacol. 2012;141:338–344. doi: 10.1016/j.jep.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 15.Ye M, Yan Y, Ni X, Qiao L. Studies on the chemical constituents of the herba of Cuscuta chinensis. Zhong Yao Cai. 2001;24:339–341. In Chinese. [PubMed] [Google Scholar]
- 16.Ye M, Yan YN, Qiao L, Ni XM. Studies on chemical constituents of Cuscuta chinensis. Zhongguo Zhong Yao Za Zhi. 2002;27:115–117. In Chinese. [PubMed] [Google Scholar]
- 17.Baek A, Park HJ, Na SJ, Shim DS, Moon JS, Yang Y, Choi YC. The expression of BAFF in the muscles of patients with dermatomyositis. J Neuroimmunol. 2012;249:96–100. doi: 10.1016/j.jneuroim.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 18.VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–626. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- 19.Schallreuter KU, Moore J, Wood JM, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. The journal of investigative dermatology. 1999;4:91–96. doi: 10.1038/sj.jidsp.5640189. Symposium proceedings/the Society for Investigative Dermatology, Inc and European Society for Dermatological Research. [DOI] [PubMed] [Google Scholar]
- 20.Li CP, Li JH, He SY, Li P, Zhong XL. Roles of Fas/Fasl, Bcl-2/Bax, and Caspase-8 in rat nonalcoholic fatty liver disease pathogenesis. Genet Mol Res. 2014;13:3991–3999. doi: 10.4238/2014.May.23.10. [DOI] [PubMed] [Google Scholar]
- 21.Sharifi S, Barar J, Hejazi MS, Samadi N. Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of breast cancer cells to paclitaxel. Asian Pac J Cancer Prev. 2014;15:8617–8622. doi: 10.7314/APJCP.2014.15.20.8617. [DOI] [PubMed] [Google Scholar]
- 22.CV SB, Babar SM, Song EJ, Oh E, Yoo YS. Kinetic analysis of the MAPK and PI3K/Akt signaling pathways. Mol Cells. 2008;25:397–406. [PubMed] [Google Scholar]
- 23.Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH, Kim BW, Choi HY, Jeong MY, Cho SG. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta. 2006;1763:958–968. doi: 10.1016/j.bbamcr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 26.Wang TJ, An J, Chen XH, Deng QD, Yang L. Assessment of Cuscuta chinensis seeds effect on melanogenesis: Comparison of water and ethanol fractions in vitro and in vivo. J Ethnopharmacol. 2014;154:240–248. doi: 10.1016/j.jep.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Zhang X, Mu K, Feng J, Niu X, Liu C, Dang Q. Effects of herb extracts on melanocyte adhesion and migration. Zhongguo pifu xingbingxue zazhi. 2004;18:526–527. In Chinese. [Google Scholar]
- 28.Sabine VS, Crozier C, Brookes CL, Drake C, Piper T, van de Velde CJ, Hasenburg A, Kieback DG, Markopoulos C, Dirix L, et al. Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol. 2014;32:2951–2958. doi: 10.1200/JCO.2013.53.8272. [DOI] [PubMed] [Google Scholar]
- 29.Toda M, Kuo CH, Borman SK, Richardson RM, Inoko A, Inagaki M, Collins A, Schneider K, Ono SJ. Evidence that formation of vimentin mitogen-activated protein kinase (MAPK) complex mediates mast cell activation following FcεRI/CC chemokine receptor 1 cross-talk. J Biol Chem. 2012;287:24516–24524. doi: 10.1074/jbc.M111.319624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavalcanti BC, da Costa PM, Carvalho AA, Rodrigues FA, Amorim RC, Silva EC, Pohlit AM, Costa-Lotufo LV, Moraes MO, Pessoa C. Involvement of intrinsic mitochondrial pathway in neosergeolide-induced apoptosis of human HL-60 leukemia cells: The role of mitochondrial permeability transition pore and DNA damage. Pharm Biol. 2012;50:980–993. doi: 10.3109/13880209.2012.654921. [DOI] [PubMed] [Google Scholar]
- 31.Huang WJ, Bi LY, Li ZZ, Zhang X, Ye Y. Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the IGF-1/IGF-1R signaling pathway. Pharm Biol. 2013 doi: 10.3109/13880209.2013.842600. [DOI] [PubMed] [Google Scholar]