Abstract
Cordyceps militaris (CM), an entomopathogenic fungus belonging to the class ascomycetes, possesses various pharmacological activities, including cytotoxic effects, on various types of human tumor cells. The present study investigated the anti-hepatocellular carcinoma (HCC) and anti-breast cancer effects of CM in in vitro and in vivo models. CM aqueous extract reduced cell viability, suppressed cell proliferation, inhibited cell migration ability, caused the over-release of lactate dehydrogenase, induced mitochondrial dysfunction and enhanced apoptotic rates in MCF-7 and HepG2 cells. The expression levels of cleaved poly (ADP ribose) polymerase and caspase-3, biomarkers of apoptosis, were increased following treatment with CM aqueous extract for 24 h. Furthermore, in the MCF-7 and HepG2 cells, enhanced levels of B cell-associated X protein and cleaved caspase-8 were observed in the CM-treated cells. Finally, the antitumor activities of CM in HCC and breast cancer were also confirmed in MCF-7- and HepG2-xengraft nude mice models. Collectively, the data obtained in the present study suggested that the cytotoxic effects of CM aqueous extract on HCC and breast cancer are associated with the caspase-dependent mitochondrial pathway.
Keywords: Cordyceps militaris, hepatocellular carcinoma, breast cancer, apoptosis, mitochondrial, caspase
Introduction
Breast cancer is reported to be the most common type of cancer diagnosed among women (1). Despite advances in medicine, the disease remains a substantial health problem worldwide. According to statistics, 1,500,000 cases of breast cancer were diagnosed in 2010, representing almost one quarter of all the cases of cancer diagnosed in women (2). Primary liver cancer, particularly hepatocellular carcinoma (HCC), continues to be a growing global health problem, and has become the third most common cause of cancer-associated mortality worldwide, accounting for a mortality rate of >800,000/year (2,3). The 5-year survival rate of HCC is <10% (4). The results of standard chemotherapy and radiotherapy for the treatment of patients with breast cancer and HCC have remained unsatisfactory (5,6). Despite efforts, no drugs have been manufactured for the satisfactory treatment of breast cancer and HCC in the past decade. Therefore, more effective alternative therapies or drugs with low side effect are required for the treatment of breast cancer and HCC.
Natural products have gradually became one of the most productive strategies in the development of antitumor agents (6), which possess potent cytotoxic abilities with fewer adverse effects. Neem leaf aqueous extract induces granulosa cell apoptosis via the mitochondria-caspase-mediated pathway (7). Parmotrema reticulatum, a tropical lichen, increases the B cell lymphoma-2 (Bcl-2)-associated X protein (Bax)/Bcl-2 ratio and activates the caspase family leading to apoptosis in MCF-7 cells (8). Cordyceps militaris (CM), an entomopathogenic fungus belonging to the class ascomycetes, is usually used as a traditional tonic in China and East Asia (9). A complex composition has been observed in the CM fruiting body, which is responsible for its various pharmacological activities (10). CM aqueous extract induces apoptotic MDA-MB-231 cell death via regulation of the phosphoinositide 3-kinase/AKT-associated mitochondrial pathway (11). CM inhibits B16-F10-xenograft tumor growth in C57BL/6 mice, associated with its angiogenic property (12). Due to activation of the caspase-associated pathway, CM aqueous extract also suppresses human premyelocytic leukemia cell growth (13). Although the cytotoxicity of CM towards MCF-7 and HepG2 cells has been reported (14,15), the underlying mechanisms remain to be elucidated.
As an energy-dependent process, during apoptosis, living cells are involved in their own death in an organized manner, which is associated with various signaling pathways (16). Mitochondrial apoptosis, one of three death signaling pathways (17), is accompanied by mitochondrial depolarization, abnormal expression of members of the Bcl-2 family, the over-release of cytochrome c, and caspase-3 activation (18,19). Caspase-3 is generally considered to be an important effector protease during apoptosis (18), the proteolytic maturation of which is catalyzed by initiator caspases (caspase-8, -9 and -10) (20). The auto-catalytic activation of procasapase-8 in extrinsic apoptosis leads to the decrease of mitochondrial membrane permeability (Δψm) (21,22).
The purpose of the present study was to investigate the in vitro and in vivo pro-apoptotic effects of CM on HCC and breast cancer. The results revealed that CM induced MCF-7 and HepG2 cell apoptosis, predominantly through the caspase-dependent mitochondrial pathway. The results of the present study indicated the potential for the addition of CM to the list of possible agents for the treatment of HCC and breast cancer.
Materials and methods
CM extract preparation
Extraction of the CM fruiting body (purchased from Qianxiang Co., Ltd., Shenyang, China) was performed at 45°C for 3 h, followed by extraction at 80°C for another 3.5 h in double distilled water (D.D. water). The merging supernatant was concentrated in an R1002B evaporator (Shanghai Shensheng Technology Co., Ltd., Shanghai, China) under reduced pressure (<10 kPa), and was further freeze-dried to produce a solid aqueous extract. Analysis of the data revealed that the CM fruiting body water extract contained 29.04% polysaccharides, 20.45% total proteins, 6.01% cordycepic acid, 0.204% adenosine and 0.347% cordycepin. The polysaccharides were measured via phenol-sulfuric acid method (23), the total proteins were analysed via Kjeldahl method (24), and the cordycepic acid, adenosine and cordycepin were analysed via high performance liquid chromatography (25).
Cell culture
HepG2 cells (human HCC line; HB-8065) and MCF-7 cells (human breast carcinoma cell line; HTB-22) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (all obtained from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) under a humidified atmosphere containing 5%/95% CO2/air at 37°C. The cultured medium was refreshed every 3 days. The cells were ready for treatment when the confluence of cells in the plates reached. All reagents used for cell culture were obtained from Invitrogen; Thermo Fisher Scientific, Inc.
Cell viability analysis
The cell viability was measured using a 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-pheny-tetrazolium-romide (MTT; Sigma-Aldrich, St. Louis, MO, USA) assay (26). The MCF-7 and HepG2 cells were seeded into 96-well plates at a density of 5,000 per well for 24 h. Following treatment with CM at doses of 0.1, 0.2, 0.4, 1.0 and 2.0 mg/ml for 24 h, the cells were incubated with 0.5 mg/ml MTT for 4 h at 37°C in the dark. Purple formazan crystals were solubilized by adding 100 µl dimethyl sulfoxide, and the absorbance was measured using an iMark microplate reader at a wavelength of 490 nm (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Lactate dehydrogenase (LDH) release analysis
The MCF-7 and HepG2 cells were seeded into 24-well plates at a density of 5×104 per well. Following treatment with CM at doses of 0.1, 0.2, 0.4, 1.0 and 2.0 mg/ml for 24 h, the cultured medium was collected and centrifuged at 1,000 × g for 5 min at 4°C. The LDH released into the culture medium was measured using an LDH assay kit (cat. no. 20141205; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Colony formation assays
Crystal violet staining was performed to examine the colony formation. Briefly, the MCF-7 and HepG2 cells were seeded into 6-well plates at a density of 5×104 cells/well. The cells were treated with CM at 0.1, 0.2, 0.4, 1.0 and 2.0 mg/ml for 7 days. The medium was replaced, either with DMEM only or the CM in complete DMEM, every 2 days. Following treatment, the cells were fixed in 75% methanol for 10 min at 4°C and stained with 0.1% crystal violet (Sigma-Aldrich) for 30 min. Images were subsequently captured.
Apoptosis analysis via Hoechst staining
The MCF-7 and HepG2 cells were seeded into 6-well plates at a density of 2×105 cells/well. The cells were treated with CM at 0.5, 1.0 and 2.0 mg/ml for 24 h. The treated cells were then incubated with Hoechst 33342 (5 µg/ml; BD Biosciences, San Jose, CA, USA) for 30 min at 37°C in the dark. Following three washes with phosphate-buffered saline (PBS), images of the altered fluorescent color in the mitochondria were captured using a fluorescent microscope (magnification, x20; charge-coupled device camera; TE2000; Nikon Corporation, Tokyo, Japan). The percentage of apoptotic cells was analyzed by measuring the fluorescence intensity using Image J software (rsb.info.nih.gov/ij/download.html) and expressed as the ratio of red to green fluorescence intensity.
Migration assay
The cells were plated in 6-well plates (4×104 cells/well) and cultured to >90% confluence, following which the cell layer was scraped with a p200 pipette tip (Shanghai Jingke Scientific Instrument Co., Ltd., Shanghai, China). The cells were treated with CM at 0.5, 1.0 and 2.0 mg/ml for 24 h, following which the distances of the migrating cells were used to evaluate the migratory ability of the cells. The width of the wound was expressed as a percentage of the control group.
Assessment of Δψm
To determine the alterations in Δψm in the cells, 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide (JC-1; Sigma-Aldrich), which selectively enters mitochondria, was used. The MCF-7 and HepG2 cells were seeded into 6-well plates at a density of 2×105 cells/well. The cells were treated with CM at doses of 0.5, 1.0 and 2.0 mg/ml for 12 h. The treated cells were then incubated with 2 µM JC-1 at 37°C for 20 min in the dark. Following three washes with PBS, alterations in the fluorescent color in the mitochondria were analyzed using fluorescent microscopy (magnification, ×20; charge-coupled device camera; TE2000; Nikon Corporation). Red fluorescence indicated healthy cells with a high Δψm, whereas green fluorescence indicated apoptotic or unhealthy cells with a low Δψm.
MCF-7- and HepG2-xenograft tumor models
The present study was approved by the ethics committee of Changchun University (Changchun, China). Male 6-week-old BALB/c athymic nude mice, purchased from Weitonglihua Laboratory Animal Technology Co., Ltd. (Beijing China; SCXK 2012-0001), were used for the in vivo experiments in the present study. The experimental animal protocol was approved by the Animal Ethics Committee of Jilin University (Changchun, China). The mice were housed in groups of two in clear plastic cages, and were maintained on a 12 h light/dark cycle at 23±1°C with water and food available ad libitum.
The tumors were generated by harvesting MCF-7 and HepG2 cells from mid-log phase cultures. A volume of 0.1 ml (1×108 cells/ml) of MCF-7 or HepG2 cell suspension was subcutaneously injected into the right side of the waist of each mouse. After 4 days, when the largest diameter of the tumors measured 2–3 mm, the mice were randomly divided into two groups (n=3 each). The mice were administered with 1 g/kg CM (treated group) or D.D. water (vehicle group) orally every other day continuously for 2 weeks. The tumor dimensions and body weights were measured every other day. Tumor volume (mm3) was estimated using the following equation: Tumor volume (mm3) = Length × (width)2 × 0.5. At the end of the experiment, the mice were sacrificed by administration of 200 mg/kg pentobarbital (Sigma-Aldrich). The tumor tissues were carefully dissected from each mouse and stored at −130°C prior to western blot analysis.
Western blot analysis
The cells were plated into 6-well plates at a density of 2×105 cells per well. The following day, the cells were treated with CM (0.5, 1.0 and 2.0 mg/ml) for 24 h. The cells or tumor tissues were lysed using radioimmunoprecipitation assay buffer (Sigma-Aldrich) containing 1% protease inhibitor cocktail (Sigma-Aldrich) and 2% phenylmethanesulfonyl fluoride (Sigma-Aldrich). Tumor tissues were homogenized using a ZW-A trace vibrator (Ronghua Instrument Manufacturing Co., Ltd.). Lysates were centrifuged at 10,000 × g for 5 mins at 4°C and the concentration of protein was determined by the Coomassie brilliant blue method (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China) The proteins (30 µg) were separated on a 12% SDS-PAGE gel [materials obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China)] and transferred electrophoretically onto nitrocellulose membranes (0.45 µm; Bio Basic, Inc., Markham, ON, Canada). The transferred membranes were then blocked with 5% bovine serum albumin (Sigma-Aldrich) for 4 h at 4°C prior to blotting with the following primary antibodies at 4°C overnight, at a dilution of 1:1,000: Monoclonal rabbit anti-cleaved poly (ADP-ribose) polymerase (PARP; cat. no. ab32064; Abcam, Cambridge, UK), polyclonal rabbit anti-cleaved caspase-3 (cat. no. ab13847; Abcam), poly-clonal rabbit anti-cleaved caspase-8 (cat. no. ab25901; Abcam), monoclonal rabbit anti-Bax (cat. no. ab32503; Abcam) and polyclonal rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (cat. no. ABS16; EMD Millipore, Billerica, MA,USA). The membranes were subsequently incubated with horseradish peroxidase-conjugated mouse anti-rabbit secondary antibody (cat. no. sc-2357; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Chemiluminescence was analyzed using Amersham ECL Western Blotting Detection reagent (cat. no. RPN2106; GE Healthcare, Buckinghamshire, UK). The intensities of the bands were quantified by scanning densitometry using Image J software.
Statistical analysis
All data are expressed as the mean ± standard deviation and were analyzed using one-way analysis of variance, followed by post-hoc multiple comparison (Dunn's test). SPSS software version 16.0 (SPSS, Inc. Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
CM exhibits cytotoxic effects in MCF-7 and HepG2 cells
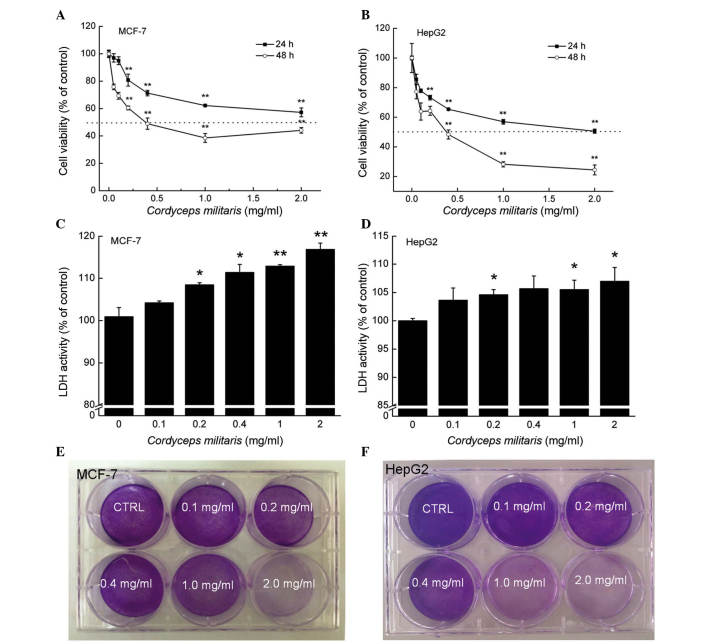
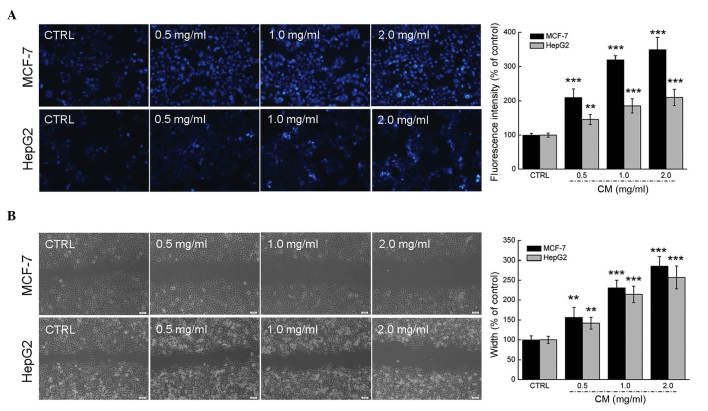
Dose- and time-dependent reductions in cell viability were observed in the MCF-7 and HepG2 cells following incubation with CM. The 24-h half maximal inhibitory concentrations of CM in the MCF-7 and HepG2 cells were ~1.096 and 0.791 mg/ml, respectively (P<0.01; Fig. 1A and B). Following exposure to CM at doses between 0.2 and 2.0 mg/ml for 24 h, 8.45–16.80% increases in LDH release were observed in the MCF-7 cells (P<0.05; Fig. 1C). Similarly, treatment with CM for 24 h (0.2–2.0 mg/ml) enhanced LDH release in the HepG2 cells by 4.6–7.0% (P<0.05; Fig. 1D). The effects of CM on the abilities of the MCF-7 and HepG2 cells to form colonies were monitored for 7 days, the marked inhibitory effect of CM on MCF-7 cell colony formation was apparent at 0.2 mg/ml, and the clonogenic ability of the MCF-7 cells was completely inhibited following treatment with 2.0 mg/ml CM (Fig. 1E). In addition, 0.4–2.0 mg/ml CM exerted significant inhibitory effects on HepG2 cell colony formation (Fig. 1F). Hoechst 33342 staining corroborated that CM at doses between 0.5 and 2.0 mg/ml markedly induced nuclear apoptosis in the MCF-7 and HepG2 cells, indicated by the enhanced intensity of blue fluorescence (Fig. 2A). Additionally, a wound healing assay was performed to observe the inhibitory effect of CM on the migratory abilities of the MCF-7 and HepG2 cell. Following incubation for 24 h, the wound areas in the untreated cells were almost healed. By contrast, the migratory abilities of the MCF-7 and HepG2 cells were significantly inhibited following CM treatment at specific doses (Fig. 2B). Collectively, these data confirmed the cytotoxic properties of CM in MCF-7 and HepG2 cells.
Figure 1.
CM induces cell damage in MCF-7 and HepG2 cells. Dose- and time-dependent suppression in cell viability was observed following treatment with CM for 24 and 48 h in (A) MCF-7 and (B) HepG2 cells. Exposure to CM for 24 h caused over-release of LDH in (C) MCF-7 and (D) HepG2 cells. Data are expressed as the mean ± standard deviation (n=6) and were analyzed using one-way analysis of variance. *P<0.05 and **P<0.01, vs. untreated cells. Treatment with CM for 7 days inhibited (E) MCF-7 and (F) HepG2 cell proliferation, detected using crystal violet staining. CM, Cordyceps militaris; LDH, lactate dehydrogenase; CTRL, control.
Figure 2.
CM increases apoptosis and inhibits migration in MCF-7 and HepG2 cells. (A) CM significantly enhanced the apoptotic rates of the MCF-7 and HepG2 cells. Apoptosis (nuclear morphology) was examined using Hoechst 33342 staining (magnification, ×20). The percentage of apoptotic cells was analyzed by measuring fluorescence intensity via Image J software. (B) CM treatment inhibited the migration ability of the MCF-7 and HepG2 cells, determined using a wound-healing assay (magnification, ×10). Data are expressed as mean ± standard deviation (n=3). **P<0.01 and ***P<0.001 vs. control group. CM, Cordyceps militaris; CTRL, control.
CM causes alterations in apoptosis, mitochondrial function and the expression levels of pro-apoptotic proteins
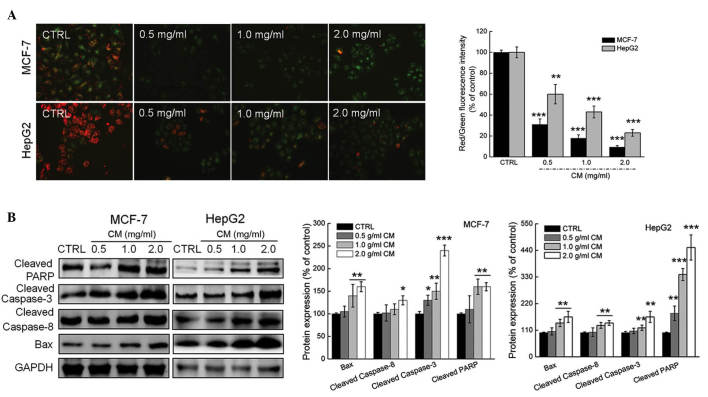
Mitochondrial function is one of the factors responsible for cell apoptosis. JC-1 staining was applied in the present study to analyze the alterations of Δψm. Incubation with CM for 24 h CM caused significant losses of Δψm in the MCF-7 and HepG2 cells, evidenced by enhanced green fluorescence intensity (Fig. 3A).
Figure 3.
Effects of CM on mitochondrial dysfunction and the expressions of pro-apoptotic proteins in MCF-7 and HepG2 cells. (A) Dissipation of Δψm was observed in cells exposed to 0.5–2.0 mg/ml CM for 12 h and quantified as the ratio of red to green fluorescence intensity. (B) CM treatment resulted in an increases in the expression levels of cleaved PARP, cleaved caspase-3 and cleaved caspase-8. The protein expression levels were quantified by densitometry analysis and normalized to the corresponding GAPDH levels. Data are expressed as the mean ± standard deviation (n=3). *P<0.05, **P<0.01, ***P<0.001 vs. control. All experiments were repeated three times. CM, Cordyceps militaris; Δψm, mitochondrial membrane potential; CTRL, control; PARP, Bax, B cell lymphoma-2-associated X protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
As one of the factors involved in the Bcl-2 family, Bax contributes to cell apoptosis and mitochondrial function (27). Treatment with CM at doses between 0.5 and 2.0 mg/ml markedly enhanced the expression levels of Bax in the MCF-7 and HepG2 cells (Fig. 3B). The activation of caspase-3 and PARP is considered as to be a hallmark of apoptosis, and this was enhanced following incubation with CM for 24 h in the present study (Fig. 3B). In addition, increases in the activation of caspase-8 were observed in the CM-treated MCF-7 and HepG2 cells (Fig. 3B). Collectively, CM induced intracellular toxicity, which was associated with its regulation of mitochondrial function and the expression of pro-apoptotic proteins.
CM inhibits MCF-7- and HepG2- xenograft tumor growth in nude mice
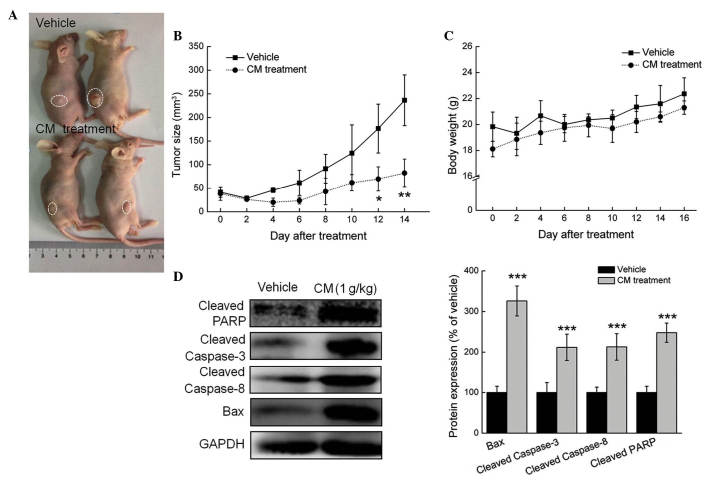
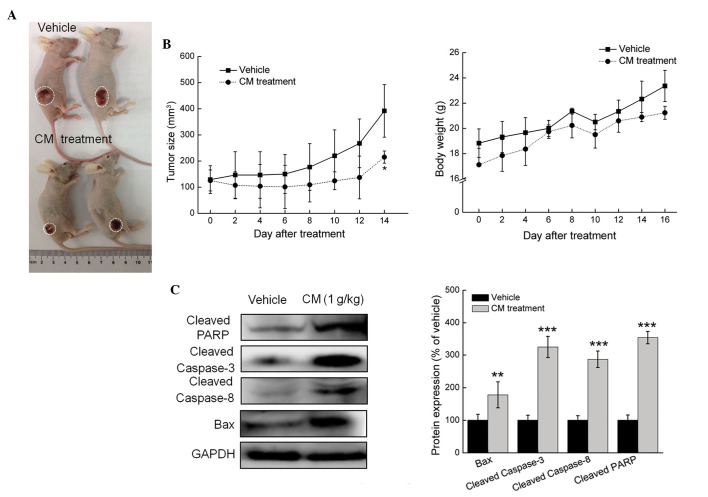
In the MCF-7- and HepG2-xenograft tumor nude mice models, tumor growth inhibition was most marked in the mice treated with 1 g/kg CM for 2 weeks (every other day), where tumor sizes reduced by almost 188.2%, compared with the vehicle-treated mice in the MCF-7 cells (P<0.05; Figs. 4A and B). Similar to the results obtained from the in vitro experiment, the administration of CM for 14 days enhanced the expression levels of cleaved PARP, cleaved caspase-3, cleaved caspase-8 and Bax in the MCF-7- and HepG2-xenograft tumor tissues, compared with the vehicle-treated mice (P<0.05; Fig. 4C). Similar results were observed in the HepG2 cells, with tumor growth inhibited by almost 82.7%, compared with the vehicle-treated mice (Fig. 5A and B), and administration of CM for 14 days enhancing the expression levels of cleaved PARP, cleaved caspase-3, cleaved caspase-8 and Bax (P<0.05; Fig. 5C). These in vivo data provided further evidence of CM-mediated antitumor activities in HCC and breast cancer.
Figure 4.
CM has a suppressive effect in the MCF-7-xenograft nude mice model. Male BALB/c athymic nude mice bearing MCF-7 tumors were treated with phosphate-buffered saline (vehicle) or CM (1 g/kg) via intraperitoneal administration every other day for 14 days. (A) Examples of tumor growth in the animals. (B) Growth curves of MCF-7-xenograft tumors in the CM-and vehicle-treated nude mice. Tumor sizes were measured every 2 days. n=4, analyzed using one-way analysis of variance. *P<0.05 and **P<0.01 vs. vehicle. (C) Body weight of MCF-7-xenografted nude mice. (D) Levels of pro-apoptotic proteins in tumor tissues, detected via western blot analysis. Protein expression levels were quantified by densitometry analysis and normalized to GAPDH. Data are expressed as the mean ± standard deviation. ***P<0.001 vs. vehicle. CM, Cordyceps militaris; CTRL, control; PARP, poly (ADP ribose) polymerase; Bax, B cell lymphoma-2-associated X protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 5.
CM has a suppressive effect in the HepG2-xenograft nude mice model. (A) Examples of tumor growth between CM- and vehicle-treated male BALB/c athymic nude mice bearing HepG2 tumors. (B) Growth curves of HepG2-xenograft tumors in CM- and vehicle-treated nude mice. Tumor sizes were measured every 2 days. Data are expressed as the mean ± standard deviation (n=4) and were analyzed using one-way analysis of variance. *P<0.05, vs. vehicle group. (C) Activation of PARP, caspase-3 and caspase-8, and the levels of Bax in tumor tissues were detected using Western blot analysis. The average fold changes in band intensity, compared with the vehicle group, were marked. **P<0.01 and ***P<0.001 vs. control. CM, Cordyceps militaris; CTRL, control; PARP, poly (ADP ribose) polymerase; Bax, B cell lymphoma-2-associated X protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
The inhibitory activities of CM on MCF-7 and HepG2 cell growth have been reported in previous studies (28–31); however, their associated mechanisms remain to be elucidated. The present study investigated the potential antitumor effects of CM on MCF-7 and HepG2 cells, and examined the possible underlying mechanisms. Compared with other reported bioactive extracts (32,33), the crude drug nature of CM suggests it may have multi-effective components, which may target various molecules. Due to the systemic targeting, less adverse side effects are expected. CM has been used for thousands of years as a crude drug and a traditional medicine in East Asia, further emphasizing its safety and minimal side effects.
Apoptosis is a physiological suicide mechanism, which occurs during normal tissue turnover. Mitochondria, triggered by diverse apoptotic stimuli, commonly exist as the predominant functional organelle for anticancer drugs (34,35). Mitochondria control the intrinsic pathway of apoptosis, in which Δψm induces the activation of caspases and other catabolic enzymes (36,37). The functional loss of mitochondria is accompanied with the dissipation of Δψm (38), which was confirmed in the present study. As reported previously, caspase-8 is located predominantly in the mitochondria, and active caspase-8 forms a complex with the BH3-interacting domain death agonist protein on the mitochondria, which leads to decreased Δψm (39–41). During this process, caspase-8 undergoes dimerization and cleaves itself to become fully activated (39). Large quantities of active caspase-8 can lead to the direct cleavage of effector caspase in the cytosol (42). Bcl-2 family members, which are located in the outer mitochondrial membrane, are essential mediators in the regulation of Δψm (27). Bax, a pro-apoptotic protein of the Bcl-2 family, acts as an important regulatory factor in mitochondria-mediated apoptosis (27). It has been reported that reactivation of the Bax gene induces mitochondrial apoptosis in cholangiocarcinoma cells (43). Collectively, the data obtained in the present study confirmed that the mitochondrial apoptotic pathway was involved in CM-meditated antitumor effects.
In addition, a reduction of Δψm promotes the release of cytochrome c from the mitochondria (44), which leads to the activation of caspase-3 and other apoptosis-inducing molecules (45). Caspase-3 is central in the induction of the apoptotic program (46), and is important for cell death in a tissue-, cell type- or death stimulus-specific manner (46). As reported, caspase-3 is essential for the characteristic changes in cell morphology and certain biochemical events associated with the execution and completion of apoptosis (46–48). Of note, caspase-3-defective embryonic stem cells exhibit marked resistance to apoptosis induced by ultraviolet irradiation and osmotic shock (49). In the present study, the activation of caspase-3 was observed, and the enhanced expression of PARP was also found. PARP is considered to be one of the most important downstream substrates of caspase-3 (50). The activity of PARP is completely dependent on the number of DNA breaks, and it is totally inactive in the absence of DNA breaks (51–53). The over-activation of PARP triggers a cell death cascade, which results in tissue damage (52). It has been reported that z-DEVD-fmk can significantly inhibit apoptosis mediated by the caspase-3/PARP apoptotic signaling pathway (50). Together, CM-mediated MCF-7 and HepG2 cell apoptosis is associated with the caspase-dependent mitochondrial pathway.
In conclusion, the present study confirmed the anti-HCC and anti-breast cancer effects of CM aqueous extract in in vitro and in vivo experiments. CM leads to the over-release of LDH, dissipation of Δψm and abnormal expression of pro-apoptotic proteins. The caspase-dependent mitochondrial pathway contributed to CM-induced cytotoxicity in the MCF-7 and HepG2 cells. These findings provide pharmacological evidence that CM possesses antitumor effects in HCC and breast cancer, offering potential as a chemotherapeutic agent.
Acknowledgments
This study was supported by the Science and Technology Key Project of Jilin Province in China (grant no. 20130201006ZY), the Natural Science foundation of P.R. China (grant no. 81402955) and the 'Twelfth Five-Year' Science and Technology Planning Project of Jilin Province in China (grant no. 2014B033).
References
- 1.Hosseini BA, Pasdaran A, Kazemi T, Shanehbandi D, Karami H, Orangi M, Baradaran B. Dichloromethane fractions of Scrophularia oxysepala extract induce apoptosis in MCF-7 human breast cancer cells. Bosn J Basic Med Sci. 2015;15:26–32. doi: 10.17305/bjbms.2015.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Bruix J. Hepatocellular carcinoma-Authors' reply. Lancet. 2012;380:470–471. doi: 10.1016/S0140-6736(12)61286-0. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PJ. Hepatocellular carcinoma: Is current therapy really altering outcome? Gut. 2002;51:459–462. doi: 10.1136/gut.51.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: A retrospective and nationwide survey in Japan. The liver cancer study group of Japan. Hepatology. 2000;32:1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 6.Chang CH, Chen SJ, Liu CY. Adjuvant treatments of breast cancer increase the risk of depressive disorders: A population-based study. J Affect Disord. 2015;182:44–49. doi: 10.1016/j.jad.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Chaube SK, Shrivastav TG, Tiwari M, Prasad S, Tripathi A, Pandey AK. Neem (Azadirachta indica L) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. Springerplus. 2014;3:464. doi: 10.1186/2193-1801-3-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghate NB, Chaudhuri D, Sarkar R, Sajem AL, Panja S, Rout J, Mandal N. An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. PLoS One. 2013;8:e82293. doi: 10.1371/journal.pone.0082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia. 2010;81:961–968. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005;57:1509–1519. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- 11.Jin CY, Kim GY, Choi YH. Induction of apoptosis by aqueous extract of Cordyceps militaris through activation of caspases and inactivation of Akt in human breast cancer MDA-MB-231 cells. J Microbiol Biotechnol. 2008;18:1997–2003. [PubMed] [Google Scholar]
- 12.Yoo HS, Shin JW, Cho JH, Son CG, Lee YW, Park SY, Cho CK. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol Sin. 2004;25:657–665. [PubMed] [Google Scholar]
- 13.Lee H, Kim YJ, Kim HW, Lee DH, Sung MK, Park T. Induction of apoptosis by Cordyceps militaris through activation of caspase-3 in leukemia HL-60 cells. Biol Pharm Bull. 2006;29:670–674. doi: 10.1248/bpb.29.670. [DOI] [PubMed] [Google Scholar]
- 14.Reis FS, Barros L, Calhelha RC, Cirić A, van Griensven LJ, Soković M, Ferreira IC. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem Toxicol. 2013;62:91–98. doi: 10.1016/j.fct.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Jing Y, Cui X, Chen Z, Huang L, Song L, Liu T, Lv W, Yu R. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr Polym. 2014;102:288–296. doi: 10.1016/j.carbpol.2013.11.061. [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa S, Shiraishi T, Kihara S, Tabuchi K. Detection of DNA strand breaks associated with apoptosis in human brain tumors. Virchows Arch. 1995;427:175–179. doi: 10.1007/BF00196523. [DOI] [PubMed] [Google Scholar]
- 17.Pintus F, Floris G, Rufini A. Nutrient availability links mitochondria, apoptosis and obesity. Aging (Albany NY) 2012;4:734–741. doi: 10.18632/aging.100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R, Liu S, Piao F, Wang Z, Qi Y, Li S, Zhang D, Shen J. 2,5-Hexanedione induced apoptosis in mesenchymal stem cells from rat bone marrow via mitochondria-dependent caspase-3 pathway. Ind Health. 2015;53:222–235. doi: 10.2486/indhealth.2014-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Wu Y, Luo K, Liu Y, Zhou M, Yan S, Shi H, Cai Y. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem Toxicol. 2013;58:61–67. doi: 10.1016/j.fct.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Hu Q, Wu D, Chen W, Yan Z, Shi Y. Proteolytic processing of the caspase-9 zymogen is required for apoptosome-mediated activation of caspase-9. J Biol Chem. 2013;288:15142–15147. doi: 10.1074/jbc.M112.441568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/S1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 22.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 23.Baharara J, Amini E. The potential of brittle star extracted polysaccharide in promoting apoptosis via intrinsic signaling pathway. Avicenna J Med Biotechnol. 2015;7:151–158. [PMC free article] [PubMed] [Google Scholar]
- 24.Kato M, Yamazaki T, Kato H, Eyama S, Goto M, Yoshioka M, Takatsu A. Development of high-purity certified reference materials for 17 proteinogenic amino acids by traceable titration methods. Anal Sci. 2015;31:805–814. doi: 10.2116/analsci.31.805. [DOI] [PubMed] [Google Scholar]
- 25.Li SP, Yang FQ, Tsim KW. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 2006;41:1571–1584. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Chan SL, Yu VC. Proteins of the bcl-2 family in apoptosis signalling: From mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol. 2004;31:119–128. doi: 10.1111/j.1440-1681.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- 28.Reis FS, Barros L, Calhelha RC, Cirić A, van Griensven LJ, Soković M, Ferreira IC. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem Toxicol. 2013;62:91–98. doi: 10.1016/j.fct.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Rao YK, Fang SH, Wu WS, Tzeng YM. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator's production and human cancer cell proliferation. J Ethnopharmacol. 2010;131:363–367. doi: 10.1016/j.jep.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Jing Y, Cui X, Chen Z, Huang L, Song L, Liu T, Lv W, Yu R. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr Polym. 2014;102:288–296. doi: 10.1016/j.carbpol.2013.11.061. [DOI] [PubMed] [Google Scholar]
- 31.Wong JH, Wang H, Ng TB. A haemagglutinin from the medicinal fungus Cordyceps militaris. Biosci Rep. 2009;29:321–327. doi: 10.1042/BSR20080153. [DOI] [PubMed] [Google Scholar]
- 32.Nourazarian SM, Nourazarian A, Majidinia M, Roshaniasl E. Effect of root extracts of medicinal herb Glycyrrhiza glabra on HSP90 gene rxpression and apoptosis in the HT-29 colon cancer cell line. Asian Pac J Cancer Prev. 2015;16:8563–8566. doi: 10.7314/APJCP.2015.16.18.8563. [DOI] [PubMed] [Google Scholar]
- 33.Hu B, An HM, Wang SS, Chen JJ, Xu L. Preventive and therapeutic effects of Chinese herbal compounds against hepatocellular carcinoma. Molecules. 2016;21:142. doi: 10.3390/molecules21020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Xie RF, Xiao QG, Li R, Shen XL, Zhu XG. Hedyotis diffusa Willd extract inhibits the growth of human glioblastoma cells by inducing mitochondrial apoptosis via AKT/ERK pathways. J Ethnopharmaco. 2014;158:404–411. doi: 10.1016/j.jep.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 37.Galluzzi L, Vitale I, Kepp O, Séror C, Hangen E, Perfettini JL, Modjtahedi N, Kroemer G. Methods to dissect mitochondrial membrane permeabilization in the course of apoptosis. Methods Enzymol. 2008;442:355–374. doi: 10.1016/S0076-6879(08)01418-3. [DOI] [PubMed] [Google Scholar]
- 38.Hisatomi T, Ishibashi T, Miller JW, Kroemer G. Pharmacological inhibition of mitochondrial membrane permeabilization for neuroprotection. Exp Neurol. 2009;218:347–352. doi: 10.1016/j.expneurol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Schug ZT, Gonzalvez F, Houtkooper RH, Vaz FM, Gottlieb E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011;18:538–548. doi: 10.1038/cdd.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyun HB, Lee WS, Go SI, Nagappan A, Park C, Han MH, Hong SH, Kim G, Kim GY, Cheong J, et al. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int J Oncol. 2015;46:2670–2678. doi: 10.3892/ijo.2015.2967. [DOI] [PubMed] [Google Scholar]
- 41.Lee JW, Park C, Han MH, Hong SH, Lee TK, Lee SH, Kim GY, Choi YH. Induction of human leukemia U937 cell apoptosis by an ethanol extract of Dendropanax morbifera Lev. Through the caspase-dependent pathway. Oncol Rep. 2013;30:1231–1238. doi: 10.3892/or.2013.2542. [DOI] [PubMed] [Google Scholar]
- 42.Lee KH, Feig C, Tchikov V, Schickel R, Hallas C, Schütze S, Peter ME, Chan AC. The role of receptor internalization in CD95 signaling. EMBO J. 2006;25:1009–1023. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu XF, Jiang H, Zhang CS, Yu SP, Wang ZQ, Su HL. Targeted drug regulation on methylation of p53-BAX mitochondrial apoptosis pathway affects the growth of cholangiocarcinoma cells. J Int Med Res. 2012;40:67–75. doi: 10.1177/147323001204000107. [DOI] [PubMed] [Google Scholar]
- 44.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 45.Bao Q, Shi Y. Apoptosome: A platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. doi: 10.1038/sj.cdd.4402028. [DOI] [PubMed] [Google Scholar]
- 46.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 47.Visagie M, Theron A, Mqoco T, Vieira W, Prudent R, Martinez A, Lafanechère L, Joubert A. Sulphamoylated 2-methoxyestradiol analogues induce apoptosis in adenocarcinoma cell lines. PLoS One. 2013;8:e71935. doi: 10.1371/journal.pone.0071935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tor YS, Yazan LS, Foo JB, Armania N, Cheah YK, Abdullah R, Imam MU, Ismail N, Ismail M. Induction of apoptosis through oxidative stress-related pathways in MCF-7, human breast cancer cells, by ethyl acetate extract of Dillenia suffruticosa. BMC Complement Altern Med. 2014;14:55. doi: 10.1186/1472-6882-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kägi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–819. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Wu Y, Wang B, Yuan X, Fang B. High levels of glucose induced the caspase-3/PARP signaling pathway, leading to apoptosis in human periodontal ligament fibroblasts. Cell Biochem Biophys. 2013;66:229–237. doi: 10.1007/s12013-012-9470-y. [DOI] [PubMed] [Google Scholar]
- 51.Benjamin RC, Gill DM. Poly (ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980;255:10502–10508. [PubMed] [Google Scholar]
- 52.Wang H, Shimoji M, Yu SW, Dawson TM, Dawson VL. Apoptosis inducing factor and PARP-mediated injury in the MPTP mouse model of Parkinson's disease. Ann N Y Acad Sci. 2003;991:132–139. doi: 10.1111/j.1749-6632.2003.tb07471.x. [DOI] [PubMed] [Google Scholar]
- 53.Shi Y, Zhou F, Jiang F, Lu H, Wang J, Cheng C. PARP inhibitor reduces proliferation and increases apoptosis in breast cancer cells. Chin J Cancer Res. 2014;26:142–147. doi: 10.3978/j.issn.1000-9604.2014.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]