Abstract
In higher eukaryotes, pre-rRNA processing occurs almost exclusively post-transcriptionally. This is not the case in rapidly dividing yeast, as the majority of nascent pre-rRNAs are processed cotranscriptionally, with cleavage at the A2 site first releasing a pre-40S ribosomal subunit followed by release of a pre-60S ribosomal subunit upon transcription termination. Ribosome assembly is driven in part by hierarchical association of assembly factors and r-proteins. Groups of proteins are thought to associate with pre-ribosomes cotranscriptionally during early assembly steps, whereas others associate later, after transcription is completed. Here we describe a previously uncharacterized phenotype observed upon disruption of ribosome assembly, in which normally late-binding proteins associate earlier, with pre-ribosomes containing 35S pre-rRNA. As previously observed by many other groups, we show that disruption of 60S subunit biogenesis results in increased amounts of 35S pre-rRNA, suggesting that a greater fraction of pre-rRNAs are processed post-transcriptionally. Surprisingly, we found that early pre-ribosomes containing 35S pre-rRNA also contain proteins previously thought to only associate with pre-ribosomes after early pre-rRNA processing steps have separated maturation of the two subunits. We believe the shift to post-transcriptional processing is ultimately due to decreased cellular division upon disruption of ribosome assembly. When cells are grown under stress or to high density, a greater fraction of pre-rRNAs are processed post-transcriptionally and follow an alternative processing pathway. Together, these results affirm the principle that ribosome assembly occurs through different, parallel assembly pathways and suggest that there is a kinetic foot-race between the formation of protein binding sites and pre-rRNA processing events.
Keywords: yeast ribosome assembly, cotranscriptional assembly, pre-rRNA processing, pre-ribosomes, hierarchical assembly
INTRODUCTION
Eukaryotic ribosome biogenesis is a complex process in which rRNA is coordinately transcribed, folded, modified, processed, and bound by ribosomal proteins (r-proteins) to produce mature ribosomal subunits. Although these processes occur in a coordinated manner, in vitro reconstitution of bacterial ribosomes has shown that there is an overall hierarchy that governs ribosome assembly (Held et al. 1973; Culver 2003; Talkington et al. 2005; Sykes and Williamson 2009; Woolford and Baserga 2013). Initial folding of rRNA promotes the association of a group of primary binding r-proteins. Biochemical and biophysical approaches have shown that binding of these r-proteins to rRNA often results in conformational changes within the rRNA that both strengthens the association of the primary binding r-proteins and creates binding sites for the subsequent association of secondary and tertiary binding r-proteins (Held et al. 1973; Nierhaus and Dohme 1974; Talkington et al. 2005; Adilakshmi et al. 2008).
The principles learned about hierarchical assembly in vitro have largely framed the way ribosome assembly is studied in vivo. In addition to the r-proteins, ribosome assembly in vivo is facilitated by more than 200 assembly factors in eukaryotes (Woolford and Baserga 2013). Purification of pre-ribosomal intermediates revealed that there is a global hierarchy of association of assembly factors with pre-ribosomes (Nissan et al. 2002). There are assembly factors that associate with pre-ribosomes very early in assembly, those that associate during the middle steps of assembly, those that bind to pre-rRNPs at the end of assembly, and those that are present in particles throughout assembly. This global hierarchy is thought to act as a series of checkpoints during ribosome biogenesis, existing to ensure that ribosome assembly occurs in the proper order, and that late steps of assembly do not occur prematurely (Strunk and Karbstein 2009).
Recently, a number of studies have shown that there is also a hierarchy of association of assembly factors during each step of biogenesis. For example, 14 assembly factors required for 27SB pre-rRNA processing stably associate with pre-ribosomes in a stepwise manner, ultimately leading to the recruitment of the GTPase Nog2 just prior to cleavage of the C2 site (Talkish et al. 2012). More recent work has shown that Nog2 shares a binding site on pre-ribosomes with the nuclear export factor Nmd3. Release of Nog2 allows binding of Nmd3, enabling pre-ribosomes to transition from the nucleus to the cytoplasm (Matsuo et al. 2014). Thus, the timely association and coordinated release of Nog2 appear to be important regulatory checkpoints in ribosome biogenesis.
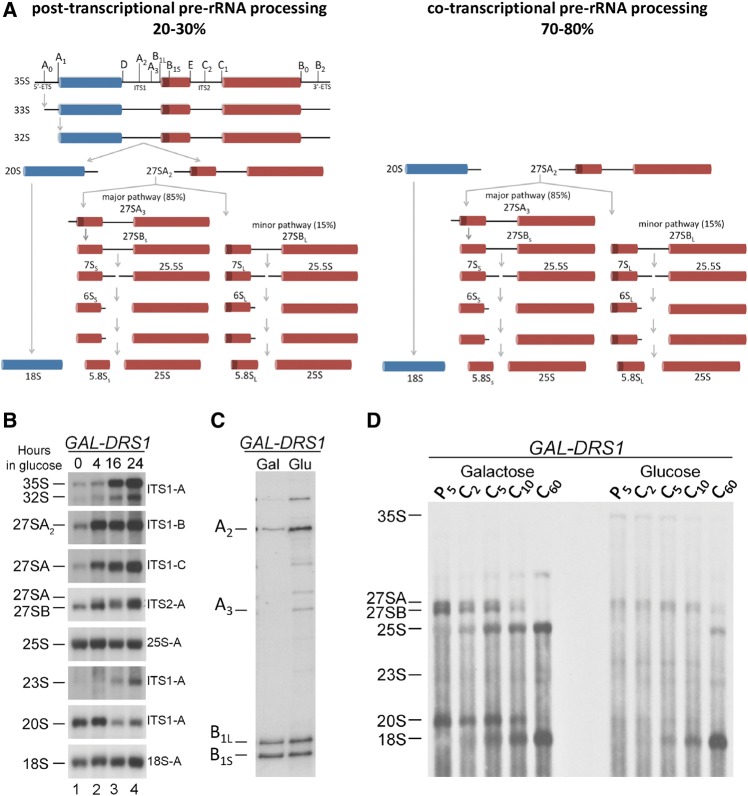
Traditionally, ribosome assembly in yeast has been portrayed as beginning with transcription of a 35S pre-rRNA and the formation of a 90S pre-ribosome in the nucleolus. The 35S pre-rRNA contains sequences for mature 18S, 5.8S, and 25S rRNAs as well as spacer sequences that are removed through a series of endo- and exonucleolytic cleavages (Fig. 1A, left). Cleavage of 35S pre-rRNA at the A0, A1, and A2 sites separates the maturation of 43S pre-ribosomes from 66S pre-ribosomes (Fernandez-Pevida et al. 2014). Although it is thought that the cleavage events that separate maturation of the two particles occur post-transcriptionally in higher eukaryotes, recently it has been shown that in yeast these early pre-rRNA processing steps occur mostly cotranscriptionally (Mougey et al. 1993; Osheim et al. 2004). In rapidly dividing yeast cells, ∼70%–80% of pre-rRNA transcripts are cleaved at the A2 site before transcription of the pre-rRNA is completed (Osheim et al. 2004; Kos and Tollervey 2010). Thus, the majority of ribosome assembly is initiated with 43S pre-ribosomes that contain 20S pre-rRNA and 66S pre-ribosomes that contain 27SA2 pre-rRNA (Fig. 1A, right), not with 90S pre-ribosomes containing 35S pre-rRNA (Fig. 1A, left). In contrast, as cells are grown to stationary phase, a greater fraction of pre-rRNAs are processed post-transcriptionally (Osheim et al. 2004), i.e., a full-length 35S pre-rRNA is produced prior to the occurrence of any pre-rRNA processing steps and separation of the two assembling subunits. Therefore, the assembly events leading up to separation of pre-40S and pre-60S particles appear to be regulated by cell growth and proliferation. However, the mechanism by which this regulation occurs, and the downstream effects on ribosome assembly, remain unknown.
FIGURE 1.
Depletion of Drs1 causes accumulation of 27SA2 and 27SA3 pre-rRNAs. (A) The pre-rRNA processing pathway in Saccharomyces cerevisiae. Ribosome assembly in yeast is traditionally portrayed as beginning with transcription of 35S pre-rRNA (left). Because a large fraction of pre-rRNAs are processed cotranscriptionally, ribosome assembly actually initiates more often with 43S pre-ribosomes containing 20S pre-rRNA and 66S pre-ribosomes containing 27SA2 pre-rRNA (right). RNAs of the 40S and 60S subunits are colored blue and red, respectively. The separately transcribed and processed 5S rRNA is not shown. (B) To assay steady-state levels of pre-rRNAs in the absence of Drs1, the GAL-HA-DRS1 (JWY8637) strain was grown in YEPGal or shifted to YEPGlu media for the indicated time-points. Total RNA was extracted from cells and separated on a 1.2% agarose-6% formaldehyde gel. Pre-rRNAs were assayed by Northern blotting. Oligonucleotide probes used are indicated on the right. Gel lanes are numbered. Quantification of triplicates of this experiment is shown in Supplemental Figure S2. (C) The steady-state levels of the 5′ ends of 27S pre-rRNAs were assayed by primer extension using an oligonucleotide probe complementary to ITS2 (de la Cruz et al. 1998). RNA was extracted from the GAL-HA-DRS1 strain grown in YEPGal or shifted to YEPGlu for 16 h. B1L and B1S represent the 5′ end of 27SBL + 7SL and 27SBS + 7SS pre-rRNAs, respectively. (D) To assay synthesis and turnover of pre-rRNA intermediates in the absence of Drs1, the GAL-HA-DRS1 strain was grown in C-met + Gal or shifted to C-met + Glu for 16 h. Cells were pulse labeled with [3H methyl]-methionine for 5 min and chased with a molar excess of unlabeled methionine for 2, 5, 10, and 60 min. Total RNA was extracted and separated on a 1.2% agarose-6% formaldehyde gel.
Here we describe studies of the assembly factor Drs1 that provide new insights into differences that occur during ribosome biogenesis when early assembly and pre-rRNA processing events occur cotranscriptionally vs. post-transcriptionally. Previously, we showed that the DEAD-box protein (DBP) Drs1 functions in 60S ribosomal subunit biogenesis and that missense mutations in Drs1 block 27SB pre-rRNA processing (Ripmaster et al. 1992; Adams et al. 2002). In contrast, here we show that depletion of Drs1 affects earlier steps of pre-rRNA processing, suggesting that Drs1 may function in more than one consecutive step of ribosome assembly. Furthermore, we show that Drs1 interacts with, and is recruited to pre-ribosomes by a subcomplex of assembly factors containing Nop7, Erb1, and Ytm1.
Most importantly, careful examination of the composition of pre-ribosomes upon depletion of Drs1 revealed a phenotype that we believe results from a partial shift from cotranscriptional pre-rRNA processing at the A0, A1, and A2 sites to post-transcriptional pre-rRNA processing (Fig. 7, below). We observed that (i) depletion of Drs1 results in a block in 27SA pre-rRNA processing, accompanied later by accumulation of 35S pre-rRNAs as cell growth and division slow down. Importantly, although production of 25S rRNA is blocked in the absence of Drs1, the post-transcriptionally processed 35S pre-rRNA is still competent to produce mature 18S rRNA. However, this occurs through a 23S pre-rRNA intermediate, produced by direct cleavage of site A3, rather than through 20S pre-rRNA produced by cleavage at the A2 site (Supplemental Fig. S3). (ii) Disruption of cotranscriptional cleavage of the nascent transcript results in the incorporation of 35S pre-rRNA into an early pre-ribosomal intermediate that contains both early- and late-associating assembly factors and r-proteins that do not coexist in the same particles when processing occurs cotranscriptionally. This was most evident by the fact that we see normally late-associating pre-60S subunit assembly factors (Nog2, Arx1, and Lsg1) associating with 35S pre-rRNA. In wild-type conditions, these assembly factors only associate with pre-ribosomes after cleavage at the A2 site has separated maturation of the pre-40S and pre-60S particles (Saveanu et al. 2001; Nissan et al. 2002; Kallstrom et al. 2003; Bradatsch et al. 2007, 2012; Dembowski et al. 2013). Furthermore, mass spectrometric analysis of the Nog2-containing particles from cells depleted of Drs1 revealed the presence of normally late-associating small subunit r-proteins S3 and S20 (Ferreira-Cerca et al. 2007). (iii) Nog2 coimmunoprecipitates components of RNA polymerase I when 35S pre-rRNA is produced. This is consistent with the observation that under these conditions Nog2 associates with 35S pre-rRNA and suggests that when pre-rRNAs are processed post-transcriptionally, Nog2 associates during transcription. (iv) The shift to post-transcriptional pre-rRNA processing is likely a result of decreased cellular growth rates upon disruption of ribosome assembly, as we were able to recapitulate the shift to post-transcriptional pre-rRNA processing by growing cells to high density or under various stressful conditions.
Together, these data show that the neighborhoods or binding sites of late-associating assembly factors have the potential to also form very early in ribosome biogenesis, before pre-rRNA processing removes spacer sequences. This suggests that during the normal course of ribosome biosynthesis, there is a kinetic foot-race between the formation of protein binding sites and the removal of pre-rRNA spacers.
RESULTS
Disruption of pre-60S ribosomal subunit assembly events leads to post-transcriptional pre-rRNA processing
We previously showed that cold-sensitive drs1 missense mutants are defective in 60S ribosomal subunit assembly due to a block in 27SB pre-rRNA processing (Ripmaster et al. 1992). However, a number of pieces of evidence suggest that Drs1 also functions in preceding steps of 60S subunit assembly together with the well-characterized Nop7 subcomplex: (i) Mutations in NOP7 are synthetically lethal with drs1 mutations, in an allele-specific manner (Adams et al. 2002). (ii) Overexpression of DRS1 can suppress temperature-sensitive growth defects of nop7 mutants, which are blocked at an earlier step of assembly than the drs1 missense mutants (Supplemental Fig. S1A). (iii) Drs1 can be purified with the Nop7-subcomplex upon turning off transcription of rRNA and disrupting assembly of ribosomes (Merl et al. 2010). (iv) Consistent with this observation, Drs1 is part of a salt-stable Nop7-subcomplex (Supplemental Fig. S1B). (v) Drs1 is dependent upon Nop7 to associate with pre-ribosomes, although Nop7 is not dependent on Drs1 (Supplemental Fig. S1C,D). (vi) Drs1 can bind the Nop7-subcomplex in vitro (Supplemental Fig. S1E).
To further investigate the earlier function of Drs1, we assayed effects on pre-rRNA processing upon depletion of Drs1, using a GAL-DRS1 strain. Upon shifting cells to glucose-containing medium to deplete Drs1, we observed a strong accumulation of 27SA2 and 27SA3 pre-rRNAs, relative to 27SB pre-rRNA (Fig. 1B,C). The accumulation of 27SA pre-rRNA was evident as early as 4 h after the shift (Fig. 1B, lane 2), even though cells were still growing at wild-type rates (data not shown). As seen upon depletion of other pre-60S subunit assembly factors, we also observed accumulation of 35S and 33S/32S pre-rRNAs at later time-points, suggesting that a greater fraction of pre-rRNAs are processed post-transcriptionally under these conditions (Sun and Woolford 1994; Zanchin et al. 1997; de la Cruz et al. 1998, 2005; Kressler et al. 1998, 1999; Zanchin and Goldfarb 1999; Burger et al. 2000; Dunbar et al. 2000; Basu et al. 2001; Daugeron et al. 2001; Pestov et al. 2001; Saveanu et al. 2001, 2003, 2007; Adams et al. 2002; Oeffinger et al. 2002; Wehner and Baserga 2002; Oeffinger and Tollervey 2003; Dez et al. 2004; Emery et al. 2004; Galani et al. 2004; Horsey et al. 2004; Rodriguez-Mateos et al. 2009). Furthermore, this was accompanied by a decrease in levels of 20S pre-rRNA and increased amounts of 23S pre-rRNA (Fig. 1B, lanes 3 and 4, and Supplemental Fig. S2). Pulse-chase analysis revealed that pre-rRNAs destined for the 60S subunit were largely unstable and turned over; however, the 35S pre-rRNA that accumulated upon Drs1 depletion could be processed to 18S rRNA, albeit not through the 20S intermediate (Fig. 1D). This suggests that 35S pre-rRNA can undergo direct cleavage at the A3 site to produce 23S and 27SA3 pre-rRNA, and that the 23S pre-rRNA is competent to undergo processing to form mature 18S rRNA (Supplemental Fig. S3, bottom).
Together, these results reveal that, like depletion of Nop7 (Adams et al. 2002), depletion of Drs1 blocks 27SA2 and 27SA3 pre-rRNA processing. Most importantly, the accumulation of 35S and 23S pre-rRNAs at late time-points suggests a partial shift from co- to post-transcriptional pre-rRNA processing.
Nop7-containing pre-ribosomes are enriched for early-associating assembly factors, but also late-associating small subunit r-proteins, upon a shift to post-transcriptional pre-rRNA processing
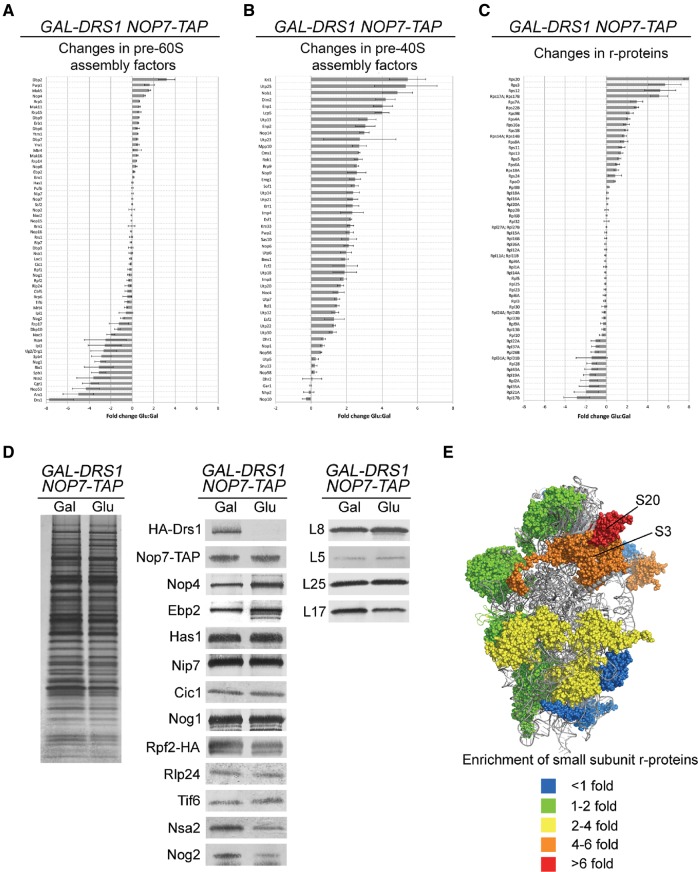
To more thoroughly understand the relationship between Drs1 and other proteins required for 27SA3 pre-rRNA processing, we investigated how the composition of 66S pre-ribosomes changes in the absence of Drs1, using a combination of semiquantitative iTRAQ mass spectrometry and Western blotting. Upon depletion of Drs1 and purification of pre-ribosomes using Nop7-TAP, we observed a number of expected changes in pre-ribosome composition compared to wild-type cells. Consistent with an early block in 60S subunit assembly, we observed accumulation of pre-60S subunit assembly factors known to associate with pre-ribosomes early in assembly (Fig. 2A,D; Dbp2, Pwp1, Mak5, Nop4, Rrp5, Mak11, Rrp15, etc.) and diminished amounts of assembly factors and r-proteins only thought to stably associate after 27SA3 pre-rRNA processing (Fig. 2A,C,D; Nsa2, Nog2, Arx1, Rsa4, Nop3, Ipi1, Rix1, Ipi3; Nissan et al. 2002). This phenotype is often observed upon blocking early steps in 60S subunit biogenesis (Lebreton et al. 2008; Sahasranaman et al. 2011; Jakovljevic et al. 2012; Shimoji et al. 2012; Dembowski et al. 2013; Gamalinda et al. 2014). Furthermore, Nop7 copurified increased amounts of 35S pre-rRNA, consistent with the accumulation of early-associating assembly factors and a shift to a greater amount of post-transcriptional pre-rRNA processing than in wild-type cells (Supplemental Fig. S4A).
FIGURE 2.
In the absence of Drs1, pre-ribosomes are enriched for early assembly factors and contain diminished amounts of late assembly factors. (A–C) Pre-ribosomes were affinity purified from the GAL-HA-DRS1 NOP7-TAP strain (JWY8636) grown in YEPGal or shifted to YEPGlu for 16 h to deplete Drs1. Purified pre-ribosomes were assayed by semiquantitative iTRAQ mass spectrometry to identify pre-60S subunit assembly factors (A), pre-40S subunit assembly factors and SSU components (B), and ribosomal proteins (C), and to compare relative amounts of each in wild-type and Drs1-depleted cells. Each data point shows the fold change of the respective protein upon depletion of Drs1. Error bars indicate the SEM. (D) To confirm iTRAQ results, pre-ribosomes were purified, separated by SDS-PAGE, and visualized by silver staining. Western blots were performed against indicated proteins. (E) The crystal structure of the yeast 40S ribosomal subunit (PDB 4V88). R-proteins that are enriched in Nop7-TAP containing pre-ribosomes are highlighted. Colors indicate the fold-change observed for each r-protein. R-proteins S3 and S20 are most enriched upon Drs1 depletion and are indicated.
Unexpectedly, we also observed copurification of increased levels of small subunit r-proteins and pre-40S subunit assembly factors upon depletion of Drs1 (Fig. 2B,C). Of the small subunit r-proteins that were enriched in Nop7-TAP particles, those most enriched were proteins that bind the 3′ domain of 18S rRNA and whose bacterial homologs have been shown to be late, tertiary binders (S20 and S3) (Fig. 2E; Mulder et al. 2010; Karbstein 2011). Since early steps of pre-rRNA processing occur cotranscriptionally in rapidly growing wild-type yeast (Osheim et al. 2004; Kos and Tollervey 2010), r-proteins S3 and S20 are thought to stably associate with the assembling 40S subunit only after release of the nascent 43S pre-ribosome by cleavage at the A2 site (Mulder et al. 2010; Strunk et al. 2011). Nop7, on the other hand, assembles into pre-ribosomes prior to A2 cleavage, and after cleavage travels with the 66S pre-ribosome. Thus, Nop7 is not predicted to be in the same pre-ribosomal particles with r-proteins S3 and S20 when pre-rRNAs are processed cotranscriptionally. The fact that we see significant enrichment of S3 and S20 in Nop7-TAP particles from cells depleted of Drs1 suggests that there is decreased cotranscriptional separation of the assembling pre-40S and pre-60S subunits. This is consistent with the observed accumulation of 35S pre-rRNA in the absence of Drs1 (Fig. 1B), and likely reflects a shift from cotranscriptional to more post-transcriptional pre-rRNA processing.
Upon disruption of cotranscriptional pre-rRNA processing, early 66S pre-ribosomes are enriched for the late-associating assembly factor Nog2
The results above suggest that, upon disruption of 60S subunit biogenesis, a greater fraction of pre-rRNAs are processed post-transcriptionally, resulting in the formation of an early pre-ribosome containing 35S pre-rRNA, associated with r-proteins (S3 and S20) that normally bind to 20S pre-rRNA after cleavage of the A2 site. This suggests that the binding sites for S3 and S20 have the potential to form prior to any pre-rRNA processing steps.
To further investigate the nature of early pre-ribosomes accumulating in the absence of Drs1, we epitope-tagged the assembly factor Ssf1 to purify pre-ribosomes upon Drs1 depletion. In contrast to Nop7, which purifies a wide range of assembly intermediates, Ssf1 associates with nascent particles early in assembly and dissociates from pre-ribosomes during the lifetime of 27SB pre-rRNA, before Nog2 joins pre-ribosomes (Fatica et al. 2002). We reasoned that by using an assembly factor bound to a much narrower subset of early intermediates than those containing Nop7, we could minimize the effect of changing the relative distribution of purified particles upon depletion of Drs1, and therefore better interpret changes in pre-ribosome composition.
We were surprised to find that when Drs1 is depleted, Ssf1-TAP pre-ribosomes coimmunoprecipitate Nog2 (Supplemental Fig. S4B). Although both Ssf1 and Nog2 copurify 27SB pre-rRNA, a number of studies have convincingly shown that in wild-type cells Ssf1 dissociates from pre-ribosomes prior to Nog2 binding, and therefore these two proteins are present in mutually exclusive populations of pre-ribosomes (Saveanu et al. 2001; Fatica et al. 2002; Kressler et al. 2008; Matsuo et al. 2014). How do we reconcile this observation with our finding that decreased amounts of Nog2 associate with Nop7-containing pre-ribosomes when Drs1 is depleted? This is likely due to changes in the relative distribution of Nop7-containing particles in wild-type vs. mutant conditions. In wild-type cells, the longest-lived Nop7-associated intermediates are particles containing 27SB pre-rRNA, a significant amount of which are also bound by Nog2. In the absence of Drs1, assembly is blocked prior to the formation of 27SB particles, likely accounting for the observed relative decrease of Nog2 in Nop7-TAP particles (Fig. 2A,D).
The fact that we could detect Nog2 copurifying with proteins that normally dissociate from pre-ribosomes before Nog2 binds suggests that even though depletion of Drs1 blocks early steps of pre-60S subunit biogenesis, assembly factors that normally bind after processing of 27SA3 pre-rRNA can now associate with earlier pre-ribosomes.
Late-associating and early-associating pre-60S subunit assembly factors are in the same pre-ribosomal particles when pre-rRNAs are processed post-transcriptionally
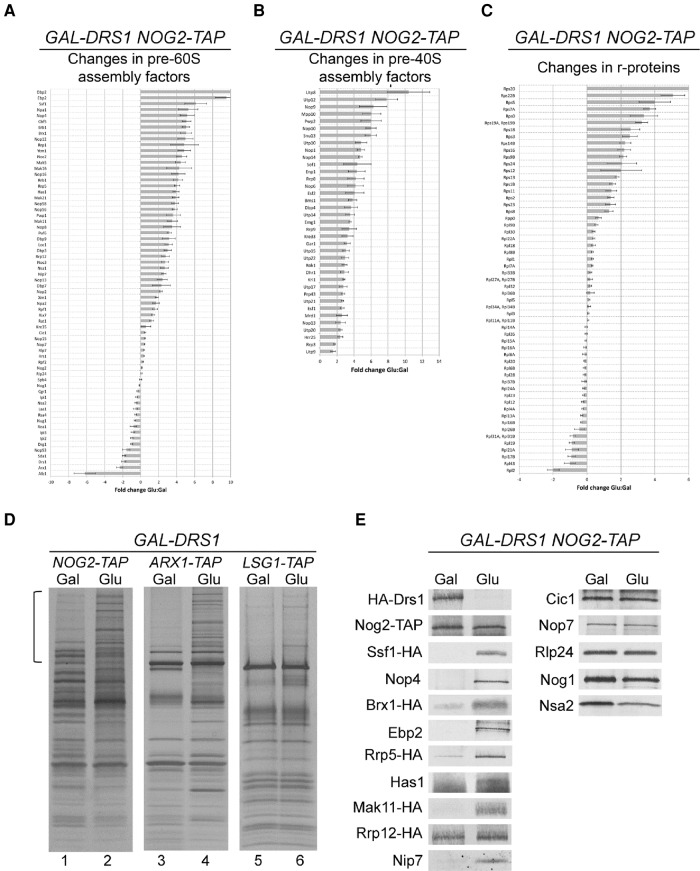
The data above suggest that in the absence of Drs1, pre-ribosomes contain both late pre-40S subunit r-proteins (S3 and S20) and late pre-60S subunit assembly factors (Nog2). To further investigate this idea, we tested whether other late-associating pre-60S subunit assembly factors might behave similarly to Nog2 and r-proteins S3 and S20. If depletion of Drs1 affects cotranscriptional cleavage of the A2 site, we might predict that not only Nog2, but also other late-associating pre-60S subunit assembly factors would copurify with assembly factors that normally dissociate from pre-ribosomes early. To test this prediction, we TAP-tagged Nog2, Arx1, and Lsg1 in the GAL-HA-DRS1 strain. Arx1, like Nog2, is primarily a nucleoplasmic assembly factor, thought to associate with 66S pre-ribosomes only after 27SB pre-rRNA is generated (Saveanu et al. 2001; Nissan et al. 2002; Bradatsch et al. 2007, 2012). Lsg1 associates with 66S pre-ribosomes in the cytoplasm (Nissan et al. 2002; Kallstrom et al. 2003). We used each of these TAP-tagged assembly factors to purify pre-ribosomes from wild-type cells and cells in which Drs1 was depleted for 16 h. As shown in Figure 3D (lanes 1, 3, and 5), pre-ribosomes purified using these late factors from wild-type cells are fairly simple particles, primarily composed of r-proteins and several assembly factors. However, when Drs1 was depleted, we saw that Nog2-, Arx1-, and Lsg1-TAP purified more complex particles, enriched for high-molecular weight assembly factors (Fig. 3D, lanes 2, 4, and 6). In wild-type cells, many of these high-molecular weight proteins (Rrp5, Rrp12, Noc1/Mak21, Erb1, Nop4, etc.) assemble and dissociate early from pre-ribosomes, before or immediately after these late TAP-tagged proteins join the nascent particle (Nissan et al. 2002; Horsey et al. 2004; Zhang et al. 2007; Matsuo et al. 2014). Thus, the fact that late factors Nog2, Arx1, and Lsg1 copurify early pre-ribosomal proteins when Drs1 is depleted is consistent with our results of late-associating Nog2 being present in early Ssf1-TAP containing pre-ribosomes upon depletion of Drs1 (Supplemental Fig. S4B).
FIGURE 3.
Late-associating and early-associating pre-60S subunit assembly factors are in the same pre-ribosomal particles when pre-rRNAs are processed post-transcriptionally. (A–C) Pre-ribosomes were affinity-purified from the GAL-HA-DRS1 NOG2-TAP strain (JWY8695) grown in YEPGal or shifted to YEPGlu for 16 h to deplete Drs1. Purified pre-ribosomes were assayed by iTRAQ mass spectrometry as described in Figure 2A. Shown is the fold change in pre-60S subunit assembly factors (A), pre-40S subunit assembly factors (B), and ribosomal proteins (C). Error bars indicate the SEM. (D) Pre-ribosomes were affinity-purified from the GAL-HA-DRS1 strain grown in YEPGal or shifted to YEPGlu using Nog2-TAP (JWY8695), Arx1-TAP (JWY8481), or Lsg1-TAP (JWY8915). Purified pre-ribosomes were separated by SDS-PAGE and visualized by silver staining. The bracket indicates the enrichment of high molecular weight assembly factors upon depletion of Drs1 that are known to be present in early 66S pre-ribosomes, but absent in late pre-ribosomes. (E) Western blotting was performed to confirm the GAL-HA-DRS1 NOG2-TAP iTRAQ data.
To investigate in more detail the changes in Nog2-containing pre-ribosomes upon depletion of Drs1, we used iTRAQ mass spectrometry and Western blotting (Fig. 3A–C,E). We found that many pre-60S subunit assembly factors were enriched in Nog2-containing particles when Drs1 was depleted, especially early assembly factors such as Ssf1, Npa1, and Rrp5, all of which have been reported to dissociate from pre-ribosomes before 27SB pre-rRNA is generated and before Nog2 binds pre-ribosomes in wild-type cells (Fig. 3A,D,E; Fatica et al. 2002; Nissan et al. 2002; Dez et al. 2004; Hierlmeier et al. 2012).
Even more striking, we observed substantial enrichment of small subunit r-proteins as well as pre-40S subunit assembly factors (Fig. 3B,C). Similar to our results with Nop7-TAP (Fig. 2), we see that Nog2-TAP pre-ribosomes purified from Drs1-depleted cells are highly enriched for the late-associating r-proteins S3 and S20 (Fig. 3C).
Late-associating pre-60S subunit assembly factors coimmunoprecipitate early pre-rRNAs upon disruption of cotranscriptional pre-rRNA processing
Thus far, our results have demonstrated that depletion of Drs1 results in decreased cotranscriptional cleavage of the A0, A1, and A2 sites (i.e., increased levels of 35S pre-rRNA, Fig. 1B,D) and in the formation of pre-ribosomes containing both pre-40S and pre-60S subunit assembly factors thought to only associate with pre-ribosomes after A2 cleavage has occurred (Fig. 3). Therefore, we would expect that these normally late-associating factors would now be associated with 35S pre-rRNA. To test this prediction, we purified pre-ribosomes from wild-type and Drs1-depleted cells using Nog2-, Arx1-, and Lsg1-TAP, and then assayed by primer extension which pre-rRNAs copurified with each assembly factor. As negative controls, we assayed pre-rRNAs that copurified with Nob1-TAP or that bound to IgG-coated beads using an extract from an untagged parent strain. Nob1 primarily associates with 43S pre-ribosomes containing 20S pre-rRNA, i.e., after A0, A1, and A2 cleavage (Fatica et al. 2003; Schafer et al. 2003; Dembowski et al. 2013). Thus, we consider the signal observed for 35S pre-rRNA coimmunoprecipitated with Nob1-TAP from wild-type cells to be background amounts (Fig. 4A; lane 7). Nog2-TAP and Arx1-TAP coimmunoprecipitated 27SB pre-rRNA, but not 35S, 27SA2, and 27SA3 pre-rRNAs from wild-type cell extracts, while Lsg1-TAP did not coimmunoprecipitate either 35S or 27S pre-rRNAs (Fig. 4A, lanes 1, 3, and 5). Interestingly, upon depletion of Drs1, we observed that Nog2-, Arx1-, and Lsg1-TAP coimmunoprecipitated above-background amounts of 35S, 27SA2, and 27SA3 pre-rRNAs (Fig. 4A, lanes 2, 4, and 6). Consistent with an early block in 27SA pre-rRNA processing, we see that Nog2-TAP and Arx1-TAP coimmunoprecipitated decreased amounts of 27SB pre-rRNA relative to 27SA pre-rRNA (Fig. 4A, lanes 2 and 4).
FIGURE 4.
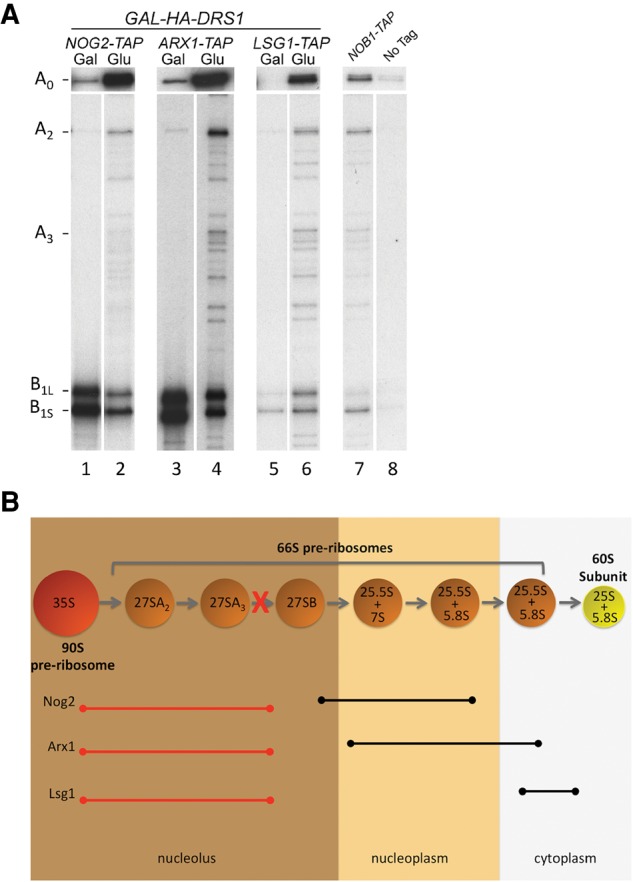
In the absence of Drs1, late-associating assembly factors copurify early pre-rRNA intermediates. (A) Pre-ribosomes were affinity-purified using Nog2-TAP (JWY8695), Arx1-TAP (JWY8481), or Lsg1-TAP (JWY8915), from the GAL-HA-DRS1 strain grown in YEPGal or shifted to YEPGlu for 16 h to deplete Drs1. RNA was extracted from purified pre-ribosomes and pre-rRNAs were detected by primer extension. An oligonucleotide complementary to the 5′-ETS was used to detect the 5′ end of 35S pre-rRNA (A0) and an oligonucleotide complementary to ITS2 was used to detect the 5′ ends of 27S pre-rRNAs (A2,A3). B1L and B1S represent the 5′ end of 27SBL + 7SL and 27SBS + 7SS pre-rRNAs, respectively. Nob1-TAP (JWY7769) and the untagged parent strain (JWY8894) serve as negative controls. (B) Model describing the timing of association of Nog2, Arx1, and Lsg1 in wild-type and Drs1-depleted cells blocked at 27SA3 pre-rRNA processing. Spheres represent consecutive pre-ribosome intermediates. Black lines indicate the pre-rRNAs associated with each assembly factor in wild-type cells. Red lines indicate the pre-rRNAs associated with each assembly factor in the absence of Drs1.
Thus, depletion of Drs1 results in decreased cotranscriptional cleavage at the A0, A1, and A2 sites. When this occurs, normally late-assembling Nog2, Arx1, and Lsg1 associate with a very different population of pre-ribosomal particles that contain 35S pre-rRNA (Fig. 4B).
Nog2-containing pre-ribosomes are enriched with RNA polymerase I components upon depletion of Drs1
Recently, a number of ribosome assembly factors were shown to copurify with RNA polymerase I (Pol I), suggesting that they are recruited to pre-ribosomes cotranscriptionally (Hierlmeier et al. 2012). Many of these factors, including Drs1 and Nop7, are proteins that associate with pre-ribosomes early in ribosome biogenesis. Late-associating proteins, such as Nog2, were not identified in a complex with Pol I, consistent with their binding pre-ribosomes after completion of transcription. In the course of our mass spectrometric analysis of pre-ribosomes isolated from cells when Drs1 is depleted, we identified subunits specific to Pol I (Rpa49 and Rpa135), but not Pol II or Pol III. When Drs1 was depleted and pre-ribosomes were purified using Nop7-TAP, no enrichment of Rpa49 and Rpa135 was seen (Fig. 5). Interestingly, when pre-ribosomes were purified from Drs1-depleted cells using Nog2-TAP, Rpa49 and Rpa135 were enriched ∼4.5-fold and ∼3.5-fold, respectively (Fig. 5), suggesting that Nog2 is now binding to nascent pre-rRNA cotranscriptionally.
FIGURE 5.
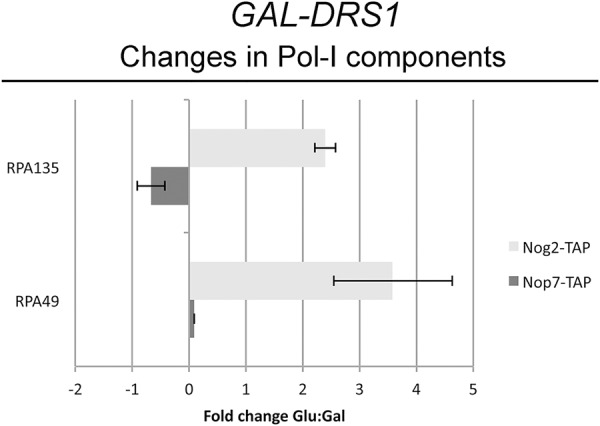
Nog2-containing pre-ribosomes are enriched with RNA polymerase I subunits upon depletion of Drs1. Pre-ribosomes were affinity-purified using either Nop7-TAP (dark gray bars) or Nog2-TAP (light gray bars) from the GAL-HA-DRS1 strain grown in YEPGal or shifted to YEPGlu to deplete Drs1. Purified pre-ribosomes were assayed by iTRAQ mass spectrometry as described in Figure 2A. Shown is the fold change in Pol I subunits identified. Error bars indicate the SEM.
In wild-type cells, Nop7 copurifies with Pol I, indicating it normally associates with pre-ribosomes cotranscriptionally (Hierlmeier et al. 2012). Thus, even upon a shift from co- to more post-transcriptional pre-rRNA processing, Nop7 still associates with pre-rRNAs cotranscriptionally. Furthermore, Pol I components are not enriched in Nop7 particles when Drs1 is depleted. Conversely, in wild-type cells, Nog2 normally associates with pre-ribosomes only after transcription is completed. The fact that we observed strong enrichment of Pol I subunits in pre-ribosomes purified using Nog2-TAP suggests that upon depletion of Drs1, Nog2 now associates with pre-ribosomes cotranscriptionally. These data, plus our finding that Nog2 can associate with 35S pre-rRNA upon depletion of Drs1, further strengthens our conclusion that depletion of Drs1, and subsequent disruption of ribosome assembly, causes a shift from co- to more post-transcriptional pre-rRNA processing. When this occurs, normally “late” associating assembly factors bind pre-ribosomes containing 35S pre-rRNA and the Pol I machinery (Fig. 7).
Co- vs. post-transcriptional pre-rRNA processing is dependent on cell growth rates and environmental stress
Electron microscopy of actively transcribed rDNA revealed that when cells are rapidly dividing, a greater fraction of pre-rRNAs are processed cotranscriptionally (Osheim et al. 2004). However, when cells are grown to high density and cell proliferation slows, nascent transcripts are more frequently processed post-transcriptionally. Based on these previous observations, and because we only observed an accumulation of 35S pre-rRNA after depletion of Drs1 for 16 h, we hypothesized that the shift from co- to post-transcriptional pre-rRNA processing is not directly due to the absence of Drs1, but rather a decrease in cellular division rates upon disruption of ribosome biogenesis. Consistent with this idea, we did not observe an accumulation of 35S pre-rRNA after a 4 h depletion of Drs1, when cells were still dividing at wild-type rates, even though the effect on 27S pre-rRNA processing was clearly evident (Fig. 1B). Furthermore, we did not observe significant accumulation of high molecular weight proteins in pre-ribosomes purified using Nog2-TAP when Drs1 was depleted for 4 h compared to 16 h (Supplemental Fig. S5).
To more thoroughly investigate the relationship between cell division rates and pre-rRNA processing, we grew cells to increasing density until stationary phase and assayed pre-rRNA processing by Northern blotting. Generally, we observed decreased steady-state amounts of both pre-rRNAs and rRNAs as cells approached stationary phase, suggesting a global down-regulation in nascent ribosome production (Fig. 6A,B). It is likely that the decrease of 25S and 18S rRNAs is due to a combination of decreased ribosome production and dilution of preexisting ribosomes by cell division. Consistent with previous work from the Warner group, the decrease in pre-rRNAs occurred slightly prior to exit from exponential growth, suggesting that cells regulate ribosome synthesis according to their estimate for the potential for growth (Ju and Warner 1994).
FIGURE 6.
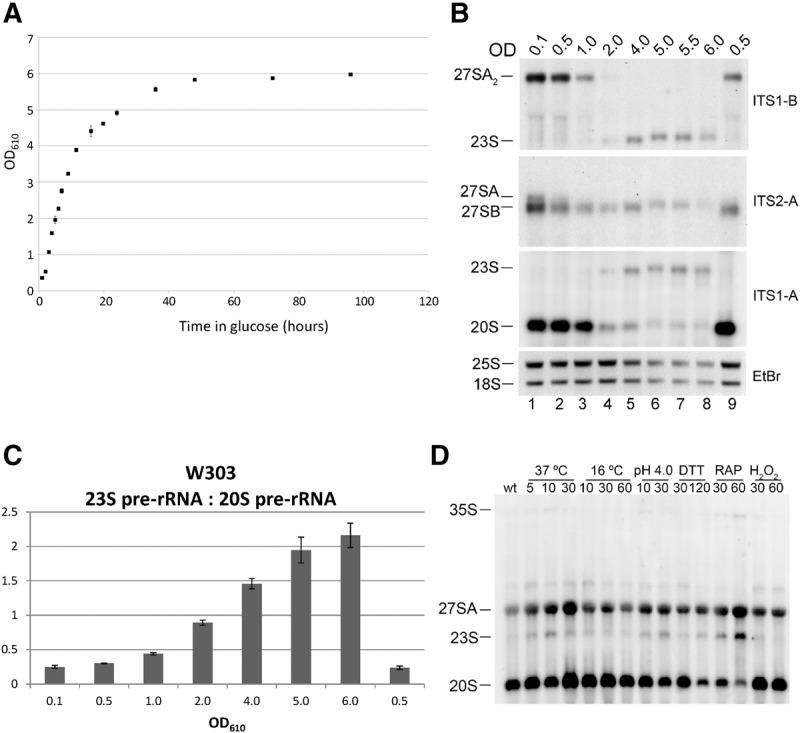
Pre-rRNA processing is dependent on cell growth. (A) The yeast strain W303 was grown in YEPGlu at 30°C from OD610 = 0.1–6.0. (B) RNA was extracted from aliquots of the culture at the indicated OD610 and separated on a 1.2% agarose-6% formaldehyde gel. Pre-rRNAs were assayed by Northern blotting with oligonucleotides indicated on the right. 25S rRNA and 18S rRNA were visualized by ethidium bromide staining. Lane 9 represents cells grown to stationary phase, diluted to OD610 = 0.01, and then grown to OD610 = 0.5. (C) The Northern blots described in B were repeated in triplicate and the ratio of 23S pre-rRNA to 20S pre-rRNA was quantified. Error bars indicate the SEM. (D) To assay the effects of different cellular environments on pre-rRNA processing, the yeast strain W303 was grown in YEPGlu at 30°C to mid-log phase and then shifted to the indicated temperature or growth condition as described by Gasch et al. (2000) and Talkish et al. (2012). After the shift, whole-cell RNA was phenol extracted, separated on a 1.2% agarose-6% formaldehyde gel, and pre-rRNAs were assayed by Northern blotting using a mixture of the oligonucleotides ITS1-A, ITS1-B, and ITS1-C.
Because 35S pre-rRNA is produced in low amounts in rapidly dividing cells, and total cellular pre-rRNA/rRNA is decreased as cells approach stationary phase, it was difficult to detect 35S pre-rRNA by Northern blotting. However, as cell division slowed, we observed a strong accumulation of 23S pre-rRNA coincident with the decrease in 20S pre-rRNA and 27SA2 pre-rRNA (Fig. 6B, lanes 4 and 5, and 6C). The 23S pre-rRNA intermediate, extending from the 5′ end of the 35S pre-rRNA to the A3 site, is generated by direct cleavage of the A3 site prior to cleavage at sites A0, A1, and A2, and thus by definition is a marker for post-transcriptional pre-rRNA processing (Supplemental Fig. S3, bottom). 23S pre-rRNA has been observed in a number of ribosome assembly mutants and had been suggested to be an aberrant intermediate. However, the 23S intermediate that accumulates in many mutants when early steps of 60S subunit assembly are blocked (including in the absence of Drs1, Fig. 1), can be processed to 18S rRNA and serves as an alternative route for maturation of the small subunit when large subunit assembly is blocked (de la Cruz et al. 1998; Adams et al. 2002; Miles et al. 2005; Hierlmeier et al. 2012; Jakovljevic et al. 2012; Talkish et al. 2014).
To further test the relationship between cell growth rate and post-transcriptional pre-rRNA processing, we also assayed pre-rRNA processing when cells were grown under a variety of conditions previously shown to trigger an environmental stress response (Gasch et al. 2000). Northern blots were performed on RNA extracted from cells responding to heat-shock, cold-shock, low pH, dithiothreitol (DTT), rapamycin, and hydrogen peroxide. In all conditions except for cold-shock and treatment with H2O2, we observed a slight accumulation of 23S pre-rRNA relative to 20S pre-rRNA (Fig. 6D). This was most evident when cells were treated with rapamycin, resulting in an observable accumulation of 35S pre-rRNA as well.
Together, these results confirm previous observations that under conditions that cause cellular stress or decreased cell division rates, a greater fraction of pre-rRNAs are processed post-transcriptionally (Osheim et al. 2004). When this occurs, a greater fraction of the resulting 35S pre-rRNA is cleaved directly at the A3 site, prior to cleavage at the A0, A1, and A2 sites (Supplemental Fig. S3).
DISCUSSION
In the course of studying the pre-60S subunit assembly factor Drs1, we observed a phenotype in which the global hierarchy of yeast ribosome assembly was changed. We do not think that this is specific to Drs1, but rather to decreased cellular growth upon disruption of ribosome assembly. Depletion of Drs1 resulted in the formation of early pre-ribosomal intermediates that contained unprocessed 35S pre-rRNA as well as r-proteins and assembly factors previously thought to only bind to pre-ribosomes after a number of pre-rRNA processing steps have taken place. We believe that these results reflect a shift from largely cotranscriptional pre-rRNA processing to a greater fraction of post-transcriptional pre-rRNA processing based on the following: (i) Depletion of Drs1 initially blocks processing of 27S pre-rRNAs; however, at later time-points when cell division decreases, there also is an accumulation of 35S pre-rRNA, suggesting that pre-rRNAs are no longer cleaved at the A0, A1, or A2 sites cotranscriptionally. (ii) The increased 35S pre-rRNA that is produced upon depletion of Drs1 is packaged into early pre-ribosomes that contain both early-associating assembly factors and r-proteins, as well as assembly factors (Nog2, Arx1, and Lsg1) and r-proteins (S3 and S20) that in wild-type cells associate with assembling subunits only after cleavage at the A2 site separates maturation of the pre-40S and pre-60S subunit intermediates. (iii) Consistent with coimmunoprecipitation of 35S pre-rRNA with Nog2 when Drs1 is depleted, we observed that Nog2 copurifies increased amounts of Pol I subunits. These results suggest that pre-rRNA processing steps are in kinetic competition with the formation of protein binding sites. When pre-rRNAs are not cleaved cotranscriptionally, the binding sites for some normally late-associating factors can form during transcription, and late-associating proteins can bind prior to completion of transcription and removal of spacer sequences.
The shift to a greater fraction of post-transcriptional pre-rRNA processing is likely due to decreased growth rates upon disruption of ribosome biogenesis, and not specific to the absence of Drs1. Growing cells to stationary phase or under environmental stress also results in decreased cotranscriptional cleavage at the A0, A1, and A2 sites (Fig. 6; Osheim et al. 2004). Interestingly, under these conditions we also observe greater amounts of 23S pre-rRNA, suggesting that nascent transcripts that are processed post-transcriptionally follow an alternative pre-rRNA processing pathway. This work raises novel ideas about ribosome assembly regarding the order and timing of pre-rRNA processing, the overall hierarchy of protein association with nascent pre-ribosomal particles, and the effects on pre-rRNA processing when early steps of assembly occur post-transcriptionally.
Disruption of ribosome assembly results in increased post-transcriptional pre-rRNA processing
Recent examination of pre-rRNA processing in yeast by electron microscopy of Miller chromatin spreads and rapid pulse-labeling approaches revealed that cleavage at the A2 site (and presumably also the A0 and A1 sites) predominantly occurs cotranscriptionally (Osheim et al. 2004; Kos and Tollervey 2010). Cotranscriptional cleavage at the A2 site occurs ∼70%–80% of the time in rapidly dividing yeast cells. Thus, only ∼20%–30% of rRNA transcription results in a 35S pre-rRNA that is processed post-transcriptionally in fast-growing wild-type cells. Furthermore, when yeast cells are grown to high density, the frequency of cotranscriptional A0, A1, and A2 cleavage decreases dramatically, suggesting that cotranscriptional pre-rRNA processing is regulated by cell growth and division (Osheim et al. 2004). Consistent with these observations, it was recently shown that pre-rRNA in C. albicans is processed cotranscriptionally in rapidly dividing cells, but processed post-transcriptionally after cells undergo the diauxic shift (Pendrak and Roberts 2011).
Depletion of Drs1, as well as other proteins that function early in the biogenesis of 60S ribosomal subunits, results in the accumulation 27SA3 pre-rRNA and a subsequent decrease in 27SB pre-rRNA (Fig. 1B; Sahasranaman et al. 2011). Interestingly, many of these early assembly mutants also exhibit increased 35S pre-rRNA (Dunbar et al. 2000; Pestov et al. 2001; Adams et al. 2002; Oeffinger et al. 2002; Oeffinger and Tollervey 2003; Horsey et al. 2004; Miles et al. 2005). Often, the initial pre-rRNA processing phenotype is revealed early upon turning off expression of the protein of interest and before cell growth rates decrease, whereas the accumulation of 35S pre-rRNA is revealed later, as cell division slows down. This later phenotype is often attributed to the sequestration of early-functioning assembly factors on stalled 66S pre-ribosomes, causing early steps in pre-rRNA processing at the A0, A1, and A2 sites to be affected, or as a quality control checkpoint (Venema and Tollervey 1999). However, a number of studies, including this one (Fig. 1D), have shown that pre-rRNAs are often turned over when ribosome assembly is blocked, particularly when early steps of 27S pre-rRNA processing are impaired. Thus, it seems likely that many of the proteins in abortive intermediates are released during turnover to be reused for another round of assembly, and unlikely that the effects observed on 35S pre-rRNA processing are only due to limited quantities of these proteins. Therefore, an equally plausible scenario is that disruption of early steps of ribosome biogenesis results in decreased cell growth and division, ultimately leading to a shift from cotranscriptional pre-rRNA processing to a greater fraction of post-transcriptional pre-rRNA processing (Figs. 1, 7). In fact, recent work from the Tollervey group revealed that disruption of the exonuclease Rat1, which functions in 27SA3 pre-rRNA processing, led to decreased cotranscriptional cleavage of nascent pre-rRNA transcripts (Axt et al. 2014).
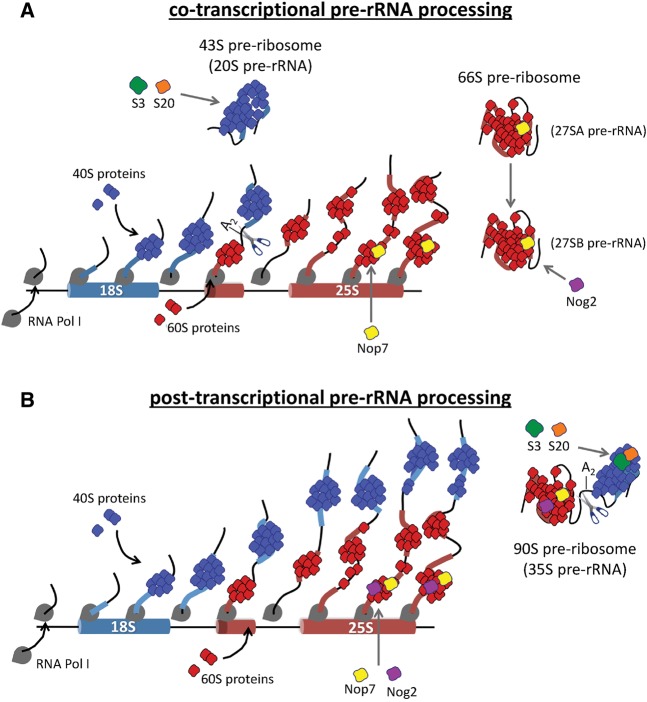
FIGURE 7.
Depletion of Drs1 causes a shift from cotranscriptional pre-rRNA processing to post-transcriptional pre-rRNA processing. (A) In wild-type yeast cells, pre-rRNAs are synthesized by Pol I (gray) and cleaved at the A2 site cotranscriptionally to first release a 43S pre-ribosome containing 20S pre-rRNA (blue). As transcription continues, pre-rRNAs are bound cotranscriptionally by Nop7 (yellow) and transcription termination releases a 66S pre-ribosome containing 27SA2 pre-rRNA (red). The 43S pre-ribosome is subsequently bound by r-proteins S3 (green) and S20 (orange). The 27SA2 pre-rRNA is rapidly processed to 27SB pre-rRNA followed by the association of Nog2 (purple). (B) Upon depletion of Drs1, pre-rRNAs are processed post-transcriptionally, generating 90S pre-ribosomes containing 35S pre-rRNA, S3, S20, and Nog2. The significant enrichment of Pol I subunits with Nog2-TAP containing pre-ribosomes (Fig. 5) indicates that Nog2 associates with pre-ribosomes during transcription in the absence of Drs1.
Co- vs. post-transcriptional pre-rRNA processing is linked to cell growth conditions
The accumulation of 35S pre-rRNA upon slowed cell growth that occurs in many different pre-60S subunit assembly mutants, and previous observations of cotranscriptional vs. post-transcriptional pre-rRNA processing by Miller chromatin spreads (Axt et al. 2014), suggest that the frequency of cotranscriptional pre-rRNA processing is linked to cell growth. Here we further investigate pre-rRNA processing by Northern blotting when cells are grown from exponential to stationary phase. As cell division slows, we observe decreased formation of 27SA2 and 20S pre-rRNAs, consistent with decreased cotranscriptional cleavage at the A0, A1, and A2 sites. This is not simply due to a reduction in the synthesis of new ribosomes, as the levels of 27SB pre-rRNA and mature rRNAs did not change significantly at time-points where 27SA2 and 20S pre-rRNAs were severely diminished (Fig. 6A,B, lane 4).
Interestingly, the decrease in 27SA2 and 20S pre-rRNA levels as cells approached stationary phase is accompanied by an increase in 23S pre-rRNA. This is also observed upon shifting yeast cells from ideal growth conditions to stressful conditions (Fig. 6D). 23S pre-rRNA is produced by direct cleavage of the A3 site in ITS1, prior to cleavage at sites A0, A1, and A2, and thus by definition is derived post-transcriptionally. There is much debate about whether 23S pre-rRNA is a genuine or aberrant pre-rRNA processing intermediate. However, recent studies, including this one (Fig. 1), have shown that 23S pre-rRNA is likely an alternative, on-pathway intermediate to mature 18S rRNA (de la Cruz et al. 1998; Adams et al. 2002; Miles et al. 2005; Hierlmeier et al. 2012; Jakovljevic et al. 2012; Talkish et al. 2014). Depletion of Drs1, like many pre-60S subunit assembly factors that function during processing of 27S pre-rRNA, results in a dramatic decrease in 20S pre-rRNA with a corresponding increase in 35S and 23S pre-rRNA levels (Fig. 1). However, 18S rRNA is still produced at wild-type levels, indicating that maturation of pre-40S subunits can occur through the 23S pre-rRNA intermediate. Consistent with this, it was recently shown in Arabidopsis that early processing steps in the 5′ external transcribed spacer sequence can be bypassed and still result in normal levels of mature rRNAs (Weis et al. 2015).
Together, the above results with pre-60S subunit assembly factor mutants show that as cell growth decreases, so does the frequency of cotranscriptional separation of pre-40S and pre-60S subunit assembly intermediates. Furthermore, a greater fraction of post-transcriptionally processed pre-rRNAs are cleaved directly at the A3 site to produce 23S pre-rRNA, serving as an alternative intermediate to mature 18S rRNA. This suggests that there are fundamental differences in the assembly of nascent particles when pre-rRNAs are processed post-transcriptionally vs. cotranscriptionally. However, the assembly events that dictate co- vs. post-transcriptional processing, and how they are related to cell growth rates, are still unknown.
Transcription and assembly
Why might pre-rRNAs be processed differently cotranscriptionally vs. post-transcriptionally? One possibility is that changes in cell growth rates lead to changes in RNA polymerase I activity, ultimately leading to different RNA folding and assembly pathways. Work from Schneider and colleagues revealed that there is indeed a relationship between Pol I transcription rates and pre-rRNA processing (Schneider et al. 2007). Mutations in Pol I that slow elongation resulted in a number of pre-rRNA processing defects, including accumulation of post-transcriptionally derived 23S pre-rRNA. Furthermore, rRNA is transcribed from tandemly repeated rDNA genes, each of which is occupied by upwards of 120 Pol I molecules (Osheim et al. 2004; Viktorovskaya and Schneider 2015). However, variation in the density of Pol I molecules along the transcript suggests polymerase pausing (El Hage et al. 2010; Turowski and Tollervey 2015). Chromatin-immunoprecipitation studies revealed that Pol I occupancy was highest over the 5′ external transcribed spacer, but also revealed a peak of occupancy across ITS1, near the A2 and A3 sites (El Hage et al. 2010). Thus, an interesting possibility is that high rates of transcription in rapidly dividing yeast leads to a pile-up or pausing of Pol I over ITS1. This may allow ITS1 to fold in a way that promotes cotranscriptional cleavage of ITS1 at the A2 site. Alternatively, in slowly dividing cells where there is less of a demand for the synthesis of new ribosomes, decreased Pol I occupancy might lead to differences in transcription rates. This may cause ITS1 to fold in a way that either inhibits cotranscriptional cleavage at the A0, A1, and A2 sites or promotes post-transcriptional processing at the A3 site.
The occupancy and activity of Pol I might also influence the way other regions of the rRNA fold, leading to the formation of late-protein binding sites before any pre-rRNA processing steps occur. Our results show that disruption of ribosome assembly upon depletion of Drs1 results in the formation of an intermediate that contains unprocessed 35S pre-rRNA associated with assembly factors (Nog2, Arx1, and Lsg1) and r-proteins (S3 and S20) previously only thought to bind post-transcriptionally. Furthermore, mass spectrometric analysis of these particles revealed that Nog2 can associate cotranscriptionally when pre-rRNAs are processed post-transcriptionally. Together, these results suggest there is a kinetic foot-race between the processing of pre-rRNAs and the folding and formation of assembly factor and r-protein binding sites.
MATERIALS AND METHODS
Construction and growth of yeast strains
Yeast strains used in this study are listed in Supplemental Table S1. Unless otherwise noted, yeast were grown at 30°C in YEPGlu (2% dextrose, 2% peptone, and 1% yeast extract) or YEPGal (2% galactose, 2% peptone, and 1% yeast extract). Cells were harvested at mid-log phase, at 3–5 × 107 cells/mL, unless otherwise indicated.
To assay pre-rRNA processing when cells are grown under different environmental stressors, the yeast strain W303 was used. Cells were grown to mid-log phase in YEPGlu at 30°C and then shifted for the indicated time to either 16°C or 37°C, low pH (4.0), or media supplemented with dithiothreitol (DTT, 2.5 mM), rapamycin (RAP, 200 ng/mL), or hydrogen peroxide (H2O2; 0.3 mM). After treatment, cells were harvested by centrifugation, flash-frozen, and RNA was extracted for Northern blotting.
Yeast strains conditional for expression of Drs1 were constructed as previously described (Longtine et al. 1998). Briefly, sequences containing the selectable marker TRP1, plus the GAL1 promoter followed by an ATG initiator codon and sequences encoding three copies of the hemaglutinin (3HA) epitope were amplified by PCR. The PCR products were transformed into yeast and transformants were screened for correct integration of the GAL1 promoter and 3HA tag by Western blotting with anti-HA antisera. Strains containing the GAL1 promoter fused with the DRS1 ORF were grown at 30°C in YEPGal to 3–5 × 107 cells/mL, or were grown in YEPGal and then shifted to YEPGlu for indicated times, to 3–5 × 107 cells/mL, to deplete Drs1 in vivo.
Yeast strains expressing C-terminal TAP-tagged proteins or C-terminal 3HA-tagged proteins were created by PCR of the tag sequences and a selectable marker (URA3 for the TAP tag and HIS3 or kanMX6 for the 3HA tag), transformation, and selection as described in Rigaut et al. (1999) or Longtine et al. (1998), respectively.
Assaying pre-rRNA processing and turnover
Steady-state levels of pre-rRNAs and rRNAs were assayed by Northern blotting and primer extension, and synthesis and turnover of pre-rRNAs were monitored by pulse-chase assays, as previously described (Horsey et al. 2004; Jakovljevic et al. 2012), with the following modifications. Prior to loading on gels, RNA was quantified using a Nano Drop 2000C spectrophotometer (Thermo Fisher Scientific). Five micrograms of RNA was used for Northern blotting and primer extension. Sequences of oligonucleotides used for primer extension and Northern blotting are available upon request. The radioactive signal in bands on Northern blots was quantified using ImageJ.
For pulse-chase assays, cells were pulse labeled with [3H methyl]-methionine for 5 min and chased with a molar excess of unlabeled methionine for 2, 5, 10, and 60 min. Total RNA was extracted and equal counts per min of each sample was loaded and separated on a 1.2% agarose-6% formaldehyde gel. RNA was transferred to a nitrocellulose membrane (Bio-Rad) and was detected by exposure to Biomax MS film (Carestream).
Affinity purification of pre-ribosomes
Ribosome assembly intermediates were affinity-purified from whole-cell extracts using magnetic Dynabeads (Invitrogen) as described in Sahasranaman et al. (2011). RNA enriched in these purified pre-ribosomes was extracted, as described in Sahasranaman et al. (2011), and was assayed by primer extension as described in Horsey et al. (2004).
Protein extraction, SDS-PAGE, and Western blot analysis
Proteins in whole cell extracts were prepared for gel electrophoresis as previously described in Ausubel et al. (1994). Proteins were isolated from affinity-purified pre-ribosomes by precipitation with 10% TCA, and were resuspended in SDS sample buffer. Proteins were resolved by SDS-PAGE on 4%–20% Tris-Glycine Novex precast gels (Invitrogen), and stained with silver by standard methods. Proteins from whole-cell extracts or from purified pre-ribosomes were assayed by Western blot analysis with the following modifications. To enable detection of multiple different proteins on the same blot, after electroblotting, nitrocellulose membranes were cut into smaller pieces based on previously established mobility of different proteins. Each section of membrane was then probed with the appropriate antibody and developed. TAP-tagged proteins were detected using alkaline phosphatase conjugated to IgG (Pierce). HA-tagged proteins were identified with mouse monoclonal antibody 12CA5. Myc-tagged proteins were identified with mouse monoclonal antibody 9e10 (Developmental Studies Hybridoma Bank). Otherwise, antibodies specific for r-proteins or ribosomal assembly factors were used. To assay Nog2 protein by Western blotting, NuPage 4%–12% Bis-Tris gels (Invitrogen) were used, since Nog2 comigrates with IgG on 4%–20% Novex gels. AP-conjugated anti-mouse or anti-rabbit secondary antibodies (Promega) were used, and colorimetric detection was performed using NBT and BCIP (Promega) to visualize the signal.
Affinity purification of pre-ribosomes and analysis by iTRAQ mass spectrometry
For semiquantitative iTRAQ mass spectrometry, pre-ribosomes were purified as described above. Pre-ribosomes were purified from 1 L of cell culture at 3–5 × 107 cells/mL. Purified pre-ribosomes were eluted from the IgG Dynabeads, precipitated with 10% TCA, and resuspended in 20 µL of 20 mM HEPES pH 7.4. iTRAQ labeling and quantification were performed as previously described in Jiang et al. (2007).
PyMOL
PyMOL images of the structure of yeast 60S and 40S ribosomal subunits were generated using PDB file 4V88 (Ben-Shem et al. 2011).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the following people for generously providing antibodies: Michael McAlear (Ebp2), Jesus de la Cruz and Patrick Linder (Has1), David Goldfarb (Nip7), Elizabeth Tosta (Cic1), Cosmin Saveanu and Micheline Fromont-Racine (Rlp24, Nsa2, Tif6, and Nog2), Arlen Johnson (rpL8), K. Siegers (rpL25), and Sabine Rospert (rpL17). This work was supported by National Institutes of Health grant GM28301 (J.L.W.), National Institutes of Health grant 1R01GM077628 (J.R.M.), funds from the David Scaife Family Charitable Foundation (award 141RA01; J.L.W., J.J., and J.T.), the Richard King Mellon Foundation Presidential Graduate Fellowship in the Life Sciences (J.T.), the Semon H. Stupakoff Scholarship (J.T.), and the Roche/ARCS Foundation Scholar Award (S.B.).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.055780.115.
REFERENCES
- Adams CC, Jakovljevic J, Roman J, Harnpicharnchai P, Woolford JL Jr. 2002. Saccharomyces cerevisiae nucleolar protein Nop7p is necessary for biogenesis of 60S ribosomal subunits. RNA 8: 150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adilakshmi T, Bellur DL, Woodson SA. 2008. Concurrent nucleation of 16S folding and induced fit in 30S ribosome assembly. Nature 455: 1268–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. 1994. Current protocols in molecular biology. Wiley, New York. [Google Scholar]
- Axt K, French SL, Beyer AL, Tollervey D. 2014. Kinetic analysis demonstrates a requirement for the Rat1 exonuclease in cotranscriptional pre-rRNA cleavage. PLoS One 9: e85703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu U, Si K, Warner JR, Maitra U. 2001. The Saccharomyces cerevisiae TIF6 gene encoding translation initiation factor 6 is required for 60S ribosomal subunit biogenesis. Mol Cell Biol 21: 1453–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. 2011. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334: 1524–1529. [DOI] [PubMed] [Google Scholar]
- Bradatsch B, Katahira J, Kowalinski E, Bange G, Yao W, Sekimoto T, Baumgartel V, Boese G, Bassler J, Wild K, et al. 2007. Arx1 functions as an unorthodox nuclear export receptor for the 60S preribosomal subunit. Mol Cell 27: 767–779. [DOI] [PubMed] [Google Scholar]
- Bradatsch B, Leidig C, Granneman S, Gnadig M, Tollervey D, Bottcher B, Beckmann R, Hurt E. 2012. Structure of the pre-60S ribosomal subunit with nuclear export factor Arx1 bound at the exit tunnel. Nat Struct Mol Biol 19: 1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger F, Daugeron MC, Linder P. 2000. Dbp10p, a putative RNA helicase from Saccharomyces cerevisiae, is required for ribosome biogenesis. Nucleic Acids Res 28: 2315–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver GM. 2003. Assembly of the 30S ribosomal subunit. Biopolymers 68: 234–249. [DOI] [PubMed] [Google Scholar]
- Daugeron MC, Kressler D, Linder P. 2001. Dbp9p, a putative ATP-dependent RNA helicase involved in 60S-ribosomal-subunit biogenesis, functionally interacts with Dbp6p. RNA 7: 1317–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Rojo M, Tollervey D, Linder P. 1998. Spb4p, an essential putative RNA helicase, is required for a late step in the assembly of 60S ribosomal subunits in Saccharomyces cerevisiae. RNA 4: 1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz J, Sanz-Martinez E, Remacha M. 2005. The essential WD-repeat protein Rsa4p is required for rRNA processing and intra-nuclear transport of 60S ribosomal subunits. Nucleic Acids Res 33: 5728–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembowski JA, Kuo B, Woolford JL Jr. 2013. Has1 regulates consecutive maturation and processing steps for assembly of 60S ribosomal subunits. Nucleic Acids Res 41: 7889–7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dez C, Froment C, Noaillac-Depeyre J, Monsarrat B, Caizergues-Ferrer M, Henry Y. 2004. Npa1p, a component of very early pre-60S ribosomal particles, associates with a subset of small nucleolar RNPs required for peptidyl transferase center modification. Mol Cell Biol 24: 6324–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar DA, Dragon F, Lee SJ, Baserga SJ. 2000. A nucleolar protein related to ribosomal protein L7 is required for an early step in large ribosomal subunit biogenesis. Proc Natl Acad Sci 97: 13027–13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage A, French SL, Beyer AL, Tollervey D. 2010. Loss of Topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes Dev 24: 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, de la Cruz J, Rocak S, Deloche O, Linder P. 2004. Has1p, a member of the DEAD-box family, is required for 40S ribosomal subunit biogenesis in Saccharomyces cerevisiae. Mol Microbiol 52: 141–158. [DOI] [PubMed] [Google Scholar]
- Fatica A, Cronshaw AD, Dlakic M, Tollervey D. 2002. Ssf1p prevents premature processing of an early pre-60S ribosomal particle. Mol Cell 9: 341–351. [DOI] [PubMed] [Google Scholar]
- Fatica A, Oeffinger M, Dlakic M, Tollervey D. 2003. Nob1p is required for cleavage of the 3′ end of 18S rRNA. Mol Cell Biol 23: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Pevida A, Kressler D, de la Cruz J. 2014. Processing of preribosomal RNA in Saccharomyces cerevisiae. Wiley Interdiscip Rev RNA 6: 191–209. [DOI] [PubMed] [Google Scholar]
- Ferreira-Cerca S, Poll G, Kuhn H, Neueder A, Jakob S, Tschochner H, Milkereit P. 2007. Analysis of the in vivo assembly pathway of eukaryotic 40S ribosomal proteins. Mol Cell 28: 446–457. [DOI] [PubMed] [Google Scholar]
- Galani K, Nissan TA, Petfalski E, Tollervey D, Hurt E. 2004. Rea1, a dynein-related nuclear AAA-ATPase, is involved in late rRNA processing and nuclear export of 60 S subunits. J Biol Chem 279: 55411–55418. [DOI] [PubMed] [Google Scholar]
- Gamalinda M, Ohmayer U, Jakovljevic J, Kumcuoglu B, Woolford J, Mbom B, Lin L, Woolford JL Jr. 2014. A hierarchical model for assembly of eukaryotic 60S ribosomal subunit domains. Genes Dev 28: 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11: 4241–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held WA, Mizushima S, Nomura M. 1973. Reconstitution of Escherichia coli 30 S ribosomal subunits from purified molecular components. J Biol Chem 248: 5720–5730. [PubMed] [Google Scholar]
- Hierlmeier T, Merl J, Sauert M, Perez-Fernandez J, Schultz P, Bruckmann A, Hamperl S, Ohmayer U, Rachel R, Jacob A, et al. 2012. Rrp5p, Noc1p and Noc2p form a protein module which is part of early large ribosomal subunit precursors in S. cerevisiae. Nucleic Acids Res 41: 1191–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsey EW, Jakovljevic J, Miles TD, Harnpicharnchai P, Woolford JL Jr. 2004. Role of the yeast Rrp1 protein in the dynamics of pre-ribosome maturation. RNA 10: 813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovljevic J, Gamalinda M, Talkish J, Alexander L, Linneman J, Milkereit P, Woolford JL Jr. 2012. Ribosomal proteins L7 and L8 function in concert with six A3 assembly factors to propagate assembly of domain I of 25S rRNA in yeast 60S ribosomal subunits. RNA 18: 1805–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Sullivan SM, Walker AK, Strahler JR, Andrews PC, Maddock JR. 2007. Identification of novel Escherichia coli ribosome-associated proteins using isobaric tags and multidimensional protein identification techniques. J Bacteriol 189: 3434–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Q, Warner JR. 1994. Ribosome synthesis during the growth cycle of Saccharomyces cerevisiae. Yeast 10: 151–157. [DOI] [PubMed] [Google Scholar]
- Kallstrom G, Hedges J, Johnson A. 2003. The putative GTPases Nog1p and Lsg1p are required for 60S ribosomal subunit biogenesis and are localized to the nucleus and cytoplasm, respectively. Mol Cell Biol 23: 4344–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbstein K. 2011. Inside the 40S ribosome assembly machinery. Curr Opin Chem Biol 15: 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos M, Tollervey D. 2010. Yeast pre-rRNA processing and modification occur cotranscriptionally. Mol Cell 37: 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D, de la Cruz J, Rojo M, Linder P. 1998. Dbp6p is an essential putative ATP-dependent RNA helicase required for 60S-ribosomal-subunit assembly in Saccharomyces cerevisiae. Mol Cell Biol 18: 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D, Rojo M, Linder P, Cruz J. 1999. Spb1p is a putative methyltransferase required for 60S ribosomal subunit biogenesis in Saccharomyces cerevisiae. Nucleic Acids Res 27: 4598–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D, Roser D, Pertschy B, Hurt E. 2008. The AAA ATPase Rix7 powers progression of ribosome biogenesis by stripping Nsa1 from pre-60S particles. J Cell Biol 181: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton A, Rousselle JC, Lenormand P, Namane A, Jacquier A, Fromont-Racine M, Saveanu C. 2008. 60S ribosomal subunit assembly dynamics defined by semi-quantitative mass spectrometry of purified complexes. Nucleic Acids Res 36: 4988–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Granneman S, Thoms M, Manikas RG, Tollervey D, Hurt E. 2014. Coupled GTPase and remodelling ATPase activities form a checkpoint for ribosome export. Nature 505: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merl J, Jakob S, Ridinger K, Hierlmeier T, Deutzmann R, Milkereit P, Tschochner H. 2010. Analysis of ribosome biogenesis factor-modules in yeast cells depleted from pre-ribosomes. Nucleic Acids Res 38: 3068–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles TD, Jakovljevic J, Horsey EW, Harnpicharnchai P, Tang L, Woolford JL Jr. 2005. Ytm1, Nop7, and Erb1 form a complex necessary for maturation of yeast 66S preribosomes. Mol Cell Biol 25: 10419–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougey EB, O'Reilly M, Osheim Y, Miller OL Jr, Beyer A, Sollner-Webb B. 1993. The terminal balls characteristic of eukaryotic rRNA transcription units in chromatin spreads are rRNA processing complexes. Genes Dev 7: 1609–1619. [DOI] [PubMed] [Google Scholar]
- Mulder AM, Yoshioka C, Beck AH, Bunner AE, Milligan RA, Potter CS, Carragher B, Williamson JR. 2010. Visualizing ribosome biogenesis: parallel assembly pathways for the 30S subunit. Science 330: 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus KH, Dohme F. 1974. Total reconstitution of functionally active 50S ribosomal subunits from Escherichia coli. Proc Natl Acad Sci 71: 4713–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissan TA, Bassler J, Petfalski E, Tollervey D, Hurt E. 2002. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J 21: 5539–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger M, Tollervey D. 2003. Yeast Nop15p is an RNA-binding protein required for pre-rRNA processing and cytokinesis. EMBO J 22: 6573–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger M, Leung A, Lamond A, Tollervey D. 2002. Yeast Pescadillo is required for multiple activities during 60S ribosomal subunit synthesis. RNA 8: 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim YN, French SL, Keck KM, Champion EA, Spasov K, Dragon F, Baserga SJ, Beyer AL. 2004. Pre-18S ribosomal RNA is structurally compacted into the SSU processome prior to being cleaved from nascent transcripts in Saccharomyces cerevisiae. Mol Cell 16: 943–954. [DOI] [PubMed] [Google Scholar]
- Pendrak ML, Roberts DD. 2011. Ribosomal RNA processing in Candida albicans. RNA 17: 2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestov DG, Stockelman MG, Strezoska Z, Lau LF. 2001. ERB1, the yeast homolog of mammalian Bop1, is an essential gene required for maturation of the 25S and 5.8S ribosomal RNAs. Nucleic Acids Res 29: 3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol 17: 1030–1032. [DOI] [PubMed] [Google Scholar]
- Ripmaster TL, Vaughn GP, Woolford JL Jr. 1992. A putative ATP-dependent RNA helicase involved in Saccharomyces cerevisiae ribosome assembly. Proc Natl Acad Sci 89: 11131–11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mateos M, Garcia-Gomez JJ, Francisco-Velilla R, Remacha M, de la Cruz J, Ballesta JP. 2009. Role and dynamics of the ribosomal protein P0 and its related trans-acting factor Mrt4 during ribosome assembly in Saccharomyces cerevisiae. Nucleic Acids Res 37: 7519–7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasranaman A, Dembowski J, Strahler J, Andrews P, Maddock J, Woolford JL Jr. 2011. Assembly of Saccharomyces cerevisiae 60S ribosomal subunits: role of factors required for 27S pre-rRNA processing. EMBO J 30: 4020–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saveanu C, Bienvenu D, Namane A, Gleizes PE, Gas N, Jacquier A, Fromont-Racine M. 2001. Nog2p, a putative GTPase associated with pre-60S subunits and required for late 60S maturation steps. EMBO J 20: 6475–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saveanu C, Namane A, Gleizes PE, Lebreton A, Rousselle JC, Noaillac-Depeyre J, Gas N, Jacquier A, Fromont-Racine M. 2003. Sequential protein association with nascent 60S ribosomal particles. Mol Cell Biol 23: 4449–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saveanu C, Rousselle JC, Lenormand P, Namane A, Jacquier A, Fromont-Racine M. 2007. The p21-activated protein kinase inhibitor Skb15 and its budding yeast homologue are 60S ribosome assembly factors. Mol Cell Biol 27: 2897–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer T, Strauss D, Petfalski E, Tollervey D, Hurt E. 2003. The path from nucleolar 90S to cytoplasmic 40S pre-ribosomes. EMBO J 22: 1370–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DA, Michel A, Sikes ML, Vu L, Dodd JA, Salgia S, Osheim YN, Beyer AL, Nomura M. 2007. Transcription elongation by RNA polymerase I is linked to efficient rRNA processing and ribosome assembly. Mol Cell 26: 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji K, Jakovljevic J, Tsuchihashi K, Umeki Y, Wan K, Kawasaki S, Talkish J, Woolford JL Jr, Mizuta K. 2012. Ebp2 and Brx1 function cooperatively in 60S ribosomal subunit assembly in Saccharomyces cerevisiae. Nucleic Acids Res 40: 4574–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk BS, Karbstein K. 2009. Powering through ribosome assembly. RNA 15: 2083–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk BS, Loucks CR, Su M, Vashisth H, Cheng S, Schilling J, Brooks CL III, Karbstein K, Skiniotis G. 2011. Ribosome assembly factors prevent premature translation initiation by 40S assembly intermediates. Science 333: 1449–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Woolford JL Jr. 1994. The yeast NOP4 gene product is an essential nucleolar protein required for pre-rRNA processing and accumulation of 60S ribosomal subunits. EMBO J 13: 3127–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes MT, Williamson JR. 2009. A complex assembly landscape for the 30S ribosomal subunit. Annu Rev Biophys 38: 197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington MW, Siuzdak G, Williamson JR. 2005. An assembly landscape for the 30S ribosomal subunit. Nature 438: 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkish J, Zhang J, Jakovljevic J, Horsey EW, Woolford JL Jr. 2012. Hierarchical recruitment into nascent ribosomes of assembly factors required for 27SB pre-rRNA processing in Saccharomyces cerevisiae. Nucleic Acids Res 40: 8646–8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkish J, Campbell IW, Sahasranaman A, Jakovljevic J, Woolford JL Jr. 2014. Ribosome assembly factors Pwp1 and Nop12 are important for folding of 5.8S rRNA during ribosome biogenesis in Saccharomyces cerevisiae. Mol Cell Biol 34: 1863–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski TW, Tollervey D. 2015. Cotranscriptional events in eukaryotic ribosome synthesis. Wiley Interdiscip Rev RNA 6: 129–139. [DOI] [PubMed] [Google Scholar]
- Venema J, Tollervey D. 1999. Ribosome synthesis in Saccharomyces cerevisiae. Annu Rev Genet 33: 261–311. [DOI] [PubMed] [Google Scholar]
- Viktorovskaya OV, Schneider DA. 2015. Functional divergence of eukaryotic RNA polymerases: unique properties of RNA polymerase I suit its cellular role. Gene 556: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner KA, Baserga SJ. 2002. The σ70-like motif: a eukaryotic RNA binding domain unique to a superfamily of proteins required for ribosome biogenesis. Mol Cell 9: 329–339. [DOI] [PubMed] [Google Scholar]
- Weis BL, Palm D, Missbach S, Bohnsack MT, Schleiff E. 2015. atBRX1-1 and atBRX1-2 are involved in an alternative rRNA processing pathway in Arabidopsis thaliana. RNA 21: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford JL Jr, Baserga SJ. 2013. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 195: 643–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchin NI, Goldfarb DS. 1999. Nip7p interacts with Nop8p, an essential nucleolar protein required for 60S ribosome biogenesis, and the exosome subunit Rrp43p. Mol Cell Biol 19: 1518–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchin NI, Roberts P, DeSilva A, Sherman F, Goldfarb DS. 1997. Saccharomyces cerevisiae Nip7p is required for efficient 60S ribosome subunit biogenesis. Mol Cell Biol 17: 5001–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Harnpicharnchai P, Jakovljevic J, Tang L, Guo Y, Oeffinger M, Rout MP, Hiley SL, Hughes T, Woolford JL Jr. 2007. Assembly factors Rpf2 and Rrs1 recruit 5S rRNA and ribosomal proteins rpL5 and rpL11 into nascent ribosomes. Genes Dev 21: 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.