Abstract
The prevalence of myopia has increased in modern society due to the educational load of children. This condition is growing rapidly, especially in Asian countries where it has already reached a pandemic level. Typically, the younger the child’s age at the onset of myopia, the more rapidly the condition will progress and the greater the likelihood that it will develop the known sight-threatening complications of high myopia. This rise in incidence of severe myopia has contributed to an increased frequency of eye diseases in adulthood, which often complicate therapeutic procedures. Currently, no treatment is available to prevent myopia progression.
Stem cell therapy can potentially address two components of myopia. Regardless of the exact etiology, myopia is always associated with scleral weakness. In this context, a strategy aimed at scleral reinforcement by transplanting connective tissue-supportive mesenchymal stem cells (MSCs) is an attractive approach that could yield effective and universal therapy. Sunlight exposure appears to have a protective effect against myopia. It is postulated that this effect is mediated via local ocular production of dopamine. With a variety of dopamine-producing cells already available for the treatment of Parkinson’s disease, stem cells engineered for dopamine production could be utilized for the treatment of myopia. In this review, we further explore these concepts and present evidence from the literature to support the use of stem cell therapy for the treatment of myopia.
Keywords: stem cells, myopia, dopamine, mesenchymal, nearsightedness, eye, sclera
Introduction
Investment in medical research aimed at improving quality of life is immense. While scientific advancements have helped to eliminate many medical problems, the increasing incidence of myopia has yet to be addressed on a comprehensive scale. Myopia is a largely civilization-driven condition. It affects a sensitive population—school children—in part, due to increased educational demands fueled by excessive pressures to meet the expectations of adults, parents, and teachers. In addition, technological developments, including the ubiquitous presence of computers, tablets, and smart phones, have dramatically decreased the time children spent outdoors, and increased the time focusing on text and graphic images at close range. The price is a rapidly increasing incidence of nearsightedness/myopia, a condition in dire need of an efficient therapy.
Medical Aspects of Myopia
Almost all cases of slight myopia, and most cases of moderate myopia, are benign conditions effectively treated by vision correction with spectacles, contact lenses, or refractive surgery (in adults). While visual acuity in patients with severe myopia (> 6.0D) can also be corrected by the same means, there are other long-term consequences of this disease[1]. One such consequence includes the extensive elongation of the eyeball that can result in posterior staphyloma. This dramatic elongation accounts for a wide array of degenerative eye changes, which occur more frequently in myopic than emmetropic eyes. and are a major cause of irreversible vision loss due to progressive degenerative maculopathy, including choroidal neovascularization, as well as retinal detachment, optic disc abnormalities, glaucoma, and cataract. In addition, surgical interventions on the eyes are risky in the myopic population. In addition to the risk of vision loss, there are also potential psychological side-effects, such as distress and anxiety[2] related to “Muscae volitantes” from vitreous body degeneration and detachment that occurs more frequently in myopic eyes[3].
Myopia in children: an emerging pandemic
While myopia was once considered a hereditary condition, the current demographics clearly indicate an environmental influence as well. A seminal study among Inuit families showed that, after the introduction of mandatory education, the incidence of myopia rose to 60%, compared to virtually no nearsightedness in the uneducated parents of these children[4]. Where education is minimal in small towns in Africa[5] and South America[6], the incidence of myopia is also low. In contrast, the prevalence of myopia is alarming in urban areas of the Far East. Among 5060 Chinese university students tested in Shanghai, 95.5% were myopic and 19.5% were highly myopic[7]. A study from Taiwan also showed that 80% of students were myopic after finishing elementary school. Hong Kong, Singapore, South Korea, and Japan are other examples of cities/countries with a very high incidence of myopia[8]. A study of 6- and 7-year-old students of Chinese ethnicity living in various different geographic locations revealed a much lower prevalence of myopia in those children living in Sydney, Australia (3.3%) compared to those residing in Singapore (29.1%)[9] After a thorough analysis of lifestyle and schooling, educational pressure for early achievement has been indicated as an underlying factor in the skyrocketing rates of myopia in Asia. The increased strain on the eye to distinguish subtle differences in hanzi or kanji (Asian characters) may also be contributing to the pandemic. The correlation between myopia prevalence and severity, and educational attainment has already been demonstrated in Singapore[10]. The population of myopic children also nearly doubled in the United States from 25% in 1971–72 to 41.6% between 1999 and 2004[11].
While school screening programs and the strict requirements for sight testing for driver’s licenses may lead to increased diagnosis of myopia; the published papers cited above do not refer to health/school/administration records, but rather use enrolled study participants, ensuring the quality of sight examination. Examination methodology has remained relatively unchanged for decades, so comparisons with previous generations should be valid.
While the majority of studies show that myopia is driven by factors related to the development of civilization, such as compulsory school attendance, there is also a somewhat contradictory thesis that civilization is driven by people with myopia. Clinical observations suggest that children with myopia may have a higher IQ [12]. A possible mechanistic explanation is that muscarinic acetylcholine receptors, which are associated with high myopia [13], are also involved in memory consolidation [14]. Thus, muscarinic overstimulation may cause excessive constriction of ciliary muscles, initiating myopia, while at the same time improving memory. Thus, the evolutionary advantage to people with myopia due to higher IQ could be an additional element contributing to the emerging pandemic. However, that advantage does not seem to exist in industrialized nations in which intelligence is instead inversely correlated to the number of children a person has [15]. One quite interesting observation is that, paradoxically, more educational pressure on less academically capable populations may fuel close-work assignments, allowing little time for outdoor activities, thus exacerbating the pandemic of myopia in the industrialized world. In other words, the evolutionary pressure promoting higher intelligence is no longer at play in a society where IQ is not necessary to survive until reproductive age and where IQ is inversely correlated with the number of descendants.
Genetics of myopia
The exact mechanism of myopia development has not yet been deciphered. Traditionally, a genetic factor has been emphasized because of the observation that myopic parents give birth to myopic children. In fact, heritability was confirmed statistically in multiple studies with mono and heterozygotic twins [16–19]. Studies of 12-year-old Australian children showed that prevalence of myopia increases with the number of myopic parents. Moreover, the strong influence of ethnicity has been shown, with a higher incidence in East Asians compared to Caucasians [20]. Similarly, new methods, such as genome-wide association studies (GWAS), have identified a considerable number of loci for refractive errors. Risk score analysis, using associated single-nucleotide polymorphism (SNPs), showed that risk for myopia increases ten-fold in individuals carrying the highest genetic load [21]. While genetic heterogeneity of susceptibility to myopia has been reported, and over 30 loci identified by linkage studies, the causative gene has been found in only a few loci, and no proteins have been identified to be directly related to myopia development [22]. For example, the SCO2 mutation has been found in families from the United States of European origin. SCO2 encodes a copper homeostasis protein[23], which is not directly related to the capture of light and signal transmission from the retina to the sclera, logical targets involved in environment-induced myopia. Thus, it is clear that the genetics of myopia is complex, and its cause is likely multifactorial. Based on the discussed mechanisms, it is warranted to suggest a double-hit hypothesis, in which myopia-related foci decrease the function of myopia-related cells in the retina and/or the sclera by different, probably unrelated mechanisms. When such “weakened” cells are additionally hit with environmental factors, such as an overburden of “close work” and/or an insufficient amount of light, myopia develops. What is important is that families at high risk of developing myopia can be identified by genetic studies [24, 25], and targeted with novel anti-myopic interventional therapies.
Environmental determinants of myopia
While genetics seem to play a significant role, other studies in monkeys, whose eye anatomy closely resembles that of humans, have shown that myopia can be induced by environmental manipulation. Restricted visual space has produced myopia, especially in young animals highly susceptible to such conditions [26]. Accommodative stress was suggested as the reason for the development of myopia, as it was prevented by atropine, which produces cycloplegia, or paralysis of the ciliary muscles [27]. In fact, accentuated ciliary muscle thickness, suggesting muscular hypertrophy, may account for the inherent dysfunction in myopia [28]. The application of negative diopter spectacle lenses alters eye development in young monkeys toward myopia [29], probably by inducing accommodative eye-strain.
Myopia can also be induced by form deprivation after fusing the eyelids [30], independent of lens-induced nearsightedness. It has been shown that lack of light stimulation leads to eyeball elongation, while outdoor activities and exposure to light not only prevent myopia development, but also decrease the rate of myopia progression in children. These observations have been confirmed by meta-analysis [31]. These findings suggest that the value of sunlight has been underestimated, and that parents should be educated to encourage children to increase outdoor activities. The reduction in myopia from light exposure is thought to be mediated by local ocular dopamine production. Retinal production of dopamine in form-deprived eyes is reduced while dopamine agonists decrease axial eye growth to reduce myopia [32]. Furthermore, scleral thinning induced by form deprivation can be prevented after intravitreal injection of dopamine in rabbits [33]. Other factors are also involved. For example, during eyeball elongation induced by form deprivation, vasoactive intestinal peptide can increase retinal neurogenesis [34].
It has been also hypothesized that vitamin D can mediate sun exposure-related protection against myopia. While lower sun exposure is clearly linked to both myopia and lower vitamin D levels, the link between vitamin D concentration as an independent variable for myopia is not consistent [35, 36]. There are no studies reporting on the use of vitamin D as a drug to prevent the initiation or to prevent myopia, requiring further investigation.
Apart from the protective effect of sun exposure, the potential risk of skin carcinogenesis should also be mentioned. Intermittent/sporadic skin exposure to UV (weekend and vacation activities in indoor workers) only increases the risk of melanoma (related to sunburn), but chronic exposure (outdoor workers) can even be protective [37–39]. Basal skin carcinoma is also related to intermittent/sporadic skin exposure [40]. Although squamous cell carcinoma seems to be related to chronic exposure to UV [41], the increase of school outdoor activities is unlikely to reach an alarming risk level. In addition, there is an initiative by four European countries to reduce exposure to UV with the use of appropriate protective clothing for outdoor workers in over the years 2010–2050 [42]. Accordingly, it is possible that children can be exposed to the appropriate level of sun, at the vital developmental stage, and the exposure can be reduced in adulthood so as not to reach a pathological cumulative level of sun exposure. Thus, there is no serious risk of carcinogenesis due to an increase of outdoor activities, and it may be even protective against melanoma, since daily exposure to the sun may eliminate the sporadic/intermittent skin exposure during weekends and vacations (as children are daily exposed to the sun). Alternatively, advocating for the topical application of UVA and UVB blockers is a practical yet effective approach to protect the skin.
The challenge to prevent myopia progression in children
Myopia most commonly affects school-age children, and, once it starts, the progressive nature is almost certain. Typically, the younger the age of myopia onset, the faster it progresses. In some geographical regions, up to 40% of children suffer severe myopia [43], with a high rate of irreversible vision loss in adulthood [44]. Attempts to predict the onset of myopia in children have been only partly effective. To date, no safe and effective method to halt myopia progression has been developed. The highest success rate has been achieved by cycloplegia, using a daily instillation of 1% atropine, but the side effects, including pupil dilation, accommodation paralysis, and the necessity to use the drug for many years, outweigh the benefits. Lower doses of atropine produce fewer side effects, but are also less effective. Discontinuation of treatment often results in progressive myopia at an accelerated pace [45]. The molecular mechanism by which atropine acts has not yet been elucidated.
Several studies have shown that myopia progression is slowed by orthokeratology, a method to reshape the cornea that is based on the overnight use of special rigid contact lenses, but this method is not yet widely accepted. In addition, only 50% of the affected children qualify for such therapy, and the positive effect disappears after four years [46]. In addition, orthokeratology requires high compliance, and excellent hygiene to prevent vision-threatening complications, such as keratitis or corneal abrasions, both of which are difficult to achieve in young children. It is believed that the benefit of orthokeratology is related to a shift from peripheral relative hyperopia to myopia [47]. Using the same concept, new, dual-focus soft contact lenses were recently designed, and have been shown to slow myopia progression [48]. However, it should be emphasized that the positive effects of orthokeratology and dual-focus soft contact lenses are rather small. Although they might be functionally important for some patients, further research to discover more effective therapies for myopia is warranted.
Another therapeutic strategy used for progressive high myopia is posterior scleral reinforcement (PSR) surgery [49]. This consists of positioning an implant such as a cadaveric fascia lata strip [50] or cadaveric sclera between the ocular muscles and the posterior aspect of the globe recipient via limbal peritomy [51], or the recently proposed concept of retrobulbar injection of an enzymatically degradable semi-interpenetrating polymer network [52]. PSR can halt myopia progression, but is not devoid of complications and challenges, such as difficulty obtaining appropriate graft material in some regions of the world [53], and complications such as delayed occlusion of the cilioretinal artery [54]. Transient muscle weakness and related binocular diplopia frequently follow the procedure [51]. A preclinical study in cats has also shown an impairment of venous outflow in the retina after PSR surgery [55]. Due to the skyrocketing rate of severe myopia in urban areas of the Far East, there are several recent reports of PSR from China [56–59], but none presents a cure. Thus, this procedure is reserved for a small population of quickly progressing, severely myopic patients.
The very high prevalence of myopia in specific populations has prompted studies on emmetropic (normal vision) school children. The myopic drift has been shown, in a clinical trial (NCT00477620), to be slowed in emmetropic school children by using reading glasses for near work. This method however, (patent US 20120236256), along with the application of progressive addition lenses in myopic children, has shown only a minimal slowing effect [60].
Thus, effective therapies for myopia which include daily administration of drugs over many years, are extremely inconvenient or challenging in young children. In addition, methods that require direct contact of a foreign material with the cornea introduce the risk of irreversible changes that, in young children, could impair proper corneal development. Thus, a treatment methodology based on a single and safe procedure, with long-term or even life-long effects, is highly desirable.
Application of stem cells for the treatment of eye disorders
The therapeutic use of stem cells was initially proposed as a strategy to restore function to damaged tissues. This is the guiding principle of regenerative medicine, a field that continues to grow tremendously, fueled in part by the increasing need of ageing societies. Recently, it has been shown that the beneficial effects of stem cells extend beyond cell replacement, and include modulatory and trophic support. There has been tremendous interest in using stem cell replacement for ocular diseases, as suggested by the hundreds of papers devoted to cell therapy for disorders of both the anterior and posterior eye.
Among anterior eye disorders, corneal diseases are among the most challenging. There are multiple reportedly successful attempts to restore function to the cornea with stem cell therapy. Stem cell therapy has also been used in posterior eye disorders, such as age-related macular degeneration, retinitis pigmentosa, and glaucoma. Depending on the complexity of the structure affected by the pathological process, the challenge to restore lost function by cell therapy varies. In age-related macular degeneration, the pathology primarily affects the retinal pigment epithelium; thus, cell therapy is more attainable, and, indeed clinical studies with stem cell transplantation have resulted in positive results in the small number of patients who have been studied [61]. Retinitis pigmentosa affects mainly photoreceptors, making it a more difficult target for regeneration, but trials in animal models have resulted in functional improvement. Retinal ganglion cells (RGC) that have degenerated in glaucoma are also difficult to replace, since restorative treatment would require directing their axons via the optic nerve to the lateral geniculate body (a few centimeters away from the retina) where they synapse with the next neuron of the visual pathway.
A novel concept for the use of stem cells for the therapy of myopia
People with myopia develop adult-age eye disorders much more frequently than those with normal vision, and the incidence is even higher in people who are severely myopic. Thus, efficient therapy to halt the progression of myopia in childhood could play a protective role against the occurrence of adult-onset eye diseases, and dramatically reduce ocular morbidity (Fig. 1). As mentioned above, various strategies have been explored to halt the progression of myopia. In addition, a multitude of stem cell-based approaches have been applied for the treatment of eye diseases. However, as of to date, stem cell therapy has not been considered as a method for halting the progression of myopia, even though the presumed mechanisms of myopia progression indicate that stem cell therapy could potentially be a viable option. Here, we introduce the concept and scientific rationale for the use of stem cell therapy to treat myopia.
Figure 1.
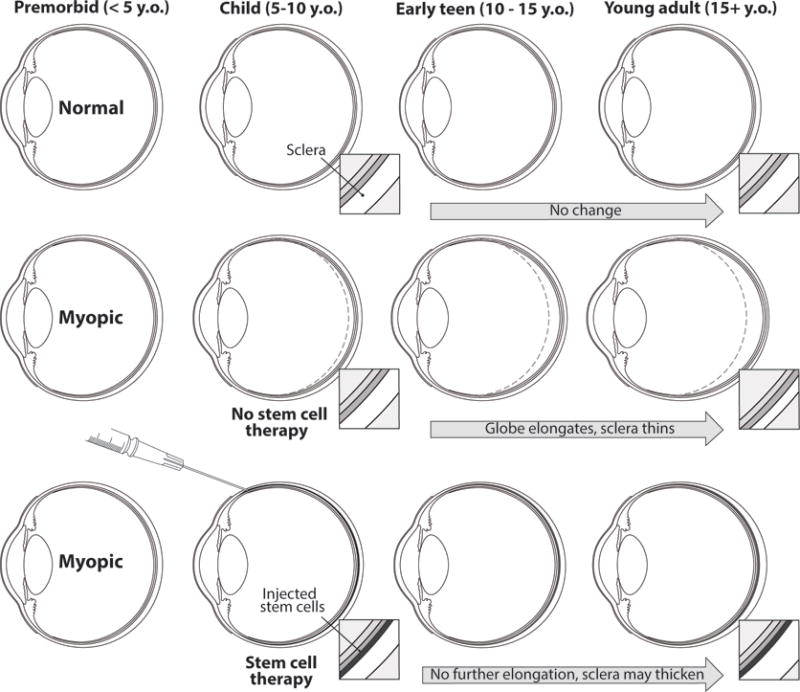
Schematic representation of severe myopia development, and the role of stem cell therapy in limiting disease progression. Note the gradual elongation of the eyeball and the scleral thinning over time, and the perspective for halting the disease progression in case of early administration of therapy.
Stem cell-based scleral support
While there are several proposed pathomechanisms that lead to myopia progression, the common feature of a myopic eye is a weak, less rigid, and thinned sclera [62] characterized by increased elasticity and reduced collagen content [63]. In this context, the development of strategies aimed at improving scleral biomechanics and preventing myopia progression is attractive because this would address the common underlying causative factor. This concept has already been used with posterior scleral reinforcement surgery, as mentioned above. While effective, this surgery can be complex, and is justified only for specific cases of severe myopia [64]. However, with a recently developed micro-needle-based, minimally invasive, safe technique, it is now possible to deposit payloads of stem cells to the back of the eye, specifically to the space between the choroid and the sclera (Fig. 2). This route has been found to be effective in the treatment of acute posterior uveitis in an animal model [65]. It has also used for the administration of biomaterials[66, 67] and tumor cells [68]. Based on this development a startup biomedical company has been established, and clinically applicable injection devices are in development, and are expected to be available in the near future [69].
Figure 2.
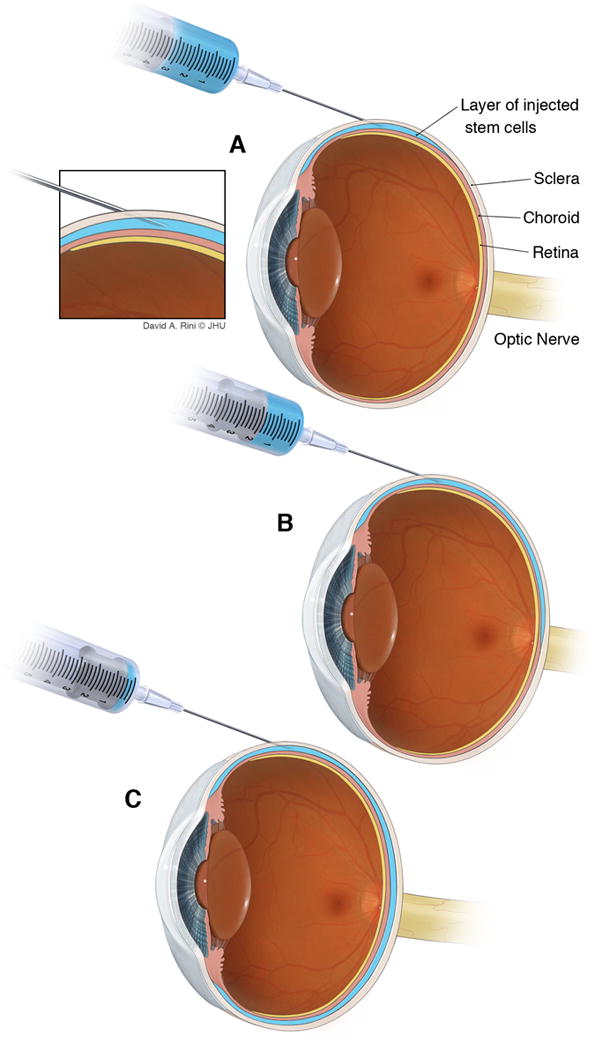
Graphic depicting the details of cell deposition (blue) within the subscleral space. A) Initial injection of the cell suspension, with gradual deposition of the payload within the subscleral space (B), which is eventually fully filled with the stem cell suspension (C). Inset shows the direct location of the needle tip with relation to the sclera, the choroid, and the ciliary body.
The major advantage of the placement of stem cells within the subscleral space is the possibility of their incorporation into the retina-sclera signaling loop. Since subscleral injection is routinely used to deliver drugs, without causing hemorrhage or retinal detachment, it is likely to become a valid delivery route for stem cells as well. The subscleral space is prone to adaptation, and, since it is flexible, as in most choroidal detachments, it will shrink with time. There are also alternative sites for targeting stem cells in order to treat myopia. One possibility is deposition of cells within the sclera itself [70], but compared to the subscleral space, the sclera has a limited capacity to expand. Therefore, multiple injections or a scleral tunnel in which to place the cells would likely be needed to achieve a sufficient supply of stem cells, which would be technically challenging given the inherently thin myopic sclera. Retrobulbar injection is a relatively safe procedure and could potentially replace subscleral injection, but it would only add mechanical stability to the tissue back to the sclera, similar to PSR, but it is not able to participate in the signaling between retina and sclera, due to the deposition of cells outside the globe. Targeting Sub-Tenon’s space would be another strategy, as it was used for delivery of chemotherapeutic drugs [71] or local anesthesia [72, 73], but it could obstruct eye lymph circulation and cause cell leakage to the lymphatic system. Therefore, that location would prevent a fine-tuned response to the dynamic needs of the eye and potentially better compliance with the vision apparatus. Thus, subscleral stem cell deposition is most promising in order to overcome the limited results of PSR, and offers hope for a more profound therapeutic effect. However, the above-mentioned alternative routes could also be considered when planning therapeutic interventions.
Mesenchymal stem cells (MSCs) have been successfully used in several clinical applications targeted at regeneration/reconstruction of connective tissue, and the reported properties of MSCs make them excellent candidates for scleral reinforcement. Technically, transplantation of autologous MSCs is a relatively easy and safe procedure, which could be routinely applied in millions of myopic children to halt the progression of disease. The strategy would include MSC derivation from bone marrow, fat, or other convenient and robust autologous sources (more research is needed to identify the most efficient cell source), cultured until the appropriate characteristics of cells are achieved, and then transplanted using a hollow micro-needle into the subscleral space. However, the naive autologous stem cells may carry a genetic load; thus, the in vitro cell repair or the use of allogeneic cells would be an alternative, but that would require immunosuppression, as MSCs are not necessarily immunoprivileged [74]. Transplanted cells would be expected to differentiate into fibroblasts that produce an extracellular matrix, to reinforce the sclera and prohibit eyeball elongation, thus preventing or halting myopia. The sclera contains MSCs [75]. Thus, an alternative approach would be to stimulate and recruit endogenous stem cells to differentiate into fibroblasts. Upon appropriate induction, they would contribute to strengthening of the sclera [75].
Stem cell-based eye signaling
While scleral reinforcement by MSCs is an attractive concept, alternative or supplementary stem cell-based therapies could also be used to prevent the progression of myopia. As mentioned above, there is dynamic cross-talk between the retina and the sclera, and one of the proposed mechanisms of myopia development is a disruption in that signaling. Dopaminergic signaling is central to this cross-talk and there is a growing body of evidence that dopamine also plays an important role in the growth of eye and regulation and myopia control [76]. Postnatal eye growth and refraction is regulated by the feedback mechanism initiated in the retina. For example, form-deprivation reduces the retinal level of dopamine, which coincides with myopia development [77]. The causative effect was further confirmed in an experiment where the local application of a dopamine agonist, apomorphine, produced an anti-myopic effect [78], which was later confirmed to be dependent on D2 receptor signaling [79]. Direct intravitreal injection of dopamine into the form-deprived rabbit eye also slowed the progression of myopia [80]. The administration of a dopamine precursor used in the treatment of Parkinson’s Disease (PD), L-Dopa, inhibits the development of form-deprivation myopia in guinea pigs [81]. In addition, the protective function of light against myopia has been shown to be abolished by dopamine antagonists [82]. Amacrine cells are a major source of dopamine in the retina [83]. Furthermore, dopamine participates in the development of lens-induced myopia [84], but dopamine agonists were not as efficacious in defocus-induced myopia as in form-deprived myopia [85]. A recent report indicates an additive effect of GABA antagonists with dopaminergic agonists to inhibit myopia development [86]. Since light induces dopamine production, it was speculated that enhanced dopamine production is the key factor by which outdoor activities prevent myopia [87]. Finally, since refractive error in adolescence is related to a low risk for schizophrenia, probably because of the low constitutive production of dopamine, additional indirect proof of dopaminergic involvement in myopia development is suggested by this genetic study [88].
Because of the evidence that dopamine plays a central role in the pathomechanism of myopia, it may be prudent to capitalize on the considerable expertise that has developed over the past few decades in stem cell-based therapy for Parkinson’s disease (PD). Highly functional dopaminergic cells were isolated from fetuses over a quarter century ago, and, more recently, from more abundant sources, such as embryonic stem cells, and the induced pluripotent stem cells. Thus, dopaminergic cells are abundantly available for possible treatment of myopia (Fig. 3). In addition, the ability to genetically engineer stem cells [89] allows for the induction of virtually any kind of cell, including MSCs, to produce dopamine. This introduces the opportunity to combine the benefits of both the supportive role of MSCs with dopaminergic signaling. It has already been shown that lentivirus-mediated transduction of MSCs, with a gene encoding tyrosine hydroxylase (TH), was effective in experimental PD [90]. The same strategy could be ideally suited to halt myopia. The system for conditional transgene silencing, similar to that employed in clinical trials for stem cell therapy of stroke [91, 92], would be an additional advantage as a safety mechanism to turn off dopamine signaling. Another option would be optogenetics [93], which could switch on dopamine production only in the presence of light, acting as a kind of light effect enhancer, but could switch off in the lack of light, preventing toxicity from prolonged exposure of the sclera to dopamine.
Figure 3.
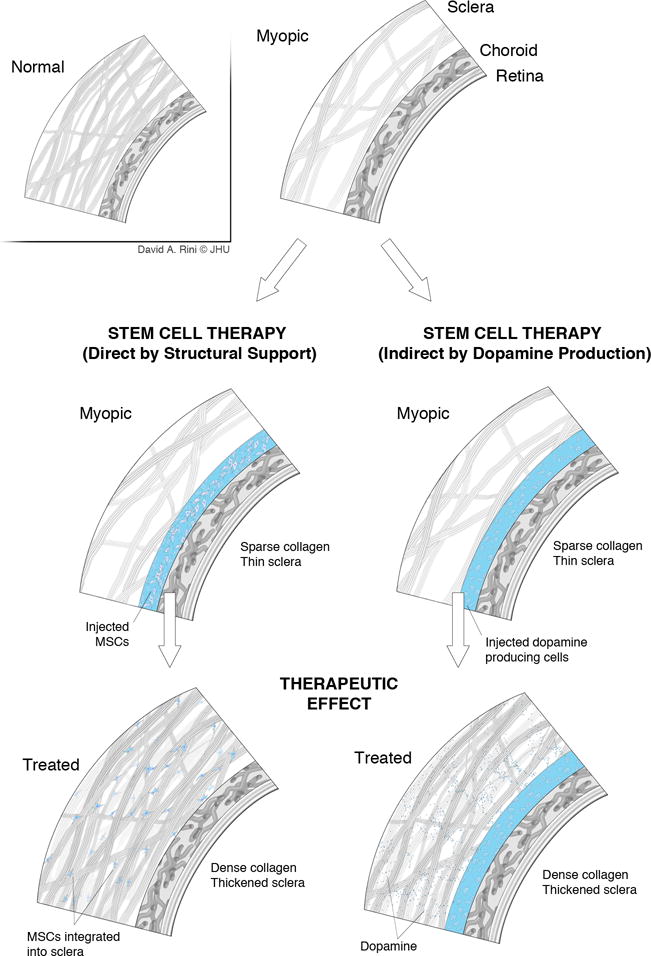
Mechanisms for the prevention of myopia progression that can be exploited with the application of stem cell-related therapy. These include the incorporation of injected MSCs into the structure of the sclera with direct mechanical support (left column), and, through the production of dopamine, indirect stimulation of the scleral tissue, which, in turn, may prevent eye elongation (right column).
In addition to dopaminergic signaling, the anti-myopic effect of bFGF, and co-transplantation of cells producing bFGF [94] could also be considered [95] to help the survival and function of dopaminergic cells. Notably, these cells do not activate TGF-beta signaling, a possible susceptibility pathway for severe myopia [96]. Finally, retinoic acid has also been proposed as a possible mediator between refractive error and compensatory eye growth, and thus, could also be potentially targeted by stem cell therapy.
Preclinical studies
The proposed stem cell-based therapies of patients with myopia need to be preceded by extensive preclinical studies to establish safety and efficacy. There are two leading models of myopia: form deprivation and lens-induced myopia. It is likely that a combination of the two would best represent the environmental conditions leading to myopia and therefore would be experimentally useful.
Due to the nature of this experimental technique, it is critical to carefully select an appropriate animal model. Although various species have been used in the past for myopia research, it would be optimal to select an animal with an eye size comparable to that of humans, and also one that is relatively low cost and deemed reasonable acceptable by society for use in research. The porcine model seems to fit these requirements and would likely be an excellent option. Additionally, in recent years, there has been significant progress in the generation of transgenic swine, particularly by the NIH National Swine Resource and Research Center (NSRRC) (http://www.nsrrc.missouri.edu/). This opens the possibility to generate pigs with targeted knockout of genes implicated in severe myopia, to further improve the preclinical study of potential therapies for myopia.
Ethics and safety
Ethics and safety are also very important issues. Obviously, at this stage, there are no data demonstrating that the proposed approach is feasible. While, initially, the method of subscleral cell injection may seem to be risky for existing eyesight, the procedure of subscleral injection is simple and cell distribution may be favorable while not endangering overall sight. Thus, the procedure may not need to be treated as a surgery, but rather as a relatively non-invasive outpatient needle injection. The choice and production of appropriate stem cells is currently still under debate, but as we learn more, the choice is expected to be more clear and perhaps even personalized, which may dramatically decrease the cost of therapy. If the method is proven safe, it may be then applied also for mild myopia to completely eradicate the “disease” our population. This maybe comparable to administration of vaccines, which are given to children in order to prevent disease occurrence at an older age. Needless to say, any of the discussed approaches should be extensively tested in preclinical models prior to their translation to patients.
An alternative approach – gene therapy?
Gene therapy is a potential alternative approach for the treatment of high myopia, but would be complex due to genetic heterogeneity. Although we are unaware of any published data on this strategy to date, there would be several challenges for this approach. Multiple loci related to myopia have been identified and in some patients, several loci may be mutated. A personalized exome-wide study would be necessary to detect the existing myopia-related genetic abnormalities and customized, patient-specific solutions would be needed to correct DNA aberrations. Although the same subscleral route could be used for delivery of exogenous genetic material, it would potentially require combinatorial use of multiple vectors. Overall, gene therapy strategies to correct the first hit of the double-hit hypothesis are likely to be cumbersome and cost-ineffective, at least at this stage of technology development. Thus, developing novel strategies to address the second hit, such as eliminating the consequences of a hostile environment by the application of stem cell therapy, are more likely to prove feasible.
Conclusions
Early onset of myopia, followed by rapid progression toward high myopia, has become a pandemic in modern, education-oriented societies. No convenient and efficient way to halt the progression of myopia currently exists. Since the pathomechanisms of myopia development are convergent with stem cell properties, this correspondence could result in novel therapeutic strategies. The weakness of the sclera in patients with myopia could be reinforced by subscleral injection of MSCs, and the preventive role of dopamine could be exploited by transplantation of MSCs that produce dopamine. Therefore, stem cell therapy represents a promising new strategy to halt the progression of myopia, particularly among the school-age population.
Acknowledgments
The authors thank Mary McAllister for editorial assistance. This work was supported by: MSCRFII-0193, MSCRFII-0052, R01 NS076573, S10 RR028955, 1R21NS081544, MSCRE-0178-00 and a Mobility Plus grant from the Polish Ministry of Science and Higher Education (MJ).
References
- 1.Gilmartin B. Myopia: precedents for research in the twenty-first century. Clin Experiment Ophthalmol. 2004;32:305–324. doi: 10.1111/j.1442-9071.2004.00831.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagle AM, Lim WY, Yap TP, et al. Utility values associated with vitreous floaters. Am J Ophthalmol. 2011;152:60–65. e61. doi: 10.1016/j.ajo.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Chuo JY, Lee TY, Hollands H, et al. Risk factors for posterior vitreous detachment: a case-control study. Am J Ophthalmol. 2006;142:931–937. doi: 10.1016/j.ajo.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Young FA, Leary GA, Baldwin WR, et al. The transmission of refractive errors within eskimo families. Am J Optom Arch Am Acad Optom. 1969;46:676–685. doi: 10.1097/00006324-196909000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Yared AW, Belaynew WT, Destaye S, et al. Prevalence of refractive errors among school children in gondar town, northwest ethiopia. Middle East Afr J Ophthalmol. 2012;19:372–376. doi: 10.4103/0974-9233.102742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moraes Ibrahim F, Moraes Ibrahim M, Pomepo de Camargo JR, et al. Visual impairment and myopia in Brazilian children: a population-based study. Optom Vis Sci. 2013;90:223–227. doi: 10.1097/OPX.0b013e31828197fd. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Zhou J, Zhao P, et al. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai. Invest Ophthalmol Vis Sci. 2012;53:7504–7509. doi: 10.1167/iovs.11-8343. [DOI] [PubMed] [Google Scholar]
- 8.Morgan IG, Rose KA. Myopia and international educational performance. Ophthalmic Physiol Opt. 2013;33:329–338. doi: 10.1111/opo.12040. [DOI] [PubMed] [Google Scholar]
- 9.Rose KA, Morgan IG, Smith W, et al. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008;126:527–530. doi: 10.1001/archopht.126.4.527. [DOI] [PubMed] [Google Scholar]
- 10.Tay MT, Au Eong KG, Ng CY, et al. Myopia and educational attainment in 421,116 young Singaporean males. Ann Acad Med Singapore. 1992;21:785–791. [PubMed] [Google Scholar]
- 11.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 12.Czepita D, Lodygowska E, Czepita M. Are children with myopia more intelligent? A literature review. Annales Academiae Medicae Stetinensis. 2008;54:13–16. discussion 16. [PubMed] [Google Scholar]
- 13.Lin HJ, Wan L, Tsai Y, et al. Muscarinic acetylcholine receptor 1 gene polymorphisms associated with high myopia. Molecular vision. 2009;15:1774–1780. [PMC free article] [PubMed] [Google Scholar]
- 14.Dobryakova YV, Gurskaya O, Markevich VA. Participation of muscarinic receptors in memory consolidation in passive avoidance learning. Acta neurobiologiae experimentalis. 2014;74:211–217. doi: 10.55782/ane-2014-1986. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa S. Intelligence and childlessness. Social science research. 2014;48:157–170. doi: 10.1016/j.ssresearch.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim MH, Zhao D, Kim W, et al. Heritability of Myopia and Ocular Biometrics in Koreans: The Healthy Twin Study. Invest Ophthalmol Vis Sci. 2013 doi: 10.1167/iovs.12-11254. [DOI] [PubMed] [Google Scholar]
- 17.Ding X, Wang D, Huang Q, et al. Distribution and heritability of peripheral eye length in Chinese children and adolescents: the Guangzhou Twin Eye Study. Invest Ophthalmol Vis Sci. 2013;54:1048–1053. doi: 10.1167/iovs.12-10066. [DOI] [PubMed] [Google Scholar]
- 18.Stankovic-Babic G, Vujanovic M, Cekic S. Identical twins with “mirror image” anisometropia and esotropia. Srp Arh Celok Lek. 2011;139:661–665. [PubMed] [Google Scholar]
- 19.Tsai MY, Lin LL, Lee V, et al. Estimation of heritability in myopic twin studies. Jpn J Ophthalmol. 2009;53:615–622. doi: 10.1007/s10384-009-0724-1. [DOI] [PubMed] [Google Scholar]
- 20.Ip JM, Huynh SC, Robaei D, et al. Ethnic differences in the impact of parental myopia: findings from a population-based study of 12-year-old Australian children. Invest Ophthalmol Vis Sci. 2007;48:2520–2528. doi: 10.1167/iovs.06-0716. [DOI] [PubMed] [Google Scholar]
- 21.Verhoeven VJ, Hysi PG, Wojciechowski R, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45:712. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawthorne FA, Young TL. Genetic contributions to myopic refractive error: Insights from human studies and supporting evidence from animal models. Experimental eye research. 2013;114:141–149. doi: 10.1016/j.exer.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Tran-Viet KN, Powell C, Barathi VA, et al. Mutations in SCO2 are associated with autosomal-dominant high-grade myopia. American journal of human genetics. 2013;92:820–826. doi: 10.1016/j.ajhg.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young TL, Ronan SM, Drahozal LA, et al. Evidence that a locus for familial high myopia maps to chromosome 18p. American journal of human genetics. 1998;63:109–119. doi: 10.1086/301907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young TL, Atwood LD, Ronan SM, et al. Further refinement of the MYP2 locus for autosomal dominant high myopia by linkage disequilibrium analysis. Ophthalmic genetics. 2001;22:69–75. doi: 10.1076/opge.22.2.69.2233. [DOI] [PubMed] [Google Scholar]
- 26.Young FA. The Effect of Restricted Visual Space on the Refractive Error of the Young Monkey Eye. Invest Ophthalmol. 1963;2:571–577. [PubMed] [Google Scholar]
- 27.Young FA. The Effect of Atropine on the Development of Myopia in Monkeys. Am J Optom Arch Am Acad Optom. 1965;42:439–449. doi: 10.1097/00006324-196508000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Jeon S, Lee WK, Lee K, et al. Diminished ciliary muscle movement on accommodation in myopia. Exp Eye Res. 2012;105:9–14. doi: 10.1016/j.exer.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 30.Smith EL, 3rd, Harwerth RS, Crawford ML, et al. Observations on the effects of form deprivation on the refractive status of the monkey. Invest Ophthalmol Vis Sci. 1987;28:1236–1245. [PubMed] [Google Scholar]
- 31.Sherwin JC, Reacher MH, Keogh RH, et al. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology. 2012;119:2141–2151. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Stone RA, Lin T, Laties AM, et al. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Z, Chen X, Ge J, et al. Effects of direct intravitreal dopamine injection on sclera and retina in form-deprived myopic rabbits. J Ocul Pharmacol Ther. 2008;24:543–550. doi: 10.1089/jop.2008.0041. [DOI] [PubMed] [Google Scholar]
- 34.Tkatchenko AV, Walsh PA, Tkatchenko TV, et al. Form deprivation modulates retinal neurogenesis in primate experimental myopia. Proc Natl Acad Sci U S A. 2006;103:4681–4686. doi: 10.1073/pnas.0600589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guggenheim JA, Williams C, Northstone K, et al. Does vitamin D mediate the protective effects of time outdoors on myopia? Findings from a prospective birth cohort. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.14-15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yazar S, Hewitt AW, Black LJ, et al. Myopia is associated with lower vitamin D status in young adults. Invest Ophthalmol Vis Sci. 2014;55:4552–4559. doi: 10.1167/iovs.14-14589. [DOI] [PubMed] [Google Scholar]
- 37.Nelemans PJ, Rampen FH, Ruiter DJ, et al. An addition to the controversy on sunlight exposure and melanoma risk: a meta-analytical approach. J Clin Epidemiol. 1995;48:1331–1342. doi: 10.1016/0895-4356(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 38.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 39.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 40.Zanetti R, Rosso S, Martinez C, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer. 2006;94:743–751. doi: 10.1038/sj.bjc.6602982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291–307. doi: 10.1111/j.1365-2133.2010.10118.x. [DOI] [PubMed] [Google Scholar]
- 42.de Vries E, Arnold M, Altsitsiadis E, et al. Potential impact of interventions resulting in reduced exposure to ultraviolet (UV) radiation (UVA and UVB) on skin cancer incidence in four European countries, 2010–2050. Br J Dermatol. 2012;167(Suppl 2):53–62. doi: 10.1111/j.1365-2133.2012.11087.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang TJ, Chiang TH, Wang TH, et al. Changes of the ocular refraction among freshmen in National Taiwan University between 1988 and 2005. Eye (Lond) 2009;23:1168–1169. doi: 10.1038/eye.2008.184. [DOI] [PubMed] [Google Scholar]
- 44.Verhoeven VJ, Wong KT, Buitendijk GH, et al. Visual Consequences of Refractive Errors in the General Population. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Tong L, Huang XL, Koh AL, et al. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 46.Hiraoka T, Kakita T, Okamoto F, et al. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. 2012;53:3913–3919. doi: 10.1167/iovs.11-8453. [DOI] [PubMed] [Google Scholar]
- 47.Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom Vis Sci. 2011;88:476–482. doi: 10.1097/OPX.0b013e31820f16fb. [DOI] [PubMed] [Google Scholar]
- 48.Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011;118:1152–1161. doi: 10.1016/j.ophtha.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 49.Thompson FB. Scleral reinforcement for high myopia. Ophthalmic Surg. 1985;16:90–94. [PubMed] [Google Scholar]
- 50.Balazs K, Bekesi L, Berta A, et al. Scleral reinforcement in progressive myopia and intraoperative ultrasound control of the cadaver fascia lata strip. Acta chirurgica Hungarica. 1997;36:14–15. [PubMed] [Google Scholar]
- 51.Ward B, Tarutta EP, Mayer MJ. The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye (Lond) 2009;23:2169–2174. doi: 10.1038/eye.2008.433. [DOI] [PubMed] [Google Scholar]
- 52.Su J, Wall ST, Healy KE, et al. Scleral reinforcement through host tissue integration with biomimetic enzymatically degradable semi-interpenetrating polymer network. Tissue engineering Part A. 2010;16:905–916. doi: 10.1089/ten.tea.2009.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curtin BJ, Whitmore WG. Long-term results of scleral reinforcement surgery. Am J Ophthalmol. 1987;103:544–548. doi: 10.1016/s0002-9394(14)74278-3. [DOI] [PubMed] [Google Scholar]
- 54.Karabatsas CH, Waldock A, Potts MJ. Cilioretinal artery occlusion following scleral reinforcement surgery. Acta ophthalmologica Scandinavica. 1997;75:316–318. doi: 10.1111/j.1600-0420.1997.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 55.Jacob-LaBarre JT, Assouline M, Conway MD, et al. Effects of scleral reinforcement on the elongation of growing cat eyes. Arch Ophthalmol. 1993;111:979–986. doi: 10.1001/archopht.1993.01090070099027. [DOI] [PubMed] [Google Scholar]
- 56.Chen M, Dai J, Chu R, et al. The efficacy and safety of modified Snyder-Thompson posterior scleral reinforcement in extensive high myopia of Chinese children. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013;251:2633–2638. doi: 10.1007/s00417-013-2429-x. [DOI] [PubMed] [Google Scholar]
- 57.Xue A, Bao F, Zheng L, et al. Posterior scleral reinforcement on progressive high myopic young patients. Optom Vis Sci. 2014;91:412–418. doi: 10.1097/OPX.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 58.Qi Y, Duan AL, You QS, et al. Posterior Scleral Reinforcement and Vitrectomy for Myopic Foveoschisis in Extreme Myopia. Retina. 2014 doi: 10.1097/IAE.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 59.Zhu SQ, Wang QM, Xue AQ, et al. Posterior sclera reinforcement and phakic intraocular lens implantation for highly myopic amblyopia in children: a 3-year follow-up. Eye (Lond) 2014;28:1310–1314. doi: 10.1038/eye.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gwiazda JE, Hyman L, Norton TT, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2004;45:2143–2151. doi: 10.1167/iovs.03-1306. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 62.Sergienko NM, Shargorogska I. The scleral rigidity of eyes with different refractions. Graefes Arch Clin Exp Ophthalmol. 2012;250:1009–1012. doi: 10.1007/s00417-012-1973-0. [DOI] [PubMed] [Google Scholar]
- 63.McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86:E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- 64.Ji X, Wang J, Zhang J, et al. The effect of posterior scleral reinforcement for high myopia macular splitting. J Int Med Res. 2011;39:662–666. doi: 10.1177/147323001103900236. [DOI] [PubMed] [Google Scholar]
- 65.Gilger BC, Abarca EM, Salmon JH, et al. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013;54:2483–2492. doi: 10.1167/iovs.13-11747. [DOI] [PubMed] [Google Scholar]
- 66.Einmahl S, Savoldelli M, D’Hermies F, et al. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest Ophthalmol Vis Sci. 2002;43:1533–1539. [PubMed] [Google Scholar]
- 67.Tyagi P, Barros M, Stansbury JW, et al. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Molecular pharmaceutics. 2013;10:2858–2867. doi: 10.1021/mp300716t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braun RD, Gradianu M, Vistisen KS, et al. Manganese-enhanced MRI of human choroidal melanoma xenografts. Invest Ophthalmol Vis Sci. 2007;48:963–967. doi: 10.1167/iovs.06-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinbach OC. Industry Update: the latest developments in therapeutic delivery. Therapeutic delivery. 2013;4:531–535. doi: 10.4155/tde.13.32. [DOI] [PubMed] [Google Scholar]
- 70.Kim SH, Galban CJ, Lutz RJ, et al. Assessment of subconjunctival and intrascleral drug delivery to the posterior segment using dynamic contrast-enhanced magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2007;48:808–814. doi: 10.1167/iovs.06-0670. [DOI] [PubMed] [Google Scholar]
- 71.Liu HS, Refojo MF, Albert DM. Experimental combined systemic and local chemotherapy for intraocular malignancy. Arch Ophthalmol. 1980;98:905–908. doi: 10.1001/archopht.1980.01020030899019. [DOI] [PubMed] [Google Scholar]
- 72.Sharma T, Gopal L, Parikh S, et al. Parabulbar anesthesia for primary vitreoretinal surgery. Ophthalmology. 1997;104:425–428. doi: 10.1016/s0161-6420(97)30297-8. [DOI] [PubMed] [Google Scholar]
- 73.Ripart J, Lefrant JY, Vivien B, et al. Ophthalmic regional anesthesia: medial canthus episcleral (sub-tenon) anesthesia is more efficient than peribulbar anesthesia: A double-blind randomized study. Anesthesiology. 2000;92:1278–1285. doi: 10.1097/00000542-200005000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsai CL, Wu PC, Fini ME, et al. Identification of multipotent stem/progenitor cells in murine sclera. Invest Ophthalmol Vis Sci. 2011;52:5481–5487. doi: 10.1167/iovs.11-7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Stone RA, Lin T, Iuvone PM, et al. Postnatal control of ocular growth: dopaminergic mechanisms. Ciba Found Symp. 1990;155:45–57. doi: 10.1002/9780470514023.ch4. discussion 57–62. [DOI] [PubMed] [Google Scholar]
- 78.Iuvone PM, Tigges M, Stone RA, et al. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991;32:1674–1677. [PubMed] [Google Scholar]
- 79.Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- 80.Gao Q, Liu Q, Ma P, et al. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol. 2006;244:1329–1335. doi: 10.1007/s00417-006-0254-1. [DOI] [PubMed] [Google Scholar]
- 81.Mao J, Liu S, Qin W, et al. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom Vis Sci. 2010;87:53–60. doi: 10.1097/OPX.0b013e3181c12b3d. [DOI] [PubMed] [Google Scholar]
- 82.McCarthy CS, Megaw P, Devadas M, et al. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res. 2007;84:100–107. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 83.Laties AM, Stone RA. Some visual and neurochemical correlates of refractive development. Vis Neurosci. 1991;7:125–128. doi: 10.1017/s0952523800010993. [DOI] [PubMed] [Google Scholar]
- 84.Guo SS, Sivak JG, Callender MG, et al. Retinal dopamine and lens-induced refractive errors in chicks. Curr Eye Res. 1995;14:385–389. doi: 10.3109/02713689508999936. [DOI] [PubMed] [Google Scholar]
- 85.Dong F, Zhi Z, Pan M, et al. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011;17:2824–2834. [PMC free article] [PubMed] [Google Scholar]
- 86.Schmid KL, Strasberg G, Rayner CL, et al. The effects and interactions of GABAergic and dopaminergic agents in the prevention of form deprivation myopia by brief periods of normal vision. Exp Eye Res. 2013;110:88–95. doi: 10.1016/j.exer.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 87.French AN, Ashby RS, Morgan IG, et al. Time outdoors and the prevention of myopia. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 88.Caspi A, Vishne T, Reichenberg A, et al. Refractive errors and schizophrenia. Schizophr Res. 2009;107:238–241. doi: 10.1016/j.schres.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 89.Nowakowski A, Andrzejewska A, Janowski M, et al. Genetic engineering of stem cells for enhanced therapy. Acta Neurobiol Exp (Wars) 2013;73:1–18. doi: 10.55782/ane-2013-1918. [DOI] [PubMed] [Google Scholar]
- 90.Shi D, Chen G, Lv L, et al. The effect of lentivirus-mediated TH and GDNF genetic engineering mesenchymal stem cells on Parkinson’s disease rat model. Neurol Sci. 2011;32:41–51. doi: 10.1007/s10072-010-0385-3. [DOI] [PubMed] [Google Scholar]
- 91.Mack GS. ReNeuron and StemCells get green light for neural stem cell trials. Nat Biotechnol. 2011;29:95–97. doi: 10.1038/nbt0211-95. [DOI] [PubMed] [Google Scholar]
- 92.Katare R, Stroemer P, Hicks C, et al. Clinical-grade human neural stem cells promote reparative neovascularization in mouse models of hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2014;34:408–418. doi: 10.1161/ATVBAHA.113.302592. [DOI] [PubMed] [Google Scholar]
- 93.Miller G. Optogenetics. Shining new light on neural circuits. Science. 2006;314:1674–1676. doi: 10.1126/science.314.5806.1674. [DOI] [PubMed] [Google Scholar]
- 94.Liang Y, Agren L, Lyczek A, et al. Neural progenitor cell survival in mouse brain can be improved by co-transplantation of helper cells expressing bFGF under doxycycline control. Experimental neurology. 2013;247:73–79. doi: 10.1016/j.expneurol.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58:553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- 96.Mathis U, Schaeffel F. Transforming growth factor-beta in the chicken fundal layers: an immunohistochemical study. Exp Eye Res. 2010;90:780–790. doi: 10.1016/j.exer.2010.03.014. [DOI] [PubMed] [Google Scholar]


