Abstract
Estrogens have previously been shown to protect the brain against acute ischemic insults, by potentially augmenting cerebrovascular function after ischemic stroke. The current study hypothesized that treatment with sustained release of high-dose 17β-estradiol (E2) at the time of reperfusion from middle cerebral artery occlusion (MCAO) in rats would attenuate reperfusion injury, augment post-stroke angiogenesis and cerebral blood flow, and attenuate lesion volume. Female Wistar rats underwent ovariectomy, followed two weeks later by transient, two-hour right MCAO (tMCAO) and treatment with E2 (n = 13) or placebo (P; n = 12) pellets starting at reperfusion. E2 treatment resulted in significantly smaller total lesion volume, smaller lesions within striatal and cortical brain regions, and less atrophy of the ipsilateral hemisphere after six weeks of recovery. E2-treated animals exhibited accelerated recovery of contralateral forelimb sensorimotor function in the cylinder test. Magnetic resonance imaging (MRI) showed that E2 treatment reduced the formation of lesion cysts, decreased lesion volume, and increased lesional cerebral blood flow (CBF). Ktrans, a measure of vascular permeability, was increased in the lesions. This finding, which represents lesion neovascularization, was not altered by E2 treatment. Ischemic stroke–related angiogenesis and vessel formation was confirmed with immunolabeling of brain tissue and was not altered with E2 treatment. In summary, E2 treatment administered immediately following reperfusion significantly reduced lesion size, cyst formation, and brain atrophy while improving lesional CBF and accelerating recovery of functional deficits in a rat model of ischemic stroke.
Keywords: Ischemic Stroke, Estradiol, Neuroprotection, Angiogenesis, Functional Recovery, Magnetic Resonance Imaging
1. Introduction
The role of estrogen in protection against ischemic stroke began with epidemiological data demonstrating that premenopausal women have a lower incidence of stroke and cardiovascular disease compared to men of the same age (Petrea et al., 2009). Removal of female sex hormones by ovariectomy in animal models of ischemic stroke, such as the MCAO, resulted in an exacerbation of injury that could be reversed by exogenous administration of estrogen (Alkayed et al., 1998; Simpkins et al., 1997). However, clinical trials showed a lack of benefit of hormone replacement therapy in preventing primary or secondary incidence of stroke (Rossouw et al., 2002; Viscoli et al., 2001), severely hampering the potential for estrogen to be used as a prophylactic treatment for ischemic stroke.
The administration of estrogen during or after an ischemic stroke may still be able to provide neuroprotection, alter repair, and restore neurological function. Simpkins et al. (1997) showed that high dose E2 at reperfusion could increase survival to one week after stroke and reduce lesion size. While high and low doses of E2 had beneficial effects when administered prior to permanent MCAO (pMCAO), only high physiological levels were able to perform the same function when administered at the onset of ischemia (Dubal et al., 1998). Yang et al. (2000) established the window of efficacy for supraphysiologic E2 administration to be ≤ 3 hours after the onset of pMCAO, and confirmed that low endogenous levels offered no protection. Importantly, they also showed that early supraphysiologic E2 treatment could increase ipsilateral CBF 24 hours after experimental stroke, suggesting a potential cerebrovascular component for E2-mediated neuroprotection.
The ability of estrogen to alter CBF was further investigated with a single intravenous injection of high-dose Premarin after tMCAO, which reduced hypoperfusion and increased CBF during the acute period of reperfusion, resulting in a reduction of lesion volume (McCullough et al., 2001). The fast onset and transient nature of this effect suggested a non-genomic mechanism of CBF regulation. Liu et al. (2002) provided additional evidence of a non-genomic mechanism with the administration of a non-estrogen receptor binding E2 derivative. The derivative increased ipsilateral and contralateral CBF during reperfusion while also retaining antioxidant properties similar to E2. Although it is difficult to attribute decreases in lesion size to any one specific mechanism, the changes in cerebrovascular function after E2 treatment seem to be associated with improved outcomes after experimental ischemic stroke.
We have previously shown that administration of E2 one week prior to tMCAO in ovariectomized female rats enhanced post-stroke angiogenesis without significantly altering lesion size, which also resulted in an improvement in contralateral forelimb function three weeks after ischemic stroke (Ardelt et al., 2012). MRI demonstrated improvement in CBF during the fourth week of recovery with E2 pre-treatment. Estrogen may have enhanced angiogenesis by increasing the expression of angiopoietin-1 (Ardelt et al., 2005) and promoting endothelial cell survival (Guo et al., 2010) after ischemic stroke. The collective actions of E2 on cerebrovascular function represent important and novel targets for enhancing current treatment strategies.
The current study was designed to understand if E2 treatment during reperfusion could alter long-term components of cerebrovascular function, lesion volume, and post-stroke repair. For this, ovariectomized female Wistar rats underwent a two-hour right MCAO followed by reperfusion and E2 or placebo pellet implantation (Figure 1). Animals were randomly assigned to the treatment groups, and all analyses were performed in a blinded fashion. Forelimb sensorimotor impairment was assessed weekly for six weeks after experimental stroke, at which time animals were euthanized and histological sections were analyzed for lesion volume and biomarkers of post-stroke injury and repair. A subset of animals underwent MRI with contrast administration for evaluation of CBF and vascular permeability, as well as measurement of lesion volume four weeks after experimental stroke. We hypothesized that administration of high-dose E2 at reperfusion would reduce lesion volume, improve cerebrovascular function, and enhance recovery during long-term survival after transient cerebral ischemia.
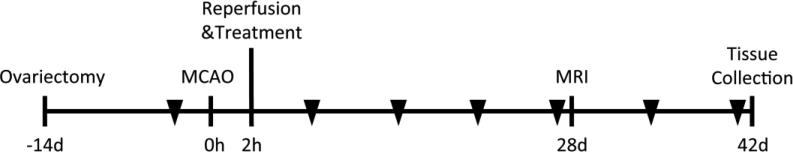
Figure 1.
Experimental outline. Female Wistar rats were ovariectomized fourteen days prior to experimental stroke. Reperfusion and treatment (placebo or 17β-estradiol pellet) took place after two hours of middle cerebral artery occlusion (MCAO). Representative rats in both groups underwent MRI analysis of lesion volume and cerebrovascular function after four weeks of recovery. Brain tissue samples were collected for histology after six weeks of recovery. Triangles represent weekly behavioral assessment of forelimb function with the cylinder test. All animals were randomly assigned to the treatment groups and all analyses were performed in a blinded fashion.
2. Results
2.1 Exclusions, and Operative and Physiologic Parameters
One animal in the E2 group, and none in the P group, died >24 hours after tMCAO. Seven animals were excluded due to the lack of ischemic lesions on histology despite having met operative flow decrease criteria: three in the E2 group and four in the P group. Ultimately, twelve rats in the P group and thirteen rats in the E2 group were included in the final analysis. Due to an error, one E2 treated animal did not undergo analysis of euthanasia serum E2 levels, vascular density, Luxol fast blue (LFB), and synaptophysin (SYP).
2.2 Estradiol Dose-Release
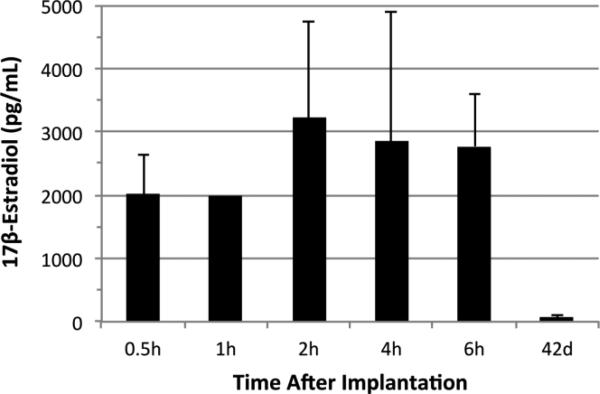
Wide variability in circulating plasma E2 has been previously reported in Innovative Research of America's (IRA) timed-release pellets (Strom et al., 2008). As timing and dose of E2 administration can influence the level of neuroprotection or neurodamage (Strom et al., 2009), it was important to understand these parameters in the current experimental paradigm. We've previously shown that the 0.72mg 60-day release IRA pellet sustains supraphysiologic levels from 1-10 days after implantation with a gradual decline to high physiologic levels by 30 days (Ardelt et al., 2012). Additional time points from the current study revealed that this pellet produces supraphysiologic levels > 2000 pg/mL as early as 30 minutes after implantation, with a peak at 2 hours of > 3200 pg/mL E2 (Figure 2). These early supraphysiologic levels have demonstrated to be neuroprotective when administered after permanent (Dubal et al., 1998; Liu et al., 2007) and transient (McCullough et al., 2001) models of focal cerebral ischemia. The administration and release time of ≤ 2.5 hours after onset of ischemia in this study is within the proposed window of efficacy for E2 treatment as assessed in the pMCAO rat model (Yang et al., 2000).
Figure 2.
Plasma levels of 17β-estradiol (E2) as measured by the Elecsys assay (mean ± sd). E2 pellets (0.72 mg, Innovative Research of America) produced supraphysiologic levels > 2000 pg/mL E2 as early as 30 minutes after implantation with a peak at 2 hours of > 3200 pg/mL. E2 levels remained elevated (> 2500 pg/mL) through 6 hours. For E2 treated MCAO animals included in this study, E2 at time of euthanasia (42d) was reduced to high physiologic levels (60 pg/mL).
2.3 Estradiol Treatment Reduces Lesion Volume and Brain Atrophy
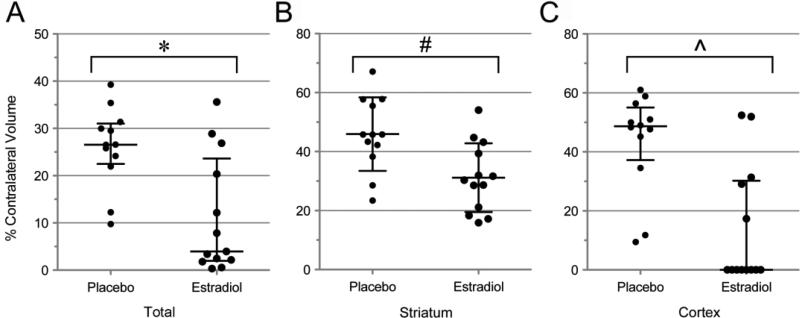
Two-hour tMCAO produced an ischemic lesion spanning the striatum and cerebral cortex in P control animals, with a median total lesion volume of 27% (Figure 3A). Treatment with E2 resulted in a significant decrease in median lesion volume to 4% of contralateral hemisphere (p = 0.007, Mann-Whitney U-test). The lesion volume data from E2 treated animals revealed a non-normal distribution, with 8/13 animals displaying a lesion volume ≤ 8% (high response group) while 5/13 animals had lesion volumes ≥ 12% (low response group). To better understand differences in the outcomes between low and high responders, a more detailed analysis of striatal and cortical lesion volumes was performed. Average striatal lesion volume was 31% in E2 treated animals (Figure 3B), which was significantly smaller than the control group at 46% (p < 0.006, unpaired t-test). E2 treated animals also had significantly smaller cortical lesions than controls (Figure 3C), with median lesion volumes of 0% and 49%, respectively (p = 0.003, Mann-Whitney U-test). All P treated animals developed prominent cortical lesions, ranging from 9-61% of the total cortical brain volume, contrary to only 5/13 E2 treated animals (p = 0.002, Fisher's exact test).
Figure 3.
A) Supraphysiologic 17β-estradiol (E2) treatment at time of reperfusion resulted in a significantly smaller total lesion volume compared to placebo (P) treated animals (27% vs. 4%, *p = 0.007, Mann-Whitney U test). The dot plot of lesion volumes for individual animals reveals non-normal distribution among the E2 treatment group, which is comprised of high and low responding animals. B) Striatal lesion volume was significantly smaller with E2 treatment (46% vs. 31%, #p = 0.006, unpaired t-test). C) E2 treatment also significantly decreased cortical lesion volume (49% vs. 0%, ^p = 0.003 Gaussian approximation, Mann-Whitney U test). Median values and 25th-75th percentile are represented in A and C, with mean ± sd for B.
High responding E2 animals never developed cortical lesions, whereas all of the low responding E2 animals did. High responding E2 animals also had significantly smaller striatal lesions (24 vs. 42% of striatum, high response vs. low response, p = 0.001, unpaired t- test). Plasma E2 levels measured at euthanasia did not differ between high response and low response E2 treatment groups, although earlier levels were not assessed (66 vs. 51 pg/mL, high response vs. low response; p = 0.577, unpaired t-test). There were also no differences in the amount of weight gain from ovariectomy to tMCAO (16 vs. 17%, high vs. low response; p = 0.89) or from tMCAO to euthanasia (−1 vs. 4%, high response vs. low response; p = 0.097, unpaired t-test).
Extensive atrophy of the ipsilateral hemisphere was observed in P animals six weeks after tMCAO, with a decrease of 11% in the ipsilateral hemisphere. E2 treatment significantly limited the extent of atrophy to 4% (p = 0.038, unpaired t-test). When the atrophy data for E2 treated animals was split into the previously described high and low responding groups, low responding animals had significantly more atrophy of the ipsilateral hemisphere than high responding animals (1% vs. 9%, high response vs. low response; p = 0.002, unpaired t-test).
2.4 Estradiol Treatment Accelerates Recovery of Forelimb Function
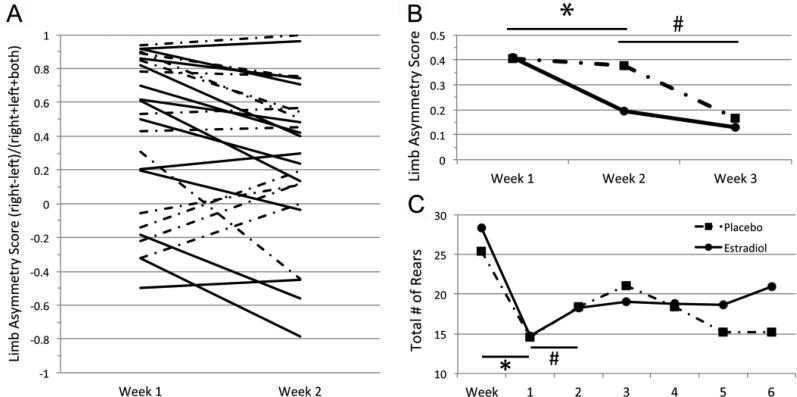
tMCAO resulted in an impairment of contralateral forelimb sensorimotor function as measured by forelimb preference upon rearing in the cylinder test. Both P and E2 groups had similar levels of impairment one week after stroke. Treatment with estradiol resulted in an improvement in forelimb function in 10/13 animals from week 1 to week 2, where only 5/12 animals showed improvement in the P control group (Figure 4A, p = 0.111, Fisher's exact test). Importantly, paired t-test revealed a significant overall improvement from week 1 to week 2 with E2 treatment (Figure 4B, p = 0.002), but not with P (p = 0.775). Significant recovery in P treated animals was delayed, occurring between weeks 2 and 3 after injury (p=0.017), when they recovered to similar levels as E2 treated animals. There were no significant changes in forelimb preference in any groups during weeks 4-6 (data not shown). Both groups of animals had significant decreases in total number of rears after tMCAO (p < 0.001 paired t-tests, Figure 4C). E2 treated animals had a significant increase in total number of rears from week 1 to week 2 (p = 0.023, paired t-test), while P treated animals did not (p = 0.178); 10/13 E2 treated and 7/12 P treated animals had increases in total number of rears from week 1 to week 2 (p = 0.411, Fisher's exact test). Although there were no differences in total number of rears between groups at any time point, there was a trend to more rears in E2 treated animals at later time points (weeks 5-6; Figure 4C).
Figure 4.
A) Contralateral forelimb function 1 and 2 weeks after tMCAO as measured by forelimb preference during exploratory behavior in the cylinder test. 17β-estradiol (E2) treatment resulted in an improvement of function in 10/13 animals between week 1 and week 2 (solid lines), while placebo (P) treatment resulted in an improvement in limb asymmetry score (LAS) in only 5/12 animals (dot-dash lines). B) Averaged LAS for E2 (solid) and P (dot-dash) treatment groups between 1 and 3 weeks after ischemic stroke. Paired t-test revealed a significant improvement for the E2 treatment group from week 1 to week 2, (* p = 0.002), but not in the P group (p = 0.775). P treated animals did recover from week 2 to week 3 after tMCAO (# p = 0.017). C) General activity as measured by average number of rears for each treatment group pre- and post-tMCAO. Both E2 and P treated animals had significant decreases in number of rears after ischemic stroke (* p < 0.001, paired t-tests). Only E2 treated animals displayed significant improvements in number of rears from week 1 to week 2 after tMCAO (# p = 0.023, paired t-test).
2.5 Vascular, White Matter, and Synaptic Organization
Ischemia-reperfusion injury resulted in an increase in CD31-labeled vascular elements in the ipsilateral striatal borderzone (penumbra) in both P and E2 treated animals six weeks after tMCAO (Table 2). Although there was a trend to increased vascular density with E2 treatment (1.64 vs. 1.43, E2 vs. P), this result did not reach statistical significance (p = 0.137; unpaired t-test). No difference was observed in vascular density between high and low responding E2 animals. E2 treatment did not have an effect on white matter myelination in the corpus callosum or anterior commissure, and there were no long-term changes in these characteristics after ischemic stroke in our model (Table 2). There were no differences in synaptophysin immunoreactivity between E2 and P treated animals.
Table 2.
Analysis of immunohistochemical staining for blood vessels, synapses, and myelin
| Ipsi/Contra Ratio | ||
|---|---|---|
| Vascular Density | Placebo | Estradiol |
| Striatum | 1.43 ± 0.35 | 1.64 ± 0.31 |
| Synaptophysin | ||
| Striatum | 1.08 ± 0.09 | 1.07 ± 0.09 |
| Hippocampus | 0.98 ± 0.12 | 0.94 ± 0.04 |
| Luxol Fast Blue | ||
| Corpus Callosum | 1.02 ± 0.03 | 1.02 ± 0.04 |
| Anterior Commissure | 1.00 ± 0.06 | 1.01 ± 0.03 |
*No significant differences were found between treatment groups. All data represented as mean ± sd.
2.6 MRI
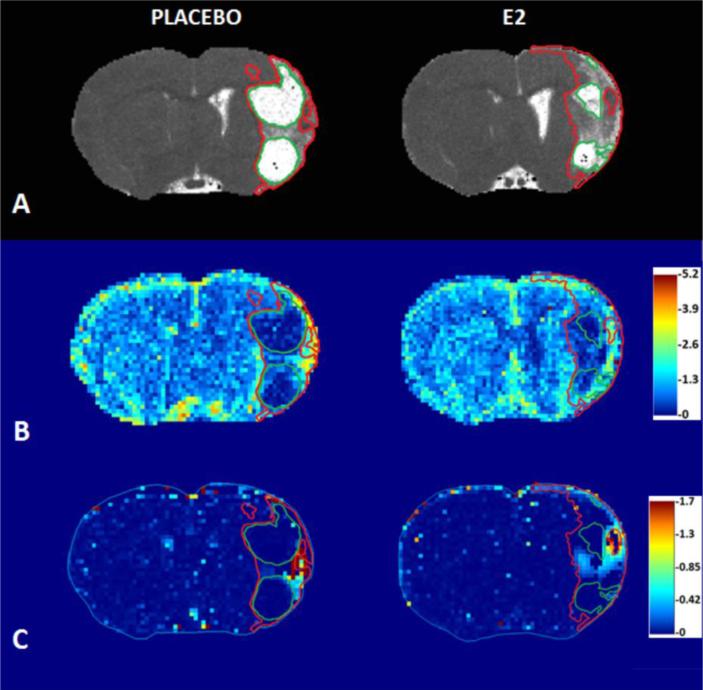
A pre-specified subset of rats from both treatment groups (n=4/group) underwent MRI analysis of lesion volume and cerebrovascular function during the fourth week after tMCAO. Total lesion volumes were determined from the multi-slice T2 image sets, both in terms of absolute volume and percent of total brain volume (Table 3), and were found to be significantly smaller for E2 treated animals versus P (58 vs. 136 mm3 and 5.9% vs. 13.8%, E2 vs. P, p < 0.05, unpaired t-test). Consistent with this, lesion volume as percent of contralateral was determined from T2 maps obtained from 3 contiguous imaging slices through the central portion of the lesion, and tended to be smaller for E2 versus P (Table 3). Over time ischemic lesions display evidence of encephalomalacia and cyst formation: as expected, T2 brain maps from the representative animals of P and E2 groups showed both the lesions (T2 values ≥ 60 msec) and cysts (T2 values ≥ 120 msec) (Figure 5A). Cysts were smaller in E2 treated rats: the mean cyst volume as percent of the lesion was reduced for E2 versus P treatment (14% vs. 53% of lesion, E2 vs. P, p = 0.004, unpaired t-test, Table 3).
Table 3.
Summary of MRI data from a subset of E2 and P treated rats 4 weeks after tMCAO (n = 4 per group each).
| MRI Lesion and Perfusion Data | Placebo, n=4 | Estradiol, n=4 | p-value | |
|---|---|---|---|---|
| Lesion Volume (mm3) | 136 ± 15 | 58 ± 60 | 0.046* | |
| Lesion Volume (% of brain volume) | 14 ± 0.01 | 6 ± 0.06 | 0.041* | |
| Lesion Volume (% contra) | 47 ± 3 | 33 ± 24 | 0.336^ | |
| Cyst (% of lesion) | 53 ± 6 | 14 ± 16 | 0.004* | |
| Total Lesion | CBF | 0.82 ± 0.09 | 1.06 ± 0.11 | 0.014* |
| CBV | 0.85 ± 0.08 | 1.01 ± 0.14 | 0.092 | |
| Ktrans (mean) | 0.09 ± 0.09 | 0.07 ± 0.06 | 0.704 | |
| Ktrans (%>0.1) | 13.3 ± 11 | 17.5 ± 13 | 0.646 | |
| Lesion w/o Cyst | CBF | 1.18 ± 0.14 | 1.12 ± 0.09 | 0.547 |
| CBV | 1.24 ± 0.11 | 1.09 ± 0.22 | 0.277 | |
| Ktrans (mean) | 0.20 ± 0.24 | 0.09 ± 0.08 | 0.389 | |
| Ktrans (%>0.1) | 22.4 ± 18 | 19.3 ± 18 | 0.806 | |
| Cyst only | CBF | 0.51 ± 0.08 | 0.71 ± 0.10 | 0.045* |
| CBV | 0.45 ± 0.07 | 0.64 ± 0.07 | 0.037* | |
| Ktrans (mean) | 0.03 ± 0.01 | 0.04 ± 0.001 | 0.213 | |
| Ktrans (%>0.1) | 6.4 ± 6 | 8.9 ± 7 | 0.671 | |
| Contralateral | Ktrans (mean) | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.491 |
| Ktrans (%>0.1) | 5.6 ± 2 | 4.9 ± 2 | 0.617 |
All data is presented as mean ± sd and analyzed with unpaired t-test
denotes unpaired t-test with Welch's correction
denotes significance by a p-value < 0.05.
CBF and CBV measures are represented as relative to the contralateral hemisphere. Ktrans is shown in terms of both its mean, and the % of pixels within the ROI above the threshold of 0.1.
Figure 5.
Representative rat brain parametric maps, obtained via MRI, from placebo (P) and 17β-estradiol (E2) treated groups. A) T2 maps, depicting lesion (red outline) and cyst (green outline), indicating smaller cyst regions in E2 treated animals. B) Cerebral blood flow (CBF) maps, from the same animals as in A, with analogous lesion and cyst ROIs, indicate decreased flow in the cyst regions (CBF color scale is relative; contralateral hues set to ~unity). C) Ktrans maps from the same animals as depicted in A and B, indicating elevated regions of Ktrans within the non-cyst component of the lesion.
Relative CBF (rCBF) maps from the same animals (Figure 5A) showed, as expected, reduced blood flow within the cystic regions (Figure 5B). E2 treated MRI animals had a significant increase in total lesion mean rCBF compared to P (1.06 vs. 0.82, E2 vs. P, p = 0.014, Table 3). A modest increase, non-significant, in total lesion mean cerebral blood volume (CBV) was also observed (1.01 vs. 0.85, E2 vs. P, p = 0.092). Mean CBF and CBV for non-cyst regions of the lesion were similar between P and E2 treatment groups, and hence the smaller % cyst in the E2 group likely contributed to the higher mean rCBF observed in the lesions of E2 treated animals. Cyst regions, which overall had substantially lower CBF and CBV than non-cyst regions, also had elevated CBF (0.71 and 0.51, p < 0.05) and CBV (0.64 and 0.45, p = 0.037) for E2 versus P, respectively, which was thought to relate to higher CBF and CBV values in regions immediately around the cysts.
Figure 5C displays Ktrans maps from the same animals as in 5A-B and shows that noncystic areas of the lesion have elevated Ktrans. This observation is consistent with the presence of leaky vasculature associated with neovascularization. Table 3 lists mean Ktrans values, as well as the % of pixels reporting Ktrans > 0.1, for the various regions. The latter parameter quantifies the extent of areas with elevated Ktrans, which tended to occur in discrete regions within the lesions (Figure 5C). The % of pixels reporting Ktrans > 0.1 was elevated in total and non-cystic lesion ROIs (15% and 21%, respectively) compared to the corresponding contralateral region (5%; p < 0.05 for both total and non-cystic lesion vs. contralateral; unpaired t-tests with Welch's correction). Although non-cystic regions of the lesion had a higher % of pixels reporting Ktrans > 0.1 than cystic regions, this was outside of significance (21% vs. 7%, p = 0.058, unpaired t-test with Welch's correction). Additionally, differences in Ktrans were not observed between P and E2 treated animals.
3. Discussion
3.1 E2 and Lesion Characteristics
The current study highlights several important observations. First, high-dose E2 treatment at reperfusion in ovariectomized female rats altered the evolution of ischemia-reperfusion injury, resulting in a significant reduction in total lesion volume, and specifically, the striatal and cortical components. Histological analysis of lesion volume was confirmed by T2-weighted MRI, and furthermore showed that E2 treatment altered the degree of encephalomalacia and cyst formation within the lesions. There was also a decrease in the extent of atrophy of the ipsilateral hemisphere with E2 treatment. These observations add to the mounting evidence showing E2-mediated neuroprotection when given after the onset of both transient and permanent models of ischemic stroke. Given that E2 was administered at reperfusion it is possible that attenuation of reperfusion injury accounted in part for the observed lesional size decrease at six weeks (Shi et al., 2001). This effect is different from that observed in our previous study in which the administration of E2 prior to tMCAO did not attenuate lesion volume at four weeks, (Ardelt et al., 2012).
E2 treatment did not result in any differences in white matter, synaptic, or vascular reorganization after experimental stroke in the current study. In both groups, ischemia-reperfusion resulted in a robust increase in vascular density in the striatal penumbra and a mild increase in synaptophysin immunoreactivity adjacent to the glial scar. It is well established that ischemic stroke induces angiogenesis in the lesion penumbra (Hayashi et al., 2003; Wei et al., 2001), and that angiogenesis and neurogenesis are coupled through multiple mechanisms (Kojima et al., 2010; Sun et al., 2003; Teng et al., 2008). Previous research has shown that estrogen pre-treatment has been able to enhance angiogenesis (Ardelt et al., 2012) and neurogenesis (Li et al., 2011; Shao et al., 2012; Suzuki et al., 2007). Nonetheless, treatment at reperfusion in the current study did not significantly augment post-stroke angiogenesis compared to P treatment. This parallels a recent finding that estrogen and progesterone immediately after reperfusion did not increase the number of vessels in the cortex over control animals fourteen days after stroke (Ulbrich et al., 2012), even though vascular endothelial growth factor mRNA was increased 24 hours after the start of treatment. Alternative explanations for the lack of difference in vascular density in E2 vs. P treated animals six weeks after ischemia are low statistical power and/or the degradation of newly formed vessels with time after injury (Yu et al., 2007). Additional research is required to determine the pro-angiogenic and neurogenic potential of E2 treatment when delivered at the time of reperfusion.
3.2 E2 and Functional Recovery
The second important finding is that E2 treatment results in an accelerated recovery of forelimb function. Ischemia-reperfusion caused extensive sensorimotor deficits to the forelimb contralateral to the injured hemisphere in both treatment groups. Rats treated with E2 had significant improvement in contralateral forelimb use between weeks one and two after tMCAO, while P treated rats had a similar improvement between weeks two and three. E2 treatment resulted in a trend towards a higher number of subjects who showed functional improvement from week one to two. tMCAO also resulted in significant decreases in number of rears per test session, interpreted as a measure of general locomotor activity. Interestingly, E2 treated rats showed a significant improvement in number of rears from week one to week two, which parallels their improvement in contralateral forelimb function. Again, treatment with E2 resulted in a higher percentage of animals improving from week one to week two in this measure over P treatment. Although P treated rats showed a similar improvement in the average number of rears during the same time frame, this result did not reach significance.
Assessment of behavioral outcomes with estrogen treatment when administered after ischemia onset has not been extensively studied. Li et al. (2004) showed enhanced recovery of forelimb function after experimental stroke with the cylinder test in both male and female mice treated with E2 at the onset of ischemia. In another study, E2 in combination with progesterone was able to alleviate sensorimotor deficits from one to fourteen days after tMCAO (Ulbrich et al., 2012). In a pMCAO model, E2 alone was also able to reduce sensorimotor deficits from six hours to fourteen days of recovery (Zheng et al., 2013). Together, these results suggest that E2 may retain its ability to limit the severity of neurologic deficits and promote recovery when given after the onset of ischemic stroke. These observations are critically important because of the potential for translation to patients who present to medical attention sometimes hours after the onset of ischemia.
3.3 E2 and Cerebrovascular Function after Stroke
Lastly, E2 treatment resulted in a significant improvement in cerebrovascular function, as measured by MRI during the fourth week of recovery. MRI was incorporated into the study to assess cerebrovascular function and corroborate histological assessment of lesion characteristics. We have previously demonstrated that MRI analyses of ischemic lesions can reliably validate histological measurements (Ardelt et al., 2012). Despite a small sample size for the prospectively selected representative E2 and P treated animals which underwent MRI (n = 4/group), and the presence of both high and low responders within the E2 group, MRI was able to corroborate significant differences in lesion volume and degree of cyst formation within ischemic lesions in the two treatment groups.
Additionally, E2 treatment after ischemic stroke resulted in improvements in rCBF and rCBV within the lesions. These differences were partly due to E2 treated animals having had significantly smaller cystic components to their lesions. In both groups, as expected, cystic portions of the lesion showed lower measures of CBF compared to non-cystic portions, and E2 treated animals had smaller cysts. Altogether, MRI data demonstrated that E2 treatment significantly reduced lesional size and cystic component with concomitant increase in rCBF.
We have previously shown that E2 treatment prior to the onset of ischemic stroke resulted in significantly improved ipsilateral rCBF and rCBV (Ardelt et al., 2012), which was attributed to the significant increase in post-stroke angiogenesis. In the current study, measurement of Ktrans indicated that vascular permeability was elevated (consistent with the presence of neovascularization) in the non-cystic components of the lesion. The mean Ktrans and the percent of lesion with Ktrans > 0.1 were not different between the two experimental groups, corroborating the lack of a significant augmentation of angiogenesis by E2 treatment in this experimental paradigm.
3.4 High and Low Responding E2 Treated Animals
Although there was an overall decrease in lesion volume with E2 treatment, we observed two different groups of “responders” to E2 treatment. High responding animals had remarkably small lesion volumes, whereas low responding animals had lesion volumes that fell within the range seen in P treated animals. After segregating animals into high and low responding groups it became apparent that high responding animals had not developed cortical lesions and displayed significantly smaller striatal lesions. In contrast, all of the low responding animals had lesions spanning both the striatum and cortex. Additionally, the high responding group had significantly less atrophy of the ipsilateral hemisphere compared to the low responding group. Further analysis showed no difference in serum E2 levels between these groups as measured at euthanasia, and there were no differences in either pre- or post-stroke weight changes. There was also no difference in vascular density between high and low E2 treated animals. Therefore, we do not believe the difference in response to E2 treatment is explained by pellet failure.
There are a number of potential explanations for the diverging response to E2 treatment within our model. Differential expression of estrogen receptors within subjects may play a role. Similarly there could be heterogeneity in vascular anatomy between subjects, including vessel size diameter as well as the extent of collateral blood flow. Variability in cerebrovascular anatomy has been attributed to strain specific susceptibility to ischemic stroke (Majid et al., 2000; Zhang et al. 2010), and even the commonly used inbred C57 BL/6 strain demonstrates marked inconsistency in circle of Willis anatomy (McColl et al. 2004). We may speculate that if the mechanism of E2 treatment involves altering cerebrovascular function, E2 treatment may be more beneficial in animals with extensive collateral blood flow. In the current study, vascular anatomy and estrogen receptor expression were not assessed.
It is interesting to note that a similar result of the variable response to E2 was observed by Liu et al. (2007) when administering a high dose of E2 subcutaneously six hours after onset of pMCAO in Sprague-Dawley rats. In both studies, the observed effect could result from differential estrogen levels at key times during lesion evolution due to differences in E2 content or release from the pellets as well as differences in estrogen metabolism in different animals. Differences in cerebrovascular function or collateral vessel anatomy in individual animals may have also contributed to this effect. Since the study by Liu et al. and the current study differed in E2 dose and experimental model, these data suggest the possibility that individual subjects may be intrinsically more or less predisposed to E2-mediated effects. Nonetheless, further research into why particular therapeutics may work in subsets of individuals will be crucial for successful translation to a vastly heterogeneous population of stroke patients.
3.5 Other Considerations
In summary, we have previously shown that E2 treatment prior to tMCAO does not offer significant neuroprotection but enhances angiogenesis, CBF, and functional recovery (Ardelt et al., 2012), while E2 treatment at the time of reperfusion attenuates lesion volume, augments CBF, and hastens functional recovery. While young animals and shorter periods of hormone deprivation save time and expenditure in the lab, aging and hormone deprivation induces many changes in both the quiescent and injured brain (Manwani et al., 2013; review by Sohrabji et al., 2013). Indeed, the ability of estrogen pretreatment to protect against stroke seems to be dependent on the length of hormone deprivation (Suzuki et al., 2007) and may be completely lost in aged mice (Cai et al., 2014). Importantly, a pilot study in 22-month old ovariectomized female Fischer 344 rats demonstrated that a single high-dose administration of E2 after tMCAO was able to reduce lesion size (Schreihofer & Ma, 2013). Additionally, Inagaki et al. (2012) showed that treatment with E2 at reperfusion in a global ischemia model reduced hippocampal neuronal death and promoted neuronal function in aged female rats after prolonged hypoestrogenicity. This would suggest that treatment with estrogen could be protective in aged subjects, which is important considering the demographic of stroke patients.
There are potential risks associated with continuous estrogen replacement, including certain cancers. In the context of ischemic stroke, the most significant risk could be inducing a hypercoagulable state. Although most studies, including the current, use long-term estrogen replacement paradigms, single dose administration of estrogen can still provide neuroprotection (McCullough et al., 2001) and may limit potential risks associated with long-term treatment. Additionally, the potential benefits of reducing morbidity and mortality from severe ischemic stroke with estrogen treatment may outweigh the potential risks.
Our results and those of others are of potential importance to patients. The current standard of care for ischemic stroke in patients is intravenous administration of tissue plasminogen activator (IV-tPA). In one preclinical study, estrogen was able to reduce lesion volume when given immediately prior to stroke onset, followed by IV-tPA treatment at reperfusion (Liu et al., 2010). When assessed in an embolic stroke model, estrogen and IV-tPA treatment three hours after stroke onset significantly reduced lesion volume over IV-tPA treatment alone. These results suggest that estrogen could be beneficial when administered in concert with IV-tPA to improve outcome from ischemic stroke in patients. Finally, given the recent publication of clinical trials showing increased reperfusion rates and improved outcomes with endovascular thromboembolectomy following IV-tPA administration in patients with large vessel occlusions (Berkhemer et al. 2015, Campbell et al. 2015, Goyal et al. 2015, Saver et al. 2015), the addition of E2 at the time of recanalization may offer additional benefit based on the results of the current study and may, therefore, require evaluation in the future.
4. Conclusion
There has been an increasing interest in using estrogens after the onset of ischemic stroke, alone or in combination with other strategies, to promote neuroprotection and functional recovery (reviews by Liu & Yang 2013, Schreihofer & Ma 2013). In the current study, E2 treatment was able to remarkably decrease lesion volume and accelerate recovery of forelimb function when administered at the time of reperfusion in an experimental stroke model in rats. These results and those of others suggest that E2 administration at the time of reperfusion, alone or in combination with IV-tPA and/or endovascular thromboembolectomy, may be beneficial in ischemic stroke. If these results were translatable to patients, they could shorten overall time in intensive care, reduce health care costs, augment rehabilitation, and improve the quality of life in stroke survivors.
5. Experimental Procedures
5.1 Animal Use Approval and Housing
All animal procedures were approved by the Institute for Care and Animal Use Committee at the University of Chicago and developed in accordance with the Animal Resource Center and NIH guidelines for the use of animals in research. All survival surgeries were performed in a designated location using aseptic technique and sterile equipment. Female Wistar rats (200-225g; Harlan, Indianapolis, IN) were housed in pairs on a 12-hour light/dark cycle and were allowed food and water ad libitum. Teklad 2018SX rat feed (Harlan, Madison, WI) was used instead of traditional feed to limit phytoestrogen intake from food consumption.
5.2 Ovariectomy
Two weeks prior to MCAO rats were ovariectomized to remove endogenous ovarian hormones. In short, anesthesia was induced with 5% isoflurane delivered with medical air (1L/min) and supplemented with medical oxygen (0.2L/min) with maintenance of anesthesia at 2%. Small, bilateral abdominal incisions were made, uterine vasculature ligated with a 3-0 silk suture, ovaries resected, and wounds closed using a 3-0 suture for the muscle layer and staples for skin. Animals were allowed to recover for one week prior to baseline behavior testing and MCAO procedure.
5.3 Transient MCAO
MCAO is a highly utilized method for modeling both transient and permanent ischemic stroke in rodents, resulting in lesions to the cortex and striatum. Rats were anesthetized with isoflurane using the above method (induction followed by maintenance) and sterilely prepped and draped. Skin was infiltrated with 1% lidocaine prior to incisions. A right groin cut-down was performed, and PE50 tubing was inserted into the femoral artery for real-time blood pressure monitoring and blood sampling for glucose and gas analysis. Body temperature was monitored rectally and maintained between 36.5 and 37.5 °C using heat lamps. The skull overlying the middle cerebral artery territory was thinned using a hand-held drill in order to monitor MCA blood flow via laser Doppler flowmetry. With the rat in the left-lateral decubitus position secured via ear bars in a Plexiglass cradle, the right neck was dissected, the common carotid artery, external carotid artery, and the pterygopalatine artery were ligated with 4-0 silk suture, while the occipital artery was cauterized. A 4-0 nylon suture with a cauterized tip was introduced into the common carotid artery and threaded into the right internal carotid artery until laser Doppler flow dropped to < 45% of baseline. Blood was sampled at 60 minutes of ischemia. The occluding suture was removed after 2 hours of ischemia to allow reperfusion of blood flow to the MCA territory. Hormone pellets were implanted (see below), wounds were sutured, and animals were allowed to recover. Rats were housed in pairs post-operatively.
5.4 Pellet Implantation, Randomization, and Blinding
Immediately after reperfusion, a subcutaneous pocket was created in the dorsal neck below the skull. A timed-release E2 or P pellet (0.72mg 60-day release; Innovative Research of America, Sarasota, FL) was implanted and the pocket closed with a sterile wound clip. On the day of MCAO procedure, animals were randomly assigned to P or E2 treatment groups, with both groups represented each session. The individual performing MCAO and implantation procedures was blinded to the pellets (AAA). Investigators remained blinded as to treatment status for the duration of the experiment, including all data collection.
5.5 Serum Estradiol Levels
We have previously reported that the IRA 0.72mg 60-day release E2 pellets produce supraphysiologic levels of circulating estradiol by one day after implantation, retain supraphysiologic levels up to 10 days, and decline to physiologic levels by one month (Ardelt et al., 2012). To verify the course of release and circulating levels of E2 immediately after implantation, rats were implanted with the pellets and euthanized at the following time points after implantation (n=2 except where listed): 0.5h, 1h (n=1), 2h, 4h, 6h. For all serum hormone measurements blood was collected at euthanasia via cardiac puncture under anesthesia, centrifuged at 5000 rpm for 20 minutes to obtain serum, and analyzed for estradiol levels using the Elecsys Estradiol Assay (Roche Diagnostics GMBH, Penzberg, Germany).
5.6 Analysis of Forelimb Function
Rats were assessed for forelimb preference using a cylinder sensorimotor test as previously described (Ardelt et al., 2012). In short, rats were placed in a 20cm diameter Plexiglas cylinder and allowed to explore the walls of the container. They were video recorded for 5 minutes. Animals were assessed for forelimb function pre-MCAO (one week after ovariectomy), and once weekly for six weeks post-MCAO. Testing was performed by the same investigator (RSC) and at the same time of day for each session in order to limit variability in circadian activity. Two different investigators (RSC, II), blinded to the hormonal status of the animals, analyzed initial paw placement upon rearing, and an average limb asymmetry score (LAS) was determined: LAS = (right-left) / (right+left+both). General locomotor activity was also evaluated for each animal by counting the total number of rears for each session during pre- and post-MCAO cylinder tests and averaging across investigators (RSC, II).
5.7.1 Magnetic Resonance Imaging
A subset of rats in the main study underwent MRI during the fourth week of recovery (n = 4/group for each treatment). Anesthesia was induced with 5% isoflurane in 100% oxygen at 1.0 L/min, and maintained throughout the procedure at 1.5% isoflurane. Contrast agents were injected via the left femoral vein, which was cannulated with PE-50 tubing. Animals were placed in a dedicated holder and positioned within the small animal MRI system (Bruker BioSpec, 9.4T, 33 cm bore, with 11.6 cm inner diameter actively shielded gradient coils; maximum constant gradient strength for all axes: 230 mT/m). An 8-element birdcage coil (4.0 cm in diameter and 4.0 cm long) was used for signal excitation and detection. Physiological parameters (respiratory rate, heart rate, and body temperature) were monitored throughout the procedure with SA Instruments MRI monitoring system (Stony Brook, NY), and body temperature was maintained to 37°C.
Axial T2-weighted MR images were obtained using the Paravision RARE spin echo sequence as previously described (Ardelt et al., 2012), except with 11 image slices and a TEeffective of 26 ms. A T2-weighted multi-slice multi-echo (MSME) spin echo sequence was implemented as previously described (Ardelt et al., 2012) except with 5 slices, and a 256 × 256 matrix. T2 maps were generated as previously described (Ardelt et al., 2012). A dual bolus DCE/DSC perfusion MRI approach was employed as previously described (Pike et al., 2009), employing a single imaging slice centered within the lesion. The DCE protocol employed a fully relaxed Paravision FLASH sequence followed by 400 rapidly acquired FLASH T1 images as previously described (Ardelt et al., 2012), employing a femoral vein injection of Magnevist (3x diluted, 2.0 μL/g, 0.33 mmol/kg; Berlex Inc., Montville, NJ) during the series. The DSC-MRI sequence was implemented after the DCE series using T2*-weighted FLASH images as previously described, (Ardelt et al., 2012), employing a femoral vein injection of the macromolecular contrast agent ferumoxytol (Feraheme; total dose, 1.8 mg Fe; AMAG Pharmaceuticals, Lexington, MA) during the image series.
5.7.2 Analysis of MR Images
a. Lesion area and volume
The total lesion volumes were determined from the multi-slice T2 images, by manually drawing regions of interest (ROIs) around regions indicating T2 intensity that was enhanced in comparison to normal parenchyma. The brain volume was determined from ROIs outlining the brain surface on 10 contiguous coronal imaging slices, starting 4 mm posterior to the anterior commissure and continuing forward. The lesion and cyst volumes are calculated as percent of contralateral or lesion, respectively, were determined from the T2 maps, employing the three imaging slices at the center of the lesion. The particle analysis tool in the JIM software package (Xinapse Systems LTD, Northants, UK), was used to identify regions with T2 values greater than 60 msec for lesion, and greater than 120 msec for cyst, to guide the manual drawing of ROIs. The percent lesion area was calculated using the indirect method, (contralateral side – nonlesioned ipsilateral side)/contralateral side)*100%, in each of three contiguous (1 mm thick) coronal slices through the central region of the lesion. Data was averaged over the three slices. Percent cyst/total lesion was determined by calculating the ratio of the total 120 ms area to the 60 ms area*100%.
b. Perfusion MRI
The ROIs mentioned above were also used for delineating mean parameter values for total lesion, cyst, and lesion excluding cyst. The calculation of ratios to contralateral hemisphere employed ROIs that were mirror images of those in the ipsilateral hemisphere, and placed in the contralateral hemisphere. Processing of perfusion MRI data was performed as previously described using the JIM software package (Pike et al., 2009; Tofts et al., 1999). Specifically, calculation of DCE-MRI parameters followed the modified model that includes a blood plasma volume term (vp) (Tofts et al., 1997). For DCE-MRI, a single vascular input function (VIF) was employed, experimentally obtained by averaging across several rat temporal veins using identical injection and experimental conditions to those employed for this study. Parametric maps were generated for Ktrans, the volume transfer constant for the contrast agent (units: min−1), which is largely an index of vascular permeability. Because of the heterogeneity of Ktrans enhancement in the lesions, we not only quantified mean Ktrans in the ROIs, but we also quantified the percentage of the ROIs that were above a threshold value (0.1), using the particle analysis tool in the JIM software package and the ROIs determined from the T2 maps. This essentially quantifies the percentage of the lesions that are characterized by Ktrans “hotspots”. Calculation of DSC-MRI perfusion parameters followed the model-independent method previously described (Ostergaard et al., 1996). Parametric maps were generated for CBF (ml blood/100 g tissue/min) and CBV (blood volume percentage of total tissue volume). Non-fitting pixels, exhibiting values of 0 or greater than 10000, were removed from CBF maps. Parameter values were reported as relative to contralateral (rCBF, rCBV, rMTT) to reduce measurement error and effects from variations in intracranial pressure, blood pressure, and depth of anesthesia. Contralateral ROIs were created by horizontally rotating the lesion ROIs by 180° and positioning to the contralateral hemisphere.
5.8 Euthanasia and Tissue Procurement
Animals were anesthetized with 5% isoflurane. As mentioned above, blood was collected via cardiac puncture under anesthesia and then centrifuged at 5000 rpm for 20 minutes to obtain serum, which was analyzed for estradiol using the Elecsys Estradiol Assay (Roche Diagnostics GMBH, Penzberg, Germany). Whole brains were extracted immediately after cardiac puncture, placed in a mold of OCT embedding compound, frozen in a 2-methylbutane bath on dry ice, and stored at -80°C. Brains were sectioned at 8μm on a cryostat (−20°C) in the coronal plane. Tissue was collected from 8 distinct bregma levels (bregma 2.28, 1.08, 0.36, −1.44, −2.16, −3.60, −5.28, −6.24) for histological estimation of lesion volume and immunolabeling.
5.9 Analysis of Lesion Volume and Brain Atrophy
Tissue sections were thawed, fixed in acetone at −20°C, and underwent hematoxylin and eosin (HE) staining using standard procedures. Slides were then imaged using a DP72 digital camera on a BX41 microscope using a 1.25x objective and a 0.5x video camera adapter (Olympus America, Center Valley, PA). All HE images were taken at the maximum pixel size of 4140×3086 with CellSens software (Olympus America, Center Valley, PA). MetaMorph (v7.7, Molecular Devices, Sunnyvale, CA) imaging analysis software was used to trace contralateral and non-lesion ipsilateral territories and to determine the area in pixels, which were then converted to mm2 (90,000 pixels/mm2). For each rat, contralateral and non-lesion ipsilateral volumes were determined by integrating the lesion area with the calculated distances between 8 distinct regions ranging from bregma 2.28 to −6.24mm (see above). Lesion volume is expressed as follows: [(contralateral volume – non-lesion ipsilateral volume) / contralateral volume] x 100%. Cortical and striatal lesion volumes were determined by isolating analysis to only appropriate brain regions and bregma levels. To determine the extent of brain atrophy, entire ipsilateral and contralateral hemisphere volumes were calculated using the same methodology as lesion volume. For each animal, atrophy of the ipsilateral hemisphere was determined using the following equation: (ipsilateral volume / contralateral volume) x 100%.
5.10 Immunolabeling
Fresh frozen tissue sections were warmed at room temperature (RT) for 10-15 minutes, fixed, and blocked in 0.1% horse serum in 1X phosphate buffered saline (PBS) at RT for 1 hour on a shaker. Primary antibodies were diluted in blocking buffer and applied to sections for 2 hours at RT or overnight at 4°C on a shaker. After sequential washes in PBS, Cy3 and/or Alexa488 conjugated secondary antibodies were applied for 1 hour at RT. All immunofluorescent labeling was followed by application of Hoechst 33258 (bis-benzimide, Sigma-Aldrich, St. Louis, MO), a non-specific nuclear fluorescent stain. Diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA) was used for the development of synaptophysin (SYP, H-93, Santa Cruz Biotechnology cat # sc-9116) and CD31 (PECAM-1, TLD-3A12, Millipore cat # MAB1393) antibodies, and included sequestration of endogenous avidin, biotin, and peroxides. Sections for immunofluorescence imaging were coverslipped in a 60% glycerol solution in PBS and stored at 4°C, while sections developed with DAB were coverslipped in Permount (Fisher Scientific, Pittsburgh, PA).
5.11 Luxol Fast Blue Staining and Analysis
Coronal sections (bregma −2.28) were thawed, fixed in 10% formalin at RT for 10 minutes, placed in a 0.1% w/v Luxol Fast Blue solution (Solvent Blue 38, Sigma-Aldrich, St. Louis, MO), and rapidly heated in a microwave to 95°C. The sections remained in the heated solution for 30 minutes, which was followed by 5 minute rinses in 95% ethanol and distilled water. Differentiation of the stain was performed in 0.05% lithium carbonate solution and in 70% ethanol (approximately 10 dips lasting 20 seconds total for each). The slides were rinsed in distilled water for 5 minutes and dehydrated using increasing ethanol concentrations (to 100%), cleared in a xylene solution, and coverslipped. Sections were imaged as described above, using a 4x objective and 1x video camera adapter (Olympus America, Center Valley, PA). Images of the ipsilateral and contralateral corpus callosum (CC) and anterior commissure (AC) were taken at the maximum pixel size of 4140×3096 with CellSens software. Degree of myelination of the CC and AC were then analyzed via integrated intensity with MetaMorph software. In short, images were grey-scaled, black-white inverted, and the intensity of the region of interest was found. For CC, a 700×700 pixel region was placed in the dorsolateral segment of the CC. For AC, a 400×400 pixel region was placed in the center of the AC. A ratio of ipsilateral to contralateral intensity of the CC and AC were determined for each animal.
5.12 Vascular Density Analysis
CD31-DAB immunolabeled sections were analyzed for vascular density as previously described (Ardelt et al. 2012). The ipsilateral borderzone and mirror-image contralateral striatum were imaged with a 20x objective, and the number of immunolabeled vascular structures was assessed using MetaMorph software. An ipsilateral/contralateral ratio was calculated for each animal, and data was averaged across treatment groups.
5.13 Statistics
Sample sizes to detect significant differences in measured outcomes were determined using previously published data (Ardelt et al., 2012), with an alpha of 0.05 and power of 0.8. Differences between treatment groups were assessed using parametric or non-parametric statistics where appropriate, and significance was assigned to a p-value of < 0.05. Data were analyzed using GraphPad Prism software (version 5.0, La Jolla, CA). Data are expressed as mean ± standard deviation (sd) or median and interquartile rankings when appropriate.
Table 1.
Operative and Physiologic Parameters
| Placebo, n = 12 | Estradiol, n = 13 | |
|---|---|---|
| Weight change, pre - post ovariectomy, % | 17 ± 9 | 17 ± 9 |
| Occlusion, % of baseline laser Doppler flow (LDF) | 33 ± 8 | 34 ± 10 |
| Intra-ischemic data: pH | 7.38 ± 0.04 | 7.37 ± 0.04 |
| pCO2, mm Hg | 41 ± 5 | 44 ± 5 |
| pO2, mm Hg | 136 ± 25 | 143 ± 15 |
| Blood glucose, mg/dl | 195 ± 35 | 200 ± 27 |
| Hematocrit, % | 34 ± 4 | 34 ± 5 |
| Mean arterial pressure, mm Hg | 91 ± 9 | 92 ± 7 |
| Temporalis temperature, °C | 36.7 (36.6 – 36.8) | 36.7 (36.7 – 36.7) ^ |
| Rectal temperature, °C | 36.7 (36.7 – 36.8) | 36.8 (36.7 – 36.8) ^ |
| Reperfusion, % baseline LDF | 109 ± 50 | 100 ± 50 |
| Weight change, tMCAO - euthanasia, % | 21 ± 8 | 1 ± 5 * |
| Blood glucose at euthanasia, mg/dl | 239 ± 51 | 174 ± 43 ** |
| Plasma estradiol at euthanasia, pg/ml | 10 ± 9 | 60 ± 51 *** |
mean ± sd, unless otherwise indicated
median (25% - 75%)
p < 0.001, unpaired t-test
p < 0.002, unpaired t-test
p < 0.008, unpaired t-test with Welch's correction
Highlights.
High-dose estradiol treatment was beneficial after experimental ischemic stroke
Estradiol treatment attenuated injury from ischemia-reperfusion
Estradiol treatment accelerated recovery of forelimb function
Acknowledgements
The current study was funded in part by NIH K08 NS050167-05, “Role of estrogen and angiogenesis in stroke prevention” (to A.A.A.). A.K. and M.M.P. are supported in part by NIH/NCI grant 1R21 CA167302-01 (to M.M.P.). This project was also supported by the National Center for Advancing Translational Science of the National Institutes of Health through grants UL1 RR024999 and UL1 TR000430. Funding sources did not have any role in the study design, data analysis, or manuscript preparation.
Abbreviations
- E2
17β-estradiol
- P
placebo
- MCAO
middle cerebral artery occlusion
- tMCAO
transient MCAO
- MRI
magnetic resonance imaging
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- IV-tPA
intravenous administration of tissue plasminogen activator
- ROI
region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
All authors were involved in performing experiments, analyzing results, and contributing to the manuscript through critical review and editing. In addition to those previously mentioned, the following are notable specific contributions: AAA performed all surgical procedures; behavior testing was performed and analyzed in a blinded fashion by RSC and II; AK and MMP analyzed and interpreted MRI data. AAA conceived the study, RSC and AAA wrote the manuscript, and all authors approved the final version of the article.
Disclosures
There are no conflicts of interest to disclose.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD, Grady PA. Gender-Linked Brain Injury in Experimental Stroke. Stroke. 1998;29:159–166. doi: 10.1161/01.str.29.1.159. doi:10.1161/01.STR.29.1.159. [DOI] [PubMed] [Google Scholar]
- Ardelt AA, Carpenter RS, Lobo MR, Zeng H, Solanki RB, Zhang A, Kulesza P, Pike MM. Estradiol modulates post-ischemic cerebral vascular remodeling and improves long-term functional outcome in a rat model of stroke. Brain Res. 2012;1461:76–86. doi: 10.1016/j.brainres.2012.04.024. doi:10.1016/j.brainres.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-α in a rodent experimental stroke model. Stroke. 2005;36:337–341. doi: 10.1161/01.STR.0000153795.38388.72. doi:10.1161/01.STR.0000153795.38388.72. [DOI] [PubMed] [Google Scholar]
- Berkhemer OA, Fransen DB, van den Berg LA, Lingsma HF. A Randomized Trial of Intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. doi:10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- Cai M, Ma YL, Qin P, Li Y, Zhang LX, Nie H, Peng Z, Dong H, Dong HL, Hou WG, Xiong LZ. The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice. Neurosci. Lett. 2014;558:115–119. doi: 10.1016/j.neulet.2013.11.007. doi:10.1016/j.neulet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N. Engl. J. Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. doi:10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J. Cereb. Blood Flow Metab. 1998;18:1253–8. doi: 10.1097/00004647-199811000-00012. doi:10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo J-H, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn S-I, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. doi:10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- Guo J, Krause DN, Horne J, Weiss JH, Li X, Duckles SP. Estrogen-receptor-mediated protection of cerebral endothelial cell viability and mitochondrial function after ischemic insult in vitro. J. Cereb. Blood Flow Metab. 2010;30:545–554. doi: 10.1038/jcbfm.2009.226. doi:10.1038/jcbfm.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J. Cereb. Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. doi:10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Kaneko N, Zukin RS, Castillo PE, Etgen AM. Estradiol attenuates ischemia-induced death of hippocampal neurons and enhances synaptic transmission in aged, long-term hormone-deprived female rats. PLoS One. 2012;7:e38018. doi: 10.1371/journal.pone.0038018. doi:10.1371/journal.pone.0038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. doi:10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, Hurn PD, McCullough LD. Estrogen enhances neurogenesis and behavioral recovery after stroke. J. Cereb. Blood Flow Metab. 2011;31:413–425. doi: 10.1038/jcbfm.2010.181. doi:10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp. Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. doi:10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu Q, He S, Simpkins JW, Yang S. Combination therapy of 17beta-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. J. Pharmacol. Exp. Ther. 2010;332:1006–1012. doi: 10.1124/jpet.109.160937. doi:10.1124/jpet.109.160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang X, Liu Q, Yang S-H, Simpkins JW. Dose dependence and therapeutic window for the neuroprotective effects of 17beta-estradiol when administered after cerebral ischemia. Neurosci. Lett. 2007;415:237–41. doi: 10.1016/j.neulet.2007.01.074. doi:10.1016/j.neulet.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yang S-H. Window of opportunity: estrogen as a treatment for ischemic stroke. Brain Res. 2013;1514:83–90. doi: 10.1016/j.brainres.2013.01.023. doi:10.1016/j.brainres.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Yang S-H, Perez E, Yi KD, Wu SS, Eberst K, Prokai L, Prokai-Tatrai K, Cai ZY, Covey DF, Day AL, Simpkins JW. Neuroprotective Effects of a Novel Non-Receptor-Binding Estrogen Analogue: In Vitro and In Vivo Analysis. Stroke. 2002;33:2485–2491. doi: 10.1161/01.str.0000030317.43597.c8. doi:10.1161/01.STR.0000030317.43597.C8. [DOI] [PubMed] [Google Scholar]
- Majid A, He YY, Gidday JM, Kaplan SS, Gonzales ER, Park TS, Fenstermacher JD, Wei L, Choi DW, Hsu CY. Differences in vulnerability to permanent focal cerebral ischemia among 3 common mouse strains. Stroke. 2000;31:2707–2714. doi: 10.1161/01.str.31.11.2707. doi:10.1161/01.STR.31.11.2707. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp. Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. doi:10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Carswell HV, McCulloch J, Horsburgh K. Extension of cerebral hypoperfusion and ischaemic pathology beyond MCA territory after intraluminal filament occlusion in C57Bl/6J mice. Brain Res. 2004;997:15–23. doi: 10.1016/j.brainres.2003.10.028. doi:10.1016/j.brainres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001 doi: 10.1161/01.str.32.3.796. doi:10.1161/01.STR.32.3.796. [DOI] [PubMed] [Google Scholar]
- Østergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn. Reson. Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. doi:10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the framingham heart study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. doi:10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike MM, Stoops CN, Langford CP, Akella NS, Nabors LB, Gillespie GY. High-resolution longitudinal assessment of flow and permeability in mouse glioma vasculature: Sequential small molecule and SPIO dynamic contrast agent MRI. Magn. Reson. Med. 2009;61:615–625. doi: 10.1002/mrm.21931. doi:10.1002/mrm.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw J. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. doi:10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Saver JL, Goyal M, Bonafe A, Diener H-C, Hacke W, Ph D, Jansen O, Ph D, Jovin TG, Lopes DK, Reddy VK, Rochemont M, De, Singer OC, Jahan R, Prime S. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N. Engl. J. Med. 2015 doi: 10.1056/NEJMoa1415061. doi:10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Ma Y. Estrogen receptors and ischemic neuroprotection: Who, what, where, and when? Brain Res. 2013;1514:107–122. doi: 10.1016/j.brainres.2013.02.051. doi:10.1016/j.brainres.2013.02.051. [DOI] [PubMed] [Google Scholar]
- Shao B, Cheng Y, Jin K. Estrogen, Neuroprotection and Neurogenesis after Ischemic Stroke. Curr. Drug Targets. 2012 doi: 10.2174/138945012799201702. doi:10.2174/138945012799201702. [DOI] [PubMed] [Google Scholar]
- Shi J, Bui JD, Yang SH, He Z, Lucas TH, Buckley DL, Blackband SJ, King MA, Day AL, Simpkins JW. Estrogens decrease reperfusion-associated cortical ischemic damage: an MRI analysis in a transient focal ischemia model. Stroke. 2001;32:987–992. doi: 10.1161/01.str.32.4.987. doi:10.1161/01.STR.32.4.987. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J. Neurosurg. 1997;87:724–30. doi: 10.3171/jns.1997.87.5.0724. doi:10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Bake S, Lewis DK. Age-related changes in brain support cells: Implications for stroke severity. Neurochem. Int. 2013;63:291–301. doi: 10.1016/j.neuint.2013.06.013. doi:10.1016/j.neuint.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E. Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: a systematic analysis. J. Cereb. Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.66. doi:10.1038/jcbfm.2009.66. [DOI] [PubMed] [Google Scholar]
- Ström JO, Theodorsson E, Theodorsson A. Order of magnitude differences between methods for maintaining physiological 17beta-oestradiol concentrations in ovariectomized rats. Scand. J. Clin. Lab. Invest. 2008;68:814–822. doi: 10.1080/00365510802409703. doi:10.1080/00365510802409703. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. Baseline. 2003;111:1843–1851. doi: 10.1172/JCI17977. doi:10.1172/JCI200317977.Introduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc. Natl. Acad. Sci. U. S. A. 2007a;104:6013–6018. doi: 10.1073/pnas.0610394104. doi:10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Böttner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J. Comp. Neurol. 2007b;500:1064–75. doi: 10.1002/cne.21240. doi:10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J. Cereb. Blood Flow Metab. 2008;28:764–71. doi: 10.1038/sj.jcbfm.9600573. doi:10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J. Magn. Reson. Imaging. 1997 doi: 10.1002/jmri.1880070113. doi:10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- Tofts PS, Brix G, Buckley DL, L Evelhoch J, Henderson E, Knopp MV, Larsson HBW, Lee T-Y, Mayr NA, Parker GJM, Port RE, Taylor J, Weisskoff RM. Estimating Kinetic Parameters From Dynamic Contrast-Enhanced T1-Weighted\tMRI of a Diffusable Tracer: Standardized Quantities and Symbols. J Magn Reson Imag. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ulbrich C, Zendedel A, Habib P, Kipp M, Beyer C, Dang J. Long-term cerebral cortex protection and behavioral stabilization by gonadal steroid hormones after transient focal hypoxia. J. Steroid Biochem. Mol. Biol. 2012;131:10–6. doi: 10.1016/j.jsbmb.2012.01.007. doi:10.1016/j.jsbmb.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. The New England journal of medicine. 2001 doi: 10.1056/NEJMoa010534. doi:10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. doi:10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Yang SH, Shi J, Day AL, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke. 2000;31:745–749. doi: 10.1161/01.str.31.3.745. discussion 749–750. doi:10.1161/01.STR.31.3.745. [DOI] [PubMed] [Google Scholar]
- Yu SW, Friedman B, Cheng Q, Lyden PD. Stroke-evoked angiogenesis results in a transient population of microvessels. J. Cereb. Blood Flow Metab. 2007;27:755–63. doi: 10.1038/sj.jcbfm.9600378. doi:10.1038/sj.jcbfm.9600378. [DOI] [PubMed] [Google Scholar]
- Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J. Cereb. Blood Flow Metab. 2010;30:923–934. doi: 10.1038/jcbfm.2010.10. doi:10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Zhang P, Li X, Lei S, Li W, He X, Zhang J, Wang N, Qi C, Chen X, Lu H, Liu Y. Post-stroke estradiol treatment enhances neurogenesis in the subventricular zone of rats after permanent focal cerebral ischemia. Neuroscience. 2013;231:82–90. doi: 10.1016/j.neuroscience.2012.11.042. doi:10.1016/j.neuroscience.2012.11.042. [DOI] [PubMed] [Google Scholar]