Abstract
Sarcocystis neurona is a significant cause of neurological disease in horses and other animals, including the threatened Southern sea otter (Enhydra lutris nereis). Opossums (Didelphis virginiana), the only known definitive hosts for S. neurona in North America, are an introduced species in California. S. neurona DNA isolated from sporocysts and/or infected tissues of 10 opossums, 6 horses, 1 cat, 23 Southern sea otters, and 1 harbor porpoise (Phocoena phocoena) with natural infections was analyzed based on 15 genetic markers, including the first internal transcribed spacer (ITS-1) region; the 25/396 marker; S. neurona surface antigen genes (snSAGs) 2, 3, and 4; and 10 different microsatellites. Based on phylogenetic analysis, most of the S. neurona strains segregated into three genetically distinct groups. Additionally, fifteen S. neurona samples from opossums and several intermediate hosts, including sea otters and horses, were found to be genetically identical across all 15 genetic markers, indicating that fatal encephalitis in Southern sea otters and equine protozoal myeloencephalitis (EPM) in horses is strongly linked to S. neurona sporocysts shed by opossums.
Keywords: Sarcocystis neurona, Opossum (Didelphis virginiana), Sea otter (Enhydra lutris), Surface antigen genes (SAGs), Microsatellite, Genetic marker
1. Introduction
It has been nearly 20 years since Sarcocystis neurona was identified as the main etiological agent of equine protozoal myeloencephalitis (EPM) in horses (Dubey et al., 1991). The economic impacts to the horse industry resulting from the treatment and prevention of S. neurona infections are substantial and EPM continues to cause significant morbidity in horses (Fayer et al., 1990; Saville et al., 2000; Dubey et al., 2001; Cohen et al., 2007). Over the past two decades numerous intermediate hosts for S. neurona have been identified, including cats (Felis catus), raccoons (Procyon lotor), harbor seals (Phoca vitulina), and sea otters (Dubey and Hamir, 2000; Lindsay et al., 2000; Miller et al., 2001). The high prevalence of infection and mortality caused by S. neurona infections in Southern sea otters (Enhydra lutris nereis) is of particular concern as this is a federally-listed threatened species (Kreuder et al., 2003; Thomas et al., 2007; Johnson et al., 2009). S. neurona infections among North American intermediate hosts are believed to result from the ingestion of S. neurona sporocysts shed by Virginia opossums (Didelphis virginiana). As an introduced species in California (Grinnell et al., 1937), opossums present a significant health risk to susceptible intermediate hosts (such as sea otters) that were not historically exposed to this pathogen.
In order to compare and accurately describe closely related pathogens, the use of multiple genetic markers is essential. Different types of genetic markers have been used to characterize S. neurona isolates from various hosts. Two of these markers, the first internal transcribed spacer (ITS-1) region in the nuclear ribosomal gene array and the 25/396 marker, have proven useful for making inter- and intraspecific comparisons among S. neurona isolates (Tanhauser et al., 1999; Elsheikha et al., 2005). The S. neurona surface antigen gene snSAG1 has also been assessed as an intraspecific genotyping marker (Hyun et al., 2003; Elsheikha and Mansfield, 2004). The prototypic member of a large superfamily of snSAG genes, snSAG1 has only recently been described (Jung et al., 2004; Howe et al., 2005). Orthologs of these snSAG genes have proved quite informative for identifying genotypic differences among strains of the related apicomplexan Toxoplasma gondii (Mondragon et al., 1998; Grigg et al., 2001). Although polymorphism is limited at the SnSAG loci, the allelic variation at these genes has allowed for the identification of multilocus genotypes for meaningful resolution of S. neurona strains (Wendte et al., in press). However, there has been inadequate resolution of relationships within S. neurona based solely on these antigen coding loci, and that has necessitated the development and application of more robust microsatellite markers to clarify the population genetic structure of S. neurona strains. Indeed, two recent studies to identify sequence length polymorphisms using microsatellites only found moderate genotypic diversity among S. neurona sampled from numerous geographical locations, respectively (Asmundsson et al., 2006; Sundar et al., 2008).
The aim of the present study was to carry out a detailed molecular characterization of S. neurona strains from opossums and numerous intermediate hosts within a localized geographical location in Central California. A total of 45 S. neurona samples were characterized using 15 genetic markers with varying levels of resolution. These included two low-resolution markers (ITS-1 and 25/396); three markers with slightly greater resolution (snSAG2, snSAG3, and snSAG4); and ten microsatellite markers that provided a high level of genetic resolution. While it has been assumed that introduced opossums are the source of S. neurona infections in horses and sea otters in California (Sundar et al., 2008), S. neurona strains from California opossums have not yet been compared against S. neurona strains from these other hosts. By focusing on a small geographical area and using a wide range of genetic markers, we show here that S. neurona sporocysts shed by California opossums are genetically synonymous with S. neurona parasites found in sea otters, horses, and a harbor porpoise from the same area.
2. Materials and methods
2.1. Parasite isolation and DNA extraction
Forty-five S. neurona strains were used in this study. Collection date, location, host information, and strain source are summarized in Table 1. The collection and isolation of some equine and the one felid isolate were described previously (Marsh et al., 1996, 2001; Turay et al., 2002). S. neurona sporocysts were obtained from intestinal scrapings and fecal samples of live-trapped and traffic-killed opossums as previously described (Rejmanek et al., 2009). In an attempt to culture S. neurona, concentrated sporocysts from intestinal scrapings or fecal samples of opossums were excysted as previously described (Murphy and Mansfield, 1999) with one minor modification; instead of shearing the treated sporocysts with a glass slide, the sporocysts were vortexed for 1 min with 500 µm glass beads to fracture the sporocyst wall. The resulting solution was then placed over monolayer cultures of monkey kidney (MA104) cells (BioWhittaker, Walkersville, MD). Isolates were grown in culture media consisting of Dulbecco’s minimum essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 2 mM l-glutamine, 50 U/ml penicillin, and 50 µG/ml streptomycin. Brain tissues from seropositive, freshly dead sea otters, a harbor porpoise, and euthanized horses were homogenized and inoculated into stationary cultures of monkey kidney cells as described (Miller et al., 2001). All cultures were maintained at 37 °C in 5% CO2 and were checked 3 times weekly for signs of parasite growth. Total genomic DNA was extracted from merozoites obtained from culture, sporocysts, and infected brain tissue using the DNeasy Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions, except that sporocysts were subjected to a freeze–thaw cycle in liquid nitrogen and boiling water prior to extraction.
Table 1.
Details of Sarcocystis neurona strains used for genetic analysis.
| Strain ID | Host/Isolate ID | Location | Year | Source |
|---|---|---|---|---|
| Opossum | ||||
| Opossum-1 | OP26 | Monterey, CA | 2005 | Sporocysts |
| Opossum-2 | OP38 | Monterey, CA | 2006 | Sporocysts |
| Opossum-3 | OP68 | Monterey, CA | 2005 | Sporocysts |
| Opossum-4 | OP134 | Rio Vista, CA | 2007 | Merozoitesa |
| Opossum-5 | OP 166 | Monterey, CA | 2007 | Sporocysts |
| Opossum-6 | OP 187 | Monterey, CA | 2008 | Merozoitesa |
| Opossum-7 | OP201 | Monterey, CA | 2008 | Merozoitesa |
| Opossum-8 | OP212 | Monterey, CA | 2008 | Merozoitesa |
| Opossum-9 | OP226 | Monterey, CA | 2008 | Merozoitesa |
| Opossum-10 | OPL49 | Rio Vista, CA | 2007 | Merozoitesa |
| Opossum-11 | OPGA3 | Georgia | 2008 | Merozoitesa |
| Opossum-12 | OPGA7 | Georgia | 2008 | Merozoitesa |
| Opossum-13 | OPBF2 | Illinois | 2008 | Merozoitesa |
| Horse | ||||
| Horse-1 | snUCD-lb | Santa Rosa, CA | 1994 | Merozoitesc |
| Horse-2 | snUCD-2b | Santa Rosa, CA | 1994 | Merozoitesc |
| Horse-3 | snUCD-3b | California | 1995 | Merozoitesc |
| Horse-4 | snMu-1d | Missouri | 1999 | Merozoitesc |
| Horse-5 | snMu-2d | Missouri | 1999 | Merozoitesc |
| Horse-6 | H1756 | Berkeley, CA | 2009 | Brain Tissue |
| Horse-7 | H1801 | Berkeley, CA | 2009 | Brain Tissue |
| Cat | ||||
| Cat | MuCAT2e | Missouri | 2000 | Merozoitesc |
| Sea Otter | ||||
| Sea Otter-1 | SO4387 | Monterey, CA | 2005 | Brain Tissue |
| Sea Otter-2 | SO4413 | Morro Bay, CA | 2005 | Brain Tissue |
| Sea Otter-3 | SO4530 | Morro Bay, CA | 2005 | BrainTissue |
| Sea Otter-4 | SO4653 | Monterey, CA | 2006 | Brain Tissue |
| Sea Otter-5 | SO4697 | Monterey, CA | 2006 | Brain Tissue |
| Sea Otter-6 | SO4711 | Monterey, CA | 2006 | Merozoitesc |
| Sea Otter-7 | SO4725 | Monterey, CA | 2006 | Merozoitesc |
| Sea Otter-8 | SO4755 | Morro Bay, CA | 2006 | Merozoitesc |
| Sea Otter-9 | SO4786 | Monterey, CA | 2006 | Brain Tissue |
| Sea Otter-10 | SO4834 | Monterey, CA | 2006 | Brain Tissue |
| Sea Otter-11 | SO4928 | Monterey, CA | 2007 | Brain Tissue |
| Sea Otter-12 | SO4970 | Monterey, CA | 2007 | Brain Tissue |
| Sea Otter-13 | SO4972 | Morro Bay, CA | 2007 | Brain Tissue |
| Sea Otter-1 4 | SO5002 | Monterey, CA | 2007 | Brain Tissue |
| Sea Otter-15 | SO5073 | Monterey, CA | 2007 | Brain Tissue |
| Sea Otter-16 | SO5110 | Monterey, CA | 2007 | Brain Tissue |
| Sea Otter-17 | SO5226 | Monterey, CA | 2008 | Brain Tissue |
| Sea Otter-18 | SO5259 | Morro Bay, CA | 2008 | Merozoitesc |
| Sea Otter-19 | SO5263 | Morro Bay, CA | 2008 | Brain Tissue |
| Sea Otter-20 | SO5274 | Monterey, CA | 2008 | Merozoitesc |
| Sea Otter-21 | SO5278 | Monterey, CA | 2008 | Brain Tissue |
| Sea Otter-22 | SO5283 | Monterey, CA | 2008 | Merozoitesc |
| Sea Otter-23 | SO5296 | Monterey, CA | 2008 | Merozoitesc |
| Harbor Porpoise | ||||
| Porpoise | HP060325 | Monterey, CA | 2006 | Merozoitesc |
Merozoites derived by inoculation of sporozoites from excysted sporocysts into cell culture.
Merozoites derived by inoculation of infected brain tissue into cell culture.
2.2. Molecular characterization
Extracted DNA was used as a template for multiple PCR reactions with primers targeting the ITS-1 region, the 25/ 396 marker, snSAG2, snSAG3, and snSAG4 genes, and 10 microsatellite regions (Table 2). The primers (ITS1DF, ITS1DR, ITS1diF, and ITS1diR) and reaction conditions for amplification of the ITS-1 region were identical to those described previously (Rejmanek et al., 2009). Amplification conditions and primers (JNB25 and JD396) for the 25/396 region were identical to those described by Tanhauser et al. (1999). To amplify the snSAG genes, multiple primers were developed based on published GenBank sequences (AY191006, AY191007, AY191008). These included primers described by Wendte et al. (in press), targeting the snSAG2 gene (snSAG2 F and snSAG2 R); a nested primer set targeting the snSAG3 gene consisting of external primers (snSAG3 extF and snSAG3 extR), and internal primers (snSAG3 intF and snSAG3); and a nested primer set targeting the snSAG4 gene consisting of external primers (snSAG4 extF and snSAG4 extR), and internal primers (snSAG4 intF and snSAG4 intR). The following reaction conditions were used for amplifying all three snSAG genes: initial denaturation at 94 °C for 4 min followed by 35 cycles at 95 °C for 40 s, 58 °C for 40 s, and 72 °C for 90 s, followed by a 5 min extension at 72 °C. Nested PCR reactions using the snSAG3 and snSAG4 primers were performed on brain tissue samples when the initial amplification signal produced too little product for sequencing. One microliter of PCR product from the first round was used as template for a second nested PCR reaction with the same amplification conditions. PCR products from positive bands were prepared for sequencing using the ExoSAP-IT PCR clean-up system (USB Corporation, Cleveland, OH) and sequenced in the forward and reverse direction at the Division of Biological Sciences DNA sequencing facility (University of California, Davis, CA). All unique sequences were deposited in GenBank (accession numbers GQ386971–GQ386980).
Table 2.
Oligonucleotide primers used for PCR and DNA sequencing reactions.
| Primer | Oligonucleotide sequence | Reference |
|---|---|---|
| ITS1DF | 5′-TACCGATTGAGTGTTCCGGTG-3′ | Rejmanek et al. (2009) |
| ITS1DR | 5′-GCAATTCACATTGCGTTTCGC-3′ | Rejmanek et al. (2009) |
| ITS1diF | 5′-CGTAACAAGGTTTCCGTAGG-3′ | Rejmanek et al. (2009) |
| ITS1diR | 5′-TTCATCGTTGCGCGAGCCAAG-3′ | Rejmanek et al. (2009) |
| JNB25 | 5′-CACACAAACACTGAAAGTCACGTACTT-3′ | Tanhauser et al. (1999) |
| JD396 | 5′-CCTGCCTCACTTCGACACAT-3′ | Tanhauser et al. (1999) |
| snSAG2F | 5′-AGCGGCGTTTTCAGATTGTA-3′ | Wendte et al. (in press) |
| snSAG2R | 5′-AAAACGAAGGCAAGTGTGCT-3′ | Wendte et al. (in press) |
| snSAG3extF | 5′-TCAAGGACGTTTTTCCCTGT-3′ | Wendte et al. (in press) |
| snSAG3extR | 5′-CTCTGCATGCTGCAATGAAT-3′ | Wendte et al. (in press) |
| snSAG3intF | 5′-CCCTGCCTTTCTGGTCTCTT-3′ | Wendte et al. (in press) |
| snSAG3intR | 5′-TTCTCCCCAAAGACCATCTG-3′ | Wendte et al. (in press) |
| snSAG4extF | 5′-ATTAATGCCACGTACTGCTG-3′ | Wendte et al. (in press) |
| snSAG4extR | 5′-TCCACCAATAGTTTAGGCTG-3′ | Wendte et al. (in press) |
| snSAG4intF | 5′-ATTGGGAACAGTTTCCATCG-3′ | Wendte et al. (in press) |
| snSAG4intR | 5′-CACCTATTCAAATGGCTGTC-3′ | Wendte et al. (in press) |
| Sn3 intF | 5′-CAGCAGGTCGTCCATTTTGG-3′ | Current study |
| Sn3 intR | 5′-ACGTGCACGTGCATTGACAC-3′ | Current study |
| Sn4 intF | 5′-TAGCGTACAGGAGGTGTACC-3′ | Current study |
| Sn7 extF | 5′-CGACAGTTCTCCCTGCTCTT-3′ | Current study |
| Sn7 extR | 5′-CATGCATCGATTTCTGATCG-3′ | Current study |
| Sn8 intF | 5′-TGGATGTTACCAGCGGAATC-3′ | Current study |
| Sn9 extF | 5′-CTGCTGCTAGCGGACTCTCT-3′ | Current study |
| Sn10 extR | 5′-GGTTCTTCAGCCACCTACGA-3′ | Current study |
| Sn D2F | 5′-GGTAGTCTCATACTTGCCAG-3′ | Current study |
| Sn D2R | 5′-CAACGCTGTTGCACCATTAC-3′ | Current study |
Amplification of microsatellites Sn2–Sn5 and Sn7–Sn11 was performed as previously described (Asmundsson and Rosenthal, 2006) with modifications; several new primers needed to be developed in order to perform nested or hemi-nested PCR on brain tissue samples that produced only weak first round amplification signals. The new primers included internal Sn3 primers (Sn3 intF and Sn3 intR); an internal Sn4 primer (Sn4 intF); external Sn7 primers (Sn7 extF and Sn7extR); an internal Sn8 primer (Sn8 intF); an external Sn9 primer (Sn9 extF); and an external Sn10 primer (Sn10 extR). The additional Sn7, Sn9, and Sn10 primers were developed by Wendte et al. (in press). The following reaction conditions were used for all nested PCR reactions: initial denaturation at 94 °C for 4 min followed by 35 cycles at 95 °C for 40 s, 59 °C for 40 s, and 72°C for 90 s, followed by a 5 min extension at 72°C. In all reactions 1 µl of PCR product from the first round was used as the template for the second round of nested PCR. In addition to the 9 microsatellite sequences described by Asmundsson and Rosenthal (2006), an additional micro-satellite (SnD2) was identified by searching the S. neurona expressed sequence tag (EST) database with the micro-satellite identification program WebSat (Martins et al., 2009). The primers (SnD2F and SnD2R) were designed based on the Genbank EST sequence (BE574449). The PCR products from positive bands were prepared for sequencing using the ExoSAP-IT PCR clean-up system (USB Corporation, Cleveland, OH) and sequenced in the forward and reverse direction at the Division of Biological Sciences DNA sequencing facility (University of California, Davis, CA). The numbers of di-nucleotide repeats at each microsatellite locus were visually counted from their respective forward and reverse sequences. Phylogenetic analysisThe DNA sequences of the ITS-1 region, the 25/396 marker, the snSAG2, snSAG3, and snSAG4 genes from 45 S. neurona isolates were edited and aligned using the program ClustalX 2.0.9 (Larkin et al., 2007). Based on the limited amount of sequence variation among our dataset, sequences from the 3 polymorphic genes (snSAG3, snSAG4 and 25/396) were combined to form a single concatenated sequence for each S. neurona strain. This was done in order to maximize the possibility of detecting phylogenetically informative differences among the S. neurona strains. Phylogenetic analyses of the concatenated sequences were performed using the program packages Phylip 3.68 (Felsenstein, 1989) and PAUP* (Swofford, 2003). Three different methods were used to resolve phylogenies: neighbor-joining using the Kimura-2 parameter model (Saitou and Nei, 1987), Fitch parsimony (Fitch, 1971), and maximum likelihood (Felsenstein and Churchill, 1996). Bootstrap support for all 3 types of analyses was based on 1000 pseudoreplicate datasets generated from the original concatenated sequence alignment. Resulting trees were visualized in Treeview 1.6.6 (Page, 1996).To visualize the relationships between all 45 S. neurona strains across the 10 microsatellites, neighbor-joining trees were reconstructed using the program Populations 1.2.30 (http://www.bioinformatics.org/project/?group_id=84). The average squared distance (ASD) model (Goldstein et al., 1995; Slatkin, 1995) which represents the squared difference in allele size summed across loci and between isolate pairs, and a similar model, Dsw (Shriver et al., 1995) which can reduce the variance sometimes encountered with the ASD model, were used to reconstruct the neighbor-joining trees using 1000 bootstrap replicates.
3. Results
Forty-five S. neurona strains from different hosts and geographical locations were analyzed in this study. A description of these strains, including their geographical origin, collection date, and source are presented in Table 1. S. neurona strains were obtained from 10 opossums, 17 sea otters, 1 harbor porpoise, 7 horses, and 1 cat.
Large portions of the ITS-1 gene; the 25/396 marker; the snSAG2, snSAG3, and SnSAG4 genes; and ten different microsatellites were amplified and sequenced from the 45 S. neurona samples. All 45 samples shared the identical sequence across 700 bp of the ITS-1 gene and 718 bp of the SnSAG2 gene. Representative sequences for the ITS-1 gene (accession # GQ386971) and the snSAG2 gene (accession # GQ386974) were deposited in Genbank. The remaining genetic markers (snSAG3, snSAG4, 25/396) and the 10 microsatellites showed varying levels of polymorphism among the 45 S. neurona samples. S. neurona genotypes observed using these 13 genetic markers are summarized in Table 3. Sequence analysis of the snSAG3 gene identified 4 unique alleles, polymorphisms and insertions/deletions (indels) were present at nucleotide positions 239, 521, 522, and 1053 within the consensus sequence (accession # GQ386977). Sequence analysis of the snSAG4 gene identified 2 unique alleles based on a single substitution at nucleotide position 494 within the consensus sequence (accession # GQ386979), and analysis of the 25/396 genetic marker identified 2 unique alleles based on the presence or absence of an AT indel at nucleotide positions 74 and 75 of the consensus sequence (accession # GQ386972). Microsatellite sequencing results (Table 3) revealed significant genotypic diversity across all 45 S. neurona samples. The number of alleles observed at each microsatellite locus ranged from 2 to 9, with an average of 4 alleles per locus. Among the 45 samples, 24 distinct genotypes were identified based on the inheritance pattern of alleles across the 15 loci examined. Several genotypes were common to multiple infected hosts including 2 opossums strains, Opossum-3 and 8, as well as 4 sea otter strains, Sea Otters 8, 18, 20, and 21. One genotype in particular was especially abundant (highlighted in Table 3), representing one third (15/45) of characterized strains. These same 15 strains also shared identical ITS-1, 25/396, SnSAG2, SnSAG3, and SnSAG4 sequences.
Table 3.
Description of Sarcocystis neurona genotypes.
| Genetic Markers Analyzed |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| snSAG3 | snSAG4 | 25/396 | Sn2 (GT)n |
Sn3 (AT)n |
Sn4 (CA)n |
Sn5 (CA)n |
Sn7 (CA)n |
Sn8 (CA)n |
Sn9 (GT)n |
Sn10 (AT)n |
Sn11 (CA)n |
SnD2 (TA)n |
|||||
| Strain | 239 | 521 | 522 | 1053 | 494 | 74 | 75 | ||||||||||
| ID | G | A | T | T | C | - | - | ||||||||||
| Opossum-1 | C | . | . | C | . | . | . | 9 | 10 | 13 | 10 | 20 | 10 | 14 | 9 | 14 | 11 |
| Opossum-2 | C | . | . | C | . | . | . | 9 | 10 | 13 | 10 | 20 | 10 | 14 | 9 | 14 | 11 |
| Opossum-3 | C | - | - | C | G | . | . | 9 | 11 | 13 | 9 | 20 | 10 | 14 | 9 | 15 | 13 |
| Opossum-4 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 10 | 13 | 15 |
| Opossum-5 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Opossum-6 | C | . | . | C | G | . | . | 9 | 11 | 13 | 9 | 20 | 10 | 14 | 9 | 15 | 13 |
| Opossum-7 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 17 |
| Opossum-8 | C | - | - | C | G | . | . | 9 | 11 | 13 | 9 | 20 | 10 | 14 | 9 | 15 | 13 |
| Opossum-9 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Opossum-10 | C | . | . | C | . | . | . | 9 | 10 | 13 | 9 | 17 | 10 | 18 | 9 | 13 | 16 |
| Opossum-11 | C | . | . | C | G | . | . | 10 | 12 | 13 | 9 | 23 | 10 | 14 | 11 | 14 | 10 |
| Opossum-12 | C | - | - | C | G | . | . | 10 | 12 | 13 | 9 | 23 | 10 | 14 | 11 | 14 | 10 |
| Opossum-13 | C | . | . | C | G | A | T | 10 | 11 | 13 | 10 | 16 | 10 | 14 | 10 | 14 | 13 |
| Sea Otter-1 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 17 | 11 | 13 | 16 |
| Sea Otter-2 | . | . | . | . | G | . | . | 9 | 11 | 12 | 9 | 18 | 10 | 17 | 10 | 13 | 9 |
| Sea Otter-3 | C | . | . | C | . | . | . | 10 | 11 | 12 | 9 | 21 | 10 | 16 | 9 | 14 | 15 |
| Sea Otter-4 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Sea Otter-5 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Sea Otter-6 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 15 |
| Sea Otter-7 | C | . | . | C | G | . | . | 10 | 10 | 13 | 9 | 18 | 10 | 14 | 9 | 14 | 12 |
| Sea Otter-8 | C | . | . | C | . | . | . | 10 | 11 | 13 | 9 | 22 | 10 | 17 | 9 | 14 | 15 |
| Sea Otter-9 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 12 |
| Sea Otter-10 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 16 | 10 | 18 | 11 | 13 | 16 |
| Sea Otter-11 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Sea Otter-12 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Sea Otter-13 | C | . | . | C | G | . | . | 9 | 11 | 13 | 9 | 19 | 10 | 14 | 9 | 15 | 13 |
| Sea Otter-14 | C | . | . | C | G | . | . | 9 | 11 | 13 | 9 | 19 | 10 | 14 | 9 | 15 | 13 |
| Sea Otter-15 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Sea Otter-16 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Sea Otter-17 | . | . | . | . | G | . | . | 9 | 13 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Sea Otter-18 | C | . | . | C | . | . | . | 10 | 11 | 13 | 9 | 22 | 10 | 17 | 9 | 14 | 15 |
| Sea Otter-19 | C | . | . | C | . | . | . | 10 | 11 | 12 | 9 | 21 | 10 | 17 | 9 | 14 | 15 |
| Sea Otter-20 | C | . | . | C | . | . | . | 10 | 11 | 13 | 9 | 22 | 10 | 17 | 9 | 14 | 15 |
| Sea Otter-21 | C | . | . | C | . | . | . | 10 | 11 | 13 | 9 | 22 | 10 | 17 | 9 | 14 | 15 |
| Sea Otter-22 | . | - | - | . | . | . | . | 9 | 11 | 12 | 9 | 18 | 10 | 17 | 9 | 13 | 11 |
| Sea Otter-23 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Porpoise | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Horse-1 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Horse-2 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Horse-3 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Horse-4 | C | . | . | C | G | . | . | 10 | 16 | 13 | 9 | 19 | 11 | 14 | 10 | 14 | 10 |
| Horse-5 | C | . | . | C | . | . | . | 9 | 11 | 12 | 9 | 18 | 10 | 17 | 10 | 16 | 17 |
| Horse-6 | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
| Horse-7 | C | - | - | C | G | A | T | 9 | 13 | 13 | 10 | 16 | 10 | 14 | 9 | 14 | 12 |
| Cat | . | . | . | . | . | . | . | 9 | 12 | 12 | 9 | 17 | 10 | 18 | 11 | 13 | 16 |
The nucleotides shown beneath the snSAG3, snSAG4, and the 25/396 genetic markers indicate polymorphic sites among the S. neurona strains. The numerical positions annotated refer to the numbered sites in GenBank sequences (GQ386977, GQ386979, and GQ386972, respectively). The consensus sequence was defined as the one with the most common nucleotides among sampled isolates. Periods (.) indicate identity with the consensus sequence and dashes (-) indicate deletions. The columns under each microsatellite genetic marker (locus Sn2–SnD2) indicate the number of di-nucleotide repeats observed in each sample. Highlighted strains form a genetically cohesive group.
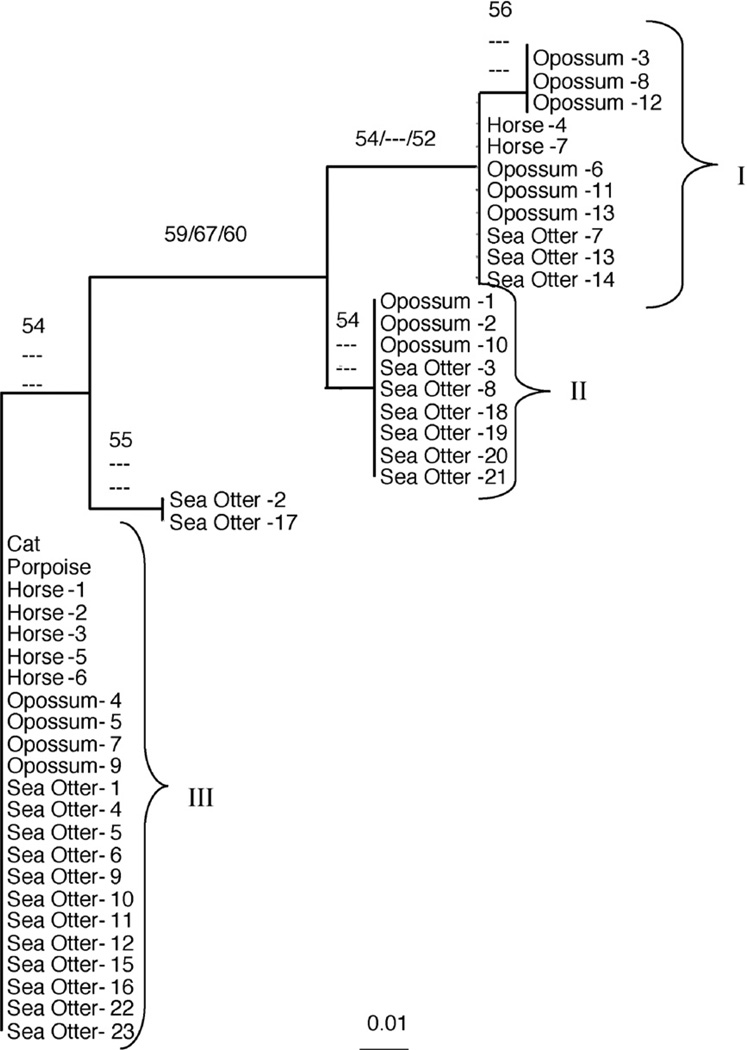
Based on the low number of polymorphisms detected across the 5 genetic loci, a concatenated sequence, formed by combining the 3 polymorphic sequences (snSAG3, snSAG4, and 25/396) into a single contiguous sequence, was created for all 45 S. neurona strains for phylogenetic analyses. The resulting 2119 bp sequences were used to generate phylogenetic trees by neighbor-joining, parsimony, and maximum likelihood methods. All 3 analytical methods yielded similar tree topologies in both PAUP* and Philip 3.68 (data not shown). The only difference was that PAUP* collapsed any clusters with less than 50% bootstrap support. A neighbor-joining tree generated by PAUP*, which includes parsimony and maximum likelihood bootstrap values for branches that had greater than 50% bootstrap support, is depicted in Fig. 1. Neighbor-joining and maximum likelihood analysis identified 3 distinct groups (I, II, and III). The parsimony analysis did not show enough support for a partitioning between groups I and II. Groups I and III consisted of S. neurona strains derived from opossums and multiple intermediate hosts, including horses and sea otters. Group II consisted of S. neurona strains derived exclusively from opossums and sea otters.
Fig. 1.
Phylogenetic relationships among Sarcocystis neurona strains from various hosts inferred from concatenated sequences comprising the snSAG3, snSAG4 genes, and the 25/396 marker. The numbers above the nodes represent bootstrap confidence values for 1000 replicates based on neighbor-joining, parsimony, and maximum likelihood analysis. Only bootstrap values exceeding 50% are shown. The branch lengths are proportional to the amount of inferred evolutionary change. The scale bar indicates the number of nucleotide substitutions per site. I, II, and III represent phylogenetically distinct groups of S. neurona strains. Details of the individual strains are given in Tables 1 and 2.
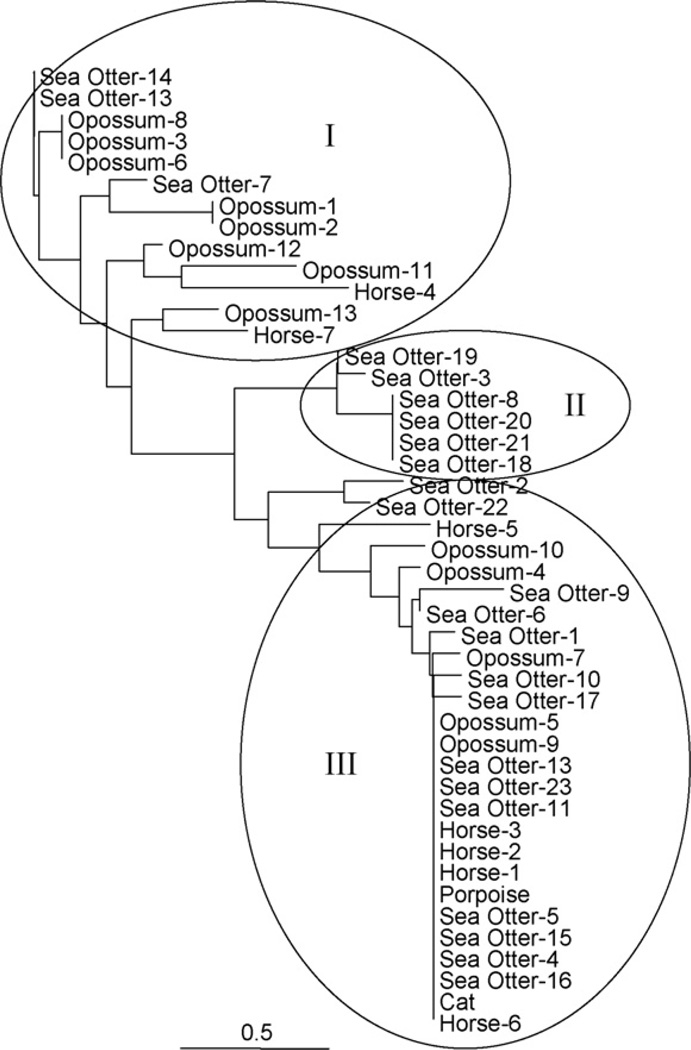
To examine the genotypic variation among the 45 S. neurona strains based on differences within the microsatellite regions, neighbor-joining trees were reconstructed according to inter-individual distances. Although bootstrap support values for the tree topologies based on either the ASD or Dsw model, were not significant, the neighbor-joining trees reconstructed according to either model were nearly identical, differing insignificantly in the lengths of several branches. The neighbor-joining tree based on the Dsw model is depicted in Fig. 2. Based on the tree topology, individual S. neurona strains could be grouped into one of 3 distinct groups (I, II, and III). With the exception of 3 strains, these groups were identical in composition to the 3 groups identified by neighbor-joining, parsimony, and maximum likelihood tree building methods based on variations within the concatenated 25/396, snSAG3, and SnSAG4 sequences (Fig. 1). The only differences were that 2 opossum S. neurona strains (opossum-1 and opossum-2) were placed in group I, and 1 opossum strain (opossum-10) was placed in group III in the microsatellite-generated tree.
Fig. 2.
Neighbor-joining tree of Sarcocystis neurona strains inferred from 10 microsatellite sequences using the Dsw model with 1000 bootstrap replicates. The branch lengths are proportional to the amount of inferred evolutionary change. The scale bar indicates the level of divergence. I, II, and III represent phylogenetically distinct groups of S. neurona strains. Details of individual strains are given in Tables 1 and 2.
4. Discussion
In this study, we assessed the relationships among 45 S. neurona strains from multiple hosts, including opossums, horses, sea otters, a harbor porpoise, and a cat, using 15 unique genetic markers. This allowed us to draw more reliable conclusions about the diversity of those strains and helped determine the underlying population structure of the group as a whole. The findings also enabled us to conclude that fatal infections caused by S. neurona in terrestrial and marine intermediate hosts in California likely originate from S. neurona sporocysts shed by opossums.
In previous studies, both the ITS-1 gene and the 25/396 marker have been useful in differentiating S. neurona from other closely related Sarcocystis species (Elsheikha et al., 2005; Tanhauser et al., 1999). These genes are useful diagnostic markers because they show little intraspecific variation. In the current study, all 45 S. neurona samples, including those from disparate geographical locations, exhibited identical sequences across 700 bp of the ITS-1 gene, providing further evidence for the stability of this gene and its utility as a diagnostic marker for S. neurona. The 25/39 marker also proved to be surprisingly non-polymorphic across all 45 S. neurona strains with only two strains (Opossum-13 and Horse-7) showing a single TA indel difference from the other 43 S. neurona strains analyzed. The same TA indel has previously been identified within a group of S. neurona isolates from Michigan (Elsheikha et al., 2005). In that study, the authors showed evidence to support partitioning S. neurona strains into either “Northern” or “Southern” geographic groups in the U.S.A. based in part by the presence or absence of the TA insertion. According to that grouping, Opossum-13 and Horse-7 would be placed within the “Northern” group while the rest of the S. neurona samples in the present study would be placed in the “Southern” group. This might be an accurate designation for Opossum-13 that was from Illinois, a state that borders Michigan, but Horse-7 and all of the other S. neurona samples with a “Southern” group designation in our study were from central California. This finding suggests that both the “Northern” and “Southern” geographic groups may co-exist within the state, which is not surprising given the significant latitudinal distribution (1200 km) of California.
Although the existence of the S. neurona surface antigen gene family was described a number of years ago (Jung et al., 2004; Howe et al., 2005), few studies have explored the utility of these genes as genetic markers. Based on the complete absence of snSAG1 genes in certain S. neurona strains (Howe et al., 2008), we elected to compare our isolates only at those snSAGs that are thought to be present in all strains (snSAG2, snSAG3, and snSAG4). The relative lack of polymorphism among the 45 S. neurona samples across all 3 snSAGs was consistent with what has been found in other studies (Wendte et al. in press). However, this study identified a new indel at positions 521 and 522 at SnSAG3, indicating a greater allelic variation at this locus than was reported by Wendte et al. (in press). Intriguingly, the SAG allelic variation among T. gondii strains is significantly greater among commonly isolated strains (Grigg et al., 2001). Both the T. gondii and S. neurona SAG orthologs function in parasite adhesion and host cell invasion (Dzierszinski et al., 2000; Lekutis et al., 2001; Howe et al., 2005), so it is unclear why one family is more polymorphic. A potential reason for this finding may stem from the fact that T. gondii exhibits a much greater range of recognized intermediate hosts (Tenter et al., 2000) when compared to S. neurona (Elsheikha, 2009). Polymorphisms among individual SAGs and the diversity expressed among the 160+ T. gondii surface antigens may account for the broad host range of T. gondii (Boothroyd, 2009). Similar to S. neurona, the related apicomplexan, Neospora caninum, has a limited host range and minimal nucleotide variation among its SAGs (Marsh et al., 1999). Another potential reason for the lack of significant variation observed among the snSAGs in this study is that our sample size may not have been sufficiently large or geographically dispersed to capture the true allelic variation that may potentially exist among extant S. neurona strains. Still, several identical inheritance patterns of the alleles present were consistently observed among our set of 45 S. neurona strains. Collectively, these differences resulted in clustering of nearly all of the S. neurona strains into 3 distinct phylogenetic groups. Although bootstrap support for the 3 groups was not particularly strong (52–67%), in part because only 5 polymorphisms were identified across 2119 bp of sequence, the same groups were consistently generated by different tree building methods (neighbor-joining, parsimony, and maximum likelihood). Each group contained S. neurona strains from opossums and one or more intermediate hosts, suggesting that sporocysts shed by opossums would be a likely source of infection.
In order to thoroughly investigate the genetic relationships among our S. neurona samples at the highest level of resolution, we further characterized all 45 S. neurona samples across 10 microsatellite loci. Neighbor-joining trees based on several distance matrix models supported a partitioning of nearly all the S. neurona samples into the same 3 groups that were generated by phylogenetic analyses among the single-copy 25/396, snSAG3, and snSAG4 gene sequences. The fact that 40 of the 45 S. neurona strains analyzed clustered into the same 3 groups regardless of the analytic method employed suggests that, like T. gondii, which has predominantly 3 distinct lineages (Howe and Sibley, 1995), S. neurona strains exhibit extensive linkage disequilibrium and may comprise a limited set of highly similar, apparently clonal lineages. A recent study comparing S. neurona genotypes within the U.S.A. using a similar set of microsatellite markers showed that many S. neurona isolates partitioned into genetically distinct groups (Sundar et al., 2008; Wendte et al., in press). Sundar et al. (2008) compared 34 S. neurona samples from 3 states and Wendte et al. (in press) compared 30 samples mainly from California. Both found that genetic partitioning was closely tied to geographical origin. In contrast, we found little evidence in our study to suggest geographical partitioning of S. neurona genotypes. However, our dataset included a large proportion of S. neurona strains from a more limited geography. Direct comparison of S. neurona genotypes between these two studies is not possible due to the different methods used to enumerate microsatellites. The apparent lack of geographic partitioning among S. neurona strains in our study may be due to the introduction of opossums to Western North America and their rapid expansion throughout California (Grinnell et al., 1937). Opossums were repeatedly introduced to California from different locations (Arkansas, Tennessee, and Missouri) over many years and often to the same areas in Central and Southern California (Grinnell et al., 1937). With each release, different S. neurona genotypes may have been introduced into the state. Potential intermediate hosts for S. neurona, such as cats (Dubey and Hamir, 2000), dogs (Cooley et al., 2007), and horses (Dubey et al., 1991), brought to California, may be another route for introduction of new S. neurona genotypes. It is perhaps not surprising that geographic partitioning of unique S. neurona genotypes was not observed in our S. neurona strains from California.
Of particular interest in this study was the identification of 15 S. neurona strains that were identical at all 10 microsatellites and across all 5 gene sequences. This group was identical to genotype II (10 samples) reported by Wendte et al. (in press) that was restricted to Monterey Bay, CA in their study. In contrast, our cluster of strains came from a variety of hosts in different geographic areas and included opossums, sea otters, horses, a harbor porpoise, and a cat, collected over a 14-year time span. While 10 of these samples came from Monterey Bay, 4 others were from different locations in Central California, and the cat was from Missouri, suggesting this genotype may be more widespread. It is interesting that 4 dissimilar hosts with unique diets and ecological niches were all infected with a strain of S. neurona that was being shed by several opossums. This finding indicates that, similar to T. gondii oocysts (Lindsay et al., 2003), S. neurona sporocysts can persist both on land and in the near-shore marine environment. Additionally, the success of this particular strain would suggest that it may possess an adaptation conferring a selective advantage over other S. neurona strains. A study by Butcher et al. (2002) suggested that S. neurona strain differences may account for variable infection rates in intermediate hosts. Another beneficial attribute that would increase the success of one particular S. neurona strain would be enhanced survival of sporocysts in the terrestrial or marine environment. Enhanced sporocyst survival could occur by multiple possible mechanisms including an increased tolerance to temperature variation, desiccation, ultra-violet light, or salinity. Previous studies have shown that S. neurona sporocysts are able to maintain viability following exposure to elevated temperatures (55 °C), various chemical treatments, and prolonged storage of up to 7 years (Dubey et al., 2002; Elsheikha et al., 2004).
Finally, the discovery of several genetically cohesive groups of S. neurona, including one group containing 15 identical samples from different hosts and locations over many years, suggests that S. neurona may be capable of propagating in the environment as a stable, non-recombinant clone. In a previous study, Asmundsson et al. (2006) also found several genetically identical S. neurona isolates from disparate geographical locations using similar microsatellites. Clonal propagation is thought to account for the 3 predominant lineages of T. gondii found in humans and domestic animals (Howe and Sibley, 1995). It is believed that T. gondii utilizes sexual recombination to produce novel strains that have the potential to quickly sweep through the environment (Grigg and Sundar, 2009). Successful clonal propagation may also require, or at least benefit from, an asexual mode of transmission that circumvents the definitive host, as is well documented for T. gondii (Miller et al., 1972; Owen and Trees, 1998; Su et al., 2003), but which has so far not been shown for S. neurona. It has long been assumed that among Sarcocystis species, only sporocysts are infective to intermediate hosts (Dubey et al., 1989). This is based primarily on the fact that most Sarcocystis species have a carnivorous definitive host and an herbivorous intermediate host (Dubey et al., 1989). However, S. neurona has multiple carnivorous and omnivorous intermediate hosts which gives it ample opportunity for asexual transmission through carnivory (Dubey and Hamir, 2000). Although results from this and other studies (Asmundsson et al., 2006) suggest that S. neurona possesses a population structure similar to T. gondii, characterization of the population genetics of S. neurona is still in its infancy, and more isolates will need to be characterized at additional genetic loci before claims of asexual transmission among intermediate hosts can be tested.
In conclusion, we have shown that opossums inhabiting the Central California coast are the likely source of infective S. neurona sporocysts for several marine and terrestrial species including horses, sea otters, and harbor porpoises. We have also provided preliminary evidence to support a population genetic structure of Central California S. neurona strains comprised primarily of 3 distinct groups. However, the overall lack of strain variation observed suggests that these tenuous groupings will likely be refined with the characterization of additional strains at other loci.
Acknowledgments
This research was supported in part through funding from the Moss Foundation, Morris Animal Foundation, UC Davis Center for Equine Health, the UC Davis Wildlife Health Center, the Prescott Fund and by the Intramural Research Program of the NIH and NIAID (MEG). MEG is a scholar of the Canadian Institute for Advanced Research (CIFAR) Program for Integrated Microbial Biodiversity. We would like to thank the staff of the California Department of Fish and Game Marine Wildlife Veterinary Care and Research Center for their assistance in obtaining sea otter and opossum samples, Antoinette Marsh for providing several S. neurona isolates, Frances Gulland, David Casper, and Elizabeth Wheeler for providing harbor porpoise tissues, Kevin Keel and the Brookfield Zoo for providing multiple opossum intestinal samples, Amandeep Nandra and Jered Wendte for sharing unpublished data and primer designs, and Jonna Mazet for intellectual feedback on the manuscript.
References
- Asmundsson IM, Dubey JP, Rosenthal BM. A genetically diverse but distinct North American population of Sarcocystis neurona includes an overrepresented clone described by 12 microsatellite alleles. Infect. Gene Evol. 2006;6:352–360. doi: 10.1016/j.meegid.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Asmundsson IM, Rosenthal BM. Isolation and characterization of microsatellite markers from Sarcocystis neurona, a causative agent of equine protozoal myeloencephalitis. Mol. Ecol. Notes. 2006;6:8–10. [Google Scholar]
- Boothroyd JC. Expansion of host range as a driving force in the evolution of Toxoplasma. Mem. Inst. Oswaldo. Cruz. 2009;104:179–184. doi: 10.1590/s0074-02762009000200009. [DOI] [PubMed] [Google Scholar]
- Butcher M, Lakritz J, Halaney A, Branson K, Gupta GD, Kreeger J, Marsh AE. Experimental inoculation of domestic cats (Felis domesticus) with Sarcocystis neurona or S. neurona-like merozoites. Vet. Parasitol. 2002;107:1–14. doi: 10.1016/s0304-4017(02)00107-3. [DOI] [PubMed] [Google Scholar]
- Cohen ND, Mackay RJ, Toby E, Andrews FM, Barr BS, Beech J, Bernard WV, Clark CK, Divers TJ, Furr MO, Kohn CW, Levy M, Reed SM, Seahorn TL, Slovis NM. A multicenter case–control study of risk factors for equine protozoal myeloencephalitis. J. Am. Vet. Med. Assoc. 2007;231:1857–1863. doi: 10.2460/javma.231.12.1857. [DOI] [PubMed] [Google Scholar]
- Cooley AJ, Barr B, Rejmanek D. Sarcocystis neurona encephalitis in a dog. Vet. Pathol. 2007;44:956–961. doi: 10.1354/vp.44-6-956. [DOI] [PubMed] [Google Scholar]
- Dzierszinski F, Mortuaire M, Cesbron-Delauw MF, Tomavo S. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol. Microbiol. 2000;37:574–582. doi: 10.1046/j.1365-2958.2000.02014.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Davis SW, Speer CA, Bowman DD, de Lahunta A, Granstrom DE, Topper MJ, Hamir AN, Cummings JF, Suter MM. Sarcocystis neurona n. sp. (Protozoa: Apicomplexa), the etiologic agent of equine protozoal myeloencephalitis. J. Parasitol. 1991;77:212–218. [PubMed] [Google Scholar]
- Dubey JP, Hamir AN. Immunohistochemical confirmation of Sarcocystis neurona infections in raccoons, mink, cat, skunk, and pony. J. Parasitol. 2000;86:1150–1152. doi: 10.1645/0022-3395(2000)086[1150:ICOSNI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Saville WJ, Reed SM, Granstrom DE, Speer CA. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM) Vet. Parasitol. 2001;95:89–131. doi: 10.1016/s0304-4017(00)00384-8. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Saville WJ, Sreekumar C, Shen SK, Lindsay OS, Pena HF, Vianna MC, Gennari SM, Reed SM. Effects of high temperature and disinfectants on the viability of Sarcocystis neurona sporocysts. J. Parasitol. 2002;88:1252–1254. doi: 10.1645/0022-3395(2002)088[1252:EOHTAD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Speer CA, Fayer R, editors. Sarcocystosis of Animals and Man. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- Elsheikha HM, Mansfield LS. Sarcocystis neurona major surface antigen gene 1 (SAG1) shows evidence of having evolved under positive selection pressure. Parasitol. Res. 2004;94:452–459. doi: 10.1007/s00436-004-1237-y. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Murphy AJ, Mansfield LS. Viability of Sarcocystis neurona sporocysts after long-term storage. Vet. Parasitol. 2004;123:257–264. doi: 10.1016/j.vetpar.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Murphy AJ, Mansfield LS. Phylogenetic congruence of Sarcocystis neurona Dubey et al., 1991 (Apicomplexa: Sarcocystidae) in the United States based on sequence analysis and restriction fragment length polymorphism (RFLP) Syst. Parasitol. 2005;61:191–202. doi: 10.1007/s11230-005-3163-5. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM. Has Sarcocystis neurona Dubey et al., 1991 (Sporozoa: Apicomplexa: Sarcocystidae) cospeciated with its intermediate hosts? Vet. Parasitol. 2009;163:307–314. doi: 10.1016/j.vetpar.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Fayer R, Mayhew IG, Baird JD, Dill SG, Foreman JH, Fox JC, Higgins RJ, Reed SM, Ruoff WW, Sweeney RW, Tuttle P. Epidemiology of equine protozoal myeloencephalitis in North America based on histologically confirmed cases. J. Vet. Intern. Med. 1990;4:54–57. doi: 10.1111/j.1939-1676.1990.tb03103.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP—Phylogeny Inference Package (Version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- Felsenstein J, Churchill GA. A hidden Markov model approach to variation among sites in rate of evolution. Mol. Biol. Evol. 1996;13:93–104. doi: 10.1093/oxfordjournals.molbev.a025575. [DOI] [PubMed] [Google Scholar]
- Fitch WM. Toward defining the course of evolution: minimum change for a specified tree topology. Syst. Zool. 1971;20:406–416. [Google Scholar]
- Goldstein DB, Ruiz Linares A, Cavalli-Sforza LL, Feldman MW. An evaluation of genetic distances for use with microsatellite loci. Genetics. 1995;139:463–471. doi: 10.1093/genetics/139.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 2001;184:633–639. doi: 10.1086/322800. [DOI] [PubMed] [Google Scholar]
- Grigg ME, Sundar N. Sexual recombination punctuated by outbreaks and clonal expansions predicts Toxoplasma gondii population genetics. Int. J. Parasitol. 2009 Jul;39(8):925–933. doi: 10.1016/j.ijpara.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell J, Dixon JS, Linsdale JM, editors. Fur-bearing Mammals of California. Berkeley, CA: University of California Press; 1937. pp. 47–60. [Google Scholar]
- Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Howe DK, Gaji RY, Mroz-Barrett M, Gubbels MJ, Striepen B, Stamper S. Sarcocystis neurona merozoites express a family of immunogenic surface antigens that are orthologues of the Toxoplasma gondii surface antigens (SAGs) and SAG-related sequences. Infect. Immun. 2005;73:1023–1033. doi: 10.1128/IAI.73.2.1023-1033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DK, Gaji RY, Marsh AE, Patil BA, Saville WJ, Lindsay DS, Dubey JP, Granstrom DE. Strains of Sarcocystis neurona exhibit differences in their surface antigens, including the absence of the major surface antigen SnSAG1. Int. J. Parasitol. 2008;38:623–631. doi: 10.1016/j.ijpara.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Hyun C, Gupta GD, Marsh AE. Sequence comparison of Sarcocystis neurona surface antigen from multiple isolates. Vet. Parasitol. 2003;112:11–20. doi: 10.1016/s0304-4017(02)00392-8. [DOI] [PubMed] [Google Scholar]
- Johnson CK, Tinker MT, Estes JA, Conrad PA, Staedler M, Miller MA, Jessup DA, Mazet JA. Prey choice and habitat use drive sea otter pathogen exposure in a resource-limited coastal system. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2242–2247. doi: 10.1073/pnas.0806449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Lee CY, Grigg ME. The SRS superfamily of Toxoplasma surface proteins. Int. J. Parasitol. 2004;4:285–296. doi: 10.1016/j.ijpara.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kreuder C, Miller MA, Jessup DA, Lowenstine LJ, Harris MD, Ames JA, Carpenter TE, Conrad PA, Mazet JA. Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998–2001. J. Wildl. Dis. 2003;39:495–509. doi: 10.7589/0090-3558-39.3.495. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lekutis C, Ferguson DJ, Grigg ME, Camps M, Boothroyd JC. Surface antigens of Toxoplasma gondii: variations on a theme. Int. J. Parasitol. 2001;31:1285–1292. doi: 10.1016/s0020-7519(01)00261-2. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Thomas NJ, Dubey JP. Biological characterisation of Sarcocystis neurona isolated from a Southern sea otter (Enhydra lutris nereis) Int. J. Parasitol. 2000;30:617–624. doi: 10.1016/s0020-7519(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Collins MV, Mitchell SM, Cole RA, Flick GJ, Wetch CN, Lindquist A, Dubey JP. Sporulation and survival of Toxoplasma gondii oocysts in seawater. J. Eukaryot. Microbiol. 2003;50(Suppl):687–688. doi: 10.1111/j.1550-7408.2003.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Barr BC, Madigan J, Lakritz J, Conrad PA. Sequence analysis and polymerase chain reaction amplification of small subunit ribosomal DNA from Sarcocystis neurona. Am. J. Vet. Res. 1996;57:975–981. [PubMed] [Google Scholar]
- Marsh AE, Howe DK, Wang G, Barr BC, Cannon N, Conrad PA. Differentiation of Neospora hughesi from Neospora caninum based on their immunodominant surface antigen, SAG1 and SRS2. Int. J. Parasitol. 1999;29:1575–1582. doi: 10.1016/s0020-7519(99)00120-4. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Johnson PJ, Ramos-Vara J, Johnson GC. Characterization of a Sarcocystis neurona isolate from a Missouri horse with equine protozoal myeloencephalitis. Vet. Parasitol. 2001;95:143–154. doi: 10.1016/s0304-4017(00)00386-1. [DOI] [PubMed] [Google Scholar]
- Martins WS, Lucas DCS, Neves KFS, Bertioli DJ. WebSat—A web software for microsatellite marker development. Bioinformation. 2009;3:282–283. doi: 10.6026/97320630003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Sverlow K, Crosbie PR, Barr BC, Lowenstine LJ, Gulland FM, Packham A, Conrad PA. Isolation and characterization of two parasitic protozoa from a Pacific harbor seal (Phoca vitulina richardsi) with meningoencephalomyelitis. J. Parasitol. 2001;87:816–822. doi: 10.1645/0022-3395(2001)087[0816:IACOTP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Miller NL, Frenkel JK, Dubey JP. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. J. Parasitol. 1972;58:928–937. [PubMed] [Google Scholar]
- Mondragon R, Howe DK, Dubey JP, Sibley LD. Genotypic analysis of Toxoplasma gondii isolates from pigs. J. Parasitol. 1998;84:639–641. [PubMed] [Google Scholar]
- Murphy AJ, Mansfield LS. Simplified technique for isolation, excystation, and culture of Sarcocystis species from opossums. J. Parasitol. 1999;85:979–981. [PubMed] [Google Scholar]
- Owen MR, Trees AJ. Vertical transmission of Toxoplasma gondii from chronically infected house (Mus musculus) and field (Apodemus sylvaticus) mice determined by polymerase chain reaction. Parasitology. 1998 Apr;116(Pt 4):299–304. doi: 10.1017/s003118209700231x. [DOI] [PubMed] [Google Scholar]
- Page RDM. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Rejmanek DR, VanWormer E, Miller MA, Maze JK, Nichelason AE, Melli AC, Packham AE, Jessup DA, Conrad PA. Prevalence and risk factors associated with Sarcocystis neurona infections in opossums (Didelphis virginiana) from central California. Vet. Parasitol. 2009;166:8–14. doi: 10.1016/j.vetpar.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Saville WJ, Reed SM, Morley PS, Granstrom DE, Kohn CW, Hinchcliff KW, Wittum TE. Analysis of risk factors for the development of equine protozoal myeloencephalitis in horses. J. Am. Vet. Med. Assoc. 2000;217:1174–1180. doi: 10.2460/javma.2000.217.1174. [DOI] [PubMed] [Google Scholar]
- Shriver MD, Jin L, Boerwinkle E, Deka R, Ferrell RE, Chakraborty R. A novel measure of genetic distance for highly polymorphic tandem repeat loci. Mol. Biol. Evol. 1995;12:914–920. doi: 10.1093/oxfordjournals.molbev.a040268. [DOI] [PubMed] [Google Scholar]
- Slatkin M. A measure of population subdivision based on microsatellite allele frequencies. Genetics. 1995;139:457–462. doi: 10.1093/genetics/139.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- Sundar N, Asmundsson IM, Thomas NJ, Samuel MD, Dubey JP, Rosenthal BM. Modest genetic differentiation among North American populations of Sarcocystis neurona may reflect expansion in its geographic range. Vet. Parasitol. 2008 Mar;152(1–2):8–15. doi: 10.1016/j.vetpar.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Swofford D. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, MA: Sinauer Associates; 2003. [Google Scholar]
- Tanhauser SM, Yowell CA, Cutler TJ, Greiner EC, MacKay RJ, Dame JB. Multiple DNA markers differentiate Sarcocystis neurona and Sarcrocystis falcatula. J. Parasitol. 1999;85:221–228. [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NJ, Dubey JP, Lindsay DS, Cole RA, Meteyer CU. Protozoal meningoencephalitis in sea otters (Enhydra lutris): a histopathological and immunohistochemical study of naturally occurring cases. J. Comp. Pathol. 2007;137:102–121. doi: 10.1016/j.jcpa.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Turay HO, Barr BC, Caldwell A, Branson KR, Cockrell MK, Marsh AE. Sarcocystis neurona reacting antibodies in Missouri feral domestic cats (Felis domesticus) and their role as an intermediate host. Parasitol. Res. 2002;88:38–43. doi: 10.1007/s004360100503. [DOI] [PubMed] [Google Scholar]
- Wendte J, Nandra AK, Miller MA, Crosbie P, Conrad PA, Grigg ME. Limited genetic diversity among Sarcocystis neurona strains infecting southern sea otters. Vet. Parasitol. doi: 10.1016/j.vetpar.2009.12.020. in press. available online: December 22, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]