Abstract
Mitochondrial dysfunction and oxidative stress are known to occur following acute seizure activity but their contribution during epileptogenesis is largely unknown. The goal of this study was to determine the extent of mitochondrial oxidative stress, changes to redox status, and mitochondrial DNA (mtDNA) damage during epileptogenesis in the lithium-pilocarpine model of temporal lobe epilepsy. Mitochondrial oxidative stress, changes in tissue and mitochondrial redox status, and mtDNA damage were assessed in the hippocampus and neocortex of Sprague–Dawley rats at time points (24 h to 3 months) following lithium-pilocarpine administration. A time-dependent increase in mitochondrial hydrogen peroxide (H2O2) production coincident with increased mtDNA lesion frequency in the hippocampus was observed during epileptogenesis. Acute increases (24–48 h) in H2O2 production and mtDNA lesion frequency were dependent on the severity of convulsive seizure activity during initial status epilepticus. Tissue levels of GSH, GSH/GSSG, coenzyme A (CoASH), and CoASH/CoASSG were persistently impaired at all measured time points throughout epileptogenesis, that is, acutely (24–48 h), during the ‘latent period’ (48 h to 7 days), and chronic epilepsy (21 days to 3 months). Together with our previous work, these results demonstrate the model independence of mitochondrial oxidative stress, genomic instability, and persistent impairment of mitochondrial specific redox status during epileptogenesis. Lasting impairment of mitochondrial and tissue redox status during the latent period, in addition to the acute and chronic phases of epileptogenesis, suggests that redox-dependent processes may contribute to the progression of epileptogenesis in experimental temporal lobe epilepsy.
Keywords: mitochondrial dysfunction, mitochondrial oxidative stress, temporal lobe epilepsy
Temporal lobe epilepsy (TLE) is a recent addition to the neurological disorders in which mitochondrial oxidative stress and dysfunction have been suggested to be contributing factors (Schapira et al. 1990; Kunz et al. 2000; Cock et al. 2002; Patel 2004). The mechanisms by which mitochondria control neuronal injury and seizure susceptibility associated with TLE have not been fully elucidated, but the role of oxidative stress appears to be critical (Cock et al. 2002; Liang and Patel 2004). Mitochondria are a significant source of reactive oxygen species (ROS), uniquely vulnerable to oxidative damage, and have been suggested to be an important contributor to mechanisms of neurodegenerative disorders and seizure-induced brain damage, for example, excitotoxicity (Dugan et al. 1995; Patel et al. 1996) and inflammation (Roberts et al. 2009; Victor et al. 2009). Catalytic removal of ROS has been demonstrated to prevent status epilepticus (SE)-induced cell loss (Rong et al. 1999; Liang et al. 2000), and patients with TLE have demonstrated dramatic metabolic and bioenergetic changes as well as mitochondrial electron transport chain (ETC) complex I deficiency which could lead to decreased ATP production and neuronal damage (Kunz et al. 2000; Pan et al. 2008). SE has been demonstrated to produce acute increases in mitochondrial oxidative stress and subsequent damage to sensitive targets such as excessive ROS production, increased oxidation of cellular macromolecules, lipid per-oxidation, impaired mitochondrial Ca2+ sequestering, mitochondrial DNA (mtDNA) damage, decreased activity of ETC complexes I and III, and increased nitric oxide (NO) and peroxynitrite (ONOO−) generation at time points preceding neuronal death in susceptible brain regions (Griffiths et al. 1984; Cheng and Sun 1994; Bruce and Baudry 1995; Kunz et al. 1999; Erakovic et al. 2000; Frantseva et al. 2000; Liang et al. 2000; Patel et al. 2001, 2008; Milatovic et al. 2002; Chuang et al. 2004; Sleven et al. 2006; Jarrett et al. 2008). Evidence of mitochondrial dysfunction, that is, decreased ETC complex I and IV activity, subunit expression, and ultrastructural damage to mitochondria during chronic epilepsy has recently emerged from animal studies (Kudin et al. 2002; Chuang et al. 2004; Gao et al. 2007; Jarrett et al. 2008). However, the question of whether mitochondrial oxidative stress and consequent damage contribute to epileptogenesis and TLE remains to be fully explored.
Recent work from our laboratory has demonstrated a time-dependent increase in mitochondrial oxidative stress, oxidative damage to mtDNA, and decreased mtDNA repair capacity (Jarrett et al. 2008) prior to the onset of spontaneous seizures following kainic acid (KA)-induced epileptogenesis. A key finding of this study was that failure of adaptive responses to ongoing oxidative stress in the brain during epileptogenesis, such as mtDNA repair, could lead to an increase in seizure susceptibility. Two important questions, however, remain to be answered. First, these observations raise the question of whether mitochondrial oxidative stress and dysfunction occur during epileptogenesis across animal models. The KA model has been used to demonstrate mostly acute oxidative alterations associated with seizure activity (Bruce and Baudry 1995; Gluck et al. 2000; Liang et al. 2000, 2007; Patel et al. 2001; Chuang et al. 2004; Liang and Patel 2006), but the model specificity and chronic occurrence of these changes is largely unknown. The systemic injection of the muscarinic cholinergic agonist, pilocarpine (Pilo), with lithium-chloride (Li) pre-treatment has been used to induce SE and as a chronic model of TLE in rodents (Honchar et al. 1983; Williams and Jope 1994; Marinho et al. 1998) which replicates key features of the human condition such as spontaneous seizures, specific patterns of neuronal cell loss in the hippocampus, astrogliosis, and axonal reorganization (Hinterkeuser et al. 2000; Ben-Ari 2001; Lehmann et al. 2001). The lithium-pilocarpine (Li-Pilo) model is particularly valuable to understanding the mechanisms of oxidative stress-induced epileptogenesis because its mechanism lacks direct activation of glutamate receptor-induced neuronal excitotoxicity.
A second important question that emerges from our previous work is whether mitochondrial and/or cellular redox status is impaired during the latent period so as to facilitate the progression of chronic epilepsy. In our previous study, we observed extramitochondrial hydrogen peroxide (H2O2) production, an index of oxidative stress, during acute and chronic phases of epileptogenesis but not during the latent period (Jarrett et al. 2008). The latter is most likely because extramitochondrial release of H2O2 requires robust increases in intramitochondrial superoxide (O2•−) and H2O2 that exceeds the capacity of mitochondrial antioxidant defenses. Therefore, a more sensitive index of intracellular oxidative stress is required to detect redox changes that may be occurring during the latent period of epileptogenesis. Redox couples such as GSH, the most abundant intracellular non-enzymatic oxidant defense in the body catalyzing cellular H2O2 removal (Meister and Anderson 1983), and its disulfide (GSSG) serve as biomarkers of oxidative stress and damage (Reed and Savage 1995; Liang and Patel 2006). Coenzyme A (CoASH) and its disulfide with GSH (CoASSG), which are primarily compartmentalized within mitochondria, can be measured as a marker of mitochondrial specific redox status (Liang and Patel 2006). Following KA-induced SE, we have shown a significant decrease in mitochondrial GSH/GSSG and tissue CoASH/CoASSG up to 7 days in hippocampal tissue (Liang and Patel 2006), but it is unknown whether chronic alterations in tissue and mitochondrial redox status persist throughout epileptogenesis. The goal of this study was to determine whether mitochondrial oxidative stress, mitochondrial specific changes in redox couples (GSH/GSSG and CoASH/CoASSG), and mtDNA damage occurred during Li-Pilo induced epileptogenesis.
Methods
Lithium-pilocarpine administration
Animal housing was conducted in compliance with the University of Colorado Denver Anschutz Medical Campus procedures and protocols. Intraperitoneal (i.p.) injections were performed on adult male Sprague–Dawley rats with lithium-chloride (Sigma 310468; Sigma, St Louis, MO, USA) (50.8 mg/mL; 127 mg/kg) followed by methyl-scopolamine (Sigma S8502) (0.8 mg/mL; 1 mg/kg) 19–24 h later to attenuate the peripheral effects of pilocarpine treatment. Thirty minutes later rats were injected with either saline or pilocarpine-hydrochloride (Sigma P6503) (50 mg/mL; 30 mg/kg) to induce status epilepticus (SE) which was attenuated after 90 min by injecting diazepam (Hospira Inc., Lake Forest, IL, USA) (5 mg/mL; 10 mg/kg). Age-matched control rats received all treatments (lithium, methyl-scopolamine, diazepam), but saline instead of pilocarpine. A subset of rats were killed at 24–48 h that did not receive diazepam treatment, allowing SE in these animals to continue unabated. Rats treated with diazepam were killed 24 h, 48 h, 96 h, 7 days, 21 days, and 3 months following SE.
Monitoring of behavioral seizures
Behavioral seizure severity during SE was evaluated by direct observation for 6 h after the initial treatment and scored based on a modified Racine scale (Racine 1972) with only motor seizures being considered (Class I and II seizures were not scored). Briefly, motor seizure severity was characterized as follows: class III, animals displayed forelimb clonus with a lordotic posture; class IV, animals reared with concomitant forelimb clonus; and class V animals had a Class IV seizure and fell over. Only rats having at least one class III convulsive seizure during each consecutive 1-h period up to 3 h were included in the study, although most rats had multiple seizures per 1-h period. Spontaneous seizures from a group of 12 Li-Pilo-treated and control rats were monitored by direct observation by an observer blind to treatment group from 24 h to 3 months following SE for random intervals totaling 8–10 h a week for seizure severity and duration used for data in Fig. 1. Seizure frequency was not quantified based on the observation protocol used. All Li-Pilo-treated rats used in this study for endpoints ≥ 7 days post-SE were observed to have a minimum of two spontaneous seizures during the observation period (24 h to 3 months) and defined as being chronically epileptic (Hellier et al. 1998). All rats used in this study were observed for ≥2 h before being killed to ensure a seizure free period.
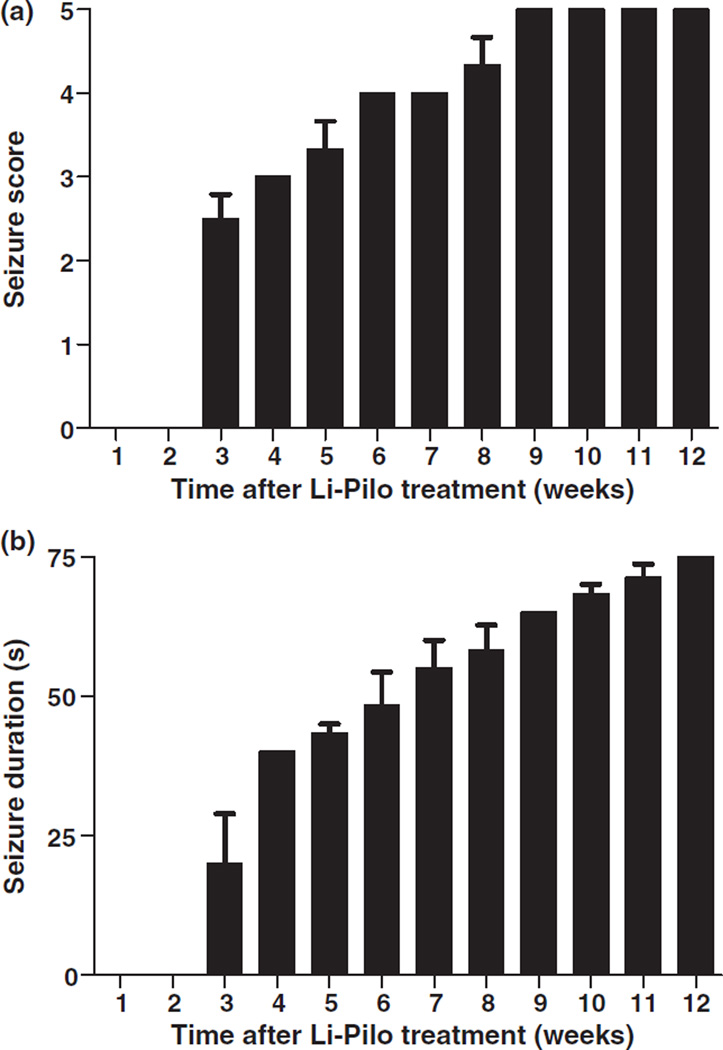
Fig. 1.
Time-dependent increase in seizure activity following Li-Pilo-induced epileptogenesis. Rats were observed to have spontaneous motor seizures by 3 weeks post-SE which increased in (a) severity and (b) duration up to 12 weeks. Bars represent mean ± SEM, n = 12.
mtDNA damage
Rats were killed at the indicated time points following SE and brain regions dissected and stored at −80°C. Genomic DNA was extracted from brain tissue using a commercially available Qiagen genomictip kit (Qiagen Inc., Valencia, CA, USA). mtDNA damage was quantified using the quantitative PCR (QPCR) by comparing the relative efficiency of the amplification of the large mtDNA gene fragment (13.4 kb) and normalized to 250 bp fragments from saline-treated controls which have a statistically negligible chance of sustaining oxidative stress-induced damage. The PCR conditions used in this study were based on previously reported sequences for mtDNA primers, with minor modifications (Ayala-Torres et al. 2000; Jarrett and Boulton, 2005). The primer sequences were as follows for the rat mitochondrial genome 5′-GGCAATTAAGAG-TGGGATGGAGCGAA-3′ and 5′-AAAATCCCCGCAAACAAT-GACCACCC-3′. QPCR was performed on a DNA Engine thermal cycler with all reactions being a total volume of 100 µL containing 15 ng total genomic DNA, 1 unit of XL rTth polymerase, 3.3 XL PCR buffer II (containing potassium acetate, glycerol and dimethylsulfoxide) and final concentrations of 200 µM dNTP’s, 1.2 mM Mg(OAC)2 and 0.1 µM primers.
The gene fragments were amplified using the following thermocycling profile: PCR was initiated with the addition of the 1 unit of XL rTth polymerase when samples had reached a temperature of 75°C followed by an initial penetration for 1 min at 94°C, cycles of denaturation at 94°C for 30 s and primer extension at 60°C for 13 min. Following the PCR cycles a final extension at 72°C for 10 min was performed. The mitochondrial gene products underwent 28 cycles of thermo-cycling. After the completion of the QPCR the gene products were resolved on a 1% agarose gel and digitally photographed on a UV transilluminator (UVi Tec, Cambridge, UK). The intensity of the PCR product bands was quantified with Scion Image analysis software (Scion Corp., Version Beta 4.0.2, Frederick, MD, USA).
Isolation of mitochondrial fractions
Hippocampi from each rat were pooled and homogenized with a Dounce tissue grinder (Wheaton, Milville, NJ, USA) in an isolation buffer (70 mM sucrose, 210 mM mannitol, 5 mM Tris–HCl, 1 mM EDTA; pH 7.4) and diluted 1 : 1 in 24% Percoll. Homogenates were centrifuged at 30 700 g at 4°C for 10 min. The sediment was subjected to Percoll gradient (19% on 40%) centrifugation at 30 700 g at 4°C for 10 min. The material located at the interface of the lowest two layers was slowly diluted 1 : 4 with mitochondrial isolation buffer containing 1 mg/mL bovine serum albumin and centrifuged at 6700 g at 4°C for 10 min to obtain final pellets consisting of respiring mitochondria. The final pellet was used immediately for H2O2 production studies.
Seizure-induced mitochondrial H2O2 production
H2O2 formation in mitochondrial fractions was measured using an Amplex Red reagent kit (Molecular Probes, Eugene, OR, USA) as previously described (Castello et al. 2007). Briefly, fluorometric detection of H2O2 production was achieved using the horseradish peroxidase-linked Amplex Ultra Red fluorometric assay. Isolated mitochondria (10 µg) were added to a 96-well plate containing 100 µL of reaction buffer containing 0.1 U/mL horseradish peroxidase, 50 µM Amplex UltraRed, and 2.5 mM malate plus 10 mM glutamate. A microplate reader equipped for excitation in the range of 530–560 nm and fluorescence emission detection at 590 nm was used to determine the production.
HPLC determination of GSH and GSSG
The measurement of GSH and GSSG was performed with an ESA (Chelmsford, MA, USA) 5600 CoulArray HPLC equipped with eight electrochemical detector cells as previously described in the literature (Lakritz et al. 1997; Liang and Patel 2006). The potentials of the electrochemical detector were set at 0/150/300/450/570/690/780/850 mV. Analyte separation was conducted on a 5 µM, 150 × 4.6 mm C-18 reversed-phase YMC ODS-A column (Waters Com., Milford, MA, USA). The mobile phase was composed of 100 mM sodium phosphate, 1% methanol; pH 3.0 and a flow rate of 0.6 mL/min was maintained. Samples were sonicated in ice cold 0.1 N perchloric acid and centrifuged at 16 000 g at 4°C for 10 min. Aliquots (20 µL) of the supernatant were subjected to HPLC. The GSSG peak was identified by the addition of glutathione reductase (GR) and H2O2 to the samples. Following a 30-min incubation with 0.15 unit/mL GR, the GSSG peak was decreased by 90%, whereas a 30-min incubation with 1 mM H2O2 resulted in a doubling of the GSSG peak (data not shown). The absolute ratios of tissue GSH/GSSG were consistent with previously reported values measured by enzymatic recycling methods (Sian et al. 1997).
Measurement of Reduced CoA (CoASH) and its GSH disulfide (CoASSG)
CoASH and CoASSG were determined by the HPLC method described previously by Rogers et al. (2000). Frozen hippocampus (50 mg) was placed in 200 µL 0.1 M sodium phosphate and 28.5 mM N-ethylmaleimide (NEM) buffer (pH 7.4) and sonicated immediately prior to thawing. The tissue was acidified with equal volumes of 4% HClO4 and centrifuged at 12 000 g at 4°C for 20 min. Aliquots of supernatant were separated on a Zorbax SB-C18 column using the following mobile phases: (A) 10% methanol and 10 mM tetrabutylammonium hydrogen sulfate (pH 5.0), (B) 85% methanol and 10 mM tetrabutylammonium hydrogen sulfate (pH 5.0). The gradient was 5 min at 100% A, 0% B, followed by a 30 min linear gradient to 40% A and 60% B. Peak height was quantified by UV detection at 254 nm. Briefly, the CoASSG standard was prepared by GSH (100 nmol) and CoASH (100 nmol) dissolved in 0.1 M sodium phosphate buffer, pH 7.4. Diamide (100 nmol) was added to a final volume of 1 mL followed by the addition of NEM (1000 nmol) and 10 µL H3PO4. The mixture was analyzed by HPLC. The retention time of the CoASSG peak preceded the CoASH-NEM peak by 2 min.
Results
Spontaneous behavioral seizures
A time-dependent increase in seizure severity and duration was observed in a representative subgroup of rats following Li-Pilo-induced SE, with the first spontaneous motor seizure noted by 3 weeks (Fig. 1). By 9 weeks following SE all observed rats were having spontaneous seizures characterized by bi-lateral clonus and loss of balance [P5 seizures based on a modified Racine scale (Racine 1972)]. Approximately 85% of Li-Pilo-treated rats were confirmed to have recurrent and spontaneous motor seizures and were included in the study. Saline-treated controls did not show any spontaneous seizure activity. It is likely that unobserved electrographic and/or behavioral seizures occurred prior to 3 weeks. Seizure frequency was not reported based on the need for 24/7 video/EEG monitoring to accurately quantify this parameter.
Mitochondrial oxidative stress during epileptogenesis
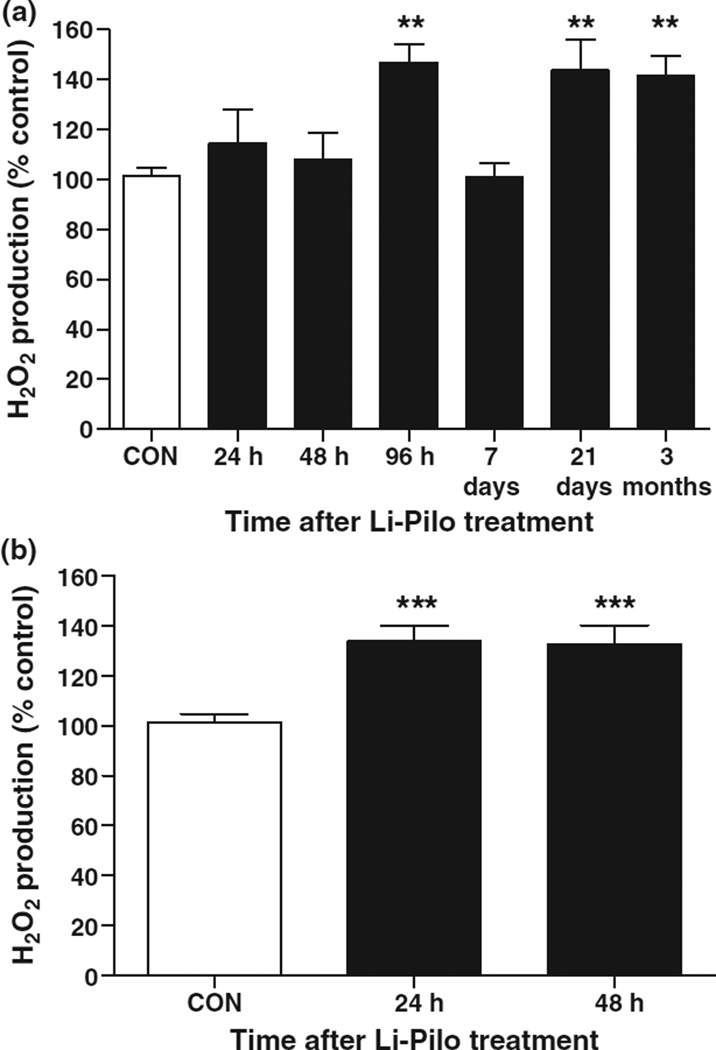
Oxidative stress was evaluated by measuring steady-state levels of H2O2 production in isolated hippocampal mitochondria to investigate the mechanism underlying oxidative damage associated with epileptogenesis. Li-Pilo-induced SE produced an initial increase in mitochondrial H2O2 production in the hippocampus that peaked by 96 h and returned to control levels at 7 days, during the so-called ‘latent period,’ and then significantly increased again coincident with spontaneous seizures at 21 days and 3 months during chronic epilepsy (Fig. 2a). KA-induced SE has been demonstrated to result in higher acute increases in H2O2 production than observed here (Jarrett et al. 2008). As diazepam was used to attenuate motor seizures in the current study 90 min following SE, it was hypothesized that the relative lack of increased H2O2 production at 24–48 h was because of less severe and/or frequent seizure activity. Hence, steady-state levels of mitochondrial H2O2 in the hippocampus of rats not treated with diazepam were measured. Figure 2(b) illustrates that levels of H2O2 production increased significantly from controls at 24–48 h when seizures were permitted to continue unabated during SE suggesting the seizure dependence of increased mitochondrial oxidative stress.
Fig. 2.
Mitochondrial oxidative stress increased in a time-dependent manner following Li-Pilo-induced epileptogenesis. (a) Levels of steady-state mitochondrial H2O2 production from the hippocampus expressed as a percentage change compared to controls from 24 h to 3 months post-SE from rats receiving diazepam 90 min following the onset of SE. Bars represent mean ± SEM, n = 5–6 per time point. **p ≤ 0.01, one-way anova. (b) Levels of steady-state mitochondrial H2O2 production from the hippocampus up to 48 h post-SE in a subset of rats not receiving diazepam to attenuate motor seizures during SE. Note that mitochondrial H2O2 production increased significantly when seizures were allowed to continue unabated during SE. Bars represent mean ± SEM, n = 5 per time point, ***p < 0.001, one-way anova.
Redox status during epileptogenesis
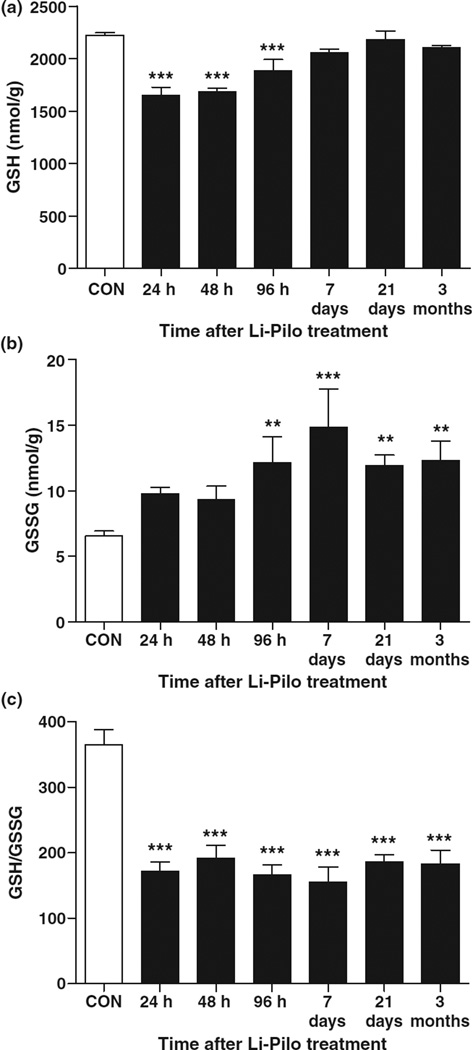
The measurement of two subcellular redox couples was conducted as an indication of hippocampal tissue and mitochondrial redox status and biomarker of oxidative stress during epileptogenesis (Reed and Savage 1995). GSH catalyzes the removal of H2O2 in cells and is the most abundant intracellular non-enzymatic oxidant defense in the body (Meister and Anderson 1983). GSH is recycled from GSSG, its disulfide redox partner, by GR and NADPH oxidation. Li-Pilo treated rats showed a significant deficiency in hippocampal tissue GSH until 96 h post-SE, and remained decreased throughout the period of epileptogenesis although not reaching statistical significance (Fig. 3a). GSSG levels markedly increased following Li-Pilo treatment, particularly at 96 h and throughout the period of epileptogenesis and chronic epilepsy (Fig. 3b). GSH/GSSG decreased throughout epileptogenesis and chronic epilepsy (Fig. 3c), suggesting that redox-dependent processes may contribute to the progression of epileptogenesis in experimental TLE.
Fig. 3.
Persistent alterations to redox status in the hippocampus throughout epileptogenesis. (a) GSH, (b) GSSG, and (c) GSH/GSSG from the hippocampus of rats at time points following Li-Pilo-induced SE versus saline-treated controls. Bars represent mean ± SEM. n = 5–6 per time point, **p < 0.01, ***p < 0.001, one-way anova.
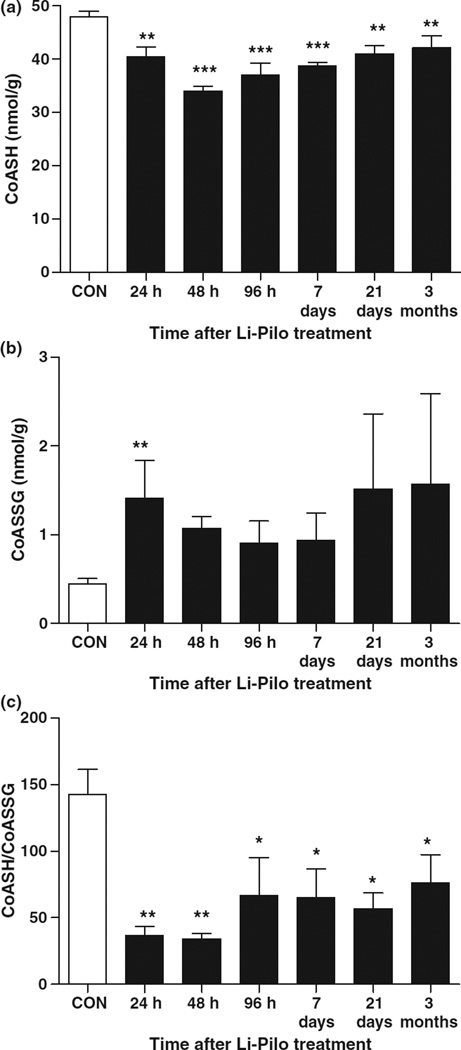
Although GSH/GSSG measurements are an important tool in the measurement of redox status and oxidative stress, non-specific GSH oxidation may occur during subcellular fractionation. This may be overcome by measurement of the thiol form of coenzyme A (CoASH) and its disulfide with GSH (CoASSG), which are primarily compartmentalized within mitochondria and exchange thiol/disulfide with GSH and GSSG (Wong et al. 2001; O’Donovan et al. 2002). Previous work in our laboratory comparing SE-induced alteration of GSH/GSSG from isolated hippocampal mitochondria with CoASH/CoASSG from whole hippocampal tissue suggests identical magnitude and time-course of the two redox couples (Liang and Patel 2006). Therefore, CoASH and CoASSG in tissue, which provides a reliable intramitochondrial redox status and overcomes artifactual changes in GSH and GSSG formation during subcellular fractionation were measured. It should be noted that tissue measurements can produce increased variability as seen at the 21 days and 3 months CoASSG time points (Fig. 4b), as well as an underestimation of changes. Following Li-Pilo treatment, CoASH levels and CoASH/CoASSG were significantly decreased as early as 24 h and persisted throughout epileptogenesis and chronic epilepsy (Fig. 4a and c) suggesting a role for mitochondrial specific oxidative stress and dysfunction in increasing seizure susceptibility and TLE onset.
Fig. 4.
Persistent alterations to mitochondrial specific redox status in the hippocampus throughout epileptogenesis. (a) CoASH, (b) CoASSG, and (c) CoASH/CoASSG in the hippocampus of rats at time points following Li-Pilo-induced SE versus saline-treated controls. Bars represent mean ± SEM. n = 5–6 per time point, *p < 0.05, **p < 0.01, ***p < 0.001, one-way anova.
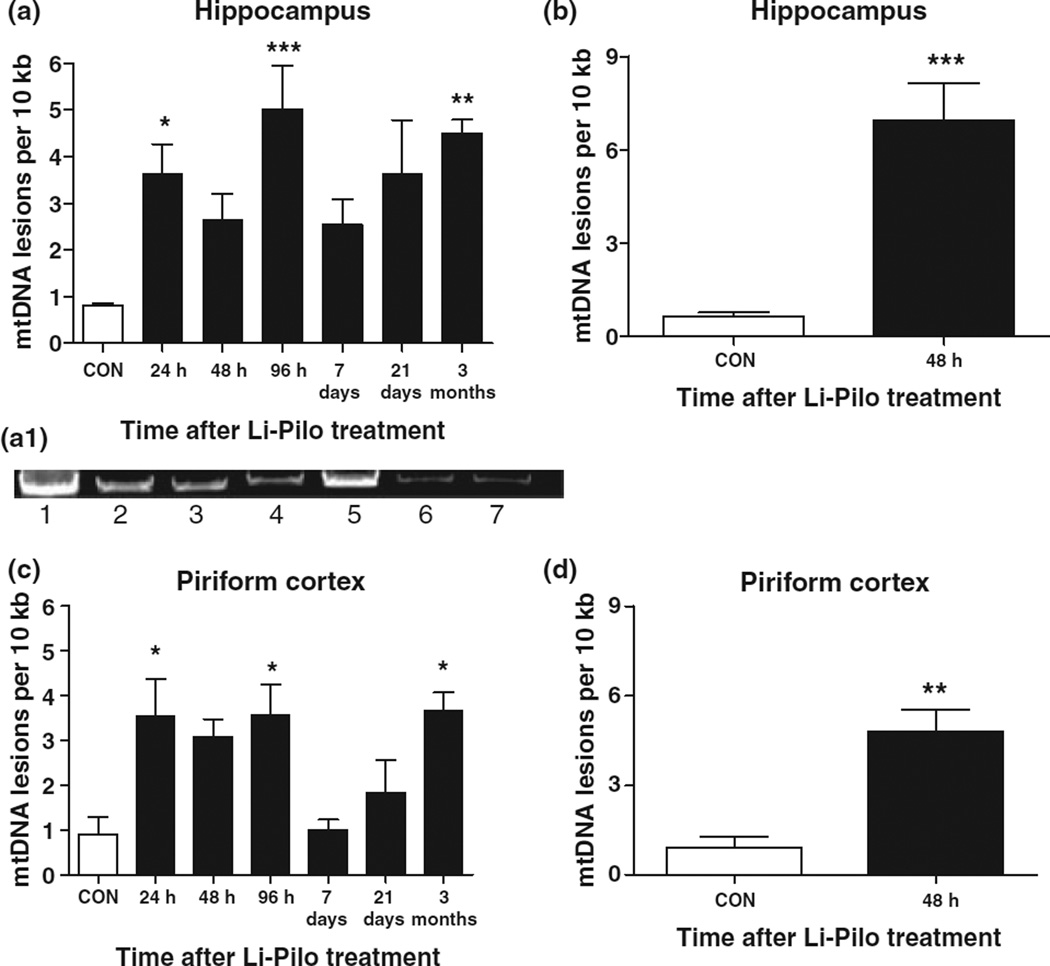
mtDNA lesion frequency during epileptogenesis
Mitochondrial oxidative stress can produce mtDNA lesions and damage. Using a sensitive QPCR assay specific to a 13.4 kb mitochondrial fragment, it was determined whether mtDNA damage occurred in the hippocampus and piriform cortex during Li-Pilo-induced epileptogenesis. Figure 5(a,a1) illustrates that mtDNA lesion frequency increased acutely in the hippocampus following Li-Pilo-induced SE, decreased during the so-called ‘latent period,’ and increased in a time-dependent manner thereafter through the period of chronic epilepsy. This biphasic increase in oxidative mtDNA damage as a function of time coincided with the biphasic increase in mitochondrial H2O2 production in the hippocampus (Fig. 2). In order to determine the seizure dependence of oxidative damage to mtDNA, a subset of rats were not treated with diazepam to allow motor seizures to continue during SE. Figure 5(b) indicates that mtDNA lesion frequency in the hippocampus increased significantly at 24 h (data not shown) and 48 h when seizures continued unabated. Because of the widespread effects of pilocarpine in the rodent brain, mtDNA lesion frequency was also determined in the piriform cortex during epileptogenesis (Fig. 5c) where it followed the same biphasic pattern as observed in the hippocampus but was not as severe. In a group of rats whose motor seizures during SE continued without diazepam treatment (Fig. 5d), mtDNA lesion frequency increased significantly at 24 h (data not shown) and 48 h.
Fig. 5.
mtDNA damage increased in a time-dependent manner during Li-Pilo-induced epileptogenesis. mtDNA lesion frequency determined using QPCR specific to a 13.4 kb mitochondrial fragment in the (a,a1) hippocampus and (c) piriform cortex during Li-Pilo-induced epileptogenesis in rats that received diazepam to attenuate SE. Panel (a1) is a representative agarose gel derived from QPCR analysis of hippocampal tissue after Lil-Pilo treatment. Lane 1, CON; lane 2, 24 h; lane 3, 48 h; lane 4, 96 h; lane 5, 7 days; lane 6, 21 days; lane 7, 3 months. mtDNA lesion frequency at 48 h from rats that did not receive diazepam are shown in the (b) hippocampus and (d) piriform cortex. Note that lesion frequency increased significantly at 48 h when motor seizures were allowed to continue during SE without diazepam treatment. Bars represent mean ± SEM, n = 5–10. *p < 0.05, **p < 0.01, ***p < 0.001, one-way anova.
Discussion
Summary
This study demonstrated that tissue and mitochondrial redox status in the hippocampus is persistently altered throughout Li-Pilo-induced epileptogenesis and provided evidence supporting the role of mitochondrial oxidative stress and mtDNA damage in the onset of TLE. Specifically, Li-Pilo treated rats showed (i) a biphasic increase in steady-state levels of mitochondrial H2O2 as a function of time during epileptogenesis contingent on the severity of SE, (ii) persistent alterations to GSH/GSSG and CoASH/CoASSG throughout epileptogenesis, and (iii) increased mtDNA lesion frequency that coincided with mitochondrial oxidative stress production in the brain. Together, these findings suggest that mitochondrial oxidative stress, mtDNA damage, and persistently altered redox status are active during epileptogenesis in the Li-Pilo animal model, and may contribute to the progression of TLE.
Behavioral seizures and mitochondrial oxidative stress during Li-Pilo-induced epileptogenesis
Motor seizure severity and duration progressively increased following Li-Pilo-induced SE with the first spontaneous seizure noted by 3 weeks. It is likely that electrographic and convulsive seizure events went undetected based on the observation protocol, particularly as non-convulsive electrographic seizures are known to precede the first convulsive motor seizure (Bertram and Cornett 1993, 1994). However, the results presented here confirm the presence of a ‘latent period’ and the typical increase in seizure frequency and duration consistent with the KA model and other experimental and human studies (French et al. 1993; Spencer and Spencer 1994; Hopkins and Shorvon 1995; Hellier et al. 1998; Jarrett et al. 2008; Williams et al. 2009) indicating the progressive nature of TLE (Engel et al. 1989; French et al. 1993). By observing and killing animals at varying time points throughout epileptogenesis, and not only acutely following SE treatment, the present study demonstrated oxidative mechanisms which may contribute to TLE onset and not merely be a consequence of chemoconvulsant treatment and/or SE.
Intramitochondrial O2•− flux can be measured in isolated mitochondria following spontaneous or enzymatic dismutation to H2O2. The Amplex Red assay is a reliable measure of steady-state levels of H2O2 production, however, a number of factors may lead to an underestimation of its accurate concentration and levels of mitochondrial oxidative stress. First, the Amplex Red assay detects extramitochondrial levels of H2O2 which have been attenuated by diffusion through the mitochondrial membranes making it susceptible to degradation and scavenging by endogenous antioxidants such as GSH and peroxidases. Also, not all O2•− is converted to H2O2 and can alternatively react with other electron acceptors, form ONOO− in the presence of nitric oxide, and hydroxyl radical (OH•) through Fenton chemistry. Thus, there is a threshold of intramitochondrial O2•− formation that must be achieved before extramitochondrial measurement of H2O2 as a marker of mitochondrial oxidative stress can be made (Murphy 2009), and this may result in an underestimation of mitochondrial oxidative stress.
ROS function as second messengers in signal transduction but are also mediators of oxidative stress, damage, and inflammation. Seizure-induced overproduction of O2•− by the mitochondrial ETC (Liang et al. 2000) or extracellular sources such as the microglial NOX2 (Gao et al. 2003; Wu et al. 2003) can lead to the generation of more highly reactive species which may alter critical proteins involved in controlling neuronal excitability making them more susceptible to degradation by intracellular proteolytic systems and thereby increasing seizure susceptibility during epileptogenesis. While many of the acute oxidative consequences of seizures on cellular constituents have been demonstrated (Bruce and Baudry 1995; Gluck et al. 2000; Liang et al. 2000; Patel et al. 2001; Tejada et al. 2007), the role of oxidative stress and mitochondrial dysfunction during epileptogenesis and as a contributor to TLE has been relatively unexplored. This study supports findings that mitochondrial oxidative stress and dysfunction are active during epileptogenesis and may contribute to the progression of chronic epilepsy (Patel 2004; Jarrett et al. 2008). In comparison with KA-induced epileptogenesis (Jarrett et al. 2008), Li-Pilo-induced epileptogenesis in this study resulted in a slower onset and lower magnitude of mitochondrial H2O2 production. The demonstration that removing diazepam treatment following SE markedly increased Li-Pilo-induced mitochondrial H2O2 production is sufficient to explain the slower onset of mitochondrial oxidative stress. The direct activation of glutamate receptors and resultant excitotoxicity produced by KA treatment may result in a higher magnitude of H2O2 production compared to Li-Pilo-induced cholinergic mechanisms. The overall pattern of increased mitochondrial oxidative stress, however, was similar between the two models. It is possible that levels of H2O2 production decreased at 7 days in both models because of an increase in neuronal death, however, the majority of neuronal death is thought to occur prior to that time point and measured redox couples in the present study were not similarly affected at 7 days.
Persistent alterations to redox status during epileptogenesis
An alternative to the measurement of oxidized cellular targets as markers of oxidative stress is to directly measure a subcellular redox couple as a sensitive index of redox status. GSH and CoASH have been demonstrated to decrease acutely following KA treatment (Skaper et al. 1999; Gilberti and Trombetta 2000; Ong et al. 2000; Liang and Patel 2006), but there is a lack of evidence demonstrating their levels during epileptogenesis and as a contributor to TLE. Hippocampal tissue GSH/GSSG following Li-Pilo-induced SE demonstrated in the present study decreased by 24 h and remained decreased throughout epileptogenesis. Similar experiments from the KA model demonstrated a significant decrease in hippocampal tissue GSH/GSSG only at 48 h in a 7-day study, while mitochondrial GSH/GSSG showed more dramatic and persistent decreases (Liang and Patel 2006). Also, CoASH and CoASH/CoASSG decreased in the KA model as early as 8 h and remained altered up until 7 days. Thus, redox status seems to be altered independent of animal models, although the effects of KA-induced epileptogenesis produced a profound and rather selective impairment of the mitochondrial redox status, while Li-Pilo-induced epileptogenesis resulted in persistent impairment of both mitochondrial and tissue redox status.
Measurement of intramitochondrial GSH and GSSG is complicated by in situ oxidation of GSH and disulfide exchange reactions occurring during the isolation procedure. This limitation is overcome by measurement of the thiol form of CoASH and its disulfide with GSH, CoASSG, which are primarily compartmentalized within mitochondria and exchange thiol/disulfide with GSH and GSSG (Wong et al. 2001; O’Donovan et al. 2002). The finding presented here that CoASH/CoASSG was persistently altered throughout epileptogenesis is consistent with the notion that mitochondria contribute disproportionately to SE-induced oxidative stress. Also, it was demonstrated that GSH/GSSG and CoASH/CoASSG were both decreased even when measurements of H2O2 production and mtDNA returned to control levels. Additionally, the changes in redox state during epileptogenesis observed here began prior to the reported occurrence of neuronal death in the hippocampus (Liang et al. 2000) and may contribute to it. Neuronal loss that occurs beyond the initial acute period following Li-Pilo-induced epileptogenesis may result in decreased GSH or CoASH levels, however, it cannot account for increased GSSG and CoASSG levels. It is possible that surviving neurons and/or other cell types such as astrocytes could contribute to the observed redox changes. Astrocytes may be an important source of the H2O2 and redox imbalance observed during pilocarpine-induced epileptogenesis given that non-synaptic mitochondria used in our studies originate largely from glial cells (Rendon and Masmoudi 1985; Brown et al. 2006). These persistent alterations to mitochondrial redox status during epileptogenesis could cause significant mitochondrial dysfunction (Jain et al. 1991; Werner and Cohen 1993) affecting neuronal excitability through ETC dysfunction and decreased ATP production. Thus, the early onset and persistent decrease in mitochondrial redox status in the hippocampus suggests its ongoing contribution to epileptogenesis.
mtDNA damage during epileptogenesis
Epileptic seizures are present in several mtDNA disorders arising from mutations and stochastic mtDNA injury has been associated with acquired epilepsy (Wallace et al. 1992, 1994; Cock et al. 1999; Deschauer et al. 2003; Horvath et al. 2006). mtDNA lack protective histones, is located in close proximity to the inner mitochondrial membrane where ROS are generated, lacks introns, has a high transcription rate leading to an increased probability of mutations and/or deletions, and possesses less efficient repair mechanisms then nDNA under certain circumstances (Yakes and Van Houten 1997; Jarrett et al. 2008) making the mitochondrial genome particularly vulnerable to ROS-induced damage (Bohr et al., 2002). Increased production of ROS causing mtDNA damage could lead to decreased activities of mitochondrial ETC complexes containing mtDNA-encoded subunits and selectively diminishing ATP production. This study demonstrates an initial rise in mtDNA damage, a return to control levels during the so-called ‘latent period,’ and re-emergence of mtDNA damage coincident with the onset of spontaneous seizures confirming previous observations that mitochondrial dysfunction occurs during chronic epilepsy (Kunz et al. 2000; Kudin et al. 2002; Kann et al. 2005; Jarrett et al. 2008). The increase in mtDNA lesion frequency largely coincided with mitochondrial H2O2 production, suggesting that mitochondrial oxidative stress is contributing to mtDNA damage. Although a similar pattern of mtDNA lesion frequency was observed in the hippocampus and piriform cortex, the damage was more severe in the former possibly because of relatively increased focal seizure activity. When motor seizures were allowed to continue during SE without diazepam treatment a significant increase in mtDNA lesion frequency occurred up to 48 h suggesting the seizure dependence of oxidative-induced mtDNA damage. It is possible that the apparent lack of mtDNA damage at 7 days is because of increased repair mechanisms that have been demonstrated following SE (Jarrett et al. 2008). It has not been determined whether and to what extent oxidative mtDNA damage may increase seizure susceptibility but it is conceivable that alterations in ATP production and increases in neuronal excitability through mechanisms such as altered Na+/K+ ATPase could be active during epileptogenesis. The seizure-induced accumulation of oxidative mtDNA lesions and resultant somatic mtDNA mutations during epileptogenesis could, over time, render the brain more susceptible to subsequent seizures in TLE.
Conclusions
The findings of the present study suggest that persistent impairment of mitochondrial and tissue redox status during the ‘latent period’ and acute and chronic phases of epileptogenesis may contribute to the progression of experimental TLE. Mitochondrial H2O2 production and mtDNA damage showed similar increases during epileptogenesis which both decreased during the ‘latent period.’ However, mitochondrial and tissue redox status remained impaired throughout epileptogenesis including the ‘latent period’ suggesting an oxidized environment which could alter critical macro-molecules involved in neuronal excitability and seizure susceptibility via redox signaling. The present study suggests that mitochondrial oxidative stress and dysfunction are active mechanisms during epileptogenesis that if attenuated may contribute to decreasing seizure susceptibility in TLE and provide an alternative therapy for patients refractory to current anti-epileptic drugs.
Abbreviations used
- ETC
electron transport chain
- GR
glutathione reductase
- KA
kainic acid
- Li-Pilo
lithium-pilocarpine
- mtDNA
mitochondrial DNA
- NEM
N-ethylmaleimide
- QPCR
quantitative PCR
- ROS
reactive oxygen species
- SE
status epilepticus
- TLE
temporal lobe epilepsy
References
- Ayala-Torres S, Chen Y, Svoboda T, Rosenblatt J, Van Houten B. Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods. 2000;22:135–147. doi: 10.1006/meth.2000.1054. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Cell death and synaptic reorganizations produced by seizures. Epilepsia. 2001;42(Suppl 3):5–7. doi: 10.1046/j.1528-1157.2001.042suppl.3005.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Cornett J. The ontogeny of seizures in a rat model of limbic epilepsy: evidence for a kindling process in the development of chronic spontaneous seizures. Brain Res. 1993;625:295–300. doi: 10.1016/0006-8993(93)91071-y. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Cornett JF. The evolution of a rat model of chronic spontaneous limbic seizures. Brain Res. 1994;661:157–162. doi: 10.1016/0006-8993(94)91192-4. [DOI] [PubMed] [Google Scholar]
- Bohr VA, Stevnsner T, de Souza-Pinto NC. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286:127–134. doi: 10.1016/s0378-1119(01)00813-7. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J. Biol. Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Radic. Biol. Med. 1995;18:993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J. Biol. Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Sun AY. Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem. Res. 1994;19:1557–1564. doi: 10.1007/BF00969006. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Chang AY, Lin JW, Hsu SP, Chan SH. Mitochondrial dysfunction and ultrastructural damage in the hippocampus during kainic acid-induced status epilepticus in the rat. Epilepsia. 2004;45:1202–1209. doi: 10.1111/j.0013-9580.2004.18204.x. [DOI] [PubMed] [Google Scholar]
- Cock HR, Cooper JM, Schapira AH. Functional consequences of the 3460-bp mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. J. Neurol. Sci. 1999;165:10–17. doi: 10.1016/s0022-510x(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Cock HR, Tong X, Hargreaves IP, et al. Mitochondrial dysfunction associated with neuronal death following status epilepticus in rat. Epilepsy Res. 2002;48:157–168. doi: 10.1016/s0920-1211(01)00334-5. [DOI] [PubMed] [Google Scholar]
- Deschauer M, Bamberg C, Claus D, Zierz S, Turnbull DM, Taylor RW. Late-onset encephalopathy associated with a C11777A mutation of mitochondrial DNA. Neurology. 2003;60:1357–1359. doi: 10.1212/01.wnl.0000055869.99975.4b. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J. Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Babb TL, Crandall PH. Surgical treatment of epilepsy: opportunities for research into basic mechanisms of human brain function. Acta Neurochir. Suppl. (Wien) 1989;46:3–8. doi: 10.1007/978-3-7091-9029-6_1. [DOI] [PubMed] [Google Scholar]
- Erakovic V, Zupan G, Varljen J, Laginja J, Simonic A. Lithium plus pilocarpine induced status epilepticus-biochemical changes. Neurosci. Res. 2000;36:157–166. doi: 10.1016/s0168-0102(99)00120-0. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Velazquez JL, Hwang PA, Carlen PL. Free radical production correlates with cell death in an in vitro model of epilepsy. Eur. J. Neurosci. 2000;12:1431–1439. doi: 10.1046/j.1460-9568.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann. Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Gao J, Chi ZF, Liu XW, Shan PY, Wang R. Mitochondrial dysfunction and ultrastructural damage in the hippocampus of pilocarpine-induced epileptic rat. Neurosci. Lett. 2007;411:152–157. doi: 10.1016/j.neulet.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Gilberti EA, Trombetta LD. The relationship between stress protein induction and the oxidative defense system in the rat hippocampus following kainic acid administration. Toxicol. Lett. 2000;116:17–26. doi: 10.1016/s0378-4274(00)00197-1. [DOI] [PubMed] [Google Scholar]
- Gluck MR, Jayatilleke E, Shaw S, Rowan AJ, Haroutunian V. CNS oxidative stress associated with the kainic acid rodent model of experimental epilepsy. Epilepsy Res. 2000;39:63–71. doi: 10.1016/s0920-1211(99)00111-4. [DOI] [PubMed] [Google Scholar]
- Griffiths T, Evans MC, Meldrum BS. Status epilepticus: the reversibility of calcium loading and acute neuronal pathological changes in the rat hippocampus. Neuroscience. 1984;12:557–567. doi: 10.1016/0306-4522(84)90073-3. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Hinterkeuser S, Schroder W, Hager G, Seifert G, Blumcke I, Elger CE, Schramm J, Steinhauser C. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur. J. Neurosci. 2000;12:2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Honchar MP, Olney JW, Sherman WR. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983;220:323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- Hopkins A, Shorvon S. Computed tomography in first uncomplicated generalised seizure. Is grossly oversold. BMJ. 1995;310:1007–1008. doi: 10.1136/bmj.310.6985.1007c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R, Hudson G, Ferrari G, et al. Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain. 2006;129:1674–1684. doi: 10.1093/brain/awl088. [DOI] [PubMed] [Google Scholar]
- Jain A, Martensson J, Stole E, Auld PA, Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc. Natl Acad. Sci. USA. 1991;88:1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Boutlon ME. Antioxidant up-regulation and increased nuclear DNA protection play key roles in adaptation to oxidative stress in epithelial cells. Free Radic. Biol. Med. 2005;38:1382–1391. doi: 10.1016/j.freeradbiomed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Jarrett SG, Liang LP, Hellier JL, Staley KJ, Patel M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol Dis. 2008;30:130–138. doi: 10.1016/j.nbd.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O, Kovacs R, Njunting M, Behrens CJ, Otahal J, Lehmann TN, Gabriel S, Heinemann U. Metabolic dysfunction during neuronal activation in the ex vivo hippocampus from chronic epileptic rats and humans. Brain. 2005;128:2396–2407. doi: 10.1093/brain/awh568. [DOI] [PubMed] [Google Scholar]
- Kudin AP, Kudina TA, Seyfried J, Vielhaber S, Beck H, Elger CE, Kunz WS. Seizure-dependent modulation of mitochondrial oxidative phosphorylation in rat hippocampus. Eur. J. Neurosci. 2002;15:1105–1114. doi: 10.1046/j.1460-9568.2002.01947.x. [DOI] [PubMed] [Google Scholar]
- Kunz WS, Goussakov IV, Beck H, Elger CE. Altered mitochondrial oxidative phosphorylation in hippocampal slices of kainite-treated rats. Brain Res. 1999;826:236–242. doi: 10.1016/s0006-8993(99)01279-2. [DOI] [PubMed] [Google Scholar]
- Kunz WS, Kudin AP, Vielhaber S, Blumcke I, Zuschratter W, Schramm J, Beck H, Elger CE. Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann. Neurol. 2000;48:766–773. [PubMed] [Google Scholar]
- Lakritz J, Plopper CG, Buckpitt AR. Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue samples. Anal. Biochem. 1997;247:63–68. doi: 10.1006/abio.1997.2032. [DOI] [PubMed] [Google Scholar]
- Lehmann TN, Gabriel S, Eilers A, Njunting M, Kovacs R, Schulze K, Lanksch WR, Heinemann U. Fluorescent tracer in pilocarpine-treated rats shows widespread aberrant hippocampal neuronal connectivity. Eur. J. Neurosci. 2001;14:83–95. doi: 10.1046/j.0953-816x.2001.01632.x. [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M. Mitochondrial oxidative stress and increased seizure susceptibility in Sod2(−/+) mice. Free Radic. Biol. Med. 2004;36:542–554. doi: 10.1016/j.freeradbiomed.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M. Seizure-induced changes in mitochondrial redox status. Free Radic. Biol. Med. 2006;40:316–322. doi: 10.1016/j.freeradbiomed.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainite-induced hippocampal damage. Neuroscience. 2000;101:563–570. doi: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- Liang LP, Huang J, Fulton R, Day BJ, Patel M. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine neurotoxicity in vivo. J. Neurosci. 2007;27:4326–4333. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho MM, de Sousa FC, de Bruin VM, Vale MR, Viana GS. Effects of lithium, alone or associated with pilocarpine, on muscarinic and dopaminergic receptors and on phosphoinositide metabolism in rat hippocampus and striatum. Neurochem. Int. 1998;33:299–306. doi: 10.1016/s0197-0186(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Gupta RC, Dettbarn WD. Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Res. 2002;957:330–337. doi: 10.1016/s0006-8993(02)03669-7. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan DJ, Rogers LK, Kelley DK, Welty SE, Ramsay PL, Smith CV. CoASH and CoASSG levels in lungs of hyperoxic rats as potential biomarkers of intramitochondrial oxidant stresses. Pediatr. Res. 2002;51:346–353. doi: 10.1203/00006450-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Ong WY, Hu CY, Hjelle OP, Ottersen OP, Halliwell B. Changes in glutathione in the hippocampus of rats injected with kainate: depletion in neurons and upregulation in glia. Exp. Brain Res. 2000;132:510–516. doi: 10.1007/s002210000347. [DOI] [PubMed] [Google Scholar]
- Pan JW, Williamson A, Cavus I, Hetherington HP, Zaveri H, Petroff OA, Spencer DD. Neurometabolism in human epilepsy. Epilepsia. 2008;49(Suppl 3):31–41. doi: 10.1111/j.1528-1167.2008.01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic. Biol. Med. 2004;37:1951–1962. doi: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16:345–355. doi: 10.1016/s0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- Patel M, Liang LP, Roberts LJ., 2nd Enhanced hippocampal F2-isoprostane formation following kainite-induced seizures. J. Neurochem. 2001;79:1065–1069. doi: 10.1046/j.1471-4159.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- Patel M, Liang LP, Hou H, Williams BB, Kmiec M, Swartz HM, Fessel JP, Roberts LJ., 2nd Seizure-induced formation of isofurans: novel products of lipid peroxidation whose formation is positively modulated by oxygen tension. J. Neurochem. 2008;104:264–270. doi: 10.1111/j.1471-4159.2007.04974.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. I After-discharge threshold. Electroencephalogr. Clin. Neurophysiol. 1972;32:269–279. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- Reed DJ, Savage MK. Influence of metabolic inhibitors on mitochondrial permeability transition and glutathione status. Biochim. Biophys. Acta. 1995;1271:43–50. doi: 10.1016/0925-4439(95)00008-r. [DOI] [PubMed] [Google Scholar]
- Rendon A, Masmoudi A. Purification of non-synaptic and synaptic mitochondria and plasma membranes from rat brain by a rapid Percoll gradient procedure. J. Neurosci. Methods. 1985;14:41–51. doi: 10.1016/0165-0270(85)90113-x. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EM, Doorn JA, Slikker W. Nitrative and oxidative stress in toxicology and disease. Toxicol. Sci. 2009;112:4–16. doi: 10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LK, Valentine CJ, Szczpyka M, Smith CV. Effects of hepatotoxic doses of acetaminophen and furosemide on tissue concentrations of CoASH and CoASSG in vivo. Chem. Res. Toxicol. 2000;13:873–882. doi: 10.1021/tx0000926. [DOI] [PubMed] [Google Scholar]
- Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc. Natl Acad. Sci. USA. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Cohen G, Jenner PG, Marsden CD. Comparison of HPLC and enzymatic recycling assays for the measurement of oxidized glutathione in rat brain. J. Pharm. Pharmacol. 1997;49:332–335. doi: 10.1111/j.2042-7158.1997.tb06807.x. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Floreani M, Ceccon M, Facci L, Giusti P. Excitotoxicity, oxidative stress, and the neuroprotective potential of melatonin. Ann. N Y Acad. Sci. 1999;890:107–118. doi: 10.1111/j.1749-6632.1999.tb07985.x. [DOI] [PubMed] [Google Scholar]
- Sleven H, Gibbs JE, Heales S, Thom M, Cock HR. Depletion of reduced glutathione precedes inactivation of mitochondrial enzymes following limbic status epilepticus in the rat hippocampus. Neurochem. Int. 2006;48:75–82. doi: 10.1016/j.neuint.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Tejada S, Sureda A, Roca C, Gamundi A, Esteban S. Antioxidant response and oxidative damage in brain cortex after high dose of pilocarpine. Brain Res. Bull. 2007;71:372–375. doi: 10.1016/j.brainresbull.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Victor VM, Espulgues JV, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect. Disord. Drug Targets. 2009;9:376–389. doi: 10.2174/187152609788922519. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Lott MT, Shoffner JM, Brown MD. Diseases resulting from mitochondrial DNA point mutations. J. Inherit. Metab. Dis. 1992;15:472–479. doi: 10.1007/BF01799605. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Lott MT, Shoffner JM, Ballinger S. Mitochondrial DNA mutations in epilepsy and neurological disease. Epilepsia. 1994;35(Suppl 1):S43–S50. doi: 10.1111/j.1528-1157.1994.tb05928.x. [DOI] [PubMed] [Google Scholar]
- Werner P, Cohen G. Glutathione disulfide (GSSG) as a marker of oxidative injury to brain mitochondria. Ann. N Y Acad. Sci. 1993;679:364–369. doi: 10.1111/j.1749-6632.1993.tb18323.x. [DOI] [PubMed] [Google Scholar]
- Williams MB, Jope RS. Protein synthesis inhibitors attenuate seizures induced in rats by lithium plus pilocarpine. Exp. Neurol. 1994;129:169–173. doi: 10.1006/exnr.1994.1158. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J. Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YL, Smith CV, McMicken HW, Rogers LK, Welty SE. Mitochondrial thiol status in the liver is altered by exposure to hyperoxia. Toxicol. Lett. 2001;123:179–193. doi: 10.1016/s0378-4274(01)00397-6. [DOI] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl Acad. Sci. USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl Acad. Sci. USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]