Abstract
Heart diseases can affect intraventricular blood flow patterns. Real-time imaging of blood flow patterns is challenging because it requires both a high frame rate and a large field of view. To date, standard Doppler techniques can only perform blood flow estimation with high temporal resolution within small regions of interest. In this work, we used ultrafast imaging to map in 2D human left ventricular blood flow patterns during the whole cardiac cycle. Cylindrical waves were transmitted at 4800 Hz with a transthoracic phased array probe to achieve ultrafast Doppler imaging of the left ventricle. The high spatio-temporal sampling of ultrafast imaging permits to rely on a much more effective wall filtering and to increase sensitivity when mapping blood flow patterns during the pre-ejection, ejection, early diastole, diastasis and late diastole phases of the heart cycle. The superior sensitivity and temporal resolution of ultrafast Doppler imaging makes it a promising tool for the noninvasive study of intraventricular hemodynamic function.
Keywords: Ultrafast imaging, Ultrafast Doppler, Heart, Blood flow, Left ventricle, cylindrical waves
I. Introduction
Major cardiovascular diseases including congestive heart failure, coronary artery disease, hypertension and cardiomyopathies are associated with left ventricular dysfunction [1]. The accurate assessment of ventricular function during all phases of the heart cycle is therefore a central issue in cardiac imaging. Since its introduction in the late 1970s, Doppler echocardiography [2, 3] has emerged as an important tool for the noninvasive hemodynamic characterization of the heart and the management of cardiac patients. Intracardiac blood flow, pressures and pressure gradients can be derived from Doppler examinations to assess systolic and diastolic performance, as well as valve function. Overall, Doppler Echocardiography provided new insights in the pathophysiology of myocardial diseases [4–7] and contributes to clinical decision making [8].
Conventional Doppler echocardiography relies on line per line focused beam transmissions to scan the medium of interest [2]. Transthoracically, the left ventricle is typically imaged at a rate of 20 to 100 frames per second, which leads to a tradeoff between the field of view and the temporal resolution achievable. Color flow imaging (CFI) has been introduced in clinical practice to map blood flow in a large region of interest but is currently limited by its low frame rate and angle dependency, while pulse wave (PW) Doppler relies on a much higher number of time samples along a single line to characterize local hemodynamics. The evaluation of left ventricular blood flow pattern is currently limited given the need for the practitioner to choose for one of these two Doppler modalities.
An alternative technique is contrast-enhanced echocardiography, mostly used for left ventricle endocardial border detection in patients with suboptimal echocardiograms [10], or to assess myocardial perfusion [11]. It was shown that ultrasound contrast agents can be used as reporters of intraventricular blood motion using particle imaging velocimetry [12]. Yet, the U.S. Food and Drug Administration only approves ultrasound contrast perfusion for left ventricular opacification and contrast-enhanced ultrasound strategies disrupt the noninvasive character of echocardiography.
Recently, the introduction of ultrafast scanners has offered new insight in medical ultrasonic imaging [13–19]. Ultrafast plane wave imaging allows for the acquisition of hundreds of temporal samples per second over a large field of view, therefore breaking the usual tradeoff between field of view and temporal resolution. The high spatio-temporal resolution of ultrafast ultrasound acquisitions offers the possibility to image and quantify blood flow simultaneously over a larger region of interest, hence providing more accurate hemodynamic information. This ultrasound imaging approach gathering the advantages of CFI and PW Doppler in a single mode was introduced under the terminology Ultrafast Doppler Imaging [20–21].
Here we report the implementation of Ultrafast Doppler Imaging (UFD) on a transthoracic phased-array probe using cylindrical waves [22–26], and present its application to the characterization of human left ventricular hemodynamics. We provide a detailed temporal analysis of all phases of the heart cycle (ejection phase, early diastole, diastasis, late-diastole, pre-ejection phase) by processing the UFD data in terms of both CFI and PW Doppler. Results demonstrate the capacity of UFD to describe intraventricular transient flows with a millisecond resolution, in agreement with earlier findings [20, 27–29]. UFD provides an enhanced characterization of left ventricular hemodynamic function without the use of ultrasound contrast agents.
II. Methods
A. Experimental setup
We made use of an ultrasound phased array probe (2.75MHz, 60% Bandwidth, 64 elements, Vermon, Tours, France) connected to an ultrafast scanner (Aixplorer, SuperSonic Imagine, Aix-en-provence, France). Data were acquired on a 27 year old healthy male volunteer who displayed a normal echocardiogram and did not report any cardiac history. To show the robustness of UFD, a second set of data was acquired on a patient with poor echogenicity. The patient was a 62 year old male with ischemic cardiomyopathy and left ventricular apical akinesis. An experienced cardiologist held the probe against the patient’s chest in order to obtain a 4 chamber apical view of the left ventricle. The patient’s heart cycle was monitored using an electrocardiography (ECG) device. The ultrasound acquisitions were triggered on the R-wave of the ECG signal.
The study was approved by the Comité de Protection des Personnes (CPP) and the national agency for health (ANSM); authorization n°2009-A01324-53. The subject was informed of the nature and aims of the study and signed a consent form.
B. Ultrafast cylindrical wave imaging
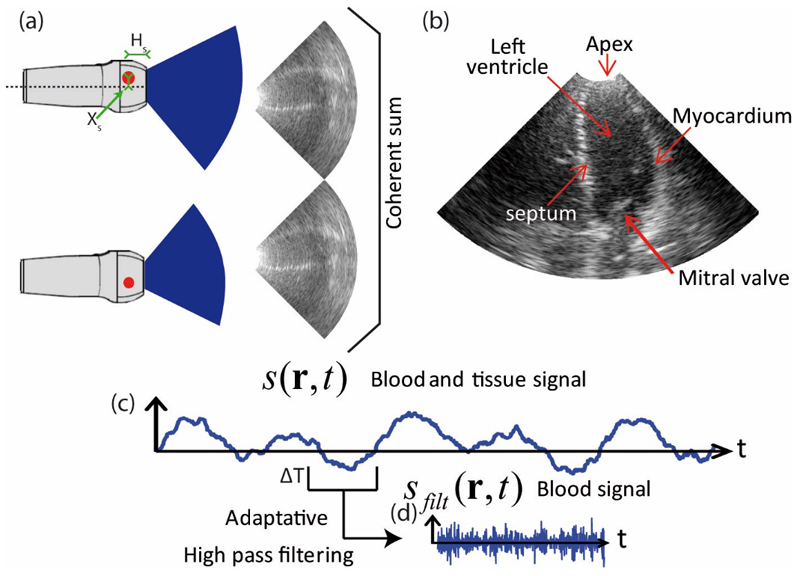
In order to image the heart with a high frame rate over a large field of view, we used cylindrical (diverging) waves emerging from a virtual source placed behind the probe [26]. We used spatial compounding to improve the resolution of Bmode images. Figure 1.a illustrates spatial compounding with two virtual sources generating two cylindrical wave images. Each image has individually a poor quality in term of contrast and resolution, while their coherent sum provides an ultrasound image of superior quality (see figure 1.b).
Figure 1.
a) Two cylindrical wave ultrasound images of the left ventricle arising from two different virtual sources. b) Enhanced ultrasound image of the left ventricle resulting from the spatial compounding of the pair of cylindrical wave images. c) Time signal of a spatial pixel containing blood and tissue signal. d) Blood signal of a time window obtained after wall filtering (ΔT=25ms).
Ultrafast ultrasound imaging presents the advantage of being tunable, as the operator can adjust the tradeoff between ultrasound image quality and frame rate for a given investigation depth. In this manuscript, two different imaging sequences were used: one for UFD and one for B-Mode imaging. For UFD imaging, the heart was insonified at a 4800 Hz frame rate during 1.5s with cylindrical waves arising from a single virtual source position. Subsequently, the heart was insonified for ultrafast Bmode imaging during 1.5s with 21 virtual sources, leading to a frame rate of 229 Hz after spatial compounding. Both sequences had the same pulse repetition frequency (4800Hz) but the first UFD sequence, using a single virtual source position, was designed to maximize frame rate, whereas the second sequence dedicated to Bmode imaging was designed to maximize imaging quality via spatial compounding.
C. Wall filtering for UFD sequence
CFI was computed with the dataset of the first (high frame rate) UDF sequence. For each spatial pixel r, we can compute a 7200 sample points temporal signal s(r,t) containing blood and tissue signal (see Figure 1c). For each time window of 120 sample points (ΔT=25ms) that we slide every 30 sample points (6.25ms), we applied a wall filter (high pass 4th order Butterworth) with an adaptive cut off frequency to remove the tissue signal (see figure 1d). The heart cycle was analyzed into 5 phases (see Results section). For the ejection phase and the early diastole phase, the cutoff frequency was set to 400Hz, while in the other phases, the cutoff frequency was set to 250Hz. PW Doppler spectra were computed along the whole heart cycle with a 400Hz cutoff frequency.
D. Color Flow Imaging processing
For each spatial pixel and for each time window of size ΔT we computed the Doppler frequency using the first moment of the power Doppler spectrum (see equation (1)). We obtained a set of 221 color Doppler images describing one heart cycle. We used the color mode convention: flow coming towards the probe was colored red and flow going downwards the probe was colored blue.
| (1) |
Spatio-temporal analysis of the blood flow
The transient transmitral flow propagation during early filling was analysed to show how spatial and temporal information can be combined using UFD. Flow propagation velocity has been investigated extensively with color M-mode to characterize the early filling of the left ventricle. Using UFD, the 2D acquisition allows us to compute the propagation velocity not only in the direction of an M-mode line, but also along an arbitrary direction in the 2D plane. The local axial acceleration field was computed by differentiating the mean velocity in each pixel of the map as a function of time. Then, the time of the acceleration peak during the early filling of the left ventricle was computed at each location. Finally, the direction of the flow propagation was determined as the direction of the lowest gradient of the timing map.
III. Results
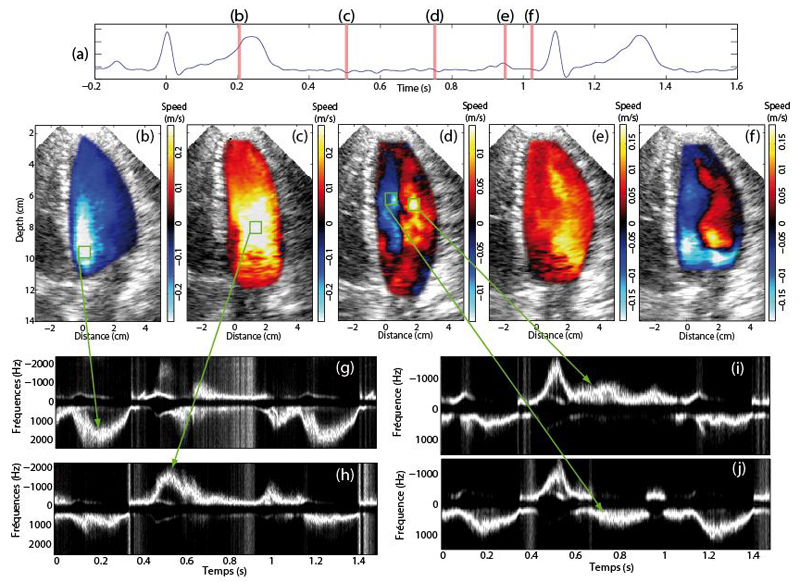
Color flow maps and PW Doppler spectrum of the healthy volunteer are shown on figure 2 for the main phases of the cardiac cycle [30]. During the ejection phase (Figure 2.b) the blood is expulsed in the aorta after the opening of the aortic valve and the mitral valve is closed, the blood is flowing downwards in the direction of the aorta. As ultrafast Doppler permits to use the same acquisition to compute a post-processed PW Doppler spectrum (see figure 3g). We noticed a high axial velocity (high frequency on the PW Doppler spectrum) of the blood approaching the aortic valve.
Figure 2.
Ultrafast Color flow images of the healthy volunteer displayed at several phases of the cardiac cycle. a) ECG signal. CFI of the left ventricle during b) the ejection phase, c) early diastole, d) diastasis e) late diastole and f) early systole. PW Doppler spectra were computed at four locations indicated by the green square boxes.
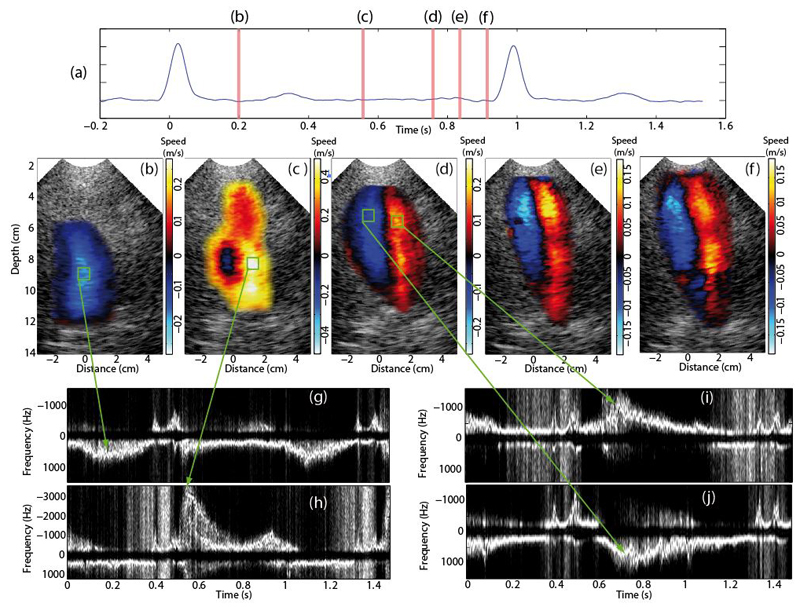
Figure 3.
Ultrafast Color flow images of the patient displayed at several phases of the cardiac cycle. a) ECG signal. CFI of the left ventricle during b) the ejection phase, c) early diastole, d) diastasis e) late diastole and f) early systole. PW Doppler spectra were computed at four locations indicated by the green square boxes.
Figure 2.c shows the early diastole phase that takes place after the closing of the aortic valve and the opening of the mitral valve. The first part of the filling of the left ventricle begins with a flow of high velocity in the direction of the apex due to low pressure inside the left ventricle. By computing a PW Doppler spectrum in a spatial window located at the maximum blood velocity of this phase (see figure 2.h), indicated that the maximum blood flow velocity during the diastole is reached during this phase. In addition, the magnitude of the transmitral flow velocity in early diastole was of the same order than the blood velocity through the aortic valve during the ejection phase.
In the diastasis phase, the filling of the left ventricle continues and a vortex starts to form. Figure 2.d shows flow distribution inside the entire left ventricle. The late diastole phase is observed just before the closure of the mitral valve and corresponds to the final part of the left ventricle filling. Fig. 2e shows a global motion of the blood flow towards the apex, the vortex has disappeared. We performed a standard evaluation of the mitral valve inflow and measured an E/A ratio of 1.4.
Finally, when the heart starts contracting, the mitral valve is closing and the aortic valve is still closed. This phase is called the pre-ejection phase. Figure 2f shows the apparition of a vortex which starts to re-direct the blood towards the aortic valve to prepare for the ejection phase. Note that as the mitral valve is hyper echogenic and moving fast during this phase, it produces an artifact in the CFI image as seen in figure 2f.
The same analysis was performed on the patient’s data. Despite the low echogenicity, CFI maps were computed successfully (see figure 3). Both flow patterns and velocities were found to be very different compared to the healthy volunteer case. A much lower velocity was measured in the ejection phase (mean velocity of 0.15 m/s), while the filling flow in early diastole was very high (mean velocity of 0.5 m/s). Contrary to the healthy patient, we observed the establishment of the vortex during the early filling phase. Moreover the vortex pattern in diastasis was found to remain during the entire diastole. No early systole phase could be observed.
Spatio-temporal analysis of blood flow
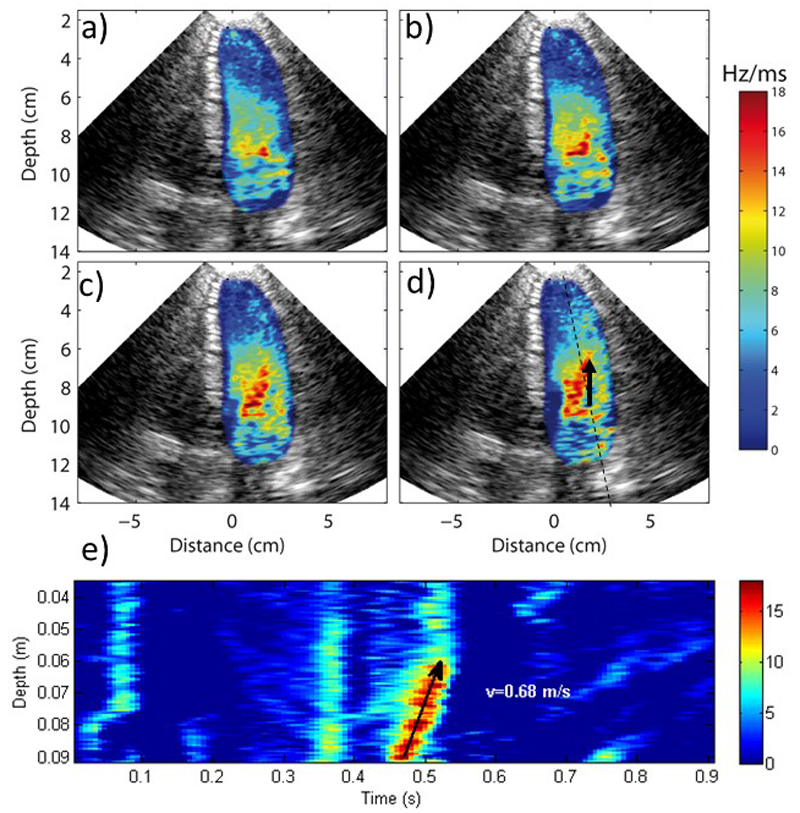
Finally, the transient transmitral flow propagation during early filling was analysed as an example of how spatial and temporal information can be combined using Ultrafast Doppler Imaging. Fig. 4 shows several acceleration maps every 13.3 ms during the early filling of the left ventricle. The propagation of the acceleration peak is visualized in 2D: the peak is initially located at the output of the mitral valve as seen in Fig. 4a), then the acceleration peak propagates in all the directions at various velocities. The largest distance reached after 40 ms was identified in Fig. 4d), determining the direction of maximum velocity propagation (indicated by the black arrow in Fig. 4.d). Fig. 4e) displays the acceleration progression along this direction as a function of time. We derived an average velocity of 0.68ms from the slope of the peak acceleration position as a function of time. A similar analysis was performed using only one line of the acquisition which is equivalent to a so-called Color M-mode. A flow propagation velocity of 0.62 m/s was measured with the M-mode. This analysis assumes the flow propagation to be in the direction of the M-mode line which is wrong as shown on Figure 4.d. Therefore an error of 9% is done on the flow propagation velocity. In this example the error was small but it could be larger if the angle between the two directions is higher.
Figure 4.
Progression of local acceleration during early filling. a), b), c) and d) shows the acceleration maps every 13ms. The black arrow shows the direction of the maximal propagation speed, which is not exactly aligned to scan line that would be performed in M-mode. e) Spatio-temporal analysis of the acceleration peak progression and flow propagation velocity.
IV. Discussion and conclusions
In this study we investigated the ability of Ultrafast Doppler Imaging to map ventricular blood flow with high temporal resolution and over a full two dimensional sector view. A conventional transthoracic imaging probe was used to acquire the apical view of the left ventricle of a healthy volunteer. Two ultrafast imaging sequences were implemented with cylindrical wave transmits on a programmable scanner to acquire 2D ultrasound images at a frame rate of 4800 images/s.
Ultrasound data were analyzed over small temporal sliding windows to provide quantitative information on the blood flow and two types of quantitative information were computed. The full Doppler spectrum was obtained at each pixel of the 2D image, and the mean velocity was extracted from the Doppler spectrum to compute 2D color flow maps. It should be noted that PW Doppler information and color flow maps were provided by the same acquisition at every location in the image simultaneously and at high frame rate. This is a major advantage of the technique as it can provide quantitative spatio-temporal information on the blood flow velocity. To illustrate this capability, we have presented both examples of color flow maps at different periods of the cardiac cycle (ejection, filling and diastasis phases) and examples of PW Doppler at different locations (transaortic and transmitral flows and mid-ventricular flows). Ultrafast Doppler imaging was capable of mapping and quantifying high blood flow velocities (ejection and filling phases), but also complex flow patterns during diastasis. Flow patterns observed during the diastolic filling of the left ventricle revealed the presence of turbulences and vortices. Several studies have highlighted the importance of vortex patterns to minimize energy dissipation during ventricular filling [30] and have suggested that vortices may be impaired in patient with abnormal heart filling [32]. Ultrafast Doppler imaging may emerge as a new tool to better analyze these vortex patterns with high temporal resolution color flow imaging. Ultrafast Doppler data could also be further analyzed to provide vector flow mapping of the vortex [33].
In addition to CFI and PW Doppler, ultrafast Doppler imaging can also provide spatio-temporal analysis of transient flow propagation. For example, the flow propagation velocity has been investigated extensively over the past decades to analyze the spatio-temporal pattern of the transmitral flow [34]. This index quantifies the rate of propagation of early filling flow velocity peak and was shown to change in patients with abnormal ventricular filling. With conventional echocardiography this index is obtained by color M-mode which provides the 1D propagation of peak velocity along this M-mode beam. This is an important limitation, because the M-mode beam is not necessarily parallel to the flow direction which can lead to important errors on the flow propagation velocity. In this study, we have shown the possibility to obtain 2D information of the flow propagation and to quantify the flow propagation the direction of maximum velocity.
This study focused on the analysis of blood flow velocities acquired with an ultrafast imaging sequence. However, tissue velocities can also be computed from the same acquisition data set as described by Papadacci et al. [26]. Tissue velocity analyses were already reported in several studies [23][35][26] in order to map tissue velocities or strain at very high frame rate and measure electromechanical and mechanical wave speed. Therefore, such an ultrafast imaging sequence can provide at very high frame rate both tissue and blood velocity maps simultaneously within a single cardiac cycle as shown by Osmanski et al. [36].
One limitation of the ultrafast imaging sequence used in this study, is the low contrast of the Bmode image. This low contrast is due to choice of a single cylindrical wave transmit to limit aliasing and reach the maximum frame rate. Ejection and filling peak velocities can reach several m/s which requires the highest frame rate possible. In other cardiac phases with lower blood velocities, the frame rate could be decreased and the contrast increased by adding more cylindrical waves as described by Papadacci et al. [26]. Another limitation of this study is that only axial velocities were computed in 2D which represents only a small part of the complex 3D blood flow distribution in the ventricle. Finally, like PW Doppler, UFD is sensitive to aliasing. For measuring flow with a magnitude over 1m/s in the left ventricular outflow track for example, a continuous wave Doppler should be performed.
Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007–2013) / ERC Grant Agreement n°311025
References
- [1].Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA: the journal of the American Medical Association. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- [2].Anavekar NS, Oh JK. Doppler echocardiography: A contemporary review. Journal of Cardiology. 2009;54(3):347–358. doi: 10.1016/j.jjcc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- [3].Evans DH, Jensen JA, Nielsen MB. Ultrasonic colour Doppler imaging. Interface Focus. 2011;1(4):490–502. doi: 10.1098/rsfs.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. Journal of the American College of Cardiology. 1997;30(1):8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- [5].Khouri SJ, Maly GT, Suh DD, Walsh TE. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004 Mar;17(3):290–297. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- [6].Thomas JD, Popović ZB. Assessment of Left Ventricular Function by Cardiac Ultrasound. J Am Coll Cardiol. 2006 Nov;48(10):2012–2025. doi: 10.1016/j.jacc.2006.06.071. [DOI] [PubMed] [Google Scholar]
- [7].Harizi RC, B J. Diastolic function of the heart in clinical cardiology. Arch Intern Med. 1988 Jan;148(1):99–09. [PubMed] [Google Scholar]
- [8].Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. Eur J Echocardiogr. 2009 Mar;10(2):165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- [9].Vivien Gibbs, Cole David, Sassano Antonio. Ultrasound Physics and Technology: How, Why, and when. Elsevier Health Sciences; 2009. [Google Scholar]
- [10].Frinking PJ, Bouakaz A, Kirkhorn J, Ten Cate FJ, de Jong N. Ultrasound contrast imaging: current and new potential methods. Ultrasound Med Biol. 2000 Jul;26(6):965–975. doi: 10.1016/s0301-5629(00)00229-5. [DOI] [PubMed] [Google Scholar]
- [11].Porter TR, Xie F. Myocardial perfusion imaging with contrast ultrasound. JACC: Cardiovascular Imaging. 2010;3(2):176–187. doi: 10.1016/j.jcmg.2009.09.024. [DOI] [PubMed] [Google Scholar]
- [12].Sengupta PP, Khandheria BK, Korinek J, Jahangir A, Yoshifuku S, Milosevic I, Belohlavek M. Left ventricular isovolumic flow sequence during sinus and paced rhythms: new insights from use of high-resolution Doppler and ultrasonic digital particle imaging velocimetry. J Am Coll Cardiol. 2007 Feb;49(8):899–908. doi: 10.1016/j.jacc.2006.07.075. [DOI] [PubMed] [Google Scholar]
- [13].Sandrin L, Catheline S, Tanter M, Hennequin X, Fink M. Time-resolved pulsed elastography with ultrafast ultrasonic imaging. Ultrason Imaging. 1999 Oct;21(4):259–272. doi: 10.1177/016173469902100402. [DOI] [PubMed] [Google Scholar]
- [14].Tanter M, Bercoff J, Sandrin L, Fink M. Ultrafast compound imaging for 2-D motion vector estimation: application to transient elastography. Ieee Trans Ultrason Ferroelectr Freq Control. 2002;49(10):1363–1374. doi: 10.1109/tuffc.2002.1041078. [DOI] [PubMed] [Google Scholar]
- [15].Sandrin L, Tanter M, Catheline S, Fink M. Shear modulus imaging with 2-D transient elastography. Ieee Trans Ultrason Ferroelectr Freq Control. 2002;49(4):426–435. doi: 10.1109/58.996560. [DOI] [PubMed] [Google Scholar]
- [16].Lu J-y, Cheng J, Jing W. High frame rate imaging system for limited diffraction array beam imaging with square-wave aperture weightings high frame rate imaging system for limited diffraction array beam imaging with square-wave aperture weightings. Ieee Trans Ultrason Ferroelectr Freq Control. 2006;53(10):1796–1812. doi: 10.1109/tuffc.2006.112. [DOI] [PubMed] [Google Scholar]
- [17].Carlson J, Ing R-K, Bercoff J, Tanter M. Vortex imaging using two-dimensional ultrasonic speckle correlation. 2001 IEEE Ultrasonics Symposium. 2001;1:559–562. [Google Scholar]
- [18].Mace E, Montaldo G, Osmanski B, Cohen I, Fink M, Tanter M. Functional ultrasound imaging of the brain: theory and basic principles. Ieee Trans Ultrason Ferroelectr Freq Control. 2013;60(3):492–506. doi: 10.1109/TUFFC.2013.2592. [DOI] [PubMed] [Google Scholar]
- [19].Cobbold R. Doppler ultrasound: Physics, instrumentation, and clinical applications: D.H. Evans, W.N. McDicken, R. Skidmore, and J.P. Woodcock John Wiley & Sons, Chichester; 1989, 297 pages, £ 47.50. J Biomed Eng. 1989 Nov;11(6):528. [Google Scholar]
- [20].Bercoff J, Montaldo G, Loupas T, Savery D, Meziere F, Fink M, Tanter M. Ultrafast compound doppler imaging: providing full blood flow characterization. Ieee Trans Ultrason Ferroelectr Freq Control. 2011;58(1):134–147. doi: 10.1109/TUFFC.2011.1780. [DOI] [PubMed] [Google Scholar]
- [21].Ekroll IK, Swillens A, Segers P, Dahl T, Torp H, Lovstakken L. Simultaneous quantification of flow and tissue velocities based on multi-angle plane wave imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2013 Apr;60(4):727–38. doi: 10.1109/TUFFC.2013.2621. [DOI] [PubMed] [Google Scholar]
- [22].Jensen JA, Nikolov SI, Gammelmark KL, Pedersen MH. Synthetic aperture ultrasound imaging. Ultrasonics. 2006 Décembre;44(Supplement):e5–e15. doi: 10.1016/j.ultras.2006.07.017. [DOI] [PubMed] [Google Scholar]
- [23].Couade M, Pernot M, Tanter M, Messas E, Bel A, Ba M, Hagege A-A, Fink M. Ultrafast imaging of the heart using circular wave synthetic imaging with phased arrays. presented at the Ultrasonics Symposium (IUS), 2009 IEEE International; 2009. pp. 515–518. [Google Scholar]
- [24].Nikolov SI, Kortbek J, Jensen JA. Practical applications of synthetic aperture imaging. presented at the 2010 IEEE Ultrasonics Symposium (IUS); 2010. pp. 350–358. [Google Scholar]
- [25].Lockwood GR, Talman JR, Brunke SS. Real-time 3-D ultrasound imaging using sparse synthetic aperture beamforming. Ieee Trans Ultrason Ferroelectr Freq Control. 1998;45(4):980–988. doi: 10.1109/58.710573. [DOI] [PubMed] [Google Scholar]
- [26].Papadacci C, Pernot M, Couade M, Fink M, Tanter M. High Contrast Ultrafast Imaging of the Heart. Ieee Trans Ultrason Ferroelectr Freq Control. 2014 doi: 10.1109/TUFFC.2014.6722614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Udesen J, Gran F, Hansen KL, Jensen JA, Thomsen C, Nielsen MB. High frame-rate blood vector velocity imaging using plane waves: simulations and preliminary experiments. Ieee Trans Ultrason Ferroelectr Freq Control. 2008 Aug;55(8):1729–1743. doi: 10.1109/TUFFC.2008.858. [DOI] [PubMed] [Google Scholar]
- [28].Nikolov SI, Jensen JA. In-vivo synthetic aperture flow imaging in medical ultrasound. Ieee Trans Ultrason Ferroelectr Freq Control. 2003;50(7):848–856. doi: 10.1109/tuffc.2003.1214504. [DOI] [PubMed] [Google Scholar]
- [29].Hergum T, Bjastad TG, Lvstakken L, Kristoffersen K, Torp H. Reducing color flow artifacts caused by parallel beamforming. Ieee Trans Ultrason Ferroelectr Freq Control. 2010;57(4):830–838. doi: 10.1109/TUFFC.2010.1488. [DOI] [PubMed] [Google Scholar]
- [30].Klabunde RE. Cardiovascular physiology concepts. 2nd edition. Lipincott Williams & Wilkins; 2012. [Google Scholar]
- [31].Pierrakos O, Vlachos PP. The effect of vortex formation on left ventricular filling and mitral valve efficiency. J Biomech Eng. 2006 Aug;128(4):527–39. doi: 10.1115/1.2205863. [DOI] [PubMed] [Google Scholar]
- [32].Mehregan F, Tournoux F, Muth S, Pibarot P, Rieu R, Cloutier G, Garcia D. Doppler vortography: a color Doppler approach to quantification of intraventricular blood flow vortices. Ultrasound Med Biol. 2014 Jan;40(1):210–21. doi: 10.1016/j.ultrasmedbio.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Uejima T, Koike A, Sawada H, Aizawa T, Ohtsuki S, Tanaka M, Furukawa TT, Fraser AG. A new echocardiographic method for identifying vortex flow in the left ventricle: numerical validation. Ultrasound in Medicine & Biology. 2010 May;36(5) doi: 10.1016/j.ultrasmedbio.2010.02.017. [DOI] [PubMed] [Google Scholar]
- [34].Takatsuji H, Mikami T, Urasawa K, Teranishi J, Onozuka H, Takagi C, Makita Y, Matsuo H, Kusuoka H, Kitabatake AA. new approach for evaluation of left ventricular diastolic function: spatial and temporal analysis of left ventricular filling flow propagation by color M-mode Doppler echocardiography. J Am Coll Cardiol. 1996 Feb;27(2):365–71. doi: 10.1016/0735-1097(96)81240-x. [DOI] [PubMed] [Google Scholar]
- [35].Provost J, Nguyen VT, Legrand D, Okrasinski S, Costet A, Gambhir A, Garan H, Konofagou EE. Electromechanical wave imaging for arrhythmias. Phys Med Biol. 2011 Nov 21;56(22):L1–11. doi: 10.1088/0031-9155/56/22/F01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Osmanski BF, Pernot M, Montaldo G, Bel A, Messas E, Tanter M. Ultrafast Doppler imaging of blood flow dynamics in the myocardium. Medical Imaging, IEEE Transactions. 2012;31(8):1661–1668. doi: 10.1109/TMI.2012.2203316. [DOI] [PubMed] [Google Scholar]