Abstract
Background. Bacterial vaginosis (BV) may increase women's susceptibility to sexually transmitted infections (STIs). In a randomized trial of periodic presumptive treatment (PPT) to reduce vaginal infections, we observed a significant reduction in BV. We further assessed the intervention effect on incident Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium infection.
Methods. Nonpregnant, human immunodeficiency virus–uninfected women from the United States and Kenya received intravaginal metronidazole (750 mg) plus miconazole (200 mg) or placebo for 5 consecutive nights each month for 12 months. Genital fluid specimens were collected every other month. Poisson regression models were used to assess the intervention effect on STI acquisition.
Results. Of 234 women enrolled, 221 had specimens available for analysis. Incidence of any bacterial STI (C. trachomatis, N. gonorrhoeae, or M. genitalium infection) was lower in the intervention arm, compared with the placebo arm (incidence rate ratio [IRR], 0.54; 95% confidence interval [CI], .32–.91). When assessed individually, reductions in STI incidences were similar but not statistically significant (IRRs, 0.50 [95% confidence interval {CI}, .20–1.23] for C. trachomatis infection, 0.56 [95% CI, .19–1.67] for N. gonorrhoeae infection, and 0.66 [95% CI, .38–1.15] for M. genitalium infection).
Conclusions. In addition to reducing BV, this PPT intervention may also reduce the risk of bacterial STI among women. Because BV is highly prevalent, often persists, and frequently recurs after treatment, interventions that reduce BV over extended periods could play a role in decreasing STI incidence globally.
Keywords: bacterial vaginosis, Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, vaginal health interventions, periodic presumptive treatment
With >500 million new cases of sexually transmitted infections (STIs) each year [1, 2], the development of innovative strategies for STI prevention is a global public health priority. Chlamydia trachomatis infection and Neisseria gonorrhoeae infection are the most prevalent bacterial STIs, and there is emerging evidence in support of Mycoplasma genitalium as a sexually transmitted pathogen in women [1, 3]. C. trachomatis, N. gonorrhoeae, and M. genitalium often go undetected because many infections are asymptomatic [4, 5]. These infections are common among reproductive-aged women and are associated with serious adverse reproductive health outcomes, including cervicitis, pelvic inflammatory disease (PID), infertility, ectopic pregnancy, neonatal infections, and human immunodeficiency virus (HIV) acquisition [3, 6–8].
The vaginal microenvironment plays an important role in mediating STI susceptibility [9]. Several prospective studies have reported an association between abnormal vaginal microbiota, in particular bacterial vaginosis (BV) and depletion of Lactobacillus species, and increased risk of STI acquisition [10–15]. In contrast, a healthy vaginal microbiota consisting primarily of Lactobacillus species has been associated with a lower risk of STI acquisition [13, 14, 16]. Furthermore, in an open-label trial involving 107 US women with asymptomatic BV determined on the basis of the Nugent score, those who received intravaginal metronidazole gel for 5 days, followed by twice weekly use for 6 months, had a lower incidence of STI (defined as a combined outcome of C. trachomatis infection, N. gonorrhoeae infection, T. vaginalis infection, herpes simplex virus [HSV] infection, or PID) while receiving treatment, compared with those who received standard of care treatment [10]. The results from this study provide preliminary evidence in support of the hypothesis that improving vaginal health through treatment of asymptomatic BV could reduce STI incidence. However, further investigation to confirm these findings is warranted, including additional research among women from resource-limited settings.
We recently completed a randomized, double-blinded, placebo-controlled trial of monthly periodic presumptive treatment for 12 months to reduce bacterial vaginosis and vulvovaginal candidiasis among US and Kenyan women [17]. Over 1 year, the intervention significantly reduced the proportion of visits in which BV was detected, compared with placebo; the proportion of visits in which vulvovaginal candidiasis was detected were similar by study arm. Using data from this multisite randomized trial, we assessed the effect of the intervention on the acquisition of C. trachomatis, N. gonorrhoeae, and M. genitalium.
METHODS
Study Population and Procedures
This is a secondary analysis of data from women participating in the Preventing Vaginal Infections (PVI) trial, a double-blinded, randomized, controlled trial that assessed the effect of monthly periodic presumptive treatment (PPT) by using topical metronidazole (750 mg) with miconazole (200 mg) intravaginal suppositories versus matching placebo nightly for 5 consecutive nights each month for 12 months to reduce rates of BV and vulvovaginal candidiasis (clinical trials registration NCT01230814). Detailed methods and results for the trial have been described previously [17]. Briefly, 234 high-risk women from 3 sites in Kenya and 1 site in the United States were enrolled between May 2011 and August 2012. Eligible women were 18–45 years of age, HIV-1 uninfected, not pregnant or breastfeeding, and sexually active (defined as a self-reported frequency of at least 4 episodes of vaginal sex in the past month) and had BV by Nugent's score [18] and/or VVC by saline/potassium hydroxide wet preparation plus positive results of culture for yeast on Sabouraud agar, and/or Trichomonas vaginalis infection detected by a saline wet preparation at screening. Eligible participants were randomly assigned in equal proportions to receive either the intervention or matching placebo. All participants provided written informed consent at screening and enrollment. Participants were asked to provide separate consent for the storage and future testing of biological specimens, including those used for the present analysis. The trial was approved by the human subjects research committees at Kenyatta National Hospital (Nairobi, Kenya), the University of Washington (Seattle), and the University of Alabama at Birmingham.
At enrollment, structured face-to-face interviews were conducted to collect data on demographic, clinical, and behavioral characteristics. Participants also received C. trachomatis and N. gonorrhoeae testing at enrollment, and those with a diagnosis of either infection were treated according to local guidelines. At monthly follow-up visits, data were collected on sexual behaviors, intravaginal practices, contraceptive use, study product use, and genital tract symptoms. Participants underwent adherence counseling, and a urine pregnancy test was performed. Nonpregnant participants received a 1-month supply of study product and free male condoms. Participants with symptomatic vulvovaginitis, vaginal discharge, or vaginal itching received treatment with open-label oral metronidazole (400 mg or 500 mg) twice daily for 7 days plus single-dose oral fluconazole (150 mg), in addition to study product. In addition, during follow-up visits at months 2, 4, 6, 8, 10, and 12, participants underwent a physical examination, including a pelvic speculum examination with collection of genital swab specimens for diagnosis of genital tract infections. If a participant missed an examination visit, a physical examination was performed at her next follow-up visit.
Laboratory Procedures
Genital fluid was collected using the Hologic/Gen-Probe Aptima Combo 2 system (Hologic/GenProbe; San Diego, California) at enrollment and follow-up months 2, 4, 6, 8, 10, and 12. Specimens collected at enrollment were tested for the presence of C. trachomatis and N. gonorrhoeae in accordance with the manufacturer's recommendations. The remainder of the enrollment specimen and all follow-up specimens were stored at −80°C for future testing. At the completion of the study, stored follow-up specimens were tested for C. trachomatis and N. gonorrhoeae per the manufacturer's recommendations; for T. vaginalis, using the Hologic/Gen-Probe ASR assay; and for M. genitalium, using a research-use only transcription TMA assay with reagents provided by Hologic/Gen-Probe as part of their ongoing research program. M. genitalium assay results with a value of >40 000 relative light units were considered positive [19]. Vaginal Gram-stained slides were evaluated for BV by using the Nugent score [18]. Saline and potassium hydroxide wet preparations were examined for the presence of motile trichomonads, clue cells, and yeast. Yeast culture was performed on Sabouraud agar with a germ tube test to identify presumptive Candida albicans. At enrollment, blood specimens were collected through venipuncture for HSV-2 testing, using the HerpeSelect assay (Focus Technologies). An OD of >2.1 was considered positive for participants in Kenya [20]. Enrollment specimens from US participants were tested using kits that produced a dichotomous result (positive vs negative).
Statistical Analysis
Our primary objective was to assess the effect of the PVI trial intervention on incident bacterial STIs during follow-up (combined outcome of incident C. trachomatis, N. gonorrhoeae, or M. genitalium infection) in the subset of PVI trial participants who consented to future testing of stored specimens. We also conducted a secondary analysis to assess the effect of the intervention on C. trachomatis, N. gonorrhoeae, and M. genitalium infection, each as a separate outcome. For each analysis, the population under study was restricted to participants who were negative for all STIs (in the combined end point analysis) or for the STI of interest (in analyses of individual STIs) at enrollment. We calculated the incidence of the combined STI outcome and each individual STI, with follow-up time censored following the first incident infection. Since the sample size and number of STI outcomes were small, we constructed Poisson regression models to assess the effect of the intervention on the combined and individual bacterial STI outcomes. Differences in duration of participant follow-up were assessed using the Wilcoxon rank sum test. All statistical tests were assessed using a 2-sided significance level of 0.05. Analyses were conducted using Stata, version 14.0 (StataCorp, College Station, Texas).
RESULTS
Of the 234 participants enrolled, 221 (94%) returned for follow-up visits and provided consent for future testing of stored specimens; 111 (50%) were in the intervention arm, and 110 (50%) were in the placebo arm. Participant follow-up did not differ by study arm, with median of 11.2 months (interquartile range [IQR], 11.1–11.6 months) in the intervention arm, compared with 11.4 months (IQR, 11.2–11.7 months) in the placebo arm (P = .12). Demographic, behavioral, and clinical characteristics at enrollment are presented by study arm in Table 1. Characteristics of participants in the intervention arm were generally similar to those in the placebo arm.
Table 1.
Baseline Characteristics of Participants in the Preventing Vaginal Infections Trial Who Were Eligible for Secondary Analysis
| Characteristic | Placebo Group (n = 110) | Intervention Group (n = 111) |
|---|---|---|
| Demographic characteristic | ||
| Age, y | ||
| Overall | 29 (23–34) | 30 (24–34) |
| <25 | 29 (26) | 33 (30) |
| Education duration, y | 11 (8–12) | 10 (8–13) |
| Partnership status | ||
| Married or living with a partner | 29 (26) | 34 (31) |
| Separated, divorced, or widowed | 48 (44) | 39 (35) |
| Never married | 33 (30) | 38 (34) |
| Study site | ||
| Birmingham, AL | 26 (24) | 27 (24) |
| Kangemi area, Nairobi, Kenya | 27 (25) | 28 (25) |
| Korogocho area, Nairobi, Kenya | 28 (25) | 28 (25) |
| Mombasa, Kenya | 29 (26) | 28 (25) |
| Health history | ||
| Live births, no. | 2 (1–3) | 2 (1–3) |
| Current family planning method | ||
| None | 16 (15) | 23 (21) |
| Condoms only | 30 (27) | 31 (28) |
| Oral contraceptives | 12 (11) | 11 (10) |
| Injectable contraceptives | 25 (23) | 24 (22) |
| IUD | 10 (9) | 4 (4) |
| Implant | 9 (8) | 5 (5) |
| Tubal ligation | 5 (5) | 10 (9) |
| Othera | 3 (3) | 3 (3) |
| Currently smoke cigarettes | 10 (9) | 20 (18) |
| Vaginal washing in the past month | 55 (50) | 56 (50) |
| Sexual behavior | ||
| Ever engaged in sex in exchange for goods/money/services | 60 (55) | 59 (53) |
| Vaginal sex acts in the past week, no. | 2 (1–4) | 2 (1–3) |
| Condom use during sex in past week | ||
| Always | 53 (48) | 42 (38) |
| Intermittentlyb | 11 (10) | 12 (11) |
| Never | 26 (24) | 36 (32) |
| No sex reported | 20 (18) | 21 (19) |
| Sex partners in past week, no. | 1 (1–2) | 1 (1–2) |
| New sex partner in past week | 23 (21) | 22 (20) |
| Ever had anal sex | 13 (12) | 12 (11) |
| Clinical characteristic | ||
| C. trachomatis infection | 8 (7) | 8 (7) |
| N. gonorrhoeae infection | 0 (0) | 3 (3) |
| M. genitalium infectionc | 9 (8) | 16 (14) |
| HSV-2 seropositived | 68 (62) | 71 (64) |
| T. vaginalis infectionc | 6 (5) | 10 (9) |
| Vulvovaginal candidiasis | 24 (22) | 28 (25) |
| Bacterial vaginosise | 40 (36) | 41 (37) |
| Intermediate microbiotae | 20 (18) | 24 (22) |
| Normal microbiotae | 50 (45) | 46 (41) |
| Cervicitisf | 14 (13) | 18 (16) |
Data are no. (%) of women or median (interquartile range).
Abbreviations: C. trachomatis, Chlamydia trachomatis; HSV-2, herpes simplex virus type 2; IUD, intrauterine device; M. genitalium, Mycoplasma genitalium; N. gonorrhoeae, Neisseria gonorrhoeae.
a Included the fertility awareness method (n = 2), vasectomy by partner (n = 1), Essure use (n = 1), withdrawal (n = 1), and herbal pills (n = 1).
b Defined as <100% but >0% of occasions. No condom use was reported among women who reported sex in the past week.
c One intervention participant was missing results at enrollment.
d Kenyan participants with an OD of >2.1 were considered seropositive.
e Assessed by the Nugent score, with 7–10 indicating bacterial vaginosis, 4–6 indicating intermediate microbiota, and 0–3 indicating normal microbiota.
f Defined as ≥30 polymorphonuclear leukocytes per high-powered field. One participant was missing a baseline result.
The prevalences of C. trachomatis, N. gonorrhoeae, and M. genitalium infections by study arm at enrollment are presented in Table 1. Of these 3 STIs, M. genitalium was the most commonly detected at baseline, with an overall prevalence of 11.3%, followed by C. trachomatis (7.2%), and N. gonorrhoeae (1.3%). Incidences and effect estimates for each STI outcome are presented in Table 2. Consistent with the baseline STI prevalence, the overall incidence of individual bacterial STIs was highest for M. genitalium, with 52 incident cases detected during 155.0 person-years of follow-up (33.6 cases per 100 person-years), followed by C. trachomatis (11.7 cases per 100 person-years) and N. gonorrhoeae (7.2 cases per 100 person-years). The overall incidence of C. trachomatis, N. gonorrhoeae, or M. genitalium infection was 46.5 cases per 100 person-years.
Table 2.
Effect of the Intervention on Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium Acquisition
| STI Outcome, Study Group | Women, No. | Events, No. | Person-Years, No. | Incidencea (95% CI) | IRRb (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Combined STIs | ||||||
| Overallc | 177 | 63 | 135.6 | 46.5 (36.3–59.5) | ||
| Intervention | 84 | 22 | 67.4 | 32.6 (21.5–49.6) | 0.54 (.32–.91) | .02 |
| Placebo | 93 | 41 | 68.2 | 60.1 (44.3–81.7) | 1.00 | |
| Individual STIs | ||||||
| C. trachomatis infection | ||||||
| Overall | 205 | 21 | 179.6 | 11.7 (7.6–17.9) | ||
| Intervention | 103 | 7 | 90.0 | 7.8 (3.7–16.3) | 0.50 (.20–1.23) | .13 |
| Placebo | 102 | 14 | 89.6 | 15.6 (9.3–26.4) | 1.00 | |
| N. gonorrhoeae infection | ||||||
| Overall | 218 | 14 | 193.3 | 7.2 (4.3–12.2) | ||
| Intervention | 108 | 5 | 96.3 | 5.2 (2.2–12.5) | 0.56 (.19–1.67) | .30 |
| Placebo | 110 | 9 | 96.9 | 9.3 (4.8–17.8) | 1.00 | |
| M. genitalium infectiond | ||||||
| Overall | 195 | 52 | 155.0 | 33.6 (25.6–44.0) | ||
| Intervention | 94 | 20 | 75.5 | 26.5 (17.1–41.1) | 0.66 (.38–1.15) | .14 |
| Placebo | 101 | 32 | 79.5 | 40.3 (28.5–56.9) | 1.00 | |
Data are for the first incident STI, with follow-up censored at the time of first infection. Each model excludes participants with the STI(s) of interest present at baseline.
Abbreviations: CI, confidence interval; STI, sexually transmitted infection.
a Data denote the number of new STIs per 100 person-years and only include the first STI detected.
b Incidence rate ratios (IRRs) were calculated by Poisson regression models.
c Defined as C. trachomatis or N. gonorrhoeae or M. genitalium infection.
d In addition to the 25 participants with baseline M. genitalium infection, 1 participant was missing baseline results and excluded from the analysis, leaving 195 participants for analysis.
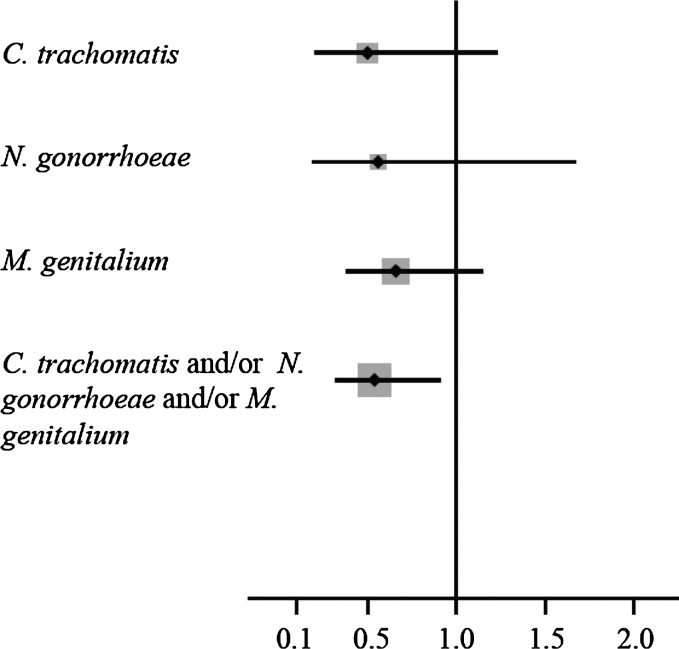
Among participants who were negative for C. trachomatis, N. gonorrhoeae, or M. genitalium at baseline, we observed a significant reduction in the combined outcome of any incident bacterial STI for participants in the intervention arm, compared with those in the placebo arm (incidence rate ratio, 0.54; 95% confidence interval, .32–.91; Table 2). The magnitude of the intervention effect was similar when each bacterial STI outcome was assessed separately, although the individual STI reductions were not statistically significant (Figure 1 and Table 2).
Figure 1.
Forest plot of incident rate ratios and 95% confidence intervals for outcomes of individual and combined sexually transmitted infections due to Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium.
DISCUSSION
Monthly periodic presumptive treatment with vaginal suppositories coformulated with metronidazole and miconazole, an intervention that significantly reduced BV over 12 months of follow-up [17], may also reduce the risk of bacterial STIs among women. We observed nearly a 50% reduction in the combined outcome of C. trachomatis, N. gonorrhoeae, or M. genitalium infection among women in the intervention arm, compared with those in the placebo arm. Similar magnitudes of reduction were observed in incident C. trachomatis, N. gonorrhoeae, and M. genitalium infections when assessed as separate outcomes, although the small sample size in this secondary analysis precluded detection of significant associations. These data support the hypothesis that the vaginal microenvironment influences bacterial STI susceptibility and interventions that improve vaginal health by shifting the microbiota away from bacterial vaginosis and that could lead to reductions in highly prevalent bacterial STIs.
With an estimated 211 million new infections globally each year, C. trachomatis infection and N. gonorrhoeae infection are the most prevalent bacterial STIs [1]. These infections are associated with adverse reproductive health outcomes, including PID, chronic pelvic pain, ectopic pregnancy, infertility, and HIV acquisition [3, 4, 6, 7, 21, 22]. The majority of infections in women are asymptomatic, and targeted screening approaches have been used to identify those with infection [23, 24]. However, uptake of screening remains low worldwide [25, 26], and cost and limited diagnostic capacity make this approach impractical in resource-limited settings. While efforts to improve bacterial STI screening and diagnostic assays are ongoing, the high STI incidence in many settings emphasizes the need for novel, effective prevention strategies. Such strategies may be even more effective in decreasing STI-associated sequelae as compared to detection and treatment.
There is a growing body of evidence in support of M. genitalium as a sexually transmitted pathogen that may be causally associated with adverse outcomes in women [3]. The prevalence of M. genitalium infection in our study was high, and as illustrated here and by others, it can be detected at a frequency equal to or greater than that of other bacterial STIs [27]. Highly sensitive diagnostic tests for M. genitalium have only recently become commercially available and are not yet widely used in clinical settings, limiting the opportunity for pathogen-specific detection and treatment, as well as for global surveillance. Data are limited and conflicting regarding the relationship between BV and M. genitalium. Several studies have reported an increased risk of M. genitalium infection among women with BV [15, 28], while others have observed no association [29–31]. Finding from the present study demonstrate a decrease in M. genitalium infection among women in the intervention arm, where there was also a significant reduction in BV [17]. An alternative hypothesis for our observation could be that the incidence of M. genitalium infection was reduced as a result of treatment with metronidazole. However, metronidazole has no known effect on M. genitalium [32]. In the absence of a direct effect of metronidazole on M. genitalium, our findings lend support to the hypothesis that reductions in BV due to the intervention may decrease susceptibility to M. genitalium infection.
The vaginal microbiome has been well characterized though cultivation-based and molecular methods. Robust data from epidemiological studies indicate that the vaginal microbiota influences STI susceptibility [10–14]. However, mechanistic data illustrating how the presence of BV or BV-associated bacteria modifies STI susceptibility are limited. Immunologic, enzymatic, and metabolic mechanisms could operate independently or in combination to enhance STI acquisition. Several lines of evidence provide a strong foundation for a biologic relationship between BV and increased STI susceptibility. An acidic vaginal pH and the presence of Lactobacillus species have been associated with decreased in vitro activity of C. trachomatis and N. gonorrhoeae [33, 34]. Cervical mucus has the ability to trap pathogens. However, this mucus barrier may be compromised by mucin-degrading enzymes such as sialidase and mucinase, which are produced by BV-associated bacteria. Loss of the protective mucus provides pathogens with unhindered access to target cells, increasing epithelial binding potential. Sialidase also cleaves terminal sialic acids from glycoproteins, exposing other sugars on their carbohydrate side chains, which can be used as an energy source for bacteria. Other mechanisms, such as use of indole, which is produced by several BV-associated species, by C. trachomatis could enable the bacteria to overcome the bactericidal effects of interferon γ [36]. Biologic mechanisms potentially driving the association between BV and STI acquisition require further investigation.
Our analysis included a number of strengths, including data collected as part of a multisite, multicountry clinical trial with high adherence and retention. The intervention was evaluated among women with a recent vaginal infection, since they would be most likely to benefit from a vaginal health intervention, and STI outcomes were assessed at regular intervals by using highly sensitive assays. Nonetheless, our findings should be interpreted in the context of several limitations. Study participants were predominantly African or African American and were recruited from populations at high-risk for STIs, including HIV infection. The prevalence of BV is frequently higher among African and African American women, compared with other races [36]. It is unclear if the magnitude of the intervention effect would be the same in other populations where the burden of BV may be lower. The PVI trial was powered to assess the effect of the PPT intervention on BV and vulvovaginal candidiasis. As a result, statistical power was insufficient to adequately assess individual STI outcomes. However, we did observe a significant effect of the intervention on any bacterial STI (C. trachomatis, N. gonorrhoeae, or M. genitalium infection).
Despite evidence from epidemiologic and in vitro studies demonstrating a relationship between BV and STI acquisition, only 1 randomized trial of BV therapy has evaluated the effect of BV treatment on STI incidence. Schwebke et al reported that the incidence of STIs was lower among those who received weekly intravaginal metronidazole gel, compared with standard of care [10]. Our findings from the PVI trial, combined with those from Schwebke et al, provide evidence in support of the hypothesis that interventions that improve vaginal health decrease susceptibility to bacterial STIs. Additional clinical trials designed specifically to assess the effect of vaginal health interventions on STI outcomes are essential to determine if there is a causal relationship between BV and an increased risk of STI acquisition. Such trials should evaluate interventions with demonstrated effectiveness in improving vaginal health (ie, decreasing BV) and be designed to assess the effectiveness of the intervention on STI outcomes. If BV increases susceptibility to STIs, this causal link could warrant shifts in clinical approaches to managing asymptomatic BV, for which treatment is not currently recommended for nonpregnant women [8]. Interventions that improve vaginal health could play an important role in decreasing the STI risk in individual women, as well as informing public health approaches to decrease the STI incidence globally.
Notes
Acknowledgments. We thank the women who participated in this study; the PVI study team and study sites, for their tireless work on data and sample collection; FHI 360, for their work on data management and study operations; and Carolyn Deal, PhD, from the National Institutes of Health Department of Microbiology and Infectious Disease, for her guidance and support of this work.
J. E. B. and R. S. M. conceptualized the article and analysis plan. J. E. B. conducted the analysis in collaboration with J. L. and R. S. M. J. E. B. drafted the initial report, and L. E. M., J. L., and R. S. M. contributed to the content and revisions. O. A., J. K., J. Schwebke, J. Shafi, C. R., and E. K. contributed to data collection. All authors contributed to article content and approved the final manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (contract HHSN266200400073C [to the Preventing Vaginal Infections trial], through the Sexually Transmitted Infections Clinical Trials Group, and grant R01-AI099106 [for C. trachomatis and N. gonorrhoeae testing]), the American Sexually Transmitted Disease Association (developmental award for M. genitalium testing), and the National Institutes of Health (grant P30-AI27757 [for infrastructural and logistical support at the Mombasa site], through the University of Washington Center for AIDS Research).
Potential conflicts of interests. J. E. B. received donated assay reagents for M. genitalium testing from Hologic/Gen-Probe and honoraria for consulting from Symbiomix. R. S. M. has received honoraria for invited lectures and consulting, as well as donated study product for the PVI trial, from Embil Pharmaceutical Company. R. S. M. currently receives research funding from Hologic/Gen-Probe. J. S. has received consultancy payments from Akesis, Hologic, Symbiomix, and Starpharma and has grants/pending grants from Akesis, BD Diagnostic, Hologic, Cepheid, Quidel, Symbiomix, Starpharma, and Viamet. L. E. M. has received donated reagents and test kits from Hologic/Gen-Probe and honoraria for scientific advisory board membership from Hologic/Gen-Probe and Qiagen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections—2008. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 2.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015; 10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium Infection and Female Reproductive Tract Disease: A Meta-analysis. Clin Infect Dis 2015; 61:418–26. [DOI] [PubMed] [Google Scholar]
- 4.Stamm W. Chlamydia trachomatis infections of the adult. In: Holmes K, Sparling P, Stamm W et al. eds. Sexually transmitted diseases. New York: McGraw-Hill, 2008. [Google Scholar]
- 5.WHO. Expert consultation and review of the latest evidence to update guidelines for the management of sexually transmitted infections. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 6.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 1992; 19:61–77. [PubMed] [Google Scholar]
- 7.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010; 201(suppl 2):S134–55. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2015; 64:1–138. [PubMed] [Google Scholar]
- 9.Buve A, Jespers V, Crucitti T, Fichorova RN. The vaginal microbiota and susceptibility to HIV. AIDS 2014; 28:2333–44. [DOI] [PubMed] [Google Scholar]
- 10.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol 2007; 196:517, e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brotman RM, Klebanoff MA, Nansel TR et al. Bacterial vaginosis assessed by Gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 2010; 202:1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allsworth JE, Peipert JF. Severity of bacterial vaginosis and the risk of sexually transmitted infection. Am J Obstet Gynecol 2011; 205:113.e1–113.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin HL Jr, Richardson BA, Nyange PM et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 180:1863–8. [DOI] [PubMed] [Google Scholar]
- 14.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 2003; 36:663–8. [DOI] [PubMed] [Google Scholar]
- 15.Oakeshott P, Aghaizu A, Hay P et al. Is Mycoplasma genitalium in women the “New Chlamydia?” A community-based prospective cohort study. Clin Infect Dis 2010; 51:1160–6. [DOI] [PubMed] [Google Scholar]
- 16.Hillier SL, Krohn MA, Klebanoff SJ, Eschenbach DA. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstet Gynecol 1992; 79:369–73. [DOI] [PubMed] [Google Scholar]
- 17.McClelland RS, Balkus JE, Lee J et al. Randomized trial of periodic presumptive treatment with high-dose intravaginal metronidazole and miconazole to prevent vaginal infections in HIV-negative women. J Infect Dis 2014; 211:1875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardick J, Giles J, Hardick A, Hsieh YH, Quinn T, Gaydos C. Performance of the gen-probe transcription-mediated [corrected] amplification research assay compared to that of a multitarget real-time PCR for Mycoplasma genitalium detection. J Clin Microbiol 2006; 44:1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mujugira A, Morrow RA, Celum C et al. Performance of the Focus HerpeSelect-2 enzyme immunoassay for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sex Transm Infect 2011; 87:238–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook EW, Handsfield H. Gonococcal infections in the adult. In: Holmes K, Sparling P, Stamm W et al. eds. Sexually transmitted diseases. New York: McGraw-Hill, 2008:627–45. [Google Scholar]
- 22.Laga M, Manoka A, Kivuvu M et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 1993; 7:95–102. [DOI] [PubMed] [Google Scholar]
- 23.Meyers DS, Halvorson H, Luckhaupt S. Screening for chlamydial infection: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med 2007; 147:135–42. [DOI] [PubMed] [Google Scholar]
- 24.Screening for gonorrhea: recommendation statement. Ann Fam Med 2005; 3:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamm WE. Chlamydia screening: expanding the scope. Ann Intern Med 2004; 141:570–2. [DOI] [PubMed] [Google Scholar]
- 26.Hoover K, Tao G, Kent C. Low rates of both asymptomatic chlamydia screening and diagnostic testing of women in US outpatient clinics. Obstet Gynecol 2008; 112:891–8. [DOI] [PubMed] [Google Scholar]
- 27.McGowin CL, Anderson-Smits C. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog 2011; 7:e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manhart L, McClelland R, Baeten J et al. Incident Mycoplasm genitalium infection among Kenyan women is associated with bacterial vaginosis [abstract P3.37]. Presented at: International Society for STD Research, London, 28 June–1 July 2009. [Google Scholar]
- 29.Cohen CR, Nosek M, Meier A et al. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis 2007; 34:274–9. [DOI] [PubMed] [Google Scholar]
- 30.Casin I, Vexiau-Robert D, De La Salmoniere P, Eche A, Grandry B, Janier M. High prevalence of Mycoplasma genitalium in the lower genitourinary tract of women attending a sexually transmitted disease clinic in Paris, France. Sex Transm Dis 2002; 29:353–9. [DOI] [PubMed] [Google Scholar]
- 31.Short VL, Totten PA, Ness RB, Astete SG, Kelsey SF, Haggerty CL. Clinical presentation of Mycoplasma genitalium Infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin Infect Dis 2009; 48:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manhart LE, Jensen JS, Bradshaw CS, Golden MR, Martin DH. Efficacy of antimicrobial therapy for Mycoplasma genitalium infections. Clin Infect Dis 2015; 61(suppl 8):S802–17. [DOI] [PubMed] [Google Scholar]
- 33.Saigh JH, Sanders CC, Sanders WE Jr. Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: evidence for a protective effect against infection. Infect Immun 1978; 19:704–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasin B, Pang M, Wagar EA, Lehrer RI. Examination of Chlamydia trachomatis infection in environments mimicking normal and abnormal vaginal pH. Sex Transm Dis 2002; 29:514–9. [DOI] [PubMed] [Google Scholar]
- 35.Aiyar A, Quayle AJ, Buckner LR et al. Influence of the tryptophan-indole-IFNγ axis on human genital Chlamydia trachomatis infection: role of vaginal co-infections. Front Cell Infect Microbiol 2014; 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109:114–20. [DOI] [PubMed] [Google Scholar]