Abstract
Background. We sought to understand why some children respond poorly to vaccinations in the first year of life.
Methods. A total of 499 children (6–36 months old) provided serum and peripheral blood mononuclear cell samples after their primary and booster vaccination. Vaccine antigen-specific antibody levels were analyzed with enzyme-linked immunosorbent assay, and frequency of memory B cells, functional T-cell responses, and antigen-presenting cell responses were assessed in peripheral blood mononuclear cell samples with flow cytometric analysis.
Results. Eleven percent of children were low vaccine responders, defined a priori as those with subprotective immunoglobulin G antibody levels to ≥66% of vaccines tested. Low vaccine responders generated fewer memory B cells, had reduced activation by CD4+ and CD8+ T cells on polyclonal stimulation, and displayed lower major histocompatibility complex II expression by antigen-presenting cells.
Conclusions. We conclude that subprotective vaccine responses in infants are associated with a distinct immunologic profile.
Keywords: vaccines, B cells, T cells, antigen presenting cells, infants
Infants and young children receive multiple priming doses and booster vaccinations in the first and second years of life to prevent infections by viral and bacterial pathogens. About 10% of infants do not respond to at least 1 vaccine with protective antibody levels after primary vaccination at 2, 4, and 6 months of age [1, 2]. This observation has been presumed to be due to biologic variation, but hyporesponsiveness to vaccination stimuli has not been systematically studied.
In infants and young children, the generation of antibody responses to vaccines and infections, generation of B-cell and T-cell memory and adequacy of professional antigen-presenting cell (APC) function are known to be generally lower than observed in older children or adults [3]. During a longitudinal, prospective study we found that 11% of infants were low vaccine responders (LVRs) after routine primary series vaccinations at 2, 4, and 6 months of age. They were characterized by antibody titers below the established protective level for several routine pediatric vaccines. Low vaccine responses are well known to occur in some infants, but the immunologic mechanisms underlying these responses have not been investigated in detail. Because antibody production depends on the interaction between professional APCs, T cells, and B cells, we sought to understand the immunologic profile of these children by examining their immune cells from peripheral blood ex vivo. A better understanding of the reasons for subprotective vaccine responses in infants will contribute to the development of more effective vaccines for both neonates and young children.
METHODS
Subjects
Eligible healthy, full-term-birth children between the ages of 6 and 30 months were prospectively enrolled from a middle class, suburban sociodemographic population in Rochester, New York, in a longitudinal study; exclusion criteria included immunodeficiency, chronic otitis media, and treatment with steroids or other immunomodulatory agents [4, 5]. Infants were enrolled at age 6 months on completion of their primary series of vaccinations, and blood samples were obtained at age 6, 9, 12, 15, 18, 24, and 30–36 months. The protocol and informed consent document were approved by the Rochester General Hospital Institutional Review Board.
Vaccinations and Minimal Protective Antibody Levels
All 499 children received age-appropriate vaccinations with products approved by the US Food and Drug Administration. Specifically, diphtheria toxoid (DT) and acellular pertussis and polyribosylribitol phosphate (PRP)–tetanus toxoid (TT) conjugate vaccines (Sanofi Pasteur or GlaxoSmithKline) were administered as 3 doses at age 2, 4, and 6 months, along with other routine vaccinations.
The minimum protective antibody level for diphtheria toxoid (DT) and TT when measured with enzyme-linked immunosorbent assay is 0.1 IU/mL; for Haemophilus influenza type b polysaccharide, the PRP concentration is 0.15 μg/mL [6, 7]. Correlates of protection for acellular pertussis vaccine antigens have not been established; however, a titer of 8 ELISA units (ELU)/mL has been proposed for pertussis toxoid (PT), pertactin (PRN), and filamentous hemagglutinin (FHA) [8]. Immunoglobulin G antibody levels to DT, TT, PT, FHA, PRN, and PRP were measured by enzyme-linked immunosorbent assays, as described elsewhere [9, 10]. Vaccine antigens used in the study were gifts from Sanofi Pasteur and GlaxoSmithKline.
LVR Group Definition
We performed a preliminary survey of antibody levels in 107 children against 13 vaccine antigens: DT, TT, PT, FHA, PRN, PRP hepatitis B, Streptococcus pneumoniae capsular polysaccharides 6B, 14, and 23F, and polio serotypes 1, 2, and 3 at age 9–12 months. We defined low vaccine responders (LVRs) as those infants who had subprotective antibody responses to >50% of tested vaccine antigens; all others were normal vaccine responders (NVRs). Statistical analysis of these responses showed that a subprotective response to ≥4 of 6 antigens (≥66% among DT, TT, PT, FHA, PRN, and PRP) could be used to distinguish LVRs from NVRs in a manner that was not significantly different from analysis of all 13 antigens. Therefore, antibody responses to these 6 antigens were measured in all 499 children.
T-Cell Analysis
Cell stimulation and intracellular cytokine profiling procedures were standardized in our laboratory after adaptation from elsewhere [11–13]. Peripheral blood mononuclear cells (PBMCs) were plated at 1 × 106 cells/mL and stimulated with anti-CD3– and anti-CD28–coated beads (Invitrogen), with unstimulated negative controls. Additional anti-CD28 and anti-CD49d (BD) were added to all wells to provide costimulation. PBMCs were then incubated for 2 hours at 37°C. Golgi transport inhibitors (brefeldin A and monensin; BD), were added and incubation continued for an additional 4 hours. Cells were then stained according to protocols described elsewhere [11–13] for surface markers (CD3, CD4, CD8a, CD45RA, and CD69, Biolegend) and cytokines (interferon [IFN] γ, interleukin 2 [IL-2], tumor necrosis factor [TNF] α, and interleukin 4; Biolegend). Flow cytometry analysis was initially performed using FlowJo software(Version 10). Lymphocytes were identified based on forward- and side-scatter properties, followed by sequential gating on CD3+, CD4+CD8+, and CD69+ subpopulations, and the cytokine producing cell numbers were calculated as a fraction of total CD4+ or CD8+ T cells in each sample. Flow cytometry data were also analyzed using the automated algorithm SWIFT (scalable weighted iterative flow-clustering technique) [14]. Activated cell clusters were identified by comparing the stimulated and unstimulated samples using the Wilcoxon test, followed by the Benjamini–Hochberg correction [15] for multiple comparisons (5% false discovery rate).
B-Cell Analysis
Frozen PBMCs were thawed and plated at 1 × 106 cells/mL in Roswell Park Memorial Institute medium (supplemented with 10% fetal bovine serum, 10 000 IU/mL each of penicillin and streptomycin, and 50 µmol/L β-mercaptoethanol) and cultured with 3 µg/mL CpG ODN-2006 (Invitrogen); 10 ng/mL IL-2, 10 ng/mL interleukin 10 (IL-10), 10 ng/mL interleukin 15, and 100 ng/mL interleukin 21 (Cell Sciences) for 5 days to increase B-cell frequency, as described elsewhere [16]. After incubation the cells were washed with phosphate-buffered saline and stained with live-dead stain (Live-Dead Aqua; Life Technologies) along with anti-CD19 and anti-CD27 antibodies (BioLegend). Flow cytometry results were analyzed using FlowJo Version 10, as described above.
APC Analysis
Expression of major histocompatibility complex (MHC) II was measured by flow cytometry on monocytes (HLA-DR+CD14+CD16+), conventional dendritic cells (cDCs) (HLA-DR+CD14lowCD16lowCD11chiCD303−), and plasmacytoid dendritic cells (pDCs) (HLA-DR+CD14lowCD16lowCD11c−CD303+) (BioLegend) after overnight rest followed by 6 hours culture without Toll-like receptor (TLR) agonists. PBMCs were also stimulated with 1 µg/mL TLR7/8 agonist R848 (Invivogen) for 24 hours. Cell pellets were stabilized using RNAlater (Sigma Aldrich) and stored at −20°C. RNA was extracted using an RNeasy kit and reverse transcribed using the RT2 First Strand Kit (Qiagen). Samples were analyzed with real-time polymerase chain reaction using primers for TLRs, intracellular signaling molecules, and cytokines (SA Biosciences). Results were normalized to the average of 18S ribosomal RNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) values and calculated as relative expression (2−ΔCt) or fold change (2−ΔΔCt) [17, 18].
Statistics
Longitudinal models for log-transformed titer responses were fit by generalized estimating equations using exchangeable correlation structure to model subject-level repeated measures in the R computing environment (www.r-project.org). The Mann–Whitney U test was used to compare samples (comparisons performed with GraphPad Prism Version 6; GraphPad).
RESULTS
Defining the LVR Population Based on Antibody Levels
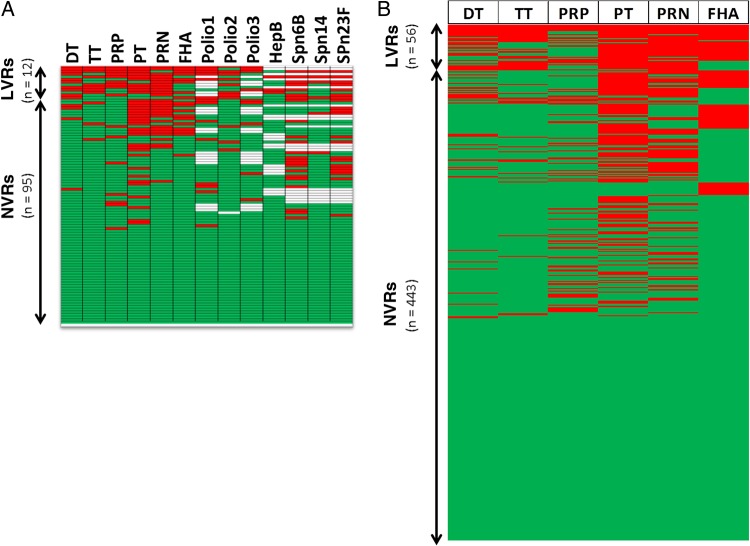
All 499 children in our study population received primary vaccines according to the US immunization schedule. First, we measured antibody titers against 13 antigens in a preliminary sample of 107 children, and we found that a definable subset of these children had subprotective responses to >50% of these antigens (Figure 1A). This same subset of children could be recognized on the basis of subprotective responses to ≥4 vaccine antigens (66%) of 6 (PT, PRN, FHA, DT, TT, and PRP). Antibody titers against these 6 vaccine antigens were measured in the serum samples of all 499 children. We detected subprotective antibody levels against PT (in 34.9% of children), PRN (27.1%), FHA (17.6%), PRP (15.4%), DT (12.6%), and TT (10.6%). LVR children (those with subprotective antibody titers against ≥4 [66%] of the 6 tested vaccine antigens) constituted 11.2% of the population (56 children) (Figure 1B). Of LVRs, 68% (38 children) went on to attain protective levels to all the tested vaccines by the age of 3 years; the others did not provide samples (data not shown).
Figure 1.
Serum antibody titers against vaccine antigens. A, Immunoglobulin (Ig) G titers from 107 children against 13 vaccine antigens. Low vaccine responders (LVRs) were defined as those with subprotective responses against ≥50% of tested antigens. Six of these vaccine antigens can be used to define LVR equally well. B, IgG titers from 499 children (56 LVRs and 443 normal vaccine responders [NVRs]) against vaccine antigens. LVR children are defined as those with subprotective antibody responses against ≥4 of the 6 tested vaccine antigens (DT, TT, PRP, PT, PRN, and FHA). Each row represents a single child. Green indicates above protection; red, below protection; white, not tested. Abbreviations: DT, diphtheria toxoid; FHA, filamentous hemagglutinin; HepB, hepatitis B; PRN, pertactin; PRP, polyribosylribitol phosphate; PT, pertussis toxoid; Spn, Streptococcus pneumoniae; TT, tetanus toxoid.
T-Cell Responses
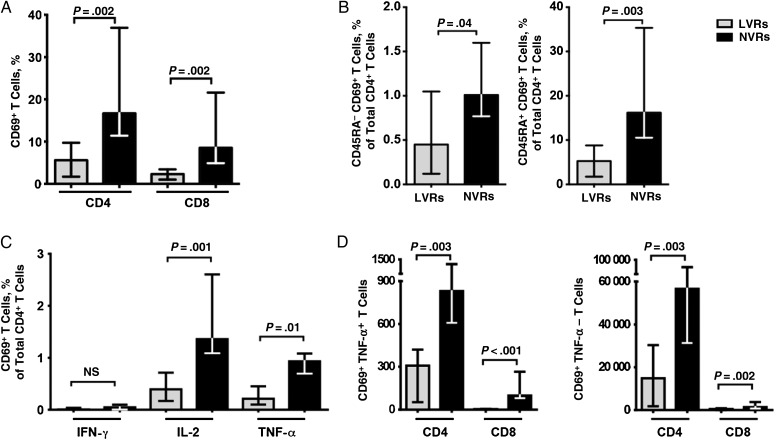
We examined in vitro T-cell responses to polyclonal stimulation with anti-CD3/anti-CD28 antibodies. Significantly lower percentages of total CD4+ and CD8+ T cells became activated after polyclonal stimulation in LVRs compared with NVRs, as measured by expression of the activation marker CD69 (both P = .002) (Figure 2A). Strikingly, this reduction was observed in both memory (CD45RA−) and naive (CD45RA+) subsets of CD4+ T cells (Figure 2B). The frequencies of activated memory CD4+ T cells producing IL-2 and TNF-α were also significantly lower among LVRs (P = .001 and P = .01, respectively; Figure 2C).
Figure 2.
Reduced T-cell function in children who were low vaccine responders (LVRs). A, Diminished CD69 expression by bulk CD4+ and CD8+ T cells from LVR children in response to polyclonal anti-CD3/CD28 stimulation. B, Diminished CD69 expression by both memory and naive CD4+ T-cell subsets from LVR children after polyclonal stimulation. C, Reduced expression of interleukin 1 (IL-2) and tumor necrosis factor (TNF) α by activated CD4+ T cells from LVR children. D, Computational SWIFT (scalable weighted iterative flow-clustering technique) analysis reveals reduced TNF-α expression by activated CD4+ and CD8+ T cells from LVR children. Bars depict medians and interquartile ranges for 10 LVRs and 10 NVRs. Abbreviations: IFN, interferon; NS, not significant; NVRs, normal vaccine responders.
At the same time, T-cell activation was analyzed by the automated, unbiased algorithm SWIFT. Significant differences in cell counts between LVR and NVR were identified in 37 cell populations (P < .05); 23 comprised activated naive T cells. These were grouped into CD4+ and CD8+ populations, with or without expression of TNF-α. By secondary analysis of these 23 clusters, the numbers of activated cells in all 4 cell types were all significantly lower in LVRs (each P < .01) than in NVRs (Figure 2D).
Memory B-Cell Frequency
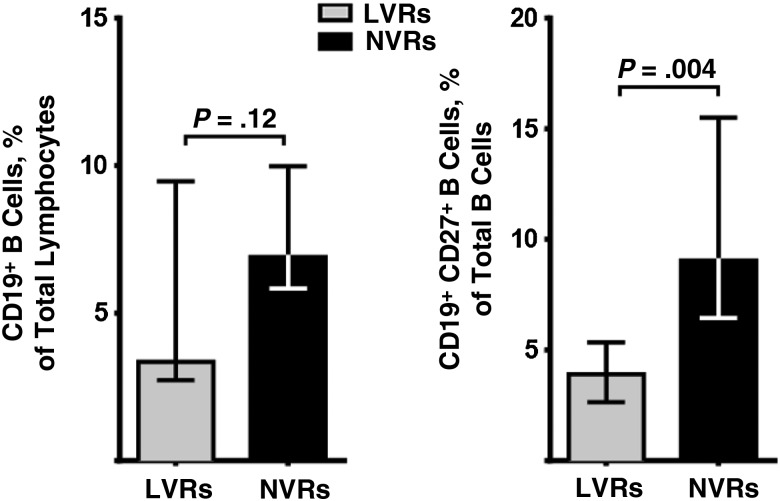
We examined B-cell counts and functional marker expression after 5 days of in vitro culture of whole PBMC in the presence of CpG ODN-2006. We found that total B-cell counts did not significantly differ between LVR and NVR groups. However, CD19+CD27+ memory B-cell frequencies were significantly lower (P = .004) in LVRs (Figure 3).
Figure 3.
Total B-cell counts in peripheral blood mononuclear cells from low vaccine responders (LVRs) and normal vaccine responders (NVRs) are comparable, but memory B-cell frequency is significantly lower in LVRs (n = 10; bars represent medians and interquartile ranges).
Professional APC Responses
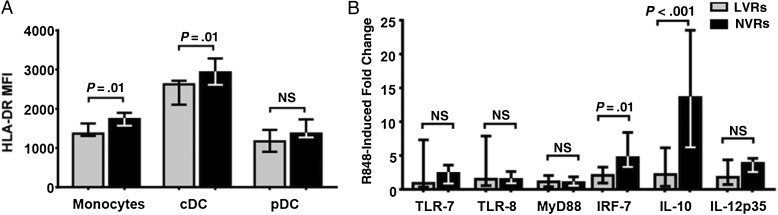
We examined APC responses by stimulating whole PBMC with resiquimod (R848), a synthetic TLR7/8 agonist that can simultaneously activate all 3 APC subsets, because human pDCs express TLR7, whereas monocytes and cDCs express TLR8 [19]. MHC II (HLA-DR) expression on monocytes and cDCs (but not pDCs) was significantly lower in LVRs (P = .01; Figure 4A). However, the frequencies of monocyte and dendritic cell subsets were not significantly different.
Figure 4.
Diminished antigen-presenting cell (APC) responses in low vaccine responder (LVRs). A, Baseline major histocompatibility complex II mean fluorescence intensity (MFI) of HLA-DR in peripheral blood unstimulated APC subsets from LVRs (n = 9) and normal vaccine responder (NVRs; n = 17) assessed with flow cytometry. B, Fold change of Toll-like receptor (TLR) signaling pathway genes in response to 24-hour stimulation with 1 µg/mL R848 (n = 14; bars represent medians and interquartile ranges). Abbreviations: cDCs, conventional dendritic cells; IL-10, interleukin 10; IL-12p35, interleukin 12p35; IRF7, interferon regulatory factor 7; MyD88, myeloid differentiation primary response gene 88; NS, not significant; pDCs, plasmacytoid dendritic cells.
To determine whether LVR subjects had defects in TLR and downstream signaling defects, we evaluated the mRNA baseline and stimulated expression levels of innate transcription factors, including Toll-like receptor (TLR) 2, TLR4, TLR7, myeloid differentiation primary response gene 88 (MyD88), TIR-domain-containing adapter-inducing interferon-β (TRIF), interleukin-1 receptor-associated kinase 4 (IRAK4), interferon regulatory factor (IRF) 3, and IRF7, as well as cytokine transcripts interleukin 12p35, IL-10, and IFN-β. Induction of IL-10 and IRF7, a critical transcription factor of the TLR7-MyD88-IRF7-IFN-α/β signaling pathway [20], was significantly lower in LVR children (Figure 4B). This suggests potential defects in the NF-κB pathway as TLR7 signals through MyD88-dependent activation of IRF7 and NF-κB [20]. None of the TLRs or intracellular innate transcription factors tested differed significantly in baseline expression levels.
DISCUSSION
We have conducted a number of experiments on systemic immune cell function in children with low antibody responses to pediatric vaccine antigens. These children display distinctive features in all 3 major immune cell lineages: LVR infants had reduced memory B-cell frequency, diminished naive and memory T-cell activation in response to polyclonal stimulation, and lower MHC II expression on APCs. Thus, our data suggest that it may be possible to classify LVRs as an immunologically distinct subpopulation of children.
Pichichero et al [5] have published elsewhere a description of stringently defined otitis-prone children, who have poor immune responses to natural antigen exposure, reflected in a propensity toward asymptomatic nasopharyngeal colonization and middle ear infections [9, 10, 21–24]. After identifying LVRs, we found that 23% of stringently defined otitis-prone children were LVRs, compared with 11% of the population at large, indicating that there may be a connection between recurrent otitis media and the inability to mount effective vaccine responses. Importantly, prior studies of such children showed that suboptimal levels of pneumococcal antigen–specific memory B cells and serum antibodies were associated with more frequent pneumococcal infections [10, 23].
In addition to protective humoral responses, there is growing evidence that both B cells and antigen-specific CD4+ T cells contribute to long-lasting protective immunity after vaccination [25–27]. In the present study, we found a clear reduction in the ability of naive CD4+ T cells in LVRs to respond to polyclonal stimulation and produce cytokines. This reduction was also observed in memory subsets of CD4+ and CD8+ T cells. This finding suggests that there is an intrinsic defect in LVR T cells. In particular, the nonresponsiveness of naive T cells strongly suggests that this defect does not depend on patient-specific vaccination and infection histories, because these cells by definition have not been affected by previous antigen exposure.
The ability to generate a protective response depends heavily on effective costimulation and antigen presentation by APCs [28–30]. The current study showed lower MHC II expression by resting APCs, indicating that APCs from LVRs might have a constitutively diminished ability to activate T cells. On stimulation with the TLR7/8 agonist R848, we observed significantly reduced transcriptional up-regulation of IRF7, a transcription factor heavily involved in the regulation of type I IFN responses in APC [31]. We also observed reduced up regulation of IL-10, which might have implications for B-cell class switching [32].
The majority of LVR children attain protective antibody titers against the vaccine antigens tested in this study by age 2–3 years; a limitation of our study is that we were unable to obtain measurements on the remaining children at later ages. There are 2 possible explanations for the observed tendency of LVR children to attain normal antibody responses as they age. First, LVR children may have delayed maturation of the immune system; that is, they may remain in a neonatal-like immune state for longer but eventually normalize. Our observations of diminished type I IFN signaling in LVR children are consistent with this hypothesis, because (1) neonates are well known to have diminished type I immune responses [28, 33] and (2) in samples from older children, we observe no differences in type I IFN signaling between LVR and NVR children (unpublished results). Alternatively, there may be a specific mechanism common to all LVR children that reduces responses in very young children but is masked by compensatory effects later in life. Whether the LVR defect is temporary or permanent, this phenotype could be triggered by many different risk factors, including genetic effects, infection history, and environmental effects, and further study of this population is necessary to answer these questions.
The question also arises whether LVR children represent merely the tail end of a biologic continuum. Whether or not this ultimately proves to be the case, our data show that this group can be distinguished by several distinctive immunologic markers. The reasons for the well-known pattern of vaccines failing to generate protective antibody levels in approximately 10% of children have not been closely examined. This study is the first to demonstrate that these suboptimal vaccine responses are connected with differences in immune cell functions. Better understanding of these immunologic differences may contribute to improvements in vaccine design and administration.
We observed that LVR children among our study subjects experience more frequent viral upper respiratory tract infections, middle ear infections, and lobar pneumonia (data not shown), indicating that their immune status may render them more susceptible to various infections. Although individual LVR children do not have protective responses to vaccination, they are probably protected from infection by herd immunity. In the United States and other countries where parental refusal of vaccines has increased, herd immunity may become threatened, which could have serious consequences for this population. We plan to study the LVR cohort further, anticipating that insights gained may help to identify methods to improve less protective vaccines and/or to develop tailored vaccines for LVR children.
Notes
Acknowledgments. We thank the staff at Legacy Pediatrics and the children and parents who agreed to participate in this study. We also thank Dr Robert Zagursky for helpful comments and discussion.
Financial support. This work was supported by the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (grant R01-08671). Sanofi Pasteur and GlaxoSmithKline provided vaccine antigens as gifts for the assays performed.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1.Fay KE, Lai J, Bocchini JA Jr. Update on childhood and adolescent immunizations: selected review of US recommendations and literature: part 1. Curr Op Pediatr 2011; 23:460–9. [DOI] [PubMed] [Google Scholar]
- 2.PrabhuDas M, Adkins B, Gans H et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol 2011; 12:189–94. [DOI] [PubMed] [Google Scholar]
- 3.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res 2009; 65:98R–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedel V, Zilora S, Bogaard D, Casey JR, Pichichero ME. Five-year prospective study of paediatric acute otitis media in Rochester, NY: modelling analysis of the risk of pneumococcal colonization in the nasopharynx and infection. Epidemiol Infect 2014; 142:2186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis prone. Pediatr Infect Dis J 2013; 32:1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47:401–9. [DOI] [PubMed] [Google Scholar]
- 7.Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis 2013; 56:1458–65. [DOI] [PubMed] [Google Scholar]
- 8.Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998; 16:1907–16. [DOI] [PubMed] [Google Scholar]
- 9.Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan MN, Almudevar A. Antibody response to Haemophilus influenzae outer membrane protein D, P6, and OMP26 after nasopharyngeal colonization and acute otitis media in children. Vaccine 2010; 28:7184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichichero ME, Kaur R, Casey JR, Xu Q, Almudevar A, Ochs M. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum Vaccin Immunother 2012; 8:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc 2006; 1:1507–16. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis 2011; 204:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver JM, Yang H, Roumanes D et al. Increase in IFNγ-IL-2+ cells in recent human CD4 T cell responses to 2009 pandemic H1N1 influenza. PLoS One 2013; 8:e57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosmann TR, Naim I, Rebhahn J et al. SWIFT-scalable clustering for automated identification of rare cell populations in large, high-dimensional flow cytometry datasets, part 2: biological evaluation. Cytometry A 2014; 85:422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 1995; 57:289–300. [Google Scholar]
- 16.Buisman AM, de Rond CG, Ozturk K, Ten Hulscher HI, van Binnendijk RS. Long-term presence of memory B-cells specific for different vaccine components. Vaccine 2009; 28:179–86. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001; 25:402–8. [DOI] [PubMed] [Google Scholar]
- 18.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008; 3:1101–8. [DOI] [PubMed] [Google Scholar]
- 19.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol 2009; 39:26–35. [DOI] [PubMed] [Google Scholar]
- 20.Love AC, Schwartz I, Petzke MM. Borrelia burgdorferi RNA induces type I and III interferons via Toll-like receptor 7 and contributes to production of NF-κB-dependent cytokines. Infect Immun 2014; 82:2405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J 2011; 30:645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine 2011; 29:1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis 2012; 205:1225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surendran N, Nicolosi T, Kaur R, Pichichero ME. Peripheral blood antigen presenting cell responses in otitis-prone and non-otitis-prone infants. Innate Immun 2016; 22:63–71. [DOI] [PubMed] [Google Scholar]
- 25.Feunou PF, Bertout J, Locht C. T- and B-cell-mediated protection induced by novel, live attenuated pertussis vaccine in mice: cross protection against parapertussis. PLoS One 2010; 5:e10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leef M, Elkins KL, Barbic J, Shahin RD. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J Exp Med 2000; 191:1841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe DN, Kirimanjeswara GS, Harvill ET. Clearance of Bordetella parapertussis from the lower respiratory tract requires humoral and cellular immunity. Infect Immun 2005; 73:6508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol 2014; 10:1171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matos I, Mizenina O, Lubkin A, Steinman RM, Idoyaga J. Targeting antigens to dendritic cells in vivo induces protective immunity. PLoS One 2013; 8:e67453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trumpfheller C, Longhi MP, Caskey M et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med 2012; 271:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautier G, Humbert M, Deauvieau F et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 2005; 201:1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerutti A, Qiao X, He B. Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. Immunol Cell Biol 2005; 83:554–62. [DOI] [PubMed] [Google Scholar]
- 33.Power Coombs MR, Kronforst K, Levy O. Neonatal host defense against staphylococcal infections. Clin Dev Immunol 2013; doi:10.1155/2013/826303. [DOI] [PMC free article] [PubMed] [Google Scholar]