Abstract
Background. Malaria control strategies depend on identifying individuals with parasitemia, who may be asymptomatic but retain the ability to transmit disease. Population-level survey data on parasitemia are limited and traditionally exclude adults and human immunodeficiency virus (HIV)–infected individuals.
Methods. We performed a cross-sectional survey of residents aged 18 months to 94 years in Nankoma, Uganda. Blood specimens were collected using the dried blood spot technique from 9629 residents (87.6%), and samples from a subset of 4131 were tested for malaria parasites, using loop-mediated isothermal amplification. Population-level prevalence was estimated using a weighted proportion, and predictors of parasitemia were identified using a multivariate Poisson regression model.
Results. The community prevalence of parasitemia was 83.8% (95% confidence interval [CI], 82.9%–84.6%). Parasite prevalence was highest among children aged 5–14 years (94.7%) and lowest among adults (61.9%). In analysis that controlled for age, HIV-infected individuals with an undetectable viral load had a lower risk of parasitemia, compared with HIV-uninfected individuals (adjusted relative risk, 0.16; 95% CI, .10–.27; P < .001).
Conclusions. In a rural Ugandan community, 2 years after distribution of long-lasting insecticide-treated bed nets, the prevalence of malaria parasitemia was high across all ages, peaking in school-aged children. Persons with well-controlled HIV infection had a lower risk of parasitemia, presumably reflecting access to HIV care.
Keywords: malaria, parasitemia, HIV, LAMP
After decades of policies aimed at controlling malaria in Africa, ambitious new goals focusing on malaria elimination require a paradigm shift from identifying only symptomatic cases to finding all patients with parasitemia [1–3]. In areas of high endemicity, a majority of individuals with parasitemia may be asymptomatic owing to the development of partial immunity [4, 5]. These asymptomatic carriers retain the ability to transmit disease [6, 7] and create a reservoir of parasites that drives transmission.
Parasite prevalence is defined as the proportion of a population with detectable parasites in their blood. Prevalence is generally determined on the basis of data from cross-sectional surveys, and it provides valuable information about the burden of malaria and the level of transmission intensity. Parasite prevalence is the cornerstone of efforts to map malaria endemicity worldwide [8], which are increasingly being used to estimate the impact of malaria control interventions [9]. Parasite prevalence has been shown to have significant heterogeneity over a small spatial scale [10, 11], which has potential implications for targeted malaria interventions [12].
Although critical to malaria control and elimination efforts, there are several limitations in the current estimates of parasite prevalence. Parasite prevalence may vary substantially across age [13], but survey data in Africa have largely focused on children and excluded adolescents and adults. In Uganda, national survey data on parasite prevalence, collected in 2009 and 2014, included findings only for children <5 years of age [14, 15]. Because of the small number of children surveyed within each geographic region, these data lack the precision to detect spatial heterogeneity within communities. Furthermore, estimates of parasite prevalence are heavily dependent on the type of diagnostic test used. Most cross-sectional surveys rely on microscopy or rapid diagnostic tests (RDTs), which have limited sensitivity at detecting parasitemia levels of <100 parasites/µL and likely miss a large portion of the parasite reservoir [16, 17]. Molecular assays such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) are more sensitive in detecting low-density infections, with a lower limit of detection of 1–5 parasite/µL [18]. LAMP requires less specialized infrastructure and equipment than PCR and therefore offers the additional advantage of its usability in field settings [19, 20].
There are limited contemporary data on associations between human immunodeficiency virus (HIV) infection and malaria, despite the significant overlap in these 2 epidemics. Older studies involving a range of African populations have reported that HIV infection is associated with an increased risk of parasitemia, clinical malaria, and severe malaria [21–23]. These associations tend to be stronger with increasing levels of immunosuppression [23, 24]. However, many of these studies were conducted before the widespread use of combination antiretroviral therapy (cART) and trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis. More recently, some studies have reported that TMP-SMX prophylaxis is associated with a lower risk of parasitemia among HIV-infected and exposed patients [25, 26], while others have reported no protection [27].
To collect population-level data on malaria parasite prevalence, we performed a cross-sectional survey as part of a universal HIV testing campaign in a 10 992-person community in Eastern Uganda with high malaria transmission intensity, 2 years following a universal long-lasting insecticide-treated bed net (LLIN) distribution campaign. LAMP was used to detect malaria parasites in a stratified random sample of >4000 participants to generate precise estimates of parasite prevalence across all ages and evaluate the spatial heterogeneity of parasitemia. Independent predictors of parasitemia were identified from available covariates of interest, including sex, HIV status, CD4+ T-cell count, and HIV load.
METHODS
Ethics and Protocol
The study protocol was approved by the Makerere University School of Medicine Research and Ethics Committee, the University of California–San Francisco Committee on Human Research, and the Uganda National Council for Science and Technology.
Study Site and Population
Nankoma (population, 10 992 persons) is a rural community in southeastern Uganda, where malaria transmission is perennial [28]. The annual entomological inoculation rate in a nearby rural community was estimated in 2011–2012 to be 310 infectious bites per person per year [29]. LLINs were distributed in the district in September 2012 as part of nationwide, universal bed net distribution campaign.
Study selection criteria included (1) enrollment in The Sustainable East Africa Research on Community Health Study (SEARCH), an ongoing cluster-randomized trial of community-wide health interventions (including universal access to efavirenz-based cART), (2) residence in Nankoma, (3) age of ≥18 months, and (4) willingness to undergo finger prick for collection of blood specimens via the dried blood spot technique (clinical trial information about SEARCH is available at: https://clinicaltrials.gov/ct2/show/NCT01864603). Children <18 months of age were excluded from finger prick–based laboratory testing owing to standard protocols for HIV testing in infants; infants born to HIV-infected mothers were referred to an early infant diagnosis program for antigen-based testing.
Sample Collection and Sample Size
Sample collection took place between September and December 2014, at a community health campaign. Design and implementation of health campaigns has been described previously [30]. Briefly, community residents were invited to attend a health campaign in their village, which offered diagnostic, treatment, and referral services, including rapid HIV testing. In addition to the usual program of diagnostic and treatment services, all participants provided verbal consent for finger prick and collection of blood specimens by the dried blood spot technique for determination of malaria parasitemia, regardless of symptoms. The dried blood spot technique involved spotting 100 µL of whole blood onto filter paper, drying the blood completely, and transporting the dried samples to a local laboratory within 48 hours for storage in a −20°C freezer.
Laboratory Methods
DNA extraction for LAMP was performed using Chelex-based extraction, as previously described [31]. LAMP was performed using Eiken Loopamp Malaria Pan Detection Kit reaction tubes, which contain vacuum-dried reagents specific for amplification of Plasmodium species mitochondrial DNA, per the manufacturer's guidelines (the complete standard operating procedure is available at: http://www.finddiagnostics.org/programs/malaria-afs/lamp/standard_procedures/index.html). DNA extraction and amplification took place in Uganda. Three positive controls (a 10 parasites/µL blood spot extraction product, a 1 parasite/µL blood spot extraction product, and a Loopamp kit–positive control) and 2 negative controls (a 0 parasites/µL extraction product and a Loopamp-negative control) were included with each cycle of LAMP reactions, and LAMP reactions were repeated if controls did not produce their expected results. A LAMP reaction was considered positive for Plasmodium species DNA if visible fluorescence was observed. A random subset of 80 LAMP-positive and 80 LAMP-negative samples was selected for quality control testing. For these 160 samples, LAMP was performed in triplicate to ensure the internal validity of results, and the extracted DNA was shipped to San Francisco for confirmatory testing with cytochrome b gene PCR, as previously described [32, 33].
Laboratory methods for rapid HIV antibody testing and finger prick–based measurement of HIV load and CD4+ T-cell count have been described previously [34]. The lower limit of detection for HIV RNA using this method is 486 copies/mL, and samples below this threshold were considered to have an undetectable viral load.
Data Management and Statistical Analysis
Data were analyzed using Stata (version 13; StataCorp, College Station, Texas). The primary outcome of interest was the detection of malaria parasites, defined as a positive LAMP reaction. All HIV-infected participants were included in LAMP. For HIV-uninfected persons, 800 samples were selected by equal-allocation stratified random sampling per each of 5 prespecified age groups (18 months–5 years, 6–10 years, 11–25 years, 26–45 years, and >45 years). These age groups were chosen to ensure adequate numbers of adolescents and adults in the study. Sample size calculations were based on the assumption of a parasite prevalence of 30% among children <5 years old [14], which demonstrated that a sample size of 4300 would provide 80% power to have a precision of 2.2% around a point estimate of overall parasite prevalence. By using weighted analysis, a point estimate and 95% confidence interval (CI) of parasite prevalence was generated for the entire study population. We used a locally weighted scatterplot smoothed curve to display the parasite prevalence across age. We used a multivariate Poisson regression model to assess for independent predictors of malaria parasitemia for the total population and the subset of HIV-infected individuals. A P value of <.05 was considered statistically significant. SaTScan software was used to detect spatial clusters of malaria parasitemia within the study area, using a Bernoulli model. A circular window was used to systematically scan the area, with the maximum proportion of the population that a cluster could contain set at 50%. Statistical significance of the clusters was explored by means of 999 Monte Carlo replications. Maps were produced using ArcGIS software (version 10.2; ESRI).
RESULTS
Characteristics of Study Participants
A total of 10 992 stable residents of Nankoma were identified from the census population, of whom 10 487 (95.4%) were ≥18 months of age, and 9629 (87.6%) had blood samples obtained using the dried blood technique that were available for LAMP. Samples from all 131 HIV-infected individuals and a random subset of 4000 HIV-uninfected individuals were successfully tested for the presence of malaria parasites, using LAMP (Figure 1). Table 1 shows characteristics of the 4131 study participants selected for LAMP, stratified by HIV status. HIV-infected individuals were significantly older (median age, 40 vs 16 years; P < .001) and more likely to be female (67.9% vs 52.4%; P < .001). Among the 131 HIV-infected participants, 107 (81.7%) were receiving cART and had an undetectable viral load, 13 (9.9%) were receiving cART and had a detectable viral load, 9 (6.9%) were not receiving cART and had a detectable viral load, and 2 (2.0%) had missing viral load data. Among those with a detectable viral load, the median viral load was 56 506 copies/mL. HIV-infected participants had a median CD4+ T-cell count of 559 cells/µL, and 7 persons (5.3%) had a CD4+ T-cell count of <200 cells/µL.
Figure 1.
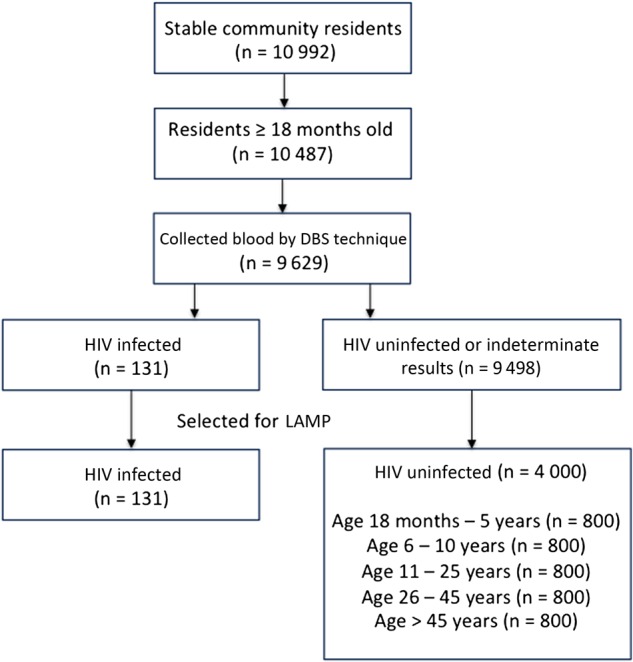
Flow diagram of the study. The diagram summarizes enrollment, sample collection, and selection of a subset of samples for loop-mediated isothermal amplification (LAMP) testing. Among 10 992 stable community residents, we included all 10 487 residents ≥18 months of age, of whom blood specimens were collected from 9629 by the dried blood spot technique (DBS). Of these specimens, 131 were from human immunodeficiency virus (HIV)–infected individuals, and 9498 were from HIV-uninfected individuals. All samples from HIV-infected participants were selected for LAMP, as well as a stratified random sample, based on 5 prespecified age groups, of 4000 samples from HIV-uninfected participants.
Table 1.
Characteristics of Study Participants Selected for Loop-Mediated Isothermal Amplification, by Human Immunodeficiency Virus (HIV) Status
| Characteristic | HIV Uninfected (n = 4000) | HIV Infected (n = 131) |
|---|---|---|
| Age, y | 16 (7–40) | 40 (31–50) |
| Female sex | 2094 (52.4) | 89 (67.9) |
| cART and HIV VL statusa | ||
| Receiving cART, undetectable load | NA | 107 (81.7) |
| Receiving cART, detectable load | NA | 13 (9.9) |
| Not receiving cART, detectable load | NA | 9 (6.9) |
| HIV VL (if detectable)a | NA | 56 506 (5904–168 661) |
| CD4+ T-cell count, cells/µLa | ||
| ≥500 | NA | 76 (58.0) |
| 200–499 | NA | 46 (35.1) |
| <200 | NA | 7 (5.3) |
| Overall | NA | 559 (402–783) |
Data are median (interquartile range) or no. (%) of subjects.
Abbreviations: cART, combination antiretroviral therapy; NA, not applicable; VL, viral load.
a Data are missing for 2 participants.
LAMP Results
Of 4131 blood samples obtained using the dried blood technique that were tested using LAMP, 3285 (79.5%) were positive for malaria parasites. Based on our sampling framework and the age distribution in the study population, the overall prevalence of malaria parasitemia was estimated to be 83.8% (95% CI, 82.9%–84.6%).
A subset of 160 samples, comprising a random subset of 80 with positive and 80 with negative LAMP reactions, was selected for LAMP in triplicate, followed by cytochrome b gene PCR. Among the 160 samples, 147 (91.9%) had concordance among all 3 LAMP results. In the remaining group of 13 discordant samples, 11 of 13 (84.6%) were initially LAMP negative but had follow-up LAMP that yielded positive reactions. By using PCR as the gold standard, LAMP had a sensitivity of 80.9% (76 of 94 samples) and a specificity of 93.9% (62 of 66 samples).
Risk Factors Associated With Malaria Parasitemia
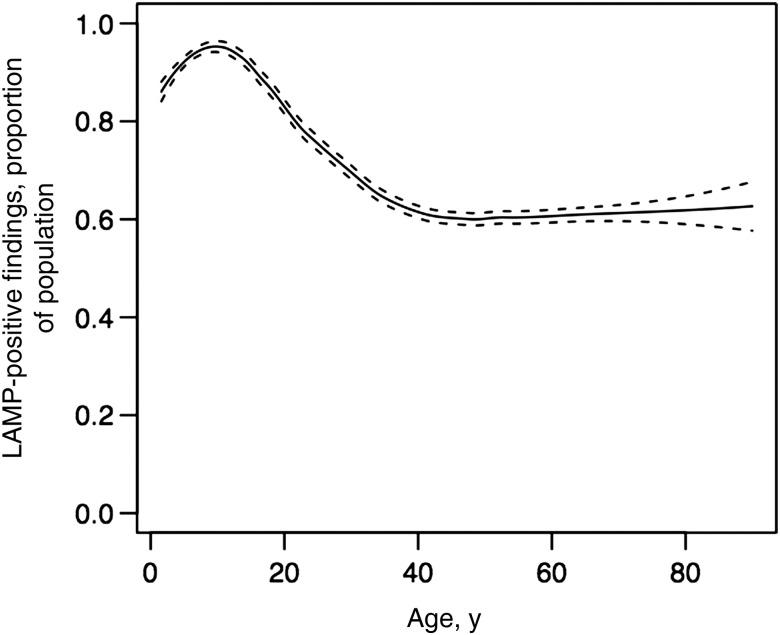
Figure 2 shows the proportion of the sampled population with parasitemia, by age. Among children <5 years old, each increased year of age was associated with a 2.7% increase in the risk of parasitemia. Between the ages of 5 and 14 years, there was no significant change in the risk of parasitemia for an additional year of age. The risk of parasitemia steadily declined between 15 and 39 years, with each increased year of age associated with a 1.4% reduction in the risk of parasitemia. For persons aged ≥40 years, there was no significant change in risk with increasing age. Based on age categories, parasite prevalence was highest among children aged 5–14 years (94.7%) and lowest among adults aged >40 years (61.9%; Table 2). Female sex was associated with a modest but statistically significantly lower risk of parasitemia, after adjustment for age and HIV status (adjusted relative risk [aRR], 0.96; 95% CI, .93–.99; P = .005). Compared with HIV-uninfected individuals, HIV-infected individuals with a detectable viral load had a similar risk of parasitemia (aRR, 0.84; 95% CI, .59–1.20; P = .341), whereas HIV-infected individuals with an undetectable viral load had a much lower risk of parasitemia (aRR, 0.16; 95% CI, .10–.27; P < .001), after adjustment for other covariates of interest (Table 2).
Figure 2.
Proportion of sampled population with parasitemia, by age. The fitted line represents locally weighted scatterplot smoothed curve, showing the trend in the proportion of study participants with malaria parasitemia with increasing age, with 95% confidence intervals shown in dashed lines. Abbreviation: LAMP, loop-mediated amplification.
Table 2.
Association Between Covariates of Interest and Infection With Malaria Parasites
| Covariate | LAMP Positive, Proportion (%) | Univariate RR (95% CI) | P Value | Multivariate RR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, y | |||||
| <5 | 560/628 (89.2) | 0.94 (.91–.97) | <.001 | 0.94 (.91–.97) | <.001 |
| 5–14 | 1241/1311 (94.7) | 1.0 (reference) | … | 1.0 (reference) | … |
| 15–19 | 221/260 (85.0) | 0.90 (.85–.95) | <.001 | 0.90 (.85–.95) | <.001 |
| 20–29 | 318/432 (73.6) | 0.78 (.73–.82) | <.001 | 0.80 (.76–.85) | <.001 |
| 30–39 | 288/439 (65.6) | 0.69 (.65–.74) | <.001 | 0.74 (.69–.79) | <.001 |
| ≥40 | 657/1061 (61.9) | 0.66 (.62–.69) | <.001 | 0.69 (.65–.72) | <.001 |
| Sex | |||||
| Male | 1609/1948 (82.6) | 1.0 (reference) | 1.0 (reference) | ||
| Female | 1676/2183 (76.8) | 0.93 (.90–.96) | <.001 | 0.96 (.93–.99) | .005 |
| HIV infection and load status | |||||
| Uninfected | 3259/4000 (81.5) | 1.0 (reference) | 1.0 (reference) | ||
| Infected, detectable load | 13/22 (59.1) | 0.73 (.52–1.04) | .083 | 0.84 (.59–1.20) | .341 |
| Infected, undetectable load | 12/107 (11.2) | 0.14 (.08–.22) | <.001 | 0.16 (.10–.27) | <.001 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; LAMP, loop-mediated amplification; RR, relative risk.
Among HIV-infected individuals, those ≥20 years of age had a much lower risk of parasitemia than those <20 years of age (Table 3). After adjustment for age and sex, having an undetectable viral load (aRR, 0.25; 95% CI, .12–.54; P < .001) and a CD4+ T-cell count of ≥500 cells/µL as compared to <200 cells/µL (aRR, 0.29; 95% CI, .12–.70; P = .006) were independently associated with a lower risk of parasitemia (Table 3).
Table 3.
Association Between Covariates of Interest and Infection With Malaria Parasites Among Human Immunodeficiency Virus (HIV)–Infected Persons
| Covariate | LAMP Positive, Proportion (%) | Univariate RR (95% CI) | P Value | Multivariate RR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, y | |||||
| <20 | 7/9 (77.8) | 1.0 (reference) | 1.0 (reference) | ||
| 20–29 | 3/21 (14.3) | 0.17 (.05–.56) | .003 | 0.20 (.05–.74) | .016 |
| 30–39 | 5/35 (14.3) | 0.18 (.07–.43) | <.001 | 0.22 (.06–.84) | .026 |
| ≥40 | 11/66 (16.7) | 0.22 (.11–.41) | <.001 | 0.23 (.08–.63) | .005 |
| Sex | |||||
| Male | 11/42 (26.2) | 1.0 (reference) | 1.0 (reference) | ||
| Female | 15/89 (16.9) | 0.69 (.34–1.39) | .300 | 0.74 (.36–1.53) | .416 |
| HIV VL status | |||||
| Detectable | 13/22 (59.1) | 1.0 (reference) | 1.0 (reference) | ||
| Undetectable | 12/107 (11.2) | 0.22 (.12–.40) | <.001 | 0.25 (.12–.54) | <.001 |
| CD4+ T-cell count, cells/µL | |||||
| <200 | 4/7 (57.1) | 1.0 (reference) | 1.0 (reference) | ||
| 200–499 | 10/46 (21.7) | 0.38 (.16–.90) | .028 | 0.37 (.15–.90) | .028 |
| ≥500 | 10/76 (13.2) | 0.24 (.10–.57) | .001 | 0.29 (.12–.70) | .006 |
Abbreviations: CI, confidence interval; LAMP, loop-mediated amplification; RR, relative risk; VL, viral load.
Spatial Clustering
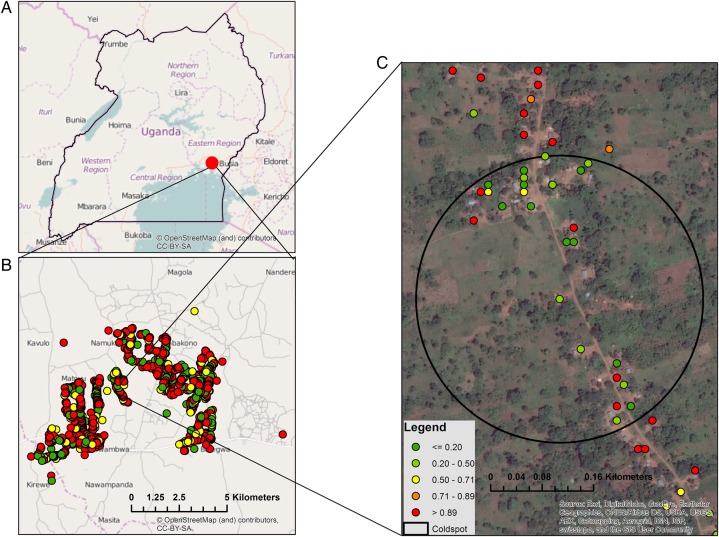
Because of the overall high parasite prevalence within the study community, SaTScan was used to explore areas of spatial heterogeneity in terms of so-called cold spots, defined as clusters of households with a lower parasite prevalence than would be expected by chance. Complete global positioning system data were available for 4066 of 4131 tested participants (98.4%), who lived in 1642 households in an area of approximately 90 km2. SaTScan revealed 1 statistically significant cold spot, which included 27 households and 65 study participants in an area of 0.15 km2 (Figure 3). Within the cold spot, parasite prevalence was 49.2%, compared with 79.5% in the remainder of the study area, corresponding to an unadjusted risk of 0.61 (95% CI, .46–.82; P = .001) and a risk of 0.66 (95% CI, .51–.85; P = .002) after adjustment for age, sex, and HIV status. Maps produced with data from ESRI ArcGIS demonstrated no visually apparent geographic feature, aquatic body, or urban area within this spatial cluster.
Figure 3.
A, Location of the study site in Uganda. B, Locations of households within the study site, with color denoting the proportion of household residents who had positive loop-mediated amplification findings. C, Location of a statistically significant cold spot and associated households.
DISCUSSION
We report here the population-level prevalence of malaria parasitemia among HIV-infected and uninfected persons in a rural Ugandan community with intense malaria transmission, 2 years following an LLIN distribution campaign. We observed an extremely high parasite prevalence in this community, using a sensitive molecular diagnostic test. The Uganda Malaria Indicator Survey, conducted in the same year as our survey in this region, reported a parasite prevalence of 36.5%, based on microscopy, and 49.2%, based on RDTs, among children <5 years of age [15]. In contrast, our study estimates a parasite prevalence of 89.2% among children <5 years of age (Table 2). These findings are consistent with those of several other studies, conducted across a range of settings, which reported that microscopy and RDTs substantially underestimate the true prevalence of infection [20, 35].
The use of molecular techniques has led to consistently higher estimates of parasite prevalence than microscopy [36], although the majority of published prevalence data are based on PCR findings. In an area in Eastern Uganda near our study site, the PCR-based parasite prevalence from community surveys conducted from 2012 to 2013 was 63.2%, 80.2%, and 45.7% among persons <5 years, 5–15 years, and >15 years of age, respectively. [37]. Our study is among the first to use LAMP to estimate parasite prevalence in older children and adults [19, 38], and its large sample size allowed us to provide precise estimates across an entire community in a high-transmission setting. We demonstrate that parasite prevalence peaks in school-aged children and then declines after adolescence, plateauing around age 40 years. This pattern is consistent with the gradual development of antimalarial immunity with increasing age and has been described in other studies [13, 37]. However, because estimates of prevalence depend on the limit of detection of the chosen diagnostic test, it is also possible these patterns reflect an inability to detect low-density infections in adults [39, 40].
Our quality control experiments indicate that LAMP, as compared to PCR, may be slightly less sensitive but very specific for malaria parasite infection. We therefore are confident that our reported prevalence, based on LAMP positivity, does not overestimate the true prevalence of parasitemia in this community. This study confirms that when using a sensitive diagnostic test, a much higher proportion of the population is infected than has been previously reported for the region. Although we found modest heterogeneity in the risk of parasitemia between the sexes and within one small cold spot, our most notable finding was that the majority of community residents, regardless of their age or other demographic characteristics, were infected with malaria parasites.
It should be emphasized that this survey took place in the context of standard malaria control interventions, including presumed high LLIN coverage following a universal distribution campaign, and use of artemisinin-based combination therapy for the treatment of malaria, which has been the policy in Uganda since 2004. A 22-year longitudinal study of malaria prevalence in a community in Senegal recently described a stepwise decrease in parasite prevalence within 2 years of introduction of non–chloroquine-based malaria treatment and then again within 2 years of distribution of LLINs [41]. Although we do not have a preintervention comparator group, we have demonstrated an extremely high parasite prevalence 2 years after universal LLIN distribution in our community. Recent data indicate that the scale up of LLINs was the largest contributor to the marked reduction in the malaria burden in sub-Saharan Africa observed between 2000 and 2015 [9]. However, this study was modeled to estimate the impact of interventions on the incidence of clinical disease, rather than the prevalence of asymptomatic infections. Our study joins a body of work that suggests that LLIN distribution, while an important component of malaria control, is alone unlikely to be sufficient for making a lasting impact on the parasite reservoir and malaria transmission in settings of high endemicity [42, 43].
Interestingly, HIV-infected participants with an undetectable viral load had a >6-fold lower risk of being infected with malaria parasites, compared with HIV-uninfected participants. This is in contrast to findings published before widespread availability of cART and TMP-SMX prophylaxis, which reported an increased risk of parasitemia and the clinical consequences of malaria among HIV-infected persons [21,23,44]. The apparent protective effect of HIV in this study was likely a result of early HIV diagnosis and linkage to care, a primary intervention in the parent study. TMP-SMX prophylaxis, which has previously been shown to reduce the burden of malaria in HIV-infected adults and HIV-exposed children [25, 45], may be part of the causal pathway that protects people with well-controlled HIV infection from parasitemia. We observed that, among HIV-infected participants, those with undetectable viral loads and higher CD4+ T-cell counts were at lower risk of parasitemia, a finding consistent with those of previous reports that the burden of malaria increases with advanced HIV disease and greater immunosuppression [21, 23]. Interestingly, a recent cross-sectional study suggests that TMP-SMX use is not associated with reduced rates of parasitemia in HIV-infected individuals in Mozambique [27]. In an era when early access to cART is becoming the standard of care in Africa [46], more work is needed to clarify the effect of HIV therapeutics on malaria parasitemia.
This study had several limitations. Microscopy results were unavailable, precluding the ability to estimate parasite density or directly compare the diagnostic accuracy of microscopy to that of LAMP. Given the cross-sectional study design and lack of clinical data, we were unable to describe the proportion of parasitemic participants who went on to develop symptomatic malaria. Although TMP-SMX was prescribed for all HIV-infected persons in care, TMP-SMX was not directly measured in the blood, limiting our ability to make conclusions about the protective effect of this medication.
We cannot make inferences about transmission on the basis of this study, but our work raises questions about the infectivity of persons with submicroscopic parasitemia. Data from sub-Saharan Africa suggest that children with submicroscopic parasitemia are capable of infecting mosquitoes [47, 48], but future longitudinal studies are needed to better characterize the implications of submicroscopic parasitemia, how it relates to clinical outcomes such as malaria and anemia, and the role it plays in driving transmission.
A better understanding of the epidemiology of malaria parasitemia is critical to allocating resources and assessing the impact of malaria control efforts. It is unlikely that current microscopy- and RDT-based survey data adequately represent the true parasite prevalence in areas of high endemicity. LAMP is a sensitive diagnostic test and usable in field settings, providing a powerful tool for estimating parasite prevalence. A program of malaria control efforts that targets mainly young children and pregnant women will miss a large portion of the parasite reservoir and may shift the burden of infection to older age groups [49]. Progress toward malaria elimination in high-transmission areas will require a multifaceted approach, including interventions that target high parasite prevalence among all age strata.
Notes
Acknowledgments. We thank all study participants for their cooperation; all members of the SEARCH study team and Tororo laboratory staff, for their dedication and hard work; and M. Conrad and P. Rosenthal, for technical support and providing standardized parasite blood spots.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH; grants U01AI099959 and K24 AI051982 to D. H.); and the NIH/University of California–San Francisco Gladstone Institute of Virology and Immunology Center for AIDS Research (mentored scientist award P30-AI027763 to S. K.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kern SE, Tiono AB, Makanga M et al. Community screening and treatment of asymptomatic carriers of Plasmodium falciparum with artemether-lumefantrine to reduce malaria disease burden: a modelling and simulation analysis. Malar J 2011; 10:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogutu B, Tiono AB, Makanga M et al. Treatment of asymptomatic carriers with artemether-lumefantrine: an opportunity to reduce the burden of malaria? Malar J 2010; 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturrock HJW, Hsiang MS, Cohen JM et al. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med 2013; 10:e1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John CC, Tande AJ, Moormann AM et al. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis 2008; 197:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langhorne J, Ndungu FM, Sponaas A-M, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 2008; 9:725–32. [DOI] [PubMed] [Google Scholar]
- 6.Bousema JT, Gouagna LC, Drakeley CJ et al. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J 2004; 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman RE, Kumpitak C, Ponlawat A et al. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol 2004; 41:201–8. [DOI] [PubMed] [Google Scholar]
- 8.Hay SI, Guerra CA, Gething PW et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med 2009; 6:e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt S, Weiss DJ, Cameron E et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousema T, Drakeley C, Gesase S et al. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis 2010; 201:1764–74. [DOI] [PubMed] [Google Scholar]
- 11.Kigozi SP, Pindolia DK, Smith DL et al. Associations between urbanicity and malaria at local scales in Uganda. Malar J 2015; 14:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bousema T, Griffin JT, Sauerwein RW et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med 2012; 9:e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeka A, Nankabirwa J, Mpimbaza A et al. Factors associated with malaria parasitemia, anemia and serological responses in a spectrum of epidemiological settings in Uganda. PLoS One 2015; 10:e0118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uganda Malaria Control Programme, Ministry of Health. Uganda malaria indicator survey 2009. http://dhsprogram.com/pubs/pdf/MIS6/MIS6.pdf Accessed August 2014.
- 15.Uganda Malaria Control Programme, Ministry of Health. Uganda malaria indicator survey 2014–15. Key indicators report. http://dhsprogram.com/pubs/pdf/PR64/PR64.pdf Accessed September 2015.
- 16.Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol 2014; 30:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic Infection in Plasmodium falciparum-Endemic Populations: A Systematic Review and Meta-Analysis. J Infect Dis 2009; 200:1509–17. [DOI] [PubMed] [Google Scholar]
- 18.Oriero EC, Jacobs J, Van geertruyden J-P, Nwakanma D, D'Alessandro U. Molecular-based isothermal tests for field diagnosis of malaria and their potential contribution to malaria elimination. J Antimicrob Chemother 2014; 70:dku343–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook J, Aydin-Schmidt B, González IJ et al. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J 2015; 14:1509–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins H, Gonzalez IJ, Polley SD et al. Highly Sensitive Detection of Malaria Parasitemia in a Malaria-Endemic Setting: Performance of a New Loop-Mediated Isothermal Amplification Kit in a Remote Clinic in Uganda. J Infect Dis 2013; 208:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen C, Karstaedt A, Frean J et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis 2005; 41:1631–7. [DOI] [PubMed] [Google Scholar]
- 22.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS 2004; 18:547–54. [DOI] [PubMed] [Google Scholar]
- 23.Whitworth J, Morgan D, Quigley M et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet 2000; 356:1051–6. [DOI] [PubMed] [Google Scholar]
- 24.Iroezindu MO, Agaba EI, Okeke EN et al. Prevalence of malaria parasitaemia in adult HIV-infected patients in Jos, North-central Nigeria. Niger J Med 2012; 21:209–13. [PubMed] [Google Scholar]
- 25.Sandison TG, Homsy J, Arinaitwe E et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ 2011; 342:d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mermin J, Ekwaru JP, Liechty CA et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet 2006; 367:1256–61. [DOI] [PubMed] [Google Scholar]
- 27.Noormahomed EV, Orlov M, do Rosario V et al. A cross-sectional study of sub-clinical Plasmodium falciparum infection in HIV-1 infected and uninfected populations in Mozambique, South-Eastern Africa. Malar J 2012; 11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okello PE, Van Bortel W, Byaruhanga AM et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg 2006; 75:219–25. [PubMed] [Google Scholar]
- 29.Kamya MR, Arinaitwe E, Wanzira H et al. Malaria Transmission, Infection, and Disease at Three Sites with Varied Transmission Intensity in Uganda: Implications for Malaria Control. Am J Trop Med Hyg 2015; 92:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamie G, Kwarisiima D, Clark TD et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One 2012; 7:e43400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 1995; 52:565–8. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz A, Baidjoe A, Rosenthal PJ, Dorsey G, Bousema T, Greenhouse B. The Effect of Storage and Extraction Methods on Amplification of Plasmodium falciparum DNA from Dried Blood Spots. Am J Trop Med Hyg 2015; 92:922–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snounou G. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. In: Clapp JP, ed. Species diagnostics protocols: PCR and other nucleic acid methods. Totowa, NJ: Humana Press, 1995:263–92. [DOI] [PubMed] [Google Scholar]
- 34.Jain V, Liegler T, Kabami J et al. Assessment of Population-Based HIV RNA Levels in a Rural East African Setting Using a Fingerprick-Based Blood Collection Method. Clin Infect Dis 2013; 56:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aydin-Schmidt B, Xu W, González IJ et al. Loop mediated isothermal amplification (LAMP) accurately detects malaria DNA from filter paper blood samples of low density parasitaemias. PLoS One 2014; 9:e103905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu L, van den Hoogen LL, Slater H et al. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 2015; 528:S86–93. [DOI] [PubMed] [Google Scholar]
- 37.Nankabirwa JI, Yeka A, Arinaitwe E et al. Estimating malaria parasite prevalence from community surveys in Uganda: a comparison of microscopy, rapid diagnostic tests and polymerase chain reaction. Malar J 2015; 14:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oriero EC, Okebe J, Jacobs J, Van geertruyden J-P, Nwakanma D, D'Alessandro U. Diagnostic performance of a novel loop-mediated isothermal amplification (LAMP) assay targeting the apicoplast genome for malaria diagnosis in a field setting in sub-Saharan Africa. Malar J 2015; 14:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 2015; 12:e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imwong M, Nguyen TN, Tripura R et al. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand–Myanmar border areas, Cambodia, and Vietnam. Malar J 2015; 14:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trape J-F, Tall A, Sokhna C et al. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22-year longitudinal study. Lancet Infect Dis 2014; 14:476–88. [DOI] [PubMed] [Google Scholar]
- 42.Walldorf JA, Cohee LM, Coalson JE et al. School-age children are a reservoir of malaria infection in Malawi. PLoS One 2015; 10:e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou G, Afrane YA, Vardo-Zalik AM et al. Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: the fall and rise of malaria. PLoS One 2011; 6:e20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ezeamama AE, Spiegelman D, Hertzmark E et al. HIV infection and the incidence of malaria among HIV-exposed children from Tanzania. J Infect Dis 2012; 205:1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suthar AB, Vitoria MA, Nagata JM et al. Co-trimoxazole prophylaxis in adults, including pregnant women, with HIV: a systematic review and meta-analysis. Lancet HIV 2015; 2:e137–50. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Guideline on when to start antiretroviral therapy and pre-exposure prophylaxis for HIV. http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en Accessed October 2015. [PubMed]
- 47.Ouédraogo AL, Bousema T, Schneider P et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One 2009; 4:e8410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider P, Bousema JT, Gouagna LC et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg 2007; 76:470–4. [PubMed] [Google Scholar]
- 49.Mharakurwa S, Mutambu SL, Mberikunashe J et al. Changes in the burden of malaria following scale up of malaria control interventions in Mutasa District, Zimbabwe. Malar J 2013; 12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]