Abstract
Background. Prime-boost regimens comprising ALVAC-HIV (prime) and human immunodeficiency virus type 1 (HIV) Env (boost) induce HIV-specific neutralizing antibody and cell-mediated immune responses, but the impact of boost schedule and adjuvant requires further definition.
Methods. A phase 1 trial was conducted. In part A (open label), 19 volunteers received oligomeric glycoprotein 160 from HIV strains MN and LAI-2 (ogp160 MN/LAI-2) with dose escalation (25, 50, 100 μg) and either polyphosphazene (pP) or alum adjuvant. In part B, 72 volunteers received either placebo (n=12) or recombinant canarypox virus expressing HIV antigens (ALVAC-HIV [vCP205]) with different doses and schedules of ogp160 MN/LAI-2 in pP or alum (n = 60).
Results. The vaccines were safe and well tolerated, with no vaccine-related serious adverse events. Anti–gp70 V1V2 antibody responses were detected in 17 of 19 part A volunteers (89%) and 10%–100% of part B volunteers. Use of a peripheral blood mononuclear cell–based assay revealed that US-1 primary isolate neutralization was induced in 2 of 19 recipients of ogp160 protein alone (10.5%) and 5 of 49 prime-boost volunteers (10.2%). Among ogp160 recipients, those who received pP were more likely than those who received alum to have serum that neutralized tier 2 viruses (12% vs 0%; P = .015).
Conclusions. Administration of ogp160 with pP induces primary isolate tier 2 neutralizing antibody responses in a small percentage of volunteers, demonstrating proof of concept and underscoring the importance of further optimization of prime-boost strategies for HIV infection prevention.
Clinical Trials Registration. NCT00004579.
Keywords: HIV, vaccine, prime-boost, neutralizing antibody, adjuvant, polyphosphazene
A phase 3 trial conducted in Thailand (RV144) provided the first evidence that an human immunodeficiency virus type 1 (HIV) vaccine could confer protective efficacy. The vaccine regimen consisted of an ALVAC-HIV (vCP1521) prime and a glycoprotein 120 (gp120) AIDSVAX B/E boost. The modified intent-to-treat analysis showed an estimated 31.2% efficacy after 42 months. However, efficacy was 60% at 12 months, suggesting waning protection that paralleled decay in the humoral response after vaccination [1, 2]. Infection risk correlated inversely with the level of plasma immunoglobulin G (IgG) to the gp120 V1V2 conformational epitope and directly with the level of plasma immunoglobulin A (IgA) to HIV Envs [3]. IgG binding antibody responses to scaffolded V1V2 constructs derived from various HIV subtypes and to linear V2 epitopes also inversely correlate with risk [4–6]. HIV vaccine regimens occasionally induce antibody responses capable of neutralizing resistant tier 2 viruses in the TZM-bl [7] or A3R5 [8] neutralization assays but not in the peripheral blood mononuclear cell (PBMC)–based assay [9–11]. Stabilized prefusion Env intermediates were shown to elicit autologous but not heterologous neutralizing antibodies to tier 2 virus following immunization of rabbits and to a lesser extent in macaques [12], but human data are not yet available.
Immunization with Env induces robust responses followed by contraction of circulating antibody and memory B cells [13]. Adjuvants may augment the magnitude and durability of such responses. In guinea pigs, oligometric gp160 from HIV strains MN and LAI-2 (ogp160 MN/LAI-2) elicited robust antibody responses at doses ranging from 5 µg to 40 µg. Responses increased significantly with the addition of alum or polyphosphazene (pP; Avant Immunotherapeutics, Needham, Massachusetts) [14] adjuvant, and high levels of neutralization against MN were detected. Similar observations were made in macaques immunized with 50 µg and 100 µg of ogp160 MN/LAI-2 (Dr Thomas VanCott, unpublished data). We conducted a randomized phase 1 clinical trial to assess the safety and immunogenicity of ogp160 MN/LAI-2 adjuvanted with either pP or alum and following priming with live recombinant canarypox vector (ALVAC-HIV [vCP205]).
MATERIALS AND METHODS
ALVAC-HIV (vCP205)
ALVAC-HIV (vCP205) is a recombinant canarypox virus containing genes encoding subtype B HIV MN gp120 Env and subtype B HIV LAI gp41 Env, Gag, and Pro. Testing of ALVAC candidate vaccines encoding a variety of different genes provided no evidence of toxicity in mammals or humans [15–17]. Freeze-dried vaccine was reconstituted in sterile water, and doses were administered intramuscularly at 106.42 50% tissue-culture infective doses (TCID50). ALVAC-HIV placebo consisted of identical buffer and freeze-drying medium as an active product.
ogp160 MN/LAI-2
ogp160 MN/LAI-2 is a HIV subtype B, CXCR4-tropic Env glycoprotein expressed by VV.TG.9150 vaccinia virus on BHK21 cells (Transgene, Strasbourg, France), as described previously [18]. The placebo control for ogp160 MN/LAI-2 was sterile sodium chloride. pP is a synthetic polymeric water-soluble adjuvant whose properties were recently reviewed [14]. Alum is a widely used aluminum hydroxide–containing adjuvant [19]. Each protein dose contained 0.6 mg of aluminum delivered as 2.4 mg of aluminum hydroxide.
Study Design, Vaccination, and Recruitment
The protocol and informed consent were approved by the Human Use Review Committee, Walter Reed Army Institute of Research (WRAIR). All volunteers provided written informed consent prior to trial activities.
The study design is illustrated in Supplementary Figure 1. Part A was an open-label dose-escalation study of ogp160, and part B was a placebo-controlled, randomized, double-blinded study of vCP205 with various doses of ogp160 in alum or pP. In both parts A and B, vaccinations were given on days 0, 28, 84, and 168. There were 14 study visits over 336 days. Volunteers were healthy, male and female (nonpregnant and nonlactating) US residents aged 18–55 years at low risk for HIV infection.
Safety Assessment and Clinical Laboratory Evaluations
Volunteers returned 1 week after each vaccination for reactogenicity assessment, adverse event (AE) elicitation, and physical examination. Safety laboratory evaluations were performed at 11 of 14 study visits, including urine pregnancy tests before each injection, and on study days 252 and 336. HIV testing was performed at screening and on study days 0, 168, 196, and 336.
HIV Testing Algorithm
Enzyme immunoassay (EIA) screening was performed using Genetic Systems rLAV (Biorad Laboratories, Redmond, Washington). Reactive samples underwent duplicate EIA testing, using the Vironostika HIV Microelisa System (Organon Teknika, Durham, North Carolina). All samples underwent testing by HIV Western blot (Biorad Laboratories) or Cambridge HIV Western blot (Calypte Biomedical, Alameda, California), with findings interpreted according to the respective instructions of each kit. HIV nucleic acid tests differentiated HIV infection from vaccine-induced seroreactivity in Western blot–positive samples. Plasma was assessed for HIV RNA by using the Roche Amplicor HIV Monitor Test, version 1.0 (Roche Molecular Systems, Indianapolis, Indiana). PBMCs from each vaccinated subject were tested with the Roche Amplicor HIV DNA Test, versions 1.0 and 1.5 (Roche Molecular Systems, Pleasanton, California).
Immunogenicity Assessments
Serum specimens and PBMCs were collected at baseline and at 10 subsequent visits through 6 months after final vaccination. Immunology assays were performed blinded to treatment allocation at the US Military HIV Research Program and the Duke University School of Medicine. Proliferative responses were assessed by incorporation of tritiated thymidine into lymphocytes after incubation with ogp160 MN/LAI-2 and gp160 TH023 LAI-DID Env proteins [1]. Cytotoxic T lymphocytes (CTLs) were expanded by incubation of PBMCs with recombinant vaccinia viruses expressing strain MN env and strain LAI gag and pol for 14 days. CTL activity was measured by chromium release (lysis) of autologous transformed B cells expressing strain MN env, strain LAI gag, or strain pol LAI antigens coincubated with the expanded CTLs [11]. Binding antibodies to HIV gp120/gp140 proteins and V1/V2 scaffolds chosen to assess either vaccine homologous responses or responses to antigens similar to those identified to be correlates of risk in RV144 were measured at days 0 and 182 by a custom multiplex assay as previously described [4, 5, 20]. Additional details are presented in the Supplementary Materials.
Neutralizing antibody activity was measured using 4 assay platforms. Cell line–based assay were used to evaluate replication competent, cell line–adapted HIV neutralization of CXCR4 and CCR5-using viruses in the H9 assay [7–9], using H9-adapted MN, and the A3R5 assay [14–17], using A3R5-adapted US1 “G,” respectively. The TZMbl assay [21] was used with 293T-produced pseudovirus, subtype B US1 and SF162 and subtype C GS015, to evaluate inhibition of viral entry. Primary cell–based PBMC assays [21] were used to evaluate primary isolate neutralization, using subtype B US1, US4, and BZ167 and subtype B/CRF01_AE recombinant CM237. Differences between assay platforms have been previously described [22]. Sera obtained on day 0 and the day of peak immunogenicity (day 182) were screened at 1:10 dilution; neutralization at day 182 was determined as ≥50% inhibition of viral growth when using day 0 values as baseline. Sera with neutralizing activity were further titered in the H9 and A3R5 assays and, in some cases, the PBMC assay.
Statistical Analysis
Laboratory staff remained blinded to the vaccine regimen during analysis. The Fisher exact test was used for comparison of proportions of volunteers exhibiting neutralizing antibodies and lymphoproliferative responses. The significance of association between HLA alleles and CTL responses was assessed using the Fisher exact test with the Bonferroni correction. Comparison of geometric mean titer (GMT) and mean fluorescent intensity antibody responses was performed using a 2-sided Wilcoxon test. P values of <.05 were considered statistically significant.
RESULTS
Enrollment, Participant Flow, and Demographic Characteristics
A total of 224 prospective volunteers were screened. The most common reasons for screen failure (n = 123) were volunteer refusal (n = 32), abnormal laboratory findings (n = 28), and loss to follow-up (n = 27). Forty-one women and 60 men were enrolled; 36% of volunteers were African American, and 59% were white. The median age was 40 years (range, 19–55 years), with group median ages ranging from 32 years (in group 1) to 48 years (in group 10). Ninety-one volunteers completed vaccination and follow-up. Of 10 volunteers not completing the study, 1 was lost to follow-up, 5 had protocol deviations, 3 refused further injections (including 1 placebo recipient), and 2 had other unspecified personal reasons for withdrawal (Supplementary Figure 1).
Safety and Reactogenicity
Reactions recorded following administration of investigational product are shown in Supplementary Table 1. Local reactogenicity occurred in 50%–100% of vaccine recipients and 77% of placebo recipients (P = not significant), without a pattern correlating to dose or adjuvant administered. Pain, the most common injection-related event, was reported by 50%–100% of vaccine recipients and 69% of placebo recipients. Pain was described as moderate in 22.7%, 24.2%, and 15.4% of subunit, prime boost, and placebo recipients, respectively, with the remaining cases of pain being mild. Swelling at the injection site occurred significantly more frequently in vaccinees from groups 5, 6, 9, and 10 (60%, 40%, 39%, and 33%, respectively), compared with placebo recipients, of whom none experienced swelling (P < .05 for all comparisons).
A systemic reaction was observed in 50%–100% of vaccine recipients, compared with 62% of placebo recipients; only group 8 (system reaction incidence, 100%) had findings that differed significantly from those of the placebo group (P = .041). There was no specific correlation between reactions and dose or adjuvant administered. The most common systemic reaction was fatigue, occurring in 50%–73% of vaccine recipients, compared with 31% of placebo recipients (P = not significant). No volunteers had a recorded temperature of >37.8°C. Subjective fever was reported at 7 visits by 6 volunteers (6%), all of whom were vaccine recipients. Fever was categorized as mild in all cases and as possibly or probably related to vaccine in 3 cases and 1 case, respectively.
There were no significant differences in frequencies and patterns of AEs between vaccine and placebo recipients (data not shown). Nine serious AEs were recorded; none were judged to be related to vaccine. One pregnancy was observed in a placebo recipient and was electively terminated for personal reasons, but no pregnancies occurred among vaccine recipients.
Vaccine-Induced HIV Seroreactivity
No positive results of HIV nucleic acid tests occurred at any time point. All placebo recipients tested seronegative. At week 26, of 10 recipients of vCP205 alone, only 1 had repeatedly reactive EIAs and indeterminate Western blot results. Of the subunit-only recipients, EIA reactivity was found in 94% (17 of 18); of the 17 with reactive EIAs, Western blot results were positive for 83%, indeterminate for 11%, and negative for 5%. Of the subjects receiving the prime-boost regimen, 78% (39 of 50) had repeatedly reactive EIAs and positive Western blot results, 8% had repeatedly reactive EIAs and indeterminate Western blot results, 4% had repeatedly reactive EIAs and negative Western blot results, and 10% had negative results of both EIAs and Western blots.
Humoral Immunogenicity
Binding Antibody
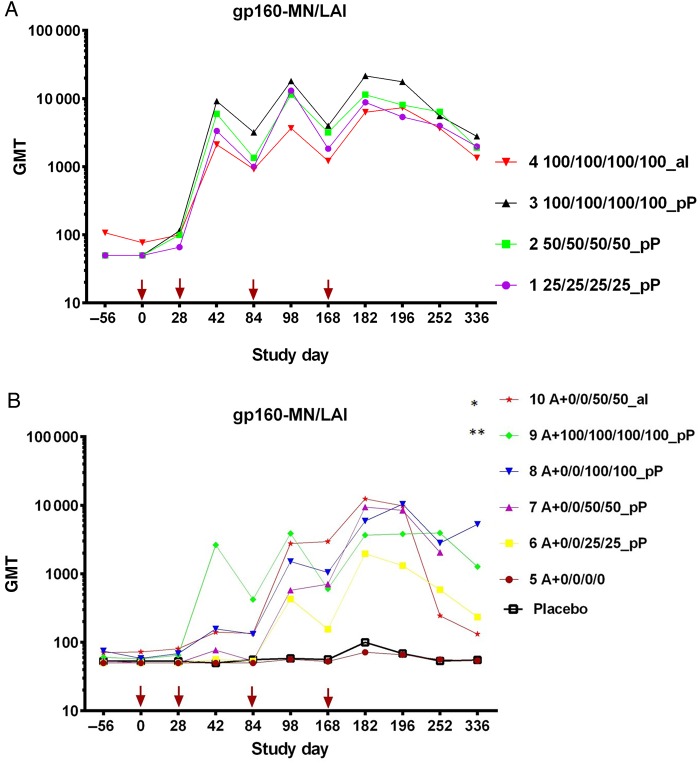
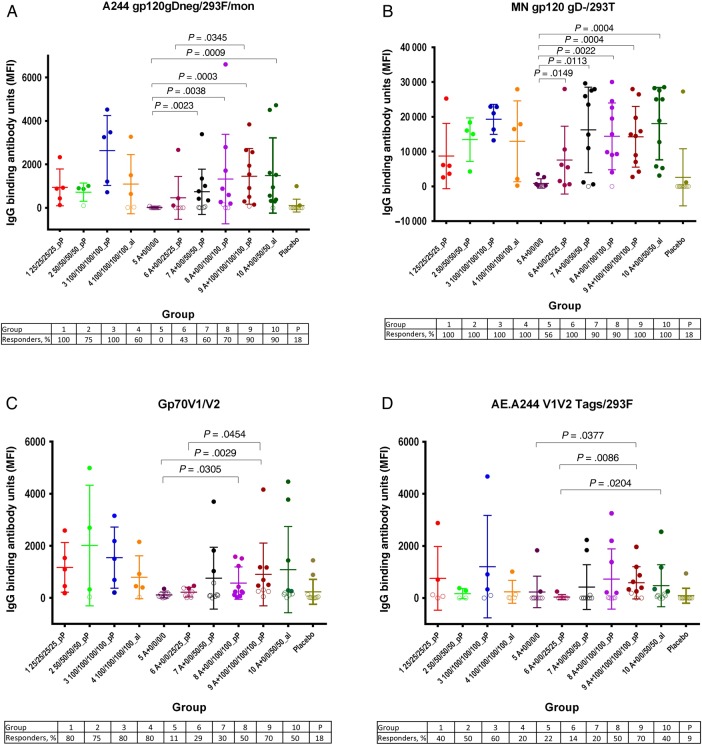
In part A (Figure 1A), at day 182, responses against ogp160 MN/LAI were not significantly different in magnitude or durability between groups 1, 2, 3, or 4. In part B (Figure 1B), vCP205 alone (group 5) did not induce detectable responses. Responses in groups 6–10 were not statistically different at day 182. However, at study day 336, between-group differences were observed: group 8 had a significantly higher response (GMT, 5276) than both group 6 (GMT, 234; P = .0003, by the Wilcoxon test) and group 10 (GMT, 132; P = .041, by the Wilcoxon test). At day 182, binding antibody responses to CRF01_AE A244 (Figure 2A) and MN (Figure 2B) gp120 were identified in the majority of volunteers. Significant differences were not observed in part A; in part B, groups 6–10 had significantly higher titers than group 5 (except for A244 gp120 recipients in group 6), and all groups had significantly higher titers than the placebo control (except A244 gp120 recipients in groups 5 and 6 and MN gp120 recipients in group 5).
Figure 1.
Binding antibody to oligomeric glycoprotein 160 (ogp160) measured by enzyme-linked immunosorbent assay end point dilution titers per study group. Vaccinations were given at study days 0, 28, 84, and 168 (arrows). A, Geometric mean titers (GMTs) of anti–gp160-MN/LAI from study part A. No significant differences in GMT were detected between groups 1, 2, and 3 or between groups 3 and 4 at any time point. B, GMTs of anti–gp160-MN/LAI from study part B. Statistically significant differences were observed in GMTs at day 336 between groups 6 and 8 (*P = .0003, by the Wilcoxon test) and groups 8 and 10 (**P = .041, by the Wilcoxon test). No samples were assayed from day 336 from group 7 volunteers.
Figure 2.
Binding antibody responses against Env antigens measured using a human immunodeficiency virus type 1 (HIV) multiplex assay at day 182. Immunoglobulin G (IgG) binding antibody units are expressed as mean fluorescent intensities (MFIs). Open circles indicate responses that failed to meet positivity criteria, whereas closed circles are those that met positivity criteria. For each group, the mean values and 95% confidence intervals are shown. P values comparing vaccine recipient groups were calculated using a 2-sided Wilcoxon test. Each panel shows results using a different capture antigen: A, A244 glycoprotein 120 (gp120); B, MN gp120; C, gp70 V1V2; and D, AE.A244 V1V2 tags.
Anti–gp70 V1V2 IgG responses were detected in 89% of volunteers (17 of 19) in part A and 10%–100% of volunteers in part B (Figure 2C). Importantly, significant differences were not observed between groups 7 and 10, which differed only by adjuvant formulation. IgG responses to CRF01_AE A244 V1V2 were less frequent (47% of responders [9 of 19] in part A and 11%–70% in part B; Figure 2D). No significant differences in responses were observed after stratification by sex or ethnicity, with the exception that white vaccine recipients responded significantly more frequently to AE.A244 V1V2 than African American recipients (21 of 87 recipients [24.1%] vs 6 of 33 recipients [18.2%]; P = .01, by the Wilcoxon test).
HIV-Specific Neutralizing Antibody
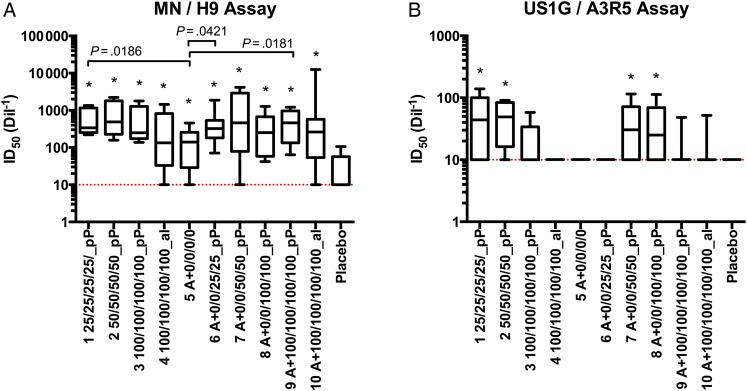
In the H9 assay, use of H9-adapted MN revealed that titers for all groups were statistically higher than those for the placebo control group (Figure 3A). Titers for group 5, which received ALVAC-HIV only, were statistically lower than those for group 1 (P = .0186), group 6 (P = .042), and group 9 (P = .018). In the A3R5 assay, use of A3R5-adapted US1 “G” revealed that titers for groups 1, 2, 7, and 8 were statistically higher than those for the placebo control group, for which no response was observed (Figure 3B). Statistical differences were not observed with respect to ogp160 dose or adjuvant formulation for either the H9 or A3R5 assay.
Figure 3.
Neutralizing antibody titers determined using 2 cell line–based neutralization assays. Sera from day 0 and day 182 were serially diluted to generate a 50% inhibitory dilution (ID50) against H9-adapted MN in the H9 assay (A) and A3R5-adapted US1 in the A3R5 assay (B). Neutralization in the postimmunization sera (day 182) was determined using the preimmunization sera (day 0) as baseline. Group means are shown; groups with titers significantly higher than the placebo control are marked with an asterisk. The dotted lines represent the negative cutoffs.
PBMC Assay
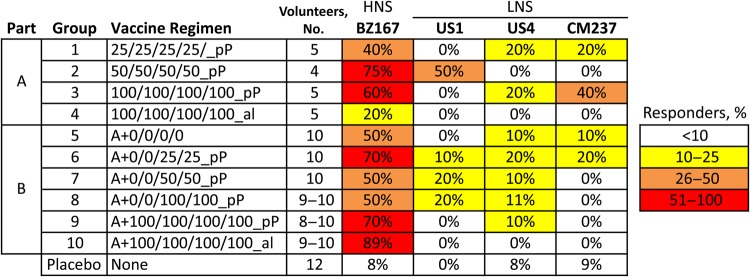
In part A, ogp160 with pP elicited antibodies capable of neutralizing the heterologous subtype B isolate, US1, in 2 of 14 volunteers (14.3%) from groups 1–3 (Figure 4). Responses did not differ significantly by race or sex. In part B, among recipients of both ALVAC and ogp160 with pP (groups 6–9), 12.5% (5 of 40) had detectable neutralizing antibody to US1 at week 26, with the 80% neutralization titer ranging from 10 to 320. Neutralization of US1 was not observed in the placebo controls or in volunteers receiving ogp160 in alum (groups 4 and 10). By comparison, neutralizing activity to BZ167, a virus highly sensitive to neutralization [23], was detected in the majority of vaccine recipients. Furthermore, 8 vaccinees neutralized CCR5-tropic CM237, a subtype BB/CRF01_AE recombinant, and/or US4, a subtype B primary isolate, in the PBMC assay. Comparisons of groups receiving ogp160 in different adjuvants revealed that, while similar response frequencies were observed against highly neutralization-sensitive subtype B viruses, significantly more-frequent responses to the less neutralization-sensitive heterologous primary isolates were observed in study vaccinees receiving ogp160 in pP, compared with those receiving alum (12% vs 0%; P = .015; Supplementary Table 2).
Figure 4.
Neutralizing antibody responses against primary human immunodeficiency virus type 1 (HIV) isolates in the peripheral blood mononuclear cell neutralizing antibody assay. Sera from day 0 and day 182 were screened at a 1:10 dilution; neutralization in the postimmunization sera (day 182) was determined using the preimmunization sera (day 0) as a baseline. The percentages indicate the number of volunteers with positive responses (ie, >50% neutralization) against each virus among those who were tested. Viruses are designated as highly neutralization sensitive (HNS) or less neutralization sensitive (LNS) to indicates their overall level of neutralization sensitivity. Statistical comparisons are presented in Supplementary Table 2.
To verify the CCR5 primary isolate neutralization detected in prevaccination and postvaccination sera, all sera showing >40% neutralization at screening were titered in a PBMC assay. Supplementary Figure 2 shows representative results of serum dilution assays for 2 prime-boost recipients (subjects BA and BQ) whose sera neutralized US1 and for 1 subject (subject BO) with no neutralization. In addition, serum obtained from subject BQ after vaccination was also able to achieve 50% neutralization (relative to preimmunization serum) of the US4 heterologous CCR5 isolate. Supplementary Figure 3 shows results of an intracellular p24 flow cytometry assay of infected cells in plasma from 1 group 8 volunteer, revealing an average neutralization level of 94%.
A subset of subjects with neutralization of US1 detected by either the PBMC or A3R5 assays was screened at a 1:20 dilution in the TZM-bl assay. Neutralizing antibody to tier 1 subtype B SF162 pseudovirus was observed in 4 of 19 subjects (21%); no neutralization was observed to tier 1 subtype B MN pseudovirus (0 of 19 subjects [0%]), tier 1 subtype C GS015 (0 of 19 subjects [0%]), or US1 pseudovirus (0 of 88 subjects [0%]).
Induction of CTL Activity
Table 1 shows the overall point prevalence and cumulative frequency of CTL responses for enrolled individuals. No CTL responses were detected in the placebo group. Cumulative CD8+ CTL responses in part B recipients were observed in 37% of vaccinated subjects. At 10 and 22 weeks after the last injection, the cumulative CTL response rates were 27% (15 of 56 subjects) and 37% (17 of 46 subjects), respectively. Eight of 17 responders (47%) had CTLs detected at multiple time points. The majority of CTL responses were directed against Env and Gag. There were no significant differences between the rates of cumulative or point response rates in each of the groups, although the number of data points was small (data not shown).
Table 1.
Cumulative CD8+ 51Cr-Release Cytotoxic T-Lymphocyte (CTL) Responses in Recipients of ALVAC-HIV (vCP205) With or Without Oligomeric Glycoprotein 160 From Human Immunodeficiency Virus Type 1 Strains MN and LAI-2 in Alum or Polyphosphazene
| Study Day | Point Prevalence, Subjects, Proportion (%) |
Cumulative Frequency, Subjects, Proportion (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Env | Gag | Pol | Any Antigen | Env | Gag | Pol | Any Antigen | |
| 0 | 0/56 (0) | 0/56 (0) | 0/56 (0) | 0/56 (0) | 0/56 (0) | 0/56 (0) | 0/56 (0) | 0/56 (0) |
| 42 | 2/53 (4) | 0/53 (0) | 0/53 (0) | 2/53 (4) | 2/53 (4) | 0/53 (0) | 0/53 (0) | 2/53 (4) |
| 98 | 0/56 (0) | 4/56 (7) | 0/56 (0) | 4/56 (7) | 2/56 (4) | 4/56 (7) | 0/56 (0) | 6/56 (11) |
| 175 | 1/56 (2) | 1/56 (2) | 0/56 (0) | 1/56 (2) | 3/56 (5) | 4/56 (7) | 0/56 (0) | 6/56 (11) |
| 182 | 3/59 (5) | 5/59 (8) | 1/59 (2) | 7/59 (12) | 5/59 (8) | 7/59 (12) | 1/59 (2) | 11/59 (19) |
| 196 | 0/59 (0) | 2/59 (3) | 0/59 (0) | 2/59 (3) | 5/59 (8) | 8/59 (14) | 1/59 (2) | 12/59 (20) |
| 252 | 3/56 (5) | 4/56 (7) | 1/56 (2) | 6/56 (11) | 8/56 (14) | 9/56 (16) | 1/56 (2) | 15/56 (27) |
| 336 | 4/46 (9) | 5/46 (11) | 0/46 (0) | 8/46 (17) | 9/46 (20) | 10/46 (22) | 1/46 (2) | 17/46 (37) |
Point prevalence and cumulative CD8-restricted CTL response data are no. of positive volunteers/total no. of vaccinated volunteers evaluated (%).
CTL responses were more often detected in ALVAC-HIV recipients with HLA-A and HLA-B alleles that express the Bw4 epitope (14 of 34; P = .003), which includes HLA-B alleles commonly associated with delayed progression to AIDS and vaccine-induced CTL responses [24]. No significant associations between B alleles and vaccine-induced CTL responses were observed in this study. Interestingly, 8 of 16 CTL responders, 6 with a response to Gag and 3 with a response to Env, possessed the A*03:01 allele (P = .04), which is associated with degenerate and promiscuous peptide recognition by CTLs and has been previously shown to restrict vaccine-induced CTL responses to Env, as well as multiple CTL epitopes in acute and nonprogressive HIV infection [24–26].
T-Cell Lymphoproliferation
Among placebo and vaccine recipients at day 0, 7%–8% had positive lymphoproliferation responses (data not shown). At week 26, 1 of 12 placebo recipients (8%) had a positive response. Baseline responses to either envelope product was low (4% responders) for all of the groups. Responses at week 26 showed immunogenicity for the protein-only group, with a peak of 60% for the highest dose (P = .05; Supplementary Table 3). The responses in the pP-adjuvanted prime-boost groups were higher than those in protein-only and alum-adjuvanted prime-boost groups, suggesting a specific effect of the pP adjuvant. ALVAC-HIV alone did not induce proliferative responses to the vaccine immunogen. Protein-only groups showed low cross-reactivity to CRF01_AE (gp160TH023/LAI-DID) envelope, while the prime-boost groups had a higher percentage of cross-subtype response (Supplementary Table 3). No responses to the CRF01_AE envelope were detected in prime-boost groups in which vaccine was adjuvanted with alum or prime alone (groups 10 and 5; Supplementary Table 3).
DISCUSSION
The vaccines and adjuvants were well tolerated, consistent with similar regimens [1, 9, 17, 27–29]. Lymphoproliferative HIV Env specific responses were seen in 53% of ogp160-only recipients and 57% of prime-boost recipients, respectively. No lymphoproliferative responses were seen in the ALVAC-HIV–only group, but we previously reported that gp120-specific CD4+ T-cell lines could be developed from vaccine recipients, which may reflect the sensitivity of this recall assay [30]. The frequency of CTL responders was modest, and review of several ALVAC-HIV prime-boost studies suggests that these data are consistent with the cumulative CTL positivity measured by the chromium release assay in other studies [11, 27, 31, 32].
Binding antibody responses in ogp160 recipients demonstrated robust responses with low durability, similar to results with gp120 adjuvanted in alum [33, 34]. There were no detectable differences between pP and alum adjuvants in subunit-only groups. In contrast, ogp160 administered in prime-boost regimens adjuvanted with pP induced significantly higher end-of-study responses than alum (fold decrease, 1.1 in group 8 and 94 in group 10), and durability improved with a higher-dose pP-adjuvanted boost (in group 8), compared with a lower-dose boost (in group 6). We observed responses against both gp70 V1V2 case A2 and AE.A244 V1V2 tags, both inverse correlates of risk in the RV144 trial [35]. Taken together with data from the HVTN505 study (DNA/Ad5) [36], these data provide further evidence that induction of anti-V1V2 responses is immunogen dependent [37]. Thus, vCP205 in combination with ogp160 MN/LAI does not induce a binding antibody duration desired for pox-protein development, but the durability of responses in pP regimens, compared with that in alum regimens, suggests a possible usefulness for pP in future protein-based regimens.
The vaccine regimens resulted in frequent EIA and Western blot seroreactivity, similar to other studies [38]. Public health implementation of these products would require careful selection of diagnostic algorithms to address the challenges of discerning vaccine-induced seroreactivity from HIV infection [39].
The most intriguing finding is detection of neutralizing antibody to heterologous, CCR5-tropic HIV measured in the PBMC-based neutralization assay and supported by reduction of cell infection in the presence of postvaccination serum. Overall, antibody capable of neutralizing at least 1 primary CCR5-tropic HIV strain was found in 10.2% of prime-boost vaccinees. Dose-specific responses were not observed in the magnitude or response rate of elicited neutralizing antibody at peak immunogenicity, findings similar to those of another study that evaluated HIV envelope vaccine [40]. Dosing and regimen may have influenced the kinetics and durability of the response, but neither was tested in this analysis.
Neutralization of primary CCR5 isolates was more frequent in recipients of ogp160 in pP, compared with alum (P = .015). This suggests that vector priming in combination with a CXCR4-tropic, T-cell line–derived oligomeric Env subunit vaccine boost can induce tier 2 neutralization in a small number of vaccinees, a response that may be broadened with improved antigen designs [12, 41] and more-potent adjuvants. This contrasts with previous studies of gp120 Env subunit vaccines [42] and prime-boost regimens involving monomeric Env boosts [43–46] that did not induce neutralization to heterologous CCR5 isolates, including US1, in PBMC assays. Sporadic weak neutralization of tier 2 viruses was detected only in Vax004 in TZMbl and A3R5 neutralization assays [8], but to our knowledge, primary isolate neutralization in PBMC assays, albeit modest, has not previously been reported in any other HIV vaccine trial in humans. Current understanding of mechanisms of neutralizing antibody induction is insufficient to engineer potently efficacious preventive vaccines, although progress toward this goal is being made [12, 41].
Previous experience with a slightly different CRF01_AE prime (vCP1521) and with oligomeric but immunodominant epitope-deleted CRF01_AE gp140 also suggested that the sequential prime-boost schedule with pP adjuvant may be associated with induction of low levels of primary isolate neutralizing antibody (Victoria Polonis, unpublished data).
Previous studies showed that neutralizing activity may be detectable in PBMC assays but not in the TZM-bl cell-pseudovirus platform [47] and vice versa. This may be due to differences in cellular chemokine receptor frequency, to chemokine production, or to sequence or structural differences of Env between PBMC-derived primary isolate and 293T-produced pseudoviruses [8, 21]. In addition, the PBMC assay may detect additional types of antibody-mediated viral inhibition, such as ADCC, as multiple cell types are present [48]. Thus, for the evaluation and prioritization of the advancement of new candidate subunits and adjuvants, assessment of neutralization by using primary cell systems in parallel with standardized cell line platforms may be prudent until correlate(s) of antibody-mediated protection can be definitively identified [3].
Taken together, the findings suggest that ogp160 subunit HIV vaccines may induce relevant neutralizing antibody to heterologous CCR5 isolates in humans and might assist in the development of an immunogen formulated with a potent adjuvant capable of generating sustainable, high-titer, broadly neutralizing antibody.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the volunteers, for their dedication, interest, and active participation in this trial; the clinical trial staff at the WRAIR and Division of Retrovirology clinical trials units, especially Sheila Taylor-Means, Robin Neilson, and Catherine Chaddic, for their support; laboratory technicians in the Division of Retrovirology, WRAIR, for their contribution to the assays described in this report; Drs Bart Haynes and Hua-Xin Liao, for protein reagents; Dr Kelly Seaton and Vicki Ashley, for binding antibody assay oversight; Robin Garner, for leading the data coordination and analysis center, which prepared the data for an end of phase 2 meeting with the Food and Drug Administration; and Dr Beryl Wessner, for serving as study pharmacist.
Disclaimer. The opinions expressed herein are those of the authors and do not represent the official position of the US Army, Department of Defense, or Department of State.
Financial support. This work was supported by the Military Infectious Diseases Research Program, US Army Medical Research and Materiel Command, through a cooperative agreement (DAMD17-98-2-7007) with the Henry M. Jackson Foundation for the Advancement of Military Medicine; and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, through the Duke University Center for AIDS Research (grant AI064518).
Potential conflicts of interest. R. E.-H. is an employee of Sanofi-Pasteur. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
- 2.Robb ML, Rerks-Ngarm S, Nitayaphan S et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis 2012; 12:531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes BF, Gilbert PB, McElrath MJ et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zolla-Pazner S, Decamp A, Gilbert PB et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014; 9:e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yates NL, Liao HX, Fong Y et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014; 6:228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottardo R, Bailer RT, Korber BT et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 2013; 8:e75665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert P, Wang M, Wrin T et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J Infect Dis 2010; 202:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montefiori DC, Karnasuta C, Huang Y et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 2012; 206:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascola JR, Snyder SW, Weislow OS et al. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis 1996; 173:340–8. [DOI] [PubMed] [Google Scholar]
- 10.Burton DR, Desrosiers RC, Doms RW et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol 2004; 5:233–6. [DOI] [PubMed] [Google Scholar]
- 11.Nitayaphan S, Pitisuttithum P, Karnasuta C et al. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J Infect Dis 2004; 190:702–6. [DOI] [PubMed] [Google Scholar]
- 12.Sanders RW, van Gils MJ, Derking R et al. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 2015; 349:aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundling C, Martinez P, Soldemo M et al. Immunization of macaques with soluble HIV type 1 and influenza virus envelope glycoproteins results in a similarly rapid contraction of peripheral B-cell responses after boosting. J Infect Dis 2013; 207:426–31. [DOI] [PubMed] [Google Scholar]
- 14.Palmer CD, Ninkovic J, Prokopowicz ZM et al. The effect of stable macromolecular complexes of ionic polyphosphazene on HIV Gag antigen and on activation of human dendritic cells and presentation to T-cells. Biomaterials 2014; 35:8876–86. [DOI] [PubMed] [Google Scholar]
- 15.Plotkin SA, Cadoz M, Meignier B et al. The safety and use of canarypox vectored vaccines. Dev Biol Stand 1995; 84:165–70. [PubMed] [Google Scholar]
- 16.Belshe RB, Stevens C, Gorse GJ et al. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus Type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J Infect Dis 2001; 183:1343–52. [DOI] [PubMed] [Google Scholar]
- 17.Pitisuttithum P, Rerks-Ngarm S, Bussaratid V et al. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS One 2011; 6:e27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pialoux G, Excler JL, Riviere Y et al. A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN/LAI). The AGIS Group, and l'Agence Nationale de Recherche sur le SIDA. AIDS Res Hum Retroviruses 1995; 11:373–81. [DOI] [PubMed] [Google Scholar]
- 19.Baylor NW, Egan W, Richman P. Aluminum salts in vaccines--US perspective. Vaccine 2002; 20(suppl 3):S18–23. [DOI] [PubMed] [Google Scholar]
- 20.Tomaras GD, Yates NL, Liu P et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 2008; 82:12449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown BK, Wieczorek L, Sanders-Buell E et al. Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology 2008; 375:529–38. [DOI] [PubMed] [Google Scholar]
- 22.Polonis VR, Brown BK, Rosa Borges A et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology 2008; 375:315–20. [DOI] [PubMed] [Google Scholar]
- 23.Yu XG, Addo MM, Rosenberg ES et al. Consistent patterns in the development and immunodominance of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses following acute HIV-1 infection. J Virol 2002; 76:8690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paris R, Bejrachandra S, Karnasuta C et al. HLA class I serotypes and cytotoxic T-lymphocyte responses among human immunodeficiency virus-1-uninfected Thai volunteers immunized with ALVAC-HIV in combination with monomeric gp120 or oligomeric gp160 protein boosting. Tissue Antigens 2004; 64:251–6. [DOI] [PubMed] [Google Scholar]
- 25.Kaslow RA, Rivers C, Tang J et al. Polymorphisms in HLA class I genes associated with both favorable prognosis of human immunodeficiency virus (HIV) type 1 infection and positive cytotoxic T-lymphocyte responses to ALVAC-HIV recombinant canarypox vaccines. J Virol 2001; 75:8681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RP, Hammond SA, Trocha A, Siliciano RF, Walker BD. Induction of a major histocompatibility complex class I-restricted cytotoxic T-lymphocyte response to a highly conserved region of human immunodeficiency virus type 1 (HIV-1) gp120 in seronegative humans immunized with a candidate HIV-1 vaccine. J Virol 1994; 68:3145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitisuttithum P, Berman PW, Phonrat B et al. Phase I/II study of a candidate vaccine designed against the B and E subtypes of HIV-1. J Acquir Immune Defic Syndr 2004; 37:1160–5. [DOI] [PubMed] [Google Scholar]
- 28.Thongcharoen P, Suriyanon V, Paris RM et al. A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. J Acquir Immune Defic Syndr 2007; 46:48–55. [DOI] [PubMed] [Google Scholar]
- 29.de Bruyn G, Rossini AJ, Chiu YL et al. Safety profile of recombinant canarypox HIV vaccines. Vaccine 2004; 22:704–13. [DOI] [PubMed] [Google Scholar]
- 30.Ratto-Kim S, Garner RP, Kim JH et al. Prospective analyses of HIV-1-specific proliferative responses, recall antigen proliferative responses, and clinical outcomes in an HIV-1-seropositive cohort. J Infect Dis 2004; 189:1988–95. [DOI] [PubMed] [Google Scholar]
- 31.Pitisuttithum P, Nitayaphan S, Thongcharoen P et al. Safety and immunogenicity of combinations of recombinant subtype E and B human immunodeficiency virus type 1 envelope glycoprotein 120 vaccines in healthy Thai adults. J Infect Dis 2003; 188:219–27. [DOI] [PubMed] [Google Scholar]
- 32.de Souza MS, Ratto-Kim S, Chuenarom W et al. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol 2012; 188:5166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitisuttithum P, Gilbert P, Gurwith M et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006; 194:1661–71. [DOI] [PubMed] [Google Scholar]
- 34.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005; 191:654–65. [DOI] [PubMed] [Google Scholar]
- 35.Zolla-Pazner S, de Camp AC, Cardozo T et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One 2013; 8:e53629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammer SM, Sobieszczyk ME, Janes H et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013; 369:2083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alam SM, Liao HX, Tomaras GD et al. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol 2013; 87:1554–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper CJ, Metch B, Dragavon J, Coombs RW, Baden LR, Force NHVTNV-IST<. Vaccine-induced HIV seropositivity/reactivity in noninfected HIV vaccine recipients. JAMA 2010; 304:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enterprise VWGoGHV Voronin Y, Zinszner H et al. HIV vaccine-induced sero-reactivity: a challenge for trial participants, researchers, and physicians. Vaccine 2015; 33:1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goepfert PA, Tomaras GD, Horton H et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine 2007; 25:510–8. [DOI] [PubMed] [Google Scholar]
- 41.Dosenovic P, von Boehmer L, Escolano A et al. Immunization for HIV-1 broadly neutralizing antibodies in human Ig knockin mice. Cell 2015; 161:1505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu R, Srivastava IK, Kuller L et al. Immunization with HIV-1 SF162-derived Envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology 2006; 349:276–89. [DOI] [PubMed] [Google Scholar]
- 43.Selvarajah S, Puffer B, Pantophlet R, Law M, Doms RW, Burton DR. Comparing antigenicity and immunogenicity of engineered gp120. J Virol 2005; 79:12148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantophlet R, Wilson IA, Burton DR. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J Virol 2003; 77:5889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol 2006; 24:739–69. [DOI] [PubMed] [Google Scholar]
- 46.Reitter JN, Means RE, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nat Med 1998; 4:679–84. [DOI] [PubMed] [Google Scholar]
- 47.Bakari M, Aboud S, Nilsson C et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine 2011; 29:8417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown BK, Wieczorek L, Kijak G et al. The role of natural killer (NK) cells and NK cell receptor polymorphisms in the assessment of HIV-1 neutralization. PLoS One 2012; 7:e29454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.