Abstract
Background. Many studies have documented lower vaccine efficacy among children in low-income countries, compared with their counterparts in high-income countries. This disparity is especially apparent with respect to oral vaccines such as rotavirus and oral polio vaccines. One potential contributing factor is the presence of maternal antenatal helminth infections, which can modulate the infant's developing immune system.
Methods. Using a multiplex immunoassay, we tested plasma immunoglobulin A (IgA) or immunoglobulin G (IgG) levels specific for antigens in 9 routinely administered childhood vaccines among 1639 children aged approximately 13 months enrolled in the ECUAVIDA (Ecuador Life) birth cohort study in Ecuador. We compared vaccine responses in 712 children of mothers who tested positive for helminth infections in the last trimester of pregnancy to responses in 927 children of mothers without helminth infection.
Results. Plasma IgA levels specific for antigens in rotavirus vaccine and oral polio vaccine containing poliovirus serotypes 1 and 3 were all significantly higher in children of helminth-infected mothers, compared with children of uninfected mothers. Plasma IgG levels specific for diphtheria, tetanus, pertussis, measles, rubella, and Haemophilus influenzae type b vaccine antigens were comparable between the 2 groups.
Conclusions. Antenatal maternal helminth infections were not associated with reduced antibody responses to infant vaccines, but rather with modestly increased IgA responses to oral vaccines.
Keywords: maternal, helminth, vaccine, immunization, IgA
Among health disparities between high-income countries and low- and middle-income countries, an issue of considerable public health importance, is the disparity in the efficacy of childhood vaccines, especially orally administered vaccines. These vaccines have the potential to save the most lives in low- and middle-income countries, where the disease burden is greatest and the associated mortality highest, but they have demonstrated poorer efficacy in these regions, particularly among the most neglected populations. This has been observed for several orally administered vaccines against enteric pathogens, such as rotavirus, cholera, typhoid, and polio vaccines [1–4].
Several hypotheses have been proposed to explain the high vaccine failure rate: for example, frequent infections with diarrheal pathogens may interfere with vaccine take, micronutrient deficiencies may impair the integrity of the gut mucosa, and differences in enteric microbiome composition may skew the mucosal immune response to vaccines [5, 6]. In the current study, we focus on maternal antenatal helminth infections as a potential modulator of infant vaccine responses. Several studies have reported reduced efficacy of vaccines in helminth-infected individuals [7–9], and there is growing evidence that perinatal helminth infection can affect the next generation [10].
We hypothesized that maternal helminth infection contributes to the poor immune responses to oral vaccines in offspring. To evaluate this, we determined plasma levels of immunoglobulin A (IgA) or immunoglobulin G (IgG) to 9 vaccine antigens in Ecuadorian children aged 13 months from a birth cohort. More than 45% of the mothers of these children tested positive for one or more helminth species during their last trimester of pregnancy; thus, we compared vaccine responses in children with and without this antenatal exposure. Here we report our unexpected findings that plasma IgA levels specific for antigens in orally administered vaccines (rotavirus vaccine and oral polio vaccine [OPV] containing poliovirus serotypes 1 [OPV1] and 3 [OPV3]) were higher in children of helminth-infected mothers, compared with those in children of uninfected mothers. Plasma IgG levels specific for antigens in parenterally administered vaccines (diphtheria, tetanus, pertussis, measles, rubella, and Haemophilus influenzae type b [Hib]) did not significantly differ by maternal helminth infection status.
METHODS
Study Design and Population
The ECUAVIDA (Ecuador Life) birth cohort study was designed to determine the effects of maternal and childhood geohelminth infections on the immune response to routine infant vaccinations and on the development of allergy in children. A total of 2404 mothers and their newborns were recruited between November 2005 and December 2009 in the Hospital Padre Alberto Buffoni (HPAB) in Quinindé, Esmeraldas Province, Ecuador. To be eligible for inclusion, newborns had to be healthy and delivered at gestation week 34 or later, and mothers had to provide at least 1 stool sample during the third trimester of pregnancy or around the time of delivery. Detailed information on the methods and design of the cohort is provided elsewhere [11]. All vaccinations were provided through the Ministry of Public Heath infant vaccination program at HPAB. Infants received trivalent OPV (Chiron) and the pentavalent vaccine containing diphtheria toxoid, Bordetella pertussis, tetanus toxoid, hepatitis B virus, and Hib (Novartis) at 2, 4, and 6 months of age and measles-mumps-rubella vaccine (Serum Institute of India) at 12 months of age. Rotavirus (Rotarix, GlaxoSmithKline) was introduced into the schedule in June 2007 and was administered at 2 and 4 months of age.
Sample and Data Collection
At least 1 stool sample was collected from the mothers during the third trimester of pregnancy and/or around the time of delivery and from the children at 13 months of age. Stool samples were examined for geohelminths, using direct saline mounts, the modified Kato-Katz method, the formol-ether concentration technique, and the carbon-coproculture method [12]. Mothers were classified as infected if any helminth was detected in a stool sample. Demographic and socioeconomic information for the household were collected from the mother within 2 weeks of delivery, using an investigator-administered questionnaire. Additional data (eg, on breast-feeding and vaccine doses) were collected at 13 months of age. Blood samples were collected at 13 months of age into tubes containing sodium heparin as anticoagulant (Vacutainer, BD Diagnostics). Plasma was separated by centrifugation and stored at −30°C before shipping on dry ice to the National Institutes of Health.
Ethics
The study was approved by the Ministry of Health in Ecuador and by the ethics committees of the Hospital Pedro Vicente Maldonado and the Universidad San Francisco de Quito (Quito, Ecuador). Mothers of all children participating in the study gave written informed consent. The ECUAVIDA study is registered as an observational study (ISRCTN 41239086).
Building the Multiplex Assay Beads
Beads were made by coupling vaccine antigens (Supplementary Table 1) to Bio-Plex Pro Magnetic COOH Beads (Biorad), using the Amine Coupling Kit (Biorad), together with EDAC and S-NHS (both from Pierce). For each antigen, 4 different protein amounts were tested (1.25 µg, 2.5 µg, 5 µg, or 10 µg per coupling reaction of 1.25 × 106 beads), and the amount with the best signal to noise ratio was selected. Beads were validated using standard sera with known titers of IgA or IgG antibodies to the antigens in question (Supplementary Table 1), and using, as a negative control, either the same sera depleted of all immunoglobulin by means of the EMD Millipore PureProteome Human Albumin/Immunoglobulin Depletion Kit (Fisher Scientific) or IgG with protein G sepharose beads (Pierce).
Multiplex Assay
Multiplex assays were performed in µClear 96-well microplates (Greiner Bio-One). Plasma samples were diluted in assay buffer (phosphate-buffered saline [PBS], 1% bovine serum albumin, and 0.05% Tween-20) at 1:25 for IgA plates (for rotavirus vaccine, OPV1, and OPV3) and 1:250 for IgG plates (for all other vaccines) and plated in duplicate plates in a volume of 50 µL. Individual vials of beads were sonicated in a water bath for 30 seconds and vortexed vigorously for another 30 seconds before pooling together in a master mix of 2000 beads of each specificity per plasma sample. A total of 50 µL of this master mix was added to each well and incubated for 1 hour at 4°C. Plates were washed 5 times with wash buffer (PBS and 0.05% Tween-20), using a magnetic plate washer (Biotek). Phycoerythrin-conjugated donkey antihuman IgG (Jackson Labs) was added at a dilution of 1:100 in 50 µL of assay buffer to the IgG plates, and phycoerythrin-conjugated goat antihuman IgA (Jackson Labs) was added at a dilution of 1:50 in 50 µL of assay buffer to the IgA plates. Plates were incubated for 1 hour at room temperature with agitation on a plate shaker and then were washed 5 times. Beads were resuspended in 100 µL of fluorescence-activated cell-sorting buffer and read on a 96-well multiplate reader (Molecular Dynamics).
Microneutralization Assay of the Antibody Response to OPV3
Titers of neutralizing antibodies to OPV3 antigen had been measured previously by microneutralization assay in a subset of 179 children by using standardized protocols at the Health Protection Agency (London, United Kingdom) [13].
Statistical Analysis
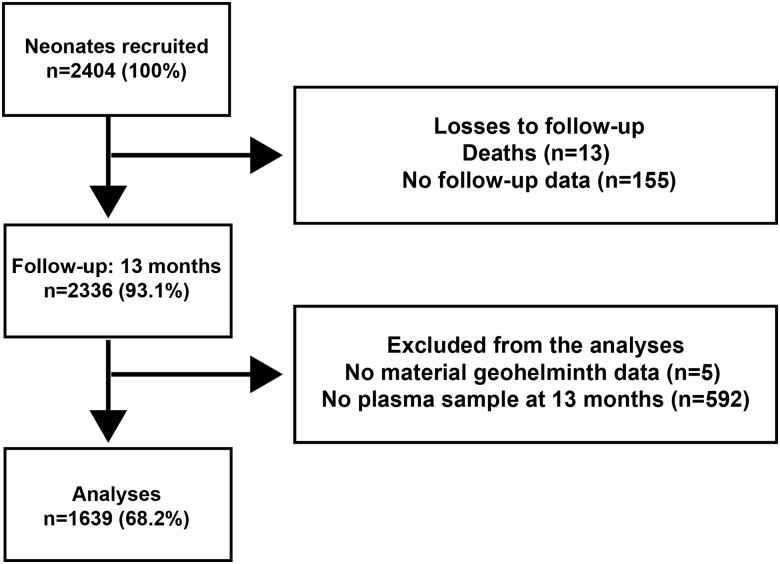
For the present analysis, only children from whom a plasma sample was collected at 13 months of age and whose mothers were tested for helminth infection were included (Figure 1). For comparing data in 2-by-2 tables, we used a central Fisher exact test and associated confidence intervals (CIs) [14]. For comparing continuous data yielded by assays, we used Spearman correlation. For the binary response regressions, we used generalized additive models (GAMs) with a logit link function that is a generalization of logistic regression. For comparison of the mean titers, we log-transformed the data and did a t test (unadjusted) or a GAM with assumed Gaussian error. For the adjusted models, we assumed that missing covariate data were missing at random and used complete data models. We adjusted analyses by using main effects for child's sex, age, helminth infection status at 13 months of age, socioeconomic status (calculated by combining socioeconomic status variables, using principal components analysis for categorical data as described previously [15]; the first component that accounted for 30.0% of variation was divided into tertiles to represent low, middle, and high socioeconomic status), whether the mother had received antiparasite treatment, mother's education level, mother's ethnicity, urban versus rural location of home, overcrowding of home (defined as ≥3 persons per sleeping room), and finally a smoothing spline with 15 degrees of freedom to model the calendar effect at the time of response measurement. For all GAMs, we used Wald-type methods to estimate CIs and P values. We prespecified that we were primarily interested in oral vaccines (ie, rotavirus vaccine and OPV), for which we corrected P values for multiple testing, using the Holm correction [16]. Analyses were done in R, version 3.1.1, using the gam R package but with a custom function to obtain Wald-type P values and CIs. Receiver operating characteristic curve (ROC) analyses and sensitivity and specificity calculations were performed using GraphPad (v5.0).
Figure 1.
Flow diagram of birth cohort participants included in this study.
RESULTS
We measured vaccine antibody responses in a sample of 1639 newborns (68.2%) of the original birth cohort of 2404. Reasons for exclusions from analysis are shown in Figure 1. The distributions of relevant study variables according to maternal helminth infections status are provided in Table 1.
Table 1.
Characteristics of Mothers and Children Enrolled in the Study and of Their Households, According to Maternal Helminth Infection Status
| Variable | Infants of Uninfected Mothers (n = 927) | Infants of Infected Mothers (n = 712) | P Value |
|---|---|---|---|
| Mother | |||
| Age, y, mean ± SD | 25.8 ± 6.1 | 24.7 ± 6.2 | <.001 |
| Ethnicity | |||
| Afro-Ecuadorian | 18.10 | 32.10 | <.001 |
| Othera | 81.90 | 67.80 | |
| Education | |||
| Illiterate | 9.20 | 17.60 | <.001 |
| Primary completed | 53.40 | 63.30 | |
| Secondary completed | 37.40 | 19.10 | |
| Helminth detection | |||
| Any | 0 | 100 | <.001 |
| Ascaris lumbricoides | 0 | 59.40 | <.001 |
| Trichuris trichiura | 0 | 59.80 | <.001 |
| Hookworm | 0 | 13.20 | <.001 |
| Strongyloides stercoralis | 0 | 8.70 | <.001 |
| Hymenolepis nana | 0 | 1.00 | .002 |
| Helminth intensityb (n = 13c) | |||
| Ascaris lumbricoides | |||
| Light | 0 | 90.00 | <.001 |
| Moderate | 0 | 9.00 | |
| Heavy | 0 | 1.00 | |
| Trichuris trichiura | |||
| Light | 0 | 92.60 | <.001 |
| Moderate | 0 | 7.00 | |
| Heavy | 0 | 0.40 | |
| Household | |||
| Residence | |||
| Urban | 67.90 | 72.60 | .037 |
| Rural | 32.10 | 27.40 | |
| Socioeconomic status | |||
| Low | 25.20 | 35.00 | <.001 |
| Medium | 33.00 | 36.50 | |
| High | 41.80 | 28.50 | |
| Overcrowding | |||
| Yes | 35.00 | 47.10 | <.001 |
| Bathroom | |||
| Field | 1.30 | 1.70 | <.001 |
| Latrine | 62.70 | 72.90 | |
| Water closet | 36.00 | 25.40 | |
| Child | |||
| Sex | |||
| Male | 49.30 | 52.10 | .26 |
| Female | 50.70 | 48.90 | |
| Gestational age, wk, mean ± SD | 39.0 ± 1.7 | 39.1 ± 1.7 | .461 |
| Breast-feeding duration, mo | |||
| <6 | 10.50 | 9.10 | .647 |
| 6–12 | 44.70 | 46.00 | |
| >12 | 44.80 | 44.90 | |
| Age at evaluation, mo, mean ± SD | 13.8 ± 1.5 | 13.8 ± 1.6 | .944 |
| Helminth detected (n = 196c) | |||
| Any | 5.90 | 18.00 | <.001 |
| Ascaris lumbricoides | 4.50 | 13.20 | <.001 |
| Trichuris trichiura | 1.10 | 5.80 | <.001 |
| Hookworm | 0.10 | 0.30 | .409 |
| Strongyloides stercoralis | 0.20 | 0.50 | .445 |
| Hymenolepis nana | 0.20 | 0.30 | .78 |
| Vaccine, doses, no. | |||
| Pentavalent (n = 51c) | |||
| 3 | 100 | 100 | |
| OPV (n = 49c) | |||
| 0 | 0.10 | 0.30 | .603 |
| 1 | 0.10 | 0 | |
| 2 | 1.20 | 1.60 | |
| 3 | 98.60 | 98.10 | |
| MMR (n = 116c) | |||
| 0 | 35.30 | 43.50 | .001 |
| 1 | 64.70 | 56.60 | |
| RV (n = 100c) | |||
| 0 | 30.80 | 34.00 | .191 |
| 1 | 13.40 | 14.80 | |
| 2 | 55.80 | 51.10 | |
Data are % of subjects, unless otherwise indicated.
Abbreviations: OPV, oral polio vaccine; SD, standard deviation.
a A total of 1236 were mestizo and 6 were Amerindian.
b Data denote World Health Organization categories of infection intensities, which are based on egg counts in stool samples [17]. For A. lumbricoides, light intensity is defined as <5000 eggs per gram of stool, moderate intensity is defined as 5000–49 999 eggs per gram of stool, and heavy intensity is defined as ≥50 000 eggs per gram of stool. For T. trichiura, light intensity is defined as <1000 eggs per gram of stool, moderate intensity is defined as 1000–9999 eggs per gram of stool, and heavy intensity is defined as ≥10 000 eggs per gram of stool.
c Data denote the number of subjects with an unknown date of helminth intensity measurement, helminth detection, or vaccination.
The children participating in the study received routine immunizations according to the standard infant vaccination schedule in Ecuador (see “Methods” section). Plasma samples were collected at approximately 13 months of age to allow completion of the schedule, including 1 dose of measles-mumps-rubella vaccine given at 12 months, and also to minimize the contribution of maternally derived antibodies. We designed a multiplex bead immunoassay that simultaneously measured antibody titers to antigens in most but not all of the vaccines received by these infants. BCG was excluded because this vaccine elicits a cellular rather than humoral response. OPV2, hepatitis B, and mumps vaccines were excluded because of a lack of availability of appropriate reagents or technical difficulties with manufacturing vaccine-specific beads. For each of the other vaccines, we chose an appropriate target antigen (Supplementary Table 1) to conjugate to Bio-Plex Pro Magnetic COOH beads from a unique region, such that all bead regions could be easily discriminated from one another when pooled together in a multiplex assay.
Of primary interest in this study were infant immune responses to the orally administered vaccines (rotavirus vaccine, OPV1, and OPV3), as these are the vaccines for which disparities in performance among children from high- and low-income countries are most pronounced. IgA responses to these 3 vaccines were therefore set as the primary end points of our study, whereas IgG responses to the remaining parenterally administered vaccines were set as secondary end points. The multiplex assay was thus ultimately divided into 2 panels, measuring IgA and IgG titers, respectively.
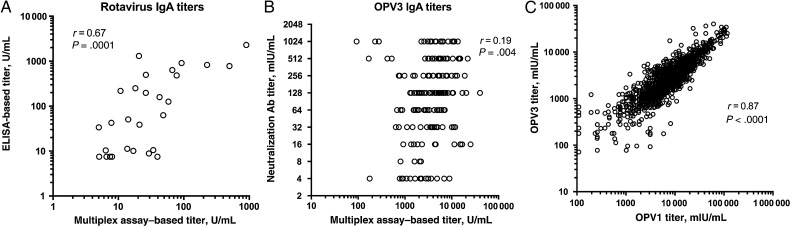
We wished to compare the performance of the beads we created to test our primary end points (rotavirus, OPV1, and OPV3) with the current gold standard tests. To this end, a small subset of our plasma samples were sent to a reference laboratory for testing by a rotavirus-specific enzyme-linked immunosorbent assay (ELISA) [18]. The IgA titers obtained with the gold standard ELISA displayed a reasonably good (r = 0.67) and highly significant (P = .0001) correlation with the IgA titers obtained with our rotavirus beads (Figure 2A). Some deviations in titers were expected because the target antigens used in the 2 assays were similar but not identical. Nevertheless, these results strongly suggest that our beads have good specificity for antirotavirus IgA and that titers derived from our beads can be interpreted in a similar manner to titers derived from the rotavirus-specific ELISA.
Figure 2.
Validating multiplex beads to assess plasma immunoglobulin A (IgA) titers after oral administration of rotavirus and polio vaccines. A, Correlation between plasma IgA titers determined by the rotavirus beads in our multiplex assay compared with those determined by the gold standard rotavirus-specific enzyme-linked immunosorbent assay (ELISA). B, Correlation of neutralization titers for poliovirus serotype 3 (OPV3) determined by standard microneutralization assays and plasma titers of total anti-OPV3 IgA by multiplex assay. C, Correlation of plasma titers of anti–poliovirus serotype 1 (OPV1) IgA and anti-OPV3 IgA determined by multiplex assays. In all panels, each circle represents 1 subject. Spearman coefficient (r) and P values represent results of a nonparametric Spearman correlation test.
The gold standard currently used to determine OPV responses for clinical applications is the microneutralization assay. When we performed OPV3 microneutralization assays on a subset of our plasma samples and compared those titers with IgA titers obtained with our OPV3 beads, we observed a positive but relatively weak association between the 2 parameters (Figure 2B), so the conclusions we can draw from our OPV1 and OPV3 beads are limited to the total IgA titers specific to each of the viruses. We also noted that OPV1 and OPV3 responses, in terms of total IgA titers, were highly correlated in our cohort of children (Figure 2C).
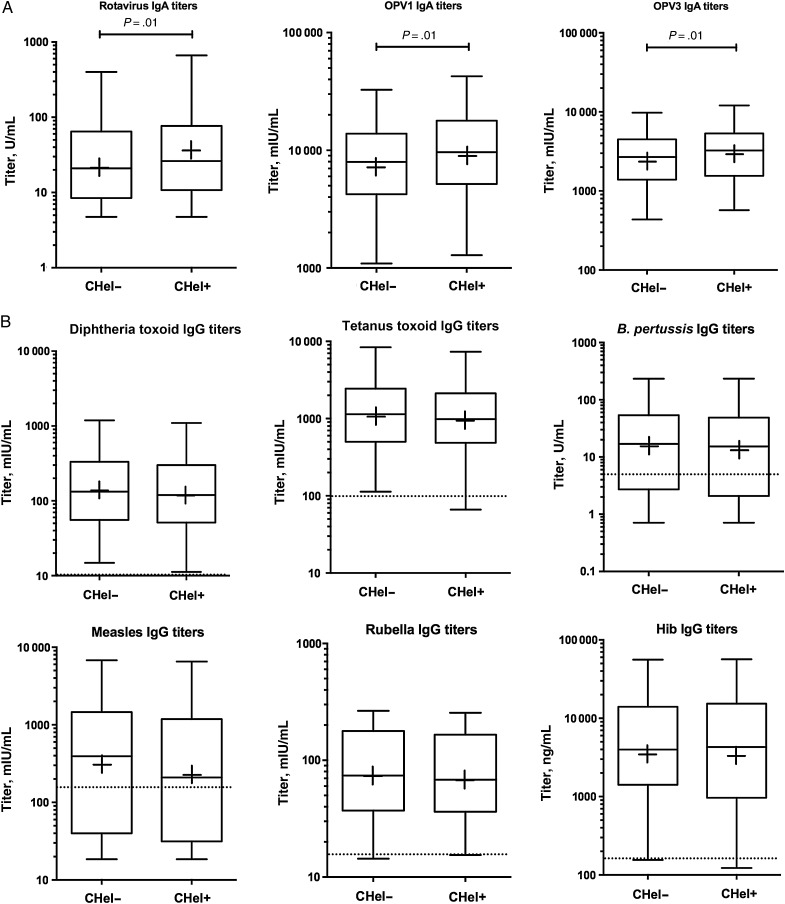
After production and validation of the beads, the entire collection of 1639 plasma samples was tested using the multiplex assay. Results were analyzed by dividing the study subjects into 2 groups: children of helminth-infected mothers and children of helminth-uninfected mothers. In our initial analysis, we considered antibody titer as a continuous variable by comparing the geometric means of the vaccine-specific antibody titers between the 2 groups of children. Unexpectedly, for all 3 orally administered vaccines represented in our panel, children of helminth-infected mothers mounted significantly higher IgA responses than children of helminth-uninfected mothers (Figure 3A), a difference that persisted after adjustment for confounders and correction for multiple comparisons (Table 2). In contrast, none of the IgG titers for any of the parenterally administered vaccines were significantly different between groups (Figure 3B and Table 2). We also compared the OPV3 microneutralization titers between the small subgroups of children for whom these data were available (94 children of helminth-uninfected mothers and 86 children of helminth-infected mothers). No significant difference was found between subgroups, but the sample sizes, which were much smaller than those used for the multiplex analyses, might have been too small to detect subtle differences.
Figure 3.
Comparison of vaccine-specific plasma antibody titers between children of mothers with helminth infection (CHel+) and children of mothers without helminth infection (CHel-). A, Plasma immunoglobulin A (IgA) titers specific for orally administered vaccines at 13 months of age (ie, rotavirus vaccine and oral polio vaccine containing poliovirus serotypes 1 [OPV1] and 3 [OPV3]). B, Plasma immunoglobulin G (IgG) titers specific for parenterally administered vaccines at 13 months of age (diphtheria, tetanus, pertussis, measles, rubella, and Haemophilus influenzae type b [Hib] vaccines). In all graphs, boxes extend between the 25th and 75th percentiles, whiskers indicate the 5th and 95th percentiles, lines indicate median titers, and plus signs indicate mean titer. Where present, dotted lines indicate minimum titers believed to be protective. Not all graphs have a dotted line because not all vaccines have an established minimum protective titer. Abbreviation: B. pertussis, Bordetella pertussis.
Table 2.
Effects of Maternal Helminth Infections on Vaccine Antibody Titers
| Antigen | Fold Change (95% CI) |
P Values |
Adjusted Fold Change (95% CI) |
P Values |
||||
|---|---|---|---|---|---|---|---|---|
| 2-Sided | Adjusted for Helminth Detection in Child at Age 13 mo | Corrected by Holm Method | Adjusted | Adjusted for Helminth Detection in Child at Age 13 mo | Corrected by Holm Method | |||
| Primary end point | ||||||||
| Rotavirus | 1.31 (1.14–1.5) | .0002 | .0041 | .0003 | 1.21 (1.05–1.4) | .0104 | .0505 | .0147 |
| OPV1 | 1.25 (1.12–1.4) | .0001 | .0045 | .0002 | 1.18 (1.06–1.33) | .0036 | .1667 | .0108 |
| OPV3 | 1.19 (1.08–1.31) | .0003 | .1577 | .0003 | 1.15 (1.04–1.27) | .0073 | .7717 | .0147 |
| Secondary end point | ||||||||
| Diphtheria toxoid | 0.88 (.77–1) | .0527 | .1215 | .2108 | 0.87 (.76–1) | .0447 | .2510 | .2679 |
| Tetanus toxoid | 0.87 (.77–.99) | .0298 | .0040 | .1489 | 0.90 (.79–1.03) | .1158 | .0028 | .5792 |
| B. pertussis | 0.9 (.75–1.08) | .2755 | .0015 | .8264 | 1.05 (.86–1.27) | .6440 | .0036 | 1.0000 |
| Measles virus | 0.72 (.58–.88) | .0015 | .3800 | .0092 | 0.99 (.82–1.18) | .8756 | .6585 | 1.0000 |
| Rubella virus | 0.96 (.87–1.05) | .3689 | .5774 | .8264 | 1.04 (.96–1.14) | .3280 | .5786 | 1.0000 |
| Hib | 0.91 (.77–1.08) | .2865 | .0483 | .8264 | 0.92 (.77–1.09) | .3346 | .0198 | 1.0000 |
Table shows fold changes representing ratios of geometric mean antibody titers for infants of infected mothers, compared with uninfected mothers. A generalized additive model was adjusted for calendar time, sex, child's age, helminth infection status at the 13-month evaluation, socioeconomic status, mother's antiparasite treatment, mother's education level, mother's ethnicity, urban versus rural residence, and overcrowding of home. In addition, for the tetanus toxoid response only, adjustments were made for the number of doses of tetanus toxoid received by the mother during pregnancy. The Holm method was used to adjust for multiple comparisons separately for primary and secondary end points.
Abbreviations: B. pertussis, Bordetella pertussis; CI, confidence interval; Hib, Haemophilus influenzae b; OPV1, poliovirus serotype 1 component of oral polio vaccine; OPV3, poliovirus serotype 3 component of oral polio vaccine.
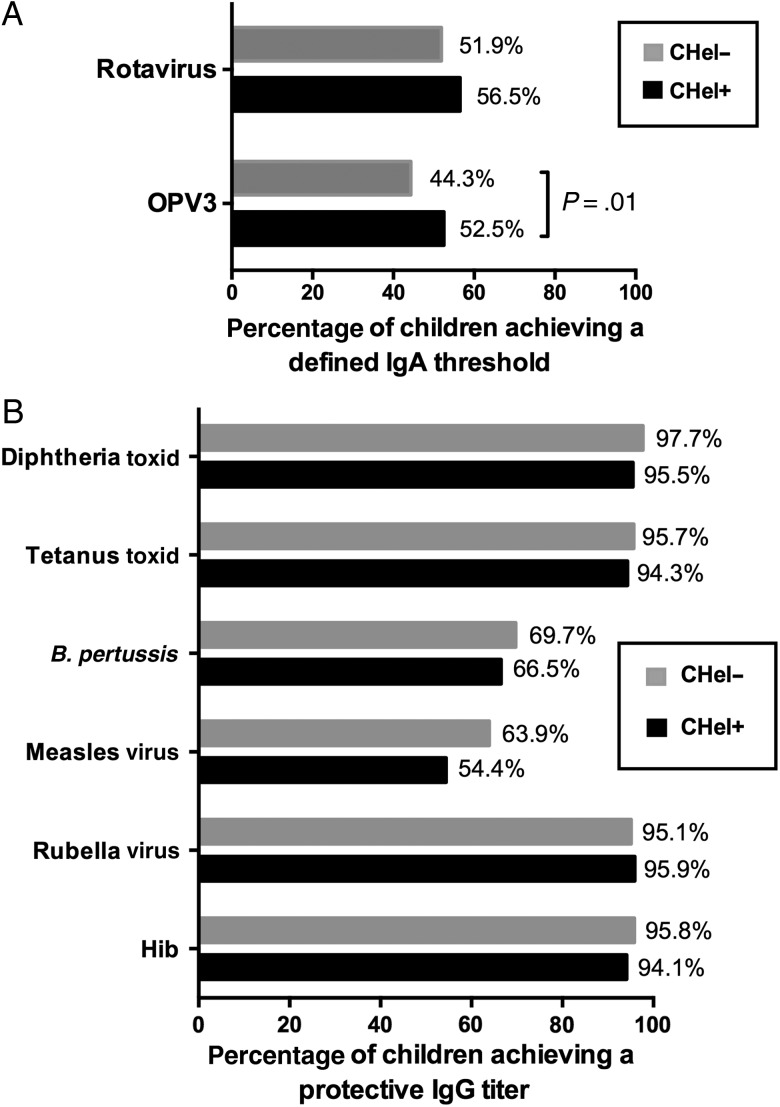
Next, we considered antibody titer as a binary variable by designating a threshold IgA or IgG titer for each vaccine and comparing the percentage of children who achieved the chosen threshold among children of helminth-infected mothers and children of helminth-uninfected mothers. For the parenterally administered vaccines included in our panel, thresholds or correlates of protection have been defined [19]; thus, these were the IgG titers chosen as the designated thresholds for these vaccines. For the orally administered vaccines, however, correlates of protection in terms of IgA titers are less clear. For the rotavirus vaccine, an arbitrary value of 20 U/mL is routinely used and considered acceptable as a cutoff in rotavirus-specific ELISAs, so we designated this value as our threshold. For the OPVs, as discussed earlier, IgA titers do not necessarily translate into protective titers. Therefore, we simply asked what percentage of children from each group had achieved a designated IgA threshold (regardless of whether it was protective), defined as the value that gave, on balance, the optimal combination of sensitivity and specificity from inspection of the ROC curve analysis when comparing the multiplex results with the microneutralization assay findings.
By this binary analysis, the difference in rotavirus take was not significant between children of helminth-infected mothers and children of helminth-uninfected mothers. In other words, although antirotavirus IgA titers among children of helminth-infected mothers were on average higher than those among children of helminth-uninfected mothers (Figure 3A and Table 2), similar proportions of subjects in each group achieved the cutoff of 20 U/mL (Figure 4A and Table 3). However, for OPV3, a significantly greater proportion of children of helminth-infected mothers achieved the designated threshold of 3100 mIU/mL, compared with children of helminth-uninfected mothers (Figure 4A and Table 3). Among the parenterally administered vaccines, there were no significant differences in vaccine take between children of helminth-infected mothers and children of helminth-uninfected mothers (Figure 4B and Table 3).
Figure 4.
Percentage of children of mothers with helminth infection (CHel+) and children of mothers without helminth infection (CHel-) achieving a defined threshold of vaccine-specific plasma antibody titers at 13 months of age. A, Primary end points: the orally administered vaccines. The threshold for antirotavirus immunoglobulin A (IgA) titer was defined as 20 U/mL, the titer often used as a cutoff in rotavirus-specific enzyme-linked immunosorbent assays. The threshold for anti–poliovirus serotype 3 (OPV3) IgA titer was defined as 3100 mIU/mL, the titer at which the sensitivity and specificity were highest, as determined by a receiver operating characteristic curve. B, Secondary end points: the parenterally administered vaccines. The thresholds for vaccine-specific immunoglobulin G (IgG) titers were as follows: anti–diphtheria toxoid, 10 mIU/mL; anti–tetanus toxoid, 10 mIU/mL; anti–Bordetella pertussis, 5 IU/mL; anti–measles virus, 120 mIU/mL; anti–rubella virus, 15 mIU/mL; and anti–Haemophilus influenzae b (Hib), 150 ng/mL. These titers were chosen because they are considered to be correlates or surrogates of protection [19].
Table 3.
Effects of Maternal Helminth Infections on Achievement of a Designated Protective Antibody Threshold Following Infant Vaccination
| Antigen | OR (95% CI) |
P Values |
Adjusted OR (95% CI) |
P Values |
||||
|---|---|---|---|---|---|---|---|---|
| 2 Sided | Adjusted for Helminth Detection in Child at Age 13 mo | Corrected by Holm Method | Adjusted | Adjusted for Helminth Detection in Child at Age 13 mo | Corrected by Holm Method | |||
| Primary end point | ||||||||
| Rotavirus | 1.3 (1.06–1.59) | .0111 | .0315 | .0111 | 1.23 (.97–1.55) | .0901 | .1399 | .0901 |
| OPV3 | 1.39 (1.14–1.7) | .0011 | .2084 | .0022 | 1.39 (1.1–1.75) | .0059 | .7609 | .0117 |
| Secondary end point | ||||||||
| Diphtheria toxoid | 0.5 (1.27–.9) | .0192 | .6624 | .0959 | 0.47 (.25–.9) | .0220 | .9061 | .1318 |
| Tetanus toxoid | 0.74 (.46–1.18) | .2225 | .0849 | .5906 | 0.85 (.48–1.50) | .5745 | .0679 | 1.0000 |
| B. pertussis | 0.86 (.69–1.06) | .1589 | .0046 | .5906 | 0.96 (.75–1.22) | .7323 | .0197 | 1.0000 |
| Measles virus | 0.67 (.54–.84) | .0003 | .4734 | .0018 | 0.91 (.66–1.24) | .5300 | .3421 | 1.0000 |
| Rubella virus | 1.22 (.72–2.1) | .5246 | .8427 | .5906 | 1.88 (.99–3.58) | .0548 | .9671 | .2738 |
| Hib | 0.7 (.44–1.12) | .1476 | .1256 | .5906 | 0.71 (.42–1.19) | .1904 | .2094 | .7615 |
A generalized additive logistic model was adjusted for calendar time, sex, child's age and helminth infection status at the 13-month evaluation, socioeconomic status, mother's antiparasite treatment, mother's education level, mother's ethnicity, urban versus rural residence, and overcrowding of home. In addition, for the tetanus toxoid response only, adjustments were made for the number of doses of tetanus toxoid received by the mother during pregnancy. The Holm method was used to adjust for multiple comparisons separately for primary and secondary end points.
Abbreviations: B. pertussis, Bordetella pertussis; CI, confidence interval; Hib, Haemophilus influenzae b; OPV3, poliovirus serotype 3 component of oral polio vaccine; OR, odds ratio.
We further analyzed whether the effect of maternal helminths on oral vaccine responses might be parasite-specific. However, when data were stratified by maternal infections with the 2 common maternal helminth infections, Ascaris lumbricoides and Trichuris trichiura, we observed similar positive associations between each of these infections and antibody titers, whether continuous or binary, indicating a more general effect of maternal helminths on infant oral vaccine responses, rather than a parasite-specific one.
DISCUSSION
Environmental factors begin influencing the developing immune system even before birth, with the result that maternal exposures in the antenatal period can have postnatal consequences for the child [20]. For example, there is evidence that soluble parasite antigens can cross the placenta and either tolerize or sensitize fetal immune responses to those antigens, thus modifying the child's susceptibility to infection with the same parasite [12, 21]. Furthermore, it is well established that helminth infections provoke a characteristic cytokine profile in the host, one that could potentially antagonize T-helper type 1 immunity required for robust responses to many vaccines. This possibility raises the concern that antenatal helminth infections in the mother may impact her infant's ability to mount protective responses to childhood vaccines.
Several previous studies have addressed this question and have reported mixed results. Researchers conducting intervention studies of maternal anthelmintic treatment in Uganda did not observe impaired vaccine responses in infants of infected mothers [22, 23]. On the contrary, maternal helminth infections were associated with an increased interferon γ (IFN-γ) response to mycobacterial antigens in infants who received BCG vaccine, although infected mothers themselves had lower IFN-γ responses than uninfected mothers. These increased IFN-γ responses were strongest for infants of mothers infected with hookworm and were reduced when infected mothers were treated with albendazole during pregnancy [24].
In contrast, researchers conducting a birth cohort study in Kenya reported lower IgG responses to Hib vaccination at 12 months of age in children of mothers infected with Wuchereria bancrofti or hookworm, compared with children of mothers without such infections. This study found no effect of maternal helminths on IgG responses to antigens in diphtheria, tetanus, and hepatitis B vaccines [25]. The contrasting results between the different vaccines examined in this study suggest that immune responses may be modulated differently depending on vaccine antigens and formulations. Furthermore, different species and intensities of helminth infections could contribute to the different findings among studies.
Our study is the first to look specifically at responses to oral vaccination in the context of maternal helminth infection. We observed higher plasma IgA responses to rotavirus vaccine and OPV in children of helminth-infected mothers, compared with children of helminth-uninfected mothers. It is known that isotype switching to IgA in the gut requires interleukin 10 (IL-10) [26]. Since one of the hallmarks of helminth infection is an elevated IL-10 level, the infected mothers are likely to have high levels of circulating IL-10. The mechanism by which a high maternal IL-10 level could translate into a high IL-10 level in the gut of the infant is unclear. One explanation might be the transfer of IL-10 across the placenta or the induction of an immune environment in the fetus that favors high circulating levels of IL-10. We have shown previously in the same cohort that cord blood plasma of children of helminth-infected mothers had elevated levels of IL-10, compared with children of helminth-uninfected mothers [27]. An alternative but unexplored hypothesis is that the cytokines are transferred through breast milk. Human milk is replete with immunomodulatory components, including cytokines [28]. Cytokine transfer from mother to offspring has been documented in mouse and pig models, and both models have shown that cytokines survive digestion in the suckling's gut and even make their way into the peripheral circulation, where they are bioactive in tissues such as the thymus [29, 30]. Thus, breast milk–derived cytokines could potentially influence how the infant mounts an immune response in the gut to oral vaccination. Whether it actually happens via this mechanism or others, we know that infants' cytokine profiles, including IL-10 level, mimic those of their mothers [31]. Therefore, children born to mothers with high levels of circulating IL-10 may also have high IL-10 levels in their gut, which would support production of IgA to orally delivered immunogens.
Strengths of our study include the prospective design from birth and the collection of a large number of sociodemographic and other relevant variables allowing us to control for potential confounders. Observation bias was reduced by using objective measures of geohelminth infection and by doing all evaluations while blinded to the child's exposure status. We were able to evaluate 68% of cohort children, with the main reason for exclusion being the unavailability of a plasma sample at 13 months of age. Such losses could lead to selection bias, although comparisons between individuals who were included and those who excluded from the analysis showed similar sociodemographic characteristics and rates of maternal helminth infections. Maternal geohelminth infections were measured in the third trimester or around the time of birth, the latter being a reasonable proxy for the former because of the chronicity of these infections.
One limitation of our study is that levels of plasma IgA do not necessarily correlate with protection for the orally administered vaccines. Even the rotavirus vaccines, for which serum IgA titers are routinely used—for lack of a better marker—as a measure of vaccine take, do not have a clearly defined correlate of protection [32]. Therefore, we cannot conclude that the children of helminth-infected mothers are better protected from these pathogens but merely that they have higher plasma IgA specific for rotavirus and OPV. What is clear, however, is that the presence of antenatal maternal helminth infection does not impair vaccine responses in infancy. Putting our study into context with others that were conducted in different settings and that examined different parameters of immune responses to different vaccines, it is clear that the cross-talk between the maternal and infant immune systems is complex and that the immune modulation by parasitic infections varies depending on the species of parasite and perhaps even the intensity of infection. A more complete picture is needed before the value of policies aiming to deworm during pregnancy can be adequately accessed.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the ECUAVIDA study team for their dedicated work; the cohort mothers and children for their enthusiastic participation; the director/s and staff of the Hospital Padre Alberto Buffoni in Quinindé, Esmeraldas Province for their support; John Patton, National Institutes of Health, and Konstantin Chumakov, Food and Drug Administration for providing reagents; Miren Iturriza-Gomara, Health Protection Agency (London, United Kingdom), for help with the microneutralization assay for OPV3; and Monica McNeal, Cincinnati Children's Hospital Medical Center for performing the rotavirus ELISAs.
Disclaimer. The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to publish.
Financial support. This work was supported by the Wellcome Trust (grant 088862/Z/09/Z) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin 2010; 6:532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallander HO, Paniagua M, Espinoza F et al. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine 2002; 21:138–45. [DOI] [PubMed] [Google Scholar]
- 3.Sur D, Ochiai RL, Bhattacharya SK et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med 2009; 361:335–44. [DOI] [PubMed] [Google Scholar]
- 4.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis 1991; 13:926–39. [DOI] [PubMed] [Google Scholar]
- 5.Korpe PS, Petri WA Jr. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med 2012; 18:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis 2009; 200(suppl 1):S39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis 1996; 173:269–72. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PJ, Chico ME, Losonsky G et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis 2000; 182:1199–206. [DOI] [PubMed] [Google Scholar]
- 9.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol 2001; 123:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis 2012; 12:330–40. [DOI] [PubMed] [Google Scholar]
- 11.Cooper PJ, Chico ME, Guadalupe I et al. Impact of early life exposures to geohelminth infections on the development of vaccine immunity, allergic sensitization, and allergic inflammatory diseases in children living in tropical Ecuador: the ECUAVIDA birth cohort study. BMC Infect Dis 2011; 11:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guadalupe I, Mitre E, Benitez S, Chico ME, Nutman TB, Cooper PJ. Evidence for in utero sensitization to Ascaris lumbricoides in newborns of mothers with ascariasis. J Infect Dis 2009; 199:1846–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Guidelines for WHO/epi collaborative studies on poliomyelitis—standard procedure for determining immunity to poliovirus using the microneutralization test. 1993. http://www.who.int/iris/handle/10665/70486. Accessed 18 March 2015.
- 14.Fay MP. Confidence intervals that match Fisher's exact or Blaker's exact tests. Biostatistics 2010; 11:373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menzies SK, Rodriguez A, Chico M et al. Risk factors for soil-transmitted helminth infections during the first 3 years of life in the tropics; findings from a birth cohort. PLoS Negl Trop Dis 2014; 8:e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright SP. Adjusted p-values for simultaneous inference. Biometrics 1992; 48:1005–13. [Google Scholar]
- 17.Saboyá MI, Catalá L, Ault SK, Nicholls RS. Prevalence and intensity of infection of Soil-transmitted Helminths in Latin America and the Caribbean Countries: Mapping at second administrative level 2000–2010. Washington, DC: Pan American Health Organization, 2011. [Google Scholar]
- 18.Ward RL, McNeal MM, Clemens JD et al. Reactivities of serotyping monoclonal antibodies with culture-adapted human rotaviruses. J Clin Microbiol 1991; 29:449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djuardi Y, Wammes LJ, Supali T, Sartono E, Yazdanbakhsh M. Immunological footprint: the development of a child's immune system in environments rich in microorganisms and parasites. Parasitology 2011; 138:1508–18. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra I, Ouma J, Wamachi A et al. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J Clin Invest 1997; 99:1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webb EL, Mawa PA, Ndibazza J et al. Effect of single-dose anthelmintic treatment during pregnancy on an infant's response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 377:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kizito D, Tweyongyere R, Namatovu A et al. Factors affecting the infant antibody response to measles immunisation in Entebbe-Uganda. BMC Public Health 2013; 13:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott AM, Namujju PB, Mawa PA et al. A randomised controlled trial of the effects of albendazole in pregnancy on maternal responses to mycobacterial antigens and infant responses to Bacille Calmette-Guerin (BCG) immunisation [ISRCTN32849447]. BMC Infect Dis 2005; 5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra I, McKibben M, Mungai P et al. Effect of antenatal parasitic infections on anti-vaccine IgG levels in children: a prospective birth cohort study in Kenya. PLoS Negl Trop Dis 2015; 9:e0003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med 1992; 175:671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta RS, Rodriguez A, Chico M et al. Maternal geohelminth infections are associated with an increased susceptibility to geohelminth infection in children: a case-control study. PLoS Negl Trop Dis 2012; 6:e1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garofalo R. Cytokines in human milk. J Pediatr 2010; 156:S36–40. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen TV, Yuan L, Azevedo MS, Jeong KI, Gonzalez AM, Saif LJ. Transfer of maternal cytokines to suckling piglets: in vivo and in vitro models with implications for immunomodulation of neonatal immunity. Vet Immunol Immunopathol 2007; 117:236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aspinall R, Prentice AM, Ngom PT. Interleukin 7 from maternal milk crosses the intestinal barrier and modulates T-cell development in offspring. PLoS One 2011; 6:e20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djuardi Y, Wibowo H, Supali T et al. Determinants of the relationship between cytokine production in pregnant women and their infants. PLoS One 2009; 4:e7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol 2012; 2:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.