Abstract
Granulomatous arteritis characterizes the pathology of giant cell arteritis, granulomatous aortitis, and intracerebral varicella zoster virus (VZV) vasculopathy. Because intracerebral VZV vasculopathy and giant cell arteritis are strongly associated with productive VZV infection in cerebral and temporal arteries, respectively, we evaluated human aortas for VZV antigen and VZV DNA. Using 3 different anti-VZV antibodies, we identified VZV antigen in 11 of 11 aortas with pathologically verified granulomatous arteritis, in 1 of 1 cases of nongranulomatous arteritis, and in 5 of 18 control aortas (28%) obtained at autopsy. The presence of VZV antigen in granulomatous aortitis was highly significant (P = .0001) as compared to control aortas, in which VZV antigen was never associated with pathology, indicating subclinical reactivation. VZV DNA was found in most aortas containing VZV antigen. The frequent clinical, radiological, and pathological aortic involvement in patients with giant cell arteritis correlates with the significant detection of VZV in granulomatous aortitis.
Keywords: VZV, granulomatous arteritis, granulomatous aortitis
(See the editorial commentary by Gershon and Gershon on pages 1859–61.)
Granulomatous arteritis is a vasculitis involving medium- and large-sized vessels, particularly the aorta, with inflammatory infiltrates composed of lymphocytes and plasma cells, along with epithelioid macrophages with or without multinucleated giant cells, as well as vessel wall damage. The most common disease that produces granulomatous arteritis is giant cell arteritis (GCA), a disorder that primarily but not exclusively affects the temporal artery (TA). The pathological changes of granulomatous arteritis are also characteristic of the intracerebral vasculopathy caused by varicella zoster virus (VZV) [1, 2]. Aortitis (ie, inflammation of the aorta) is most commonly characterized histologically by granulomatous inflammation, such as in GCA and Takayasu arteritis. Granulomatous arteritis is less frequent in other rheumatologic diseases, and only rarely is aortitis attributable to infection.
We previously reported the detection of VZV in most TAs from patients with pathologically verified GCA [3] and in most TAs of patients with clinical and laboratory features of GCA for whom TA biopsy specimens were pathologically negative for GCA [4]. Compared with normal TAs, the presence of VZV in both GCA-positive and GCA-negative TAs was highly significant. Our present search for VZV in the aortas of patients with pathologically verified granulomatous arteritis was based on the pathological recapitulation of intracerebral VZV vasculopathy and of extracranial GCA and large-vessel disease involving the aorta in granulomatous arteritis, as well as on the presence of VZV in arteries of patients with intracerebral vasculopathy and GCA. Here, we analyzed a series of aortic specimens, with pathological verification of granulomatous arteritis in 11, inflammation but not granulomatous arteritis in 1, and no inflammatory changes in 18, that were removed at autopsy from subjects who were either chronically ill and had been taking immunosuppressive drugs just before death or had received no immunomodulatory medications. All 30 aortas underwent immunohistochemical analysis for VZV antigen. When VZV antigen was found, sections containing the antigen were scraped, DNA was extracted, and polymerase chain reaction (PCR) analysis was performed with virus-specific primers.
METHODS
Human Aortas
After receiving institutional review board (IRB) approval, the surgical pathology databases at Massachusetts General Hospital from 1990 to the present were searched for mention of aorta, aortitis, and Takayasu arteritis in the surgical pathology report. In a 3-year period, 16 cases of pathologically verified aortitis were identified. Pathological rereview of the slides and paraffin blocks for these cases revealed 11 patients (5 men and 6 women aged 32–87 years) who fulfilled criteria of granulomatous arteritis. One case in an 83-year-old woman met criteria of nongranulomatous arteritis. In addition, 18 control aortas were studied, of which 5 were obtained from intervening noninflamed aortic repairs and 13 were acquired during routine autopsy from the Denver Office of the Medical Examiner and the University of Colorado Hospital with permission from next of kin. The 18 control aortas were from 8 men and 10 women aged 23–66 years. No control aortas had any inflammation or evidence of GCA.
Experimental Design
Granulomatous arteritis and control aortas were first examined immunohistochemically for VZV antigen and by PCR for VZV DNA by 2 virologists blinded to the clinical and pathological diagnoses. Sections from each aorta were then stained with hematoxylin and eosin and examined by our pathologist (P. J. B.), who was unaware of any previous pathological diagnostic or virological findings. After recording all findings, clinical features from the 11 patients with granulomatous arteritis were correlated with pathological and virological data.
Immunohistochemical Analysis of Human Aortas for VZV
Ten 5-µm sections were cut from formalin-fixed, paraffin-embedded aortas and analyzed immunohistochemically as described elsewhere [3], using a 1:500 dilution of mouse monoclonal anti-VZV gE IgG1 antibody (Santa Cruz Biotechnology, Dallas, Texas), a 1:10 000 dilution of rabbit monospecific polyclonal antibody directed against VZV IE63, or a 1:25 dilution of mouse monoclonal anti-VZV antibody directed against the Marsden strain of VZV (Abcam, Cambridge, Massachusetts). Controls for the 2 mouse anti-VZV antibodies and for the rabbit anti-VZV antibody were provided by mouse IgG1 antibody (Dako, Carpenteria, California) and rabbit anti–herpes simplex virus type 1 (HSV-1) antibody (Dako), respectively. Positive controls consisted of VZV-infected cadaveric cerebral arteries maintained for 14 days in vitro and immunostained with all 3 anti-VZV antibodies. Using light microscopy, 2 readers (D. G. and M. A. N.) blinded to diagnosis and clinical information examined each section and deemed an aorta section positive or negative for VZV antigen only when both readers agreed.
PCR Amplification of VZV DNA in FFPE Aorta Sections Containing VZV Antigen
To confirm the presence of VZV, all VZV antigen–positive sections from each of 17 aortas were scraped with a scalpel and pooled, after which DNA was extracted and analyzed by PCR for the presence of VZV DNA as described elsewhere [3]. Samples were considered positive for VZV DNA if (1) no VZV DNA was amplified in samples without added DNA, (2) GAPdH was detected in wells with aortic DNA, and (3) both PCR replicates amplified at least 10 copies of target VZV DNA per 5 µL of DNA added. VZV DNA copy number was determined using known VZV DNA concentrations as PCR standards [5].
Histopathological Analysis of Sections Adjacent to Those Stained for VZV Antigen
A 5-µm aorta section adjacent to that immunohistochemically positive for VZV antigen was stained with hematoxylin and eosin and examined by standard light microscopy by the pathologist (P. J. B.) and neurovirologists (D. G. and M. A. N.) for inflammation and tissue necrosis with giant or epithelioid cells. An aorta was deemed positive for granulomatous arteritis only when all readers agreed that all 3 criteria were met.
RESULTS
Pathological Verification of Granulomatous Arteritis
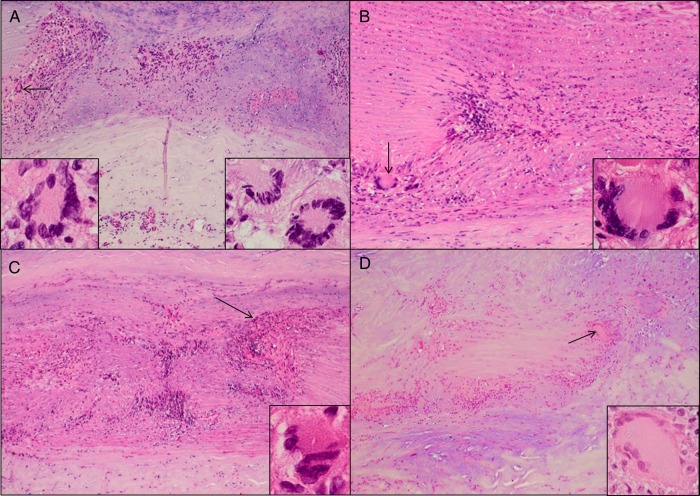
Granulomatous arteritis was verified pathologically in 11 aortas, based on the presence of inflammation and tissue necrosis with giant cells (Figure 1A–D) or epithelioid cells. One aorta was inflamed, but no giant cells or epithelioid cells were seen (nongranulomatous arteritis). None of 18 control aortas showed inflammation.
Figure 1.
Granulomatous arteritis pathology in the aorta, as shown by hematoxylin and eosin staining. A, Specimens from patient 3 exhibited inflammation in the adventitia and media, as well as medial necrosis with giant cells (arrow and insets); inflammation in the intima is not shown. B, Patient 8 showed inflammation and necrosis in the media with giant cells (arrow and inset); inflammation in the adventitia and intima is not shown. C, In patient 10, extensive inflammation and necrosis in the media with epithelioid cells (arrow and inset) was seen; inflammation in the adventitia and intima is not shown. D, In patient 5, inflammation and necrosis in the media with giant cells (arrow and inset) was seen; inflammation in the intima is not shown. All images are 600× original magnification.
VZV Antigen and VZV DNA in Granulomatous Arteritis and Control Aortas
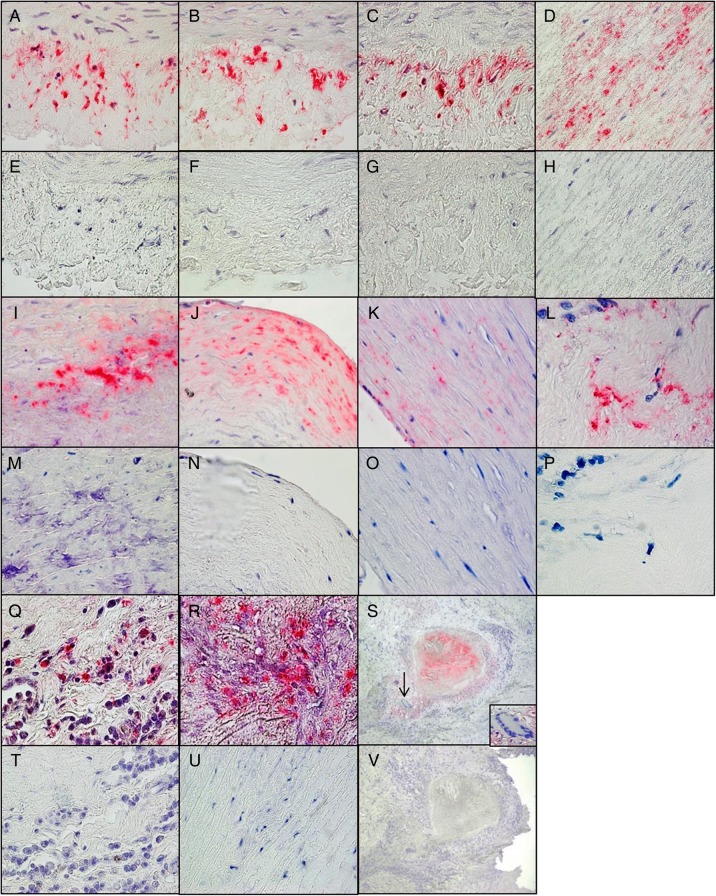
Immunohistochemical analysis indicated VZV antigen in the cytoplasm in all 11 aortas with pathologically verified granulomatous arteritis. Use of 3 different anti-VZV antibodies revealed VZV antigen in all arterial layers of the aorta (Figure 2). VZV antigen was also detected in 1 case of nongranulomatous arteritis and in 5 of 18 control aortas (28%) from subjects with no symptoms or signs of aortitis. VZV antigen was 3.60-fold more likely to be present in aortas with granulomatous arteritis than in control aortas (relative risk, 3.60; 95% confidence interval, 1.87–10.447; P = .0001). Overall, 17 aortas (11 from individuals with granulomatous arteritis, 1 from an individual with nongranulomatous arteritis, and 5 controls) contained VZV antigen.
Figure 2.
Varicella zoster virus (VZV) antigen in the aorta of patients with granulomatous arteritis. A–C, Positive control cadaveric human cerebral arteries maintained in tissue culture infected with VZV and analyzed immunohistochemically 14 days later with mouse anti-VZV gE immunoglobulin G1 (IgG1) antibody (A), mouse monoclonal antibody directed against the Marsden strain of VZV (B), and rabbit anti-VZV IE63 antibody (C) revealed VZV antigen in the arterial adventitia of the VZV-infected cadaveric arteries. E–G, No staining was seen when mouse isotype IgG1 antibody was substituted for mouse anti-VZV gE IgG1 antibody (E) or mouse monoclonal anti-Marsden strain VZV antibody (F) or when rabbit anti-HSV-1 antibody was substituted for rabbit anti-VZV IE63 antibody (G). D, H–J, M, and N, Immunohistochemical analysis of aortas from patients with granulomatous arteritis by using mouse anti-VZV gE IgG1 antibody showed VZV antigen in the media of the aorta from patient 11 (D) and in the intima of the aorta from patient 1 (I) and patient 7 (J) that was not seen using control mouse isotype IgG1 antibody (H, M, and N). K, L, O–R, T, and U, VZV antigen was also detected in the media and intima of the aorta of patient 2 after immunostaining with rabbit anti-VZV IE63 antibody (K and L) but not when control rabbit anti-HSV-1 antibody was used (O and P), as well as in the adventitia of the aorta of patient 10 (Q) and in the media of the aorta of patient 4 (R) after immunostaining with mouse monoclonal anti-Marsden strain VZV antibody but not when control mouse isotype IgG1 antibody was used (T and U). S and V, VZV antigen in the aorta of patient 1 (red) was in close proximity to inflammation, necrosis, and giant cells (inset; S) but was not seen using control mouse isotype IgG1 antibody (V). Images in panels A–R, T, and U, are 600× original magnification; images and panels S and V are 100× original magnification.
Of the 11 aortas with granulomatous arteritis that were positive for VZV antigen, 10 contained amplifiable cellular DNA, of which 7 (70%) contained VZV DNA. The 1 aorta with nongranulomatous arteritis contained both cellular DNA and VZV DNA. Of the 5 control aortas positive for VZV antigen, all had amplifiable cellular DNA, and 4 (80%) contained VZV DNA.
Clinicopathologic Correlation
Review of medical records of the 11 patients with surgical repair of an aortic aneurysm suggested the diagnosis of giant cell arteritis in 5 patients, whereas clinical criteria for the diagnosis of Takayasu arteritis were not met by any of the patients [6, 7]. See Supplementary Material for clinical vignettes of the patients.
DISCUSSION
Herein, we detected abundant VZV antigen in all of 11 aortas that exhibited the characteristic pathology of granulomatous arteritis. VZV antigen was also found in 1 case of nongranulomatous arteritis and in 5 of 18 control aortas (28%) from subjects with no symptoms or signs of aortitis. Importantly, the specificity of VZV antigen detection was confirmed using 3 different anti-VZV antibodies prepared in mouse and rabbit, which detected VZV antigen in cadaveric cerebral arteries infected with VZV. Compared with control aortas, the presence of VZV antigen in patients with granulomatous aortitis was highly significant (P < .0001). Despite formalin fixation, the detection of VZV antigen was readily corroborated by the presence of amplifiable VZV DNA in most VZV antigen–positive aortas, which further supports the specificity of the anti-VZV antibodies used in this study. Note that while the previous detection of VZV in patients with GCA required immunostaining of 50 sections of TAs, VZV antigen detection in these aortas required immunostaining of only 3–6 sections, an indication of the considerable abundance of VZV antigen in granulomatous arteritis and/or the greater amount of tissue in sections of aorta as compared to the TA.
In our earlier study that detected VZV in most TAs of patients with GCA [3], we also found VZV antigen in 18% of normal extracranial TAs, a finding paralleled herein by the detection of VZV antigen in 28% of control aortas. While some anti-VZV antibodies cross-react with blood group A antigens in ganglionic neurons [8], our immunostaining appears to be highly specific, as evidenced by using 3 different anti-VZV antibodies and by the detection of VZV antigen in skip areas of aorta rather than every section of normal aorta, and by the detection of VZV DNA in 4 of 5 normal aortas that contained VZV antigen. Moreover, no inflammation was ever seen in normal aortas that contained VZV, consistent with our findings in normal TAs acquired at autopsy, which revealed no association between VZV antigen and inflammation. Overall, the detection of VZV antigen in nonneuronal arterial cells, confirmed by the presence of VZV DNA in most control arteries, indicates VZV reactivation. Our previous detection of VZV antigen and DNA in some normal TAs showed that VZV reactivates in the absence of clinical symptoms and signs in people aged >50 years [3]; the presence of VZV in some normal aortas in the present study confirms subclinical reactivation in younger individuals [9, 10]. A difference in host immune response may account for the absence of pathology in normal aortas containing VZV antigen. For example, genetic differences in host cells affect the immune response to virus and susceptibility to HSV-1 encephalitis [11]. Owing to tissue fixation and the limited number of sections available for study, it was not possible to compare the quantity of VZV antigen in normal aortas and aortas with granulomatous arteritis.
The presence of VZV antigen in granulomatous arteritis lesions is not likely to be a bystander effect of inflammation. Indeed, our previous analyses of tissue sections and cerebrospinal fluid (CSF) from patients with inflammatory/infectious diseases indicated that inflammation does not cause VZV to reactivate and infect the inflamed region; we have never found VZV in inflammatory brain tissue [12] or in highly inflamed CSF in patients with meningitis [13]. Instead, VZV is only associated with inflammation when the virus causes the inflammatory response, as demonstrated by the presence of VZV antigen and VZV DNA in a patient with VZV pneumonitis and by giant cells and Cowdry A inclusions [14] in the intracerebral arteries of patients with VZV vasculopathy [1] and in the TAs of patients with GCA [3, 4].
Our detection of VZV in all arterial layers of the aorta is consistent with earlier findings of VZV antigen in the adventitia, media, and intima of intracerebral arteries from patients with VZV vasculopathy [15] and in TAs of patients with GCA [3]. In intracerebral vasculopathy and in GCA, arterial infection likely develops after VZV reactivates from cranial nerve ganglia, followed by transaxonal spread to the arterial adventitia and transmural spread to the media and intima. Although the exact mechanism by which VZV infects the aorta is unknown, infection likely follows VZV reactivation from thoracic sensory ganglia [16] and autonomic ganglia, where latent VZV is abundant [17], and eventual transaxonal spread to the aortic adventitia and transmural spread to the media and intima.
The detection of VZV in the aortas of patients with granulomatous arteritis provides a virological link to long-term clinical, radiologic, and pathological studies of patients with GCA with aortic involvement. For example, long-term clinical follow-up of patients with GCA showed that involvement of other extracranial arteries, especially the aorta and its main branches, resulted in an increased incidence of aortic aneurysm and dissection [18]. Furthermore, computed tomography angiography of 40 patients with newly diagnosed GCA revealed large-vessel vasculitis in 67% [19]. Importantly, an earlier systematic necropsy study revealed large-vessel inflammation involving the aorta and its main branches in nearly all 13 patients with GCA [20].
Although the exact clinical cause of granulomatous arteritis in the aortas of our 11 patients is unknown, 5 had abnormal clinical, laboratory, or imaging findings suggestive of GCA or Takayasu arteritis. Patient 1 had TA tenderness often seen in GCA, and patient 2 had an elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level, as well as stenosis of many large arteries in the chest and abdomen, as commonly seen in both GCA and in Takayasu arteritis. Patient 4 had elevated an ESR and CRP level, as well as aortic thickening, further linking GCA and large-vessel involvement. Patient 6 had headache and jaw claudication typical of GCA and polymyalgia rheumatica, with an elevated ESR and CRP level, while patient 10, treated with steroids for visual symptoms possibly due to ischemic optic neuropathy, had an elevated ESR and CRP level; in both patients, the clinical features and abnormal laboratory findings showed an association between the detection of VZV in the aorta and features of GCA. Interestingly, long-term follow-up of 66 patients with nonatheromatous aortic complications (aortitis, aortic ectasia, and/or aneurysm) revealed the relationship of aortic involvement to GCA in 48 subjects and to Takayasu arteritis in 6 subjects [21].
Overall, the pathology in patients with intracerebral vasculopathy, GCA, and granulomatous arteritis of the aorta is identical, and VZV infection is readily demonstrated. While VZV infection in the aortas of patients with granulomatous aortitis was always associated with the pathology of granulomatous arteritis and VZV infection of some control aortas was never associated with inflammation, future studies demonstrating causation are necessary. Although antiviral agents are effective in treating intracerebral VZV vasculopathy [22], their role in treating GCA and granulomatous arteritis of the aorta remains unclear, although one patient who satisfied American College of Rheumatology criteria for both GCA and Takayasu arteritis, including the presence of VZV in the TA, responded dramatically to antiviral treatment [23].
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Marina Hoffman for editorial review and Cathy Allen for manuscript preparation.
Financial support. This work was supported by the National Institutes of Health (grant AG032958 to D. G. and M. A. N.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis. NEJM case 5-1995 revisited. Neurology 1996; 47:1441–6. [DOI] [PubMed] [Google Scholar]
- 2.Nagel MA, Cohrs RJ, Mahalingam R et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging and virological features. Neurology 2008; 70:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilden D, White T, Khmeleva N et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology 2015; 84:1948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagel MA, White T, Khmeleva N et al. Analysis of varicella-zoster virus in temporal arteries biopsy positive and negative for giant cell arteritis. JAMA Neurol 2015; 72:1281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird NL, Bowlin JL, Yu X et al. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology 2014; 458–459:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunder GG, Bloch DA, Michel BA et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990; 33:1122–8. [DOI] [PubMed] [Google Scholar]
- 7.de Souza AW, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83. [DOI] [PubMed] [Google Scholar]
- 8.Zerboni L, Sobel RA, Lai M et al. Apparent expression of varicella-zoster virus proteins in latency resulting from reactivity of murine and rabbit antibodies with human blood group A determinants in sensory neurons. J Virol 2012; 86:578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol 2004; 72:174–9. [DOI] [PubMed] [Google Scholar]
- 10.Papaevangelou V, Quinlivan M, Lockwood J et al. Subclinical VZV reactivation in immunocompetent children hospitalized in the ICU associated with prolonged fever duration. Clin Microbiol Infect 2013; 19:E245–51. [DOI] [PubMed] [Google Scholar]
- 11.Bereczky-Veress B, Abdelmagid N, Piehl F et al. Influence of perineurial cells and toll-like receptors 2 and 9 on herpes simplex type 1 entry to the central nervous system in rat encephalitis. PLoS One 2010; 5:e12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgoon MP, Cohrs RJ, Bennett JL et al. Varicella zoster virus is not a disese-relevant antigen in multiple sclerosis. Ann Neurol 2009; 65:474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez N, Tyler KL, Gilden DH. Recurrent dermatomal vesicular skin lesions: a clue to diagnosis of herpes simplex virus 2 meningitis. Arch Neurol 2003; 60:868–9. [DOI] [PubMed] [Google Scholar]
- 14.Nandhagopal R, Khmeleva N, Jayakrishnan B et al. Varicella zoster virus pneumonitis and brainstem encephalitis without skin rash in an immunocompetent adult. Open Forum Infect Dis 2014; 1:ofu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagel MA, Traktinskiy I, Azarkh Y et al. Varicella zoster virus vasculopathy. Analysis of virus-infected arteries. Neurology 2011; 77:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahalingam R, Wellish MC, Dueland AN, Cohrs RJ, Gilden D. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann Neurol 1992; 31:444–8. [DOI] [PubMed] [Google Scholar]
- 17.Nagel MA, Rempel A, Huntington J, Kim F, Choe A, Gilden D. Frequency and abundance of alphaherpesvirus DNA in human thoracic sympathetic ganglia. J Virol 2014; 88:8189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans JM, O'Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis: a population-based study. Ann Intern Med 1995; 122:502–7. [DOI] [PubMed] [Google Scholar]
- 19.Prieto-González S, Arguis P, García-Martinez A et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis 2012; 71:1170–6. [DOI] [PubMed] [Google Scholar]
- 20.Ostberg G. Morphological changes in the large arteries in polymyalgia arteritica. Acta Med Scand Suppl 1972; 533:135–59. [PubMed] [Google Scholar]
- 21.Marie I, Proux A, Duhaut P et al. Long-term follow-up on aortic involvement in giant cell arteritis: a series of 48 patients. Medicine 2009; 88:182–92. [DOI] [PubMed] [Google Scholar]
- 22.Cheng-Ching E, Jones S, Hui FK et al. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci 2015; 351:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilden D, White TM, Nagae L, Gurdin WH, Boyer PJ, Nagel MA. Successful antiviral treatment of giant cell arteritis and Takayasu arteritis. JAMA Neurol 2015; 72:943–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.