Abstract
We described a comprehensive analysis of the molecular epidemiology of multidrug-resistant (MDR) P. aeruginosa. Molecular analysis included typing by Pulsed Field Gel Electrophoresis, identification of genes of interest through PCR-based assays and sequencing of target genes. Case-control study was conducted to better understand the prognostic of patients and the impact of inappropriate therapy in patients with bacteremia, as well as the risk factors of MDR infections. We observed a high rate of MDR isolates (40.7%), and 51.0% of them was independently associated with inappropriate antibiotic therapy. Bacteremia was detected in 66.9% of patients, and prolonged hospital stay was expressive in those resistant to fluoroquinolone. Plasmid-mediated quinolone resistance genes (PMQR), qnrS1 and aac(6’)Ib-cr, were detected in two different nosocomial isolates (5.3%), and the aac(6’)-Ib7 variant was detected at a high frequency (87.5%) in those negative to PMQR. The presence of mutations in gyrA and parC genes was observed in 100% and 85% of selected isolates, respectively. Isolates harboring PMQR genes or mutations in gyrA and parC were not closely related, except in those containing SPM (São Paulo metallo-β-lactamase) clone. In addition, there is no study published in Brazil to date reporting the presence of Pseudomonas aeruginosa isolates harboring both qnrS1 and aac(6’)Ib-cr genes, with alarming frequency of patients with inappropriate therapy.
Introduction
Currently, the increasing incidence of MDR P. aeruginosa is a global problem as a consequence of the ability of this microorganism to develop resistance to almost all antibiotics that are available for treatment, either by mutations present in chromosomal gene, or by horizontal gene transfer [1,2]. In Brazil, this problem is aggravated because of either the very high density of the antimicrobials use, especially β-lactams (including carbapenems) and fluoroquinolones [3,4] and the lack of affirmative action’s for prevention of these infections [5,6].
Carbapenems are a therapeutic choice for the treatment of severe infections caused by P. aeruginosa. However, its use has been threatened mainly by the increased incidence of isolates resistant to this class of antibiotics [7–9]. Among the carbapenem resistance mechanisms, there is the presence of carbapenemases, which are a heterogeneous group of β-lactamases comprising Classes A (penicillinases), B (metalloenzymes) and D (oxacillinases), with the ability to hydrolyze imipenem and meropenem in addition to other penicillins and cephalosporins [10]. The genes encoding the carbapenemases are usually located in plasmids, which significantly increase the risk of their dissemination [11,12]. The carbapenem resistance in P. aeruginosa infection occurs mainly by metallo-β-lactamase (MBL), in addition to the appearance of other β-lactamases with carbapenemase activity, including KPC (Klebsiella pneumoniae carbapenemase) and OXA-carbapenemase [7,9,13,14].
In recent years, the production of MBL by P. aeruginosa isolates has assumed epidemiological importance and is associated with high mortality rates [15]. MBLs confer resistance to carbapenems, and are generally encoded by mobile elements facilitating the spread of antibiotic resistance [16–18]. The blaSPM-1 gene has been spread in Brazilian regions by the persistent MDR P. aeruginosa, and studies have reported concerns about its worldwide spread and potential pandemic [7, 18–20]. The increased prevalence of infections and colonization by P. aeruginosa São Paulo metallo-β-lactamases (SPM)-producers suggests that the gene blaSPM-1 settled successfully in plasmids and is associated with high risk of formation of clones, which can facilitate its global spread [21, 22].
Beyond the potent in vitro anti-pseudomonal activity, the use of fluoroquinolone antibiotics has spread widely in the past decade, and its usage popularity has also facilitated the emergence of resistant and MDR strains [23]. The resistance to fluoroquinolones is mainly due to (i) the point mutations in quinolone resistance determining regions (QRDR) in the DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) genes, (ii) the presence of plasmid-mediated quinolone resistance (PMQR) determinants, and/or (iii) the decreased uptake of the drug due to the loss of a membrane-bound porin and/or drug extrusion via efflux pumps [24]. The co-existence of mutations in genes encoding for type-II topoisomerase and PMQR is often found together in microorganisms of the Enterobacteriaceae family, and the presence of PMQR determinants may promote QRDR mutations, increasing the resistance to fluoroquinolone [25, 26]. Although PMQR genes, such as qnrA, qnrB, qnrC, qnrD, qnrS, qepA and aac(6’)-Ib-cr, have been increasingly reported in bacterial pathogens within the Enterobactericeae family, they have not been detected frequently in P. aeruginosa isolates [24, 27]. To the best of our knowledge, as far as we know, this is the first description of the presence of PMQR genes in P. aeruginosa isolates in Brazil.
In addition, only a few studies have examined the consequences of resistance to antibiotics and the impact of inappropriate therapy on the outcome of patients with MDR P. aeruginosa infections. Thus, this study was performed to evaluate this factor, and to identify the risk factors in patients with MDR P. aeruginosa infections. In addition, we also aimed to determine: (i) the presence of metallo-β-lactamases genes in carbapenem-resistant isolates; (ii) the co-occurrence of PMQR determinants and altered gyrA and parC genes in fluoroquinolone-resistant isolates; and (iii) the pattern of clonal spread of P. aeruginosa in the hospital environment.
Materials and Methods
Study design and data collection
Active surveillance was conducted from May 2009 to December 2012 and from April to October 2014 for the detection of patients with P. aeruginosa infections resistant to carbapenems and fluoroquinolones at Uberlândia University Hospital (Brazil). In total, 242 episodes of P. aeruginosa infections obtained from 236 patients were included in the study. From this surveillance, two case-control studies were conducted: (i) to determine the risk factors associated with MDR P. aeruginosa infections, and (ii) to determine the risk factors associated with antimicrobial resistance and treatment outcome in patients with P. aeruginosa bacteremia. In both studies, only the first episode of each infection was considered. The demographic, clinical and epidemiological data of the patients were also obtained through review of medical records, following the model of National Healthcare Safety Network (NHSN).
Definitions
According to the Centers for Disease Control and Prevention (CDC), bacteremia was defined as the presence of viable bacteria in the blood, documented by a positive blood culture result [28]. The isolates were considered to be nosocomial if the infection occurred >48 h after admission and no clinical evidence of infection on admission existed [29]. The criteria used for defining MDR phenotype was: non-susceptible to ≥1 agent in ≥3 antimicrobial categories [30]. Previous antibiotic use was considered when the patient received therapy with any antibiotic for at least 72 h over a period of 30 days prior to the microbiological infection diagnosis [31]. The antimicrobial therapy was considered to be appropriate if the initial antibiotics, which were administered within 24 h of acquisition of a blood culture sample, included at least one antibiotic that was active in vitro [32]. The 30-day mortality was considered as the number of deaths of patients with infections during hospitalization that occurred within 30 days of the diagnosis of infection [33], and the 5-day mortality, also known as early mortality, was considered as the number of deaths within 5 days of hospitalization [34]. Hospital stays were considered prolonged if they reached or exceeded 45 days [35]. It was considered MIC50 and MIC90 represent the concentration of antimicrobial agent (μg/mL) that inhibited 50% and 90%, respectively, of the isolates tested [36].
Clinical microbiological and antibiotic resistant profile
Microbial identification and antimicrobial susceptibility tests were performed on a VITEK II system (bioMérieux, Brazil) for the following antimicrobials: aminoglycoside (gentamicin, amikacin), carbapenems (imipenem, meropenem), cephalosporin (cefepime), fluoroquinolone (ciprofloxacin) and penicillin plus β-lactamase inhibitors (piperacillin-tazobactam). Quality-control protocols were used according to the standards of the Clinical and Laboratory Standard Institute [37, 38]. The isolates with intermediate susceptibility were considered as resistant.
The minimum inhibitory concentration (MIC) and the confirmation test of resistance to imipenem (≥8 μg/mL) were performed by the E-test® method, according to the manufacturer’s guidelines (AB Biodisk, Sweden) [38]. In addition, resistance to ciprofloxacin was confirmed by broth microdilution method according to Capuano [39] with modifications, and the interpretations also were made according to CLSI [38], considering resistance to ciprofloxacin ≥4 μg/mL.
Characterization of strains harboring MBL and PMQR genes
Forty clinical P. aeruginosa fluoroquinolone-resistant isolates were selected, being obtained from 39 patients, with various clinical infections (urinary infection, pneumonia, wound infection, otitis, and bloodstream infection).
DNA extraction was performed using a PureYield™ Plasmid Miniprep System (Promega, Brazil). Amplification of the MBL and PMQR markers (blaIMP, blaVIM, blaSPM, blaGIM, blaSIM, qnrA, qnrB, qnrC, qnrD, qnrS, qepA and aac(6’)-Ib-cr) were performed using primers listed in S1 Table (available in the Supporting Information). The reaction mixture (25 μL) contained 1.0 μL DNA template (10 ng), 12.5 μL GoTaq® Green Master Mix (Promega) and 0.5 μL of each primer. Amplifications were performed in Mastercycler Personal (Eppendorf) using the following program: initial denaturation at 95°C for 2 min followed by 30 cycles of 30 seconds at 95°C, 1 min at annealing temperature (54°C for blaIMP, blaVIM, blaSPM, blaGIM and blaSIM; 51°C for qnrA, qnrB, qnrC, qnrS and qnrD; 52°C for qepA and aac(6’)-Ib-cr), 1 min at 72°C and a final extension steap of 5 min at 72°C. Multiplex PCR was performed for genotypic characterization of different MBL (blaIMP, blaVIM, blaSPM, blaGIM and blaSIM) and PMQR (qnrA, qnrB, qnrC, and qnrS) genes; and, for the others genes individual PCRs were carried out (qnrD, qepA and aac(6’)-Ib-cr). The amplified PCR products were visualized by electrophoresis in 1.5% agarose gel by the photo documentation System L-Pix EX (Loccus Biotechnology, Brazil).
Sequencing of PMQR genes and QRDRs mutations
The PCR products of target regions for QRDR mutations (gyrA and parC) and PMQR genes (qnrS and aac(6’)-Ib) were sequenced (primers listed in S1 Table), using an automatic sequencer ABI-PRISM 3100 Genetic Analyzer (Applied Biosystems, USA). Sequences were edited using SeqMan Pro alignment was carried out with MegaAlign, both of Lasergene package version 10 (DNAStar, USA) and deposited at GenBank (http://www.ncbi.nlm.nih.gov/genbank/) [accessions numbers: KT962252 (qnrS1), KT987419 (aac(6’)-Ib-cr) and KT987420 (aac(6’)-Ib7)].
Pulsed-Field Gel Electrophoresis (PFGE)
Isolates were typed according to the protocols described by Galetti [40] with modifications, following digestion of genomic DNA with SpeI restriction enzyme (Promega). DNA fragments were separated on 1% (w/v) agarose gels in 0.5x TBE [Tris–borate–ethylene diamine tetra-acetic acid (EDTA)] buffer using a CHEF DRIII apparatus (Bio-Rad, USA) with 6 V/cm, pulsed from 5 s to 40 s, for 21 h at 12°C. Gels were stained with ethidium bromide and photographed under ultraviolet light. Computer-assisted analysis was performed using BioNumerics 5.01 software (Applied Maths, Belgium). Comparison of the banding patterns was accomplished by the unweighted pair-group method with arithmetic averages (UPGMA) using the Dice similarity coefficient.
Statistical analysis
The Chi-square or Fisher’s exact test was used to compare discrete variables. The comparison of two quantitative variables was made using the Mann–Whitney test for nonparametric variables and the Student t test for parametric variables. Two-sided tests were used for all analyses. Multivariate analysis was performed using multiple logistic regression and the values were included when significance was <0.05 in univariate analysis. To determine inappropriate therapy for mortality within 30 days of hospitalization, a multiple logistic regression model was used to control for the effects of confounding variables. All p-value <0.05 was considered statistically significant. The epidemiological data were analyzed through the programs Graph Pad Prism® 5.0 (La Jolla, USA) and BioEstat 5.0 (Tefé, Brazil).
Ethical considerations
The data and the samples analyzed in the present study were obtained in accordance with the norms and approved by the Federal University of Uberlandia Ethics Committee (UFU), through license number 36601814.7.0000.5152. For this study, samples were collected at the Microbiology Laboratory of the Clinical Hospital, with no contact to the patient and with the permission of the Hospital. Moreover, this study was retrospective and there are no patient identification when performed data collection, so the ethics committee dismissed the informed consent term and clarified.
Results
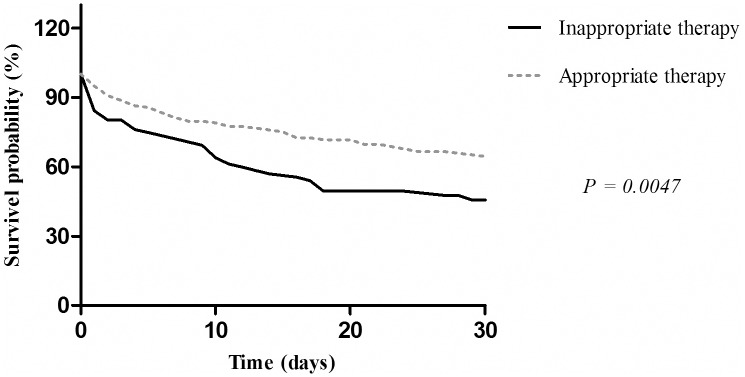
From May 2009 to December 2012 and from April to October 2014, a total of 236 non-repetitive patients with P. aeruginosa infections at the University Hospital were included in the study. The univariate analysis and independent risk factors associated with MDR P. aeruginosa infections are summarized in Table 1. According to antimicrobial susceptibility testing results, MDR P. aeruginosa infections occurred in 40.7% of the cases. Data from these patients (MDR) were compared with a sensitive P. aeruginosa infections group (non-MDR). In the whole series, prior exposure to carbapenems and inappropriate therapy as well as the co-morbidity condition (diabetes mellitus) were significant in the univariate analysis by MDR P. aeruginosa infections. The results of multivariate analyses showed that factors independently associated with MDR P. aeruginosa were patients who received inappropriate therapy. The Kaplan–Meier cumulative survival estimates (Fig 1) for patients with inappropriate versus appropriate therapy showed that the first group had a lower probability of survival than the group that received appropriate therapy (P = 0.0047). The 30-day mortality rate of the first group was 55.3%, whereas that of the second group was 34.4%. Furthermore, of the total mortality, 5-day mortality rate was 40.6% (54/133) independently of the therapy received.
Table 1. Univariate and multivariate analyses, mortality and independent risk factors associated with multidrug-resistant P. aeruginosa infections.
| Risk factor/ characteristics | Total | MDR1 | non-MDR | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|---|
| n = 236 (%) | n = 96 (%) | n = 140 (%) | OR2 (CI3 95%) | P value | OR (CI 95%) | P value | |
| Age [mean]; ±SD4 | 52.7; ±22.9 | 56.5; ±18.3 | 50.6; ±25.1 | - | 0.2111 | - | - |
| Male | 166 (70.3) | 65 (67.7) | 101 (72.1) | 0.81 (0.46–1.42) | 0.4638 | - | - |
| Female | 70 (29.7) | 31 (32.3) | 39 (27.8) | 1.23 (0.70–2.17) | 0.4638 | - | - |
| Length of hospital stay days [mean]; ±SD | 55.0; ±69.7 | 54.7; ±46.5 | 53.3; ±80.9 | - | 0.0669 | - | - |
| Invasive procedures | 210 (88.9) | 86 (89.6) | 123 (87.9) | 1.19 (0.52–2.72) | 0.6824 | - | - |
| Mechanical ventilation | 126 (53.4) | 58 (60.4) | 68 (48.6) | 1.62 (0.95–2.74) | 0.0731 | - | - |
| Tracheostomy | 120 (50.8) | 56 (58.3) | 64 (45.7) | 1.66 (0.98–2.81) | 0.0568 | - | - |
| Urinary catheter | 163 (69.1) | 71 (73.9) | 92 (65.7) | 1.48 (0.83–2.63) | 0.1783 | - | - |
| Central venous catheter | 192 (81.4) | 77 (80.2) | 115 (82.1) | 0.88 (0.45–1.71) | 0.7078 | - | - |
| Surgical drain | 41 (17.4) | 17 (17.7) | 24 (17.1) | 1.04 (0.52–2.06) | 0.9103 | - | - |
| Enteral probes/gastric nutrition | 172 (72.9) | 71 (73.9) | 101 (72.1) | 1.10 (0.61–1.97) | 0.7580 | - | - |
| Haemodialysis | 71 (30.1) | 29 (30.2) | 42 (30.0) | 1.01 (0.57–1.78) | 0.9727 | - | - |
| Co-morbidity conditions | 146 (61.9) | 57 (59.4) | 89 (63.6) | 0.84 (0.49–1.43) | 0.5144 | - | - |
| Heart failure | 60 (25.4) | 27 (28.1) | 33 (23.6) | 1.27 (0.70–2.29) | 0.4300 | - | - |
| Neoplasia | 36 (15.2) | 9 (9.4) | 27 (19.3) | 0.43 (0.19–0.97) | 0.0375 | - | - |
| Diabetes mellitus | 40 (16.9) | 22 (22.9) | 18 (12.9) | 2.01 (1.01–4.00) | 0.0430* | 1.9907 (0.97–4.09) | 0.0608 |
| Chronic renal failure | 66 (28.0) | 28 (29.2) | 38 (27.1) | 1.10 (0.62–1.97) | 0.7337 | - | - |
| Previous use of antimicrobial | 195 (82.6) | 82 (85.4) | 113 (80.7) | 1.40 (0.69–2.83) | 0.3490 | - | - |
| Carbapenems | 119 (50.4) | 56 (58.3) | 63 (45.0) | 1.71 (1.01–2.89) | 0.0442* | 0.8928 (0.51–1.55) | 0.6873 |
| Fluoroquinolone | 51 (21.6) | 24 (25.0) | 27 (19.3) | 1.41 (0.76–2.64) | 0.2753 | - | - |
| Cephalosporin (3 and 4th generation) | 161 (68.2) | 65 (67.7) | 96 (68.6) | 0.96 (0.55–1.68) | 0.8888 | - | - |
| Inappropriate therapy | 85 (36.0) | 49 (51.0) | 36 (25.7) | 3.01 (1.73–5.23) | < 0.0001* | 3.0169 (1.72–5.31) | 0.0001* |
| Mortality | |||||||
| Total mortality | 133 (56.4) | 54 (56.2) | 79 (56.4) | 0.99 (0.59–1.68) | 0.9783 | - | - |
| 30-day mortality | 99 (41.9) | 42 (43.7) | 57 (40.7) | 1.133 (0.67–1.92) | 0.6425 | - | - |
| 5-day mortality | 54 (22.9) | 25 (26.0) | 28 (20.0) | 1.408 (0.76–2.61) | 0.2746 | - | - |
1Multidrug-resistant;
2Odds ratio;
3Confidence interval;
4Standard deviation.
*P ≤ 0.05 –statistically significant for risk factor.
Fig 1. Survival curve using the Kaplan–Meier method for patients who received appropriate antimicrobial therapy compared with those who received inappropriate therapy.
P ≤ 0.05 –statistically significant.
Antimicrobial therapy and clinical outcome of patients with or without bacteremia caused by P. aeruginosa were evaluated and it can be observed that patients with bacteremia caused by isolates resistant to carbapenems, had a high 5-day mortality rate. Moreover, the time of hospital stay was significantly higher for the MDR and fluoroquinolone-resistant groups when compared with susceptible group, and the latter was also independently associated in the multivariate analyses (Table 2).
Table 2. Antimicrobial therapy and clinical outcome of patients with bacteremia caused by P. aeruginosa resistant to carbapenems, fluoroquinolones and multiresistant.
| Patients with bacteremia | Patients without bacteremia | Univariate | ||
|---|---|---|---|---|
| n = 158 (%) | n = 78 (%) | P value | OR1 (CI2 95%) | |
| Carbapenem resistant isolates3 | ||||
| Total | 70 (100.0) | 39 (100.0) | - | - |
| 5-day mortality | 22 (31.4) | 5 (12.8) | 0.0310* | 3.117 (1.073–9.051) |
| 30-day mortality | 36 (51.4) | 14 (35.9) | 0.1188 | 1.891 (0.8453–4.229) |
| Inappropriate therapy | 32 (45.7) | 24 (61.5) | 0.1131 | 0.5263 (0.2368–1.170) |
| Prolonged hospital stay5 | 40 (57.1) | 15 (38.5) | 0.0615 | 2.133 (0.9582–4.749) |
| Fluoroquinolone resistant isolates4 | ||||
| Total | 67 (100.0) | 31 (100.0) | - | - |
| 5-day mortality | 21 (31.3) | 4 (12.9) | 0.0796 | 3.082 (0.9561–9.932) |
| 30-day mortality | 33 (49.2) | 9 (29.0) | 0.0600 | 2.373 (0.9534–5.904) |
| Inappropriate therapy | 30 (44.8) | 21 (67.7) | 0.0343 | 0.3861 (0.1579–0.9440) |
| Prolonged hospital stay6 | 38 (56.7) | 7 (22.6) | 0.0016* | 4.493 (1.701–11.86) |
| Multidrug-resistant isolates | ||||
| Total | 67 (100.0) | 29 (100.0) | - | - |
| 5-day mortality | 21 (31.3) | 4 (13.8) | 0.0820 | 2.853 (0.8809–9.241) |
| 30-day mortality | 32 (47.8) | 11 (37.9) | 0.3738 | 1.496 (0.6141–3.645) |
| Inappropriate therapy | 30 (44.8) | 19 (65.5) | 0.0620 | 0.4267 (0.1727–1.055) |
| Prolonged hospital stay | 39 (58.2) | 7 (24.1) | 0.0022* | 4.378 (1.644–11.66) |
1Odds ratio;
2Confidence interval;
3Imipenem and/or meropenem;
4Ciprofloxacin and/or norfloxacin;
5Length of stay ≥ 45 days;
6Significant risk factor by multivariate analyses P = 0.0088*, OR = 3.8151 (CI = 1.40–10.38); *P ≤ 0.05 –statistically significant.
The antimicrobial resistance analysis of the 242 P. aeruginosa isolates is presented in Table 3. The resistance to carbapenem and fluoroquinolones were the most frequents, 45.9% and 42.6% respectively, followed by resistance to aminoglycosides (38.4%), cephalosporin (3rd and 4th generation) (34.3%) and piperacilin/tazobactam (24.8%). No isolate was resistant to polymyxin.
Table 3. Pseudomonas aeruginosa antimicrobial resistance and sensitivity profiles to the main antimicrobials of isolates from 242 episodes of hospital infections.
| Antimicrobials | Resistant | Sensitive | ||
|---|---|---|---|---|
| Isolates | Rate (%) | Isolates | Rate (%) | |
| Cephalosporin (3rd and 4th generation) | 83 | 34.3 | 159 | 65.7 |
| Carbapenem | 111 | 45.9 | 131 | 54.1 |
| Aminoglycoside | 93 | 38.4 | 149 | 61.6 |
| Polymyxin | 0 | 0 | 242 | 100.0 |
| Piperacilin/Tazobactam | 60 | 24.8 | 182 | 75.2 |
| Fluoroquinolone | 103 | 42.6 | 139 | 57.4 |
Table 4 summarizes the characterization of forty P. aeruginosa isolates that were resistant to fluoroquinolones (100%) and/or resistant to carbapenems (80%), with 87.5% (35/40) of nosocomial origin and 12.5% (5/40) classified as community acquired. The isolates were recovered from clinical specimens as blood (52.5%; 21/40), tracheal secretion (20.0%; 8/40), urine (15.0%; 6/40), tissue fragment (10.0%; 4/40) and otitis (2.5%; 1/40). All patients with bloodstream infection used a central venous catheter, and 50% of patients with urinary infection had a urinary catheter. Furthermore, 86.7% (13/15) of the patients with pneumonia had ventilator-associated pneumonia (VAP). Antimicrobial susceptibility results were analyzed and 87.5% (35/40) isolates was characterized as MDR. The resistance rates to carbapenem, cefepime, piperacillin/tazobactam, and aminoglycoside were 80% (32/40), 72.5% (29/40), 57.5% (23/40) and 55% (22/40), respectively (data not shown). Moreover, 40% community isolates were characterized as MDR. The minimum inhibitory concentrations of imipenem and ciprofloxacin to inhibit 90% of 38 P. aeruginosa isolates selected were ≥32 and 64 μg/mL, respectively.
Table 4. Characterization of P. aeruginosa isolates resistant to fluoroquinolones and/or carbapenems with regard to the minimum inhibitory concentration, blaSPM, blaVIM, qnrS, aac(6´)-Ib-cr and aac(6´)-Ib7 genes, quinolone resistance determining region mutations and the PFGE patterns.
| Characteristics | Positive/analyzed (%) |
|---|---|
| Phenotype | |
| Multiresistant | 35/40 (87.5) |
| Non-multiresistant | 05/40 (12.5) |
| Metallo-β-lactamase | |
| SPM | 05/32 (15.6) |
| VIM | 02/32 (06.2) |
| PMQR/aac(6′)-Ib1 | |
| qnrS1 | 01/38 (02.6) |
| aac(6′)-Ib-cr | 01/38 (02.6) |
| aac(6′)-Ib7 | 28/32 (02.6) |
| QRDR2 mutations | |
| gyrA: Thr83Ile | 20/20 (100.0) |
| parC: Ser87Leu | 16/20 (80.0) |
| parC: Glu91Lys | 01/20 (05.0) |
| gyrA + parC: Thr83Ile + Ser87Leu | 16/20 (80.0) |
| gyrA + parC: Thr83Ile + Glu91Lys | 01/20 (05.0) |
| PFGE3 patterns | |
| A | 03/21 (14.3) |
| B | 02/21 (09.5) |
| C | 02/21 (09.5) |
| D-Q4 | 01/21 (04.8) |
| Predominant clones | |
| A, B and C | 07/21 (33,3) |
| Antimicrobial | MIC5 (μg/mL) |
| Imipenem | |
| MIC50 | ≥32 |
| MIC90 | ≥32 |
| Ciprofloxacin | |
| MIC50 | 16 |
| MIC90 | 64 |
1Plasmid-mediated quinolone resistance or aminoglycoside 6′-N-acetyltransferase type Ib;
2Quinolone-resistance determining region;
3Pulsed field gel electrophoresis;
4each clone;
5Minimum inhibitory concentration (n = 38). Of the total isolates, 12.5% are of Community origin (5/40).
Multiplex PCR to MBL was conducted only for carbapenem-resistant P. aeruginosa (32/40) from nosocomial and community infections, with 85.7% (30/35) and 40.0% (2/5), respectively, and seven isolates showed amplifications consistent with MBL genes indentified as blaSPM-1 and blaVIM types.
The frequency of isolates that presented PMQR genes (qnrS1 and aac(6’)-Ib-cr) was 5.3% (2/38). The only MDR isolate of nosocomial origin that presented the qnrS1 gene was recovered from lung, with the following MIC for ciprofloxacin and imipenem, 64 μg/mL and ≥32 μg/mL, respectively. This isolate did not show to present the MBL genes tested (blaIMP, blaVIM, blaSPM, blaGIM and blaSIM). Overall, 85% (34/40) of the isolates harboured aac(6’)-Ib gene, and was recovered from blood (38.2%; 13/34), tracheal aspirate (32.4%; 11/34), urine (17.6%; 6/34) and tissue fragment (11.8%; 4/34). The sequencing results showed that only one nosocomial isolate, recovered from tissue fragment and showed non-MDR profile, presented the cr variant (aac(6’)-Ib-cr gene), with a MIC of 64 μg/mL for ciprofloxacin. Besides that, 87.5% (28/32) harboured the aac(6’)-Ib7 variant. None of the isolates harboured qnrA, qnrB, qnrC, qnrD, and qepA genes. Moreover, among PMQR-positive isolates none had community origin.
Of the 20 P. aeruginosa isolates evaluated for QRDR mutations in gyrA and parC (Thre83Ile; Ser87Leu; Glu91Lys), none presented concomitant PMQR determinants (Table 4).
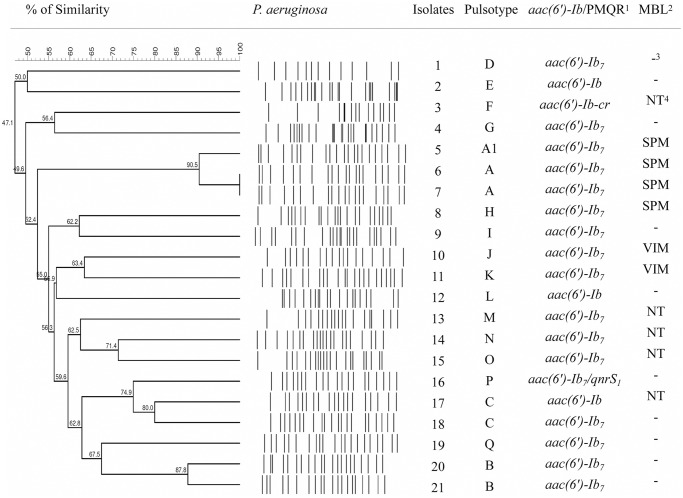
A total of 17 PFGE patterns (A-Q) of P. aeruginosa were observed among the 21 isolates analyzed, comprising 3 main clones: A (14.3%; 3/21), B (9.5%; 2/21), C (9.5%; 2/21) (Table 4 and Fig 2). The pulsotype A had two subtypes (A and A1), and they belonged to isolates that contained the blaSPM gene, while B and C had only one.
Fig 2. UPGMA dendrogram of PFGE profiles of 21 clinical P. aeruginosa isolates used in this study using the Dice coefficient under 1% tolerance and 1% optimization.
A similarity coefficient of 80% was chosen for cluster definition. 1Plasmid-mediated quinolone resistance determinants or aminoglycoside 6′-N-acetyltransferase type Ib; 2Metallo-β-lactamase; 3negative; 4not tested.
Discussion
Recent studies have shown that multi-drug resistance and several virulence determinants are key factors that contribute to the global spread of P. aeruginosa in hospitals [41–43]. The present study evaluated the risk factors for the development of infections caused by MDR P. aeruginosa, as well as those associated with antimicrobial resistance and treatment outcome in patients with bacteremia. The independent risk factors for development of MDR P. aeruginosa infections include prior use of antibiotics (carbapenems, fluoroquinolones, broad-spectrum cephalosporins, and aminoglycosides), being bedridden or in the intensive care unit, prolonged hospital stay, P. aeruginosa infection or colonization within a previous period of one year, malignant disease, mechanical ventilation, and history of chronic obstructive pulmonary disease [44, 45]. Data of univariate analysis from this study have corroborated some of these risk factors. However, only inappropriate therapy was a risk predictor independent associated for developing MDR P. aeruginosa infections, but it must be considered as such within the epidemiological context [46]. Some studies provide evidences of a general view that the development of MDR can be caused by treatment that is inappropriate, incorrect and widespread use of carbapenems [3, 46]. The pressure of carbapenems use has contributed to an explosive increase of KPC (carbapenemase-producing K. pneumoniae), which has been responsible for 70.9% of K. pneumoniae infections in the hospital of this study (data not shown).
Among patients with bacteremia (158 patients), 44.3% had isolates resistant to carbapenems, 42.4% resistant to fluoroquinolones and 42.4% with a multidrug-resistant profile. The association of bacteremia with antimicrobial resistant isolates is common, but few studies have addressed this problem systematically [47–49]. In hospitalized patients, the association between bacteremia and fluoroquinolone-resistant P. aeruginosa was observed in our study with a high frequency of patients remained hospitalized longer (56.7%), independently associated with bacteremia. Similar frequencies were also observed for those patients with MDR isolates (58.2%). In the carbapenem-resistant group, was observed a higher early mortality rate (5 days) that was statistically significant with those that had bacteremia infections. These aspects have often been observed in developing countries like Brazil [48, 50, 51], where the macro and micro-regional differences in relation to the hospitals are extremely significant, as well as the characteristics of the hospitalized population [29, 52]. Besides, the lack of the microbiology laboratories and human and financial resources, a result of poor implementation of control practices for prevention of nosocomial infections, favors the intra and inter-hospital transmission of resistant pathogens that exhibit adaptation to the environment [6, 52]. Another aspect that must be considered is that the antimicrobial use is commonly abusive, empirical and often less judicious [6, 52]. A study performed by Dantas [53] in the same university hospital of this study, showed that the density of antibiotic use was much higher when compared with hospitals with similar size in other countries, allowing MDR strains such as Gram-negative non-fermentative bacteria to emerge and spread quickly [54].
In addition to the multi-drug resistance, special attention was given in this study to P. aeruginosa resistant to carbapenems, considering the significant increase of this resistance in Latin America and widespread of different clones associated with the production enzymes of the MBL type [55–58]. The predominant MBL-encoding gene in Brazil is blaSPM-1, which has been disseminated by the MDR P. aeruginosa clone SP/ST277, considered a high-risk clone [7, 18, 59, 60]. The blaSPM-1 gene was first detected in São Paulo, and later in others cities in Brazil, moreover several studies have shown its global spread and pandemic potential, causing important morbidity and mortality in hospital infections [18, 43, 60, 61]. In our study, we observed a high frequency of SPM among the isolates, especially those belonging to clone A. In addition, two isolates harboring blaVIM were detected. An increase in the rates of P. aeruginosa isolates containing the gene blaVIM has been also observed in others hospitals in Brazil [62, 63]. The frequent data among different types of metallo-β-lactamase in Brazil, and not in other countries, especially those developed, suggest the spread of specific clones and the knowledge of these facts may contribute to improving the multidrug resistance scenario.
Besides the carbapenem resistance, resistance to fluoroquinolones has become an increasing problem, so far, only a few studies have investigated the occurrence of PMQR in P. aeruginosa [27, 29, 64, 65]. PMQR is an important phenomenon that is being disseminated worldwide and the most relevant PMQR genes to date are the aac(6’)-Ib-cr, qnr and genes encoding efflux pumps such as qepA [24]. Surprisingly, the results from this study demonstrated for the first time the presence of PMQR genes in clinical isolates of P. aeruginosa in Brazil (5.3%), as well as a very significant high frequency not shown in other studies of the aac(6’)-Ib7 variant. The PMQR rate in our study was higher than that some reported in the literature [27, 65]. According to the study reported by Jiang et al. [27], the frequency of clinical isolates carrying the aac(6’)-Ib-cr gene was 1.9% (2/106 isolates), and the total rate of PMQR determinants was 3,8% among P. aeruginosa isolates, while in the Yang and colleagues [65] study, only one in 256 P. aeruginosa isolates (0.4%) showed a PMQR gene. The higher detection frequency of these genes in our study reflects most likely the complexity of epidemiology and resistance mechanisms associated with P. aeruginosa in developing countries. The co-existence of different PMQR genes in the same clinical isolate was not observed throughout this study, although it has been reported by Jiang et al. [27].
Regarding the target site mutations in QRDR of fluoroquinolone resistant P. aeruginosa, we observed mutations consistent with those published previously in all isolates tested [66–68]. Of total, 20 Pseudomonas aeruginosa of nosocomial origin were evaluated for mutations in gyrA and parC, and none of them presented PMQR genes. However, according to literature evidences, the chromosomal QRDR mutations in gyrA and parC genes are crucial to fluoroquinolone resistance, and their association with PMQR determinants may have an additional role that contributes to resistance to fluoroquinolones [26].
Another interesting observation from this study was the identification of a mixed wound infection by P. aeruginosa and E. coli in the patient who presented P. aeruginosa harboring the PMQR gene aac(6’)-Ib-cr. In a previous evaluation of E. coli infections in the same hospital, 71.4% had aac(6’)-Ib-cr gene (data not shown). The epidemiology of P. aeruginosa in this hospital proved to be complex and can be explained by its involvement in the propagation through transference of plasmids to different species as well as by clonal spread.
PFGE results, in this study, suggested that P. aeruginosa isolates harboring PMQR genes or mutations in gyrA and parC were not closely related, except in those containing SPM clone, wherein in the literature showing results with a similarity clonal among isolates of MDR P. aeruginosa [69].
In conclusion, our results confirm previous findings regarding the dissemination of SPM-type clones in Brazil, and contribute whit additional evidence that may indicate that inappropriate therapy may be a crucial factor to the emergence of MDR isolates, besides being related to worse prognosis. Additionally, this study demonstrates for the first time in Brazil the presence of the PMQR determinants in P. aeruginosa, spreading in the hospital. Future follow-up surveillance studies of molecular epidemiology in Brazilian hospitals have crucial importance to infection-control practices and reduce the effects of these infections on hospital patients.
Supporting Information
(DOC)
Acknowledgments
The authors wish to thank Silvia Dias Oliveira (Immunology and Microbiology Laboratory, Faculty of Biosciences Pontifical, Catholic University of Rio Grande do Sul), Márcia Maria Camargo Morais (Microbial Resistance Laboratory, University of Pernambuco) and Magna Cristina de Paiva (Federal University of São João del Rei, Dona Lindu Campus, Divinópolis), who kindly provided the control strains to this study. We thank Daise Aparecida Rossi (Applied Animal Biotechnology Laboratory of the Faculty of Veterinary Medicine) for the technical support. We also thank FAPEMIG (Fundação de Amparo à Pesquisa de Minas Gerais) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the financial support and scholarships.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
FAPEMIG (Fundação de Amparo à Pesquisa de Minas Gerais) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) provided financial support and scholarships.
References
- 1.Zavascki AO, Gaspareto PB, Martins AF, Goncalves AL, Barth AL. Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing SPM-1 metallo-β-lactamase in a teaching hospital in southern Brazil. The Journal of Antimicrobial Chemotherapy 2005; 56: 1148–1151. [DOI] [PubMed] [Google Scholar]
- 2.Xavier DE, Picao RC, Girardello R, Fehlberg LCC, Gales AA. Efflux pumps expression and its association with porin down-regulation and b-lactamase production among Pseudomonas aeruginosa causing bloodstream infections in Brazil. BMC Microbiolog. 2010; 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreira MR, Guimarães MP, Rodrigues AAA, Gontijo-Filho PP. Antimicrobial use, incidence, etiology and resistance patterns in bacteria causing ventilator-associated pneumonia in a clinical-surgical intensive care unit. Revista da Sociedade Brasileira de Medicina Tropical 2013; 46: 39–44. [DOI] [PubMed] [Google Scholar]
- 4.Porto JP, Santos RO, Gontijo-Filho PP, Ribas RM. Active surveillance to determine the impact of methicillin resistance on mortality in patients with bacteremia and influences of the use of antibiotics on the development of MRSA infection. Revista da Sociedade Brasileira de Medicina Tropical 2013; 46: 713–718. 10.1590/0037-8682-0199-2013 [DOI] [PubMed] [Google Scholar]
- 5.Borges LFA, Rocha LA, Nunes MJ, Gontijo Filho PP. Low Compliance to Handwashing Program and High Nosocomial Infection in a Brazilian Hospital. Hindawi Publishing Corporation Interdisciplinary Perspectives on Infectious Diseases 2012: 579–681. 10.1155/2012/579681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padoveze MC, Fortaleza CMCB, Kiffer C, Barth AL, Carneiro ICRS, Giamberardino HIG et al. Structure for prevention of health care–associated infections in Brazilian hospitals: A countrywide study. Am J Infect Control. 2015: 1–6. 10.1016/j.ajic.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 7.Gales AC, Menezes LC, Silbert S et al. Dissemination in distinct Brazilian regions of an epidemic carbapenem-resistant Pseudomonas aeruginosa producing SPM metallo-b-lactamase. J Antimicrob Chemother. 2003; 52: 699–702. [DOI] [PubMed] [Google Scholar]
- 8.Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among gram-negative bacilli isolated from latin America: results from SENTRY antimicrobial Suveillance Program (Latin America, 2008–2010). Diagnostic Microbiology and Infections Diseases 2012; 73: 354–360. [DOI] [PubMed] [Google Scholar]
- 9.Quale J, Bratu S, Gupta J, Landman D. Interplay of efflux system, ampC and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrobial Agents and Chemotherapy 2006; 50: 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clinical Microbiology Reviews 2007; 20: 440–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gülmez D et al. Carbapenem-resistant Escherichia coli and Klebsiella pneumoniae isolates from Turkey with OXA-48-like carbapenemases and outer membrane protein loss. International Journal of Antimicrobial Agent. 2008; 31: 523–6. [DOI] [PubMed] [Google Scholar]
- 12.Gootz TD et al. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrobial Agents and Chemotherapy 2009; 53: 1998–2004. 10.1128/AAC.01355-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cezário RC, Morais LD, Ferreira JC, Costa-Pinto RM, Darini ALC, Gontijo-Filho PP. Nosocomial outbreak by imipenem-resistant metallo-β-lactamase-producing Pseudomonas aeruginosa in an adult intensive care unit in a Brazilian teaching hospital. Enfermedades Infecciosas y Microbiología Clínica 2009; 27: 269–274. 10.1016/j.eimc.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 14.Scheffer MC, Bazzo ML, Steindel M, Darini AL, Clímaco E, Dalla-Costa LM. Intrahospital spread of carbapenem-resistant Pseudomonas aeruginosa in a University Hospital in Florianópolis, Santa Catarina, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2010; 43: 367–371. [DOI] [PubMed] [Google Scholar]
- 15.Samuelsen O, Toleman MA, Sundsfjord A, Rydberg J, Leegaard TM, Walder M et al. Molecular epidemiology of metallo-beta-lactamase-producing Pseudomonas aeruginosa isolates from Norway and Sweden shows import of international clones and local clonal expansion. Antimicrobial Agents and Chemotherapy 2010; 54: 346–352. 10.1128/AAC.00824-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett PM. Integrons and gene cassettes: a genetic construction kit for bacteria. Antimicrob. Agents Chemother. 1999; 43: 1–4. [PubMed] [Google Scholar]
- 17.King D, Strynadka N. Crystal structure of New Delhi metallo-b-lactamase reveals molecular basis for antibiotic resistance. Published by Wiley-Blackwell. VC 2011 The Protein Society. Protein Science 2011; 20: 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonseca EL, Marin MA, Encinas F, Vicente ACP. Full characterization of the integrative and conjugative element carrying the metallo-b-lactamase blaSPM-1 and bicyclomycin bcr1 resistance genes found in the pandemic Pseudomonas aeruginosa clone SP/ST277. J Antimicrob Chemother. 2015. 10.1093/jac/dkv152 [DOI] [PubMed] [Google Scholar]
- 19.Salabi AE, Toleman MA, Weeks J et al. First report of the metallo-blactamase SPM-1 in Europe. Antimicrob Agents Chemother. 2010; 54: 582 10.1128/AAC.00719-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonseca EL, Freitas FS, Vicente AC. The colistin-only-sensitive Brazilian Pseudomonas aeruginosa clone SP (sequence type 277) is spread worldwide. Antimicrob Agents Chemother. 2010; 54: 2743 10.1128/AAC.00012-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodford N. Rapid Characterization of β-lactamases by Multiplex PCR. In: Gillespie SH, Mchugh TD. Antibiotic Resistance Protocols: Second Edition, Methods in Molecular Biology. 2010; 642: 181–192. [DOI] [PubMed] [Google Scholar]
- 22.Andrade LN, Woodford N, Darini ALC. International gatherings and potential for global dissemination of São Paulo metallo-β-lactamase (SPM) from Brazil. International Journal of Antimicrobial Agents 2014; 43: 195–200. [DOI] [PubMed] [Google Scholar]
- 23.Agnello M, Wong-Beringer A. Differentiation in Quinolone Resistance by Virulence Genotype in Pseudomonas aeruginosa. PLoS one 2012; 7: e42973 10.1371/journal.pone.0042973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez-Martínez JM, Cano ME, Velasco C, Martínez-Martínez L, Pascual Á. Plasmid mediated quinolone resistance: an update. J Infect Chemother. 2011; 17: 149–82. 10.1007/s10156-010-0120-2 [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Chen H, Yang Q, Chen M, Wang H. High prevalence of plasmid mediated quinolone resistance genes qnr and aac(6`)-Ib-cr in clinical isolates of Enterobacteriaceae from nine teaching hospital in China. Antimicrob Agents Chemother. 2008; 52: 4268–73. 10.1128/AAC.00830-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piekarska K, Wołkowicz T, Zacharczuk K, Rzeczkowska M, Chrost A, Bareja E et al. Co-existence of plasmid-mediated quinolone resistance determinants and mutations in gyrA and parC among fluoroquinolone-resistant clinical Enterobacteriaceae isolated in a tertiary hospital in Warsaw, Poland. International Journal of Antimicrobial Agents 2015; 45: 238–243. 10.1016/j.ijantimicag.2014.09.019 [DOI] [PubMed] [Google Scholar]
- 27.Jiang X, Yu T, Jiang X, Zhang W, Zhang L, Ma J. Emergence of plasmid-mediated quinolone resistance genes in clinical isolates of Acinetobacter baumannii and Pseudomonas aeruginosa in Henan, China. Diagn Microbiol Infect Dis. 2014; 79: 381–383. 10.1016/j.diagmicrobio.2014.03.025 [DOI] [PubMed] [Google Scholar]
- 28.CDC. Guidelines for the prevention of intravascular catheter–related infections. MMWR Morb Mortal Wkly Rep. 2002; 51: 1–36. [Google Scholar]
- 29.Bouchillon S, Hoban DJ, Badal R, Hawser S. Fluoroquinolone Resistance Among Gram-Negative Urinary Tract Pathogens: Global Smart Program Results, 2009–2010. The Open Microbiology Journal 2012; 6: 74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18: 268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 31.Gulen TA, Guner R, Celikbilek N, Keske S, Tasyaran M. Clinical importance and cost of bacteremia caused by nosocomial multi drug resistant Acinetobacter baumannii. International Journal of Infectious Diseases 2015; 38: 32–35. 10.1016/j.ijid.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 32.Gilbert DN, Moellering RC, Eliopoulos GM, Sande MA. The Sanford Guide to Antimicrobial Therapy, Sperryville, VA: Antimicrobial Therapy; 2007; 37. [Google Scholar]
- 33.Lodise TP Jr, Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, Lomaestro B, McGregor JC. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother. 2007; 51: 3510–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgos J, Luján M, Larrosa MN, Pedro-Botet ML, Fontanals D, Quesada MD, Lung M, Bermudo G, Almirante B, Falcó V. The problem of early mortality in pneumococcal pneumonia: a study of risk factors. Eur Respir J. 2015. 10.1183/09031936.00034415 [DOI] [PubMed] [Google Scholar]
- 35.Mcclaran J, Berglas RT, Franco ED. Long hospital stays and need for alternate level of care at discharge Doesfamily make a differencefor elderly patients? Canadian Family Physician 1996; 42: 449–461. [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz S, Silley P, Simjee S, Woodford N, Duijkeren EV, Johnson AP, Gaastra W. Editorial: Assessing the antimicrobial susceptibility of bacteria obtained from animals. J Antimicrob Chemother. 2010. [DOI] [PubMed] [Google Scholar]
- 37.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Document M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 38.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Document M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute; 2014. [Google Scholar]
- 39.Capuano VSC. Estudo comparativo de métodos fenotípicos e biomoleculares para determinação de resistência a antibióticos em cepas de Salmonella spp isoladas de coiro e carcaça de bovinos e produtos cárneos. Dissertação de mestrado. Universidade de São Paulo, Faculdade de Ciências Farmacêuticas. São Paulo. 2012.
- 40.Galetti R. Estudo de Pseudomonas aeruginosa produtoras de metalo-beta-lactamases e de genes envolvidos na resistência aos carbapenêmicos. 49 f. Master Thesis—Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, Ribeirão Preto, Brazil. 2010.
- 41.Beceiro A, Tomás M, Bou G. Antimicrobial Resistance and Virulence: a Successful or Deleterious Association in the Bacterial World?. Clinical Microbiology Reviews 2013; 26: 185–230. 10.1128/CMR.00059-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koutsogiannou M, Drougka E, Liakopoulos A, Jelastopulu E, Petinaki E, Anastassiou ED, Spiliopoulou I, Christofidou M. Spread of Multidrug-Resistant Pseudomonas aeruginosa Clones in a University Hospital. Journal of Clinical Microbiology 2013; 51: 665–668. 10.1128/JCM.03071-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galetti R, Andrade LN, Clímaco EC, Pitondo-Silva A, Ferreira JC, Darini ALC. Genomic diversification and virulence features in SPM-1–producing Pseudomonas aeruginosa 13 years later. Diagnostic Microbiology and Infectious Disease 2015; 82: 179–180. 10.1016/j.diagmicrobio.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 44.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-Resistant Pseudomonas aeruginosa: Risk Factors and Clinical Impact. Antimicrobial Agents and Chemotherapy 2006; 50: 43–48. 10.1128/AAC.50.1.43-48.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsch EB, Tam VH. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res. 2010; 10: 441–451. 10.1586/erp.10.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van der Werf MJ, Langendam MW, Huitric E, Manissero D. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012; 39: 1511–1519 10.1183/09031936.00125711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groß U, Amuzu SK, Ciman R, Kassimova I, Groß L, Rabsch W, Rosenberg U, Schulze M, Stich A, Zimmermann O. Bacteremia and Antimicrobial Drug Resistance over Time, Ghana. Emerging Infectious Diseases 2011; 17: 1879–1882. 10.3201/eid1710.110327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dantas RC, Ferreira ML, Gontijo-Filho PP, Ribas RM. Pseudomonas aeruginosa bacteremia: independet risk factors for mortality and impact of resistance on outcome. Journal of Medical Microbiology 2014; 63. [DOI] [PubMed] [Google Scholar]
- 49.Sligl WI, Dragan T, Smith SW. Nosocomial gram-negative bacteremia in intensive care: epidemiology, antimicrobial susceptibilities and outcomes. International Journal of Infectious Diseases 2015. 10.1016/j.ijid.2015.06.024 [DOI] [PubMed] [Google Scholar]
- 50.Conterno LO, Wey SB, Castelo A. Staphylococcus aureus Bacteremia: Comparison of Two Periods and a Predictive Model of Mortality. The Brazilian Journal of Infectious Diseases 2002; 6: 288–297. [DOI] [PubMed] [Google Scholar]
- 51.Ferreira ML, Dantas RC, Faria ALS, Gonçalves IR, Brito CS, Queiroz LL, Gontijo-Filho PP, Ribas RM. Molecular epidemiological survey of the quinolone- and carbapenem-resistant genotype and its association with the type III secretion system in Pseudomonas aeruginosa. Journal of Medical Microbiology 2015; 64: 262–271. 10.1099/jmm.0.000023 [DOI] [PubMed] [Google Scholar]
- 52.Gontijo-Filho PP. Problemas da vigilância epidemiológica de infecções hospitalares sem o uso de critérios microbiológicos no Brasil. Journal of Basic and Applied Pharmaceutical Sciences 2006; 27: 97–102. [Google Scholar]
- 53.Dantas RC. Estudo epidemiológico molecular da resistência aos carbapenêmicos em Pseudomonas aeruginosa isoladas de sangue: produção de β-lactamases, perda de porina OprD e hiperexpressão de bombas de efluxo, PhD Thesis, Universidade Federal de Uberlândia, Uberlândia. 2015.
- 54.Goel N, Wattal C, Oberoi JK, Raveendran R, Datta S, Prasad KJ. Trend analysis of antimicrobial consumption and development of resistance in non-fermenters in a tertiary care hospital in Delhi, India. J Antimicrob Chemother. 2011. [DOI] [PubMed] [Google Scholar]
- 55.Andrade SS, Jones RN, Gales AC, Sader HS. Increasing prevalence of antimicrobial resistance among Pseudomonas aeruginosa isolates in Latin American medical centres: 5 year report of the SENTRY Antimicrobial Surveillance Program (1997–2001). Journal of Antimicrobial Chemotherapy 2003; 52: 140–141. 10.1093/jac/dkg270 [DOI] [PubMed] [Google Scholar]
- 56.Baumgart AMK, Molinari MA, Silveira ACO. Prevalence of carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii in high complexity hospital. Braz J Infect Dis. 2010; 14: 433–436. [DOI] [PubMed] [Google Scholar]
- 57.Scheffer MC, Gales AC, Barth AL, Filho JRC, Dalla-Costa LM. Carbapenem-resistant Pseudomonas aeruginosa–clonal spread in Southern Brazil and in the State of Goiás. Braz J Infect Dis. 2010; 14: 508–509. [PubMed] [Google Scholar]
- 58.Akya A, Salimi A, Nomanpour B, Ahmadi K. Prevalence and Clonal Dissemination of Metallo-Beta-Lactamase-Producing Pseudomonas aeruginosa in Kermanshah. Jundishapur J Microbiol. 2015; 8: e20980 10.5812/jjm.20980v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright LL, Turton JF, Livermore DM, Hopkins KL, Woodford N. Dominance of international “high-risk clones” among metallo-beta-lactamase-producing Pseudomonas aeruginosa in the UK. J Antimicrob Chemother. 2014; 70: 103–10. 10.1093/jac/dku339 [DOI] [PubMed] [Google Scholar]
- 60.Costa LMA, Fleming MECK, Paula GR, Teixeira LA, Mondino PJJ, Mondino SSB, Mendonça-Souza CRV. Production of metallo-β-lactamase among Pseudomonas aeruginosa strains isolated in the State of Sergipe, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2015; 48: 212–215. 10.1590/0037-8682-0198-2014 [DOI] [PubMed] [Google Scholar]
- 61.Silva LV, Galdino AC, Nunes AP, Dos Santos KR, Moreira BM, Cacci LC et al. Virulence attributes in Brazilian clinical isolates of Pseudomonas aeruginosa. Int J Med Microbiol. 2014; 30: 990–1000. [DOI] [PubMed] [Google Scholar]
- 62.Sader HS, Reis AO, Silbert S, Gales AC. IMPs, VIMs and SPMs: the diversity of metallo-b-lactamases produced by carbapenem-resistant Pseudomonas aeruginosa in a Brazilian hospital. Clin Microbiol Infect. 2005; 11: 73–76. [DOI] [PubMed] [Google Scholar]
- 63.Franco MR, Caiaffa-Filho HH, Burattini MN, Rossi F. Metallo-b-lactamases among imipenem-resistant Pseudomonas aeruginosa in a Brazilian university hospital. Clinics 2010; 65: 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Deng Q, Yu Y, Cao X, Xu Q, Wan L. Analysis of the resistance mechanism and homology of carbapenems-resistant Pseudomonas aeruginosa. Zhonghua Shao Shang Za Zhi 2014; 30: 15–20. [PubMed] [Google Scholar]
- 65.Yang X, Xing B, Liang C, Ye Z, Zhang Y. Prevalence and fluoroquinolone resistance of Pseudomonas aeruginosa in a hospital of South China. Int J Clin Exp Med. 2015; 8: 1386–1390. [PMC free article] [PubMed] [Google Scholar]
- 66.Mouneimne H, Robert J, Jarlier V, Cambau E. Type II topoisomerase mutations in ciprofloxacin-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999; 43: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Higgins PG, Fluit AC, Milatovic D, Verhoef J, Schmitz FJ. Mutations in GyrA, ParC, MexR and NfxB in clinical isolates of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2003; 21: 409–413. [DOI] [PubMed] [Google Scholar]
- 68.Lee JK, Lee YS, Park YK, Kim BS. Alterations in the GyrA and GyrB subunits of topoisomerase II and the ParC and ParE subunits of topoisomerase IV in ciprofloxacin-resistant clinical isolates of Pseudomonas aeruginosa. Int J Antimicrob Agents 2005; 25: 290–295. [DOI] [PubMed] [Google Scholar]
- 69.Oliveira RA, Gales AC, Silva RC, Pereira MS, Filho JRC. Description and molecular characterization of metallo-β-lactamase SPM-1 in Pseudomonas aeruginosa clinical strains from Goiânia, Brazil. Perspectivas Médicas 2014; 25: 11–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.