Abstract
Individuals living with HIV show moderate decision-making deficits, though no prior studies have evaluated the ability to make optimal health-related decisions across the HIV healthcare continuum. Forty-three HIV+ individuals with HIV−associated neurocognitive disorders (HAND+), 50 HIV+ individuals without HAND (HAND−), and 42 HIV− participants were administered two measures of health-related decision-making as part of a comprehensive neuropsychological battery: 1) The Decisional Conflict Scale (DCS), and 2) The Modified UCSD Brief Assessment for Capacity to Consent (UBACC-T). Multiple regression analyses revealed that HAND was an independent predictor of both the DCS and the UBACC-T, such that the HAND+ sample evidenced significantly poorer scores relative to comparison groups. Within the HIV+ sample, poorer health-related decision-making was associated with worse performance on tests of episodic memory, risky decision-making, and health literacy. Findings indicate that individuals with HAND evidence moderate deficits in effectively comprehending and evaluating various health-related choices.
Keywords: HIV/AIDS, episodic memory, health literacy, neuropsychology, neurocognition
Across the HIV care continuum, individuals are regularly faced with making complex decisions that can greatly impact a host of different health outcomes. Common health-related decisions include choosing whether to get tested for HIV, engage in medical care (e.g., management of HIV and its comorbidities), as well as day-to-day decisions such as choosing whether to adhere to prescribed pharmacological (e.g., antiretroviral therapy) and behavioral (e.g., diet and exercise) treatment regimens (Gardner, McLees, Steiner, del Rio, & Burman, 2011; Centers for Disease Control, 2014). Persons infected with HIV also encounter difficult decisions related to end of life issues, such as choices related to palliative care and potentially life-sustaining interventions, the beneficial effects of which can be unclear in late-stage HIV disease (e.g., Harding et al., 2005). Such medical decisions are complicated by the fact that they are oftentimes made under highly emotional circumstances (e.g., Beattie & Barlas, 2001), involve at least some risk (Waters, McQueen, & Cameron, 2013), and carry uncertain and/or delayed rewards (e.g., variable treatment response; O’Connor, 1995). Disturbances in the neurocognitive abilities that allow one to effectively learn, understand, and evaluate the risks and benefits of various health-related choices and subsequently make informed decisions could increase risk of suboptimal health, functional, and quality of life outcomes for persons living with HIV. Indeed, neurocognitive deficits are associated with poorer health-related decision-making in other clinical samples, including diabetes mellitus type 1 (Rustad et al., 2013) and substance dependence (Turner, LaRowe, Horner, Herron, & Malcolm, 2009).
Thus, one subset of the HIV-infected population in which health-related decision-making is particularly relevant is that of HIV-associated neurocognitive disorders (HAND). Individuals with HAND evidence more severe HIV disease (e.g., lower nadir CD4 counts; Ellis et al., 2011), higher rates of medical comorbidities (e.g., cardiovascular disease; e.g., Wright et al., 2010), and poorer health-related behaviors (e.g., appointment attendance; Jacks et al., 2015) as compared to HIV+ individuals without HAND (e.g., Heaton et al., 2010). As such, it is reasonable to posit that there may be a relationship between poor health outcomes and difficulty in making optimal health-related decisions in HAND. In the current era of combination antiretroviral therapy (cART), HAND occurs in approximately 30–50% of the population and typically includes deficits in executive functions, episodic memory, psychomotor speed, and attention (Reger, Welsh, Razani, Martin, & Boone, 2002), all of which are neurocognitive abilities that play an important role in the decision-making process (Brand, Labudda, & Markowitsch, 2006). In brief, the respective contributions of these neurocognitive abilities (as detailed in a decision-making model proposed by Brand, Labudda, & Markowitsch, 2006) include the following: 1) episodic memory is necessary in early and late stages of decision-making, as one must learn and retain information related to the decision, as well remember past decision-making experiences and strategies; 2) executive functions aid in the categorization of alternatives, selection of information to be recalled, and strategy application; and 3) attention/working memory is necessary for attending to relevant information and maintaining an accurate representation of the decision situation’s features. Thus, it is reasonable to posit that HAND would be associated with poorer health-related decision-making.
Prior studies have examined decision-making in HIV exclusively in the context of gambling, with a pattern of findings suggestive of poor (i.e., “risky”) decision-making (Hardy, Hinkin, Levine, Castellon, & Lam, 2006; Martin et al., 2004; Martin et al., 2013; Thames et al., 2012), particularly in persons with HAND (Iudicello et al., 2013). These previous studies have shown significant associations between poor decision-making ability and deficits within neurocognitive domains of learning and executive functions (e.g., Iudicello et al., 2013). In addition, at the neural level, Connolly and colleagues (2014) showed that poor monetary decision-making ability in HIV was related to frontostriatal circuit dysfunction (i.e., increased activation in the basal ganglia, anterior cingulate, dorsolateral prefrontal cortex, and insula). Thus, there is both neuropsychological and neuroimaging evidence to suggest that HAND may be associated with dysfunctional decision-making in the context of health-related scenarios. As such, our aim in the current study is to examine the effects of HAND on health-related decision-making as well as its associations with neurocognition and health literacy functioning, which may act as important determinants for health-related decision-making ability in this at-risk group. Our specific hypotheses for this study were as follows: 1) Individuals with HAND will evidence significantly worse performance on measures of health-related decision-making as compared to HIV+ individuals without HAND and a HIV seronegative comparison group; 2) Within the HIV+ sample, poor performance on the health-related decision-making measures would be related to poor neurocognitive functioning within domains of learning and executive function; and 3) Within the HIV+ sample, performance on the health-related decision-making measures would correlate with performance on health literacy measures.
Methods
Participants
One hundred thirty-five participants were drawn from various cohort studies at the University of California, San Diego (UCSD) HIV Neurobehavioral Research Program (HNRP). The sample included 93 HIV+ individuals and 42 HIV seronegative comparison subjects. Based on the results of a comprehensive neuropsychological evaluation that was administered at the HNRP parent study visit, 43 participants in the HIV+ group met criteria for HAND in accordance with current Frascati criteria (Antinori et al., 2007), which is a widely used and well-validated method for HAND diagnosis (e.g., Heaton et al., 2010; cf. Gisslén, Price, & Nilsson, 2011). In brief, the following criteria are assessed in order to make a determination of HAND via the Frascati method: 1) impairment (i.e., 1 standard deviation below the mean for asymptomatic neurocognitive impairment [ANI] and mild neurocognitive disorder [MND]; 2 standard deviations below the mean for HIV-associated dementia [HAD]) in at least 2 neurocognitive domains that are frequently affected by HIV disease; 2) impairment does not meet criteria for delirium (or dementia in the case of ANI and MND); and 3) there is no evidence for a preexisting cause of HAND. The fourth criterion assesses the everyday functioning impact of cognitive impairment; those with ANI do not evidence impairment in everyday functioning, while those with MND and HAD evidence mild to marked impairment in this regard. Within the HAND group, 16 (37%) of individuals met criteria for ANI, 23 individuals (54%) for MND, and 4 individuals (9%) met criteria for HAD.
Potential study participants were not enrolled into the current study if they met any of the following criteria, which were selected based on their high potential to affect neurocognitive and neuropsychiatric functioning: severe psychiatric (e.g., schizophrenia) or neurologic illness (e.g., seizure disorder, active CNS opportunistic infections); substance dependence within one month of evaluation (as determined by the Composite International Diagnostic Interview, CIDI version 2.1; World Health Organization, 1998); Breathalyzer test positive for alcohol (i.e., blood alcohol content >0.0 on two administrations) or urine toxicology screen positive for illicit drugs (excluding marijuana) on the day of evaluation. Individuals with positive marijuana toxicology screens (n=) were not excluded as marijuana is detectable up to 30 days after last use, is commonly used in HIV disease, and medications commonly prescribed for persons with HIV (e.g., efavirenz, marinol) can produce positive toxicology results. Of note, our study design intentionally included HIV− individuals with risk factors for cognitive impairment that are also common in HIV+ persons (e.g., HCV, depression, substance use). This allows us to achieve greater specificity in attributing the observed effects specifically to HIV disease versus its common comorbidities.
Demographic, medical, psychiatric, HIV disease, and neurocognitive characteristics across the three study groups are outlined in Table 1. Both HIV+ groups had a significantly higher proportion of men than the HIV− group, and the HAND− group reported more years of education as compared to the HAND+ sample (all ps<.05). For psychiatric characteristics, the HIV+ groups had greater proportions of individuals who met criteria for lifetime affective disorder (i.e., Major Depressive and/or Generalized Anxiety Disorder) and lifetime alcohol dependence as compared to the HIV− group (ps<.05). The HIV+ groups were comparable with respect to most disease characteristics, though a greater proportion of individuals in the HAND+ group had detectable HIV RNA in plasma (p<.05).
Table 1.
Demographic, psychiatric, disease, and cognitive characteristics of the study sample.
| HIV– (n=42) |
HIV+ without HAND (n=50) |
HIV+ with HAND (n=43) |
p-value | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Age (years) | 44.3 (12.8) | 46.5 (9.6) | 45.2 (10.5) | .828 |
| Education (years) | 13.9 (2.2) | 14.1 (2.7) | 13.1 (2.1) | .093 |
| WTAR | 102.3 (12.5) | 105.9 (10.5) | 94.1 (10.6) | <.001 |
| Sex (% male) | 69.1 | 92.0 | 88.4 | .007 |
| Ethnicity (% non-Caucasian) | 47.6 | 38.0 | 51.2 | .414 |
| African American | 21.4 | 16.0 | 23.4 | – |
| Asian | 2.4 | 6.0 | 2.3 | – |
| Hispanic | 21.4 | 16.0 | 23.4 | – |
| Native American | 2.4 | 0.0 | 2.3 | – |
| Psychiatric Characteristics | ||||
| Lifetime Affective Disorder (%)a | 33.3 | 54.0 | 70.0 | .003 |
| Lifetime Alcohol Dependence (%) | 11.9 | 33.3 | 30.2 | .047 |
| Lifetime Non-alcohol Dependence (%) | 11.9 | 16.0 | 27.9 | .140 |
| HIV Disease Characteristics | ||||
| Estimated duration of infection (yrs.)b | – | 8 (3, 22) | 13 (6, 21) | .166 |
| Proportion with AIDS (%) | – | 52.1 | 57.1 | .631 |
| Proportion on cART (%) | – | 91.7 | 90.2 | .744 |
| HIV RNA log10 (% detectable) | – | 8.5 | 23.3 | .056 |
| Nadir CD4 countb | – | 223 (82, 375) | 193 (16, 305) | .286 |
| Current CD4 countb | – | 676 (438, 858) | 603 (306, 827) | .204 |
| Cognitive Characteristics | ||||
| HAND Classification: ANI (%) | – | – | 37.2 | – |
| HAND Classification: MND (%) | – | – | 53.5 | – |
| HAND Classification: HAD (%) | – | – | 9.3 | – |
Note. Data represents means and standard deviations unless otherwise noted.
Affective disorder = diagnosis of Major Depressive Disorder and/or Generalized Anxiety Disorder.
Data represent medians and interquartile ranges.
WTAR = Wechsler Test of Adult Reading. cART = combination antiretroviral therapy. ANI = asymptomatic neurocognitive impairment. MND = mild neurocognitive disorder. HAD = HIV-associated dementia.
Materials and Procedure
This study was approved by UCSD’s human research protections program, and was conducted between 2012–2014. After participants had completed an HNRP parent study visit, they were contacted by the participant accrual and retention (PAR) unit at the HNRP in order to assess their interest in participating in the current study. All participants had consented within their parent study to be contacted about additional studies in this fashion. Participants who agreed to participate provided written, informed consent, and also completed a brief questionnaire to confirm informed consent. Participants then completed a battery of health-related decision-making and health literacy measures, for which they were paid $35. The length of the assessment was approximately 3 hours.
Health-related decision-making tasks
Modified UCSD Brief Assessment for Capacity to Consent (UBACC-T)
The UBACC, a measure developed to assess capacity to consent in research studies (Jeste et al., 2007), was modified by Burton and colleagues (2012) to an evaluation of treatment appraisal (UBACC-T). Participants were presented with a hypothetical medical treatment scenario in which a friend who has an advanced, life-threatening illness was diagnosed with pneumonia and must decide whether or not to be treated with a course of antibiotics (see Appendix). The examiner read the story aloud and the printed story was placed in front of the participant for the duration of the test. Participants were then asked 10 questions related to the medical decision-making scenario to assess appraisal and comprehension (e.g., “What are some alternatives to this treatment?” and “What advice would you give to the patient regarding the antibiotic treatment?”). Individual item scores range from 0–2, with 2 points given for a plausible answer, 1 point for a partially plausible answer, and 0 points for a non-plausible answer. The total UBACC-T score range is 0–19, with higher scores reflecting better performance. The UBACC-T has demonstrated convergent construct validity (Burton et al., 2012), though to our knowledge reliability information on the UBACC-T has not yet been established.
Decisional Conflict Scale (DCS)
The DCS is a measure of self-efficacy for health-related decision-making, and has demonstrated strong reliability and construct validity within clinical populations (O’Connor, 1995). In brief, individuals are presented with a brief hypothetical scenario (see Appendix) in which they are asked to imagine that they have been experiencing mild memory and attention problems. Upon reporting their symptoms to their healthcare provider, they are presented with four treatment options: (1) medication; (2) cognitive training with a psychologist; (3) combination of options 1 and 2; or (4) no treatment. Each treatment option description includes potential risks and benefits of selecting that option. Having made a decision, participants then answered 16 questions assessing self-efficacy in relation to their decision using a Likert-type scale (range 1–5; 1=strongly agree, 5=strongly disagree), with higher total values indicating less confidence in their health-related decision-making. The DCS comprises 5 subscales: (1) Informed (e.g., “I know which options are available to me”); (2) Values clarity (e.g., “I am clear about which benefits matter most to me”); (3) Support (e.g., “I have enough support from others to make a choice”); (4) Uncertainty (e.g., “I am clear about the best choice for me”); and (5) Effective decision (e.g., “I feel I have made an informed choice”).
Neuropsychological Assessment
Risky decision-making
Participants were also administered the computerized Iowa Gambling Task (IGT; Bechara, Damasio, Damasio, & Anderson, 1994). in order to assess decision-making under monetary risk. The IGT incorporates factors such as rewards and punishments, uncertainty, implicit rule learning, and response to feedback. The goal of the IGT is to maximize profits over 100 trials, and to do so successfully participants must learn over the course of the task that two of the decks are disadvantageous (high immediate gains, occasional higher penalties) and two are advantageous (low immediate gains, occasional low penalties). The total IGT score reflects the total number of choices from advantageous decks minus the total number of choices from disadvantageous decks, whereby lower values indicate more disadvantageous (i.e., “risky”) decision-making (total score range = −100 to 100). There is a considerable body of literature to support the discriminant and convergent validity of the IGT (e.g., Buelow & Suhr, 2009), including many studies in HIV disease (e.g., Martin et al., 2004). Reliability research on the IGT shows evidence of low internal consistency (e.g., Gansler, Jerram, Vannorsdall, & Schretlen, 2011) and modest practice effects (e.g., Lin, Song, Chen, Lee, & Chiu, 2013) commonly observed on executive tasks.
Clinical neuropsychological assessment
All participants received a comprehensive neuropsychological assessment during an adjacent HNRP parent study visit, which assessed 7 neurocognitive domains in accordance with Frascati criteria for the assessment of HAND. The domains and associated measures are as follows: (1) Information processing speed: Digit Symbol subtest of the Wechsler Adult Intelligence Scale-III (WAIS-III; Tulsky et al., 1997), Trail Making Test – Part A (TMT-A; Heaton, Miller, Taylor, & Grant, 2004; Reitan & Wolfson, 1993), detection and identification response times on the Identification Task of the CogState (Collie, Maruff, Darby, & McStephen, 2003); (2) Attention/working memory: Total correct on the Paced Auditory Serial Addition Test (PASAT; Diehr, Heaton, Miller, & Grant, 1998; Gronwall, 1977), accuracy on the One Back and Two Back Tasks of the CogState; (3) Learning: Hopkins Verbal Learning Test – Revised (HVLT-R; Benedict, Schretlen, Groninger, & Brandt, 1998; Norman et al., 2011) and the Brief Visuospatial Memory Test – Revised (BVMT-R; Benedict, 1997; Norman et al., 2011) total trials 1–3 recall, accuracy on the One Card Learning Task of the Cogstate; (4) Memory: HVLT-R and BVMT-R delayed recall trials; (5) Executive Function: Perseverative responses on the Wisconsin Card Sorting Test – 64 Card Version (WCST-64; Kongs, Thompson, Iverson, & Heaton, 2000), Trail Making Test – Part B (TMT-B; Heaton, Miller, Taylor, & Grant, 2004; Reitan & Wolfson, 1993); (6) Verbal fluency: animal (Gladsjo et al., 1999) and action (Woods et al., 2005) semantic verbal fluency; and (7) Motor: Grooved Pegboard dominant and non-dominant hand (Heaton, Miller, Taylor, & Grant, 2004; Kløve, 1963).
HAND diagnosis
HIV+ participants were assigned a HAND status (i.e., HAND− or HAND+) using the global deficit score (GDS) approach (Carey et al., 2004). First, individual raw scores from the neurocognitive measures mentioned above were converted into demographically-adjusted T scores using published normative data. Resulting T scores were then transformed into deficit scores, which range from 0 (T>= 40) to 5 (T<20). GDS was calculated by averaging the deficit scores amongst all measures; a standard cutoff of GDS ≥ 0.5, which indicates that the participant demonstrated impaired performance within at least half of the domains, was used to assign a diagnosis of HAND. Additionally, for planned post hoc analyses involving relationships between health-related decision-making and neurocognitive domains, individuals were classified as “within normal limits” or “impaired” using a domain deficit score cutoff of ≥ 0.5. Within the HIV− sample, 12 individuals (30%) met criteria for impairment based on GDS criteria. As noted above, the inclusion of these individuals with common comorbidities in the study provides a clinically comparable HIV− group that allows for greater specificity with regard to the effects of HIV on health-related decision-making factors.
Health Literacy Assessment
The following four measures were administered for the assessment of both fundamental and applied aspects of health literacy, which may be important determinants of health-related decision-making: (1) Rapid Estimate of Adult Literacy in Medicine (REALM; Davis et al., 1993), a 66-item list of anatomy and illness-related words (e.g., “asthma”, “colitis”) which individuals much read aloud and correctly pronounce (total score range=0–66, whereby higher scores reflect better performance); (2) Brief Health Literacy Screen (BHLS; Chew, Bradley, & Boyko, 2004), a 3-item self-report questionnaire that measures perceived competency to perform health literacy related tasks (e.g., “How often do you have problems learning about your medical condition because of difficulty understanding written information?”; “How often do you have someone [like a family member, friend, hospital/clinic worker or caregiver] help you read hospital materials?”). The BHLS utilizes a Likert-type scale ranging from 0 (i.e., “none of the time”) to 4 (i.e., “all of the time”), whereby higher scores reflect poorer self-reported competency; (3) Expanded Numeracy Scale (ENS; Lipkus, Samsa, & Rimer, 2001), a 7-item measure that assesses competency with mathematical concepts (e.g., “If Person A’s chance of getting a disease is 1 in 100 in ten years, and Person B’s risk is double that of A’s, what is B’s risk?”; “The chance of getting a viral infection is .0005. Out of 10,000 people, how many of them are expected to get infected?”). Total scores on the ENS range from 0–7, whereby higher scores reflect better performance; and (4) Newest Vital Sign (NVS; Weiss et al., 2005), which is a 6-item measure that assesses competency to read, interpret, and act on information contained on a nutrition label (e.g., “If you are allowed to eat 60 grams of carbohydrates as a snack, how much ice cream could you have?”; “Pretend you are allergic to penicillin, peanuts, latex gloves, and bee stings. Is it safe for you to eat this ice cream?”). Total scores on the NVS range from 0–6, whereby higher scores reflect better performance.
Neuromedical and psychiatric evaluation
Data from neuromedical and psychiatric evaluations also were obtained from participants’ HNRP parent study visit. The neuromedical evaluation consisted of a blood draw and medical interview regarding the participant’s comorbid medical conditions and current medications. Diagnoses of lifetime affective disorders and substance use disorders were derived from the CIDI (World Health Organization, 1998).
Statistical method
Data were analyzed using JMP® Pro version 11.1.1, and critical alpha level was set to .05. In selecting our statistics procedures, we considered our study design, scale and distribution of the outcomes, and the potential relevance of covariates. The primary study hypotheses were tested using a series of multiple linear regression analyses, with study group as the predictor and the UBACC-T and DCS total scores as outcomes. Our criteria for determining covariates included variables that 1) significantly differed between the three study groups (as indicated in Table 1); and (2) related to the given health-related decision-making measure in the entire sample (p<.05). Accordingly, education and estimated verbal IQ score were included as covariates in the UBACC-T model, and lifetime alcohol use disorder was included in the DCS model. Although non-normal distributions were observed for total scores on both the UBACC-T (Shapiro-Wilk W=.93, p<.001) and DCS (W=.91, p<.001), analysis of regression residuals did not reveal major departures from normality, and findings did not differ when non-parametric statistics were used. Within the HIV+ group, relationships between individual neurocognitive domains and the health-related decision-making measures were conducted amongst all 7 neurocognitive domains using Wilcoxon rank-sum tests, and associated effect sizes were represented as Cohen’s d coefficients. Relationships between the health-related decision-making, the IGT, and health literacy measures were also investigated using Spearman’s rho correlations.
Table 2.
Multiple linear regressions predicting the UBACC-T and the DCS from HAND.
| F | Adj R2 | B | σB | B 95% Cl | p | |
|---|---|---|---|---|---|---|
| UBACC-T | 6.51*** | 0.15 | ||||
| Reference Group | ||||||
| [HAND+] | ||||||
| HIV− | 1.79 | 0.71 | 0.39, 3.19 | .012 | ||
| HAND− | 1.60 | 0.69 | 0.23, 2.97 | .022 | ||
| Education | 0.17 | 0.12 | −0.08, 0.41 | .186 | ||
| WRAT verbal IQ | 0.05 | 0.03 | −0.00, 0.10 | .052 | ||
|
| ||||||
| Decisional Conflict Scale (DCS) | 2.99* | 0.04 | ||||
| Reference Group | ||||||
| [HAND+] | ||||||
| HIV− | −5.78 | 2.69 | −11.10, −0.45 | .034 | ||
| HAND− | −4.12 | 2.57 | −9.20, 0.96 | .112 | ||
| LT alcohol dependence | −4.14 | 2.49 | −9.06, 0.78 | .096 | ||
Note. Adj = adjusted; B = beta weight; UBACC-T = Modified UCSD Brief Assessment for Capacity to Consent; HAND = HIV-associated neurocognitive disorders; WTAR = Wechsler Test of Adult Reading; LT = lifetime.
p < .05.
p < .01.
p < .001.
Results
Modified UCSD Brief Assessment for Capacity to Consent (UBACC-T)
As shown in Table 2, the overall UBACC-T model was significant, with the HAND+ group evidencing significantly lower scores on the UBACC-T than both the HIV− (p<.05, Cohen’s d=.64) and HAND− groups (p<.05, d=.78; see Figure 1). In order to investigate whether individuals with HAD may be driving this finding, we conducted the same analysis excluding the 4 individuals with HAD, and the significance of the results was unchanged. Within the HIV+ group, poorer performance on the UBACC-T was related to impairment in attention/working memory (p<.05, d=.58), learning (p<.05, d=.78), and memory (p<.05, d=.95), as well as risky decision-making as measured by the IGT (ρ=.24; p<.05).
Figure 1.
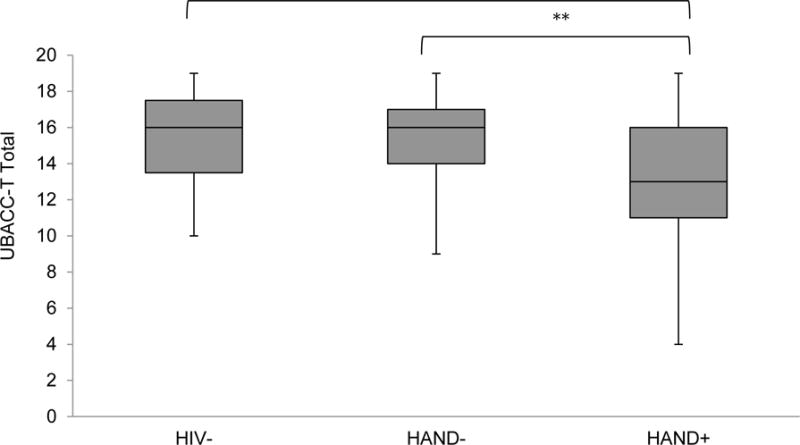
Health-related decision-making comprehension and appraisal across the study samples as measured by the UCSD Brief Assessment for Capacity to Consent to Treatment (UBACC-T total for which lower scores reflect poorer performance). Data are presented as box and whisker plots, with the middle line representing the median, the filled box the middle 50% of each subgroup, and the upper whisker and lower whisker representing, respectively, the upper and lower 25% of each subgroup.
**p< .01; ***p<.001*
Concerning correlations with health literacy measures in the HIV+ group, the UBACC-T was related to the Expanded Numeracy Scale (ρ=.29, p<.05) and Newest Vital Sign (ρ=.43, p<.05). No significant associations were observed between the UBACC-T and any of the other variables listed in Table 1.
Decisional Conflict Scale (DCS)
Figure 2 shows the distributions of DCS treatment choices across the 4 groups. No omnibus group differences were observed with respect to the treatment options (p>.05). Across all groups, a majority of individuals (65% of the entire sample) elected the psychological treatment involving neurocognitive rehabilitation.
Figure 2.
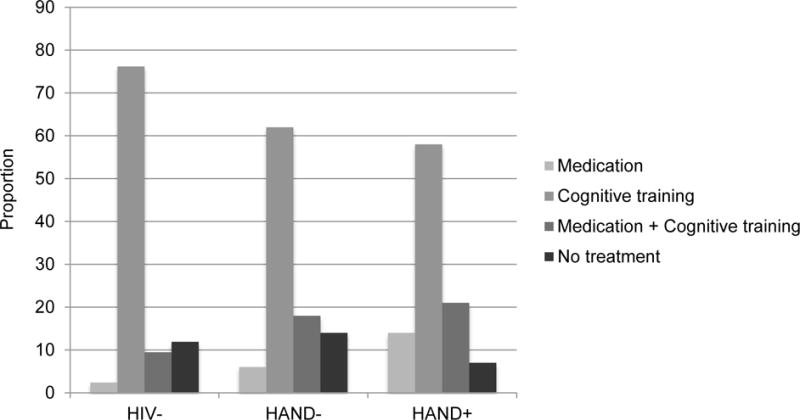
Simple bar graph displaying the proportion of specific treatment options from the Decisional Conflict Scale (DCS) that were selected by each of the study samples.
As shown in Table 2, the overall model predicting the DCS total score from HAND group was significant, with the HAND+ sample evidencing significantly higher scores on the DCS as compared to the HIV− group (p<.05, d=.53; see Figure 3). DCS scores between the HAND+ and HAND− groups did not significantly differ (p>.05, d=.32). As with the UBACC-T, we ran the same model again while excluding those individuals with HAD, and the pattern of results remained the same. Within the HIV+ group, higher scores on the DCS were related to impairment in learning (p<.05; d=.49) and risky decision-making (IGT; ρ=.23; p<.05). Follow-up Wilcoxon rank-sum tests were also conducted for the individual DCS subscales. Omnibus group differences were observed for the values clarity and support subscales (ps<.05), such that the HAND+ group endorsed less values clarity as compared to the HIV− group (p<.05, d=.54), and endorsed lower support as compared to both the HIV− (p<.05, d=.71) and HAND− (p<.05, d=.49) groups.
Figure 3.
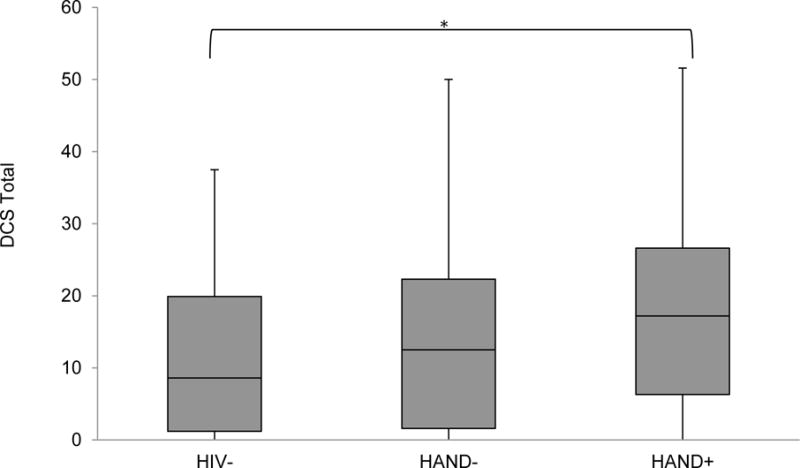
Health-related decision-making self-efficacy across the study samples as measured by the Decisional Conflict Scale (DCS, for which higher scores reflect greater conflict). Data are presented as box and whisker plots, with the middle line representing the median, the filled box the middle 50% of each subgroup, and the upper whisker and lower whisker representing, respectively, the upper and lower 25% of each subgroup.
*p<.05
Finally, concerning associations with health literacy measures, the DCS evidenced significant correlations with the Expanded Numeracy Scale (ρ=−.21, p<.05) as well as the Brief Health Literacy Screen (ρ=.23, p<.05). It was not related to either of the other health literacy measures (ps>.05) or any of the other variables listed in Table 1.
Discussion
This study sought to examine the potential effect of HAND on health-related decision-making ability, and to explore potential relationships between health-related decision-making and neurocognitive and health literacy functioning in HIV. Results demonstrated that HAND is associated with a moderate disruption in health-related decision-making, such that individuals with HAND evidenced poorer performance as compared to both HIV− and HAND− groups on a measure that required participants to understand, appraise, and ultimately decide upon a treatment option (i.e., UBACC-T). Additionally, the HAND group exhibited lower self-efficacy in their ability to make an optimal treatment choice as compared to the HIV− sample, which was driven by lower ratings of values clarity and social support. These associations between HAND and health-related decision-making were not better explained by other demographic or clinical factors. The decision-making difficulties observed in this study are consistent with the prior literature that has demonstrated poor ability to make advantageous decisions in the context of uncertain rewards and punishments in HIV and HAND (Hardy et al., 2006; Iudicello et al., 2013; Martin et al., 2004; Martin et al., 2013), as well as other clinical populations with known frontostriatal systems dysfunction (e.g., Parkinson’s disease; Perretta, Pari, & Beninger, 2005). The effect sizes that accompanied the significant associations between HAND and health-related decision-making were moderate-to-large, thereby suggesting that such difficulties are of clinical relevance.
It is important to note that these findings do not reflexively suggest that all individuals with HAND are incapable of rendering independent health-related decisions. Rather, these data suggest the presence of subtler deficits that may lead an individual with HAND to experience difficulty in making optimal health-related decisions across the HIV healthcare continuum. While decisional capacity and health-related decision-making both rely on underlying constructs of understanding, reasoning, appreciating, and expression of choice (Appelbaum & Grisso, 1988), health-related decision-making departs from capacity to consent in two fundamental ways: first, there is an increased focus on producing decisions that are consistent with patient preferences and values; second, it allows for the individual to have increased participation in the decision-making process (Rimer, Briss, Zeller, Chan, & Woolf, 2004), which is consistent with recommendations to improve healthcare quality (Institute of Medicine, 2001). Persons who suffer from mild-to-moderate cognitive impairment such as HAND may thus be placed at a disadvantage in such situations, as although they are not necessarily incapable, they may nevertheless struggle with certain aspects of the decision-making process that in turn lead to suboptimal health outcomes.
The current findings also shed light on the specific cognitive underpinnings of health-related decision-making in HIV. Herein, decisional appraisal and comprehension ability in the HIV+ sample was related to learning, memory, and attention/working memory, while decisional self-efficacy was associated solely with learning. These findings replicate prior studies in various clinical groups (e.g., schizophrenia; Palmer, Dunn, Appelbaum, & Jeste, 2004; Parkinson’s disease; Dymek, Atchison, Harrell, & Marson, 2000) showing that health-related decision-making draws heavily upon intact episodic memory functioning, which allows individuals to properly acquire and retain information regarding health-related information and various treatment options. These results also parallel prior literature on risky decision-making in HIV, in which learning shows moderate associations with decision-making ability (Hardy et al., 2006; Iudicello et al., 2013; Martin et al., 2004; Martin et al., 2013). Indeed, episodic memory is preferentially affected in HAND, and HIV-associated episodic memory deficits are strongly predictive of health-related behaviors (e.g., antiretroviral adherence; Lovejoy & Suhr, 2009). As such, the development and deployment of interventions that use episodic memory theory to support and improve health-related decision-making may be relevant to persons living with HIV. This is important because we are unaware of any theory-driven memory-based neurorehabilitation approaches to improve health-related decision-making in any clinical population.
Interestingly, poorer health-related decision-making in the HIV+ group was related only to certain aspects of executive functions, namely risky decision-making as measured by the IGT. One possible interpretation for this finding is that individuals who are more impulsive such as those with HAND (Iudicello et al., 2013) may in turn have difficulty when considering the long-term effects of various treatment options and healthcare regimens. For example, a patient may choose to not take to a prescribed ARV with adverse side effects in order to avoid immediate negative symptoms, without consideration for the potential long-term benefits of adherence. This interpretation is also consistent with our follow-up DCS analyses in which the HAND group reported lower levels of values clarity and social support; impulsivity in those with HAND may in part be a function of uncertainty about health values and a lack of social support that may bolster such values. Of note, health-related decision-making in HIV did not relate to traditional measure of executive functions in this study, which largely assessed cognitive flexibility (i.e., Trail-Making Test Part B and perseveration on the WCST). As such, assessment of other constructs such as planning and/or novel problem solving may better map on to health-related decision-making.
Findings also indicate that both aspects of health literacy (Sørensen et al., 2012) may play an important role in effective health-related decision-making in HIV. Within the HIV+ sample, both health-related decision-making tasks were associated with competency in numeracy, which is affected in HAND (Morgan et al., 2015). These findings are in accordance with studies that have shown that poor numeracy predicts poorer health outcomes, less accurate perception of health risks, and a compromised ability to make medical decisions (e.g., Reyna & Brainerd, 2007). The Newest Vital Sign, which primarily measures appraisal and application components of health literacy, was only related to the UBACC-T. This finding makes conceptual sense in that the UBACC-T also captures “higher order” aspects of health-related decision-making, including appraisal. In turn, the Brief Health Literacy Screen, which measures competency in the context of health literacy, was related solely to the DCS, an intuitive finding given the conceptual overlap between self-reported competency and self-efficacy. Accordingly, the health literacy findings reported herein suggest that while numeracy may be important in various aspects of health-related decision-making, other health literacy abilities (e.g., appraisal, competency) may play important roles at specific time points in the decision-making process. Targeting rehabilitation efforts at these health literacy abilities may potentially indirectly enhance health-related decision-making abilities in HIV.
While classic models of decision-making are largely based on cognitive processes alone (e.g., heuristics; Tversky & Kahneman, 1986), more modern models place an equally important emphasis on emotion (i.e., dual-process and fuzzy trace theories; Epstein, 1994; Reyna, 2008). Indeed, real-life health-related decisions are often made under highly emotional circumstances (e.g., end of life issues). However, the current study provided participants with a hypothetical scenario within a research laboratory setting; as such, we were limited in our ability to mimic more real-life health-related decision-making situations. In addition, the hypothetical scenario of the UBACC-T is one in which an individual must make a health-related decision for a friend, which entails a different process than making a decision for oneself, particularly with respect to the ability to take perspective and the differing emotional implications. Relatedly, the current study did not assess the real-world relevance of health-related decision-making to health behavior in HAND; future studies may with to explore whether health-related decision-making is related to health behaviors such as medication adherence, compliance with medical appointments and instructions, and engagement in daily behavioral health regimens (e.g., diet and exercise). Finally, we note a conservative bias such that all subjects were able to provide informed consent to research; it is likely that health-related decision-making findings would have been amplified had we included those individuals who were unable to evidence adequate decisional capacity.
The findings presented herein present many possible directions for future work, from extending health-related decision-making assessment to other neurological populations, to developing remediation therapies aimed at improving this important ability in those who suffer from cognitive impairment. As the prevalence of older adults living with HIV has been steadily increasing given treatment advances (e.g., Vance, 2010), investigating health-related decision-making in the context of aging and HIV may particularly relevant. Given our findings that health-related decision-making dysfunction in HIV was related to episodic memory impairment, interventions may wish to incorporate techniques such as spaced learning (Kramár et al., 2012) or memory testing on the health information that was provided, which research has shown not only assesses what one knows but also enhances later retention (Roediger & Karpicke, 2006). Health literacy may also be an important point of intervention; focusing on remediating one’s fundamental and/or critical health literacy competencies may well have the indirect effect of improving health-related decision-making outcomes as well (Sørensen et al., 2012).
Acknowledgments
The San Diego HIV Neurobehavioral Research Program [HNRP] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H., Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D., Ian Everall, FRCPsych., FRCPath., Ph.D. (Consultant); Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.); Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.
This research was supported by National Institutes of Health grants R21-MH098607, R01-MH073419, T32-DA31098, L30-DA032120, L30-DA034362 and P30-MH62512.
Appendix 1.1: Modified UCSD Brief Assessment for Capacity to Consent (UBACC-T)
Examiner Instructions
“I am going to read a scenario that describes an imaginary situation in which your friend asks for your advice in making an important medical decision. Listen carefully, because once I finish the story, I will ask you some questions about the treatment options. Any questions? Ok, listen carefully as I read you this story.”
Story
Imagine you have a good friend named Pat. Pat has a terminal illness and is receiving hospice care at her home. Her doctors think she has several weeks to several months to live. She would like to stay at home until she dies. It is difficult for Pat to swallow food or fluids.
Pat has had a lung infection (pneumonia) several times, and has been diagnosed with pneumonia again. She wants your advice. Pat needs to decide whether she should take antibiotics to treat the pneumonia or not.
Pat says that when she gets pneumonia, she coughs up phlegm, feels short of breath, and feels confused.
If Pat DOES take the antibiotics, the doctors say that there is about a 50% (1 in 2) chance that her lung infection will get better.
The antibiotics might help Pat live longer, but will not change the fact that she has a terminal illness. More than 10% of people (1 in 10) treated with antibiotics get bad diarrhea and stomach cramps. The antibiotic pills are large and would have to be swallowed twice a day with a large glass of water for 2 weeks.
If she does NOT take the antibiotics, she might die from the pneumonia.
Pat asks you, should she take the antibiotics?
Appendix 1.2: Decisional Conflict Scale (DCS)
Examiner Instructions
“I am going to read you a brief story that describes an imaginary situation in which your doctor asks you to make a decision between 4 treatment options. Listen carefully, because once I finish the story, I will ask you to make a decision about which treatment you would choose. I will also ask you to answer a few questions about how you decided what treatment to choose. Any questions? Ok, listen carefully as I read you this story.”
Story
I want you to imagine that you have been experiencing mild problems paying attention and remembering things. For example, you have noticed problems keeping up with conversations with friends and often forget to do things you wanted to do, like take out the garbage or pay your bills. When you report these symptoms to your doctor, she tells you that you have 4 possible treatment options.
Option 1 is to take a medication, which according to research has a 1 in 5 chance of improving attention and memory problems in people who have the same type of symptoms as you. However, 75% of people who take this medication also have mild side effects, like headache, dizziness, and fatigue. About 2% of people who take this medication have serious liver problems, so your doctor will have to draw blood to check for possible liver problems every month.
Option 2 is to sign up for 10 weekly sessions with a psychologist who uses computer training “brain exercises” to help people improve their attention and memory skills. One out of every 5 people who complete the brain exercises at this clinic report that their attention and memory improve. About 5% of people who start the program, however, do not finish it because it can sometimes be frustrating.
Option 3 is to do both Option 1 and Option 2 at the same time. It is not known whether doing both options at the same time is either better or worse than doing one option or the other.
Option 4 is to do nothing. About 15–20% of people with your kind of symptoms who choose not to go into treatment have worse symptoms 5 years in the future, but 5–10% of people improve and don’t show any symptoms after 5 years, while 70–80% of people don’t show any changes.
Footnotes
Conflict of Interest
Authors Katie L. Doyle, Steven Paul Woods, Erin E. Morgan, Jennifer E. Iudicello, Marizela V. Cameron, Paul Gilbert, and Jessica Beltran declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation of the University of California, San Diego and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. New England Journal of Medicine. 1988;319:1635–1638. doi: 10.1056/NEJM198812223192504. [DOI] [PubMed] [Google Scholar]
- Beattie J, Barlas S. Predicting perceived differences in tradeoff difficulty. In: Weber EU, Baron J, Loomes G, editors. Conflict and tradeoff in decision making. Cambridge: Cambridge University Press; 2001. pp. 25–64. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test-Revised. Odessa: Psychological Assessment Resources; 1997. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test-revised: normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Brand M, Labudda K, Markowitsch H. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Networks. 2006;19:1266–1276. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Burton CZ, Twamley EW, Lee LC, Palmer BW, Jeste DV, Dunn LB, Irwin SA. Undetected cognitive impairment and decision-making capacity in patients receiving hospice care. American Journal of Geriatric Psychiatry. 2012;20:306–316. doi: 10.1097/JGP.0b013e3182436987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, The HNRC Group Predictive validity of global deficit scores for detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–19. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control: National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention: Division of HIV/AIDS Prevention. Understanding the HIV Care Continuum. 2014 Retrieved from www.cdc.gov/hiv/pdf/dhap_continuum.pdf.
- Chew LD, Bradley KA, Boyko E. Brief questions to identify patients with inadequate health literacy. Family Medicine. 2004;36:588–594. [PubMed] [Google Scholar]
- Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. Journal of the International Neuropsychological Society. 2003;9:419–428. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Bischoff-Grethe A, Jordan SJ, Woods SP, Ellis RJ, Paulus MP, The Translational Methamphetamine AIDS Research Center (TMARC) Altered functional response to risky choice in HIV infection. PLoS One. 2014;9:e111583. doi: 10.1371/journal.pone.0111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, Crouch MA. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Family Medicine. 1993;25:391–395. [PubMed] [Google Scholar]
- Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): Norms for age, education, and ethnicity. Assessment. 1998;5:375–387. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- Dymek MP, Atchison P, Harrell L, Marson DC. Competency to consent to medical treatment in cognitively impaired patients with Parkinson’s disease. Neurology. 2000;56:17–24. doi: 10.1212/wnl.56.1.17. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, CHARTER Group CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S. Integration of the cognitive and the psychodynamic unconscious. American Psychologist. 1994;49:709–724. doi: 10.1037//0003-066x.49.8.709. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Jerram MW, Vannorsdall TD, Schrettlen DJ. Comparing alternative metrics to assess performance on the Iowa Gambling Task. Journal of Clinical and Experimental Neuropsychology. 2011;33:1040–1048. doi: 10.1080/13803395.2011.596820. [DOI] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for the prevention of HIV infection. Clinical Infectious Diseases. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslén M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infectious Diseases. 2011;11:356. doi: 10.1186/1471-2334-11-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Harding R, Karus D, Easterbrook P, Raveis VH, Higgginson IJ, Marconi K. Does palliative care improve outcomes for patients with HIV/AIDS? A systematic review of the evidence. Sexually Transmitted Infections. 2005;81:5–14. doi: 10.1136/sti.2004.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;3:355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Rivera Mindt M, Sadek J, Moore DJ, Bentley H, The HNRC Group The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Odessa: Psychological Assessment Resources Inc; 2004. [Google Scholar]
- Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington: National Academy Press; 2001. [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Cattie JE, Doyle KL, Grant I, The HIV Neurobehavioral Research Program Group Risky decision-making in HIV-associated neurocognitive disorders (HAND) The Clinical Neuropsychologist. 2013;27:256–275. doi: 10.1080/13854046.2012.740077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks A, Wainwright D, Salazar L, Grimes R, York M, Strutt A, Hasbun R. Neurocognitive deficits increase risk of poor retention in care among older adults with newly diagnosed HIV infection. AIDS. 2015;29:1711–1714. doi: 10.1097/QAD.0000000000000700. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Palmer BW, Appelbaum PS, Golshan S, Glorioso D, Dunn LB, Kraemer HC. A new brief instrument for assessing decisional capacity for clinical research. Archives of General Psychiatry. 2007;64:966–974. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- Kløve H. Grooved pegboard. Lafayette: Lafayette Instruments; 1963. [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test–64 Card Version. Odessa: Psychological Assessment Resources; 2000. [Google Scholar]
- Kramár EA, Babayan AH, Gavin CF, Cox CD, Jafari M, Gall CM, Lynch G. Synaptic evidence for the efficacy of spaced learning. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5121–5126. doi: 10.1073/pnas.1120700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Song T, Chen Y, Lee W, Chiu Y. Reexamining the validity and reliability of the clinical version of the Iowa Gambling Task: evidence from a normal subject group. Frontiers in Psychology. 2013;4:1–12. doi: 10.3389/fpsyg.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkus IM, Samsa G, Rimer BK. General performance on a Numeracy Scale among highly educated samples. Medical Decision Making. 2001;21:37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- Lovejoy TI, Suhr JA. The relationship between neuropsychological functioning and HAART adherence in HIV-positive adults: A systematic review. Journal of Behavioral Medicine. 2009;5:389–405. doi: 10.1007/s10865-009-9212-9. [DOI] [PubMed] [Google Scholar]
- Martin EM, DeHaan S, Vassileva J, Gonzalez R, Weller J, Bechara A. Decision making among HIV+ drug using men who have sex with men: a preliminary report from the Chicago Multicenter AIDS Cohort Study. Journal of Clinical and Experimental Neuropsychology. 2013;35:573–583. doi: 10.1080/13803395.2013.799122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Cattie JE, Blackstone K, Grant I, Woods SP, The HNRP Group Neurocognitive impairment is associated with lower health literacy among persons living with HIV infection. AIDS and Behavior. 2015;19:166–177. doi: 10.1007/s10461-014-0851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D, Jr, Cysique L, Ake C, The HNRC Group Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop color and word test, and Wisconsin Card Sorting Test–64 Card Version. Journal of Clinical and Experimental Neuropsychology. 2011;33:793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AM. Validation of a decisional conflict scale. Medical Decision Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Dunn LB, Appelbaum PS, Jeste DV. Correlates of treatment-related decision-making capacity among middle-aged and older patients with schizophrenia. Archives of General Psychiatry. 2004;61:230–236. doi: 10.1001/archpsyc.61.3.230. [DOI] [PubMed] [Google Scholar]
- Perretta JG, Pari G, Beninger RJ. Effects of Parkinson disease on two putative nondeclarative learning tasks – probabilistic classification and gambling. Cognitive and Behavioral Neurology. 2005;18:185–192. doi: 10.1097/01.wnn.0000187939.81541.1d. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. 2nd. Tucson: Neuropsychological Press; 1993. [Google Scholar]
- Reyna VF. Theories of Medical Decision Making and Health: An Evidence-Based Approach. Medical Decision Making. 2008;28:829–833. doi: 10.1177/0272989X08327069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, Brainerd CJ. The importance of mathematics in health and human judgment: Numeracy, risk communication, and medical decision-making. Learning and Individual Differences. 2007;17:147–159. [Google Scholar]
- Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: what is its role in cancer screening? Cancer. 2004;101:1214–1228. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Karpicke JD. Test-enhanced learning – taking memory tests improves long-term retention. Psychological Science. 2006;17:249–255. doi: 10.1111/j.1467-9280.2006.01693.x. [DOI] [PubMed] [Google Scholar]
- Rustad JK, Musselman DL, Skyler JS, Matheson D, Delamater A, Kenyon NS, Nemeroff CB. Decision-making in diabetes mellitus type 1. The Journal of Neuropsychiatry and Clinical Neurosciences. 2013;25:40–50. doi: 10.1176/appi.neuropsych.12010016. [DOI] [PubMed] [Google Scholar]
- Sørensen K, Van de Broucke S, Fullam J, Doyle G, Pelikan J, Slonska Z, (HLS-EU) Consortium Health Literacy Project European Health literacy and public health: A systematic review and integration of definitions and models. BMC Public Health. 2012;12:80. doi: 10.1186/1471-2458-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Streiff V, Patel SM, Panos SE, Castellon SA, Hinkin CH. The role of HIV infection, cognition, and depression in risky decision-making. Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24:340–348. doi: 10.1176/appi.neuropsych.11110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky D, Zhu J, Ledbetter MF. WAIS-III and WMS-III technical manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Turner TH, LaRowe S, Horner MD, Herron J, Malcolm R. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. Journal of Substance Abuse and Treatment. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Rational choice and the framing of decisions. Journal of Business. 1986;59:S251–78. [Google Scholar]
- Vance DE. Aging with HIV: clinical considerations for an emerging population. The American Journal of Nursing. 2010;110:42–47. doi: 10.1097/01.NAJ.0000368952.80634.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EA, McQueen A, Cameron LD. Perceived risk and its relationship to health-related decisions and behavior. In: Martin LR, DiMatteo MR, editors. The Oxford handbook of health communication, behavior change, and treatment adherence. Oxford University Press; 2013. pp. 193–213. [Google Scholar]
- Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Hale FA. Quick assessment of literacy in primary care: The newest vital sign. Annals of Family Medicine. 2005;3:514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EJ, Grund B, Robertson K, Brew BJ, Roedinger M, Bain MP, INSIGHT SMART Study Group Neurology. 2010;75:864–873. doi: 10.1212/WNL.0b013e3181f11bd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Tröster AI, The HNRC Group Action (verb) fluency: test-retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society. 2005;11:408–415. [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI, version 2.1) Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]


