Abstract
Diversity and size of the antigen-specific T cell receptor (TCR) repertoire are two critical determinants for successful control of chronic infection. Varicella zoster virus (VZV) that establishes latency during childhood is able to escape control mechanisms, in particular with increasing age. We examined the TCR diversity of VZV-reactive CD4 T cells in individuals older than 50 years by studying three identical twin pairs and three unrelated individuals before and after vaccination with live attenuated VZV. While all individuals had a small number of dominant T cell clones, the breadth of the VZV-specific repertoire differed markedly. A genetic influence was seen for the sharing of individual TCR sequences from antigen-reactive cells, but not for repertoire richness or the selection of dominant clones. VZV vaccination favored the expansion of infrequent VZV antigen-reactive TCRs including those from naïve T cells with lesser boosting of dominant T cell clones. Thus, vaccination does not reinforce the in vivo selection occurred during chronic infection but leads to a diversification of the VZV-reactive T cell repertoire. However, a single booster immunization seems insufficient to establish new clonal dominance. Our results suggest that repertoire analysis of antigen-specific TCRs can be an important read-out to assess whether a vaccination was able to generate memory cells in clonal sizes that are necessary for immune protection.
Introduction
Varicella zoster virus (VZV) belongs to the family of alpha herpes viruses that establish latent infection in humans. Cellular immunity, in particular virus-reactive CD4 T cells are critical for effective viral control (1). With progressive age, reactivation of VZV, manifesting as herpes zoster, is increasingly frequent, presumably due to a decline in VZV-specific T cell immunity (2). The size of the antigen-specific T cell compartment is one critical determinant of T cell immunity. Upon antigenic stimulation, antigen-specific naïve T cells expand rapidly more than 1000-fold. Although most of these effector T cells do not survive, long-lived memory cells are maintained at frequencies that are at least 10-fold higher than those in the naïve compartment. Indeed, VZV-reactive memory T cells are present in most individuals and decline with increasing age. The functional properties of antigen-reactive T cells represent the second dimension of immune competence. Polyfunctionality, e.g. the ability of T cells to produce different cytokines in response to antigenic stimulation, has been identified as a positive correlate of protection (3).
In addition to T cell frequency and functionality, T cell receptor (TCR) diversity is a defining hallmark of the antigen-reactive T cell repertoire (4). TCRs are highly polymorphic heterodimers. Diversity is generated through the combination of gene segments combined with insertion and deletion of single nucleotides. The potential richness of αβ TCRs, defined as the number of receptors with different sequences, is up to 1020. Next generation sequencing, combined with novel statistical approaches, now allows us to estimate the total number of different TCR β-chains in the human naïve repertoire (5, 6). Using an incidence-based nonparametric estimator by comparing the presence or absence of particular sequences in replicate samples, we estimated that healthy young adults have TCR β-chains with more than 20 million amino acid sequences (7). With increasing age this diversity contracts 2- to 5-fold. Even with this contraction, the repertoire remains highly diverse.
In addition to the available TCR repertoire, the diversity of a virus-specific T cell response is determined by the number of viral proteins that are recognized. VZV encodes 70 proteins, with at least ten ORFs transcribed during latencies of which ORF63 is most prevalent (8). T cell responses have been identified to several proteins including the glycoproteins gB, gC, gE, gI and the Immediate Early proteins IE4, IE62 and IE63 (9, 10). T cell responses to different proteins may not be equally protective, but the overall breadth of the T cell response may contribute to protection. Interestingly, vaccination appears to broaden the spectrum of viral proteins to which T cell responses can be detected (10). However, a recent vaccine study demonstrated that boosting the magnitude of the immune response to gE alone is able to improve control of viral latency (11).
Differences in the diversity of TCRs already exist at the level of the recognition of single peptide epitopes (12). A broader repertoire might be beneficial to prevent the emergence of escape mutants, as has been shown for T cell responses to viruses or to tumor antigens (13, 14). More relevant for infection with viruses having low mutation frequencies, such as VZV, a more diverse TCR repertoire to a viral peptide increases the likelihood of cross-reactivity to related viral peptides (15). Given the sequence similarities of different herpes viruses, such a mechanism may contribute to maintaining protective memory. One example of T cell cross-reactivity between VZV-, HSV- and EBV-derived peptides has been described, although broader epidemiological evidence is lacking (16). Moreover, in case of chronic CMV infection, the breadth of the viral peptide-specific TCR repertoire, but not the clonal size of the T cell response, was inversely correlated with antibody titers to CMV, a measure associated with detectable viremia (17).
The selection of TCR repertoires in epitope-specific immune responses has been addressed mostly in CD8 T cells in murine systems (18). A central question is the degree to which repertoire formation in an immune response is stochastic or deterministic. In general, during infection or vaccination, epitope-specific naive CD8 T cells are recruited into the memory compartment. However, deterministic selection mechanisms cause skewed clonal size distributions that further increase in chronic infections and recall responses (19, 20). These observations raise the question of whether vaccination induces a contraction of the virus specific repertoire owing to the disproportionate expansion of dominant clones.
In the current study we examined the TCR repertoire of VZV antigen-reactive CD4 T cells before and after vaccination with a live attenuated virus. We show large inter-individual differences in repertoire diversity that were not determined by genetic factors and may correlate with different degrees of susceptibility for zoster reactivation. Vaccination was able to diversify the antigen-specific repertoire due to the preferential expansion of small clones including those recruited from the naïve compartment.
Results
Heterogeneity in repertoire complexity of VZV antigen-reactive CD4 T cells
To determine whether the complexity of the VZV-reactive repertoire differs between individuals, we performed next generation sequencing of TRB genes. Previous studies have suggested that protection from VZV reactivation resides with CD4 T cells (1, 21), and we therefore focused on them. To identify VZV antigen-reactive T cells, CFSE-labeled PBMCs were cultured with a lysate of pOKa-infected MEL39 melanoma cells and CFSE-dim CD4 T cells were isolated (Fig. 1A). Mock stimulation with a lysate of non-infected MEL39 cells only induced minimal proliferation. Since the global CD4 memory repertoire includes approximately one million different TRB genes in a total memory pool of 1011 CD4 T cells (7), each culture was set up with ten million PBMC to avoid undersampling. We recovered between 5,000 and 10,000 CFSE-dim CD4 T cells from each culture, which corresponded to original frequencies of 0.03 to 0.21% after accounting for the number of divisions in the culture, consistent with the estimates for VZV antigen-reactive CD4 T cell frequencies obtained by other approaches (22, 23). Frequencies of mock lysate reactive T cells were one to two magnitudes lower.
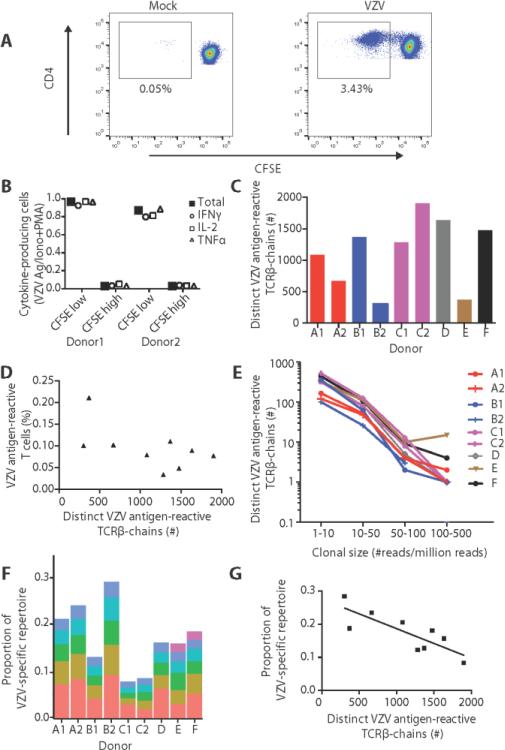
Fig. 1. The T cell receptor repertoire of VZV antigen-reactive CD4 T cells.
(A) CFSE-labeled PBMCs were stimulated with VZV lysate or mock lysate for 8 days. Scatter plots are gated on CD4 T cells and are representative of four experiments. (B) 8-day VZV lysate activated PBMCs were either restimulated with PMA and ionomycin or VZV lysate together with fresh T cell-depleted CTV color-coded PBMCs. The frequencies of cytokine-producing cells stimulated by VZV lysate in CFSElow and CFSEhigh CTVnegativeCD4 T cells were normalized for the number of cytokine-producing cells after stimulation with PMA and ionomycin. (C) The number of distinct TCRβ chains in VZV antigen-reactive CD4 T cells from three twin pairs (A, B and C) and three unrelated individuals (D, E and F) was determined. Identical twins are indicated by the same color. (D) Frequencies of VZV antigen-reactive T cells were correlated with the richness of their TCRβ chain repertoires (r=−0.5, p=0.18). (E) Total CD4 T cells were analyzed for the presence and frequencies of VZV-reactive TCR sequences. Sequences that were too infrequent to be re-identified were set at a minimum frequency of 1 in 106. Clonal size distributions are shown by plotting normalized clonal size bins vs. the log number of TCR sequences found at that particular frequency. Identical twins are shown in the same color, but different symbols. (F) The stacked bar plot shows the proportion of the VZV antigen-reactive CD4 TCRβ repertoire that the most abundant clones occupy. (G) The number of distinct VZV-reactive CD4 TCRβ chains inversely correlated with the space taken up by the most abundant clones (r=−0.8, p=0.01). Results are from two-sided non-parametric Spearman correlation.
To determine how many T cells in the proliferating cultures were truly VZV reactive, cells were restimulated with new VZV lysate in the presence of CellTrace Violet (CTV)-labeled PBMC from the original donor. Production of IL-2, IFN-γ and TNF-α was determined in CSFE-dim CTV-negative cells by intracellular cytokine staining. Control cultures were stimulated with ionomycin and PMA. Results in Figure 1B represent the ratio of cells that produced one or any of the three cytokines in response to the VZV antigen compared to polyclonally activated T cells. The data confirm that the vast majority of proliferating cells were reactive to the VZV lysate and that contamination by proliferating bystander cells was small.
The TCR repertoire of VZV antigen-reactive CD4 T cells was determined in nine healthy individuals including three identical twin pairs. Their demographic data are shown in Table S1. All of them had pre-existing VZV-specific antibodies. With the exception of individual E who had a history of VZV reactivation more than 5 years ago, nobody had a history of zoster. Libraries from two to three replicate VZV antigen-reactive cultures were generated for each individual. Approximately 43,000 to 89,000 TCRβ sequence reads were obtained for each replicate (Table S2). Estimates of richness varied greatly among the nine individuals; from 303 to 1901 distinct CD4 T cell clones (Fig. 1C). In this analysis, we used stringent criteria to designate a TCR as derived from a VZV antigen-reactive T cell (see Methods), and our estimates are therefore minimal estimates that may have missed infrequent clones. Mock lysate-reactive TCRs were mostly sorted out by these criteria. In general, it was difficult to generate sufficient numbers of mock lysate-reactive T cells for analysis. Even when we collected sufficient mock lysate-reactive T cells to generate TCR libraries of similar breadth, the number of TCRs surviving the selection criteria was ten-fold lower (23, 5 and 257) than for VZV antigen-reactive cells. Taken together, our control experiments support the notion that the number of false positive TCRs designated as VZV antigen-reactive is very small.
Age did not account for the heterogeneity in the breadth of the VZV antigen-reactive repertoires, since eight of nine individuals were between 50 and 60 years. As shown in Fig. 1D, clonal richness did not correlate with the frequencies of CD4 T cells that were driven into proliferation by stimulation with VZV lysate (frequencies were determined by correcting the percentage of CSFE-low cells for the number of divisions); i.e., a larger response to VZV in vitro was not indicative of increased richness. The three identical twin pairs did not show higher similarity in richness than unrelated individuals, suggesting that genetic factors did not substantially influence the richness of VZV-specific T cell memory (p=0.70 by a permutation test).
Since TCR sequence frequencies within the sorted population may be skewed due to differences in proliferative behavior, we used the sequences as identifiers and determined their frequencies in unstimulated ex vivo CD4 T cells to assess evenness, i.e. the clonal size distributions in VZV antigen-reactive CD4 TCR repertoires (Fig. 1E). Approximately half (range, 42-65%) of the TCRs were re-identified in a library of one million ex vivo CD4 T cells. The distribution of sequence counts followed a power law with most of the detectable VZV-specific clones relatively infrequent (1-50 reads per million reads). All individual repertoires included 5 to 6 abundant clones whose frequency was more than two standard deviations above the average clonal size. These abundant clones contributed between 8 to 28 percent of the total VZV antigen-reactive CD4 TCR repertoire (Fig. 1F). The space that the abundant clones occupied correlated inversely with the total number of VZV-specific CD4 TCRβ sequences (Fig. 1G).
TRB gene repertoire of VZV-specific CD4 T cells
To determine unique sequence features of VZV-specific CD4 TRB repertoires, we examined BV and BJ segment usage, and CDR3 sequences in VZV antigen-reactive CD4 T cells and compared them with those from naïve and memory CD4 T cells of the same individual. The use of seven TRBV gene segments was significantly increased in VZV antigen-reactive CD4 T cells, while the use of seven TRBV and one TRBJ gene was significantly decreased compared to naïve CD4 T cells at an FDR≤0.1 based on paired Wilcoxon rank sum test and Benjamini Hochberg adjustment (Table S3). A largely overlapping set of BV and BJ gene segments was found to be significantly different when VZV antigen-reactive CD4 T cells were compared with memory CD4 T cells (FDR≤0.1). We further analyzed CDR3 amino acid sequences, the major contributor of TCR diversity and antigen specificity. Length distributions, hydrophobicity and charge of CDR3 sequences were all similar in VZV-specific CD4 T cells, naïve CD4 T cells, and memory CD4 T cells (Fig. S3). Moreover, abundant clones as defined in Fig. 1F had unique amino acid sequences with no evidence of convergence.
Genetic control of naïve, memory and VZV-specific CD4 TRB repertoires
A recent twin study has shown a genetic influence on TRBV, but not J gene segment usage in the global T cell repertoire due to a mechanism other than thymic selection (24). To determine whether genetic factors impact the selection of the global antigen-reactive repertoire of memory CD4 T cells or, more specifically, the TCR repertoire to a chronic viral infection such as VZV, we compared TRBV and BJ gene segment usage in naïve, memory and VZV antigen-reactive CD4 T cells. Analysis of BV-BJ combination frequencies showed clustering of all three identical twins for both naïve and memory CD4 T cells (Fig. 2A). However, similarities in BV-BJ combination usage in VZV-specific CD4 T cells repertoires were lower in twin pair C or not present in twin pairs A and B suggesting that the genetic influence on the TRBV-BJ usage in antigen-specific T cells in the setting of a chronic infection is not a consistent feature in identical twins.
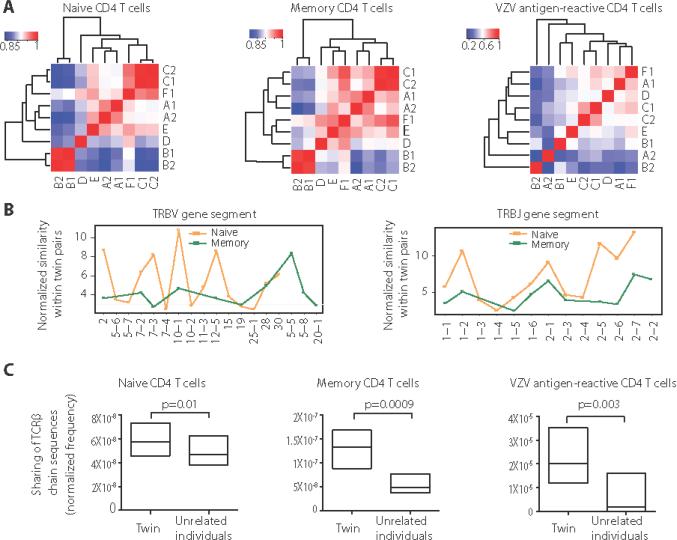
Fig. 2. Genetic impact on naïve, memory and VZV antigen-reactive CD4 TCRβ repertoires.
(A) Heatmaps show hierarchical clustering of TRBV-TRBJ combination frequencies in naïve (left panel), memory (middle panel), and VZV antigen-reactive CD4 T cells (right panel) for three twin pairs (A-C) and three unrelated individuals (D-F). The color represents pairwise Pearson correlation coefficients ranging from 0.85 to 1 for naïve and memory cells and 0.2 to 1 for VZV-reactive T cells. (B) TRBV (left) and TRBJ (right) genes that were significantly more similar in twin pairs than unrelated individuals were identified using a nonparametric permutation test (p<0.05). Similarities are expressed as the ratio of differences between unrelated individuals and differences within twins. Naïve (orange) and memory cells (green) are compared. (C) The repertoires in twins and non-twins were analyzed for the presence of TCRs with identical amino acid sequences. Frequencies of shared TCRβ chain sequences were normalized by dividing the number of shared TCRβ chain sequences within a pair by the product of the total number of distinct TCRβ chain sequences from these two individuals. Similarities were compared using nonparametric permutation test.
Selected BV and BJ segments, shown in Figure 2B, accounted for the similarities within the twin pairs. For the most part, the same set of BV and BJ segments determined twin-specific signatures for both naïve and memory T cells, suggesting that the pre-existing naïve repertoire influences the composition of the memory repertoire. However, similarity scores of different TRBV elements and to a lesser extents TRBJ elements were quite variable for naïve and memory cells. This finding is consistent with the existence of non-inherited pressures on selection and on the formation of memory.
To examine another determinant of repertoire selection that could be influenced by genetic predisposition, we assessed the extent of sharing of identical TCR β-chain amino acid sequences in naïve, memory, and VZV-specific CD4 T cells of different individuals. Data were normalized to adjust for sequencing depth as described (24, 25). For naïve CD4 T cells, the normalized number of TCRβ sequence sharing was between 4×10−8 and 7×10−8, i.e. for every 104 distinct TCRβ clones of an individual, about 2 to 3 T cells with identical TCRβ chains were found to be shared with an unrelated individual (Figure 2C). The number was slightly higher at between 4×10−8 and 1.6×10−7 for memory CD4 T cells. With 1.5×10−7 to 4×10−5, sharing was the highest for VZV-specific CD4 T cells, suggesting that antigen recognition resulted in the expansion of similar TCRβ-chain sequences. Twin analysis showed that genetic factors influenced the selection of TCR sequences in response to antigen. Identical twin pairs shared more TCRβ sequences than unrelated individuals for total naïve, total memory and VZV antigen-reactive CD4 T cells with similarities highest for VZV-reactive cells. In summary, a genetic influence was apparent for the formation of the VZV-reactive T cell repertoire; however, this was limited to a few clonotypes and did not include the clones that reached clonal dominance. For the majority of T cell clones, the virus-specific TCR repertoire was less genetically influenced than the naïve TCR repertoire.
Clonal heterogeneity in responses to VZV vaccination
To study the in vivo clonal dynamics of the CD4 T cell response to live attenuated VZV vaccination, we measured the frequencies of VZV antigen-reactive TRB clonotypes in PBMCs collected on days 7, 14 and 28 after vaccination. Sequencing depths at each time point and for each individual were similar (Table S2). Total frequencies of VZV antigen-reactive CD4 T cells were estimated by adding up frequencies of individual sequences and showed the expected time course of a vaccine-induced T cell response (Fig. 3A). Frequencies were slightly higher than the frequencies obtained from the proliferation assay, probably because the former approach is independent of whether all antigen-specific cells are activated and survive in the culture. Prior to vaccination, the frequencies of VZV antigen-reactive CD4 T cells were low, ranging from about 0.1% to 0.6% of total CD4 T cells. After vaccination, the frequencies significantly increased, peaking at 0.5% to 3.2% at day 8 or day 14 after vaccination. By day 28, the frequencies had declined, but they remained significantly higher than before vaccination. Overall kinetic changes were small, consistent with previous findings using ELISpot assays to quantify antigen-specific T cells (22, 23). Responses were variable and did not appear to be determined by genetic factors; identical twin pairs did not show higher similarity than unrelated individuals (Fig. 3A).
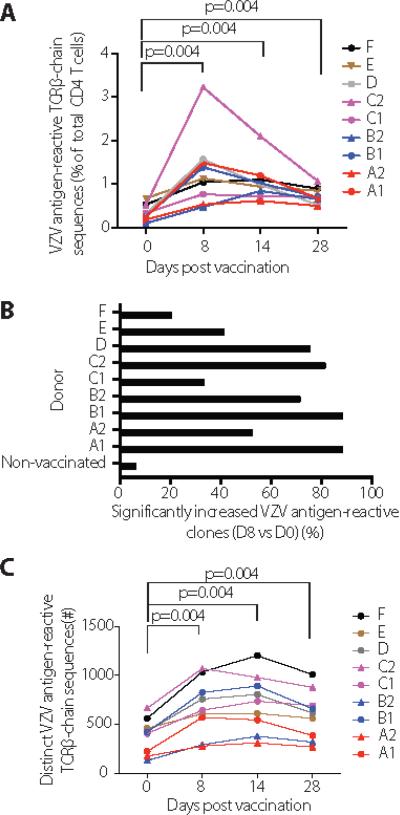
Fig. 3. Influence of vaccination on the CD4 T cell receptor repertoire of VZV-reactive cells.
VZV antigen-reactive TCRβ chain sequences were identified as described in Fig. 1 on days 0, 8, 14 and 28 and their individual frequencies in total CD4 T cells were determined. (A) Line graphs show the sum of all frequencies of detected TCRβ-chains in one individual. Unrelated individuals are indicated by different colors, twins by different symbols. (B) The percent of VZV-reactive TCRβ chains that had a significant change in clonal size between day 0 and day 8 after vaccination is shown. Changes between two time points from a non-vaccinated control individual are shown for comparison. (C) The number of unique VZV-specific TCRβ chains before and on days 8, 14, and 28 after vaccination is shown. Paired Wilcoxon-Mann-Whitney tests were performed for comparison in A and C, n=9.
Not all clones expanded equally upon vaccination. We estimated the proportion of VZV antigen-reactive CD4 T cells expanding between days 0 and 8 after vaccination by comparing the distribution of observed p-values with the uniform distribution as the null reference distribution (Figure 3B). As expected, none of the TCRs decreased in frequency after vaccination and very few TCRs changed in an individual who was not vaccinated. In contrast, in five of the nine vaccinated individuals more than 70% of TCRs responded to vaccination with significant expansion. Interestingly, the response rate was much lower in four individuals. Since we did not find a correlation between the global increase in frequencies of VZV antigen-reactive T cells and the proportion of clones expanding, we examined whether vaccination recruited new TCRs. Indeed, vaccination increased the total number of TRB sequences in the VZV-reactive repertoire in all nine individuals, indicating that it caused the expansion of infrequent VZV antigen-reactive clones and possibly diversified the repertoire (Fig. 3C).
Vaccination-induced expansion of naïve and memory VZV-specific T cells
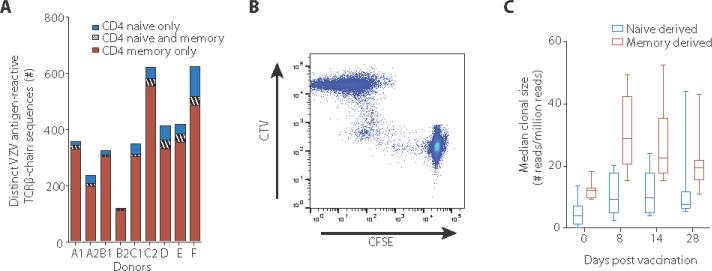
Primary VZV infection frequently occurs in childhood before the final T cell repertoire is formed. Whether clinically silent viral reinfection or reactivation continues to recruit naïve T cells throughout life into the memory repertoire is unknown. As has been shown for other antigenic systems (26), the naïve repertoire may have a reserve of VZV-specific T cells that could be mobilized by vaccination. To address this question, we determined whether VZV-specific TCRβ sequences on day 8 after vaccination can be identified in the original naïve or memory T cell repertoire on day 0. Approximately 50% of all VZV antigen-reactive TCRβ sequences could be re-identified in unstimulated CD4 T cells, the majority in memory CD4 T cells (Fig. 4A). However, naïve TCRβ sequences also contributed to the VZV-reactive TCR repertoire after vaccination. This number was small, but likely an underestimate since the probability of re-identifying sequences in the naïve repertoire is low due to its high diversity. Most of the TCR sequences that we did not detect in our experiments could therefore be derived from naïve T cells. Some VZV antigen-reactive TCRβ clones were present in both naïve and memory populations. Since CD4 memory T cells only infrequently revert to the naïve phenotype, these sequences may represent T cell clones that due to low antigen availability were only incompletely recruited to the memory compartment. To confirm that the naïve CD4 repertoire includes VZV-reactive T cells, we sorted naïve CD4+CD45RA+CCR7+ T cells from PBMC, stained them with CFSE and combined them with CTV-labeled PBMC from the same individual as a source of antigen-presenting cells. The cell mixture was stimulated with VZV lysate and cell proliferation was assessed by flow cytometry. The scatter plot shown as Figure 4B identifies a small population of naïve cells that proliferated in response to VZV antigen (Fig.4C). As expected, clonal sizes of originally naïve VZV-specific CD4 T cells were smaller than those of memory T cells. After vaccination, median clonal sizes of both populations increased significantly (Fig. 4C). These data suggest that VZV vaccination not only increased the frequencies of existing memory VZV-reactive CD4 T cell clones, but also activated naïve CD4 T cell clones, thereby increasing the richness of VZV-specific memory CD4 T cell repertoire and potentially improving protective immunity to VZV.
Fig. 4. VZV vaccination recruits antigen-specific CD4 T cells from the naïve compartment in addition to expanding memory T cells.
(A) Stacked bars show the number of distinct VZV-specific CD4 TCRβ chains that could be recovered only in the naïve or in the memory compartment and those present in both compartments before vaccination. (B) CFSE-labeled purified naïve T cells and CTV-labeled PBMCs were cultured together in the presence of VZV lysate for 8 days. The scatter plot of gated CD4 T cells identifies proliferating naïve CD4 T cells and total CD4 T cells. Data are representative of two experiments. (C) Clonal size distributions of VZV-specific TCRβ chains that were originally derived from either naïve or memory CD4 T cells are shown as box plots of median frequencies of the nine individuals. Compared to day 0, clonal sizes were significantly increased for both naïve and memory population at all time-points after vaccination (p<0.01, paired Wilcoxon-Mann-Whitney test, n=9).
Vaccination-induced diversification of the VZV-specific CD4 TCR repertoire
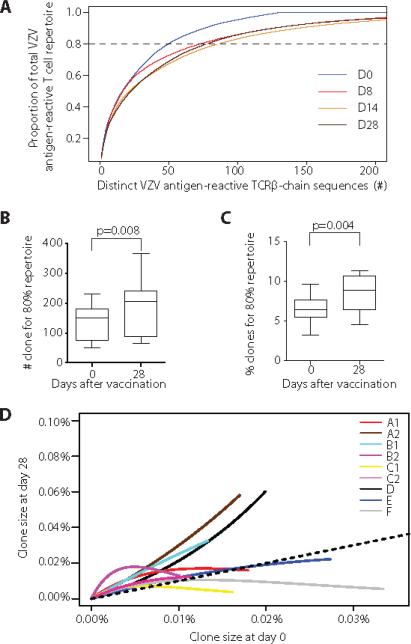
Our data indicate that VZV vaccination not only increased the frequency of VZV-reactive T cells, but also modified the composition of these cell populations. To assess whether vaccination influences repertoire diversity, we plotted fractional space against the number of T cell clones, ranked by decreasing sequence count, that take up this space. Results from a representative individual are shown in Fig. 5A; curves with a steep slope indicate repertoires with low diversity, while curves that are less steep or level off early indicate higher diversity. Vaccination induced an increase in diversity on day 8 that was maintained on days 14 and 28 after vaccination. Data for the nine individuals are summarized in Fig. 5B and 5C. The diversity index was either expressed as number of clones or the percent of clones, sorted by decreasing clonal size that took up 80% of the repertoire space. With both definitions, the diversity of VZV antigen-reactive TCRβ repertoire was increased on day 28 after vaccination compared to day 0.
Fig. 5. VZV vaccination diversifies the VZV-reactive TCR repertoire.
(A) The numbers of distinct VZV antigen-reactive TCRβ chains ordered by decreasing clonal size are plotted versus the cumulative space they occupy. Line graphs with different colors illustrate the clonal size distributions for different time points after vaccination. Data from one individual is shown. (B) The number of the largest distinct T cell clones that take up 80% of VZV-reactive CD4 TCR repertoire was determined as illustrated by the intersection with the indicated line in Fig. 5A. Results for the nine individuals before and 28 days after vaccination are shown as box plots. (C) Results are expressed as the percentage of clones that take up 80% of the antigen-specific repertoire space by dividing the number of different clones shown in Fig. 5B by the total number of clones for each time point and individual. (D) Distribution of clonal frequencies in VZV-reactive CD4 TCRβ repertoire at day 0 pre-vaccination is plotted against those at day 28 after vaccination. Each smoothed line represents the fitting curve that summarizes the distribution of clonal sizes for one individual. For comparison, lack of changes in clonal sizes is included as a dotted line. Paired Wilcoxon-Mann-Whitney tests were performed for comparison in B and C, n=9.
A better response and larger expansion of VZV antigen-reactive infrequent clones to vaccination compared to abundant clones is one possible mechanism that could contribute to an increase in diversity, thereby increasing the evenness of the repertoire. To test this hypothesis, we estimated the association between the percent increases of clonal frequencies in response to vaccination and the clone sizes for each individual based on a Poisson model and then combined the estimated association with a random effects model. In Fig. 5D, we compared clonal size distributions of VZV-reactive T cells at day 0 and day 28. Each fitted curve summarizes the relationship of clone sizes at day 0 and at day 28 for an individual. Since the frequency estimates of large clones are based on many sequence replicates and therefore robust, the slopes of the curve were supported in the small as well as the large clone regions although abundant clones were few. Only two individuals (A2, D) preferentially expanded one abundant clone. 7 out of the 9 curves were steeper in pre-vaccination small clonal size region, while they tended to be more flat for the pre-vaccination larger clonal size region indicating that percent expansion is greater for infrequent clones than abundant clones. The result from random effects model for these 9 curves confirms the existence of this pattern in the population (p=0.02).
Discussion
The ability of memory T cells to resolve or contain viral infections is determined by the frequency of antigen-specific T cells, their functionality, and the complexity of their TCR repertoire (27). In the current study, we examined the diversity of VZV-reactive CD4 T cells before and after vaccination in three pairs of identical twins and three unrelated individuals. We found large differences in the repertoire richness of VZV antigen-reactive CD4 T cells among individuals, even in identical twins. Vaccination with live attenuated VZV expanded VZV-reactive T cell clones unevenly, with a preference for infrequent and non-dominant TCRs. Vaccination therefore resulted in repertoire diversification with a smaller effect on dominant, in vivo selected T cell clones.
While inherited factors influenced the naïve and memory repertoires of TRBV-BJ combination frequencies, this effect was no longer seen for VZV-reactive T cells. However, when CDR3 sequences were included in the analysis, we saw increased sharing of identical TCR β-chain amino acid sequences in the VZV-reactive T cell response of twins compared to unrelated individuals, consistent with the selection of the VZV-specific TCR repertoire being partly under genetic control. Moreover, increased sharing of VZV-reactive compared to naïve sequences from the same twin pair indicates convergent recombination as described at the level of epitope-specific T cell responses (28). For all individuals, we find clonal dominance with a small number of clones making up a large fraction of the VZV-specific repertoire. Clonal size distributions were not influenced by genetic factors and dominant T cell clones did not show any evidence for increased sequence sharing.
Repertoire richness for VZV-reactive CD4 T cells varied widely in our study population. Comparative studies of the TCR profiles of epitope-specific CTL precursors from murine naïve CD8 T cells with those from immune mice have come to the conclusions that memory formation is not associated with a decline in richness (29) and that unevenness of clonal sizes in the memory repertoire is due to preferential expansion of some clones (30). It therefore appears unlikely that memory repertoires of VZV-specific cells generated at time of the initial infection differed in richness by an order of magnitude. The high discordance in identical twins indicates that genetic factors do not determine the different degrees of expansion during initial infection or the changes in repertoire diversity over lifetime. Contraction in richness did not correlate with frequencies of VZV-reactive CD4 T cells, suggesting that it is not a simple reflection of the loss of memory T cells with age. Moreover, contraction was caused by a preferential loss of infrequent T cell clones, consistent with the interpretation of greater selection of dominant T cell specificities. A similar observation of contraction at the epitope level has been made for the repertoire of influenza-specific CD8 T cells in older individuals suggesting that age may be a contributing factor (20). The clinical relevance of repertoire diversity has been shown for CMV infection, where the number of different TCRs reactive to a CMV peptide was more important for effective control of viral latency than their frequencies (17). Whether repertoire contraction could therefore be a predisposing factor of zoster reactivation remains to be determined. Our study was not longitudinal and the study population was too small to make any clinical correlations. However, it is interesting to note that the only individual with a previous history of zoster reactivation had the smallest repertoire.
Vaccination with the live attenuated VZV had a very uneven effect on the frequencies of different VZV-reactive TCRs. Surprisingly large clones that had reached dominance were less likely to respond than small clones. Most studies of repertoire selection in T cell responses imply that clonal dominance is a consequence of selection for higher avidity and that these T cell clones are more useful (30). The failure to expand dominant clones may explain why the zoster vaccine is of only modest benefit in older individuals (31). Clonal T cell exhaustion and failure to proliferate have been described for other chronic viral infections (32). Viruses that cause T cell exhaustion are generally thought to be highly replicative, as is the case with hepatitis C and human immunodeficiency viruses. By contrast, VZV establishes latency and should therefore not cause a high antigen load chronically stimulating T cells. However, recent publications described expression of CD38 and PD1 on VZV-specific T cells, characterizing them as activated or exhausted (33). It is also possible that these dominant clones are less responsive because they are responsive primarily to other herpes viruses and only cross-reactive to VZV with lower affinity. Cross-reactivities of VZV-reactive CD4 and CD8 T cells with other herpes viruses including HSV1, HSV2 and EBV have been described, and at least CD8 cross-reactive T cells frequently expressed markers of terminal differentiation or exhaustion (16, 34). However, this explanation appears unlikely since only four of the nine individuals vaccinated in this study had HSV1- or HSV2-specific antibodies including individuals A2 and D who had large clones, which responded to vaccination (Table S1, Figure 5D). Finally, some studies propose that generation of dominance is determined by cell-intrinsic or stochastic factors, not related to antigen-driven selection and therefore not necessarily highly useful (35).
Instead of further promoting the selection of dominant TCR sequences, VZV vaccination in this study broadened the repertoire in individuals by preferentially expanding small T cell clones including recruiting new specificities from the naïve repertoire. Larger TCR diversity in an antigen-specific T cell repertoire may provide better protection because it provides a foundation for the selection of high avidity TCRs (36, 37). Continuous recruitment of naïve T cells during chronic infection in a mouse model has been shown to increase the heterogeneity of antiviral CD8 T cell responses (38). In the case of chronic VZV infection, an untapped reservoir may remain in the naïve compartment. Mobilizing this resource to broaden the repertoire of VZV-specific T cells may therefore have long-term benefits for VZV-specific immunity. We have studied the vaccine response to a live-attenuated virus and used total VZV lysate as the antigen, and we are therefore not able to determine, which viral protein or peptides these naïve or more infrequent T cells recognize. It appears unlikely that they are reactive to epitopes in the vaccine virus that are absent in the wild-type virus, since sequence differences between the two viral strains are limited (39, 40). Repertoire studies of CD4 T cells selected on MHC/peptide tetramers would be desirable, however, peptide-specific CD4 T cells are very infrequent and population studies using MHC class II tetramers are problematic due to the polymorphic nature of MHC molecules. Such studies may be possible if a single component VZV vaccine such as the gE protein becomes available (11).
The current vaccine strategy with a single immunization is built on the premise of refreshing a recall response and is not optimal for rebuilding the memory compartment from infrequent or naïve cells and for selecting for dominance of new T cell clones; such a rebuilding of the memory repertoire would require prolonged or repeated stimulation such as is the case with infection or booster immunization. The attenuated VZV virus seems not to replicate much after vaccination - we have not found any viral sequences in peripheral blood or an inflammatory response in the days subsequent to vaccination (Table S4). Viral replication is possibly required to yield sufficient clonal expansion of the low-frequent VZV-reactive T cells. Interestingly, vaccination with the new, adjuvanted subunit vaccine that confers better protection than the currently approved live virus vaccine includes a booster after two months consistent with the interpretation that repeated immunizations are needed to reach critical frequencies of VZV-specific memory T cells (11).
Based on our study, one can envision several areas where TCR repertoire analysis of antigen-specific T cells could find clinical applications. Obviously, more studies are needed to determine whether low breadth or high clonal dominance is associated with increased risk for VZV reactivation. In this context it will be important to compare the VZV-specific repertoire after natural infection, to the repertoire that develops after primary vaccination in children who are now universally vaccinated in the US. In vaccination studies, TCR repertoire studies may emerge as an additional read-out system to determine whether a vaccine can induce a broad response, sufficient increase in clonal T cell frequencies and clonal dominance. This information will be valuable to guide the selection of adjuvants or decide on booster immunizations.
MATERIALS AND METHODS
Study design
The study was designed as an exploratory study to describe the TCR repertoire of VZV-specific T cells and to analyze longitudinal changes in repertoire diversity and clonal sizes after vaccination with live attenuated VZV (ZostavaxR, Merck). Nine participants were enrolled providing 70% power to detect a change in an outcome such the size of a particular T cell clone at the one-sided significance level of 0.1. Inclusion of three pairs of monozygotic twins from the Twin Research Registry at SRI International (41) allowed us to estimate the contribution of genetic factors in determining repertoire diversity before and after vaccination. Demographic information on the study population is provided in Table S1. Information on the isolation of VZV-reactive T cells, the sequencing of their TCR repertoire and the mathematical analysis is in the supplemental methods.
Supplementary Material
One Sentence Summary.
Adult vaccination with an attenuated herpes zoster virus broadens the T cell receptor repertoire of antigen-reactive CD4 T cells without reinforcing clonal dominance. Full Text: http://stm.sciencemag.org/cgi/content/full/8/332/332ra46?ijkey=496Lnc48LaIkM&keytype=ref&siteid=scitransmed
Acknowledgements
Funding: This work was supported by the National Institutes of Health (R01 AI108891, R01 AG045779, U19 AI057266 and I01 BX001669 to JJG, R01 AR042547, R01 AI044142, HL 117913, R01 AI108906 and P01 HL058000 to CMW and U19 AI090019 to CLD, RAO, SDB and JJG).
Footnotes
Author contributions: QQ, SDB, CMW, LT and JJG designed the study; SM, GES and CLD organized recruitment and vaccination; QQ, MMC, SLS, HN, CK and ET performed experiments, QQ, LT and JJG analyzed data with contribution from YL, CW and RAO; LT and RAO performed statistical analysis; QQ, RAO, CMW, SDB, LT and JJG wrote the paper.
Competing interests: The authors declare no competing financial interests.
Data and materials availability: TRB sequences have been deposited in the database dbGAP under accession number
References and Notes
- 1.Haberthur K, Engelmann F, Park B, Barron A, Legasse A, Dewane J, Fischer M, Kerns A, Brown M, Messaoudi I. CD4 T cell immunity is critical for the control of simian varicella virus infection in a nonhuman primate model of VZV infection. PLoS Pathog. 2011;7:e1002367. doi: 10.1371/journal.ppat.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–1609. [PubMed] [Google Scholar]
- 3.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 4.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Qi Q, Olshen RA, Weyand CM. High-throughput sequencing insights into T-cell receptor repertoire diversity in aging. Genome Med. 2015;7:117. doi: 10.1186/s13073-015-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgiou G, Ippolito GC, Beausang J, Busse CE, Wardemann H, Quake SR. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat Biotechnol. 2014;32:158–168. doi: 10.1038/nbt.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, Olshen RA, Weyand CM, Boyd SD, Goronzy JJ. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A. 2014;111:13139–13144. doi: 10.1073/pnas.1409155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen JI. The varicella-zoster virus genome. Curr Top Microbiol Immunol. 2010;342:1–14. doi: 10.1007/82_2010_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouwendijk WJ, Laing KJ, Verjans GM, Koelle DM. T-cell immunity to human alphaherpesviruses. Curr Opin Virol. 2013;3:452–460. doi: 10.1016/j.coviro.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laing KJ, Russell RM, Dong L, Schmid DS, Stern M, Magaret A, Haas JG, Johnston C, Wald A, Koelle DM. Zoster Vaccination Increases the Breadth of CD4+ T Cells Responsive to Varicella Zoster Virus. J Infect Dis. 2015;212:1022–1031. doi: 10.1093/infdis/jiv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC, Group ZOES. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 12.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price DA, West SM, Betts MR, Ruff LE, Brenchley JM, Ambrozak DR, Edghill-Smith Y, Kuroda MJ, Bogdan D, Kunstman K, Letvin NL, Franchini G, Wolinsky SM, Koup RA, Douek DC. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity. 2004;21:793–803. doi: 10.1016/j.immuni.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, Fong L. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6:238ra270. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu C, McCausland M, Sidney J, Duh FM, Rouphael N, Mehta A, Mulligan M, Carrington M, Wieland A, Sullivan NL, Weinberg A, Levin MJ, Pulendran B, Peters B, Sette A, Ahmed R. Broadly reactive human CD8 T cells that recognize an epitope conserved between VZV, HSV and EBV. PLoS Pathog. 2014;10:e1004008. doi: 10.1371/journal.ppat.1004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang GC, Dash P, McCullers JA, Doherty PC, Thomas PG. T cell receptor alphabeta diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci Transl Med. 2012;4:128ra142. doi: 10.1126/scitranslmed.3003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Gruta NL, Thomas PG. Interrogating the relationship between naive and immune antiviral T cell repertoires. Curr Opin Virol. 2013;3:447–451. doi: 10.1016/j.coviro.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cukalac T, Chadderton J, Handel A, Doherty PC, Turner SJ, Thomas PG, La Gruta NL. Reproducible selection of high avidity CD8+ T-cell clones following secondary acute virus infection. Proc Natl Acad Sci U S A. 2014;111:1485–1490. doi: 10.1073/pnas.1323736111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil A, Yassai MB, Naumov YN, Selin LK. Narrowing of human influenza A virus-specific T cell receptor alpha and beta repertoires with increasing age. J Virol. 2015;89:4102–4116. doi: 10.1128/JVI.03020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hata A, Asanuma H, Rinki M, Sharp M, Wong RM, Blume K, Arvin AM. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- 22.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A, I. Veterans Affairs Cooperative Studies Program Shingles Prevention Study, Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeulen JN, Lange JM, Tyring SK, Peters PH, Nunez M, Poland G, Levin MJ, Freeman C, Chalikonda I, Li J, Smith JG, Caulfield MJ, Stek JE, Chan IS, Vessey R, Schodel FP, Annunziato PW, Schlienger K, Silber JL. Safety, tolerability, and immunogenicity after 1 and 2 doses of zoster vaccine in healthy adults >/=60 years of age. Vaccine. 2012;30:904–910. doi: 10.1016/j.vaccine.2011.11.096. [DOI] [PubMed] [Google Scholar]
- 24.Zvyagin IV, Pogorelyy MV, Ivanova ME, Komech EA, Shugay M, Bolotin DA, Shelenkov AA, Kurnosov AA, Staroverov DB, Chudakov DM, Lebedev YB, Mamedov IZ. Distinctive properties of identical twins' TCR repertoires revealed by high-throughput sequencing. Proc Natl Acad Sci U S A. 2014;111:5980–5985. doi: 10.1073/pnas.1319389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, Liu Y, Cavanagh MM, Le Saux S, Qi Q, Roskin KM, Looney TJ, Lee JY, Dixit V, Dekker CL, Swan GE, Goronzy JJ, Boyd SD. B-cell repertoire responses to varicella-zoster vaccination in human identical twins. Proc Natl Acad Sci U S A. 2015;112:500–505. doi: 10.1073/pnas.1415875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009;206:1525–1534. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115:1678–1689. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price DA, Asher TE, Wilson NA, Nason MC, Brenchley JM, Metzler IS, Venturi V, Gostick E, Chattopadhyay PK, Roederer M, Davenport MP, Watkins DI, Douek DC. Public clonotype usage identifies protective Gag-specific CD8+ T cell responses in SIV infection. J Exp Med. 2009;206:923–936. doi: 10.1084/jem.20081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Heijst JW, Gerlach C, Swart E, Sie D, Nunes-Alves C, Kerkhoven RM, Arens R, Correia-Neves M, Schepers K, Schumacher TN. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science. 2009;325:1265–1269. doi: 10.1126/science.1175455. [DOI] [PubMed] [Google Scholar]
- 30.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–166. doi: 10.1001/jama.2010.1983. [DOI] [PubMed] [Google Scholar]
- 32.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 33.Jones L, Black AP, Malavige GN, Ogg GS. Phenotypic analysis of human CD4+ T cells specific for immediate-early 63 protein of varicella-zoster virus. Eur J Immunol. 2007;37:3393–3403. doi: 10.1002/eji.200737648. [DOI] [PubMed] [Google Scholar]
- 34.Ouwendijk WJ, Geluk A, Smits SL, Getu S, Osterhaus AD, Verjans GM. Functional characterization of ocular-derived human alphaherpesvirus cross-reactive CD4 T cells. J Immunol. 2014;192:3730–3739. doi: 10.4049/jimmunol.1302307. [DOI] [PubMed] [Google Scholar]
- 35.Nolz JC, Rai D, Badovinac VP, Harty JT. Division-linked generation of death-intermediates regulates the numerical stability of memory CD8 T cells. Proc Natl Acad Sci U S A. 2012;109:6199–6204. doi: 10.1073/pnas.1118868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong T, Stewart-Jones G, Chen N, Easterbrook P, Xu X, Papagno L, Appay V, Weekes M, Conlon C, Spina C, Little S, Screaton G, van der Merwe A, Richman DD, McMichael AJ, Jones EY, Rowland-Jones SL. HIV-specific cytotoxic T cells from long-term survivors select a unique T cell receptor. J Exp Med. 2004;200:1547–1557. doi: 10.1084/jem.20032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, Brockman MA, Miura T, Brumme ZL, Schneidewind A, Piechocka-Trocha A, Cesa KT, Sela J, Cung TD, Toth I, Pereyra F, Yu XG, Douek DC, Kaufmann DE, Allen TM, Walker BD. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat Immunol. 2012;13:691–700. doi: 10.1038/ni.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vezys V, Masopust D, Kemball CC, Barber DL, O'Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Argaw T, Cohen JI, Klutch M, Lekstrom K, Yoshikawa T, Asano Y, Krause PR. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J Infect Dis. 2000;181:1153–1157. doi: 10.1086/315335. [DOI] [PubMed] [Google Scholar]
- 40.Gomi Y, Sunamachi H, Mori Y, Nagaike K, Takahashi M, Yamanishi K. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J Virol. 2002;76:11447–11459. doi: 10.1128/JVI.76.22.11447-11459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krasnow RE, Jack LM, Lessov-Schlaggar CN, Bergen AW, Swan GE. The Twin Research Registry at SRI International. Twin Res Hum Genet. 2013;16:463–470. doi: 10.1017/thg.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.