Abstract
Background
To investigate the frequency, predictors, and clinical impact of electrographic seizures in patients with high clinical or radiologic grade non-traumatic subarachnoid hemorrhage (SAH), independent of referral bias.
Methods
We compared rates of electrographic seizures and associated clinical variables and outcomes in patients with high clinical or radiologic grade non-traumatic SAH. Rates of electrographic seizure detection before and after institution of a guideline which made continuous EEG monitoring routine in this population were compared.
Results
Electrographic seizures occurred in 17.6 % of patients monitored expressly because of clinically suspected subclinical seizures. In unselected patients, seizures still occurred in 9.6 % of all cases, and in 8.6 % of cases in which there was no a priori suspicion of seizures. The first seizure detected occurred 5.4 (IQR 2.9–7.3) days after onset of subarachnoid hemorrhage with three of eight patients (37.5 %) having the first recorded seizure more than 48 h following EEG initiation, and 2/8 (25 %) at more than 72 h following EEG initiation. High clinical grade was associated with poor outcome at time of hospital discharge; electrographic seizures were not associated with poor outcome.
Conclusions
Electrographic seizures occur at a relatively high rate in patients with non-traumatic SAH even after accounting for referral bias. The prolonged time to the first detected seizure in this cohort may reflect dynamic clinical features unique to the SAH population.
Keywords: Subarachnoid hemorrhage, Non-convulsive seizures, Continuous electroencephalography
Introduction
Although adverse outcomes associated with subarachnoid hemorrhage (SAH) have decreased by 17 % between 1973 and 2002 [1] with advancements in medical care, mortality remains high (26–36 %) [2], and functional impairment persists in almost half of all SAH patients, with 8–20 % remaining functionally dependent [2]. Seizures are believed to be a common complication following SAH, and there is evidence that seizures increase both mortality and disability [3], independent of other sources of secondary injury such as re-bleeding [4], symptomatic vasospasm [5], and delayed cerebral injury [6]. However, estimates of the risk for seizure following SAH vary widely, with reported rates ranging from as low as 1 % to as high as 28 % [7–10]. Probable factors underlying the variation in reported seizure rates include heterogeneous patient populations, practice differences in pharmacologic seizure prophylaxis, center-specific treatment guidelines, and differences in continuous electroencephalography (cEEG) monitoring and reporting practices [11]. An improved understanding of the frequency and risk factors for in-hospital seizures in patients with SAH would help clarify which patients might benefit from seizure monitoring, treatment, and prophylaxis.
We sought to determine the occurrence, predictors, and clinical impact of seizures in patients with high clinical or radiologic grade (non-traumatic) SAH by examining our single-center cohort of patients. Additionally, because estimates of seizure rates can vary significantly depending on referral bias, we investigated the impact on seizure frequency following SAH by studying two specific cohorts: (1) high-grade patients undergoing cEEG because of explicit suspicion for seizure, and (2) high-grade patients who underwent routine cEEG monitoring (regardless of suspicion for seizure) following the introduction of an institutional guideline for ischemia detection after aneurysm obliteration.
Methods
Patient Selection and Classification
We retrospectively reviewed patient cEEG and medical records from our institutional EEG Data Registry. The study was approved by the local Institutional Review Board. Inclusion criteria included (1) non-traumatic subarachnoid hemorrhage; (2) high clinical or radiologic grade, defined as Hunt Hess (HH) 4–5 or Fisher group (F) 3; as well as (3) clinical EEG performed during SAH hospitalization in patients admitted between January 2010 and August 2012.
Prior to November 2011, only patients with an explicit suspicion for seizure underwent EEG monitoring. After November 2011, institution of an ischemia monitoring guideline resulted in a long-term EEG monitoring being performed for ischemia detection in all HH4-5 and F3 patients regardless of the treating team’s suspicion for seizure. Over the course of the 10-month period of ischemia detection, 64.6 % of SAH patients were monitored. Patients who were not monitored were excluded due to low clinical or radiological grade, traumatic injury, or comfort measures goals of care. For these post-guideline cases, we also assessed whether or not the ordering provider(s) had any suspicion for seizures by reviewing the indication listed at the time cEEG monitoring was ordered, and by reviewing daily neurocritical care progress notes for any explicit clinical documentation of a suspicion for seizures, independent of the indication for ischemia monitoring.
Review of Neurophysiology and Other Clinical Data
Medical records were reviewed for any indication of tonic-clinic activity at ictus (SAH onset) by emergency care providers and/or family members. Two fellowship-trained clinical neurophysiologists reviewed all cEEGs to determine the presence or absence of in-hospital electrographic seizures. Electrographic seizures were defined using accepted criteria for “definite” seizures on cEEG [12]. Factors which might affect the risk for seizure were extracted from the medical record including age, pre-morbid hypertension, aneurysm location, neurological grade (HH4-5 vs. HH1-3), thickness of hemorrhage (F3 vs. other), the presence of co-morbid intraparenchymal hemorrhage, hydrocephalus, subsequent neuroimaging, and evidence of re-bleeding or infarction. To determine the presence of anti-epileptic drug (AED) treatment at time of EEG recording, the medication logs for each patient were reviewed. AEDs including levetiracetam or phenytoin were required to be administered within 12 h of EEG initiation, while propofol was required to be administered within 2 h to be counted as “present” at the time of EEG recording. The dosages administered were uniformly 500 mg twice daily for levetiracetam and a dose range varying between 0 and 300 mg/h for propofol (ordered uniformly as “titrate to sedation”). Phenytoin was not administered in the cohort. We pre-specified poor outcome as a discharge-modified Rankin Scale (mRS) score of 4–6 at the time of hospital discharge. mRS was determined based on discharge assessments documented by physicians, nurses, and clinical therapists.
Statistical Analysis
We pre-specified univariate analyses of the degree to which the clinical factors influenced seizure rates. Chi-square and Fisher’s Exact tests were used for binary associations. A univariate threshold of p ≤ 0.15 was selected for inclusion in a multivariate logistic regression model for predicting seizure occurrence. Age was dichotomized based on the mean value determined during univariate analysis.
Results
During the 32-month study period, 69 patients with non-traumatic SAH of high clinical or radiologic grade were admitted (Fig. 1). Prior to the ischemia monitoring era, 17 patients received cEEG over the course of 32 months. During the subsequent 10 months following initiation of the cEEG ischemia monitoring guideline for high clinical or radiologic grade patients, 52 patients received cEEG. Demographics and clinical characteristics are listed in Table 1. In comparison with patients for which ischemia detection was the sole reason for monitoring, patients referred because of an explicit suspicion for seizures tended to be older (p < 0.01), more deeply comatose (p < 0.01), and we monitored for a shorter duration (p < 0.01). There were no statistically significant group differences in the rates of intraventricular hemorrhage, hydrocephalus, aneurysm location and size, or treatment modality between these groups.
Fig. 1.
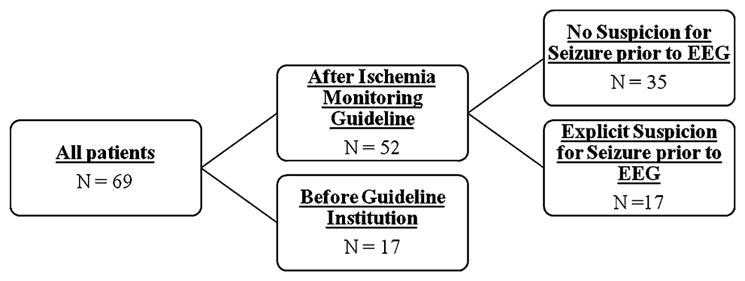
Patient groups. The total cohort included 69 patients. Prior to instituting routine cEEG monitoring for ischemia detection, 17 patients underwent cEEG monitoring for explicit suspicion for seizure. The remaining 52 patients were monitored as part of a ischemia guideline regardless of suspicion for seizure. Of these 52 patients, medical records reported a suspicion for seizure existed at the time cEEG monitoring for 17 patients. No documented clinical concern for seizure was documented at the time cEEG commenced for the remaining 35 patients monitored under the guideline
Table 1.
Patient demographics
| Patient characteristic | Suspected seizures (n = 31) | No suspicion for seizures (n = 38) | Significance OR [95 % CI] |
|---|---|---|---|
| Demographics | |||
| Female, n (%) | 20 (66.2 %) | 24 (64.4) | 0.94 [0.35–2.53] |
| Age, mean (SD) | 62.8 (13.7) | 55.1 (14.0) | t(67) = 2.30, p = 0.02* |
| Clinical presentation | |||
| Glascow coma scale, median (IQR) | 11 IQR [3–14] | 14 IQR [3–15] | z = 1.04, p = 0.30 |
| Thick cisternal blood (F 3), n (%) | 21 (67.7 %) | 30 (79.0 %) | 0.56 [0.19–1.66] |
| High clinical grade (HH 4–5), n (%) | 16 (55.2 %) | 9 (24.3 %) | 3.83 [1.34–10.93]** |
| Intraparenchymal hemorrhage, n (%) | 5 (16.1 %) | 10 (27.0 %) | 0.52 [0.16–1.73] |
| Intraventricular hemorrhage, n (%) | 20 (64.5 %) | 28 (73.7 %) | 0.65 [0.23–1.82] |
| Hydrocephalus, n (%) | 17 (54.8 %) | 21 (55.3 %) | 0.98 [0.38–2.55] |
| Size of aneurysm (mm): mean (SD) | 6.3 (4.0) | 6.7 (3.8) | t(59) = −0.40; p = 0.70 |
| MCA aneurysm Location, n (%) | 4 (12.9 %) | 4 (10.5 %) | 1.26 [0.29–5.50] |
| ACA aneurysm location, n (%) | 2 (6.5 %) | 3 (7.9 %) | 0.80 [0.13–5.15] |
| Craniotomy, n (%) | 17 (54.8 %) | 28 (73.7 %) | 0.43 [0.16–1.19] |
| Electrophysiology | |||
| Seizure at ictus, n (%) | 7 (22.6 %) | 4 (12.1 %) | 2.11 [0.55–8.01] |
| AED at time of EEG, n (%) | 15 (48.4 %) | 23 (60.5 %) | 0.61 [0.23–1.59] |
| Time to EEG from SAH (days), mean (SD) | 3.9 (3.68) | 2.7 (3.68) | t(67) = 1.88; p = 0.06 |
| cEEG duration (days), mean (SD) | 4.0 (4.27) | 7.0 (2.95) | t(67) = −3.45; p < 0.01** |
IQR inter-quartile range, MCA middle cerebral artery, ACA anterior cerebral artery, AED anti-epileptic drug
p ≤ 0.05,
p ≤ 0.01
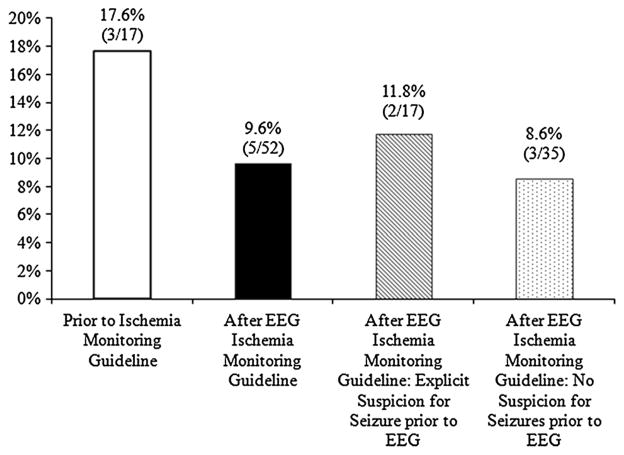
Seizures were detected in 11.6 % (8 of 69) of SAH patients (Fig. 2), the large majority of which were non-convulsive (n = 7.88 %). All electrographic seizures occurred in patients without a prior clinical seizure at the time of bleed. The rate of electrographic seizure detection dropped non-significantly from 17.6 % to 9.6 % (OR 0.5; p = 0.4) following institution of the cEEG ischemia monitoring guideline. Among patients monitored after adoption of the guideline, the seizure rate in the subset of patients with an explicit suspicion for seizures (11.8 %) was not significantly different from the seizure rate in patients without explicit suspicion for seizures by the clinical team (8.6 %). Moreover, suspicion for seizures versus lack thereof did not impact the frequency of seizures prior or following (p = 1.0) implementation of the ischemia monitoring guideline (Fig. 2). 55 % of patients (n = 38) had an AED present at time of EEG, and levetiracetam was the most commonly prescribed AED (89.5 %, 34/38).
Fig. 2.
Effect of referral bias on seizure detection rate. The rate of seizure detection is higher among patients with non-traumatic SAH undergoing cEEG monitoring because of suspected subclinical seizures (white bar) versus patients monitored per routine care regardless of suspicion for seizure (black bar). Among patients monitored after institution of ischemia monitoring, patients whose treating physician documented a clinical suspicion for seizure at the commencement of cEEG monitoring (striped bar) had a higher rate of detected seizures than in patients for whom there was no clinical suspicion (dappled bar), though in these latter patients seizure frequency was still high
In univariate analysis of factors correlating with the occurrence of seizures on cEEG, middle cerebral artery (MCA) aneurysm location was significantly associated with an increased seizure probability (OR 6.7, p < 0.05), while administration of a prophylactic anticonvulsant was associated with decreased seizure probability (OR 0.23, p < 0.01). In multivariate analysis, MCA location and prophylactic anticonvulsant use each lacked significant independent association with seizure occurrence, although a modestly significant multivariate model for seizure prediction (R2 = 0.13; p = 0.04) could be constructed with these two variables (Table 2).
Table 2.
Clinical predictors of seizures
| Patient characteristic | Without seizure (n = 61) | With seizure (n = 8) | Univariate odds OR [95 % CI] | Multivariate odds OR [95 % CI] |
|---|---|---|---|---|
| Demographics | ||||
| Age > median, n (%) | 30 (49.2 %) | 6 (75.0 %) | 3.1 [0.58–16.58] | – |
| Clinical presentation | ||||
| Thick cisternal blood (F3), n (%) | 44 (72.1 %) | 7 (87.5 %) | 2.7 [0.31–23.66] | – |
| High clinical grade (HH4-5), n (%) | 23 (39.0 %) | 2 (28.6 %) | 0.6 [0.11–3.50] | – |
| Intraparenchymal hemorrhage, n (%) | 14 (23.3 %) | 1 (12.5 %) | 0.5 [0.05–4.15] | – |
| Intraventricular hemorrhage, n (%) | 43 (70.5 %) | 5 (62.5 %) | 0.7 [0.15–3.23] | – |
| Hydrocephalus, n (%) | 33 (54.1 %) | 5 (62.5 %) | 1.4 [0.31–6.45] | – |
| MCA aneurysm location, n (%) | 5 (8.2 %) | 3 (37.5 %) | 6.7 [1.23–36.74]* | 5.0 [0.78–29.13] |
| ACA aneurysm location, n (%) | 5 (8.2 %) | 0 (0 %) | – | – |
| Craniotomy, n (%) | 40 (65.6 %) | 5 (62.5 %) | 0.9 [0.19–4.02] | – |
| Clinical suspicion for seizures, n (%) | 26 (42.6 %) | 5 (62.5 %) | 2.2 [0.49–10.24] | – |
| Electrophysiology | ||||
| AED at time of EEG, n (%) | 36 (59.0 %) | 2 (25.0 %) | 0.2 [0.04–1.24]** | 0.3 [0.04–1.58] |
| cEEG duration (days), mean (SD) | 5.5 (3.8) | 6.6 (4.6) | t(67) = 0.72, p = 0.5 | – |
MCA middle cerebral artery, ACA anterior cerebral artery, AED anti-epileptic drug
p ≤ 0.05,
p ≤ 0.01
Similar univariate and multivariate analyses were performed for factors associated with poor clinical outcome (Table 3). The multivariate logistic model for poor outcome retained thick cisternal clot (F3 grouping), high clinical grade (HH 4–5), age, explicit suspicion for seizures, and electrographic seizures (R2 = 0.28; p < 0.01). High clinical grade was the only factor of these, which remained independently associated with poor outcome.
Table 3.
Clinical predictors of poor outcome
| Patient characteristic | Good outcome (mRS 0–3) n = 21 | Poor outcome (mRS 4–6) n = 48 | Univariate OR [95 % CI] | Multivariate OR [95 % CI] |
|---|---|---|---|---|
| Demographics | ||||
| Age > median, n (%) | 8 (38.1 %) | 28 (58.3 %) | 2.3 [0.80–6.51] | 2.0 [0.50–8.01] |
| Clinical presentation | ||||
| High grade (HH 4–5), n (%) | 1 (5.0 %) | 24 (52.2 %) | 20.7 [2.56–168.0]** | 13.3 [1.58–350.0]** |
| Thick cisternal blood (F 3), n (%) | 19 (90.5 %) | 32 (66.0 %) | 0.2 [0.04–1.02]* | 0.6 [0.02–8.22] |
| Intraparenchymal hemorrhage, n (%) | 4 (19.1 %) | 11 (23.4 %) | 1.3 [0.36–4.68] | – |
| Intraventricular hemorrhage, n (%) | 15 (71.4 %) | 33 (68.8 %) | 0.9 [0.29–2.71] | – |
| Hydrocephalus, n (%) | 11 (52.4 %) | 27 (56.3 %) | 1.2 [0.42–3.27] | – |
| MCA aneurysm location, n (%) | 1 (4.8 %) | 7 (14.6 %) | 3.4 [0.40–29.68] | – |
| ACA aneurysm location, n (%) | 1 (4.8 %) | 4 (8.3 %) | 1.8 [0.19–17.32] | – |
| Craniotomy, n (%) | 15 (71.4 %) | 30 (62.5 %) | 0.7 [0.22–2.03] | – |
| Clinical suspicion for seizure, n (%) | 3 (14.3 %) | 28 (58.3 %) | 8.4 [2.17–32.41]** | 3.9 [0.87–21.36] |
| Electrophysiology | ||||
| Seizure at ictus, n (%) | 1 (5.6 %) | 10 (21.7 %) | 4.7 [0.56–39.9] | – |
| Receiving AED, n (%) | 10 (47.6 %) | 28 (58.3 %) | 1.6 [0.55–4.32] | – |
| EEG evidence of seizure, n (%) | 1 (4.8 %) | 7 (14.6 %) | 3.4 [0.39–29.68] | – |
MCA middle cerebral artery, ACA anterior cerebral artery, AED anti-epileptic drug
p ≤ 0.05,
p ≤ 0.01
ICU stays averaged 17.4 (SD = 7.5) days for patients with seizures compared to 16.5 (SD = 6.3) for patients without seizures. Similarly, hospitalization was non-significantly longer for patients with seizures, 28.4 (SD 12.2) days versus 24.0 (SD 10.0). While patients with electrographic seizures tended to have longer ICU and hospital stays, this finding was not significant (p > 0.5).
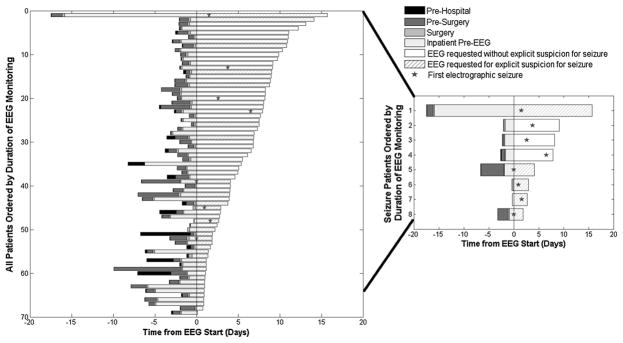
Overall, first seizure detection occurred a median of 5.4 days (IQR 2.9–7.3) from SAH ictus and 1.6 (IQR 0.7–2.9) days into cEEG recording. Three of eight patients (37.5 %) had their first recorded seizure more than 48 after EEG was initiated, and two of eight (25 %) had their first recorded seizure more than 72 h after EEG initiation (Fig. 3).
Fig. 3.
Time to seizure detection. Length of cEEG monitoring is shown, ordered by duration of cEEG monitoring. Those patients who developed a seizure are focused on within the inset
Conclusions
Our results help to clarify the natural history of in-hospital seizures among non-traumatic high-grade SAH patients, and the impact of referral bias on estimates of non-convulsive seizures in SAH. The results demonstrate that the seizures are frequent after SAH, even when unsuspected, and that seizures occur late after SAH, with more than a third of seizures first detected after 2 full days of EEG monitoring and one quarter of seizures first detected after 3 full days of EEG monitoring.
Although referral bias appears to account in part for the rates as high as 28 % reported in prior studies [13], our results demonstrate that cEEG monitoring identifies a modestly high number of patients with seizure regardless of a priori suspicion for seizures. After instituting a guideline by which cEEG monitoring to detect ischemia became routine in the ICU care for patients with non-traumatic SAH, the rate of detection of electrographic seizures decreased non-significantly and modestly (i.e., from 18 to 10 %). Even after excluding patients in whom seizures were explicitly suspected at the time cEEG monitoring was begun, seizures still occurred in 9 % of the high-grade cohort. Aneurysm location was associated with increased risk of these electrographic seizures in our sample. MCA aneurysms have been previously reported to be common among SAH patients who developed seizures [14], potentially due to a cortical injury related to the region of primary hemorrhage or subsequent craniotomy [15–17]. Contrary to previous publications, age [7, 18], seizure at ictus [9], high radiologic grade SAH [13, 19, 20], ICH, and hydrocephalus [16] among high-grade SAH patients did not independently increase the risk of in-hospital electrographic seizures, although such patients were encountered with low incidence in this single-center cohort. Similar to previous work by [21], the majority of seizures captured by cEEG were non-convulsive.
In-hospital electrographic seizures tended to occur relatively late. While previous work suggests the need for monitoring longer than 48 h in comatose patients, the current sample included three of eight patients (37.5 %) with a first seizure detected after 2 days of cEEG monitoring and two (25 %) with a first seizure detected after 3 days of cEEG monitoring. Only one of the patients with a first seizure after 48 h of cEEG monitoring was comatose, suggesting that coma may be an insufficient factor for pre-specifying the duration of monitoring.
This relatively delayed onset of non-convulsive seizures may be specific to SAH patients. Delayed time to first inhospital seizure has been reported previously for select SAH patients with prior seizures on seizure prophylaxis medication [19] and now for our heterogeneous cohort; whereas cohorts of patients with heterogeneous brain injuries yielded findings such that nearly all seizures in critically ill patients occurred within 48 h of EEG initiation [21]. A possible hypothesis that might explain this prolonged time for seizures to emerge in patients with SAH is a potential link between delayed cerebral ischemia and cortical spreading depolarizations, both of which similarly occur preferentially several days after the initial hemorrhage [22, 23]. While it is not possible to ascertain whether subclinical electrographic seizures occurred within the few days after SAH ictus and aneurysm treatment but before EEG initiation, it is notable that very few seizures occurred immediately following EEG placement.
While our data did not show that in-hospital seizures increased the risk of a poor discharge outcome independently after accounting for clinical grade and the clinician’s explicit suspicion for seizure, controlled or case-matched studies with larger numbers of seizures and more fine-grained characterization of seizure burden may be required to definitively define the true impact of seizures on SAH outcomes. The deleterious effect of seizures on outcome may also be mitigated in an environment in which seizures are detected and adequately treated.
While the rate of seizures after high-grade SAH has not previously been examined irrespective of referral bias, it is unclear that whether this single-center experience is generalizable to other institutions with different approaches to aneurysm obliteration, seizure prophylaxis, and method of sedation. In our population, surgical management was common, patients frequently received prophylactic anti-seizure medication until aneurysm obliteration, and mechanically ventilated patients were lifted from sedation every 2 h by protocol but otherwise were effectively maintained on anti-seizure treatment (i.e., propofol sedation).
Under our clinical guideline, adherence to the cEEG monitoring clinical guideline was not universal, and thus it is possible that we have underestimated the occurrence of seizures in patients with SAH, particularly in patients monitored for shorter durations or not monitored due to early withdrawal of life-sustaining therapies. However, the large majority of our patients achieved a duration of monitoring at least as long as in prior reported experiences in the critical care setting [21]. It is unlikely that seizures were missed among patients without prolonged EEG monitoring, as the average EEG monitoring period was at least 20 h longer than the average time to the first electrographic seizure detection. Although we did not have access to patient outcomes following hospital discharge, discharge outcomes are temporally closer to and are presumably impacted as much by in-hospital seizures as follow-up outcomes.
While ordering clinicians were required to prospectively document a reason for requesting cEEG monitoring before the test was performed and interpreted, retrospective identification of the cohort may have allowed for subtle referral bias in missing the nuances of reasons for EEG requisition that were documented with subtlety in the provider order entry system. We attempted to minimize this effect by performing a second layer of medical record review of physician progress note documentation to ascertain for any explicit suspicion for seizures by the clinician before EEG was initiated.
In conclusion, referral bias may enrich the likelihood of detecting seizures after SAH. Nevertheless, the likelihood of seizures remains high (10 %) in unselected patients undergoing cEEG monitoring after high-grade SAH, and nearly 9 % of patients still have electrographic seizures even when initially unsuspected by clinicians. Additionally, the prolonged time to a first seizure among the SAH cohort deserves exploration into whether delayed seizures are unique in comparison to other populations in which most seizures are detected in the first 2 days of monitoring. While the majority of patients with in-hospital electrographic seizures have poor discharge outcomes, further investigation is required to understand whether the timing, duration, and treatment of seizures modify their effect on outcome.
Footnotes
Conflict of interest
Kathryn L. O’Connor, M. Brandon Westover, Michael T. Phillips, Nicolae A. Iftimia, Deidre A. Buckley, Christopher S. Ogilvy, Mouhsin M. Shafi, and Eric S. Rosenthal declare that they have no conflict of interest.
Contributor Information
Kathryn L. O’Connor, Email: koconnor13@mgh.harvard.edu, Department of Neurology, Massachusetts General Hospital, Lunder 6 Neurosciences ICU, 55 Fruit Street, Boston, MA 02114, USA
M. Brandon Westover, Email: mwestover@mgh.harvard.edu, Department of Neurology, Massachusetts General Hospital, Lunder 6 Neurosciences ICU, 55 Fruit Street, Boston, MA 02114, USA.
Michael T. Phillips, Email: MPHILLIPS8@partners.org, Department of Neurosurgery, Massachusetts General Hospital, Boston, MA, USA
Nicolae A. Iftimia, Email: nickiftimia@gmail.com, Department of Neurology, Massachusetts General Hospital, Lunder 6 Neurosciences ICU, 55 Fruit Street, Boston, MA 02114, USA
Deidre A. Buckley, Email: dabuckle@bidmc.harvard.edu, Department of Neurosurgery, Beth Israel Deaconess Medical Center, Boston, MA, USA
Christopher S. Ogilvy, Email: cogilvy@bidmc.harvard.edu, Department of Neurosurgery, Beth Israel Deaconess Medical Center, Boston, MA, USA
Mouhsin M. Shafi, Email: mouhsin.shafi@gmail.com, Department of Neurology, Massachusetts General Hospital, Lunder 6 Neurosciences ICU, 55 Fruit Street, Boston, MA 02114, USA; Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA, USA
Eric S. Rosenthal, Email: erosenthal@partners.org, Department of Neurology, Massachusetts General Hospital, Lunder 6 Neurosciences ICU, 55 Fruit Street, Boston, MA 02114, USA
References
- 1.Nieuwkamp DJ, Setz LE, Algra A, Linn FHH, de Rooij NK, Rinkel GJE. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635–42. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 2.Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–37. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 3.Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt J, Tu B, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53–64. doi: 10.1002/ana.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sluzewski M, van Rooij WJ. Early rebleeding after coiling of ruptured cerebral aneurysms: incidence, morbidity, and risk factors. AJNR Am J Neuroradiol. 2005;26:1739–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Hoh BLBL, Topcuoglu MAMA, Singhal ABAB, Pryor JCJC, Rabinov JDJD, Rordorf GAGA, et al. Effect of clipping, craniotomy, or intravascular coiling on cerebral vasospasm and patient outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2004;55:779. doi: 10.1227/01.neu.0000137628.51839.d5. [DOI] [PubMed] [Google Scholar]
- 6.Vergouwen MDI, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42:924–9. doi: 10.1161/STROKEAHA.110.597914. [DOI] [PubMed] [Google Scholar]
- 7.Choi K-S, Chun H-J, Yi H-J, Ko Y, Kim Y-S, Kim J-M. Seizures and epilepsy following aneurysmal subarachnoid hemorrhage: incidence and risk factors. J Korean Neurosurg Soc. 2009;46:93–8. doi: 10.3340/jkns.2009.46.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanzino G, D’Urso PI, Suarez J. Seizures and anticonvulsants after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15:247–56. doi: 10.1007/s12028-011-9584-x. [DOI] [PubMed] [Google Scholar]
- 9.De Marchis GM, Pugin D, Lantigua H, Zammit C, Tadi P, Schmidt JM, et al. Tonic-clonic activity at subarachnoid hemorrhage onset: impact on complications and outcome. PLoS ONE. 2013;8:e71405. doi: 10.1371/journal.pone.0071405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart R, Byer J, Slaughter J, Hewett J, Easton D. Occurrence and implications of seizures in subarachnoid hemorrhage due to ruptured intracranial aneurysms. Neurosurgery. 1981;8:417–21. doi: 10.1227/00006123-198104000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Riordan KC, Wingerchuk DM, Wellik KE, Zimmerman RS, Sirven JI, Noe KH, et al. Anticonvulsant drug therapy after aneurysmal subarachnoid hemorrhage: a critically appraised topic. Neurologist. 2010;16:397–9. doi: 10.1097/NRL.0b013e3181efc92f. [DOI] [PubMed] [Google Scholar]
- 12.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- 13.Lin C-L, Dumont AS, Lieu A-S, Yen C-P, Hwang S-L, Kwan A-L, et al. Characterization of perioperative seizures and epilepsy following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99:978–85. doi: 10.3171/jns.2003.99.6.0978. [DOI] [PubMed] [Google Scholar]
- 14.Raper DMS, Starke RM, Komotar RJ, Allan R, Connolly ES. Seizures after aneurysmal subarachnoid hemorrhage: a systematic review of outcomes. World Neurosurg. 2012;79:682–90. doi: 10.1016/j.wneu.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Hart Y, Sneade M, Birks J, Rischmiller J, Kerr R, Molyneux A. Epilepsy after subarachnoid hemorrhage: the frequency of seizures after clip occlusion or coil embolization of a ruptured cerebral aneurysm: results from the International Subarachnoid Aneurysm Trial. J Neurosurg. 2011;115:1159–68. doi: 10.3171/2011.6.JNS101836. [DOI] [PubMed] [Google Scholar]
- 16.Keränen T, Tapaninaho A, Hernesniemi J, Vapalahti M. Late epilepsy after aneurysm operations. Neurosurgery. 1985;17:897–900. doi: 10.1227/00006123-198512000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ukkola V, Heikkinen E. Epilepsy after operative treatment of ruptured cerebral aneurysms. Acta Neurochir (Wien) 1990;106:115–8. doi: 10.1007/BF01809452. [DOI] [PubMed] [Google Scholar]
- 18.Dennis LJ, Claassen J, Hirsch LJ, Emerson RG. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery. 2002;51:1136–44. doi: 10.1097/00006123-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Rhoney DH, Tipps LB, Murry KR, Basham MC, Michael DB, Coplin WM. Anticonvulsant prophylaxis and timing of seizures after aneurysmal subarachnoid hemorrhage. Neurology. 2000;55:258–65. doi: 10.1212/wnl.55.2.258. [DOI] [PubMed] [Google Scholar]
- 20.Hasan D, Schonck R, Avezaat C, Tanghe H, van Gijn J, van der Lugt P. Epileptic seizures after subarachnoid hemorrhage. Ann Neurol. 1993;33:286–91. doi: 10.1002/ana.410330310. [DOI] [PubMed] [Google Scholar]
- 21.Claassen J, Mayer S, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 22.Dreier JP, Major S, Pannek H-W, Woitzik J, Scheel M, Wiesenthal D, et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain. 2012;135:259–75. doi: 10.1093/brain/awr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claassen J, Hirsch LJ, Kreiter KT, Du EY, Connolly ES, Emerson R, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115:2699–710. doi: 10.1016/j.clinph.2004.06.017. [DOI] [PubMed] [Google Scholar]