Abstract
Hepatitis C virus (HCV) infection is a major global health problem that continues to grow with an estimated 170 million people infected. The consequences of chronic infection can include cirrhosis, end-stage liver disease and hepatocellular carcinoma. Due to shared routes of transmission, coinfection with HIV is a significant problem and individuals infected with both viruses have poorer outcomes. There is no effective vaccine though HCVis a potentially curable persistent viral infection. For many years, the standard of care has been subcutaneous interferon-alpha and oral ribavirin for between 24 and 72 weeks of treatment. This treatment results in a sustained virological response in only about 50% of individuals and is complicated by significant adverse events. In recent years, advances in HCV cell culture has allowed a greater understanding of HCV virology and this has paved the way for the development of many new directly acting antiviral drugs that target key components of virus replication such as protease and polymerase inhibitors. We are now at a point of improved and simplified treatments for HCV that may be administered as oral regimens of short duration and with far greater tolerability than regimens of old. The remaining hurdles may be access to appropriate care and cost of treatment as the epidemic continues to grow.
Introduction
First discovered in 1989, hepatitis C virus (HCV) is a major, global health problem affecting over 170 million people worldwide.1The problem continues to grow; globally the number of people who are seropositive for anti-HCV antibodies is estimated to have increased from 2.3% to 2.8% between 1990 and 2005.2 Central and East Asia along with North Africa and the Middle East are estimated to have the highest prevalence (>3.5%) with moderate prevalence in Eastern and Western Europe (1.5%-3.5%).2 The majority of subjects who become acutely infected (~80-85%) fail to clear the virus and progress to chronic infection. This figure may be higher in subjects who are coinfected with HIV and lower in women and children.3, 4 The consequences of chronic infection can be cirrhosis, portal hypertension, liver decompensation and the development of hepatocellular carcinoma (HCC) with HCV infection ultimately causing approximately 350,000 deaths per year.5 In regions of high endemicity, chronic viral hepatitis usually accounts for >50% of HCC and cirrhosis.6 Globally, 27% of cases of cirrhosis can be attributed to HCV and 25% of HCC is attributable to HCV infection. Aside from fatal consequences, individuals chronically infected with HCV have a decreased quality of life compared to the general population.7
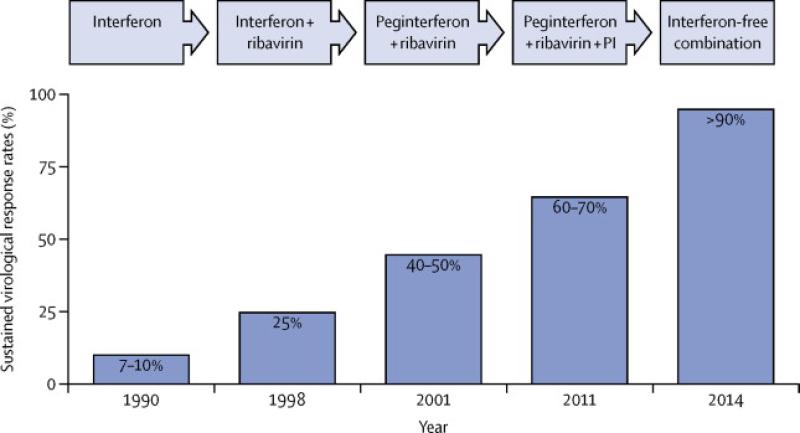
For many years, treatment for chronic HCV has been inadequate with success rates of treatment estimated at around 50%, depending on genotype. The standard-of-care until 2011 was a combination of pegylated interferon-alpha (PEG-IFN), administered subcutaneously and ribavirin (RBV) taken orally. This combination could lead to a sustained virological response (SVR) and, based on long-term follow up results, SVR means cure and chronic HCV has become the first chronic viral infection to be cured by medical therapy. However, such treatment is associated with significant adverse events and furthermore, poorly tolerated and less efficacious in subjects with advanced disease who are at most need.8 The introduction of direct acting antiviral drugs (DAAs), with two protease inhibitor drugs licensed in 2011, has improved treatment responses rates and heralded a new era of HCV treatment (figure 1).9-12 A pipeline of new DAAs are in various stages of pre-clinical and clinical development creating great optimism for the future of managing chronic HCV infection with simple, short, interferon-free, all oral regimens.13
Figure 1.
The evolution of the standard of care for HCV and improvements in sustained virological response rates. (Refs 9 to 12)
Virology
HCV is a positive single-stranded RNA virus in the Flaviridae family, within the genus Hepacivirus.The positive-sense RNA genome is 9600 nucleotides in length from which a single HCV polyprotein of 3011 amino acids is translated. This is subsequently cleaved by cellular and viral proteases into three structural proteins (core, E1 and E2) and seven non-structural (NS) proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B).14 For some time since its discovery in 1989 there were no close relatives identified in other animal species. However, in recent years there has been an explosion of data on the subject of non-primate hepaciviruses (NPHVs). Initially a related virus – canine hepacivirus - was identified as the cause of respiratory infection in dogs,15 a discovery closely followed by detection of a related NPHV in horses.16 Related viral sequences have now also been identified in rodents and bats, extending the diversity of the family enormously.17
HCV infections within human populations also show extreme genetic diversity. This is partly explained by the long evolutionary association between the virus and humans, likely several centuries if not longer.18 There are currently 7 established genotypes, although most work has focused on genotypes 1-6, which although showing global spread, have different geographical origins. Genotypes 1,2,4 and 5 are found as endemic infections in Africa, whilst genotypes 3 and 6 have evolved in Asia.19 During the last century, a number of medical interventions such as schistosomiasis eradication campaigns in Egypt have amplified specific strains to epidemic proportions and subsequently many of these have spread internationally.20 In the UK, genotype 3ais co-dominant with genotype 1, a feature which has implications for both vaccines and therapy (see below).
HCV infection also shows enormous diversity within infected hosts, existing in blood as a ‘swarm’ of related sequences or quasispecies. This diversity is a consequence of the error-prone viral polymerase and the high viral replication rate and allows for rapid adaptation to host antibody responses, cellular immune responses and many antiviral drugs. Recent analyses suggest within-host viral diversity may be even greater than had previously been estimated, likely due to hidden populations within the liver.21
For many years, it was not possible to grow HCV in tissue culture, but this is now possible following the discovery of a specific strain of HCV genotype 2 which was able to infect specific hepatoma cell lines and allow a full replication cycle.22 Culture of HCV has allowed study of entry, revealing a complex set of interactions with surface receptors, including CD81, SRB-1 (a scavenger receptor) and two tight junction proteins Occludin-1 and Claudin.23 Similarly these models have allowed critical insights into viral replication, and host-virus interactions.24 Tissue culture-derived virus has also recently facilitated imaging studies for analysis of mature virions by electron microscopy, revealing an unusually irregular structure.25 Most importantly, the ability to analyse HCV replication in tissue culture coupled with structural analysis of key proteins such as the NS3 protease and NS5b polymerase has driven the development of novel specific DAAs.26-28
Immunology
Immune responses to HCV play a critical role in determining the outcome of acute disease, and also play a more complex role in determining long-term disease progression. Acute responses to HCV include both innate and adaptive arms with clear evidence for both. Genetic evidence has implicated polymorphisms in the region of the IL28B (IFNL3) gene to strongly impact on spontaneous resolution of infection.29 IFNL3 is a lambda interferon, with sustained antiviral activity similar to that of interferon, but with a more restricted receptor distribution. Whether the polymorphisms identified influence the regulation of IFNL3 itself or impact on a nearby gene, termed IFNL4, remains to be defined.30 Similarly, genetic associations between genes in the KIR locus and acute resolution of infection point to a role for NK cell responses in viral control.31
Adaptive responses mediated by CD8+ and CD4+ T cells are also involved in acute host defence and there are strong associations reported with HLA Class II alleles in many studies, including a well-powered genome-wide association study.29 HLA Class I associations have been identified in single-source outbreaks, such as that seen in Irish women infected through a batch of Anti-D preparation. CD8+ T cell responses to peptides bound by HLA-A3 and HLA-B27 have been proposed as the mediators of such protection – these target regions of the virus where single mutations that allow immune escape also compromise viral fitness.32 Studies from infected chimpanzees indicate that both CD4+ and CD8+ T cell responses are required for full protection.33 These studies have prompted the development of T-cell based preventive vaccines: a regimen based on two recombinant vectors expressing HCV nonstructural genes – a novel adenovirus construct followed by a modified vaccinia Ankara (MVA) construct) - is now in Phase II trials in the USA.34
B cell responses to HCV leading to the generation of neutralising antibodies (NAbs), have also been intensely studied, although this is a complex area due to the extreme heterogeneity of key regions of the envelope genes – the major target for NAbs.35 The variability of regions such as the hypervariable regions (HVRs) of HCV E2 within-hosts is a consequence of antibody driven immune selection. Nevertheless, broadly cross-reactive Nabs have been described,36and further work to characterise these, especially in the context of a recently described HCV E2 crystal structure may lay the foundations for antibody-based vaccines.37
Epidemiology
HCV has long been recognized as a parenterally transmitted cause of viral hepatitis.38 Transmission via blood transfusion was a major route before the implementation of universal screening of blood in the developed world though this route remains a problem elsewhere.39 Where blood products and transplanted organs are now safe intravenous drug use (IDU) has become the major route of HCV transmission.40Epidemiological studies highlight transmission sources other than the sharing of contaminated needles, including the sharing of other drug paraphernalia such as foil and spoons.41 These findings are of vital importance in targeted prevention interventions.
Mother to child transmission (MTCT) of HCV has been less intensively studied than other chronic viral infections. The natural history of HCV in pregnancy and in infected infants born to these mothers is poorly understood. Consequently effective methods for prevention of HCV vertical transmission have not been developed. MTCT rates are estimated to be 2 to 8% in HCV monoinfected mothers but may be two to four times higher in those coinfected with HIV.42, 43There are currently no data from randomized controlled trials to support recommending caesarian section in this setting.44 The efficiency with which HCV is sexually transmitted has been controversial. However, in monogamous heterosexual couples where one partner has chronic HCV, the rate of transmission to a discordant partner is extremely low. Furthermore, HCV transmission is not associated with any particular sexual practice allowing for more unambiguous messages to be given to these couples.45
In the context of HIV infection, the epidemiology of HCV infection has changed dramatically in the last decade. Since 2000 there has been an ongoing epidemic of acute HCV infections in HIV positive men who have sex with men (MSM) that shows no sign of abating.46 There is evidence that transmission is permucosal rather than parenteral and is associated with certain sexual practices (fisting and group sex) and intranasal and intrarectal drug use.47 Sophisticated molecular epidemiological and phylogenetic studies in several European countries have identified several transmission clusters within MSM networks where sexual risks are implicated.46, 48, 49 Of particular concern in this group is the rate of reinfection after successful treatment or spontaneous clearance. As many as 25% of individuals treated for HCV will become re-infected within 2 years and these data highlight the need for effective sexual health education and preventative interventions targeted at this group.50 It further demonstrates that natural immunity is not adequate protection against a subsequent infection, even with the same genotype, and highlights the challenge in developing a prophylactic vaccine.
HIV/HCV Coinfection
HIV and HCV share routes of transmission and therefore coinfection with both viruses is a common problem, affecting an estimated 20-30% of the world's 34 million HIV-infected individuals.51 HCV-related liver disease has become a leading cause of morbidity and death in HIV patients in the era of HAART.50 The impact of HIV on the natural history of HCV is well-established and significant, affecting every aspect of the disease.51 Chronic HCV infection is more likely in HIV patients and this is associated with higher HCV viral loads.51 Coinfected patients demonstrate a faster progression to cirrhosis and end-stage liver disease though this may be attenuated by early HAART where available,52-54 and historically responded less well to interferon and ribavirin treatment regimens.55 However, attempting treatment remains important as achieving a sustained virological response dramatically reduces the incidence of liver-related morbidity and mortality in this population.56
Initiation of HAART in coinfected patients is associated with a higher risk of hepatotoxicity. However, it is felt that this is outweighed by the potential benefits of immune restoration that might lessen disease progression and thus, initiation of HAART is generally recommended early in these patients.57 Both HIV and HCV are infections associated with disorders of multiple systems. Alongside, the deleterious effect of HIV on HCV-related liver pathology, patients who have HIV-HCV coinfection have higher rates of HIV-related kidney disease,58 more global neurocognitive dysfunction,59 and the prevalence of cardiovascular disease and bone disease is higher.60-62
Diagnosis
The diagnosis of HCV relies on the detection of antibody to the virus and nucleic acid amplification tests to detect HCV RNA. Antibody tests were first approved by the US Food and Drug Administration (FDA) in 1990 and have evolved considerably since this time.63 HCV RNA is detected early in infection (~2 weeks) and will be followed by antibody seroconversion days to weeks later (~6 weeks), though the development of detectable antibody can be delayed or not occur at all in the immunocompromised such as HIV positive.64 Anti-HCV antibodies are detected by an enzyme immunoassay (EIA) or chemiluminescence immunoassay (CIA) and these are the first screening tests.63 The presence of anti-HCV antibody in the absence of detectable RNA indicates old spontaneously resolved or treated infection and, in the presence of RNA, indicates a current HCV infection. Therefore, all antibody tests that are repeatedly positive, should be assessed by a sensitive test for HCV RNA. In acute infection, RNA may be present without antibody and in immunocompromised patients (such as those with HIV) with abnormal liver function, tests to detect HCV RNA may be a more appropriate diagnostic test.
The different HCV genotypes respond with different success rates to treatment with interferon and ribavirin.11, 12 Additionally, the genotype determines the duration of therapy necessary to achieve SVR.65 Therefore, accurate genotyping of chronic infections is vital. Early genotyping was performed using assays that either determined the specificity of the antibody present or by hybridisation of amplified viral RNA from highly conserved regions of the virus. However, it is becoming apparent that the sehybridisation assays may not be specific enough and that more accurate results may be obtained from molecular sequence data for example, sequencing both C/E1 and NS5B regions (or in future whole genomes using next generation sequencing approaches) and establishing phylogenetic relatedness.66, 67
On treatment, the measurement and quantification of HCV RNA at predetermined timepoints may allow for the shortening of treatment regimens with first generation protease inhibitors (response-guided therapy) or determine that it is futile to continue with treatment due to a lack of response.68 Assays have become more sensitive and the lower limits of detection and sensitivity of quantification of HCV RNA has improvedwith consequences for response guided therapy and stopping rules. Resistance testing may become more important than previously. It is therefore important that treating clinicians remain familiar with updates in diagnostic technology and liaise closely with laboratory colleagues.68
Acute HCV
Studies on the natural history of early HCV infection have been limited due to the asymptomatic nature of the majority of acute HCV infections.69 Our understanding is improving following the study of acute cases in patients with HIV and in animal models of infections conducted in chimpanzees. 10 to 14 weeks after infection, an acute hepatitis with a corresponding increase in liver transaminase enzymes occurs.70 A characteristic that has been observed in both human and chimpanzee infection is an early peak in HCV viral RNA load followed by a dip.69 It is conservatively estimated that 15 to 20% of subjects will clear acute infection and in these, this downward trajectory in HCV viral load continues whereas chronic infection is associated with a recrudescence of viraemia.3, 69, 71 Multiple factors have been shown to be associated with spontaneous clearance of HCV infection including gender, IL28B polymorphisms, ALT levels or presence of jaundice, rate of decline in HCV-RNA, and blood IP-10 levels.72 Attempts have been made to establish scoring systems that might reliably discriminate those patients with high potential for spontaneous clearance from those that should be treated early.72 There is a balance between treating too early in patients who may go on to clear infection and delaying treatment which will result in reduced treatment efficacy. A positive HCV RNA 12 weeks into the course of acute HCV infection has been established as a helpful transition from acute into chronic infection and might help guide early treatment decisions.73
In subjects not clearing the virus, early treatment has been shown to be more effective than delayed treatment in subjects both with and without HIV. In the context of HIV infection, treatment within 3 to 6 months is recommended, where possible, by European guidelines and this is associated with improved treatment outcomes.74 The optimal treatment regimen for acute HCV infection with PEG-IFN in HIV has not been established but 24 or 48 weeks of PEG-IFN with RBV is recommended depending on the early viral kinetics.73 Treatment success rates ranging from 65 to 85% have been reported in this setting.75, 76 By contrast, in acute HCV in HIV-negative individuals, interferon-alpha, standard or pegylated, alone for up to 24 weeks may be sufficient to effect a cure in up to 98% of subjects.77, 78 Optimal treatment regimes using DAAs have not yet been defined in this context.
Natural history
HCV infection has a propensity to cause chronic hepatitis which may lead to cirrhosis, decompensated cirrhosis and HCC. The onset and accumulation of hepatic fibrosis is clinically silent in the early stages of disease, and it therefore remains difficult to accurately identify progression of the disease to cirrhosis in patient.79, 80 Annual rates of progression of hepatic fibrosis from minimal disease to cirrhosis have been modelled and estimated. The prevalence of biopsy proven cirrhosis after 20 years of infection has varied between 7% (in retrospective studies) to 18% (in clinical referred settings). The risk of cirrhosis is increased in individuals abusing alcohol, in those who acquire the disease at an older age, by concomitant obesity, in men, and in immunosuppressed HIV positive patients or in recurrent HCV following liver transplantation.81, 82
Patients with minimal fibrosis have a low risk of developing complications of liver disease over the ensuing two decades. Patients with bridging fibrosis or cirrhosis, conversely have a higher risk. It may be necessary to repeat liver biopsies in patients to determine progression. Alternatively and more practically, non-invasive blood tests, fibroelastography and hepatic imaging can be used to identify patients with advanced fibrosis to gauge indications for immediate or deferred treatment.83Extrahepatic manifestations of HCV such as cryoglobulinaemia, or HCV-associated splenic lymphoma are also indications for antiviral therapy. Treatment will reduce infectivity and transmission in individuals using intravenous drugs.
Treatment
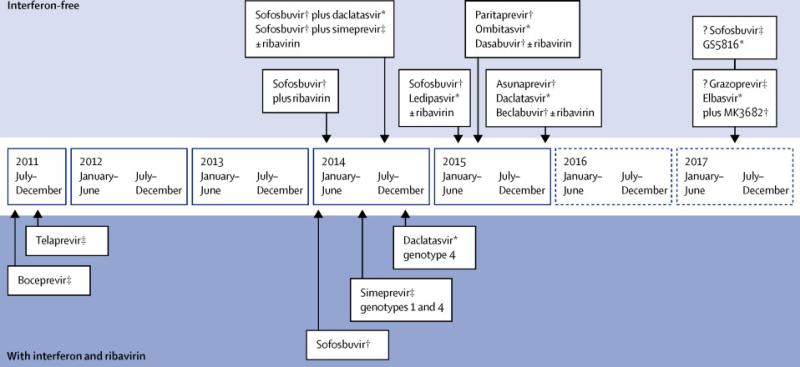
The primary goal of treatment for chronic HCV is cure, and thus prevention of progression of the disease. A sustained virological response (SVR), i.e HCV RNA < 15 iu/ml 12-24 weeks after completion of antiviral therapy is associated with an improvement in both all-cause and liver-related mortality from HCV.84, 85 PEG-IFN and RBV was previously the mainstay of treatment for all genotypes of HCV, given for up to 48 weeks, but is being rapidly superseded by the advent of direct acting antiviral agents (DAAs) (Fig. 2). There is no prophylactic vaccine but several are in development and early stage clinical trials.14
Figure 2.
The treatment of HCV in 2015 (including approved or imminently approved protease, NS5b and NS5a inhibitors).
¥ protease inhibitor *NS5a inhibitor # NS5b inhibitor
Indications for treatment
Patients with cirrhosis are at more immediate threat of complications of liver disease. Unfortunately current IFN-based treatment response rates are lower in patients with cirrhosis, and thus suboptimal, albeit they are improved by the addition of first generation protease inhibitors. Furthermore the potential risks of adverse events are greater. Recent data suggest that response rates in patients with cirrhosis can be improved by DAA regimens without IFN. Treatment may also given to patients to prevent the development of advanced fibrosis, and cirrhosis, or for extrahepatic symptoms, and to prevent transmission of the infection. The high costs of current regimens may require stratification of patients for treatment.
Current treatment genotype 1
First generation protease inhibitors (PI) improve response rates in patients with genotype 1 infection. Telaprevir and boceprevir are inhibitors of the NS3/4a HCV protease. SVR rates are improved in both treatment-naïve and treatment-experienced patients treated with a combination of telaprevir or boceprevir plus PEG-IFN and RBV.11, 12 Approximately 50 -60% of PI recipients also qualify for a reduced (six months) duration of treatment, based on achieving a rapid virological response. Lower response rates have been observed in patients with cirrhosis and those with a prior null response to PEG-IFN and RBV, i.e. those with a < 2 log10 IU/ml decline in HCV RNA by treatment week 12. Also, patients with cirrhosis require a longer duration (48 weeks) of PEG-IFN and RBV.86 Treatment with first generation PIs can be complex and cause considerable adverse events. The safety profile of prolonged IFN and first generation DAA treatment in patients with advanced cirrhosis is poor.87
A single nucleotide polymorphism upstream of the IL28B gene influences response to PEG-IFN and RBV. Higher response rates have been reported in patients inheriting the IL28B rs12979860 CC genotype.88 Several drug-drug interactions can occur.89 Resistance associated viral variants, with substitutions located in the catalytic site of the NS3 protease, have been described following telaprevir and boceprevir treatment. Stopping rules to avoid the acquisition of more complex mutations are recommended.90-92
The most common side effects of telaprevir are anaemia, pruritis, nausea, diarrhoea and anorectal discomfort. About 4% of patients develop a severe dermatitis, necessitating cessation of treatment. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS syndrome) or Stevens Johnson syndrome are rare, but reported. Boceprevir causes dysguesia and anaemia. The use of PIs is constrained in patients with decompensated cirrhosis and post-transplant HCV infection because of the risk of severe adverse effects of the IFN backbone.93
Interferon-sparing regimens with new agents for genotype 1
Treatment for genotype 1 HCV is evolving rapidly. Key viral replication targets have been identified, namely the HCV protease, NS5a protein and the NS5b RNA polymerase. In 2014, several potent antiviral inhibitors with once daily dosing and, because of improved potency, a shorter duration of treatment with PEG-IFN and RBV have been licenced. These IFN-“sparing” regimens include the addition of simeprevir (second generation PI), daclatasvir (a NS5a inhibitor) and sofosbuvir (a uridinenucleotide prodrugNS5b polymerase inhibitor) in combination with PEG-IFN and RBV for 12 to 24 weeks.94-97 High response rates have been observed in naïve genotype 1 patients: up to 90%.
Simeprevir 150 mg daily for 12 weeks with PEG-IFN and RBV for 24 weeks resulted in SVR rates in treatment naïve genotype 1 patients of 80% and 81% in the QUEST-1 and QUEST-2 studies respectively.98, 99 A rapid virological response occurs in 85% of whom 91% subsequently achieve an SVR at 12 weeks. Lower response rates were observed in patients with F3-F4 fibrosis. A Q80K mutation detectable at baseline in the NS3sequence impairs the response to simeprevir. Simeprevir with PEG-IFN and RBV resulted in SVR rates in 79% of prior relapsers compared to 36% of PEG-IFN retreated controls.100
Sofosbuvir together with PEG-IFN and RBV has been given for 12 weeks in treatment-naïve patients with genotypes 1,4,5 and 6 in the ATOMIC and NEUTRINO studies. SVR rates of 89% (genotype 1) and 82% (genotype 4) were reported in ATOMIC and 89% and 100% for genotype 1 and 4 respectively in NEUTRINO.97, 101 Smaller numbers of patients with genotype 5 and 6 were treated. Prior non-responders were not included in these clinical trials and only a limited number of patients with cirrhosis were included.
In a dose finding study 332 patients were treated MK-5172 (100, 200, 400, or 800 mg) once daily for 12 weeks together with PEG IFN and RBV. A control group received boceprevirplus PEG IFN and ribavirin. SVR rates of 89% to 91% were observed in patients given MK-5172 versus 61% of controls.Transient increases in serum aminotransferase concentrations occurred in the MK-5172 groups given 400 and 800 mg, suggesting that 100 mg is the safe dose of the protease inhibitor for utilisation in interferon free regimens.102
Interferon-free regimens for genotype 1
It is likely that IFN-sparing regimens will be displaced in 2015 by the introduction of IFN-free regimens with improved efficacy and tolerability. These regimens will comprise a protease inhibitor or a NS5a inhibitor plus a nucleoside NS5b inhibitor ± RBV; a protease inhibitor plus a NS5a inhibitor plus a NS5b non-nucleoside inhibitor ± RBV; or a protease inhibitor plus a NS5a inhibitor ± RBV.103 Updated guidelines have been published by the European Association for the Study of the Liver (EASL) and the American Association (AASLD). Sofosbuvircan also be used in combination with RBV in patients with genotype 1 infection who are intolerant to IFN, particularly in non-cirrhotic patients, and up to 72% response rates can be achieved.104
Both sofosbuvir and daclatasvir,105 or sofusbivir plus ledipasvir (without RBV) are highly effective regimens for genotype 1 naïve and prior IFN non-responders including telaprevir and boceprevir non-responder patients.103 SVR rates of 97% in treatment naïve patients, (ION-1),106 93% of treatment experienced patients (ION-2) treated with sofosbuvir and ledipasvir without ribavirin for 12 weeks and 94% of genotype 1 naïve patients treated for 8 weeks have been reported;107 the first two studies have included 15-20% of patients with cirrhosis. Anaemia was rare in the ribavirin-free arms. Clinical resistance is extremely rare, although a S282T mutation in the replicon model confers resistance to sofosbuvir.108 Although the numbers of patients are relatively small, a subgroup analysis indicated a SVR rate of 22/22 patients treated for 24 weeks versus 86% treated for 12 weeks (19/22). However, NS5a resistant variants are detected in patients who relapse after sofosubuvir in combination with a NS5a inhibitor; it is not clear whether these patients will respond to retreatment for longer period with the same regimen.
The current EMA recommendation for sofosbuvir + ledispavir (HarvoniR) for patients without cirrhosis is: for naïve patients 8 weeks in genotype 1 and 4; 24 weeks should be considered for previously treated patients with uncertain subsequent retreatment options. For patients with cirrhosis: Patients with compensated cirrhosis: 24 weeks, but 12 weeks may be considered for patients deemed at low risk for clinical disease progression and who have subsequent retreatment options. For patients with decompensated cirrhosis or who are pre or post-liver transplant: 24 weeks plus ribavirin. However the combination of sofosbuvir and ledispasvir plus RBV for 12 versus sofosbuvir plus ledispavir 24 weeks was equally efficacious in patients with well compensated cirrhosis (CPT class A): 96% and 97% SVR respectively.109
Further studies are examining the efficacy of sofosbuvir in combination with either ledipasvir and a non-nucleoside HCV NS5B inhibitor, (GS 9669) 500 mg per day or a protease inhibitor (GS 9451) 80 mg per day in naïve non-cirrhotic patients and further shortening of treatment to as little as four or six weeks.110 Phase 3 studies examining the efficacy of sofosbuvir and GS-5881- a next generation NS5a inhibitor are in progress based on the pangenotypic efficacy of this combination in preliminary studies.111
Other demonstrably effective, short duration, oral combinations include ritonavir-boosted ABT-450 (paritaprevir, a PI) (150 mg/100 mg) co-formulated together with ABT-267 25 mg once daily (an NS5a inhibitor, ombitasvir) and ABT-333 250 mg twice daily (dasabuvir, a nonnucleoside NS5b polymerase inhibitor) with RBV (weight-based). (SAPPHIRE I). Ninety-six percent of naïve genotype 1 patients responded.112 One hundred of 172 patients untreated and prior non responders with HCV genotype 1 infection and compensated cirrhosis given 24 weeks of treatment with the same regimen had a SVR: 95.9% (TURQUOISE-II)113 Virological failure was thus rare, but in this and other multiple drug regimens, the emergence of NS3, NS5a and NS5b resistance-associated variants will require an appropriate salvage therapy. In the SAPPHIRE-II study, 96% of non-cirrhotic prior non-responders to PEGIFN and RBV, including 49% who were prior null responders achieved an SVR.114 Ninety-one and 98% of patients with compensated cirrhosis responded to the same regimen given for 12 or 24 weeks respectively.113 Response rates were improved in 1a patients with a prior null response given for 24 weeks versus 12 weeks (80% versus 92%) These studies have also demonstrated that ribavirin need only be used if required, and is not advantageous in naïve non-cirrhotic 1b patients. In the (PEARL-III) study419 patients with genotype 1b infection, and 305 patients with genotype 1a infection (PEARL-IV) were given 12 weeks of ABT-450/r–ombitasvir, dasabuvir (250 mg twice daily), and ribavirin or placebo for ribavirin. SVR rates in 1b infection were 99.5% with ribavirin and 99.0% without ribavirin; in patients with 1a the SVR rates were 97.0% and 90.2%, respectively.115 A longer duration of treatment (24 weeks) with ribavirin is advisable for 1a patients with cirrhosis and a prior non response or other adverse factors. PEARL-II evaluated the efficacy of 12 weeks of treatment with the same regimen with orwithout RBV in non-cirrhotic pegIFNRBVtreatment-experienced HCV genotype 1b patients. The response rates with or without RBV were 96% and 100%.116 Licencing information is awaited.
Sofosbuvir 400 mg qd plus simeprevir 150 mg qd have been assessed in cirrhotic and noncirrhotic naïve and prior non-responder patients; In COSMOS two cohorts were studied; F0-F2 null-responders and F3-F4 naïve or null-responders.117 In the cohort of prior null-responders with early stage fibrosis, 92% of patients treated without RBV had an SVR. In cohort 2 (naive and prior null-responders with metavir F3-F4 fibrosis), 96% of patients responded.There is no apparent need for RBV with this combination of a PI and NS5b polymerase inhibitor. Prior Q80K mutations in patients with subtype 1a had little impact on treatment. Anaemia and bilirubin elevations were more common in the RBV arms. Data now obtained from large observational databases have also confirmed the efficacy of simeprevir and sofosbuvir in genotype 1 patients (TARGET and TRIO cohorts). 118, 119 Response rates of 80-94% have been observed utilising the combination of sofosbuvir and simeprevir ± RBV in treatment experienced patients, although response rates can be 10-15% lower in patients with cirrhosis. Daclatasvir and asunaprevir (a PI) is effective in patients with subtype 1b infection (HALLMARK DUAL).120
New DAA regimens including sofosbuvir plus GS5816 100mg for 12 weeks, without ribavirin,121 MK5172 (grazoprevir) and MK8742 (elbasvir) ± RBV 122 or asunaprevir, daclatasvir and BMS-791325 The combination of daclatasvir 30 mg bd, asunaprevir 200 mg bd and BMS-791325 (Beclabuvir, a non-nucleoside NS5b polymerase inhibitor) ± RBVfor 12 weeks in treatment-naïve patients with or without compensated cirrhosis, are similarly encouraging and result in cures in more than 87-93% 123,124
Lower rates of cure can occur with some regimens in subgroups particularly treatment experienced patients with cirrhosis. The need for ribavirin for subgroups treated for shorter periods is being assessed.
The dose of sofosbuvir in patients with eGRF< 30 ml /minute/1.73m2 is not yet established. No dose adjustment of daclatasvir is required for renal or hepatic impairment. The results obtained in phase 3 studies portend the advent of IFN-free regimens in 2014 for naïve and prior non-responder patients, including those with resistance to telaprevir and boceprevir and patients with cirrhosis. Refinements of treatment for decompensated cirrhosis will be ascertained in further phase 3 trials. Ultrashort regimens of four to six weeks are being studied with several combinations of next generation protease inhibitors, NS5a inhibitors and polymerase inhibitors. Appropriate IFN-free regimens may overcome the biological ineffectiveness of IFN in advanced liver disease.
These interferon free regimens are remarkably effective in patients with genotype 1 infection. Further analysis is required, but baseline factors including baseline RAVs, unfavourable IL28b genotype, viral load, subtype and cirrhosis can influence response with potent multiple DAA regimens. Longer durations of treatment (24 weeks) could be required to optimise response rates in patients with more advanced liver disease. Other factors acting in concert such as baseline viral load or baseline NS5a mutations could affect response to NS5a inhibitors although their detection does not preclude a response. In a small percentage of patients multiple-drug resistant viruses will be encountered after treatment failure or a relapse.125
Treatment for genotypes 2 to 6
PEG-IFN and RBV are effective against genotypes 2-6. Forty-eight weeks of PEG-IFN and RBV are generally required for genotypes 4, 5 and 6, although rapid virological responders may be successfully treated for 24 weeks. Twenty-four weeks of PEG-IFN/RBV are given to patients with genotype 2 and 3. SVR rates are highest in patients with genotype 2 (85-90%). SVR rates of between 43 and 70% have been recorded in patients with genotype 4 treated with PEG-IFN and ribavirin. Genotype 4 patients with low baseline viral concentrations and a rapid virological response can be treated for 24 weeks. SVR rates of 60-85% occur in genotype 6.126 It has become apparent that patients with genotype 3 infection and cirrhosis have higher relapse and thus lower response rates.
Interferon-sparing regimens
Daclatasvir has been administered with PEG-IFN and RBV to naïve patients with genotype 2 or 3 infection. SVR rates of approximately 83% in genotype 2 and 70% in genotype 3 have been reported.127 Similar encouraging results were reported in genotype 4 patients.128 Simeprevir, a second wave protease inhibitor, is active against genotype 4, particularly in treatment-naïve and relapsed patients.129 As noted above, sofosbuvir administered with 12 weeks of PEG-IFN and RBV is active against all genotypes. In the LONESTAR-2 study, sofosbuvir given in combination with PEG-IFN and RBV for 12 weeks to treatment-experienced genotype 2 or 3 patients resulting in SVR rates in 96% in genotype 2 and 83% in genotype 3 patients. Although the numbers were small, cirrhosis did not affect the response.130 Presently, genotype 3 treatment-experienced patients with cirrhosis may require 12 weeks PEG-IFN sofosbuvirand RBV plus to attain the highest response rates.
Interferon-free regimens for genotype 2 and 3
A 12-week combination of sofosbuvir and ribavirin is highly efficacious (97%) in genotypes 2, but a longer treatment period of 24 weeks is required for patients with genotype 3. In the POSITRON and FUSION studies a lower proportion of patients with genotype 3 responded to 12 weeks of therapy, and in these genotype 3 patients, responses were lower among those with cirrhosis than among those without cirrhosis.131 Response rates are similar in patients with genotype 2 irrespective of the presence of cirrhosis, but are reduced to 62% in experienced patients with genotype 3 and cirrhosis treated with sofosbuvir and ribavirin for 24 weeks.132 However, 94% SVR rates occurred in naïve, non-cirrhotic genotype 3 patients treated for 24 weeks.132 Virological failures are usually due to relapse with wild-type HCV, and discontinuations for drug-related adverse events have been rare. SVR rates of 94-100% were reported with the combination of daclatasvir plus sofosbuvir in treatment-naive patients infected with genotypes 2 or 3. In the ALLY-3 study, in which treatment-naïve and treatment-experienced genotype 3 patients were treated for 12 weeks with sofosbuvir 400 mg + daclatasvir 60 mg (without RBV) for 12 weeks 92/101 (91%) of naïve patients had an SVR, versus 44/51 (86%) of experienced patients. Overall 105/109 (96%) of non cirrhotic patients responded versus (20/32, 63% of those with cirrhosis.133 The current EMA posology recommends sofosbuvir plus daclatasvir + RBV for 24 weeks for genotype 3 patients with compensated cirrhosis and/or the treatment-experienced 104 Preliminary results of a 12 week combination of sofosbuvir and ledipasvir for patients with genotype 3 showed a lower SVR rate (16/25, 64%) versus 26/26 of those treated with sofosbuvir, ledispavir and RBV.134 The current EMA posology for patients with genotype 3 with cirrhosis and/or prior treatment failureis 24 weeks plus ribavirin.
Sofosbuvir plus GS-5816 and other combinations including MK5172 plus MK874 (or next generation NS5a inhibitors) plus next generation polymerase inhibitors are being studied. High rates of response in genotype 3 patients without cirrhosis have been reported,using 100 mg of GS5816. Lower rates were reported (88%) in genotype 3 treatment-experienced patients with cirrhosis if ribavirin was not used.121. SVR rates of > 90% have been reported for genotype 4 patients withsofosbuvir and ribavirin treated for 24 weeks. By extrapolation the combination of sofosbuvir and simeprevir or daclatasvir should be active against genotype 4.135 A 12-week dual regimen of ABT450/r + ombitasvir, ± RBV in treatment-naive patients (treatment-experienced patients all received RBV) showed excellent response rates ref Hezode C, et al. EASL 2014, London, O58, as did all-oral therapy with daclatasvir plus asunaprevir + BMS-791325 for treatment-naive patients with chronic HCV G4 infection.136
HIV/HCV coinfected patients
Telaprevir and boceprevir have both been assessed in chronic coinfected patients.137-140 Although promising SVR rates have been obtained, drug-drug interactions can be problematic. Telaprevir can be given for 12 weeks in combination with PEG-IFN and RBV to treat acute genotype 1 co-infection.141 Several promising IFN-sparing as well as IFN-free regimens have been tested in HIV/HCV coinfected patients.137 These include the combination of simeprevir together with PEG IFN and RBV, sofosbuvir plus RBV, sofosbuvir plus ledispasvir, for 12 wks in HIV/HCV-coinfected patients,142 or MK5172 plus MK8742 with or without RBV in the C-WORTHY study.143 In the PHOTON study, coinfected genotype 1, 2 and 3 treatment-naïve patients were treated with sofobuvir 400 mg daily and ribavirin for 24 weeks (genotype 1) and 12 weeks (genotype 2 and 3).144 Multiple antiretroviral therapies were permitted as sofosbuvir is cleared by renal elimination. Seventy-six percent of 114 patients with genotype 1 (treated for 24 weeks), 88% of 26 patients with genotype 2 and 67% of 42 with genotype 3 achieved SVR12 (after treatment for 12 weeks in the latter groups). Amongst the prior non-responder patients, 92% with genotype 2 and 94% of 17 genotype 3 achieved SVR12 after 24 weeks of treatment. It appears fortunately that patients who are coinfected with HIV andHCV have similar outcomes as those with HCV monoinfection.145 However, appropriate dose modifications will be required in coinfected patients. Efavirenz, etravirine and nevaripine are not recommended with daclatasvir, simeprevir or sofosbuvir. The dose of daclatasvir should be reduced to 30 mg from 60 mg if atazanavir/r is used but increased to 90 mg if dosed together with efavirenz, nevirapine or etravirine. Coadminstration of atazanavir/r, lopinavir/r or darunavir/r with simeprevir is not recommended.
Patients with decompensated cirrhosis and pre- and post-liver transplant patients
Patients with decompensated cirrhosis are not candidates for interferon therapy. A preliminary report of the use of pre-transplant sofosbuvir and RBV for up to 48 weeks, stopping on day of transplant for patients transplanted for HCV and HCC (within Milan criteria), has resulted in a 64% post-transplant SVR rate. The duration of undetectable HCV RNA pre-transplant was the best predictor of response.146 Several clinical trials are in progress. Ledispavir and sofosbuvir plus ribavirin given for 12 or 24 weeks in patients with Childs Pugh Turcotte (CPT) class B and C cirrhosis suggest that 86-90% of genotype 1 or 4 naive or experienced patients respond. An improvement in the MELD score has been observed. 147 Definitive guidance on the optimal duration of therapy in this group is critical.
Telaprevir and boceprevir have been utilised to treat post-transplant recurrent HCV.148 Preliminary reports indicated that higher SVR rates than with PEG-IFN and RBV occur. However, toxicity, particularly anaemia and sepsis, and drug-drug interactions with the calcineurin inhibitors complicate treatment.149 Fortunately, these treatments are being to be displaced by better tolerated and more effective DAA therapies. Sofosbuvir and ribavirin have been used for the treatment of recurrent post-transplant hepatitis C (all genotypes). Virological response rates of 77% after 24 weeks of treatment have been reported.150 These landmark treatments,have already been improved upon. High response rates (>90% in patients with CPT A cirrhosis have been observed with sofosbuvir and ledispavir151 or sofosbuvir plus simeprevir ± RBV.152 Excellent post-transplant SVR results were observed in a small group of gentotype 1 patients without advanced fibrosis treated with. ombitasvir/paritaprevir/ritonavir + dasabuvir. The combination plus ribavirin lead to a 7 fold and 3 fold increase in tacrolimus and cyclosporin half life but the immunosuppressive therapy is manageable although requiring dosing of tacrolimus 0.5 to 1.0 mg at one to two week intervals. 153
Conclusion
Improved, efficacious and simplified, interferon free, and for most, ribavirin free treatments for hepatitis C are now available. Detailed guidelines which will be updated at frequent intervals have being published for genotypes 1-6, and subcategories of patients. Simple all-oral regimens of short duration have now become a reality. Treatment could be expanded into groups for whom interferon was not tolerated. Emerging evidence that patients on stable opiod replacement therapy are good candidates for DAA regimens. Treatment algorithms may still be necessary with sofosbuvir and ledispavir, or the AbbVie three DAA regimen, or sofosbuvir and daclatasvir for exampleto attain the highest chance of an SVR; (a slower primary response, may perhaps indicate a need to extend the duration of therapy and may be necessary particularly in treatment experienced patients with advanced cirrhosis).154
However the next generation of DAA treatments are costly drugs. Meeting the demand for therapy of a numerically common disease with these breakthrough therapies is concerning for policy makers because of the immediate budgetary impact. The cost may limit access, thus limiting societal benefit. Stratification and prioritisation of patients based on cost-effectiveness, stage of disease, and the potential gain from treatment, may be required. Prices may decrease as several effective drugs offering a high cure are licenced. However, the epidemic continues to grow. A major hurdle currently is the identification and appropriate referral of people in need of treatment and widespread delivery in primary care. Treatment will form part of the control of the disease; however, successful treatment of an infection has never led to its eradication. The search for an effective prophylactic vaccine must continue and advances in molecular vaccinology are paving the way for progress in this era.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Search strategy and selection criteria
We searched MEDLINE and PUBMED databases using the search terms HCV and hepatitis C virus as well as epidemiology, clinical manifestation, virology, diagnosis, biopsy, treatment, drugs, immunology or vaccines. We selected publications mostly from the past 5 years, but did not exclude commonly referenced and highly regarded older publications.
REFERENCES
- 1.Szabo E, Lotz G, Paska C, Kiss A, Schaff Z. Viral hepatitis: new data on hepatitis C infection. Pathology oncology research : POR. 2003;9(4):215–21. doi: 10.1007/BF02893380. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.Thomson EC, Fleming VM, Main J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2011;60(6):837–45. doi: 10.1136/gut.2010.217166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nature reviews Gastroenterology & hepatology. 2013;10(9):553–62. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 5.Zaltron S, Spinetti A, Biasi L, Baiguera C, Castelli F. Chronic HCV infection: epidemiological and clinical relevance. BMC infectious diseases. 2012;12(Suppl 2):S2. doi: 10.1186/1471-2334-12-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of hepatology. 2006;45(4):529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Bezemer G, Van Gool AR, Verheij-Hart E, et al. Long-term effects of treatment and response in patients with chronic hepatitis C on quality of life. An international, multicenter, randomized, controlled study. BMC gastroenterology. 2012;12:11. doi: 10.1186/1471-230X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. The New England journal of medicine. 2002;347(13):975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 9.Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352(9138):1426–32. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 10.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 11.Poordad F, McCone J, Jr., Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. The New England journal of medicine. 2011;364(13):1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. The New England journal of medicine. 2011;364(25):2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 13.Asselah T, Marcellin P. Interferon free therapy with direct acting antivirals for HCV. Liver international : official journal of the International Association for the Study of the Liver. 2013;33(Suppl 1):93–104. doi: 10.1111/liv.12076. [DOI] [PubMed] [Google Scholar]
- 14.Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C virus: closing in on an evasive target. Expert review of vaccines. 2011;10(5):659–72. doi: 10.1586/erv.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor A, Simmonds P, Gerold G, et al. Characterization of a canine homolog of hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(28):11608–13. doi: 10.1073/pnas.1101794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons S, Kapoor A, Sharp C, et al. Nonprimate hepaciviruses in domestic horses, United kingdom. Emerging infectious diseases. 2012;18(12):1976–82. doi: 10.3201/eid1812.120498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor A, Simmonds P, Scheel TK, et al. Identification of rodent homologs of hepatitis C virus and pegiviruses. mBio. 2013;4(2):e00216–13. doi: 10.1128/mBio.00216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pybus OG, Barnes E, Taggart R, et al. Genetic history of hepatitis C virus in East Asia. Journal of virology. 2009;83(2):1071–82. doi: 10.1128/JVI.01501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. The Journal of general virology. 2004;85(Pt 11):3173–88. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 20.Pybus OG, Charleston MA, Gupta S, Rambaut A, Holmes EC, Harvey PH. The epidemic behavior of the hepatitis C virus. Science. 2001;292(5525):2323–5. doi: 10.1126/science.1058321. [DOI] [PubMed] [Google Scholar]
- 21.Gray RR, Salemi M, Klenerman P, Pybus OG. A new evolutionary model for hepatitis C virus chronic infection. PLoS pathogens. 2012;8(5):e1002656. doi: 10.1371/journal.ppat.1002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakita T, Pietschmann T, Kato T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nature medicine. 2005;11(7):791–6. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith LW, Wilson GK, Fletcher NF, McKeating JA. Hepatitis C virus entry: beyond receptors. Reviews in medical virology. 2012;22(3):182–93. doi: 10.1002/rmv.723. [DOI] [PubMed] [Google Scholar]
- 24.Shulla A, Randall G. Hepatitis C virus-host interactions, replication, and viral assembly. Current opinion in virology. 2012;2(6):725–32. doi: 10.1016/j.coviro.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catanese MT, Uryu K, Kopp M, et al. Ultrastructural analysis of hepatitis C virus particles. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(23):9505–10. doi: 10.1073/pnas.1307527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JL, Morgenstern KA, Lin C, et al. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell. 1996;87(2):343–55. doi: 10.1016/s0092-8674(00)81351-3. [DOI] [PubMed] [Google Scholar]
- 27.Love RA, Parge HE, Wickersham JA, et al. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell. 1996;87(2):331–42. doi: 10.1016/s0092-8674(00)81350-1. [DOI] [PubMed] [Google Scholar]
- 28.Lesburg CA, Cable MB, Ferrari E, Hong Z, Mannarino AF, Weber PC. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nature structural biology. 1999;6(10):937–43. doi: 10.1038/13305. [DOI] [PubMed] [Google Scholar]
- 29.Duggal P, Thio CL, Wojcik GL, et al. Genome-wide association study of spontaneous resolution of hepatitis C virus infection: data from multiple cohorts. Annals of internal medicine. 2013;158(4):235–45. doi: 10.7326/0003-4819-158-4-201302190-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nature genetics. 2013;45(2):164–71. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 32.Fitzmaurice K, Petrovic D, Ramamurthy N, et al. Molecular footprints reveal the impact of the protective HLA-A*03 allele in hepatitis C virus infection. Gut. 2011;60(11):1563–71. doi: 10.1136/gut.2010.228403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. The Journal of experimental medicine. 2003;197(12):1645–55. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes E, Folgori A, Capone S, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Science translational medicine. 2012;4(115):115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wahid A, Dubuisson J. Virus-neutralizing antibodies to hepatitis C virus. Journal of viral hepatitis. 2013;20(6):369–76. doi: 10.1111/jvh.12094. [DOI] [PubMed] [Google Scholar]
- 36.Giang E, Dorner M, Prentoe JC, et al. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(16):6205–10. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong L, Giang E, Nieusma T, et al. Structure of hepatitis C virus envelope glycoprotein E2 antigenic site 412 to 423 in complex with antibody AP33. Journal of virology. 2012;86(23):13085–8. doi: 10.1128/JVI.01939-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(Suppl 1):S3–9. doi: 10.1093/cid/cis393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou S, Dorsey KA, Notari EP, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion. 2010;50(7):1495–504. doi: 10.1111/j.1537-2995.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- 40.Cornberg M, Razavi HA, Alberti A, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver international : official journal of the International Association for the Study of the Liver. 2011;31(Suppl 2):30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 41.Thorpe LE, Ouellet LJ, Hershow R, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. American journal of epidemiology. 2002;155(7):645–53. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 42.Prasad MR, Honegger JR. Hepatitis C virus in pregnancy. American journal of perinatology. 2013;30(2):149–59. doi: 10.1055/s-0033-1334459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain S, Goharkhay N, Saade G, Hankins GD, Anderson GD. Hepatitis C in pregnancy. American journal of perinatology. 2007;24(4):251–6. doi: 10.1055/s-2007-970181. [DOI] [PubMed] [Google Scholar]
- 44.McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane database of systematic reviews. 2006;(4):CD005546. doi: 10.1002/14651858.CD005546.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57(3):881–9. doi: 10.1002/hep.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danta M, Rodger AJ. Transmission of HCV in HIV-positive populations. Current opinion in HIV and AIDS. 2011;6(6):451–8. doi: 10.1097/COH.0b013e32834b4974. [DOI] [PubMed] [Google Scholar]
- 47.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. Aids. 2007;21(8):983–91. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 48.Serpaggi J, Chaix ML, Batisse D, et al. Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. Aids. 2006;20(2):233–40. doi: 10.1097/01.aids.0000200541.40633.56. [DOI] [PubMed] [Google Scholar]
- 49.van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136(5):1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin TC, Martin NK, Hickman M, et al. HCV reinfection incidence and treatment outcome among HIV-positive MSM in London. Aids. 2013 doi: 10.1097/QAD.0b013e32836381cc. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez MD, Sherman KE. HIV/hepatitis C coinfection natural history and disease progression. Current opinion in HIV and AIDS. 2011;6(6):478–82. doi: 10.1097/COH.0b013e32834bd365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Dieguez M, Montes ML, Pascual-Pareja JF, et al. The natural history of liver cirrhosis in HIV-hepatitis C virus-coinfected patients. Aids. 2011;25(7):899–904. doi: 10.1097/QAD.0b013e3283454174. [DOI] [PubMed] [Google Scholar]
- 53.Macias J, Berenguer J, Japon MA, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50(4):1056–63. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 54.Tovo CV, Becker SC, Almeida PR, Galperim B, Chaves S. Progression of liver fibrosis in monoinfected patients by hepatitis C virus and coinfected by HCV and human immunodeficiency virus. Arquivos de gastroenterologia. 2013;50(1):19–22. doi: 10.1590/s0004-28032013000100005. [DOI] [PubMed] [Google Scholar]
- 55.Sulkowski MS. Current management of hepatitis C virus infection in patients with HIV co-infection. The Journal of infectious diseases. 2013;207(Suppl 1):S26–32. doi: 10.1093/infdis/jis764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50(2):407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 57.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA : the journal of the American Medical Association. 2010;304(3):321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 58.Wyatt CM, Malvestutto C, Coca SG, Klotman PE, Parikh CR. The impact of hepatitis C virus coinfection on HIV-related kidney disease: a systematic review and meta-analysis. Aids. 2008;22(14):1799–807. doi: 10.1097/QAD.0b013e32830e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals co-infected with HIV and hepatitis C. Journal of addictive diseases. 2008;27(2):11–7. doi: 10.1300/j069v27n02_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV medicine. 2010;11(7):462–8. doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 61.Collin F, Duval X, Le Moing V, et al. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. Aids. 2009;23(8):1021–4. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Current HIV/AIDS reports. 2011;8(1):12–22. doi: 10.1007/s11904-010-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Almeida Ponde RA. Enzyme-linked immunosorbent/chemiluminescence assays, recombinant immunoblot assays and nucleic acid tests in the diagnosis of HCV infection. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2013 doi: 10.1007/s10096-013-1857-1. [DOI] [PubMed] [Google Scholar]
- 64.Klenerman P, Kim A. HCV-HIV coinfection: simple messages from a complex disease. PLoS medicine. 2007;4(10):e240. doi: 10.1371/journal.pmed.0040240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez V, Gomes-Fernandes M, Bascunana E, et al. Accuracy of a commercially available assay for HCV genotyping and subtyping in the clinical practice. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2013 doi: 10.1016/j.jcv.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Avo AP, Agua-Doce I, Andrade A, Padua E. Hepatitis C virus subtyping based on sequencing of the C/E1 and NS5B genomic regions in comparison to a commercially available line probe assay. Journal of medical virology. 2013;85(5):815–22. doi: 10.1002/jmv.23545. [DOI] [PubMed] [Google Scholar]
- 67.Batty EM, Wong TH, Trebes A, et al. A modified RNA-Seq approach for whole genome sequencing of RNA viruses from faecal and blood samples. PloS one. 2013;8(6):e66129. doi: 10.1371/journal.pone.0066129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cobb B, Pockros PJ, Vilchez RA, Vierling JM. HCV RNA viral load assessments in the era of direct-acting antivirals. The American journal of gastroenterology. 2013;108(4):471–5. doi: 10.1038/ajg.2012.248. [DOI] [PubMed] [Google Scholar]
- 69.Thomson EC, Smith JA, Klenerman P. The natural history of early hepatitis C virus evolution; lessons from a global outbreak in human immunodeficiency virus-1-infected individuals. The Journal of general virology. 2011;92(Pt 10):2227–36. doi: 10.1099/vir.0.033910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Racanelli V, Rehermann B. Hepatitis C virus infection: when silence is deception. Trends in immunology. 2003;24(8):456–64. doi: 10.1016/s1471-4906(03)00178-9. [DOI] [PubMed] [Google Scholar]
- 71.Loomba R, Rivera MM, McBurney R, et al. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Alimentary pharmacology & therapeutics. 2011;33(5):559–65. doi: 10.1111/j.1365-2036.2010.04549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beinhardt S, Anna Payer B, Datz C, et al. A Diagnostic Score for the Prediction of Spontaneous Resolution of Acute Hepatitis C Virus Infection. Journal of hepatology. 2013 doi: 10.1016/j.jhep.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 73.European ATNAHCICP Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. Aids. 2011;25(4):399–409. doi: 10.1097/QAD.0b013e328343443b. [DOI] [PubMed] [Google Scholar]
- 74.Deterding K, Gruner N, Buggisch P, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. The Lancet infectious diseases. 2013;13(6):497–506. doi: 10.1016/S1473-3099(13)70059-8. [DOI] [PubMed] [Google Scholar]
- 75.Webster DP, Wojcikiewicz T, Keller M, et al. Spontaneous clearance and treatment of acute hepatitis C infection in HIV-positive men with 48 weeks of interferon-alpha and ribavirin. International journal of STD & AIDS. 2013 doi: 10.1177/0956462412472317. [DOI] [PubMed] [Google Scholar]
- 76.Gilleece YC, Browne RE, Asboe D, et al. Transmission of hepatitis C virus among HIV-positive homosexual men and response to a 24-week course of pegylated interferon and ribavirin. Journal of acquired immune deficiency syndromes. 2005;40(1):41–6. doi: 10.1097/01.qai.0000174930.64145.a9. [DOI] [PubMed] [Google Scholar]
- 77.Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125(1):80–8. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 78.Wiegand J, Buggisch P, Boecher W, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43(2):250–6. doi: 10.1002/hep.21043. [DOI] [PubMed] [Google Scholar]
- 79.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 80.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349(9055):825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 81.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–31. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 82.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. Aids. 2008;22(15):1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 83.Castera L, Le Bail B, Roudot-Thoraval F, et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. Journal of hepatology. 2009;50(1):59–68. doi: 10.1016/j.jhep.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 84.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA : the journal of the American Medical Association. 2012;308(24):2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 85.Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45(3):579–87. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 86.Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. The New England journal of medicine. 2011;364(25):2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 87.Hezode C, Fontaine H, Dorival C, et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147(1):132–42. e4. doi: 10.1053/j.gastro.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 88.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 89.Kiser JJ, Burton JR, Jr., Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nature reviews Gastroenterology & hepatology. 2013;10(10):596–606. doi: 10.1038/nrgastro.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chevaliez S. Antiviral activity of the new DAAs for the treatment of hepatitis C virus infection: virology and resistance. Clinics and research in hepatology and gastroenterology. 2011;35(Suppl 2):S46–51. doi: 10.1016/S2210-7401(11)70007-9. [DOI] [PubMed] [Google Scholar]
- 91.Jacobson IM, Marcellin P, Zeuzem S, et al. Refinement of stopping rules during treatment of hepatitis C genotype 1 infection with boceprevir and peginterferon/ribavirin. Hepatology. 2012;56(2):567–75. doi: 10.1002/hep.25865. [DOI] [PubMed] [Google Scholar]
- 92.Pawlotsky JM. Treatment failure and resistance with direct-acting antiviral drugs against hepatitis C virus. Hepatology. 2011;53(5):1742–51. doi: 10.1002/hep.24262. [DOI] [PubMed] [Google Scholar]
- 93.Jacobson IM, Pawlotsky JM, Afdhal NH, et al. A practical guide for the use of boceprevir and telaprevir for the treatment of hepatitis C. Journal of viral hepatitis. 2012;19(Suppl 2):1–26. doi: 10.1111/j.1365-2893.2012.01590.x. [DOI] [PubMed] [Google Scholar]
- 94.Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: The randomized PILLAR study. Hepatology. 2013;58(6):1918–29. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sulkowski MS, Asselah T, Lalezari J, et al. Faldaprevir combined with pegylated interferon alfa-2a and ribavirin in treatment-naive patients with chronic genotype 1 HCV: SILEN-C1 trial. Hepatology. 2013;57(6):2143–54. doi: 10.1002/hep.26276. [DOI] [PubMed] [Google Scholar]
- 96.Pol S, Ghalib RH, Rustgi VK, et al. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. The Lancet infectious diseases. 2012;12(9):671–7. doi: 10.1016/S1473-3099(12)70138-X. [DOI] [PubMed] [Google Scholar]
- 97.Kowdley KV, Lawitz E, Crespo I, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381(9883):2100–7. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 98.Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384(9941):403–13. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 99.Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384(9941):414–26. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 100.Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146(7):1669–79. e3. doi: 10.1053/j.gastro.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 101.Lawitz E, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. The New England journal of medicine. 2013;369(7):678–9. doi: 10.1056/NEJMc1307641. [DOI] [PubMed] [Google Scholar]
- 102.Manns MP, Vierling JM, Bacon BR, et al. The combination of MK-5172, peginterferon, and ribavirin is effective in treatment-naive patients with hepatitis C virus genotype 1 infection without cirrhosis. Gastroenterology. 2014;147(2):366–76. e6. doi: 10.1053/j.gastro.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 103.Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 104.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. The New England journal of medicine. 2014;370(3):211–21. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 105.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Sustained virologic response with daclatasvir plus sofosbuvir ± ribavirin (RBV) in chronic HCV genotype (GT) 1-infected patients who previously failed telaprevir (TVR) or boceprevir (BOC) Journal of hepatology. 2013;58(Suppl. 1):S570. [Google Scholar]
- 106.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. The New England journal of medicine. 2014;370(16):1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 107.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. The New England journal of medicine. 2014;370(20):1879–88. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 108.Membreno FE, Lawitz EJ. The HCV NS5B nucleoside and non-nucleoside inhibitors. Clinics in liver disease. 2011;15(3):611–26. doi: 10.1016/j.cld.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 109.Bourliere M, Bronowicki JP, De Ledinghen V. Ledispavir/sofosbuvir fixed dose combination is safe and efficacious in cirrhotic patients who have previously failed protease inhibitor based triple therapy. Hepatology. 2014;60(4 Supplement):LB6. [Google Scholar]
- 110.Kohli A, Sims Z, Marti M, et al. Combination Oral, Ribavirin Free, Antiviral Therapy to Optimize Treatment Outcomes for Hepatitis C GT-1 Treatment Naïve Patients: Interim Results from the NIAID SYNERGY Trial. AASLD 2013; Washington DC, USA: 2013. [Google Scholar]
- 111.Everson G, Tran T, Towner W, et al. Safety and efficacy of treatment with the interferon-free combination of sobosbuvir + GS-5816 for 12 weeks in treatment naïve patients with genotype 1-6 HCV infection. EASL 2014; London: 2014. [Google Scholar]
- 112.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/rombitasvir and dasabuvir with ribavirin. The New England journal of medicine. 2014;370(17):1594–603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 113.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. The New England journal of medicine. 2014;370(21):1973–82. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 114.Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. The New England journal of medicine. 2014;370(17):1604–14. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 115.Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. The New England journal of medicine. 2014;370(21):1983–92. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 116.Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147(2):359–65. e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 117.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. The Lancet. 384(9956):1756–65. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 118.Jensen D, O'Leary J, Pockros P. Safety and efficacy of sofosbuvir containing regimens for hepatitis C: A real world experience in a diverse longitudinal observational cohort. Hepatology. 2014;60(4 (Suppl):219A. [Google Scholar]
- 119.Dietrich D, Bacon B, Flamm S. Sofosbuvir and simeprevir based regimens in the TRIO network: Academic and community treatment of a real world heterogenous population . Hepatology. 2014;60(4 (Suppl)) [Google Scholar]
- 120.Manns M, Pol S, Jacobson IM, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014 doi: 10.1016/S0140-6736(14)61059-X. [DOI] [PubMed] [Google Scholar]
- 121.Pianko S, Flamm S, Shiffman M, et al. High Efficacy of Treatment with Sofosbuvir+GS-5816 ±Ribavirin for 12 Weeks in Treatment Experienced Patients with Genotype 1 or 3 HCV Infection. Hepatology. 2014;60(4 (SUPPL)) [Google Scholar]
- 122.Lawitz E, Gane E, Pearlman B, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. The Lancet. doi: 10.1016/S0140-6736(14)61795-5. In Press, Corrected Proof, Available online 11 November 2014 2014; In Press, Corrected Proof, Available online. [DOI] [PubMed] [Google Scholar]
- 123.Everson GT, Sims KD, Thuluvath PJ, et al. Phase 2b study of the interferon-free and ribavirin-free combination of daclatasvir, asunaprevir, and BMS-791325 for 12 weeks in treatment-naïve patients with chronic HCV genotype 1 infection. AASLD 2013; Washington DC, USA; 2013. 2013. [Google Scholar]
- 124.Muir A, Poordad F, Lalezari J. All oral fixed dose combination with daclatasvir asunaprevir and BMS 791325 plus or minus ribavirin for patients with chronic hepatitis C genotype 1 infection and compensated cirrhosis: Unity -2 phase 3 SVR12 results. Hepatoloogy. 2014;60(4 (Suppl)) [Google Scholar]
- 125.Plaza Z, Soriano V, Vispo E, et al. Prevalence of natural polymorphisms at the HCV NS5A gene associated with resistance to daclatasvir, an NS5A inhibitor. Antiviral therapy. 2012;17(5):921–6. doi: 10.3851/IMP2091. [DOI] [PubMed] [Google Scholar]
- 126.Antaki N, Craxi A, Kamal S, et al. The neglected hepatitis C virus genotypes 4, 5 and 6: an international consensus report. Liver international : official journal of the International Association for the Study of the Liver. 2010;30(3):342–55. doi: 10.1111/j.1478-3231.2009.02188.x. [DOI] [PubMed] [Google Scholar]
- 127.Dore GJ, Lawitz E, Hezode C, et al. Daclatasvir combined with peginterferon alfa-2A and ribavirin for 12 or 16 weeks in patients with HCV genotype 2 or 3 infection: COMMAND GT2/3 STUDY. Journal of hepatology. 2013;58(Suppl. 1) [Google Scholar]
- 128.McPhee F, Zhou N, Ueland J, et al. Pre-existence, emergence and persistence of HCV genotype 4 NS5A resistance variants from the phase 2B COMMAND-1 study: Daclatasvir plus peginterferon-alfa/ribavirin in treatment-naive patients. Journal of hepatology. 2013;58(Suppl. 1):S492. [Google Scholar]
- 129.Moreno C, Hezode C, Marcellin P, et al. Once daily Simeprevir (TMC435) with Peginterferon/Ribavirin in treatment-naive or treatment-experienced chronic HCV genotype 4-infected patients: final results of a phase III trial. EASL 2014; London: 2014. [Google Scholar]
- 130.Lawitz E E, Poordad F, Brainard DM, et al. Sofosbuvir in Combination With PegIFN and Ribavirin for 12 Weeks Provides High SVR Rates in HCV-Infected Genotype 2 or 3 Treatment Experienced Patients with and without Compensated Cirrhosis: Results from the LONESTAR-2 Study. AASLD 2013; Washington DC, USA: 2013. 2013. [Google Scholar]
- 131.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. The New England journal of medicine. 2013;368(20):1867–77. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 132.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. The New England journal of medicine. 2014;370(21):1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 133.Nelson DR. All oral 12 week combination treatment with daclatasvir and sofosbuvir in patients infected with HCV genotype 3: ALLY-3 phase 3 study. Hepatology. 2014;60(Suppl S1) doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gane E, Hyland RH, An D, et al. Sofosbuvir/Ledipisvir fixed dose combination is safe and effective in difficult-to-treat populations including genotype-3 patients, decompensated genotype-1 patients, and genotype-1 patients with prior sofosbuvir treatment experience. EASL 2014; London: 2014. [Google Scholar]
- 135.Ruane PJ, Ain D, Riad J, et al. Sofosbuvir plus Ribavirin in the Treatment of Chronic HCV Genotype 4 Infection in Patients of Egyptian Ancestry. AASLD 2013; Washington DC, USA: 2013. [DOI] [PubMed] [Google Scholar]
- 136.Hassanein T, Sims KD, Bennett M, et al. All-oral therapy with daclatasvir in combination with asunaprevir and BMS-791325 for treatment-naive patients with chronic HCV genotype 4 infection. EASL 2014; London: 2014. [Google Scholar]
- 137.Rockstroh JK, Bhagani S. Managing HIV/hepatitis C co-infection in the era of direct acting antivirals. BMC medicine. 2013;11:234. doi: 10.1186/1741-7015-11-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lacombe K, Valin N, Stitou H, et al. Efficacy and tolerance of telaprevir in HIV-hepatitis C virus genotype 1-coinfected patients failing previous antihepatitis C virus therapy: 24-week results. Aids. 2013;27(8):1356–9. doi: 10.1097/QAD.0b013e32836138d0. [DOI] [PubMed] [Google Scholar]
- 139.Sulkowski MS, Sherman KE, Dieterich DT, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Annals of internal medicine. 2013;159(2):86–96. doi: 10.7326/0003-4819-159-2-201307160-00654. [DOI] [PubMed] [Google Scholar]
- 140.Chastain CA, Naggie S. Treatment of Genotype 1 HCV Infection in the HIV Coinfected Patient in 2014. Current HIV/AIDS reports. 2013;10(4):408–19. doi: 10.1007/s11904-013-0182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fierer DS, Dieterich DT, Mullen MP, et al. Telaprevir in the Treatment of Acute Hepatitis C Virus Infection in HIV-Infected Men. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 doi: 10.1093/cid/cit799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Osinusi A, Townsend K, Nelson A, et al. Use of Sofosbuvir/Ledipasvir fixed dose combination for treatment of HCV genotype-1 infection in patients coinfected with HIV (Interim results) EASL 2014; 2014. [Google Scholar]
- 143.Sulkowski M, Mallolas J, Bourliere M, et al. Efficacy and safety of the all-oral regimen, MK-5172/MK-8742 +/− RBV for 12 weeks in GT1 HCV/HIV co-infected patients: the C-WORTHY study. EASL 2014; 2014. [Google Scholar]
- 144.Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA : the journal of the American Medical Association. 2014;312(4):353–61. doi: 10.1001/jama.2014.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sulkowski MS, Rodriguez-Torres M, Lalezari JP, et al. All-Oral Therapy With Sofosbuvir Plus Ribavirin For the Treatment of HCV Genotype 1, 2, and 3 Infection in Patients Co-infected With HIV (PHOTON-1) AASLD 2013; Washington DC, USA: 2013. [Google Scholar]
- 146.Curry MP, Forns X, Chung RT, et al. Pretransplant Sofosbuvir and Ribavirin to Prevent Recurrence of HCV Infection after Liver Transplantation. AASLD 2013; Washington DC, USA: 2013. [DOI] [PubMed] [Google Scholar]
- 147.Flamm S, Everson G, Charlton M, et al. Ledipasvir/Sofosbuvir with Ribavirin for the Treatment of HCV in Patients with Decompensated Cirrhosis: Preliminary Results of a Prospective, Multicenter Study. Hepatology. 2014;60(4 (SUPPL)) [Google Scholar]
- 148.Coilly A, Roche B, Dumortier J, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: A multicenter experience. Journal of hepatology. 2014;60(1):78–86. doi: 10.1016/j.jhep.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 149.Charlton M. Telaprevir, boceprevir, cytochrome P450 and immunosuppressive agents--a potentially lethal cocktail. Hepatology. 2011;54(1):3–5. doi: 10.1002/hep.24470. [DOI] [PubMed] [Google Scholar]
- 150.Charlton M, Gane E, Manns MP, et al. Sofosbuvir and Ribavirin for Treatment of Compensated Recurrent Hepatitis C Virus Infection After Liver Transplantation. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 151.Reddy R, Everson G, Flamm S, et al. Ledipasvir/Sofosbuvir with Ribavirin for the Treatment of HCV in Patients with Post Transplant Recurrence: Preliminary Results of a Prospective, Multicenter Study. Hepatology. 2014;60(4 (SUPPL)):49A. [Google Scholar]
- 152.Brown R, Reddy KR, O'Leary J. Safety and efficacy of new DAA therapy for hepatitis C post transplant: interval results from the HCV TARGET longitudinal observational study. Hepatology. 2014;60 [Google Scholar]
- 153.Kwo PY, Mantry PS, Coakley E, et al. An Interferon-free Antiviral Regimen for HCV after Liver Transplantation. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 154.Afdhal N, Everson G, Calleja J, et al. EASL. Vol. 2014. London: 2014. Sofosbuvir and ribavirin for the treatment of chronic HCV with cirrhosis and portal hypertension with and without decompensation: Early virological response and safety. [Google Scholar]