Abstract
Objective
Nearly one third of patients presenting with mesial temporal lobe epilepsy (MTLE), the most prevalent lesion-related epileptic disorder in adulthood, do not respond to currently available antiepileptic medications. Thus, there is a need to identify and characterize new antiepileptic drugs. In this study, we used the pilocarpine model of MTLE to establish the effects of a third generation drug, lacosamide (LCM), on seizures, interictal spikes and high-frequency oscillations (HFOs, ripples: 80–200 Hz, fast ripples: 250–500 Hz).
Methods
Sprague–Dawley rats (250–300 g) were injected with pilocarpine to induce a status epilepticus (SE) that was pharmacologically terminated after 1 h. Eight pilocarpine-treated rats were then injected with LCM (30 mg/kg, i.p.) 4 h after SE and daily for 14 days. Eight pilocarpine-treated rats were used as controls and treated with saline. Three days after SE, all rats were implanted with bipolar electrodes in the hippocampal CA3 region, entorhinal cortex (EC), dentate gyrus (DG) and subiculum and EEG-video monitored from day 4 to day 14 after SE.
Results
LCM-treated animals showed lower rates of seizures (0.21 (±0.11) seizures/day) than controls (2.6 (±0.57), p < 0.05), and a longer latent period (LCM: 11 (±1) days, controls: 6.25 (±1), p < 0.05). Rates of interictal spikes in LCM-treated rats were significantly lower than in controls in CA3 and subiculum (p < 0.05). Rates of ripples and fast ripples associated with interictal spikes in CA3 and subiculum as well as rates of fast ripples occurring outside of interictal spikes in CA3 were also significantly lower in LCM-treated animals. In controls, interictal spikes and associated HFOs correlated to seizure clustering, while this was not the case for isolated HFOs.
Significance
Our findings show that early treatment with LCM has powerful anti-ictogenic properties in the pilocarpine model of MTLE. These effects are accompanied by decreased rates of interictal spikes and associated HFOs. Isolated HFOs were also modulated by LCM, in a manner that appeared to be unrelated to its antiictogenic effects. These results thus suggest that distinct mechanisms may underlie interictal-associated and isolated HFOs in the pilocarpine model of MTLE.
Keywords: Epilepsy, Seizures, Interictal spikes, High-frequency oscillations, Hippocampus
Introduction
Lacosamide (LCM, (R)-2-acetamido-N-benzyl-3-methoxypro-pionamide) is a voltage-gated Na+ channel blocker that modulates the slow inactivation component of Na+ channels (Errington et al., 2008; Niespodziany et al., 2013). LCM is effective in patients with pharmacoresistant focal epilepsy (Ben-Menachem et al., 2007; Cross and Curran, 2009; Halasz et al., 2009), and in animal models such as the maximal electroshock model, the amygdala kindling model and the 6 Hz model of psychomotor seizures(Brandt et al., 2006; Stohr et al., 2007). However, its effect on status epilepticus (SE)-induced models of epilepsy remains undefined.
Mesial temporal lobe epilepsy (MTLE) is characterised by the occurrence of pharmacoresistant seizures many years after a brain insult, such as febrile seizures or encephalitis (Cendes et al., 1993; French et al., 1993). These seizures originate from hippocampal and/or parahippocampal structures (Spencer, 2002; Swanson, 1995). Many antiepileptic drugs are currently available to control seizures, but approximately one third of MTLE patients are refractory to medication thus making MTLE one of the most refractory forms of focal epilepsy in adults (Engel et al., 2012). Costly, and at times impracticable, surgical resection of the epileptic tissue remains the only therapeutic alternative in these cases.
In this study, we used the pilocarpine model of MTLE (Curia et al., 2008; Turski et al., 1983) to address the impact of LCM on the occurrence of seizures and interictal spikes. We investigated whether LCM treatment over a period of 14 days after the initial SE influences epileptic activity in temporal lobe regions such as the CA3 region of the hippocampus, entorhinal cortex (EC), dentate gyrus (DG) and subiculum. Each of these regions have indeed been identified as a key structure for the generation of interictal and ictal activities in human and animal studies (Bragin et al., 1999b; Cohen et al., 2002; Huberfeld et al., 2011; Levesque et al., 2011, 2012; Salami et al., 2014; Spencer and Spencer, 1994). Moreover, since high-frequency oscillations (HFOs, ripples: 80–200 Hz, fast ripples: 250–500 Hz) are thought to represent biomarkers of pathological network activity that may sustain epileptogenesis and ictogenesis in MTLE (Jefferys et al., 2012), we studied their occurrence in pilocarpine controls and LCM-treated animals.
Materials and methods
Ethical approval
All procedures were approved by the Canadian Council of Animal Care and all efforts were made to minimize the number of animals and their suffering.
Animal preparation
Twenty adult male rats (Sprague–Dawley, 250–300 g, around P60) were purchased from Charles River (St-Constant, Qc, Canada) and housed under controlled conditions, at 22 (±2) °C and 12 h light/12 h dark cycle (lights on from 7:00 A.M. to 7:00 P.M.) with food and water ad libitum.
Pilocarpine and lacosamide treatment
SE was induced by a systemic injection of pilocarpine as previously described (Bortel et al., 2010; Levesque et al., 2011; Salami et al., 2014). Briefly, peripheral effects were reduced by scopolamine methylnitrate pre-treatment (1 mg/kg, i.p., Sigma-Aldrich, Canada) 30 min before a single dose of pilocarpine hydrochloride was injected i.p. (380 mg/kg; Sigma-Aldrich, Canada). Continuous stage 5 seizures (Racine scale, (Racine, 1972)) defined SE that was interrupted by diazepam (5 mg/kg, s.c.; CDMV, Canada) and ketamine (50 mg/kg, s.c.; CDMV, Canada) after 1 h (Martin and Kapur, 2008). The mortality rate after pilocarpine injection was around 15%. In addition, 10% of pilocarpine-treated animals did not develop SE, allowing the recording of 16 rats. Approximately 4 h after SE termination, eight rats received a single injection of LCM (30 mg/kg i.p.), diluted in fresh saline. The same procedure was repeated every day for 14 continuous days. A single daily injection was compatible with pharmacokinetic properties in rats, according to preclinical studies (Bialer et al., 2002; Brandt et al., 2006; Hovinga, 2003) and reduced animal discomfort. Eight rats were used as controls and treated with saline. Injections were always performed at the same time of the day (10 AM).
Stereotaxic implantation of depth electrodes
Three days after SE, all rats received topical lidocaine (5%; Odan, Canada), before placement in the stereotaxic frame. An incision was done to expose the skull plate from Bregma to Lambda, after careful assessment of the level of anaesthesia (isoflurane 2% in 100% O2). Anchor screws (2.4 mm length, n = 4) were fixed to the skull which was drilled to allow the implantation of four bipolar electrodes (20–30 kΩ; 5–10 mm length; distance between exposed tips: 500 μm, MS303/2-B/spc, Plastics One, VA, USA). Electrodes were implanted in the right CA3 subfield of the ventral hippocampus (AP: −4.4, ML: −4, DV: 7.8), right medial EC (AP: −8.6, ML: −5.2, DV: 6.8), left ventral subiculum (AP: −6.8, ML: +4, DV: 6) and left DG (AP: −4.4, ML: +2.4, DV: 3.4) (Paxinos, 1998). A reference electrode was placed under the left frontal bone. Preventive antibiotherapy (enrofloxacine, s.c., 10 mg/kg) and post-operative analgesic were systematically administered (ketoprofen 5 mg/kg, s.c. Merail, Canada and buprenorphine 0.01–0.05 mg/kg, s.c. CDMV, Canada) along with rehydration (2 ml of 0.9% sterile saline, s.c. repeated every 12 h if necessary).
EEG recordings and seizures detection
Video-EEG monitoring (24 h/day, 7 days/weeks) occurred in custom-made Plexiglas boxes (30 × 30 × 40 cm), after a 24 h recovery period, until day 11 after surgery. Each animal was connected to a multichannel cable and electrical swivel (Commutator SL 18 C, HRS Scientific, Canada) and local field potentials were amplified via an interface kit (Mobile 36ch LTM ProAmp, Stellate, Montreal, QC, Canada), low-pass filtered at 500 Hz and sampled at 2 kHz per channel. Recordings were performed for 11 days (from day 4 to day 14) after SE. Day and night video monitoring using infrared cameras were time-stamped for integration with the electrophysiological data using monitoring software (Harmonie, Stellate, Montreal, QC, Canada). Temperature and day-night cycle were controlled (22 ± 2 °C, 12 h light/dark schedule), food and water were provided ad libitum. All traces and video files were reviewed manually to detect seizures, based on the occurrence of an abrupt change from baseline (increase in frequency and/or amplitude) with concordant behaviour. The latent period was defined as the time between the end of the SE and the occurrence of the first spontaneous seizure (convulsive or non-convulsive).
Detection of interictal spikes
Two epochs of 10 min of non-REM sleep were selected for each day and each animal, since interictal spikes and HFOs are known to occur at higher rates during these time periods (Bagshaw et al., 2009; Montplaisir et al., 1985; Staba et al., 2004) as well as because possible contamination of the EEG by movement artefacts would be reduced. Moreover, to minimize the effect of seizure occurrence, we selected traces at least 1 h before or after seizures. To account for a possible light-dark cycle effect, we selected for each day one epoch during the light period (from 7 AM to 7 PM) and a second epoch during the dark period (from 7 PM to 7 AM), results obtained from the two epochs were averaged in order to obtain a single value per day per animal. Interictal spikes were detected based on threshold crossings (mean and standard deviation (SD)), calculated over the entire period for the 10 min epoch. Every event detected by the automated method was visually reviewed and all events caused by movement artefacts were excluded. The rate of interictal spikes (number of events/s) was then calculated for each region (CA3, EC, DG and subiculum).
Detection of high-frequency oscillations
The same time-periods used to analyse interictal spikes were used for the analysis of HFOs, because these events are prominent during the non-REM sleep stage (Bagshaw et al., 2009; Staba et al., 2004). A multi-parametric algorithm was employed to identify oscillations in each frequency range (80–200 Hz and 250–500 Hz), using routines based on standardized functions (Matlab Signal Processing Toolbox). Raw EEG recordings were bandpass-filtered in the 80–200 Hz and in the 250–500 Hz frequency range using a finite impulse response filter; zero-phase digital filtering was used to avoid phase distortion. Filtered EEGs from each region were then normalized using their own average root mean square (RMS) value. To be considered as an HFO candidate, oscillatory events in each frequency band had to show at least four consecutive cycles having amplitude of 3 SD above the mean. The time lag between two consecutive cycles had to be between 5 and 12.5 ms for ripples and between 2 and 4 ms for fast ripples (Levesque et al., 2011). If an HFO co-occurred within a time window of ±300 ms from the peak of an interictal spike, we considered that it co-occurred with an interictal spike. The remaining detected HFOs were considered as isolated HFOs.
Statistical analyses
Statistical analyses were performed in Matlab R2013a using the Statistics Toolbox. Since values were not normally distributed (as measured with the Kolmogorov–Sminorv goodness-of-fit hypothesis test), Wilcoxon rank sum tests were used to compare rates of interictal spikes and HFOs from the control and LCM-treated group in each region (CA3, EC, DG and subiculum). We expected that LCM would induce significantly lower rates of interictal spikes with HFOs in LCM-treated animals compared to controls. Linear regressions were applied between rates of interictal spikes, rates of HFOs co-occurring with spikes, rates of isolated HFOs and rates of seizures. To test the predictive value of each interictal event (namely, interictal spikes, interictal spikes associated HFOs and isolated HFOs) with regards to seizure occurrence, we performed a linear regression with seizure rates shifted forward in time (Levesque et al., 2011). Fisher’s exact test was used to compare percentage of seizing animal. The level of significance was set at p < 0.05. Results are expressed as mean (±standard error of mean).
Results
Seizure occurrence
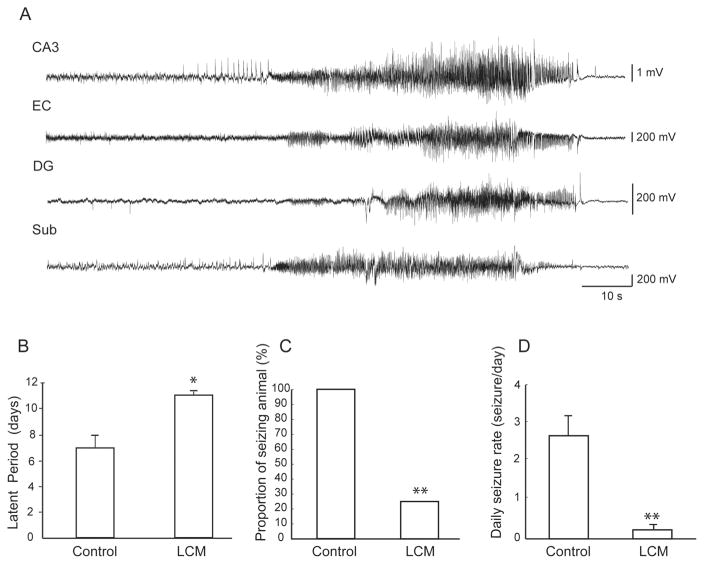
In controls, a total of 352 seizures were recorded between day 4 and day 14 after SE, whereas in LCM-treated animals, a total of 3 seizures were detected. Fig. 1A shows an example of a seizure detected in a control animal on the four electrodes. The latent period was significantly longer in LCM-treated animals (Fig. 1B). Both the proportion of seizing animals and the daily rate of seizure were significantly higher in the control group (Fig. 1C and D). The light-dark cycle did not modulate the occurrence of seizures, since 48% of seizures occurred during the dark period compared to 52% during the light period.
Figure 1.
Effect of lacosamide on the occurrence of seizures—A. Representative example of a seizure recorded in control animals in the right CA3, right EC, left DG and left subiculum. B. Bar graph showing the average duration of the latent period in controls and in LCM-treated animals. LCM-treated animals showed a significantly longer latent period (* p < 0.05). C. Bar graph representing the proportion of seizing animals in each group, this proportion was significantly higher in the control group (Fisher’s exact test, ** p < 0.01). D. Bar graph showing average daily seizure rates in animals with seizures in each group. CA3: CA3 region of the hippocampus, EC: entorhinal cortex, DG: dentate gyrus, Sub: subiculum.
Interictal spikes
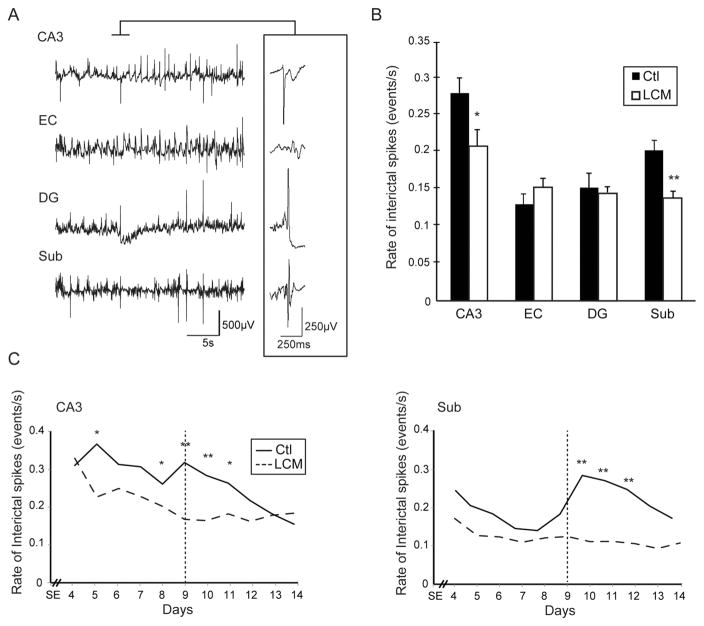
In both groups, all animals showed interictal spikes in all regions of the temporal lobe that were recorded. Fig. 2A shows a typical example of a seizure recorded from a control animal. For the analysis of interictal spike rates, we selected 6 controls and 6 LCM-treated animals, including the LCM-treated rats that were seizing (the remaining 2 LCM-treated rats were excluded due to the poor signal quality). Seizures in the 6 selected controls occurred in a cluster between day 7 and day 10 after SE (Fig. 2C, the vertical dotted line represents the average peak of seizure occurrence in the control group). A total of 22 h of interictal activity was analysed for each group (two 10 min epochs in each animal from each day of recording between day 4 and day 14 after SE). The analysis was performed over 38,165 interictal spikes in the control group and 36,415 interictal spikes in the LCM-treated group. Fig. 2B shows the mean rates of interictal spikes, averaged over the entire recording period for each structure. The rates of interictal spikes in CA3 and subiculum were significantly lower in the LCM-treated group than in controls (p < 0.05 and p < 0.001). Interictal spike rates were significantly lower in LCM-treated animals at day 5 and between day 8 and 11 after SE in CA3 and between day 10 and day 12 in the subiculum (Fig. 2C). In both regions, rates of ripples associated with interictal spikes were not significantly correlated over time with seizure occurrence in controls (data not shown), but as it was observed for interictal spike rates, they significantly decreased over time in CA3 (r = −0.93, p < 0.01).
Figure 2.
Effect of lacosamide on interictal spikes—A. Example of EEG wideband recordings from a control animal showing interictal spikes occurring in the four structures. The inset shows one interictal spike occurring simultaneously in CA3, DG and Sub. B. Bar graph showing the average rates of interictal spikes in both groups. LCM-treated animals showed significantly lower rates than controls in CA3 and in the subiculum (* p < 0.05, ** p < 0.01). C. Rates of interictal spikes from day 4 to day 14 after SE in controls and in LCM-treated animals, in CA3 and subiculum (* p < 0.05, ** p < 0.01) (vertical dotted lines define the peak of seizure occurrence in control animals).
High-frequency oscillations associated with interictal spikes
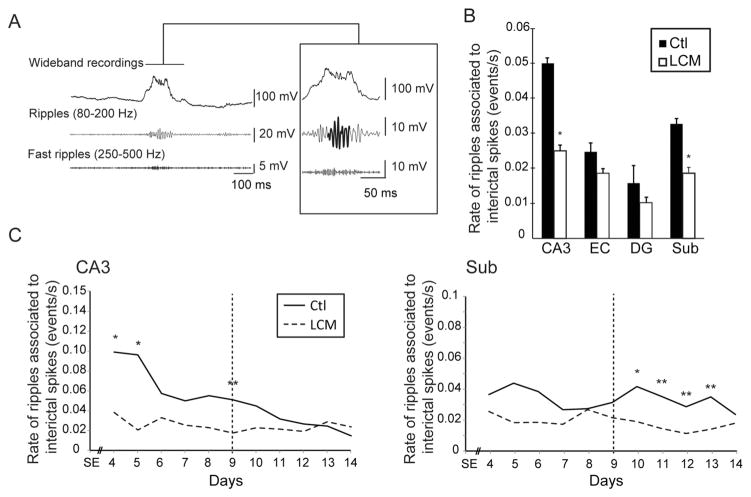
As previously observed in MTLE patients and animal models (Bragin et al., 1999a; Jacobs et al., 2010; Levesque et al., 2011; Salami et al., 2014), HFOs co-occurred with interictal spikes (Fig. 3A). LCM-treated animals showed significantly lower rates of ripples associated to interictal spikes in CA3 and subiculum compared to controls (p < 0.01) (Fig. 3B). In CA3, ripple rates were significantly lower in LCM-treated animals at day 4, 5 and 9 whereas they were significantly lower in the subiculum between days 10 and 13, (p < 0.05 and p < 0.01) (Fig. 3C). In both regions, rates of ripples associated with interictal spikes were not significantly correlated over time with seizure occurrence in controls (data not shown), but as it was observed for interictal spike rates, they significantly decreased over time in CA3 (r = −0.93, p < 0.01).
Figure 3.
Effect of lacosamide on interictal spikes associated ripples—A. Example of a ripple associated with an interictal spike in a control animal. B. Bar graph showing the average rates of ripples associated with interictal spikes in all regions. Rates of ripples associated with interictal spikes were significantly lower in CA3 and in the subiculum of LCM-treated animals compared to controls. C. Average rates of ripples associated with interictal spikes from day 4 to day 14 after SE in controls and LCM-treated animals, in CA3 and subiculum (* p < 0.05, ** p < 0.01) (vertical dotted lines define the averaged peak of seizure occurrence in control animals, around day 9 after SE).
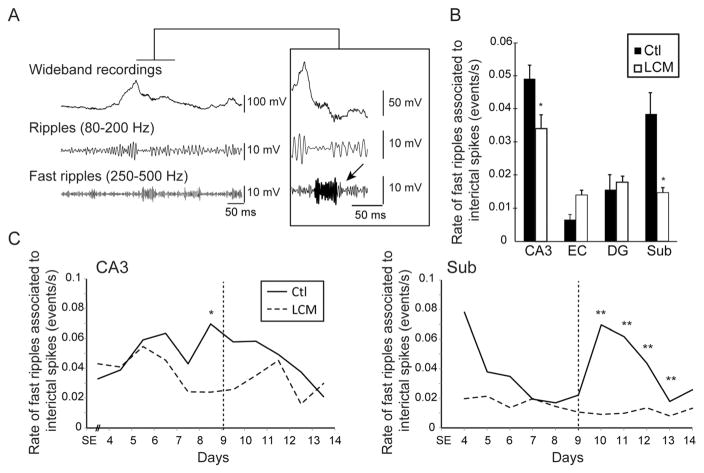
Similar results were obtained for fast ripples associated with interictal spikes (Fig. 4A). In both CA3 and subiculum, LCM-treated animals showed significantly lower rates of fast ripples associated with interictal spikes compared to controls (p < 0.05) (Fig. 4B). Moreover, at day 9, when seizures reached a peak in occurrence in controls, rates of fast ripples associated with interictal spikes were significantly lower in the CA3 of LCM-treated animals (p < 0.05) (Fig. 4C). A linear regression between rates of fast ripples associated with interictal spikes in CA3 and seizure rates in controls revealed a significant correlation (r = 0.62, p < 0.05), indicating that in this area an increase in the occurrence of fast ripples associated with interictal spikes was related to an increase in seizure rate, as previously reported (Levesque et al., 2011). In the subiculum, fast ripples associated with interictal spikes were significantly lower in LCM-treated animals between day 10 and 13, which followed the day during which a peak in seizure rate occurred (day 9) (Fig. 4C). A time-shifted linear regression analysis between rates of fast ripples associated with interictal spikes and rates of seizures in controls showed a significant correlation, (r = 0.72, p < 0.05), indicating that, as previously reported (Levesque et al., 2011), high fast ripple activity in this structure increases after seizure clustering.
Figure 4.
Effect of lacosamide on interictal spikes associated fast ripples—A. Example of a fast ripple (arrow) associated with an interictal spike in a control animal. B. Bar graph showing the average rates of fast ripples associated with interictal spikes in all regions. Note that rates of fast ripples associated with interictal spikes were significantly lower in CA3 and in the subiculum of LCM-treated animals compared to controls. C. Average rates of fast ripples associated with interictal spikes from day 4 to day 14 after SE in controls and LCM-treated animals, in CA3 and subiculum (* p < 0.05, ** p < 0.01) (vertical dotted lines represent the average peak of seizure occurrence in control animals, around day 9 after SE). D. Linear regression analysis showing that in CA3, rates of fast ripples associated to interictal spikes were significantly correlated with seizure occurrence. In the subiculum, high rates of fast ripples associated to interictal spikes followed seizure clustering (+2 days).
Isolated high-frequency oscillations
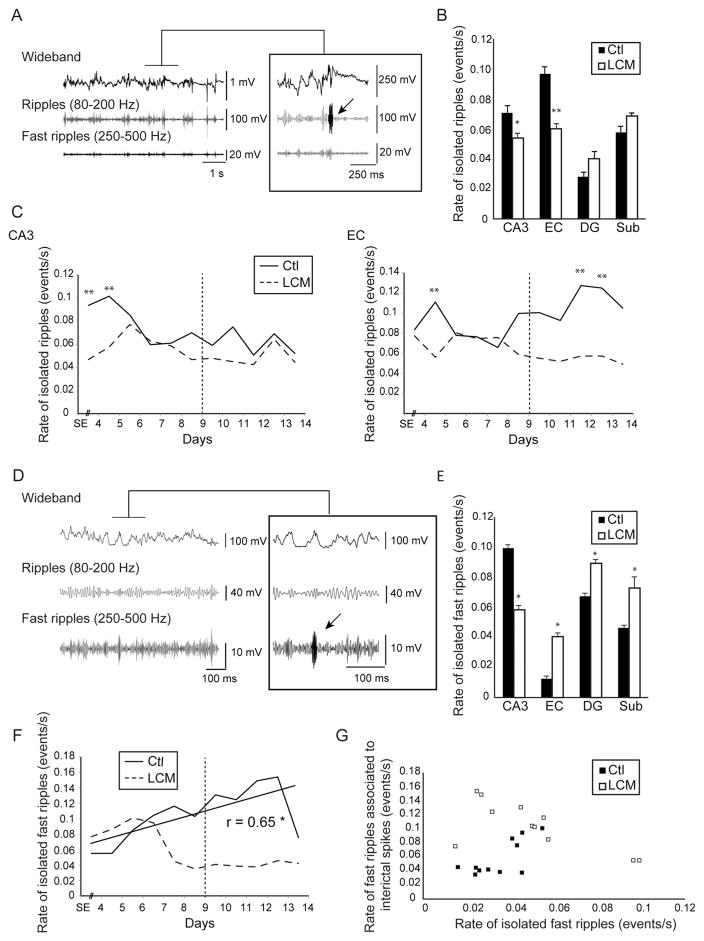
Ripples occurring outside of interictal spikes were recorded in both groups in all regions (Fig. 5A), but LCM-treated animals showed significantly lower rates in CA3 (p < 0.05) and EC (p < 0.01) compared to controls (Fig. 5B). The analysis of isolated ripples over time showed that their rates of occurrence in CA3 were significantly lower in LCM-treated animals at day 4 and 5 after SE (Fig. 5C). In EC, they showed lower rates compared to controls at day 5, 12 and 13 after SE (Fig. 5C). No significant correlation was found however between the occurrence of seizures and isolated ripples in CA3 or in EC in controls indicating that isolated ripples in these regions do not predict periods of high seizure occurrence. We performed a linear regression analysis between isolated ripples and ripples that occurred in association with interictal spikes in order to test the hypothesis that the two types of ripple activity display similar patterns of occurrence over time. Results showed that in CA3, ripples associated with interictal spikes and rates of isolated ripples were significantly correlated over time (r = 0.82, p < 0.01) (data not shown). Such a correlation was not observed in EC.
Figure 5.
Effect of lacosamide on isolated high-frequency oscillations—A. Example of an isolated ripple (arrow) recorded in a control animal. B. Bar graph showing the average rate of isolated ripples in both groups. Note that significantly lower rates of isolated ripples were observed in CA3 and EC in the LCM-treated group compared to the control group. C. Average rates of isolated ripples in CA3 and EC from day 4 to day 14 after SE in both groups. D. Representative example of an isolated fast ripple (arrow) recorded in a control animal. E. Bar graph showing the average rate of isolated fast ripples in both groups. Note that significantly lower rates of isolated fast ripples were observed in CA3 in the LCM-treated group compared to the control group. F. Average rates of isolated fast ripples in CA3 from day 4 to day 14 after SE in both groups. No significant differences were observed between groups. (* p < 0.05, ** p < 0.01) (Vertical dotted lines define the averaged peak of seizure occurrence in control animals, around day 9 after SE). G. Scatter plot showing the relation between fast ripples associated with interictal spikes and isolated fast ripples in CA3. No significant relationship was found in both groups.
As illustrated in Fig. 5E, isolated fast ripples occurred at significantly lower rates in the CA3 of LCM-treated animals compared to controls (p < 0.05). They also significantly increased over time (r = 0.65, p < 0.05) but were not correlated with seizure occurrence (Fig. 5F). Moreover, in both groups, isolated fast ripples and fast ripples associated with interictal spikes were not correlated, indicating that they displayed distinct patterns of occurrence over time (Fig. 5G). Thus, our results show that contrary to what was observed for fast ripples associated to interictal spikes in CA3, isolated fast ripples are not modulated by seizures even though they are sensitive to LCM. In addition, in EC, DG and subiculum, rates of isolated fast ripples were significantly higher in controls compared to LCM-treated animals (p < 0.05).
Discussion
The main results of our study can be summarized as follows. First, only 25% of LCM-treated animals showed seizures compared to all controls. LCM-treated animals also showed significantly lower seizure rates and a longer latent period. Second, rates of interictal spikes in CA3 and subiculum were significantly lower in LCM-treated animals than in controls. Third, rates of ripples associated with interictal spikes and rates of isolated ripples were lower in LCM-treated rats and were correlated over time in CA3. Fourth, rates of fast ripples associated with interictal spikes and rates of isolated fast ripples were significantly lower in LCM-treated in CA3; however, the two types of fast ripples were not correlated with each other, and only fast ripples associated with interictal spikes were correlated with seizure occurrence.
Only 25% of animals treated with LCM showed seizures compared to all controls. In addition, these LCM-treated epileptic rats had a significant smaller number of seizures. Our results are in line with clinical studies showing that LCM has anti-ictogenic properties in patients with partial-onset seizures (Chung et al., 2010; Li et al., 2013); however the percentage of patients reaching seizure control in placebo-controlled trials does not exceed 50%. The pilocarpine model of MTLE is known to reproduce the level of pharmacoresistance that is characteristic of this epileptic syndrome (Curia et al., 2008). Further studies are needed to confirm the efficiency of LCM in the pilocarpine model over time, but the anti-ictogenic efficacy of LCM identified in our study suggests that targeting the latent period may represent an interesting strategy in MTLE, assuming that predictive tools may become available in the future to identify patients at risk of developing this syndrome.
We have also observed that LCM increased the duration of the latent period in treated animals compared to controls. Brandt et al. (2006) have also reported an effect of LCM on the duration of the latent period, since they observed that it can significantly delay kindling following stimulation of the basolateral amygdala. However, the effect of LCM treatment on seizure severity and the duration of afterdischarges disappeared when those animals were re-kindled after a treatment-free period of 2.5 month. In addition, a recent study found a neuroprotective effect of LCM on hippocampal and parahippocampal structures when administered after a SE induced with prolonged stimulation of the basolateral amygdala, without however any effect on the duration of the latent period (Licko et al., 2013). Although the methods used to monitor seizures are different between the study of Licko et al. (2013) and our study, these results highlight the need for additional studies addressing the potential disease-modifying properties of LCM.
We have observed that in both CA3 and subiculum, interictal spike rates in LCM-treated animals were significantly lower than in controls. To date, no study has specifically explored the effect of LCM on interictal spikes in patients with MTLE. However, in patients with pharmacoresistant focal epilepsy, Li et al. (2013) found a non-significant reduction of interictal epileptiform activity after acute intravenous LCM treatment, whereas no effect were observed by Giorgi et al. (2013) following adjunctive per os treatment with LCM. Moreover, it has been reported in the perforant path stimulation model that LCM induces a decrease in interictal spike rates in the dentate gyrus 6 weeks after SE (Wasterlain et al., 2011). Therefore, our study is the first to provide evidence of the ability of LCM to decrease interictal spike occurrence in CA3 and subiculum in a well-established animal model of MTLE. It should be emphasized that these two limbic areas are known to play important roles in MTLE. Indeed, the CA3 region is highly sensitive to neuronal damage induced by the initial SE (Ben-Ari et al., 1980). It is one of the first regions to show epileptiform activity during the latent period (Lothman et al., 1981) and is often the onset zone of spontaneous seizures in pilocarpine-treated animals (Bortel et al., 2010; Levesque et al., 2011, 2012). Pathological network activity in this region is thus likely to lead to excessive neuronal synchronization and ictogenesis.
As previously reported by Levesque et al. (2011), fast ripples, but not ripples, associated with interictal spikes in CA3 were tightly linked to seizure occurrence. Similar results were reported in a study performed on epileptic patients in which HFOs associated with interictal spikes recorded in the hippocampus and para-hippocampal structures were shown to directly correlate with seizure occurrence (Zijlmans et al., 2009). Since fast ripples are thought to reflect the hypersynchronous firing of principal (glutamatergic) neurons in seizure onset zones (Jefferys et al., 2012), we are inclined to propose that LCM prevents excessive neuronal synchronization in the hippocampus, which thus decreases fast ripple activity and seizure occurrence in the pilocarpine model of MTLE.
Fast ripples associated with interictal spikes in the subiculum only increased in occurrence after seizure clustering in controls, suggesting that they were modulated by seizure occurrence. The subiculum is known to play an important role in the pilocarpine model of MTLE and in the human condition. It was proposed that the high vulnerability of subicular GABAergic interneurons and the hyperexcitability of subicular networks following a pilocarpine-induced SE might contribute to epileptogenesis and ictogenesis in this model (de Guzman et al., 2006; Knopp et al., 2008). In humans, studies performed on resected tissue from patients with temporal lobe epilepsy also suggest that changes in subicular network activity might contribute to the generation of ictal (Huberfeld et al., 2011) and interictal activity in the sclerotic (Cohen et al., 2002) and non-sclerotic tissue (Wozny et al., 2005). In vivo, depth recordings from the subiculum in MTLE patients have revealed the existence of focal and highly synchronous subicular interictal spikes (Fabo et al., 2008).
Rates of isolated HFOs behaved differently than HFOs associated to interictal spikes. They were not linked to the occurrence of seizures in controls and were reduced in occurrence under LCM. These results are in line with those obtained in humans, in which it was shown that isolated HFOs increase after medication reduction whereas HFOs co-occurring with interictal spikes do not increase (Zijlmans et al., 2009). Our results further suggest that among isolated HFOs, isolated fast ripples may represent a unique category of epileptic biomarkers, since their occurrence was neither correlated with seizure occurrence nor with fast ripples associated to interictal spikes. Moreover, in controls but not in LCM-treated animals, rates of isolated fast ripples linearly increased over time. This increase could be explained by the progressive neuronal loss in the hippocampus after SE. Indeed, a reduction of hippocampal volume was found in pilocarpine-treated animals after SE (Nairismagi et al., 2006; Niessen et al., 2005; Polli et al., 2014) and high rates of fast ripples were linked to small hippocampal volumes in epileptic patients (Staba et al., 2007). We also recently found that in CA3, isolated fast ripples, but not fast ripples associated to interictal spikes, occur at high rates during the chronic period (Salami et al., 2014). Thus, isolated fast ripples and fast ripples associated to interictal spikes may herald different pathophysiological mechanisms underlying MTLE that are differentially affected by anti-epileptic drugs.
In CA3, rates of ripples associated with interictal spikes and rates of isolated ripples were lower in LCM-treated animals than in controls. Both ripples subtypes showed significant correlation with each other in this structure, but did not correlate with seizure occurrence. Ripples are thought to reflect the summation of post-synaptic inhibitory potentials (Ylinen et al., 1995). Ripples associated with interictal spikes could thus mirror the inhibitory barrage following interictal hypersynchronization (Ulbert et al., 2004). In CA3, isolated ripples were correlated to ripples associated to interictal spikes, suggesting either that they reflect the same mechanism or that some interictal spikes did not cross the threshold and were not detected. In treated animals, lower rates of isolated ripples compared to controls were found in CA3 and EC; whereas isolated fast ripples were lower only in CA3. These findings support the view that ripples and fast ripples may arise from different networks (Staba et al., 2007). Human studies suggest that fast ripples may be more reliable markers for the identification of the seizure onset zone compared to ripples (Jacobs et al., 2008; Staba et al., 2002) and that isolated HFOs and HFOs associated with interictal spikes may behave differently with regards to seizure occurrence and medication withdrawal (Zijlmans et al., 2009). We thus propose that isolated fast ripples in CA3 may indeed represent another biomarker of disease activity as well as a target for anti-epileptic drug development.
We have also observed that isolated fast ripples occur at higher rates in EC, DG and subiculum in controls compared to LCM-treated animals. This result was unexpected and is actually difficult to interpret. It could be due to the fact that LCM reduces the amplitude of interictal spikes associated to fast ripples and that they are missed by the automatic detector. The HFOs that are detected are thus analysed as isolated fast ripples. However, this explanation seems unlikely since all detected interictal spikes and HFOs were visually analysed and rates of interictal spikes were similar in occurrence in LCM-treated animals compared to controls in EC and DG. Moreover, no significant correlation was observed between rates of isolated fast ripples and rates of fast ripples associated to interictal spikes. These results thus merit further investigation.
Conclusions
We have provided evidence that early LCM treatment has effective anti-ictogenic properties in the pilocarpine model of MTLE. These effects are accompanied by decreases in interictal spike rates and HFO occurrence in the hippocampus. We have also found that in the hippocampus, isolated HFOs and HFOs associated with interictal spikes may represent biomarkers of distinct network mechanisms that are differentially affected by anti-epileptic medication. In particular, we have further shown that isolated fast ripples in CA3 may represent a reliable biomarker of disease activity. Future studies should be aimed at demonstrating an enduring efficiency of LCM treatment, when administered early in the epileptogenic process.
Acknowledgments
This study was supported by an investigator initiated grant from UCB Pharma as well as by operating grants from the Canadian Institutes of Health Research (8109 and 74609) and the Savoy Foundation. CB is a recipient of a Student Scholarship from the Savoy Foundation.
Footnotes
Disclosure
MA received an investigator initiated grant from UCB Pharma. The remaining authors have no conflict of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP, Meldrum BS. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980;191:79–97. doi: 10.1016/0006-8993(80)90316-9. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48:1308–1317. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Loiseau P, Perucca E. Progress report on new antiepileptic drugs: a summary of the Sixth Eilat Conference (EILAT VI) Epilepsy Res. 2002;51:31–71. doi: 10.1016/s0920-1211(02)00106-7. [DOI] [PubMed] [Google Scholar]
- Bortel A, Levesque M, Biagini G, Gotman J, Avoli M. Convulsive status epilepticus duration as determinant for epileptogenesis and interictal discharge generation in the rat limbic system. Neurobiol Dis. 2010;40:478–489. doi: 10.1016/j.nbd.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999a;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid—treated rats with chronic seizures. Epilepsia. 1999b;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Brandt C, Heile A, Potschka H, Stoehr T, Loscher W. Effects of the novel antiepileptic drug lacosamide on the development of amygdala kindling in rats. Epilepsia. 2006;47:1803–1809. doi: 10.1111/j.1528-1167.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, Jones-Gotman M, Olivier A, Andermann E, Robitaille Y, Lopes-Cendes I, et al. Early childhood prolonged febrile convulsions, atrophy and sclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology. 1993;43:1083–1087. doi: 10.1212/wnl.43.6.1083. [DOI] [PubMed] [Google Scholar]
- Chung S, Sperling MR, Biton V, Krauss G, Hebert D, Rudd GD, Doty P, Group SPS. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51:958–967. doi: 10.1111/j.1528-1167.2009.02496.x. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Cross SA, Curran MP. Lacosamide: in partial-onset seizures. Drugs. 2009;69:449–459. doi: 10.2165/00003495-200969040-00005. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman P, Inaba Y, Biagini G, Baldelli E, Mollinari C, Merlo D, Avoli M. Subiculum network excitability is increased in a rodent model of temporal lobe epilepsy. Hippocampus. 2006;16:843–860. doi: 10.1002/hipo.20215. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K Early Randomized Surgical Epilepsy Trial Study G. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington AC, Stohr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol. 2008;73:157–169. doi: 10.1124/mol.107.039867. [DOI] [PubMed] [Google Scholar]
- Fabo D, Magloczky Z, Wittner L, Pek A, Eross L, Czirjak S, Vajda J, Solyom A, Rasonyi G, Szucs A, Kelemen A, Juhos V, Grand L, Dombovari B, Halasz P, Freund TF, Halgren E, Karmos G, Ulbert I. Properties of in vivo interictal spike generation in the human subiculum. Brain. 2008;131:485–499. doi: 10.1093/brain/awm297. a journal of neurology. [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Pizzanelli C, Pelliccia V, Di Coscio E, Maestri M, Guida M, Iacopini E, Iudice A, Bonanni E. A clinical-EEG study of sleepiness and psychological symptoms in pharmacoresistant epilepsy patients treated with lacosamide. Epilepsy Res Treat. 2013 doi: 10.1155/2013/593149. http://dx.doi.org/10.1155/2013/593149. Article ID 593149. [DOI] [PMC free article] [PubMed]
- Halasz P, Kalviainen R, Mazurkiewicz-Beldzinska M, Rosenow F, Doty P, Hebert D, Sullivan T, Group SPS. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50:443–453. doi: 10.1111/j.1528-1167.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- Hovinga CA. SPM-927 (Schwarz Pharma) IDrugs. 2003;6:479–485. the investigational drugs journal. [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci. 2011;14:627–634. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp A, Frahm C, Fidzinski P, Witte OW, Behr J. Loss of GABAergic neurons in the subiculum and its functional implications in temporal lobe epilepsy. Brain. 2008;131:1516–1527. doi: 10.1093/brain/awn095. a journal of neurology. [DOI] [PubMed] [Google Scholar]
- Levesque M, Bortel A, Gotman J, Avoli M. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2011;42:231–241. doi: 10.1016/j.nbd.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. (the official journal of the Society for Neuroscience) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Stefan H, Matzen J, Rampp S, Heinze HJ, Schmitt FC. Rapid loading of intravenous lacosamide: efficacy and practicability during presurgical video-EEG monitoring. Epilepsia. 2013;54:75–80. doi: 10.1111/j.1528-1167.2012.03651.x. [DOI] [PubMed] [Google Scholar]
- Licko T, Seeger N, Zellinger C, Russmann V, Matagne A, Potschka H. Lacosamide treatment following status epilepticus attenuates neuronal cell loss and alterations in hippocampal neurogenesis in a rat electrical status epilepticus model. Epilepsia. 2013;54:1176–1185. doi: 10.1111/epi.12196. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Collins RC, Ferrendelli JA. Kainic acid-induced limbic seizures: electrophysiologic studies. Neurology. 1981;31:806–812. doi: 10.1212/wnl.31.7.806. [DOI] [PubMed] [Google Scholar]
- Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49:248–255. doi: 10.1111/j.1528-1167.2007.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montplaisir J, Laverdière M, Saint-Hilaire JM. Sleep and epilepsy. In: Gotman J, et al., editors. Long-Term Monitoring in Epilepsy. Suppl No 37. Elsevier Science; Amsterdam: 1985. pp. 215–239. [PubMed] [Google Scholar]
- Nairismagi J, Pitkanen A, Kettunen MI, Kauppinen RA, Kubova H. Status epilepticus in 12-day-old rats leads to temporal lobe neurodegeneration and volume reduction: a histologic and MRI study. Epilepsia. 2006;47:479–488. doi: 10.1111/j.1528-1167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- Niespodziany, et al. Effects of lacosamide versus classical sodium channel blocking antiepileptic drugs on sodium current slow inactivation parameters. J Neurosci Res. 2013;91:436–443. doi: 10.1002/jnr.23136. [DOI] [PubMed] [Google Scholar]
- Niessen HG, Angenstein F, Vielhaber S, Frisch C, Kudin A, Elger CE, Heinze HJ, Scheich H, Kunz WS. Volumetric magnetic resonance imaging of functionally relevant structural alterations in chronic epilepsy after pilocarpine-induced status epilepticus in rats. Epilepsia. 2005;46:1021–1026. doi: 10.1111/j.1528-1167.2005.60704.x. [DOI] [PubMed] [Google Scholar]
- Paxinos GWC. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Polli RS, Malheiros JM, Dos Santos R, Hamani C, Longo BM, Tannus A, Mello LE, Covolan L. Changes in hippocampal volume are correlated with cell loss but not with seizure frequency in two chronic models of temporal lobe epilepsy. Front Neurol. 2014;5:111. doi: 10.3389/fneur.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Salami P, Levesque M, Benini R, Behr C, Gotman J, Avoli M. Dynamics of interictal spikes and high-frequency oscillations during epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2014;67C:97–106. doi: 10.1016/j.nbd.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal–hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, Ogren J, Fried I, Wilson CL, Engel J., Jr Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Stohr T, Kupferberg HJ, Stables JP, Choi D, Harris RH, Kohn H, Walton N, White HS. Lacosamide, a novel anti-convulsant drug, shows efficacy with a wide safety margin in rodent models for epilepsy. Epilepsy Res. 2007;74:147–154. doi: 10.1016/j.eplepsyres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Swanson TH. The pathophysiology of human mesial temporal lobe epilepsy. J Clin Neurophysiol. 1995;12:2–22. official publication of the American Electroencephalographic Society. [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Heit G, Madsen J, Karmos G, Halgren E. Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia. 2004;45(Suppl 4):48–56. doi: 10.1111/j.0013-9580.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Stohr T, Matagne A. The acute and chronic effects of the novel anticonvulsant lacosamide in an experimental model of status epilepticus. Epilepsy Res. 2011;94:10–17. doi: 10.1016/j.eplepsyres.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozny C, Gabriel S, Jandova K, Schulze K, Heinemann U, Behr J. Entorhinal cortex entrains epileptiform activity in CA1 in pilocarpine-treated rats. Neurobiol Dis. 2005;19:451–460. doi: 10.1016/j.nbd.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. the official journal of the Society for Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]