Abstract
In vitro studies performed on brain slices demonstrate that the potassium channel blocker 4-aminopyridine (4AP, 50 μM) discloses electrographic seizure activity and interictal discharges. These epileptiform patterns have been further analyzed here in a isolated whole guinea pig brain in vitro by using field potential recordings in olfactory and limbic structures. In 8 of 13 experiments runs of fast oscillatory activity (fast runs, FRs) in the piriform cortex (PC) propagated to the lateral entorhinal cortex (EC), hippocampus and occasionally to the medial EC. Early and late FRs were asynchronous in the hemispheres showed different duration [1.78 ± 0.51 and 27.95 ± 4.55 (SD) s, respectively], frequency of occurrence (1.82 ± 0.49 and 34.16 ± 6.03 s) and frequency content (20–40 vs. 40–60 Hz). Preictal spikes independent from the FRs appeared in the hippocampus/EC and developed into ictal-like discharges that did not propagate to the PC. Ictal-like activity consisted of fast activity with onset either in the hippocampus (n = 6) or in the mEC (n = 2), followed by irregular spiking and sequences of diffusely synchronous bursts. Perfusion of the N-methyl-D-aspartate receptor antagonist 2-amino-5-phosphonopentanoic acid (100 μM) did not prevent FRs, increased the duration of limbic ictal-like discharges and favored their propagation to olfactory structures. The AMPA receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (50 μM) blocked ictal-like events and reduced FRs. In conclusion, 4AP-induced epileptiform activities are asynchronous and independent in olfactory and hippocampal-entorhinal regions. Epileptiform discharges in the isolated guinea pig brain show different pharmacological properties compared with rodent in vitro slices.
INTRODUCTION
In vitro brain preparations have been extensively used to study the mechanisms underlying neuronal hyperexcitability and epileptiform synchronization. Pharmacological treatment of isolated adult hippocampal slices with pro-epileptic agents induces interictal- and afterdischarge-like activities but infrequently causes the appearance of electrographic seizures. However, prolonged epileptiform events sharing electrographic similarities with the seizure discharges seen in patients with temporal lobe epilepsy can be induced in extended brain slice preparations that include hippo- and parahippocampal areas (de Curtis and Gnatkovsky 2009).
Studies performed in rodent combined slices with the K+ channel blocker, 4-aminopyridine (4AP), have demonstrated that ictal discharges dependent on N-methyl-D-aspartate (NMDA) receptor activation initiate in parahippocampal areas such as the entorhinal cortex (EC) or the amygdala and secondarily propagate to the hippocampus proper (reviewed by Avoli 2002). Two types of interictal events were identified in these experiments: one type was independent of glutamatergic transmission, was abolished by GABAA receptor antagonists and could emerge from any hippocampal or parahippocampal area; the other type was driven by CA3 networks and could control the propensity of parahippocampal structures to generate ictal discharges (Avoli et al. 2002; Barbarosie and Avoli 1997). Hence in the in vitro brain slice preparation generation of seizure-like discharges is a feature of parahippocampal structures, where it is caused by NMDA receptor activation and can be controlled by hippocampus-driven interictal activity.
Experiments performed in the isolated guinea pig brain preparation during brief applications of the GABAA receptor antagonist bicuculline have shown that seizure-like discharges can indifferently initiate either in the hippocampus (CA1 subiculum) or in the medial EC. In this preparation, long-range connections among limbic brain structures, inter-hemispheric connections, and the extracellular milieu are functionally preserved. Therefore we tested here whether the initiation and propagation patterns of epileptiform discharges induced in rodent brain slices by 4AP could be reproduced in the intact guinea pig whole-brain maintained in vitro.
METHODS
Adult Hartley guinea pigs (150–200 g) were anesthetized with sodium thiopental (125 mg/kg ip, Farmotal, Milan, Pharmacia) and were trans-cardially perfused with a cold (10°C) carboxygenated (95% O2-5% CO2) saline solution (composition, in mM: 126 NaCl, 3 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26 NaHCO3, 15 glucose, 2.1 HEPES, and 3% dextran M.W. 70000, pH = 7.1). Following decapitation, brains were dissected out and transferred into the recording chamber. A polyethylene cannula was inserted into the basilar artery, and brain perfusion was restored with the above solution (15°C; pH 7.3) at 6 ml/min via a peristaltic pump (Minipulse 3, Gilson, France). The temperature of both chamber and perfusate was slowly raised to 32°C (de Curtis et al. 1991, 1998; Muhletaler et al. 1993). This temperature was maintained for the entire experiment as substitute for anesthesia (Pearcy and Virtue 1959). The number of animals used and their suffering was minimized according to the International guidelines on ethical use of animals. The experimental protocol (for details, see) was reviewed and approved by the Committee on Animal Care and Use and by Ethics Committee of the Fondazione Istituto Neurologico Carlo Besta.
Responses evoked in the olfactory-limbic regions by stimulation of the lateral olfactory tract (LOT) were monitored during the experiment (see Fig. 1A). Previous studies demonstrated that LOT-evoked responses show a high stability in this region in healthy isolated brains (Biella and de Curtis 2000); therefore we utilized this test to confirm the viability of the preparation. Stimuli were delivered through an isolation unit driven by a pulse generator (Grass Telefactor S88, Warwick, RI). Stimulating and recording electrodes were positioned under direct visual control with a stereoscopic microscope. Extracellular recordings were simultaneously performed in the II/III layers of piriform cortex (PC), III layers of lateral and deep layers of medial entorhinal cortex (l-EC and m-EC, respectively) and CA1 hippocampal region (CA1) with NaCl-filled micropipettes (5–8 μm tip diameter, 5–10 MΩ resistance). In most experiments, recordings were obtained from both hemispheres (e.g., experiment shown in Fig. 1A). Signals were amplified with a multichannel differential amplifier (Biomedical Engineering, Thornwood, NY), digitized via an AT-MIO-64E3 National A/D Board (National Instrument, Milan, Italy), and analyzed with a custom-made software (ELPHO) developed in Labview by V. Gnadtkovsky.
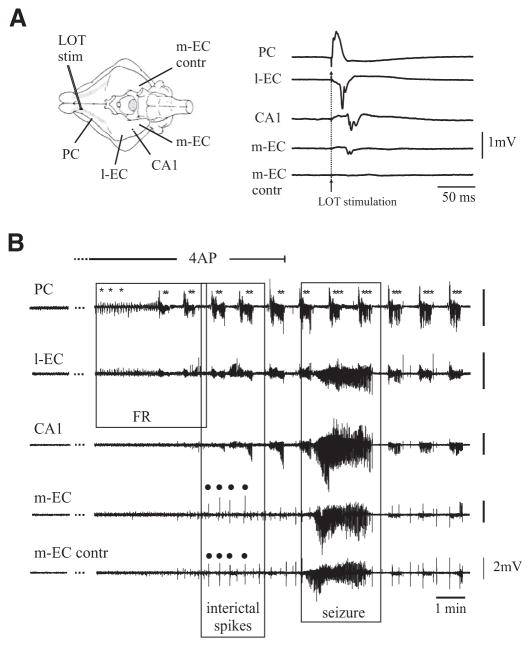
FIG. 1.
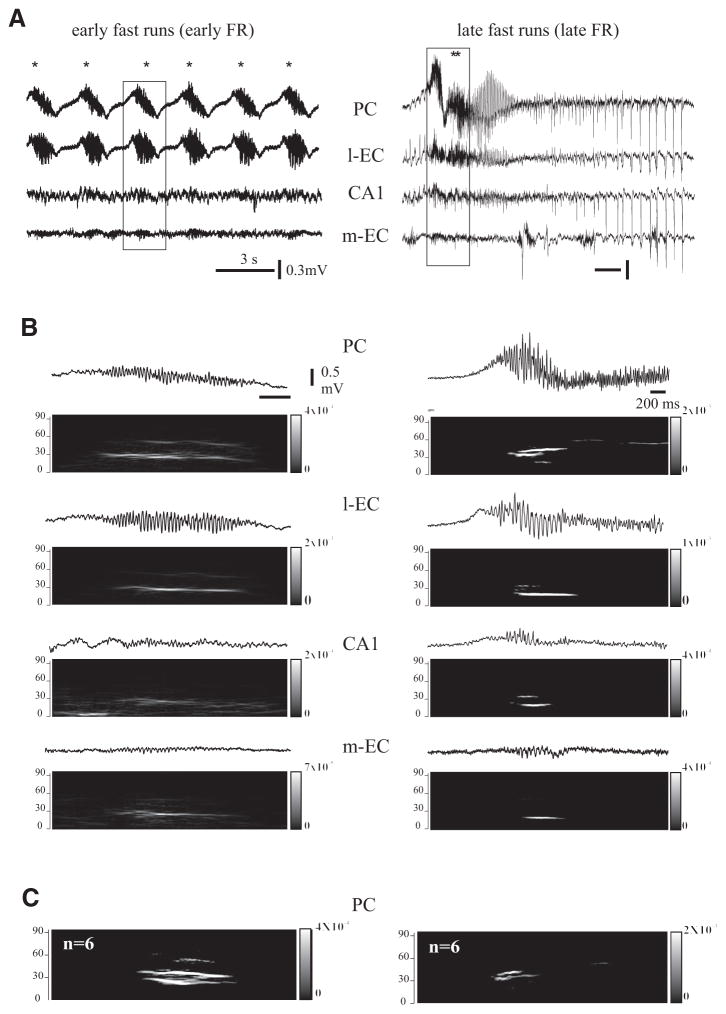
A: scheme of the isolated guinea pig brain maintained in vitro with the position of the stimulating electrode on the lateral olfactory tract (LOT) and the recording electrodes positioned in piriform cortex (PC), lateral entorhinal cortex (l-EC), CA1 area of the hippocampus, medial entorhinal cortex (m-EC), and contralateral medial entorhinal cortex (m-EC contr). LOT stimulation was performed to test the viability of the brain and the evoked responses in the different structures are shown (right). B: arterial perfusion with 4-aminopyridine (4AP, 50 μM) induces a highly reproducible pattern. Spontaneous activity begins with early fast runs (FRs) originating in the PC (PC trace, *), that are followed by late FR (PC trace, **). Note that differently from the early FRs, late FRs propagate to both l-EC and hippocampus CA1. Later on interictal spikes appear in m-EC (m-EC trace, ●) and propagate bilaterally before the seizure onset. Note also that the seizure propagates to the contralateral hemisphere but spares the PC that continues to exhibit FR activity (***). The period of application of 4AP is indicated by the line above the electrophysiological traces.
To evaluate the frequency content of extracellular signals recorded in PC, l-EC, CA1, and m-EC, amplitude spectra (Vrms) were computed with a fast Fourier transform (FFT) algorithm. To visualize how the frequency content of the field potential signals evolves over time, joint time-frequency analysis was applied. Correlation coefficient (r) of the field potential signals recorded in PC versus l-EC, CA1, and m-EC was calculated in sequences of 40 ms time windows shifted by 20 ms intervals. The same method was applied to evaluate the correlation coefficient in all the possible combinations among PC, l-EC, CA1, and m-EC (see Fig. 3). The correlation coefficient analysis was performed with a 2 s window during runs of fast oscillatory activity. To focus on the difference between mean correlation coefficients, data were presented as square values of r (r2). Statistical analyses were performed using Student’s t-test for independent samples.
FIG. 3.
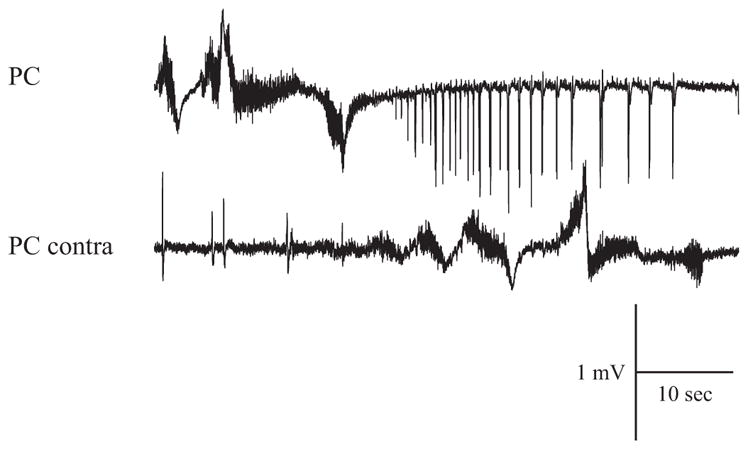
Indipendent FRs recorded in PC and contralateral PC.
The following chemicals, acquired either from Sigma-Aldrich (Milan, Italy) or from Tocris Cookson (Ellisville, MO), were arterially perfused in the isolated brains: 4AP (50 μM), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 50 μM), 6,7-dinitroquinoxaline-2,3-dione (DNQX, 50 μM), and 2-amino-5-phosphonopentanoic acid (AP5, 100 μM).
RESULTS
Patterns of spontaneous activity induced by 4AP in the in vitro isolated guinea pig brain
First, we analyzed the spontaneous activity induced by arterial perfusion of 50 μM 4AP (n = 13).
This concentration of 4AP is commonly used in experiments performed in rat brain slices (review by Avoli 2002) and was effective in inducing epileptiform activities in the isolated guinea pig brain (Uva et al. 2009). Recording of LOT-evoked field potential activity from PC, l-EC, m-EC and from CA1 hippocampal subfield were performed (Fig. 1A). In 10 experiments, the activity was monitored in both hemispheres, from PC, CA1, or m-EC. After careful evaluation of activities in different cortical and subcortical regions of the brain that include neocortex, thalamus, and basal nuclei, we selected these brain areas because they generate epileptiform events. Olfactory-limbic structures are activated in the isolated brain also in the bicuculline and pilocarpine models (Gnatkovsky et al. 2008; Uva et al. 2005, 2008). In these olfactory-limbic cortices, small amplitude background activity in the theta/gamma range was commonly observed before perfusing 4AP (see Fig. 4A, left). As illustrated in Fig. 1B (*), brief runs of fast oscillatory activity (thereafter termed fast runs, FRs) were observed in the PC within 4.5 ± 1.2 (mean ± SE) min (n = 5 of 13) after the beginning of 4AP perfusion. In five experiments, neither FR nor epileptiform activity was observed in the olfactory region (PC). FRs activity was detected by visual inspection on off-line digitized traces. It propagated to the l-EC (n = 8 of 13), to the hippocampus (n = 5 of 13), and, less frequently, to the m-EC (n = 4 of 13).
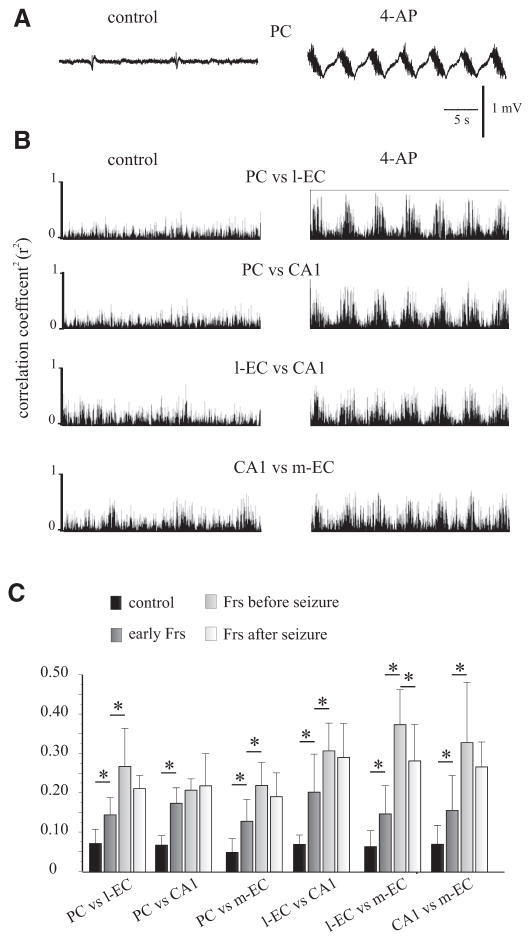
FIG. 4.
Correlation analysis of the early FRs. A: field potential activity recorded in the PC before the onset of FRs (control) and during early FRs induced by 4AP. B: representation of the correlation coefficient (r2) of PC vs. l-EC, PC vs. CA1, l-EC vs. CA1, and CA1 vs. m-EC were evaluated during a period of 30 s in control condition and during the early FRs. Note the increase of r2 with the appearance of FRs. C: histogram representation of r2 during control (black), early-FRs (dark gray), late-FRs before seizure (gray), and FRs after seizure (white). Note that r2 increases in all the possible pairs of structures from the control phase to the l-FRs recorded before seizure onset in 4 different experiments.
These initial brief FRs lasted 1–2 s and progressively evolved in longer events that were superimposed on a slow potential (** in Figs. 1B and 2A). The propagation of these prolonged FRs to the m-EC (n = 5 of 8) was more consistent than for the early FRs observed at the onset of 4AP administration.
Simultaneous bilateral recordings in the PC (n = 2 of 2), m-EC (n = 3 of 6), and CA1 area (n = 2 of 4) demonstrated late FRs that occurred independently and asynchronously in the two hemispheres (Figs. 3 and 6). After 6.5 ± 4.3 min of 4AP perfusion, interictal spikes were observed bilaterally in the m-EC (n = 6 of 13) and in the CA1 region of the hippocampus (n = 4 of 13; dots in Figs. 1B and 4–6). Approximately 2 min after the appearance of interictal spikes, 4AP induced seizure-like discharges in limbic cortices, i.e., the hippocampus and EC (n = 8 of 13; Fig. 1B, but see also Figs. 5 and 6). Interestingly, the appearance of FR in the olfactory cortices and the generation of epileptiform events in the limbic region were independent and may occur at different times after the onset of 4AP perfusion (see DISCUSSION). The differences in the initiation of these segregated patterns is illustrated by comparing Figs. 1 and 5.
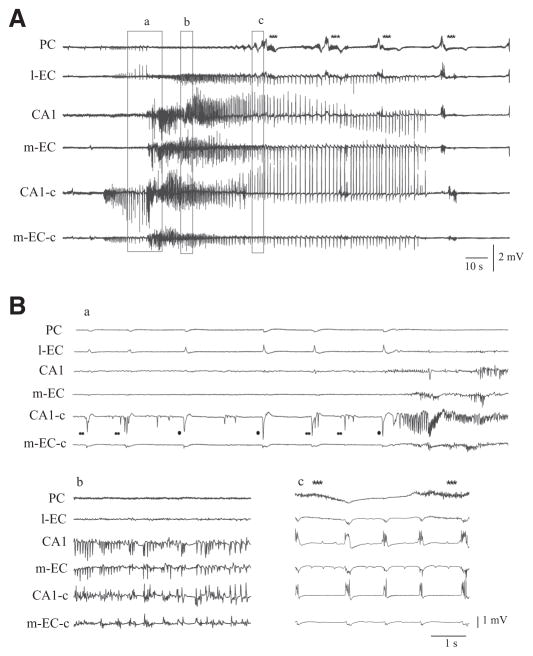
FIG. 6.
4AP-induced epileptiform activity in the in vitro isolated guinea pig brain. A: the entire sequence of epileptiform activity recorded simultaneously in olfactory and limbic structures is shown. B: expansion of sections as indicated in A. Note in a that preictal spikes are recorded in the contralateral hemisphere (CA1-c and m-EC-c). Some of these interictal events (●) resemble the GABA-mediated potentials reported in a previous work during co-perfusion of 4AP and glutamate receptor antagonists. Note that after the preictal GABA-mediated spikes (●) and complex spikes (● ●) fast onset activity is recorded but not in the olfactory areas. Note also in b that irregular spiking appears later on, whereas in c, hypersynchronous burst activity originates in the hippocampus with components that propagate to the EC. Note also in c that FRs appear in the PC during the burst activity (***).
FIG. 5.
A: late FRs were followed by interictal spikes (◀). We compared these spike (a, ◀) with the preictal spikes (b, ●). B: expansions of the 2 sections shown in A. Note that the interictal spikes that follow the late FRs originate in PC and propagated to l-EC, CA1, and m-EC. In contrast, the preictal spikes originate in m-EC and propagated to CA1 and l-EC, without invading the PC. C: further expansion of spikes shown in B.
The early brief FRs lasted 1.78 ± 0.51 s (n = 8 of 13) and recurred every 1.82 ± 0.49 s, whereas the late, prolonged FRs extended over 27.95 ± 4.55 s and recurred at intervals of 34.16 ± 6.03 s. In four of eight experiments, brief FRs were observed in isolation in the PC at the very onset of 4AP perfusion. Both brief and prolonged FRs were larger in amplitude in PC and l-EC, two cortical regions that are closely interconnected (Haberly and Price 1978; Krettek and Price 1977; Luskin and Price 1983). The gradual enhancement of FR amplitude over time in the CA1 region and in the m-EC also suggested that FRs propagated from olfactory areas (PC and l-EC) to the caudal limbic structures (Fig. 2). As illustrated by the experiment shown in Fig. 1B (***), FRs continued to occur during and after seizure activity in the EC-hippocampal region. In five of eight experiments, the frequency of recurrence and the general features of late FRs were not affected by seizures occurring in the limbic cortices (*** in Figs. 1B, 6 and 7A). In three experiments, however, FRs in PC disappeared during the electrographic seizures.
FIG. 2.
A: early (*) and late (**) FRs induced by 4AP recorded in PC, l-EC, CA1, and m-EC. In both cases, the fast activity propagates from the PC to the m-EC. B: expansions of early FRs and late FRs in all the recorded structures and relative joint time-frequency analysis. Note that early FRs are mainly characterized by frequency around 20 Hz, whereas in the late FRs, peak frequency was higher than 30 Hz. With the joint time-frequency analysis it is possible to follow the spread of FR from PC to m-EC. C: average of 6 early and late FRs recorded in PC.
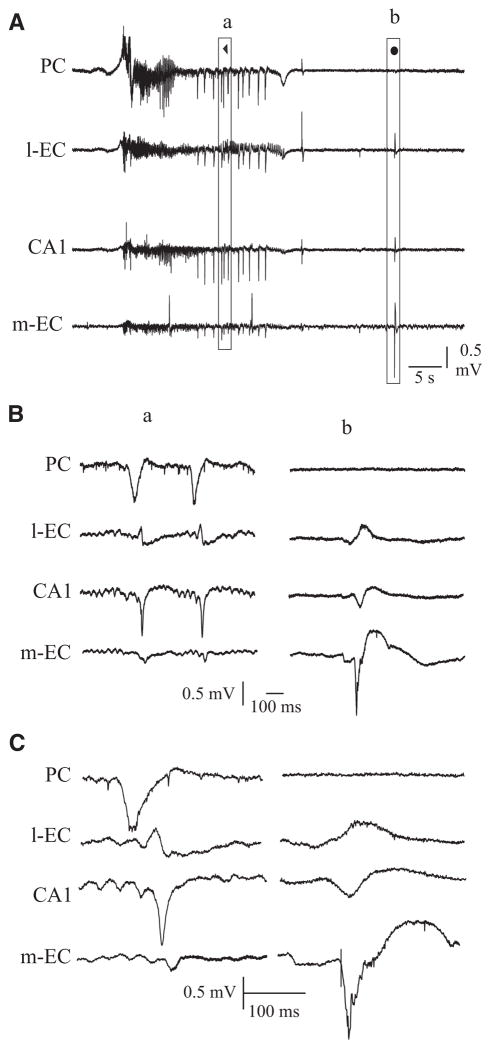
FIG. 7.
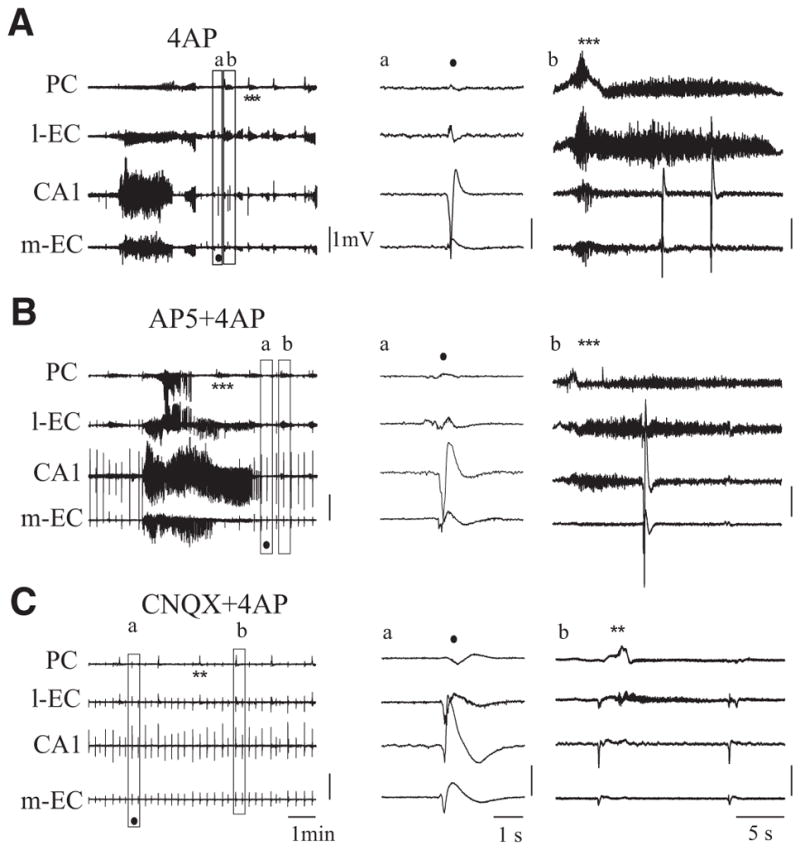
A: field activity occurring during arterial perfusion with 4AP. B: co-perfusion of 4AP and 2-amino-5-phosphonopentanoic acid (AP5) induces a prolongation of the seizure activity that spreads to PC. C: co-perfusion of 4AP and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) blocks seizure-like activity while interictal spikes (●) and low-amplitude FRs continue to occur. In A–C, examples of interictal spikes and FRs are expanded in a and b. **, late FRs; ***, FRs during and after seizure; ●, interictal spikes).
Single experiment and averaged power spectrum analysis of both early and late FRs demonstrated prominent frequency content in the low gamma range (i.e., 20–40 Hz) in all recording sites (Fig. 2, B and C). The frequency of the oscillations in late FRs increased with time from 20–40 to 40–60 Hz (Fig. 2B, right). Late FRs were superimposed on a complex slow wave and terminated with a sequence of highly synchronous spikes at low frequency (4–8 Hz; Figs. 2A, right, and 5, right). Square correlation coefficient (r2) between pairs of recorded structures is presented in Fig. 4. The average mean r2 measured during the early FRs showed a significant increase compared with pre-4AP conditions in all pairs of recorded sites (P < 0.05). Moreover, further significant increases of correlation were observed comparing early and late FRs recorded before seizure activity in all but PC/CA1 pair (n = 4 of 8; Fig. 4C).
The comparison between the correlation coefficient of late FRs before and after seizures in the limbic region showed a consistent but nonsignificant decrease of r2 in all pairs with the exception of PC/CA1. The spikes seen at the end of FRs (Fig. 5A, ◀) were well defined in PC and propagated to l-EC (12.59 ± 8.79 ms), CA1 (30.5 ± 8.93 ms), and m-EC (34.3±,7.29 ms) as demonstrated by the time delays shown in the expanded traces in Fig. 5, B and C.
Interictal spikes initiated in both hippocampus and m-EC 6.5 ± 4.3 min after the onset of 4AP perfusion and propagated bilaterally within the hippocampal-parahippocampal region. These spikes occurred independently from the PC spikes observed at the end of the FRs and their features resembled the GABA-mediated preictal potentials previously described in rodent slices (Perreault and Avoli 1992) and in the isolated guinea pig brain (Uva at el 2009). The hippocampal interictal activity developed into more complex spikes organized in burst-like events and consistently evolved into seizure discharges (• •in Fig. 6).
The onset of the ictal seizure-like discharges in the limbic cortices was characterized by the disappearance of FRs in m-EC and CA1 (but not in PC and l-EC) and by the occurrence of fast activity at 10–60 Hz that started either in the hippocampus (n = 6 of 13) or in the m-EC (n = 2 of 13) and propagated bilaterally throughout hippocampal and parahippocampal structures (n = 8 of 13, Figs. 1B and 6Ba). In five of eight experiments, the ictal discharge that initiated in the hippocampus/m-EC did not propagate to the PC, where FRs continued to be generated independently (*** in top traces in Figs. 1, 6A, and 7A). Seizure activity continued with irregular spiking (Fig. 6Bb) that progressively organized in bursts of high-amplitude spikes separated by brief silent intervals (Bc). Burst sequences synchronously propagated to the hippocampus and m-EC. During the late phase of the seizure, the interburst intervals became gradually longer and postictal depression ensued (Fig. 6A). In those experiments in which bilateral recordings were performed, seizure initiated in one hemisphere and consistently induced bilateral propagation to contralateral limbic cortices (n = 8 of 10; see Fig. 6A, bottom).
Pharmacological characteristics of the 4AP-induced epileptiform discharges
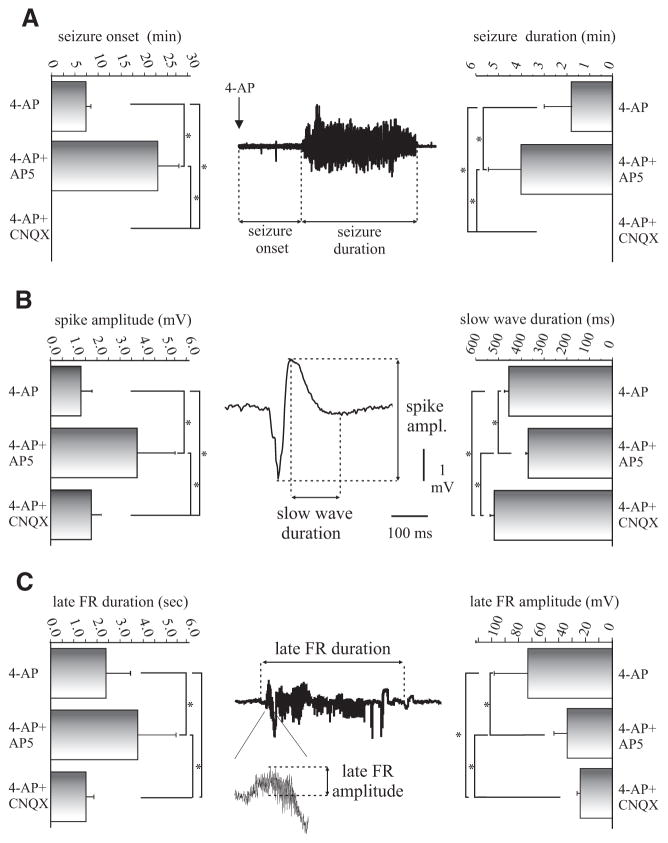
Next we analyzed the involvement of different glutamate receptors in generating and sustaining 4AP–induced epileptiform activity. As illustrated in Fig. 7B, NMDA receptors blockade with AP5 (100 μM, 30 min) and subsequent co-application of 4AP (50 μM) did not abolish seizure generation in four of five experiments. This procedure neither altered FRs in the olfactory cortices nor blocked spontaneous interictal events in CA1, m-EC and l-EC (* and • in Fig. 7B). The amplitude of interictal potentials in CA1 during co-perfusion of AP5 and 4AP increased (3.74 ± 1.72 mV, n = 5 of 5) when compared with those observed with 4AP alone (1.3 ± 0.48 mV, n = 4 of 13; * in Figs. 7, A and B, and Fig. 8). During co-perfusion of 4AP and AP5, the delay to the first limbic seizure was longer (25 ± 4.97 min; n = 4 of 5) than with application of 4AP alone (8.1 ± 1.17 min; n = 8 of 13). In addition, the duration of the limbic seizures increased from 1.91 ± 1.3 min in control (i.e., 4AP only) to 3.1 ± 1.1 min when AP5 was co-applied with 4AP. Finally, seizure activity propagated to the olfactory cortices during NMDA blockade (n = 4 of 5; Fig. 7, A and B).
FIG. 8.
A: seizures onset and duration recorded in CA1. Co-perfusion of 4AP and AP5 100 μM induced an increase statistically significant both in time onset and duration of ictal events, whereas 4AP+CNQX 50 μM completely abolished seizure occurrence. B: duration and amplitude of the slow wave of the interictal spikes recorded in CA1. Co-perfusion of 4AP and AP5 and CNQX induced, respectively, a decrease and an increase statistically significant of the duration of the preictal spike slow wave compared with 4AP alone. The amplitude of the peak of the interictal slow wave was increased during co-perfusion with 4AP+AP5 and 4AP+CNQX compared with 4AP alone (P < 0.05). C: duration and amplitude of late FRs recorded in PC. Co-perfusion of 4AP and AP5 induced, respectively, an increase and a decrease statistically significant of both duration and amplitude of FRs. Otherwise 4AP+CNQX induced a decrease in the duration of FRs and a statistically significant decreased in amplitude of FRs.
Prolonged (over 60 min) co-perfusion of 4AP with AMPA receptors antagonists, either CNQX (50 μM; n = 2) or DNQX (50 μM; n = 2), completely abolished ictal epileptiform discharges (Fig. 7C) but did not affect limbic interictal spikes that were spared by AMPA receptor antagonists (dots in Fig. 7C) (see Uva et al. 2009). The average measurement of the spike amplitude and the duration of the slow wave that follows the interictal spike recorded in the CA1 region in control condition and during selective blockade of NMDA or AMPA receptors are shown in Fig. 8. FRs persisted during AMPA receptor blockade, but they were shorter in duration and of lower amplitude than during 4AP perfusion alone (* in Fig. 7, A and C). Figure 8 illustrates the average measurement of late FR duration and amplitude recorded in PC, of seizure onset and duration, of the spike amplitude and the duration of the slow wave that follows the interictal spike recorded in the CA1 region before and during selective blockade of NMDA or AMPA receptors.
DISCUSSION
Arterial perfusions of the in vitro guinea pig brain with 4AP induced a sequence of epileptiform activities recorded in olfactory (PC and l-EC) and limbic (CA1 and m-EC) cortices. The common pattern consisted of runs of fast oscillatory activity (FRs) originating in PC and propagating through the olfactory/limbic system and interictal and ictal epileptiform discharges in the hippocampal/entorhinal circuit. Both these events represent epileptiform phenomena generated by different structures and appear to occur independently. The time of appearance of the specific patterns recorded either in the olfactory region (PC and lateral EC) or in the limbic cortices (hippocampus and medial EC) could be different in distinct experiments, reinforcing the concept that these two cortical systems and the synchronous patterns sustained by their intrinsic networks are separate and independent. In spite of the different delay-to-onset after the initiation of 4AP application, the sequence of events observed within either one of the systems is quite stereotyped; hyperexcitability induced by 4AP expresses through different patterns of synchronization (FRs, spikes, seizures) that are system-dependent and -specific. Interactions between olfactory and limbic regions may occur when excitability is highly enhanced after the repetition of seizure-like events (see also Librizzi and de Curtis 2003). This could be due to the disinhibition and progressive entrainment of weak synaptic interactions between the two systems.
FRs were characterized by brief sequences of oscillatory activity originating in the PC and propagating along the olfactory pathway to the l-EC, the hippocampus, and the m-EC. FRs features changed over time during 4AP perfusion. Early FRs appeared first in the PC, were brief (~1–2 s) and showed frequency range between 20 and 40 Hz. Late FRs were characterized by faster oscillations at 40–60 Hz, superimposed to a slow potential and were terminated by a series of large amplitude spikes. We analyzed the correlation between all the possible combination of the recorded structures during FRs and found an increase in the mean of r2 (r = correlation coefficient) from the baseline to the late FRs, suggesting a gradual entrainment of synchronization in successive synaptic stations along the olfactory-limbic pathway. This progressive increase in correlation between cortical structures could be due either to a mechanism of synaptic plasticity that takes place during the development of FR events or to the progressive reinforcement of synaptic networks mediated by the intensification of the effect exerted by 4AP on neurotransmitter release from presynaptic terminals (Perreault and Avoli 1991; Thesleff 1980).
The early effects induced by arterial perfusion of 4AP mimics the network oscillations evoked by olfactory inputs in the olfactory system. Odor-evoked oscillations in the beta frequency range at 10 and 20 Hz have been described in anesthetized animals (Boeijinga and Van Groen 1984; Bressler and Freeman 1980; Ketchum and Haberly 1993; Neville and Haberly 2003) and in the PC of the in vitro whole guinea pig brain that was isolated with the olfactory epithelium preserved. One of these studies (Neville and Haberly 2003) showed a progressive enhancement of gamma frequency (50–100 Hz) to the detriment of beta activity (15–40 Hz) in the rat olfactory bulbs and PC in response to an increased concentration of odorant stimulation. The persistent action of prolonged 4AP perfusions could mimic an enhanced power of the sensory input, and might explain the shift of the main peak frequency in the late FRs from 20–40 to 40–60 Hz. The observations that the complex FRs with higher oscillation rate are always preceded by early FRs and that the late FRs can propagate to limbic system is in line with the interpretation that treatment with 4AP progressively enhances olfactory synaptic interactions in a way that may mimic different intensities of olfactory stimulation.
Prolonged perfusions with 4AP (>7 min) induced a transition toward hypersynchronous and long-lasting sequences of oscillatory activities that progressively acquire the features of periodic epileptiform discharges. It should be, however, emphasized that this epileptiform pattern was entirely different from the electrographic seizure activity observed in more caudal cortical areas. Indeed, ~2 min after FR onset, ictal discharges were recorded in the hippocampal/entorhinal region, here designated as limbic cortices. During limbic epileptiform discharges, FRs continued to occur in the olfactory cortex confirming that the two types of epileptiform discharge are generated by different and segregated networks.
The epileptiform activity in limbic cortices started with the generation of interictal spikes in both hippocampus and m-EC that preceded the outburst of ictal discharges. Unlike the spikes observed after late FRs that spread from PC to the limbic system, these limbic spikes originated in m-EC/CA1 and eventually propagated to l-EC. The analysis of the preictal spikes revealed similarities with the GABAergic spikes described in rat slices of hippocampus and neocortex (reviewed by Avoli et al. 2002) and in a previous study performed on the isolated guinea pig brain (Uva et al. 2009). Interestingly we recently demonstrated that partial disinhibition induced by a short arterial perfusion with 50 μM bicuculline in the isolated guinea pig brain correlated with the activation of seizures in the hippocampal-EC region that were preceded by preictal spikes. Intracellular recordings have also demonstrated that these preictal spikes correlate with an inhibitory potential in the principal mEC cells and to robust firing of putative interneurons (Gnatkovsky et al. 2008). These findings and the observation of GABA-mediated interictal spikes in the 4AP model (Uva et al. 2009) confirmed in the present study, strongly suggest that the preictal spikes can be sustained by GABAergic networks as suggested by a large number of reports obtained on in vitro models of seizures recorded in limbic structures, in the neocortex, and in human temporal lobe tissue obtained from epilepsy surgery.
By comparing the features of the epileptiform activity induced by arterial perfusion of 50 μM 4AP and 50 μM bicuculline (Uva et al. 2005), we observed that the olfactory structures (PC and l-EC) were involved earlier in both models, suggesting a lower threshold to interictal activity generation in these structures. In both models, the hippocampal-entorhinal loop is activated later through the generation of preictal spikes and ictal discharges that are independent from the epileptic network events occurring in the olfactory region. Ictal onset is characterized by fast oscillatory activity at either 10–60 Hz (4AP model) and 20–30 Hz (bicuculline model) in hippocampus/m-EC, followed by a transitional phase with irregular spiking that leads to hyper-synchronous, high-amplitude bursting. During the bursts phase of seizures, interictal spikes and FRs in the PC were preserved in both models (Librizzi and de Curtis 2003; Uva et al. 2009). Therefore we conclude that there are common features in the development of the spontaneous epileptiform activity in olfactory and limbic cortices in the two models in spite of the different mechanisms of the two proepileptic agents. Interestingly the fast activity observed both in olfactory and limbic cortices closely resemble the most common seizure onset pattern observed in the temporal lobe of humans during focal seizures (for review, see de Curtis and Gnatkovsky 2009). Intracerebral electrode recordings performed during presurgical studies in patients with drug-resistant temporal lobe epilepsy demonstrated that the ictal discharge is initiated by fast oscillations at 20–40 Hz that are preceded by large-amplitude interictal spikes.
Previous studies have analyzed the effect of 4AP in the neocortical slices of guinea pig and rat. This study demonstrated in the guinea pig neocortex a higher propensity to generate seizure-like events that were abolished by NMDA receptor antagonism. Contradictory findings are available in the literature on the pharmacological sensitivity of seizure events induced by 4AP in the rat hippocampus. The application of the NMDA receptor antagonist AP5 in dorsal hippocampal rat slices did not abolish the ictal-like discharges induced by 4AP, whereas data obtained by recordings in EC and hippocampus from rat complex slices showed that CPP is able to block the seizures but not the interictal activity, and CNQX abolished all the forms of epileptiform discharges (Avoli et al. 1996). Finally, experiments performed on slices from young rodents showed the inability of the NMDA receptor block to abolish the epileptiform discharges.
In our guinea pig preparation, NMDA blockade with AP5 did not prevent the seizure-like activity and CNQX blocked the ictal discharges but did not affect interictal spikes. Moreover, NMDA receptor blockade prolongs seizure-like events and also promotes their propagation to the olfactory regions. The propagation of seizures from EC to PC was possibly due to the fact that seizures in EC-hippocampus are reinforced and prolonged by AP5 application. This last finding could be the consequence of the prolongation of seizures that may reinforce the epileptogenic network and could enhance the diffusion of seizure discharges. Overall, our study along with previous experiments reviewed in the preceding text suggest that pharmacological manipulations of epileptiform discharges induced by 4AP depend on several factors including brain structure, animal species, and type of preparation in which epileptiform discharges are analyzed.
Acknowledgments
GRANTS
The study was supported by the Italian Health Ministry, Telethon Foundation Grant GGP07278, and Canadian Institutes of Health Research Grant MOP 8109.
References
- Aram JA, Michelson HB, Wong RKS. Synchronized GABAergic IPSPs recorded in the neocortex after blockade of synaptic transmission mediated by excitatory amino acids. J Neurophysiol. 1991;65:1034–1041. doi: 10.1152/jn.1991.65.5.1034. [DOI] [PubMed] [Google Scholar]
- Avoli M. GABA-mediated synchronous potentials and seizure generation. Epilepsia. 1996;37:1035–1042. doi: 10.1111/j.1528-1157.1996.tb01022.x. [DOI] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, D’Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network pharmachological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Progr Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biella G, de Curtis M. Olfactory inputs activate the medial entorhinal cortex via the hippocampus. J Neurophysiol. 2000;83:1924–1931. doi: 10.1152/jn.2000.83.4.1924. [DOI] [PubMed] [Google Scholar]
- Biella GR, Gnatkovsky V, Takashima I, Kajiwara R, Iijima T, de Curtis M. Olfactory input to the parahippocampal region of the isolated guinea pig brain reveals weak entorhinal-to-perirhinal interactions. Eur J Neurosci. 2003;18:95–101. doi: 10.1046/j.1460-9568.2003.02730.x. [DOI] [PubMed] [Google Scholar]
- Biella G, Uva L, de Curtis M. Propagation of neronal acvity along the neocortical-perirhinal-entorhinal pathway in the guinea pig. J Neurosci. 2002;22:9972–9979. doi: 10.1523/JNEUROSCI.22-22-09972.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeijinga PH, Van Groen Th. Inputs from the olfactory bulb and olfactory cortex to the entorhinal cortex in the cat. Exp Brain Res. 1984;57:40–48. doi: 10.1007/BF00231130. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr Clin Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- Calcagnotto ME, Barbarosie M, Avoli M. Hippocampus-entorhinal cortex loop and seizure generation in the young rodent limbic system. J Neurophysiol. 2000;83:3183–3187. doi: 10.1152/jn.2000.83.5.3183. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Biella G, Buccellati C, Folco G. Simultaneous investigation of the neuronal and vascular compartments in the guinea pig brain isolated in vitro. Brain Res Protoc. 1998;3:221–228. doi: 10.1016/s1385-299x(98)00044-0. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. Re-considering the mechanisms of focal ictogenesis: the role of low-voltage fast activity. Epilepsia. 2009;50:2514–2525. doi: 10.1111/j.1528-1167.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Pare D, Llinas RR. The electrophysiology of the olfactory-hippocampal circuit in the isolated and perfused adult mammalian brain in vitro. Hippocampus. 1991;1:341–354. doi: 10.1002/hipo.450010402. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Biella GR, de Curtis M. Interactions between parahippocampal subfields in the isolated guinea pig brain. Soc Neurosci Abstr. 2003;719:7. doi: 10.1046/j.1460-9568.2003.02730.x. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, de Curtis M. Hippocampus-mediated activation of superficial and deep layer neurons in the medial entorhinal cortex of the isolated guinea pig brain. J Neurosci. 2006;26:873–881. doi: 10.1523/JNEUROSCI.4365-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Uva L, de Curtis M. Topographic distribution of direct and hippocampus- mediated entorhinal cortex activity evoked by olfactory tract stimulation. Eur J Neurosci. 2004;20:1897–1905. doi: 10.1111/j.1460-9568.2004.03627.x. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Wendling F, de Curtis M. Cellular correlates of spontaneous periodic events in the medial entorhinal cortex of the in vitro isolated guinea pig brain. Eur J Neurosci. 2007;26:302–311. doi: 10.1111/j.1460-9568.2007.05667.x. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. II. Systems originating in the olfactory peduncle. J Comp Neurol. 1978;181:781–807. doi: 10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Haberly LB. Role of synaptic excitation in the generation of bursting-induced epileptiform potentials in the endopiriform nucleus and piriform cortex. J Neurophysiol. 1993;70:2550–2561. doi: 10.1152/jn.1993.70.6.2550. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Sato T, Shimizu A, Tsutsui K, de Curtis M, Iijima T. Odor-driven activity in the olfactory cortex of an in vitro isolated guinea pig whole brain with olfactory epithelium. J Neurophysiol. 2007;97:670–679. doi: 10.1152/jn.01366.2005. [DOI] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum KL, Haberly LB. Synaptic events that generate fast oscillations in piriform cortex. J Neurosci. 1993;13:3980–3985. doi: 10.1523/JNEUROSCI.13-09-03980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhling R, Vreugdenhl M, Bracci E, Jefferys JGR. Ictal epileptiform activity is facilitated by hippocampal GABAA receptor-mediated oscillations. J Neurosci. 2000;20:6820–6829. doi: 10.1523/JNEUROSCI.20-18-06820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and the subiculum in the rat and cat. J Comp Neurol. 1977;172:723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- Librizzi L, de Curtis M. Epileptiform ictal discharges are prevented by periodic interictal spiking in the olfactory cortex. Ann Neurol. 2003;53:382–389. doi: 10.1002/ana.10471. [DOI] [PubMed] [Google Scholar]
- Ling DS, Benardo LS. Recruitment of GABAA inhibition in rat neocortex is limited and not NMDA dependent. J Neurophysiol. 1995;74:2329–2335. doi: 10.1152/jn.1995.74.6.2329. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictallike discharges in the rat entorhinal cortex. J Neurophysiol. 1998;79:352–360. doi: 10.1152/jn.1998.79.1.352. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Mattia D, Hwa GG, Avoli M. Epileptiform activity induced by 4-aminopyridine in guinea-pig and rat neocortices. Neurosci Lett. 1993;154:157–160. doi: 10.1016/0304-3940(93)90195-q. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Plant JR, Kelly ME. Dorsal hippocampal kindling produces long-lasting changes in the origin of spontaneous discharges in the piriform vs perirhinal cortex in vitro. Epil Res. 2000;39:191–200. doi: 10.1016/s0920-1211(99)00120-5. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RKS. Synchronization of inhibitory neurones in the guinea-pig hippocampus in vitro. J Physiol. 1994;477:35–45. doi: 10.1113/jphysiol.1994.sp020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Traub RD, Wong RK. Spread of synchronous firing in longitudinal slices from the CA3 region of the hippocampus. J Neurophysiol. 1996;60:1481–1496. doi: 10.1152/jn.1988.60.4.1481. [DOI] [PubMed] [Google Scholar]
- Muhletaler M, de Curtis M, Walton K, Llinas R. The isolated and perfused brain of the guinea-pig in vitro. Eur J Neurosci. 1993;5:915–926. doi: 10.1111/j.1460-9568.1993.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience. 1996;72:399– 408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol. 2003;90:3921–3930. doi: 10.1152/jn.00475.2003. [DOI] [PubMed] [Google Scholar]
- Pearcy WC, Virtue RW. The electroencephalogram in hypothermia with circulatory arrest. Anesthesiology. 1959;20:341–347. doi: 10.1097/00000542-195905000-00014. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992;12:104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piredda S, Gale K. A crucial epileptogenic site in the deep prepiriform cortex. Nature. 1985;317:623–625. doi: 10.1038/317623a0. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FJ, Johnston D. Epileptiform activity in the hippocampus produced by tetraethylammonium. J Neurophysiol. 1990;64:1077–1088. doi: 10.1152/jn.1990.64.4.1077. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Penicillin-induced epileptiform activity in the hippocampal in vitro prepatation. Ann Neurol. 1977;1:463–469. doi: 10.1002/ana.410010510. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Jones RSG, Mody I, Heinemann U. Epileptiform activity induced by lowering extracellular magnesium in combined hippocampal-entorhinal cortex slices, modification by receptors for norepinephrine and N-methyl-d-aspartate. Epil Res. 1987;1:53–62. doi: 10.1016/0920-1211(87)90051-9. [DOI] [PubMed] [Google Scholar]
- Thesleff S. Aminopyridines and synaptic transmission. Neuroscience. 1980;5:1413– 1419. doi: 10.1016/0306-4522(80)90002-0. [DOI] [PubMed] [Google Scholar]
- Traub RD, Borck C, Colling SB, Jefferys JG. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Traub RD, Colling SB, Jefferys JG. Cellular mechanisms of 4-aminopyridine-induced synchronized after-discharges in the rat hippocampal slice. J Physiol. 1995;489:127–140. doi: 10.1113/jphysiol.1995.sp021036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva L, Avoli M, de Curtis M. Synchronous GABA-receptor-dependent potentials in limbic areas of the in-vitro isolated adult guinea pig brain. Eur J Neurosci. 2009;29:911–920. doi: 10.1111/j.1460-9568.2009.06672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva L, de Curtis M. Polysynaptic olfactory pathway to the ipsi- and contralateral entorhinal cortex mediated via the hippocampus. Neuroscience. 2005;130:249–258. doi: 10.1016/j.neuroscience.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Uva L, Librizzi L, Wendling F, de Curtis M. Propagation dynamics of epileptiform activity acutely induced by bicuculline in the hippocampal-parahippocampal region of the isolated guinea pig brain. Epilepsia. 2005;46:1914–1925. doi: 10.1111/j.1528-1167.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Uva L, Librizzi L, Noe F, Marchi N, Janigro D, Vezzani A, Chepurnov S, de Curtis M. Acute induction of epileptiform discharges by pilocarpine in the in vitro isolated guinea pig brain requires enhancement of BBB permeability. Neuroscience. 2008;151:303–312. doi: 10.1016/j.neuroscience.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther H, Lambert JDC, Jones RSG, Heinemann U, Hamon B. Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett. 1986;69:165–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- Wilson WA, Swartzwelder HS, Anderson WW, Lewis DV. Seizure activity in vitro, a dual focus model. Epil Res. 1988;2:289–293. doi: 10.1016/0920-1211(88)90036-8. [DOI] [PubMed] [Google Scholar]
- Yang L, Benardo LS. Laminar properties of 4-aminopyridine-induced synchronous network activities in rat neocortex. Neuroscience. 2002;111:303–313. doi: 10.1016/s0306-4522(01)00622-4. [DOI] [PubMed] [Google Scholar]