Summary
Purpose
The K+ channel blocker 4-aminopyridine (4AP) induces epileptiform synchronization in brain slices maintained in vitro without interfering with γ-aminobutyric acid (GABA)A receptor–mediated inhibition and, actually, even enhancing it. The hypothesis that similar electrographic epileptiform patterns occur in vivo following systemic 4AP injection was tested here.
Methods
Sprague-Dawley rats (n = 13) were implanted with bipolar electrodes aimed at the hippocampal CA3 region, entorhinal cortex, subiculum, dentate gyrus, and amygdala. They were then injected with a single dose of 4AP (4–5 mg/kg, i.p.), and video-monitoring/electroencephalography (EEG) recordings were performed.
Key Findings
4AP induced convulsive or nonconvulsive seizures in 12 of 13 rats, along with generalized fascicular twitching, wet-dog shakes, and myoclonic jerks. On EEG, we observed in 7 (58.3%) of 12 animals long-lasting interictal spikes from the subiculum before the occurrence of the first seizure. Once seizures had started, interictal spikes occurred in all areas with no fixed site of origin. Most seizures (41/60, 68.3%) were characterized by a low-voltage fast-activity onset pattern and were convulsive (48/60, 80%). 4AP also induced highly rhythmic theta (6–11 Hz) oscillations in CA3 and entorhinal cortex before seizure occurrence.
Significance
Our study shows that systemic 4AP administration in vivo can enhance theta oscillations and induce slow interictal spikes and low-voltage fast-onset seizures similar to those reported in brain slices. We propose that these effects may reflect, at least in part, enhanced GABAA receptor–mediated inhibition as reported in in vitro studies.
Keywords: Epileptiform synchronization, GABA, Ictogenesis, Limbic system, Theta rhythm
Knowledge of the fundamental mechanisms leading to epileptiform synchronization has greatly advanced during the last three decades thanks to the use of the in vitro brain-slice preparation. Early studies—which were often carried out in “isolated” hippocampus slices—provided detailed information on the cellular and pharmacologic correlates of short-lasting (interictal-like) discharges induced by γ-aminobutyric acid (GABA)A-receptor antagonists (see for review, Avoli & De Curtis, 2011). Later, in vitro experiments conducted in extended brain slices including parahippocampal areas have shown that both interictal-like and long-lasting (ictal- or seizure-like) discharges occur following experimental procedures that do not fully block GABAA receptor–mediated inhibition (e.g., increased [K+] or removal of Mg2+ in the bathing medium) and even enhance it (e.g., bath application of the K+ channel blocker 4-aminopyridine [4AP]; see for review, Avoli & De Curtis, 2011).
Aminopyridines can induce seizures in humans as reported following accidental overdose (Schwam, 2011). Moreover, local application of 3AP to the somatosensory cortex elicits tonic seizures (Szente & Pongrácz, 1979), whereas intraperitoneal administration of 4AP in rodents causes generalized tremors and tonic–clonic seizures (Mihály et al., 1990). Depth electrode recordings in rats treated with systemic 4AP have also shown generalized tonic seizures that were associated with epileptiform discharges occurring in hippocampus, amygdala, and neocortex (Fragoso-Veloz et al., 1990). In contrast, local application of 4AP to the hippocampus or entorhinal cortex (EC) induced local hypersynchronous activity but not convulsive seizures (Fragoso-Veloz et al., 1990; Martín & Pozo, 2003; Medina-Ceja et al., 2008; Medina-Ceja & Ventura-Mejía, 2010).
Despite these in vivo findings, it remains unclear how interictal and ictal epileptiform discharges induced by systemic 4AP interrelate in multiple depth structures of the temporal lobe as well as whether and how these electrographic events correlate with specific behavioral symptoms. Therefore, we obtained depth electroencephalography (EEG) recordings from the hippocampal CA3 region, EC, subiculum, dentate gyrus, and amygdala along with video monitoring to study in Sprague-Dawley rats the effects induced by systemic 4AP injection. We report here that intraperitoneal administration of 4AP induces highly rhythmic theta (6–11 Hz) oscillations, a specific type of slow interictal discharge in the subiculum, and seizure activity that is often characterized by low-voltage fast-onset pattern (LVF).
Materials and Methods
Ethical approval
All procedures were approved by the Canadian Council of Animal Care, and all efforts were made to minimize the number of animals used and their suffering.
Animal preparation
Sprague-Dawley rats (250–300 g), obtained from Charles River Laboratories (St-Constant, QC, Canada), were housed under controlled environmental conditions (22 [±2]°C and 12 h light/12 h dark cycle) with food and water ad libitum. On the day of surgery, animals were anesthetized with isoflurane (3%) in 100% O2. They were then positioned in a stereotaxic frame so that lambda and bregma were in the same horizontal plane. An incision was made in the skin to expose the skull plate. Four stainless steel screws (2.4 mm length) were fixed to the skull and used as anchors. Up to five small holes were drilled in order to implant bipolar electrodes (20–30 kΩ, 30–50 mm length, distance between exposed tips: 500 μm) made by gluing two insulated copper wires. Electrodes were implanted in temporal lobe regions, that is, the CA3 region of the ventral hippocampus (anteroposterior [AP]: −4.4, mediolateral [ML]: ±4.6, dorsoventral [DV]: −7.6), medial EC (AP: −6.7, ML: ±4.6, DV: −8.4), ventral subiculum (AP: −6, ML: ±4.4, DV: −8.6), dentate gyrus (AP: −4.4, ML: ±2.4, DV: −3.5), and amygdala (AP: −2, ML: ±4.5, DV: −9). In order to rule out a cortical origin of seizures, we implanted additional animals (n = 2) with electrodes in temporal lobe regions and in the neocortex (AP: −1, ML: ±1.1, DV: 1.5). Screws and electrode pins were connected with a connector and fastened to the skull with dental cement. A cortical screw placed in the frontal bone was used as the reference, and a second screw, placed on the opposite side of the frontal region, was used as ground. After surgery, animals received topic application of chloramphenicol (Erfa, Montreal, QC, Canada) and lidocaine (5%; Odan, Pointe-Claire, QC, Canada) and were injected with carprofen (5 mg/kg, s.c.; Merail, Montreal, QC, Canada), buprenorphine (0.01–0.05 mg/kg, s.c., repeated every 12 h if necessary; CDMV, Saint-Hyacinthe, QC, Canada), and 2 ml of 0.9% sterile saline (s.c.).
EEG recordings and systemic injection of 4AP
Following surgery, rats were housed individually in custom-made acrylic glass boxes (30 × 30 × 40 cm) and allowed to habituate to the environment for 24 h. Animals were placed in controlled conditions (22 ± 2°C, 12-h light/dark schedule) and provided with food and water ad libitum. On the day before 4AP injection, the pin connector was connected to multichannel cables and electrical swivels (Slip ring T13EEG; Air Precision, Le Plessis Robinson, France; or Commutator SL 18C; HRS Scientific, Montreal, QC, Canada), and EEG-video monitoring was performed. EEG signals were amplified with an interface kit (Mobile 36ch LTM ProAmp; Stellate, Montreal, QC, Canada), and sampled at 2 kHz per channel. Infrared cameras were used to record video files that were time-stamped for integration with the electrophysiologic data using monitoring software (Harmonie; Stellate, Montreal, QC, Canada). On the day of 4AP (4–5 mg/kg, i.p.) injection, EEG-video recordings were obtained up to 4 h after injection.
Detection of seizures and seizure-onset zones
EEG signals were extracted from the Stellate Harmonie software and exported to Matlab for offline analysis. Seizure-onset zones were identified by visually analyzing EEG traces and spectrograms. The occurrence of activity between 5 and 20 Hz was considered as the onset of an ictal event (Fig. 3). Seizure-onset zones could be localized in one region or in two regions simultaneously. If the seizure started in more than three regions simultaneously, it was termed as “widespread.” Seizures were also classified as nonconvulsive (stage 0–2 of the Racine’s scale) or convulsive (stage 3–5; Racine, 1972).
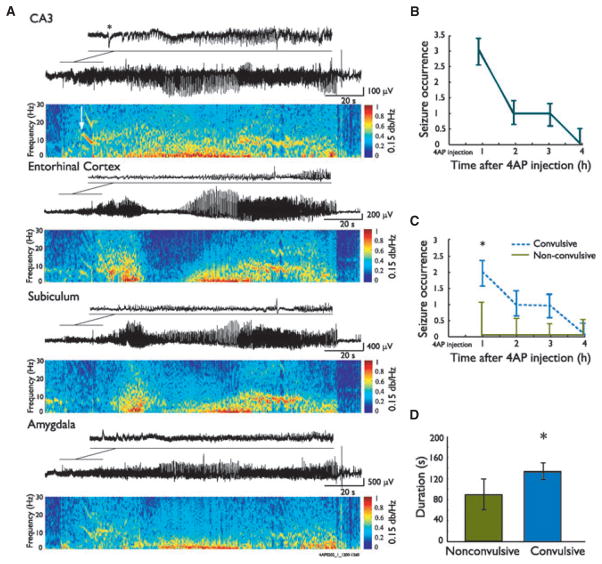
Figure 3.
Ictal activity induced by systemic 4AP administration. (A) Representative seizure recorded after 4AP systemic administration from four structures simultaneously. Note at seizure onset the occurrence of a negative spike (asterisk) in the CA3 region of the hippocampus, followed by the occurrence of fast activity in the 5–20 Hz range; this spike is mirrored by a positive-going spike in the amygdala. The white arrow in the power spectrogram indicates seizure onset time, based on the first occurrence of fast activity. During this seizure, fast activity first occurred in CA3, which was considered as the seizure-onset zone. (B) Rate of seizures per hour recorded after 4AP in 12 animals. (C) Duration of convulsive and nonconvulsive seizures over time after 4AP in these 12 rats. Note that the occurrence of convulsive seizures was higher during the first hour after 4AP. (D) Bar graph showing the duration of convulsive and nonconvulsive seizures recorded from these 12 animals; note that convulsive seizures lasted significantly longer compared to nonconvulsive seizures. Medians and 95% confidence intervals are shown. *p < 0.005.
Detection of theta (6–11 Hz) oscillations
EEG recordings were analyzed using the spectrogram method, and calculated using the discrete short-time Fourier transform. Data sampled at 2 kHz were decimated by a factor of 15 after being low-pass filtered by a 100th order finite impulse response filter. The spectrogram was then elaborated from the data set and separated into 1-s intervals (134 points) to which a Hamming window was applied. The discrete Fourier transforms were evaluated over 1,024 points with zero padding.
The EEG was analyzed to identify time periods of strong theta oscillations. To enhance the detection of significant oscillations, a gamma correction with a factor of 0.15 was applied to the spectrogram to improve their contrast to random noise. A multiparametric algorithm was then used to identify oscillations in the 6–11 Hz band of the spectrogram. For each time slot, the algorithm identified the peak frequency in the band of interest and calculated the energy within a 1-Hz band centered on this peak. The energy within the peak must have been at least 40% of the largest peak within a 1-s window (time domain criterion), and contain at least 60% of the band energy at that time (frequency domain criterion) to be considered a valid candidate. An oscillation event was then identified by a succession of peaks with a specific track in time and frequency. Moreover, the relative peak intensities must not have varied in time by >100% per second, successive peak frequencies must not have varied by more than 7.5 Hz per second, and there must have been a continuous track of candidate peaks at least 5 s long.
Statistical tests
Because values were not normally distributed, non-parametric tests were used and are mentioned in the text when applied. The level of significance was set at p < 0.05. Statistical analyses were performed in Matlab 7.9.0 (Math-Works, Natick, MA, U.S.A.) using the Statistics Toolbox. Values are expressed as medians (95% confidence intervals) when compared with nonparametric tests; otherwise they are expressed as means (±standard error of mean).
Results
Preictal and interictal behavior
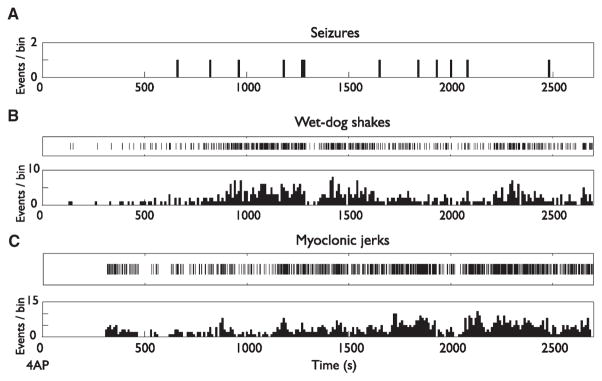
Increased exploratory behavior, widespread fasciculation with wet-dog shakes, as well as myoclonic jerks of the rear and forelimbs or of the entire body were observed in all animals 4.6 (±0.8) min after 4AP injection. The frequency of these behavioral symptoms increased progressively over time before reaching a peak approximately 20 min following 4AP injection. Moreover, we observed a high occurrence of wet-dog shakes shortly after 4AP administration and increases in their occurrence between seizures (Fig. 1A,B). In contrast, myoclonic jerks increased in occurrence later compared to wet-dog shakes (Fig. 1C). Two rats died 30 min after injection and one died 2.5 h after injection during strong convulsive seizures, giving a mortality rate of 16.7%.
Figure 1.
Development of seizures, wet-dog shakes, and myoclonic jerks following administration of 4AP in four rats. (A) Occurrence of seizures over time after 4AP administration. (B, C) Raster plots and corresponding frequency distribution histograms showing the occurrence of behavioral symptoms over time. Note that occurrence of wet-dog shakes (B) is higher after 4AP administration compared to myoclonic jerks that increased in occurrence later after 4AP administration (C).
4AP-induced epileptiform activities on EEG
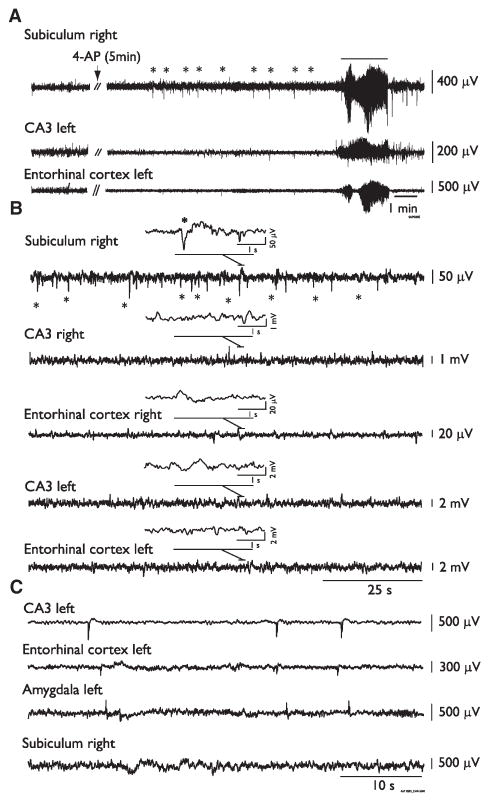
Interictal spikes were the first EEG abnormality to occur 11.4 (±8.5) min after 4AP injection. In 7 (58.3%) of 12 rats, this initial interictal activity was observed in the subiculum and consisted of long-lasting events (duration = 3.26 ± 0.12 s, n = 5 animals; Fig. 2A,B). The initial EEG change seen in the remaining animals after 4AP injection consisted of interictal spikes occurring in any region (not illustrated). Following the first seizure occurrence, interictal spikes were recorded from all regions (Fig. 2C). In addition to having shorter duration (0.74 ± 0.45 s) than those initially recorded in subiculum, these interictal spikes had no fixed site of origin.
Figure 2.
Interictal activity induced by systemic 4AP administration. (A) EEG recordings obtained from an animal before and after the systemic administration of 4AP (arrow); note the early appearance of interictal spikes in the subiculum (asterisks) that precede the occurrence of the first seizure 12 min after injection. (B) EEG recordings from a different rat after the administration of 4AP demonstrate at low speed the occurrence of interictal spikes only in the subiculum before the occurrence of the first seizure. Insets show simultaneous recordings (4 s) in all regions during an interictal spike occurring in the subiculum (asterisk). (C) Representative recordings showing interictal spikes occurring in all regions once seizures started to occur. These interictal spikes had no fixed site of origin.
Systemic injection of 4AP was effective in inducing seizures in 12 of 13 rats (n = 60 seizures). The remaining rat showed only subicular long-lasting interictal spikes that were later followed by the appearance of short interictal spikes in all regions, with no fixed site of origin. Most seizures (41/60, 68.3%) were characterized by an LVF pattern, that is, they started with a negative or a positive spike followed by oscillatory activity in the 5–20 Hz frequency range and, later on, by high-amplitude low-frequency spikes (Fig. 3A). The remaining seizures were hypersynchronous-onset seizures (HYP; 16/60, 26.7%), since they were characterized at onset by a pattern of focal (preictal) spiking at a frequency of approximately 2 Hz (not illustrated). The remaining seizures could not be classified (3/60, 5%). Most seizures (48/60, 80%) were convulsive (stage 3–5 on the Racine scale); they were first characterized by behavioral arrest, followed by a short and discrete tonic phase, characterized by a mild extension of the head, with an axial twist of both head and neck and/or a tonic elevation of the tail. This was followed by a clonic phase, characterized by myoclonic activity of rear and forelimbs, which could evolve to rearing, running, and loss of balance (Racine stage 5). Seizure termination was characterized by immobility, during which the animal laid on his side.
Nonconvulsive seizures were characterized by automatisms (chewing, sniffing, swallowing) and an increase of occurrence of exploratory behaviors, followed by discrete tonic (head and trunk extension) symptoms. Wet-dog shakes were observed following both convulsive and nonconvulsive seizures. Fifty percent of the animals showed only convulsive seizures, whereas one rat showed only nonconvulsive seizures. The remaining rats showed both types of seizures, but convulsive seizures were more frequently observed, as they represented on average 72.1 (±12.1)% of seizures in these animals (n = 5).
The latency between 4AP injection and the occurrence of the first seizure was 23.6 (±19.8) min (n = 59 seizures observed in 12 animals), and successive seizures occurred on average every 28 (±24.5) min, giving a rate of 1.3 (±1.02) seizures per hour. The average duration of seizures was 123.9 (±48.7) s. Seizure rates were highest during the first hour and decreased progressively over time (Fig. 3B). Moreover, during the first hour after 4AP, rates of convulsive seizures were significantly higher compared to rates of nonconvulsive seizures (Wilcoxon signed-rank test, p < 0.05; Fig. 3C); this difference was not present from the second to the fourth hours after 4AP, and the occurrence of both seizure types decreased progressively over time. Convulsive seizures lasted longer than nonconvulsive seizures (Wilcoxon rank-sum test, p < 0.05; Fig. 3D).
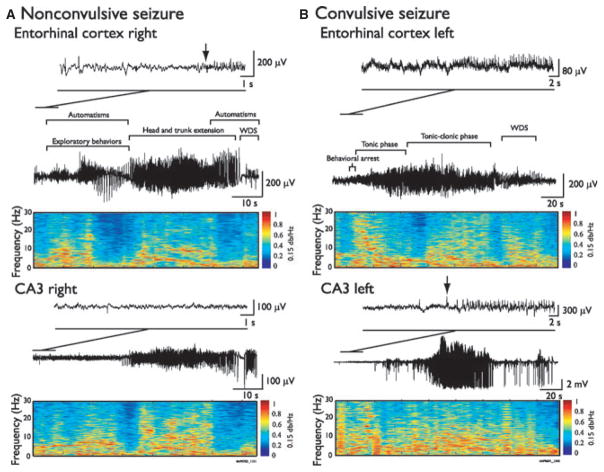
On the EEG, convulsive and nonconvulsive seizures showed similar patterns of activity (Fig. 4). However, careful analysis of nonconvulsive seizures showed that mild behavioral symptoms (automatisms, increased exploratory behaviors) could co-occur with the onset of local epileptiform activity in the seizure-onset zone. For instance, Fig. 4A shows a nonconvulsive seizure characterized by initial symptoms such as automatisms and sustained exploratory behaviors, with the onset of fast activity (5–20 Hz) in the EC. Head and trunk extension then occurred when epileptiform activity spread outside of the seizure-onset zone. During convulsive seizures, as shown in Fig. 4B, the tonic and clonic phases were associated with widespread and high-amplitude epileptiform activity across the seizure-onset zone and ipsilateral regions.
Figure 4.
EEG-behavioral correlates of ictal events induced by systemic 4AP administration. Representative EEG recordings of a nonconvulsive (A) and a convulsive seizure (B) in the ipsilateral CA3 and EC, with corresponding behavioral symptoms. Arrows indicate seizure-onset time. In A, automatisms and an increase of exploratory behaviors occurred during the nonconvulsive seizure after onset in EC, whereas head and trunk extension occurred when the epileptiform activity was also observed in the ipsilateral CA3. Panel B shows a convulsive seizure with a tonic phase, characterized by head and trunk extension, followed by a tonic–clonic phase, characterized by a head and trunk extension associated with rearing and falling. Both the tonic and the tonic–clonic phase were associated to epileptiform activity in both CA3 and EC. Both seizure types were followed by wet-dog shakes (WDS).
Seizures induced by 4AP were initiated from mesial temporal lobe regions. A propagation of ictal activity to the cortex occurred late after seizure onset, and was associated with the convulsive phase of the seizure (Fig. S1). In temporal lobe regions, seizures most often started from the EC (20/60, 33.3%), whereas the remaining seizures started from the CA3 area, (9/60, 15%), the subiculum (7/60, 11.7%), or the amygdala (2/60, 3.3%). In 13.3% of seizures (8/60), the onset zone was located in CA3 and in another structure, simultaneously. Finally, 22% (13/60) of seizures were labeled as “widespread,” since they started on all channels simultaneously.
Although theta oscillations (6–11 Hz) were observed in all regions following 4AP administration, we analyzed oscillatory activity in the CA3 region and EC, since (1) it was more prominent in these regions than in other areas, and (2) these areas were mostly involved as seizure-onset zones. We identified a total of 919 runs of theta oscillations (n = 514 and 405 in EC and CA3, respectively). Compared to the baseline period (before 4AP), theta oscillations became highly sustained following 4AP (Fig. 5). They did not appear, however, to be related to any behavioral symptoms. The duration of theta oscillations in CA3 (median 18, 95% confidence interval [CI] 2.8 s) was not significantly different from the duration of theta oscillations in EC (median 18, 95% CI2.6 s), although they were significantly longer than periods of theta oscillations recorded before 4AP (CA3: median 7, 95% CI 3 s, EC: median 11, 95% CI 6.4 s, Wilcoxon rank sum test, p < 0.01, n = 3 animals).
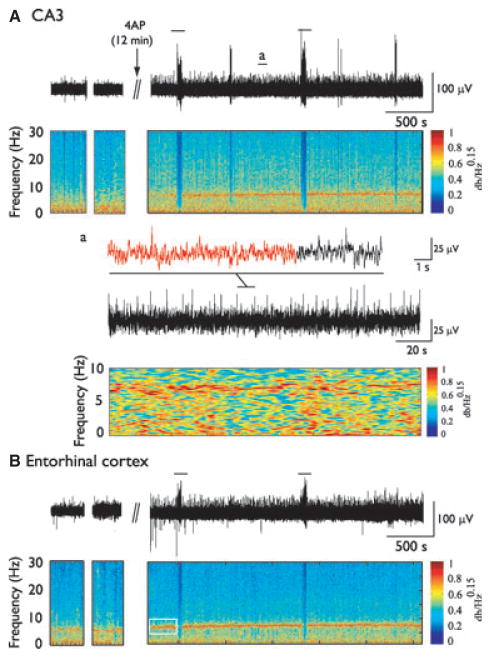
Figure 5.
Theta oscillations are potentiated by systemic injection of 4AP. EEG recordings and corresponding spectrograms obtained from CA3 (A) and the EC (B) before and after 4AP injection (arrow). Solid lines indicate seizures. EEG samples obtained before 4AP correspond to periods when the rat was immobile. Note the highly rhythmic and sustained activity in the theta frequency range (6–11 Hz) after 4AP compared to before injection. Note that in that case, especially in EC, highly rhythmic theta oscillations occurred before first seizure occurrence (white rectangle). Inset (a) shows an example of theta oscillations from CA3 on a shorter time scale, with the corresponding spectrogram. Theta oscillations were not associated with any behavioral symptoms.
Discussion
The main findings of our study can be summarized as follows: (1) systemic injection of 4AP induces initially behavioral symptoms such as widespread fasciculations, wet-dog shakes, and myoclonic jerks; (2) long-lasting interictal spikes appear in the subiculum before the occurrence of the first seizure, whereas shorter interictal spikes with no fixed site of origin recur once seizures appear; (3) most seizures are characterized by an LVF onset pattern, are convulsive, and initiate from the EC or the CA3 region of the hippocampus; and (4) highly rhythmic activities in the theta (6–11 Hz) frequency band are disclosed by 4AP injection.
Initial 4AP- induced behavioral symptoms
As previously reported (Fragoso-Veloz & Tapia, 1992; Világi et al., 2009), we have found here that systemic 4AP administration induces wet-dog shakes, myoclonic jerks, fascicular twitching, forepaw tremor, chewing, myoclonus, and tonic–clonic seizures. The detailed mechanisms underlying such variety of symptoms remain unknown, although generalized fascicular twitching should presumably reflect enhanced neurotransmission at the neuromuscular junction (cf., Fastier & McDowall, 1958), as also suggested by the use of 4AP in the treatment of myasthenia gravis (Miller, 1979) and botulism (Ball et al., 1979). On the other hand, other symptoms may be linked to central effects induced by 4AP. For instance, wet-dog shakes that occurred early after injection could reflect an effect of 4AP on the limbic system, since these symptoms can be reproduced by electrical or pharmacologic stimulation of the hippocampus (Lerner-Natoli et al., 1984; Leung & Shen, 2006), the lateral septum, or the amygdala (Le Gal La Salle & Cavalheiro, 1981). Wet-dog shakes are also thought to be dependent on GABAergic processes (Tuff et al., 1983; Leung & Shen, 2006), making this behavior another GABAergic correlate of 4AP action. However, serotoninergic processes in limbic structures and dopaminergic processes in mesodiencephalic structures have also been linked to wet-dog shakes (Wang et al., 2005; Leung & Shen, 2006).
Occurrence of long interictal spikes in the subiculum before first seizure occurrence
The subiculum, which represents the major output of the hippocampus, plays a central role in modulating neuronal activity from the hippocampus to the EC and to other cortical and subcortical regions (Menendez de la Prida et al., 2006). Several studies have shown that subiculum generates interictal spikes and may thus serve as a generator of epileptiform activity or as a gate for the propagation of epileptic activity to other limbic and extralimbic areas (Benini & Avoli, 2005; Menendez de la Prida et al., 2006). In addition, Cohen et al. (2002) have reported that interictal spikes can occur in the human subiculum in an in vitro brain slice preparation without any epileptiform activity in the CA1–CA3 regions of the hippocampus. These interictal spikes were shown to rely on depolarizing GABAA-receptor signaling contributed by the synchronous activation of interneurons. Similar findings have been obtained in human neocortical temporal lobe tissue (Köhling et al., 1998). The local generation of subicular interictal spikes that occurred following 4AP in our study is thus compatible with these previous in vitro findings.
Theta oscillations induced by 4AP in CA3 and entorhinal cortex
We have also observed that 4AP induces sustained and highly rhythmic runs of theta oscillations. These effects were more pronounced in EC and CA3, the two regions that were involved as seizure-onset zones in our experiments. These results–which are consistent with those obtained in vivo (Fragoso-Veloz et al., 1990) and in vitro (Henderson et al., 2010), following 4AP administration as well as during kindling stimulation of the perforant path (Popova et al., 2008; Kitchigina & Butuzova, 2009)—suggest that theta oscillations may have a role in ictogenesis (Butuzova & Kitchigina, 2008). Of interest, theta oscillations are believed to result from the interaction between pyramidal cells and interneurons, and more specifically on GABAergic inputs to pyramidal cells (Klausberger, 2009). In line with this view Buzsáki (2002) has reported that theta oscillations are abolished by the GABAA-receptor antagonist picrotoxin. Therefore, we are inclined to propose that the occurrence of high amplitude and highly rhythmic theta oscillations following 4AP rests on the ability of this drug to increase GABAergic transmission (Avoli & De Curtis, 2011).
4AP-induced ictal events and seizure-onset zones
As in early in vitro 4AP studies (Avoli et al., 1996; Benini et al., 2003), we have found that seizures recorded in vivo frequently initiated in the EC. This finding is also in line with what was reported in experiments performed in combined hippocampus-EC slices bathed in Mg2+-free medium (Jones & Lambert, 1990; Dreier & Heinemann, 1991) and with evidence obtained in vivo by stimulating the hippocampus (Stringer & Lothman, 1992). In addition, dysfunctional EC has been identified in patients with temporal lobe epilepsy (Rutecki et al., 1989; Deutsch et al., 1991; Spencer & Spencer, 1994). However, we also identified that some seizures initiated in CA3 or in CA3 and another region simultaneously. This evidence is in keeping with what has been reported in vivo in chronic models of temporal lobe epilepsy (Ben-Ari & Cossart, 2000; Bortel et al., 2010; Lévesque et al., 2011).
Systemic administration of 4AP induced seizure-onset patterns that could be classified as HYP or LVF. Similar findings have been recently obtained in animal models of temporal lobe epilepsy (Bragin et al., 2005; Lévesque et al., 2012) and in patients with epilepsy (Velasco et al., 2000; Ogren et al., 2009). However, 4AP could induce a higher proportion of LVF compared to HYP seizures (68.3% and 26.7%, respectively). Of interest, similar LVF ictal-like discharges have been identified in most of the in vitro experiments performed with 4AP, as well as that intracellular recordings from principal neurons have shown that these ictal events are shortly preceded (and thus presumably initiated) by a long-lasting GABAergic potential that reflects the synchronous activity of interneurons (cf., Avoli & De Curtis, 2011). Furthermore, we have recently reported that in vivo and in vitro, LVF seizures are associated with an increased occurrence of ripples (80–200 Hz; Lévesque et al., 2012; Panuccio et al., 2012), which are thought to reflect summated inhibitory postsynaptic potentials (IPSPs) generated by principal cells in response to inhibitory interneuron firing (Jefferys et al., 2012). Therefore, the in vivo prevalence of LVF seizures indicates that also in this type of preparation 4AP may enhance GABAergic mechanisms.
It is also well established that 4AP blocks Kv1 channels (Yao & Tseng, 1994; Coetzee et al., 1999; Gutman et al., 2005), thus increasing action potential duration in nerve cells including interneurons (Zhang & McBain, 1995). In line with this mechanism of action, Smart et al. (1998) have found that spontaneous seizures and signs of peripheral neuronal hyperexcitability are observed in the Kv1.1 null mice model. This evidence, along with the results reported here, points at the role of K+ channels in epilepsy and at their relevance as targets for antiepileptic drugs (Wickenden, 2002).
Supplementary Material
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR grants 8109 and 74609). ML was recipient of a postdoctoral fellowship from the Savoy Foundation.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional Supporting Information may be found in the online version of this article:
Figure S1. Ictal activity induced by 4AP in mesial temporal lobe and neocortical regions.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Avoli M, De Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996;15:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball AP, Hopkinson RB, Farrell ID, Hutchison JG, Paul R, Watson RD, Page AJF, Parker RGF, Edwards CW, Snow M, Scott DK, Leone-Ganado A, Hastings A, Ghosh AC, Gilbert RJ. Human botulism caused by Clostridium botulinum type E: the Birmingham outbreak. Q J Med. 1979;48:473–491. [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Benini R, Avoli M. Rat subicular networks gate hippocampal output activity in an in vitro model of limbic seizures. J Physiol. 2005;566:885–900. doi: 10.1113/jphysiol.2005.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benini R, D’Antuono M, Pralong E, Avoli M. Involvement of amygdala networks in epileptiform synchronization in vitro. Neuroscience. 2003;120:75–84. doi: 10.1016/s0306-4522(03)00262-8. [DOI] [PubMed] [Google Scholar]
- Bortel A, Lévesque M, Biagini G, Gotman J, Avoli M. Convulsive status as a determinant for epileptogenesis and interictal discharge generation in the rat limbic system. Neurobiol Dis. 2010;40:478–489. doi: 10.1016/j.nbd.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Butuzova MV, Kitchigina VF. Repeated blockade of GABAA receptors in the medial septal region induces epileptiform activity in the hippocampus. Neurosci Lett. 2008;434:133–138. doi: 10.1016/j.neulet.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;31:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormak T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, de Vega-Saenz ME, Rudy B. Molecular diversity ok K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Deutsch C, Spencer S, Robbins R, Cicchetti D, Spencer D. Interictal spikes and hippocampal somatostatin levels in temporal lobe epilepsy. Epilepsia. 1991;32:174–178. doi: 10.1111/j.1528-1157.1991.tb05241.x. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Heinemann U. Regional and time-dependent variations of low Mg2+-induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- Fastier FN, McDowall DM. A comparison of the pharmacological properties of the three isomeric aminopyridines. Aust J Exp Biol Med Sci. 1958;36:365–371. doi: 10.1038/icb.1958.39. [DOI] [PubMed] [Google Scholar]
- Fragoso-Veloz J, Tapia R. NMDA receptor antagonists protect against seizures and wet-dog shakes induced by 4-aminopyridine. Eur J Pharmacol. 1992;221:275–280. doi: 10.1016/0014-2999(92)90713-e. [DOI] [PubMed] [Google Scholar]
- Fragoso-Veloz J, Massieu L, Alvarado R, Tapia R. Seizures and wet-dog shakes induced by 4-Aminopyridine, and their potentiation by nifedipine. Eur J Pharmacol. 1990;178:275–284. doi: 10.1016/0014-2999(90)90106-g. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer Z, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Henderson Z, Lu CB, Janzsó G, Matto N, McKinley CE, Yanagawa Y, Halasy K. Distribution and role of Kv3.1b in neurons in the medial septum band diagonal complex. Neuroscience. 2010;166:952–969. doi: 10.1016/j.neuroscience.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Jefferys JG, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RSG, Lambert JDC. The role of excitatory amino acid receptors in the propagation of epileptiform discharges from the entorhinal cortex to the dentate gyrus in vitro. Exp Brain Res. 1990;80:310–322. doi: 10.1007/BF00228158. [DOI] [PubMed] [Google Scholar]
- Kitchigina VF, Butuzova MV. Theta activity of septal neurons during different epileptic phases: the same frequency but different significance? Exp Neurol. 2009;216:449–458. doi: 10.1016/j.expneurol.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30:947–957. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- Köhling R, Lücke A, Straub H, Speckmann EJ, Tuxhorn I, Wolf P, Pannek H, Oppel F. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain. 1998;121:1073–1087. doi: 10.1093/brain/121.6.1073. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Cavalheiro EA. Stimulation of septal and amygdaloid nuclei: EEG and behavioral responses during early development of kindling with special reference to wet dog shakes. Exp Neurol. 1981;74:717–727. doi: 10.1016/0014-4886(81)90246-6. [DOI] [PubMed] [Google Scholar]
- Lerner-Natoli M, Rondouin G, Baldy-Moulinier M. Evolution of wet dog shakes during kindling in rats: comparison between hippocampal and amygdala kindling. Exp Neurol. 1984;83:1–12. doi: 10.1016/0014-4886(84)90040-2. [DOI] [PubMed] [Google Scholar]
- Leung LS, Shen B. Hippocampal partial kindling decreased hippocampal GABAB receptor efficacy and wet dog shakes in rats. Behav Brain Res. 2006;173:274–281. doi: 10.1016/j.bbr.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Bortel A, Gotman J, Avoli M. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2011;42:231–241. doi: 10.1016/j.nbd.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, Avoli M. Two seizure onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín ED, Pozo M. Valproate supresses status epilepticus induced by 4-Aminopyridine in CA1 hippocampus region. Epilepsia. 2003;44:1375–1379. doi: 10.1046/j.1528-1157.2003.11603.x. [DOI] [PubMed] [Google Scholar]
- Medina-Ceja L, Ventura-Mejía C. Differential effects of trimethylamine and quinine on seizures induced by 4-aminopyridine administration in the entorhinal cortex of vigilant rats. Seizure. 2010;19:507–513. doi: 10.1016/j.seizure.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Medina-Ceja L, Cordero-Romero A, Morales-Villagrán A. Antiepileptic effect of carbonexolone on seizures induced by 4-aminopyridine: a study in the rat hippocampus and entorhinal cortex. Brain Res. 2008;1187:74–81. doi: 10.1016/j.brainres.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Totterdell S, Gigg J, Miles R. The subiculum comes of age. Hippocampus. 2006;16:916–923. doi: 10.1002/hipo.20220. [DOI] [PubMed] [Google Scholar]
- Mihály A, Bencsik K, Solymosi T. Naltrexone potentiates 4-aminopyridine seizures in the rat. J Neural Transm Gen Sect. 1990;79:59–67. doi: 10.1007/BF01251001. [DOI] [PubMed] [Google Scholar]
- Miller RD. Recent developments with muscles relaxants and their antagonists. Can Anaesth Soc J. 1979;26:83–93. doi: 10.1007/BF03013775. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Wilson CL, Bragin A, Lin JJ, Salamon N, Dutton RA, Luders E, Fileds TA, Fried I, Toga AW, Thompson PM, Engel J, Staba RJ. Three-dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66:783–791. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuccio G, Sanchez G, Lévesque M, Salami P, De Curtis M, Avoli M. On the ictogenic properties of the piriform cortex. Epilepsia. 2012;53:459–468. doi: 10.1111/j.1528-1167.2012.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova IY, Sinelnikova W, Kitchigina VF. Disturbance of the correlation between hippocampal and septal EEGs during epileptogenesis. Neurosci Lett. 2008;19:228–233. doi: 10.1016/j.neulet.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Grossman RG, Armstrong D, Irish-Loewen S. Electrophysiological connections between the hippocampus and entorhinal cortex in patients with complex partial seizures. J Neurosurg. 1989;70:667–675. doi: 10.3171/jns.1989.70.5.0667. [DOI] [PubMed] [Google Scholar]
- Schwam E. Severe accidental overdose of 4-aminopyridine due to compounding pharmacy error. J Emerg Med. 2011;41:51–54. doi: 10.1016/j.jemermed.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the KV1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Stringer JL, Lothman EW. Reverberatory seizure discharges in hippocampal-parahippocampal circuits. Exp Neurol. 1992;116:198–203. doi: 10.1016/0014-4886(92)90168-p. [DOI] [PubMed] [Google Scholar]
- Szente M, Pongrácz F. Aminopyridine-induced seizure activity. Electroencephalogr Clin Neurophysiol. 1979;46:605–608. doi: 10.1016/0013-4694(79)90014-2. [DOI] [PubMed] [Google Scholar]
- Tuff LP, Racine RJ, Mishra RK. The effects of kindling on GABA-mediated inhibition in the dentate gyrus of the rat. II. Receptor binding. Brain Res. 1983;277:91–98. doi: 10.1016/0006-8993(83)90910-1. [DOI] [PubMed] [Google Scholar]
- Velasco AL, Wilson CL, Babb TL, Engel J. Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plast. 2000;7:49–63. doi: 10.1155/NP.2000.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Világi I, Dobó E, Borbély S, Czégé D, Molnár E, Mihály A. Repeated 4-aminopyridine induced seizures diminish the efficacy of glutamatergic transmission in the neocortex. Exp Neurol. 2009;219:136–145. doi: 10.1016/j.expneurol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wang HL, Xiang XH, Guo Y, Wu WR, Cao DY, Wang HS, Zhao Y. Ionotropic glutamatergic neurotransmission in the ventral tegmental area modulates DeltaFosB expression in the nucleus accumbens and abstinence syndrome in morphine withdrawal rats. Eur J Pharm. 2005;527:94–104. doi: 10.1016/j.ejphar.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Wickenden AD. Potassium channels as anti-epileptic drug targets. Neuropharmacology. 2002;43:1055–1060. doi: 10.1016/s0028-3908(02)00237-x. [DOI] [PubMed] [Google Scholar]
- Yao JA, Tseng GN. Modulation of 4-AP block of a mammalian Atype K channel clone by channel gating and membrane voltage. J Biophys. 1994;67:130–142. doi: 10.1016/S0006-3495(94)80462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McBain CJ. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurons. J Physiol. 1995;488:661–672. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.