Abstract
The kainic acid model of temporal lobe epilepsy has greatly contributed to the understanding of the molecular, cellular and pharmacological mechanisms underlying epileptogenesis and ictogenesis. This model presents with neuropathological and electroencephalographic features that are seen in patients with temporal lobe epilepsy. It is also characterized by a latent period that follows the initial precipitating injury (i.e., status epilepticus) until the appearance of recurrent seizures, as observed in the human condition. Finally, the kainic acid model can be reproduced in a variety of species using either systemic, intrahippocampal or intra-amygdaloid administrations. In this review, we describe the various methodological procedures and evaluate their differences with respect to the behavioral, electroencephalographic and neuropathological correlates. In addition, we compare the kainic acid model with other animal models of temporal lobe epilepsy such as the pilocarpine and the kindling model. We conclude that the kainic acid model is a reliable tool for understanding temporal lobe epilepsy, provided that the differences existing between methodological procedures are taken into account.
Keywords: Kainic acid, Animal models of temporal lobe epilepsy, Intracerebral administration, Systemic administration
1. Introduction
According to the World Health Organization, epilepsy is the most prevalent neurological disorder, with a prevalence of over 50 million and an incidence of 2.4 million per year. Partial epileptic disorders represent 60% of these cases, with temporal lobe epilepsy (TLE) being the most common type. Symptoms in TLE consist of partial seizures that originate from the hippocampus, entorhinal cortex or amygdala many years after an initial brain insult such as status epilepticus (SE), encephalitis or febrile convulsions. Many antiepileptic drugs are currently available to control or to reduce seizure occurrence, but approximately one third of patients are refractory to medication, making TLE one of the most refractory form of partial epilepsy in adults (Engel et al., 2012). In such patients, surgical resection of the epileptic tissue remains the only therapeutic alternative. However, the seizure onset zone and possible post-surgical neurological deficits must be assessed with multiple and costly tests including pre-surgical invasive procedures such as intracranial EEG recordings.
Patients with TLE typically show hippocampal sclerosis, characterized by selective neuronal loss in the CA1/CA3 region of the hippocampus and the hilus, along with granule cell dispersion and aberrant mossy fiber sprouting in the molecular layer of the dentate gyrus (Berkovic et al., 1991; Buckmaster, 2012; Gloor, 1997; Jackson et al., 1990; Thorn, 1997). Removing the sclerotic hippocampus will reduce seizure occurrence but still approximately 30% of patients are not seizure-free after surgery because of insufficient resection of the epileptic tissue. Indeed, it was hypothesized that TLE may involve a broad extrahippocampal, or even extratemporal, network (Bartolomei et al., 2008; Harroud et al., 2012; Najm et al., 2013; Spencer, 2002; Spencer and Spencer, 1994) since performing a total resection of the hippocampus is more effective in controlling seizures compared to a lobectomy restricted to only the anterior part of the temporal lobe (Wyler et al., 1995). Finally, in some patients, the seizure onset zone cannot be clearly identified and thus they become poor candidates for surgical treatment.
Taken together, these clinical findings put further emphasis on the need to use experimental models of TLE to replicate the histopathological, electroencephalographic and behavioral features encountered in this neurological disorder in order to answer many unresolved questions; these include the localization and extent of the seizure onset zone as well as the mechanisms underlying epileptogenesis. The identification of the seizure onset zone is especially important for the surgical treatment of TLE while understanding epileptogenesis is crucial for establishing the evolution of this epileptic disorder since “seizures may beget seizures”, by inducing additional neuronal damage or aberrant synaptogenesis (Ben-Ari et al., 2008).
Although there is no experimental model that reproduces all the features of TLE, some models have been extensively used over the past decades because of their high level of similarity with human epilepsy. One of these is the kainic acid (KA) model, that was originally discovered by Ben-Ari (Ben-Ari and Lagowska, 1978; Ben-Ari et al., 1979a). In these initial studies, they showed that intra-amydaloid injections of KA induce behavioral seizures and produce neuropathological lesions that are similar to those occurring in patients with TLE (i.e., neuronal degeneration in the CA3 region of the dorsal hippocampus).
In this paper, we will review the results obtained from in vivo studies in which KA was administered intracerebrally or systemically. For each method, we will summarize their associated behavioral, electroencephalographic and neuropathological features. In addition, we will compare the epileptogenic properties of KA following intracerebral or systemic injection as well as the influence of the age of the animals on both KA-induced seizures and associated neuropathological changes. Finally, we will compare the KA model to two other models of TLE, namely the pilocarpine model and the kindling model.
2. Kainic acid
KA is a cyclic analog of L-glutamate and an agonist of ionotropic KA receptors. It was isolated and extracted in the early 1950s, from a red algae (Digenea simplex) found in tropical and sub-tropical waters (Murakami et al., 1953). It was named digenic acid but this term was later changed to KA in order to avoid confusion with the other derivatives of Digenea (Nadler, 1979). KA was first meant to be used as an ascaricide to eradicate ascariasis, a disease caused by the parasitic worm Ascaris lumbricoides. However, it was later found that it induced in rats prolonged excitatory responses in cortical neurons following microiontophoretic application (Shinozaki and Konishi, 1970). It became thus clear that KA could be used as a potent analog of glutamate, and that it could induce robust depolarizations and eventually cell death, a central phenomenon to TLE (Bloss and Hunter, 2010; Vincent and Mulle, 2009). This opened the path to the identification of new glutamate receptor subtypes, a better characterization of glutamatergic structures and pathways, and eventually the development of a new animal model of temporal lobe epilepsy characterized by a latent period followed by refractory spontaneous seizures, as in human TLE (Ben-Ari, 1985; Ben-Ari and Cossart, 2000; Nadler, 1981). The use of KA also led to a better understanding of various neurodegenerative disorders such as Parkinson’s disease (Meredith et al., 2009), Huntington’s disease (Coyle and Schwarcz, 1976; Coyle et al., 1977), Alzheimer’s disease (Hynd et al., 2004), amyotrophic lateral sclerosis (Redler and Dokholyan, 2012) and multiple sclerosis (Pitt et al., 2000). These neurological conditions, will, however, not be discussed in this review.
2.1. Kainic acid receptors
During the past thirty years, many studies have successfully mapped the localization of KA receptors (KARs). They can be found at different levels of expression in the amygdala (Rogawski et al., 2003), entorhinal cortex (Patel et al., 1986), basal ganglia (Jin and Smith, 2011) and cerebellum (Wisden and Seeburg, 1993). They are also highly expressed in the hippocampus where they are located both presynaptically and postsynaptically (Bloss and Hunter, 2010). KA1 subunits (actually known as GluK4, according to the International Union of Basic Science and Clinical Pharmacology Database (IUPHAR-DB)) (Sharman et al., 2013) are highly expressed in CA3 pyramidal cells but only weakly expressed in CA1 pyramidal cells (Bahn et al., 1994; Werner et al., 1991; Wisden and Seeburg, 1993). KA2 (GluK5) subunits are instead highly expressed in both CA1 and CA3 pyramidal cells (Bahn et al., 1994; Wisden and Seeburg, 1993). Therefore, the high affinity of KA1 and KA2 receptors to glutamate and their high rates of expression in the CA3 region of the hippocampus render this region highly susceptible to the excitotoxic damage induced by KA and often makes the hippocampus the seizure onset zone in this model (Ben-Ari and Cossart, 2000; Lévesque et al., 2009; Lothman et al., 1981). More specifically, the ictogenic properties of CA3 and, more generally, its capacity to generate synchronized activities following KA administration may be attributed to the activation of pyramidal neurons via the high-affinity KARs in the mossy fiber synaptic region that correspond to the stratum lucidum (Ben-Ari and Cossart, 2000).
Other KAR subunits (such as the GluR5 and GluR6 subunits or GluK1 and GluK2, respectively) also contribute to the excitatory action of KA; GluR6 are highly expressed in CA3 pyramidal cells (Bahn et al., 1994) while GluR5 are highly expressed in GABAergic interneurons in both CA1 and CA3 subfields (Bloss and Hunter, 2010). In line with this view it has been shown that ablation of the GluR5 subunit in knock-out (KO) mice makes the hippocampus more susceptible to the epileptogenic action of KA whereas the ablation of GluR6 subunits prevents the induction of epileptiform bursts by KA in vitro (Fisahn et al., 2004). GluR6 KO mice are also less susceptible to generate seizures following systemic administration of KA (Mulle et al., 1998). Moreover, the overexpression of GluR6 in the rat hippocampus by injection of a viral vector (HSVGluR6) induce seizures and modify over time and at a distance from the injection site the electrophysiological phenotype of CA1 pyramidal cells, making them hyperexcitable to synaptic stimulation (Telfeian et al., 2000). In epileptic patients, GluR5 and GluR6 mRNA levels were shown to decrease as a consequence of seizures (Mathern et al., 1998) but recent studies found upregulation of GluR5 mRNA levels in the hippocampus, but not in the neocortex, of patients with TLE compared to controls (Li et al., 2010).
It should also be emphasized that, although previous studies established the involvement of NMDA and AMPA receptors in mossy fiber sprouting observed in histological samples from the brain of epileptic patients and epileptic animals (Babb et al., 1996; Okazaki et al., 1999; Ying et al., 1998), recent studies suggest that KARs significantly contribute to this phenomenon. Mossy fiber sprouting in epileptic patients is correlated to an increase of GluR5 upregulation (Li et al., 2010), and such up-regulation even precedes mossy fiber sprouting in KA-treated rats (Bernard et al., 1999). These data suggest that KARs could be highly involved in the generation of epileptiform activity recorded from these new and functionally aberrant synapses. Accordingly, in these epileptic animals, synchronized network-driven activities in the dentate gyrus can be inhibited by pharmacological blockade of KA receptors (Epsztein et al., 2005).
3. Intracerebral administration of KA
One of the first studies showing an effect of KA on hippocampal neurons was published by Nadler et al. (1978). These experiments demonstrated that intraventricular injections of KA (0.5 nmol) in Sprague–Dawley rats caused one to three days after treatment pyramidal cell degeneration in CA3 at the rostral pole of the hippocampus whereas higher doses (0.8 μg) induced neuronal loss in more caudal regions of the hippocampus. Doses that were higher than 0.8 μg induced neurodegeneration in CA1 and CA2. Subsequent studies by Ben-Ari and colleagues (Ben-Ari and Lagowska, 1978; Ben-Ari et al., 1979a, 1980a, 1980b; Tremblay et al., 1983) showed that similar patterns of hippocampal neurodegeneration along with behavioral seizures could be induced by intra-amygdaloid administration of KA. Cepeda et al. (1982) reported later that intra-amygdaloid injections of KA in baboons could induce bilateral hippocampal lesions and status epilepticus (SE). These findings thus suggested that intracerebral injections of KA in the hippocampus or amygdala could represent a model of temporal lobe epilepsy since it reproduced the typical histopathological changes seen in epileptic patients (Ben-Ari et al., 1979a; Nadler, 1979).
3.1. Behavioral manifestations
The acute behavioral manifestations that follow the intracerebral administration of KA are similar to those observed following systemic injection of pilocarpine, another commonly used model of epilepsy (Curia et al., 2008). In rats, intrahippocampal administration of KA at doses ranging between 0.4 and 2.0 μg is usually effective in inducing a convulsive SE after 5–60 min following the injection (Bragin et al., 1999, 2000, 2004, 2009; Carriero et al., 2012; Raedt et al., 2009). Acute seizures are characterized by immobility followed by facial clonus (stage 1 according to (Racine, 1972), masticatory movements and head nodding (stage 2), wet-dog shakes, unilateral or bilateral forelimb clonus (stage 4) and rearing and falling (stage 5)) (Mouri et al., 2008; Pernot et al., 2011; Raedt et al., 2009). In contrast, in guinea pigs, intrahippocampal administrations of KA induce non-convulsive seizures (Carriero et al., 2012).
Acute seizures induced by intra-amygdaloid injections of KA (0.4–2 μg) in rats are characterized by similar symptoms to those observed following intrahippocampal injections (Ben-Ari et al., 1979a; Gurbanova et al., 2008), but additional symptoms such as salivation and exophthalmos can occur (Ben-Ari et al., 1980b; Gurbanova et al., 2008). In monkeys, however, intracerebral administration of KA (0.5–10 μg/μl of saline) in the hippocampus or in the amygdala does not produce generalized seizures but it is associated to focal seizures accompanied with movement arrests and oral automatisms (sniffing, chewing, facial movements, deviation of the eyes or contraversive head turning) (Menini et al., 1980). These behavioral differences have been attributed to the independence between limbic and neocortical circuits in the monkey brain (Menini et al., 1980).
3.2. Electroencephalographic features
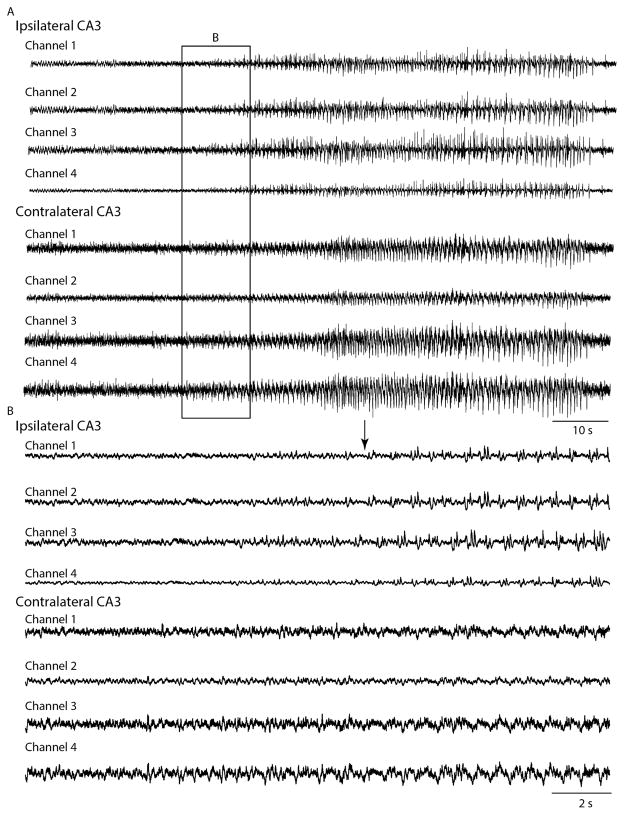
Intrahippocampal administration of KA induces epileptiform discharges that become visible on hippocampal recordings within 10 min after the injection (Cavalheiro et al., 1982; Raedt et al., 2009). Before seizures occurrence, EEG changes (paroxysmal bursts, interictal spikes, gamma oscillations (30–50 Hz)) are seen in the injected hippocampus (French et al., 1982; Jinde et al., 2009; Medvedev et al., 2000). This is followed by the occurrence of seizures that start in the hippocampus (Bragin et al., 2009; Carriero et al., 2012; Mathern et al., 1993) (Fig. 1). These seizures may then propagate to the ipsilateral amygdala (Akaike et al., 2001), and eventually to the contralateral amygdala, hippocampus and frontal cortex (Leite et al., 1996; Longo and Mello, 1999; Riban et al., 2002; Tanaka et al., 1982).
Fig. 1.
(A) EEG depth recordings acquired with single wires of two micro-wire bundles implanted in the CA3 region of the hippocampus ipsilateral and contralateral to the site of KA intrahippocampal (CA3) injection (0.4 μM/0.2 μL) in a Long Evans rat. Each micro-wire contains 4 wires (channels 1–4). This seizure occurred approximately 6 h after the intracerebral administration of KA. (B) Onset of the seizure is shown on an expanded time scale. Note that ictal activity starts in the ipsilateral CA3 region of the hippocampus (arrow).
Courtesy of Dr. K. Moxon and Ms S. Karunakaran.
A similar pattern of propagation occurs following intra-amygdaloid injections of KA. Accordingly, early studies have shown that epileptiform activity initiates in the amygdala (the site of injection) and then propagates to the cortex, ipsilateral hippocampus, contralateral amygdala and contralateral hippocampus (Ben-Ari et al., 1979a, 1980b). Intra-amygdaloid injection of KA in dogs induces similar results since a secondary seizure focus, in the contralateral amygdala or in the hippocampus, is observed (Hasegawa et al., 2002). These results suggest, as it was proposed initially by Ben-Ari et al. (1981), that the hippocampal formation plays a major role in the onset and propagation of KA-induced epileptiform activity even if the administration of KA is performed at sites that are distant from the hippocampus.
3.3. Neuropathological changes
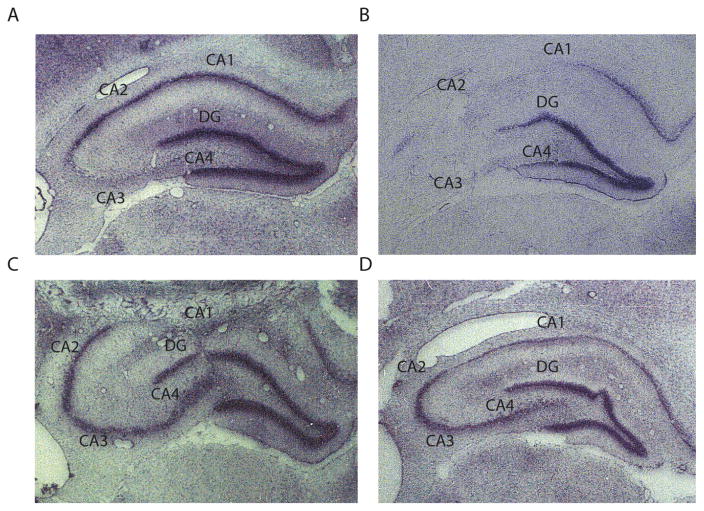
The hippocampus is highly susceptible to neuropathological changes following local administration of KA, even when it is not the injection site (Ben-Ari and Cossart, 2000) (Table 1). For instance, intraventricular administration of KA preferentially destroys the CA3 and CA4 regions of the hippocampus but leaves the CA1 region and the dentate gyrus almost intact (Lancaster and Wheal, 1982; Nadler and Cuthbertson, 1980) (Fig. 2A and B). However, there is a substantial loss of parvalbumin- and somatostatin-expressing GABAergic interneurons in CA1 (Best et al., 1993, 1994; Morin et al., 1998). This loss is especially marked in the stratus oriens of CA1, which may result in a reduction in the efficacy of feedback inhibition on pyramidal cells (Lacaille et al., 1987; Maccaferri and McBain, 1995; Morin et al., 1998) and possibly the development of a chronic epileptic state in this model.
Table 1.
Effects of intracerebral and systemic administrations of KA on the survival rate, the duration of the latent period and the neuropathological changes seen in the acute phase (<48 h after SE) and at a later stage (more than 2 days after SE).
| Mode of administration | Mortality rate | Latent period (days) | Neuropathological changes (<48 h after SE) | Neuropathological changes (>2 days after SE) | References |
|---|---|---|---|---|---|
| Intra-amygdaloid | ±55% | 10–40 | Ipsilateral CA1, CA3 and CA4 region of the hippocampus. Resistance of the CA2 region and of dentate granule cells. | Entire hippocampus, contralateral amygdala, extra-temporal regions. | Araki et al. (2002), Ben-Ari et al. (1980b), Cavalheiro et al. (1982), Dunleavy et al. (2010), Gurbanova et al. (2008), Li et al. (2012), Mouri et al. (2008), Represa et al. (1987) |
| Intrahippocampal | ±12% | 5–30 | Ipsilateral CA3 and CA4 region of the hippocampus, with CA1 being less affected. | Entire hippocampus, dispersion of cells in the granular and molecular layer of the dentate gyrus. | Arabadzisz et al. (2005), Carriero et al. (2012), Daniels et al. (1990), Nadler and Cuthbertson (1980) and Raedt et al. (2009) |
| Systemic | ±17% | 10–30 | Bilateral damage in the CA1, CA3 and CA4 regions of the hippocampus and in extrahippocampal regions. | Massive bilateral neuronal loss in the entire hippocampus and in extra-temporal regions, dispersion of granule cell layer in the dentate gyrus. | Ben-Ari et al. (1980a), Drexel et al. (2012), Haas et al. (2001), Heggli and Malthe-Sørenssen (1982), Kar et al. (1997), Sperk et al. (1985), Sloviter and Damiano (1981), Strain and Tasker (1991), Suarez et al. (2012) and Zhang et al. (2002) |
Fig. 2.
Lesions in the hippocampus following intraventricular administration of KA. (A) Histological sample from the hippocampus following 3.75 nmol (0.8 μg) of KA. Note the neuronal loss in CA3 and CA4 and the preservation of CA1 and dentate gyrus. (B) Sample from the hippocampus following 0.47 nmol (0.1 μg) of KA. Note the almost complete degeneration of CA2, CA3 and CA4 and again the preservation of CA1 and dentate gyrus. (C) Sample showing the preservation of the CA3/CA4 regions of the hippocampus following an intraventricular administration of KA 3 days after transection of the mossy fibers. (D) Sample showing the preservation of CA3/CA4 regions from KA after combined lesions of the mossy fibers and entorhinal cortex.
Courtesy of Dr. J. Victor Nadler.
Using unilateral intrahippocampal administration of KA produces nearly identical results. In the ipsilateral side to injection, there is CA3 pyramidal cell loss, even if KA injections are performed in distal sites (such as CA1), further suggesting that neuronal damage in CA3 is caused by the propagation of ictal activity to this region (Cavalheiro et al., 1982; Magloczky and Freund, 1993; Théorêt et al., 1988). A loss of GABAergic interneurons is nonetheless occurring in the dentate gyrus and CA1, with a selective loss of parvalbumin and calbindin-D28k immunoreactive interneurons (Bouilleret et al., 2000). Overtime, there is a near complete degeneration of the hippocampus with enlargement of the granule cell layer of the dentate gyrus, 2 weeks to one month after intrahippocampal administration of KA (Bouilleret et al., 1999; Davenport et al., 1990). Interestingly, transection of the hippocampal mossy fibers and combined lesions of the entorhinal cortex and mossy fibers protect the CA3/CA4 regions from KA-induced damage, presumably by preventing the spread of ictal activity to these regions (Nadler and Cuthbertson, 1980; Okazaki and Nadler, 1988) (Fig. 2C and D).
Using intra-amygdaloid administration of KA, Ben-Ari et al. (1980b) found that early after injection, neuronal loss occurs at the injection site and in the ipsilateral dorsal CA3 region. Successive studies have shown that pyramidal cells of the ipsilateral hippocampal CA3/CA4 region are especially vulnerable to KA compared to CA1, in the early post-treatment phase (up to 48 h) (Araki et al., 2002; Cavalheiro et al., 1982; Kasugai et al., 2007; Lancaster and Wheal, 1984; Mouri et al., 2008; Represa et al., 1987; Shinoda et al., 2004) (Table 1). On the other hand, damage in the contralateral hippocampus and extrahippocampal structures, such as the thalamus, the contralateral amygdala, the cingulate cortex and the neocortex occur after a prolonged survival (i.e., 4 days) (Ben-Ari et al., 1980a). Lesions outside the amygdala were however not attributed to the toxin itself but to the SE it induces (Ben-Ari, 1985; Ben-Ari et al., 1979b), since administration of diazepam before the KA administration in the amygdala did not modulate the extent of lesions at the site of injection but prevented distant hippocampal damage (Ben-Ari et al., 1978). These results have led to the hypothesis that, lesions occurring at sites that are distal from the injection site (such as the hippocampus) result from the propagation of seizure activity. This hypothesis was supported by the ability of transection of the perforant path to diminish the behavioral impact of intra-amygdaloid injections of KA and to reduce the extent of damage in the hippocampus and extrahippocampal regions (Ben-Ari et al., 1980a).
Berger et al. (1989) also reported that intra-amygdaloid injections of KA do not necessarily lead to histopathological changes in the hippocampus and extrahippocampal regions, provided that there is no SE. Finally, it was shown that low doses (0.4 μg) of KA are associated to focal amygdaloid seizures and damage in the CA3a region of the hippocampus whereas high doses (1.6 μg) induce a generalized SE that is associated to degeneration of the entire CA3/CA4 region (Ben-Ari et al., 1980a). Therefore, the pathological changes in regions that are distal from the injection site depend on the propagation of epileptic activity to these regions and not the toxin itself.
3.4. Epileptogenesis
Administration of KA in the hippocampus leads to the occurrence of spontaneous seizures between 5 days to one month after SE (Carriero et al., 2012; Cavalheiro et al., 1982; Gouder et al., 2003; Gröticke et al., 2008; Raedt et al., 2009; Riban et al., 2002; Tanaka et al., 1982; White et al., 2010) (Table 1). However, before the onset of the first spontaneous seizure and within one week after SE, abnormalities in the EEG of KA-treated rodents occur. Interictal spikes can be recorded from the ipsilateral and contralateral hippocampus, as well as in regions outside of the hippocampus (Bragin et al., 1999; Leite et al., 1996). Later, recurrent spontaneous seizures occur; they usually start from the injected hippocampus and propagate to the contralateral hippocampus and other cortical regions (Leite et al., 1996). There are, however, inter-species differences regarding the behavioral symptoms associated to the spontaneous recurrent seizures that should be taken into account: in mice and guinea pigs, seizures are always non-convulsive whereas in rats they are non-convulsive in the early phase of the chronic period but become convulsive during the late phase (Bouilleret et al., 1999; Bragin et al., 1999; Li et al., 2012).
Besides interictal spikes, altered patterns of rhythmicity occur in the KA model during the latent phase. For instance, there is a complete disappearance of the theta rhythm in the hippocampus ipsilateral to the site of injection in KA-treated mice (Riban et al., 2002), which could be related to changes in the synaptic properties of stratum oriens-lacunosum-moleculare (O-LM) interneurons. These neurons are known to receive excitatory glutamatergic inputs that are mediated by KA receptors (Dugladze et al., 2007) and they are known to contribute to the generation of hippocampal theta rhythms (Buzsáki, 2002; Goldin et al., 2007; Klausberger et al., 2003).
Oscillations in the gamma frequency range (30–80 Hz) are also present during the chronic period in the KA model, and Bragin et al. (2005) have reported that they precede the occurrence of spontaneous seizures, especially those characterized by low-voltage fast onset. In vitro, gamma activity occurs in the CA3 region of the hippocampus of chronic epileptic mice after a unilateral hippocampal injection of KA and the discharge frequency of dendrite-inhibiting OLM interneurons changes from the theta to the gamma frequency band in these epileptic mice (Dugladze et al., 2007). It was therefore proposed that these changes in rhythmogenesis reflected the altered firing properties of these interneurons. In brain slices from non-epileptic animals, high-amplitude gamma oscillations in CA3 can also be induced acutely by bath application of KA (Hájos et al., 2000; Lu et al., 2011).
Intra-amygdaloid injections of KA in the amygdala produce results that are similar to those observed following intrahippocampal administrations. The latent period lasts approximately 10–40 days (Gurbanova et al., 2008; Li et al., 2008; Mouri et al., 2008; Tanaka et al., 1985) (Table 1). Regarding the behavioral symptoms associated to seizures, there is also a difference between species since spontaneous non-convulsive seizures occur in mice (Li et al., 2008, 2012) whereas spontaneous convulsive seizures occur in rats (Gurbanova et al., 2008). Spontaneous seizures may originate from different sites, but their onset is usually located in the injected amygdala or in the ipsilateral hippocampus. In their initial studies, Tanaka et al. (1985) reported that seizures originate from the amygdala ipsilateral to the side of injection, approximately 14–20 days after SE, while after 30–60 days, seizure onset zones are located in both the ipsilateral and contralateral amygdala. However, further studies have shown that spontaneous seizures originate from the CA3 region of the hippocampus (Li et al., 2008). Later, using the same protocol, these authors identified two independent foci of seizure onset, one located in the amygdala and the other in the ipsilateral CA3 region of the hippocampus, which is characterized by prominent and acute cell loss (Li et al., 2012). These results further support the hypothesis that regions (for instance the CA3) that are distal from the site of injection are more likely to become seizure onset zones because of a reorganization of neural networks induced by SE. Moreover, it has been reported that a pronounced loss of inhibition characterizes CA1 within 4 weeks after SE; these findings have been interpreted to reflect the CA3 damage leading to loss of inputs on CA1 interneurons (Ashwood and Wheal, 1986; Ashwood et al., 1986; Cornish and Wheal, 1989; Franck and Schwartzkroin, 1985; Meier et al., 1992).
4. Systemic administration of KA
4.1. Behavioral manifestations
The main advantage of using systemic administrations of KA compared to intracerebral administrations are that many animals can be injected at one time and it does not require from the experimenter to perform surgical procedures, which therefore eliminates post-surgical complications that could affect the animal’s health or damage to the brain tissue made by the cannula. However, its main disadvantages are that one has no control on the bio-availability of KA in the brain and some animals may require multiple systemic injections before SE (2005). Possible bias may thus be introduced when comparing animals that have undergone SE after one injection to animals that in which multiple injections of KA were performed.
The mortality rate following systemic administration of KA in rats is between 5 and 30% (Friedman et al., 1994; Hellier and Dudek, 1999; Hellier et al., 1998; Sharma et al., 2008; Sperk et al., 1983; Williams et al., 2006) (Table 1). Single injections in the range of 6–15 mg/kg produce SE but using multiple doses of 5 mg/kg/h until the occurrence of SE can reduce the mortality rate (Sharma et al., 2007). Moreover, Hellier and Dudek (2005) suggested that a full (5 mg/kg) or a half dose (2.5 mg/kg) of KA should be administered depending on the severity of the behavioral symptoms induced by the initial injection.
According to several investigators, SE occurs approximately 1 h after injection in 93% of animals, and it can be stopped with a combination of diazepam (20 mg/kg) and ketamine (50 mg/kg) (Giorgi et al., 2005; Sharma et al., 2008; Stafstrom et al., 1992; Tabb et al., 2007). During this time-period, KA-treated animals show automatisms and a catatonic posture that often progresses to myoclonic twitching of the head, forelimbs and rearlimbs (Friedman et al., 1994; Sperk et al., 1983; Strain and Tasker, 1991). Typically, before and after SE, KA-treated rats also develop wet-dog shakes (Velísek et al., 1992). Interestingly, the decrease in occurrence of wet-dog shakes is associated to an increase of seizure occurrence (i.e., time-periods during which there is a low occurrence of wet-dog shakes are characterized by high seizure occurrence) (Ben-Ari et al., 1981; Collins et al., 1980).
4.2. Electroencephalographic features
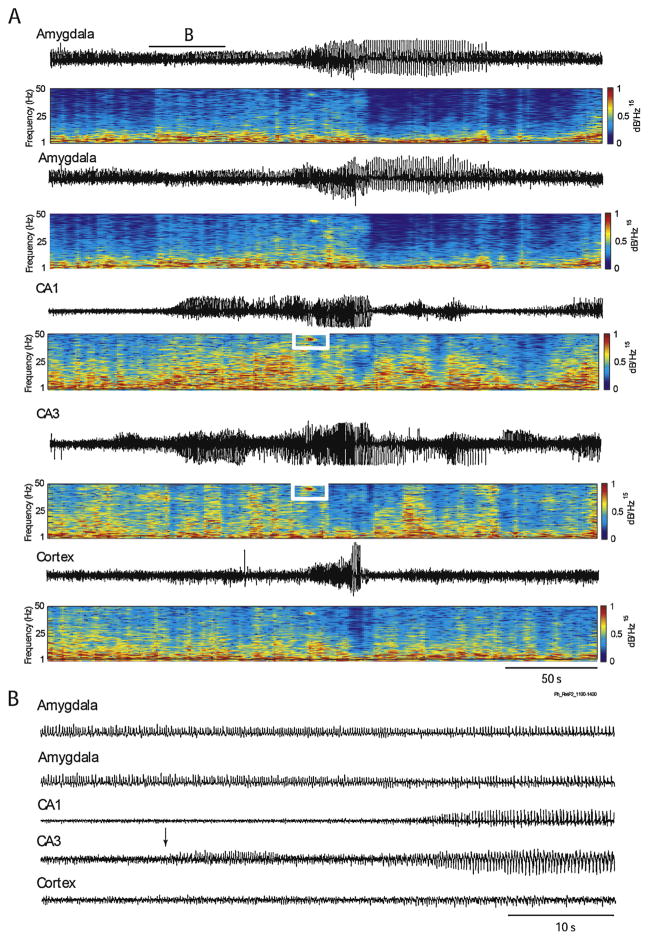
Epileptiform EEG patterns occur during the first 30 min following the systemic administration of KA (i.p., i.v. or s.c.). Ben-Ari et al. (1981) first described in the entorhinal cortex of KA-treated rats interictal spikes that were associated to the occurrence of wet-dog shakes. Ictal discharges then appeared in the CA3 region and in the amygdala, followed by propagation to the thalamus, the CA1 region and the frontal cortex. These ictal discharges were, however, observed only on the EEG signal and were not associated to any clinical signs, besides wet-dog shakes. Similar results have been obtained by Lothman et al. (1981), who found that epileptiform activity first occurs in the hippocampus following a systemic injection of KA in rats, without any detectable clinical symptoms. Besides interictal spikes, hippocampal EEG activity in these experiments was characterized by rhythmic patterns in the gamma frequency range (25–30 Hz). Medvedev et al. (2000) have later confirmed this phenomenon in the KA model and found that the EEG recorded from the hippocampus is characterized by runs of gamma oscillations (30–40 Hz) followed by sporadic spikes before the occurrence of clinical signs. Lévesque et al. (2009) have also reported hippocampal gamma oscillations during seizure activity acutely induced by KA, and showed that the hippocampus was often the seizure onset zone (Fig. 3). Overall, these results suggest that, as with the intracerebral administration of KA, the hippocampus occupies a central role in the onset of seizures in this model (Ben-Ari and Cossart, 2000; Ben-Ari et al., 1981).
Fig. 3.
(A) EEG depth recordings performed in a Sprague–Dawley rat showing a seizure recorded simultaneously in the amygdala, hippocampus and neocortex approximately 2 h after systemic administration of KA (6 mg/kg, i.p.). Gamma oscillations (30–80 Hz) (white rectangles) occurs in the CA1 and CA3 regions of the hippocampus. (B) Onset of the seizure is shown on an expanded time scale. Note that the seizure starts in the CA3 region (arrow).
Courtesy of Dr. Lionel Carmant.
4.3. Neuropathological changes
Lesions induced by the systemic administration of KA are similar to those seen following intracerebral administration (Schwob et al., 1980), although the extent of neuronal damage is greater. Following systemic injection, there is a loss of pyramidal cells in the CA1, CA3 and CA4 regions of the hippocampus (Haas et al., 2001; Kar et al., 1997; Suarez et al., 2012) but parvalbumin-positive interneurons are also highly sensitive to KA, since they degenerate in the CA1 region, the entorhinal cortex and the subiculum (Best et al., 1993, 1994; Drexel et al., 2011). Interestingly, interneurons in the stratum oriens-alveus of CA1 can be damaged by KA in the absence of damage to pyramidal cells, especially in young animals (P20) (Renaud et al., 2002; Sanon et al., 2005). In the amygdala, the density of GABAergic interneurons are also significantly reduced in the basolateral part (Fritsch et al., 2009; Tuunanen et al., 1996), with somatostatin-expressing interneurons being mainly affected (Tuunanen et al., 1996).
Systemic injections also induce neuronal loss bilaterally and in extra-temporal regions. Neurodegeneration in layer III of the entorhinal cortex, proximal subiculum, claustrum, thalamus, caudate putamen and the cerebral cortex occur within a 24 h period after SE (Drexel et al., 2012). Animals surviving longer time periods (more than 48 h) after SE show bilateral gliosis, brain edema or shrinkage of nerve cells in the piriform and entorhinal cortices, olfactory bulb, substantia nigra, thalamus and mesencephalon, and cell layer dispersion in the dentate gyrus (Ben-Ari et al., 1980a) (Table 1). Interestingly, these neuropathological changes mainly occur in animals presenting robust convulsions during SE (Sperk et al., 1985), thus supporting the view that damage in extrahippocampal regions is not caused by the toxin itself but depends on the propagation of epileptiform activity.
4.4. Epileptogenesis
In 60–80% of animals that survive the systemic administration of KA, non-convulsive seizures occur 10–30 days after treatment (Chauvière et al., 2012; Cherubini et al., 1983; Drexel et al., 2012; Lado, 2006; Sharma et al., 2008; White et al., 2010; Williams et al., 2006) (Table 1). The occurrence of non-convulsive seizures in the early phase of the latent period puts further emphasis on the need to perform EEG monitoring following KA treatment since the estimation of the duration of the latent period can differ substantially between animals that are only video-monitored and those that are implanted for EEG recordings. For instance, according to Hellier et al. (1998), who studied only the behavior and thus the occurrence of convulsive seizures, the latent period was estimated to be 77 ± 38 days. In contrast, when EEG-video monitoring recordings were performed, the latent period lasted approximately 14 days (Lado, 2006; Williams et al., 2009).
Similar to what was reported following intracerebral application of KA, systemic injections induce a latent period characterized by interictal spikes. Interestingly, in animals that develop chronic epilepsy, rates of occurrence of interictal spikes during the latent period are higher compared to rats that do not become epileptic, and epileptic rats are more likely to show clusters of interictal spikes, suggesting that in the KA model, these events are markers of pathological network activity in limbic networks (White et al., 2010). A recent study by Chauvière et al. (2012) has identified two distinct types of interictal spikes during the latent period: one that is characterized by a spike followed by a long-lasting wave (type 1) and a second one with a spike but no wave (type 2). These authors have found that the occurrence of type 1 spikes decreases during the latent period whereas the occurrence of type 2 spikes increases, reaching their minimum/maximum values just before the onset of the chronic period. These results further suggest that in the KA model the pattern of interictal spikes occurring during the latent period may reflect underlying epileptogenic mechanisms.
5. Age specificity
It is known that brains in young animals are hyperexcitable when compared to adults. For instance, Holmes and Thompson (1988) showed that young rats (P12) exhibit more severe SE following intraperitoneal injection of KA compared to adult animals (P27). This difference could not be explained by the degree of damage induced by SE since no histological lesions were found in the two groups. Moreover, animals in both groups developed spontaneous seizures. Stafstrom et al. (1992) have also reported an effect of age on the latency between the intracerebral administration of KA in the hippocampus and the onset of SE: younger rats (P5–P10) showed a SE after a shorter time-period compared to older rats (P20–P60). Golden et al. (1995) later obtained similar findings using systemic subcutaneous injections of KA and found that rats aged from P35–40 showed a shorter latency to SE compared to older rats (P70–90). Finally, Mikati et al. (2003) reported higher mortality rates in P15 rats compared to P35 rats following 5–15 mg/kg of KA administered intraperitoneally.
When older animals are compared, P33–37 rats are more resistant to KA than P90 rats (Albala et al., 1984). Similar results have been obtained by Sarkisian et al. (1997) who showed that P20 rats have a longer latency to acute seizure onset and lower mortality rates following repetitive systemic intraperitoneal injections of KA compared to older rats (P60). Dawson and Wallace (1992) also observed significantly more acute seizures and more severe symptoms in aged Long–Evans female rats (30-month old) compared to younger ones (6-month old) following intraperitoneal injections of KA. These results are similar to those obtained by Wozniak et al. (1991) who showed that rats aged from 12 to 13 months and from 22 to 25 months were more sensitive to KA compared to rats aged from 5 to 6 months. All together, these studies suggest that KA age specificity shows a U pattern, with young (up to P15) and old rats (from P60 and older) being more sensitive to KA (shorter latency to SE and more severe seizures) compared to rats aged from approximately P20 to P60.
Many hypotheses have been formulated to explain these age-related differences. However, the mechanisms underlying this phenomenon remain unclear. For instance, both depolarizing effects of GABA and intense synaptogenesis have been proposed to contribute to the high susceptibility to seizures in immature brains (Ben-Ari, 2002; Ben-Ari and Holmes, 2005; Ben-Ari et al., 1989). Due to high intracellular concentrations of chloride, the immature brain is more prone to seizures because GABA can exert an excitatory and depolarizing action on neurons (Ben-Ari, 2002; Ben-Ari et al., 2007; Cherubini et al., 1991; Kahle et al., 2008). Thus, in the event of environmental insults, congenital abnormalities or genetic factors, brain excitability is enhanced and seizures are more likely to occur in young subjects (Ben-Ari et al., 2007; Dzhala and Staley, 2003; Khazipov et al., 2004). However, both enhanced excitability and seizure occurrence are not necessarily associated to neuronal damage, as seen in mature brains. For instance, the administration of KA in animals at an early age (less than 3 weeks of age) induces severe tonic–clonic seizures but leaves the brain almost intact (Nitecka et al., 1984). This phenomenon was attributed to the delayed maturation of the dentate gyrus and of the mossy fibers, and the lack of inputs from the entorhinal cortex to the hippocampus since ictal activity is unable to propagate along mossy fibers and to reach the CA3 region of the hippocampus (Nitecka et al., 1984).
However, even though seizures do not cause damage in early age, they interfere with brain development, by inducing long-term alterations in GABAergic synapses. This aspect has been documented through abnormal GABAergic signaling (Bortolato et al., 2010). More precisely, in young rats, epileptiform activity induced by KA in one hippocampus would be able to produce a secondary mirror focus contralaterally, possibly through the intracellular accumulation of chloride and a shift in the reversal potential of GABA in distal brain regions (Dzhala et al., 2012; Khalilov et al., 2003). The existence of a secondary mirror focus could contribute to the development of a chronic epileptic state.
6. Comparison with other animal models of mesial temporal lobe epilepsy
6.1. The pilocarpine model
The electroencephalographic features and the neuropathological alterations seen in pilocarpine-treated animals are similar to what is reported with KA. Following pilocarpine intrahippocampal administration, the first epileptiform discharge occurs in the hippocampus and there is mossy fiber sprouting in the inner molecular layer of the dentate gyrus (Furtado et al., 2002). With systemic administration of pilocarpine, more extensive lesions are observed since there are morphological changes in the thalamus, substantia nigra, dentate hilus, cerebral cortex, olfactory cortices and the amygdala (Rose Priel et al., 1996). This has been considered as one of the drawback of the pilocarpine model (Sloviter, 2005), but one has to consider that (1) systemic KA also induces massive damage in extrahippocampal regions and (2) patients with TLE also show neuronal damage outside of the hippocampus (Davis et al., 2002). Finally, following systemic injections of pilocarpine, the hippocampus is often the onset zone as in the KA model (Arida et al., 1999; Bortel et al., 2010; Cavalheiro et al., 1991; Curia et al., 2008; Goffin et al., 2007; Lévesque et al., 2011, 2012; Turski et al., 1983).
Pilocarpine treatment is also associated with enhanced permeability of the blood–brain barrier, and such an effect was proposed to contribute to the generation of SE following pilocarpine injection (Marchi et al., 2007). Recent studies have indeed shown that in the isolated guinea-pig brain preparation, arterial perfusion of pilocarpine induces epileptiform activity only when it is co-administered with compounds that enhance blood–brain barrier permeability, such as bradykinin or histamine (Uva et al., 2008). In contrast, when administered alone, at concentrations that normally cause SE in vivo, it is not sufficient to induce epileptiform activity (Uva et al., 2008). Similar to pilocarpine, KA can enhance the permeability of the blood brain barrier. This phenomenon was initially reported in a study by Zucker et al. (1983), who discovered that animals experiencing severe limbic seizures present with enhanced permeability of the blood bran barrier in limbic regions between 2 and 24 h after SE. Further studies have however shown that the permeability of the blood brain barrier can be observed within 1 h after its administration and before the occurrence of SE (Saija et al., 1992). The increased permeability of the blood brain barrier would lead to an enhanced release of glutamate in the hippocampus that would eventually favor seizure occurrence (Pont et al., 1995). Thus, in both models, the opening of the blood brain barrier may be perceived as a precipitating factor in inducing acute seizures (Marchi et al., 2011).
Finally, the main advantage of the pilocarpine model over the KA model is its reliability. We have observed that almost all rats injected with 380 mg/kg, i.p. of pilocarpine (and that survive the initial SE) will develop spontaneous seizures, independently of the duration of the SE (from 30 min to 2 h) (Bortel et al., 2010; Lévesque et al., 2011). Few injections are also necessary, since if a single initial dose of pilocarpine does not induce seizures after 30 min, usually one or two additional half doses (190 mg/kg, i.p.) can be given, which thus requires approximately a maximum of 4 h from the initial injection to the end of SE. In the KA model, animals often need to experience a SE of more than 3 h in order to show spontaneous seizures and as already stated, multiple injections at one hour interval may be necessary to induce a SE, which could make the duration of the experiment last between 6 and 8 h (2005) (Hellier et al., 1998).
6.2. Electrical kindling
Repetitive electrical stimulation protocols repeated over several days can induce a chronic permanent hyperexcitable state that can be associated to spontaneous seizures. This procedure is known as kindling (Goddard et al., 1969). One of the main advantage of kindling over the KA model is that a specific region of the brain can be targeted and the parameters can be easily manipulated by the experimenter (Raol and Brooks-Kayal, 2012). Ictogenesis is thus partially controlled, along with neural networks that may be involved in seizure generation as multiple sites can be stimulated simultaneously (McNamara, 1984). However, compared to both the KA and pilocarpine models, lesions are often not visible (Raol and Brooks-Kayal, 2012; Sharma et al., 2007). In fact, when damage is observed following kindling, it occurs in animals who survive multiple stage 5 seizures and it is characterized by decreased neuronal densities in CA1, CA3, entorhinal cortex and endopiriform nucleus (Cavazos et al., 1994). However, kindling is not associated to hippocampal sclerosis, lesions do not resemble those seen in MTLE and spontaneous seizures rarely occur, which casts doubt on the validity of this model (Sharma et al., 2007). The kindling method is also time-consuming, since each animal has to go under repetitive electrical stimulation sessions.
7. Conclusive remarks
After three decades of active epilepsy research, the KA model is still one of the most widely used animal models of TLE. It has allowed investigators to study ictogenesis and epileptogenesis from single neurons to networks, and results obtained do support the hypothesis that epilepsy results from a complex interaction between aberrant network activity and morphological changes. In this paper, we have reviewed evidence supporting the view that the KA model is a highly isomorphic model of the human disease, independently of the method of the administration. The intracerebral and systemic procedures yield similar results in terms of latency to SE, behavioral symptoms, duration of the latent period and electroencephalographic features of the latent and chronic periods. The main difference lies in the extent of neuropathological damage that is induced by each route of administration, with the intracerebral administration producing unilateral temporal lobe lesions as observed in humans with TLE (Engel, 1996; Sloviter, 2005). The choice of either one or the other method will thus depend on the questions that need to be answered: intracerebral administration of KA may be used to investigate the effect of epileptiform activities in circumscribed pathological networks and the effect of seizures on surrounding and presumably healthy tissue, whereas systemic administration should be employed to study the selective vulnerability of multiple brain regions to the agent and to the occurrence of a more widespread epileptic disease. We have also highlighted the differences that exist between species and age specificity related to this model. Further studies are needed to understand the discrepancies between the behavioral symptoms that are specific to each species and why the age of the animal affects the ability of KA to induce seizures. However, as it was suggested, the answer may lie in the different patterns of connectivity and the effect of age on the organization of neural networks.
In conclusion, the KA model has provided a better understanding of the processes underlying TLE and may have contributed to the development of more efficient targeted therapeutic drugs. It is likely that with the advent of new technologies – which are providing a more in-depth look at the molecular, neurophysiological and neuroanatomical aspects – the KA model will remain a widely used animal model of TLE.
Acknowledgments
This review was supported by the Canadian Institutes of Health Research (CIHR grants 8109 and 74609). ML was recipient of a post-doctoral fellowship from the Savoy Foundation. We thank Dr Lionel Carmant, Dr J Victor Nadler, Dr Karen Moxon and Ms Suganya Karunakaran for providing the EEG recordings and histological samples shown in this review. We also thank Dr. Philippe Séguéla for making constructive comments on the manuscript.
Footnotes
Conflicts of interest
None of the authors has any conflict of interest to disclose.
References
- Akaike K, Tanaka S, Tojo H, Fukumoto S, Imamura S, Takigawa M. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Res. 2001;900:65–71. doi: 10.1016/s0006-8993(01)02252-1. [DOI] [PubMed] [Google Scholar]
- Albala BJ, Moshé SL, Okada R. Kainic-acid-induced seizures: a developmental study. Brain Res. 1984;315:139–148. doi: 10.1016/0165-3806(84)90085-3. [DOI] [PubMed] [Google Scholar]
- Araki T, Simon RP, Taki W, Lan JQ, Henshall DC. Characterization of neuronal death induced by focally evoked limbic seizures in the C57BL/6 mouse. J Neurosci Res. 2002;69:614–621. doi: 10.1002/jnr.10356. [DOI] [PubMed] [Google Scholar]
- Arabadzisz D, Antal K, Parpan F, Emri Z, Fritschy JM. Epileptogenesis and chronic seizures in a mouse model of temporal lobe epilepsy are associated with distinct EEG patterns and selective neurochemical alterations in the contralateral hippocampus. Exp Neurol. 2005;194:76–90. doi: 10.1016/j.expneurol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Arida RM, Scorza FA, Peres CA, Cavalheiro EA. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res. 1999;34:99–107. doi: 10.1016/s0920-1211(98)00092-8. [DOI] [PubMed] [Google Scholar]
- Ashwood TJ, Wheal HV. Loss of inhibition in the CA1 region of the kainic acid lesioned hippocampus is not associated with changes in postsynaptic responses to GABA. Brain Res. 1986;367:390–394. doi: 10.1016/0006-8993(86)91625-2. [DOI] [PubMed] [Google Scholar]
- Ashwood TJ, Lancaster B, Wheal HV. Intracellular electrophysiology of CA1 pyramidal neurones in slices of the kainic acid lesioned hippocampus of the rat. Exp Brain Res Exp Hirnforsch Expérimentation Cérébrale. 1986;62:189–198. doi: 10.1007/BF00237415. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. The multiple facets of gamma-aminobutyric acid dysfunction in epilepsy. Curr Opin Neurol. 2005;18:141–145. doi: 10.1097/01.wco.0000162855.75391.6a. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Lagowska J. Epileptogenic action of intra-amygdaloid injection of kainic acid. Comptes Rendus Hebd Séances Acad Sci Série Sci Nat. 1978;287:813–816. [PubMed] [Google Scholar]
- Ben-Ari Y, Lagowska Y, Le Gal La Salle G, Tremblay E, Ottersen OP, Naquet R. Diazepam pretreatment reduces distant hippocampal damage induced by intra-amygdaloid injections of kainic acid. Eur J Pharmacol. 1978;52:419–420. doi: 10.1016/0014-2999(78)90302-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Lagowska J, Tremblay E, Le Gal La Salle G. A new model of focal status epilepticus: intra-amygdaloid application of kainic acid elicits repetitive secondarily generalized convulsive seizures. Brain Res. 1979a;163:176–179. doi: 10.1016/0006-8993(79)90163-x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP. Primary and secondary cerebral lesions produced by kainic acid injections in the rat. Comptes Rendus Hebd Séances Acad Sci Série Sci Nat. 1979b;288:991–994. [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP, Meldrum BS. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980a;191:79–97. doi: 10.1016/0006-8993(80)90316-9. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience. 1980b;5:515–528. doi: 10.1016/0306-4522(80)90049-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Crepel V, Represa A. Seizures beget seizures in temporal lobe epilepsies: the boomerang effects of newly formed aberrant kainatergic synapses. Epilepsy Curr Am Epilepsy Soc. 2008;8:68–72. doi: 10.1111/j.1535-7511.2008.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Mathern GW, Leite JP, Pretorius JK, Yeoman KM, Kuhlman PA. Glutamate AMPA receptors in the fascia dentata of human and kainate rat hippocampal epilepsy. Epilepsy Res. 1996;26:193–205. doi: 10.1016/s0920-1211(96)00053-8. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci Off J Soc Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain. 2008;131:1818–1830. doi: 10.1093/brain/awn111. [DOI] [PubMed] [Google Scholar]
- Berger ML, Lassmann H, Hornykiewicz O. Limbic seizures without brain damage after injection of low doses of kainic acid into the amygdala of freely moving rats. Brain Res. 1989;489:261–272. doi: 10.1016/0006-8993(89)90859-7. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Andermann F, Olivier A, Ethier R, Melanson D, Robitaille Y, Kuzniecky R, Peters T, Feindel W. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann Neurol. 1991;29:175–182. doi: 10.1002/ana.410290210. [DOI] [PubMed] [Google Scholar]
- Bernard A, Ferhat L, Dessi F, Charton G, Represa A, Ben-Ari Y, Khrestchatisky M. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur J Neurosci. 1999;11:604–616. doi: 10.1046/j.1460-9568.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- Best N, Mitchell J, Baimbridge KG, Wheal HV. Changes in parvalbumin-immunoreactive neurons in the rat hippocampus following a kainic acid lesion. Neurosci Lett. 1993;155:1–6. doi: 10.1016/0304-3940(93)90660-d. [DOI] [PubMed] [Google Scholar]
- Best N, Mitchell J, Wheal HV. Ultrastructure of parvalbumin-immunoreactive neurons in the CA1 area of the rat hippocampus following a kainic acid injection. Acta Neuropathol (Berl) 1994;87:187–195. doi: 10.1007/BF00296189. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Hunter RG. Hippocampal kainate receptors. Vitam Horm. 2010;82:167–184. doi: 10.1016/S0083-6729(10)82009-6. [DOI] [PubMed] [Google Scholar]
- Bortel A, Lévesque M, Biagini G, Gotman J, Avoli M. Convulsive status epilepticus duration as determinant for epileptogenesis and interictal discharge generation in the rat limbic system. Neurobiol Dis. 2010;40:478–489. doi: 10.1016/j.nbd.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Barberini L, Puligheddu M, Muroni A, Maleci A, Ennas F, Gioi G, Serra A, Piga M, Marrosu F. Involvement of GABA in mirror focus: a case report. Epilepsy Res. 2010;90:300–303. doi: 10.1016/j.eplepsyres.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience. 1999;89:717–729. doi: 10.1016/s0306-4522(98)00401-1. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy JM. Early loss of interneurons and delayed subunit-specific changes in GABAA-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus. 2000;10:305–324. doi: 10.1002/1098-1063(2000)10:3<305::AID-HIPO11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Vizentin E, Mathern GW. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999;40:1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41(Suppl 6):S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Engel J., Jr The cause of the imbalance in the neuronal network leading to seizure activity can be predicted by the electrographic pattern of the seizure onset. J Neurosci Off J Soc Neurosci. 2009;29:3660–3671. doi: 10.1523/JNEUROSCI.5309-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS. Mossy fiber sprouting in the dentate gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information (US); Bethesda, MD: 2012. [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Carriero G, Arcieri S, Cattalini A, Corsi L, Gnatkovsky V, de Curtis M. A guinea pig model of mesial temporal lobe epilepsy following nonconvulsive status epilepticus induced by unilateral intrahippocampal injection of kainic acid. Epilepsia. 2012;53:1917–1927. doi: 10.1111/j.1528-1167.2012.03669.x. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Riche DA, Le Gal La Salle G. Long-term effects of intrahippocampal kainic acid injection in rats: a method for inducing spontaneous recurrent seizures. Electroencephalogr Clin Neurophysiol. 1982;53:581–589. doi: 10.1016/0013-4694(82)90134-1. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci Off J Soc Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Tanaka T, Riche D, Naquet R. Limbic status epilepticus: behaviour and sleep alterations after intra-amygdaloid kainic acid microinjections in Papio Papio baboons. Electroencephalogr Clin Neurophysiol. 1982;54:603–613. doi: 10.1016/0013-4694(82)90114-6. [DOI] [PubMed] [Google Scholar]
- Chauvière L, Doublet T, Ghestem A, Siyoucef SS, Wendling F, Huys R, Jirsa V, Bartolomei F, Bernard C. Changes in interictal spike features precede the onset of temporal lobe epilepsy. Ann Neurol. 2012;71:805–814. doi: 10.1002/ana.23549. [DOI] [PubMed] [Google Scholar]
- Cherubini E, De Feo MR, Mecarelli O, Ricci GF. Behavioral and electrographic patterns induced by systemic administration of kainic acid in developing rats. Brain Res. 1983;285:69–77. doi: 10.1016/0165-3806(83)90110-4. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Collins RC, McLean M, Olney J. Cerebral metabolic response to systemic kainic acid: 14-C-deoxyglucose studies. Life Sci. 1980;27:855–862. doi: 10.1016/0024-3205(80)90080-6. [DOI] [PubMed] [Google Scholar]
- Cornish SM, Wheal HV. Long-term loss of paired pulse inhibition in the kainic acid-lesioned hippocampus of the rat. Neuroscience. 1989;28:563–571. doi: 10.1016/0306-4522(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington’s chorea. Nature. 1976;263:244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Schwarcz R, Bennett JP, Campochiaro P. Clinical, neuropathologic and pharmacologic aspects of Huntington’s disease: correlates with a new animal model. Prog Neuropsychopharmacol. 1977;1:13–30. doi: 10.1016/0364-7722(77)90025-x. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RSG, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WM, Jaffer A, Engelbrecht AH, Russell VA, Taljaard JJ. The effect of intrahippocampal injection of kainic acid on corticosterone release in rats. Neurochem Res. 1990;15:495–499. doi: 10.1007/BF00966206. [DOI] [PubMed] [Google Scholar]
- Davenport CJ, Brown WJ, Babb TL. GABAergic neurons are spared after intrahippocampal kainate in the rat. Epilepsy Res. 1990;5:28–42. doi: 10.1016/0920-1211(90)90063-2. [DOI] [PubMed] [Google Scholar]
- Davis KL, Charney D, Coyle JT, Nemeroff C. Neuropsychopharmacology: The Fifth Generation of Progress: An Official Publication of the American College of Neuropsychopharmacology. Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2002. [Google Scholar]
- Dawson R, Jr, Wallace DR. Kainic acid-induced seizures in aged rats: neurochemical correlates. Brain Res Bull. 1992;29:459–468. doi: 10.1016/0361-9230(92)90083-a. [DOI] [PubMed] [Google Scholar]
- Drexel M, Preidt AP, Kirchmair E, Sperk G. Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reuniens degenerate in the subiculum after kainic acid-induced seizures. Neuroscience. 2011;189:316–329. doi: 10.1016/j.neuroscience.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexel M, Preidt AP, Sperk G. Sequel of spontaneous seizures after kainic acid-induced status epilepticus and associated neuropathological changes in the subiculum and entorhinal cortex. Neuropharmacology. 2012;63:806–817. doi: 10.1016/j.neuropharm.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugladze T, Vida I, Tort AB, Gross A, Otahal J, Heinemann U, Kopell NJ, Gloveli T. Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc Natl Acad Sci USA. 2007;104:17530–17535. doi: 10.1073/pnas.0708301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy M, Shinoda S, Schindler C, Ewart C, Dolan R, Gobbo OL, Kerskens CM, Henshall DC. Experimental neonatal status epilepticus and the development of temporal lobe epilepsy with unilateral hippocampal sclerosis. Am J Pathol. 2010;176:330–342. doi: 10.2353/ajpath.2010.090119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus. J Neurosci. 2003;23:1840–1846. doi: 10.1523/JNEUROSCI.23-05-01840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala V, Valeeva G, Glykys J, Khazipov R, Staley K. Traumatic alterations in GABA signaling disrupt hippocampal network activity in the developing brain. J Neurosci Off J Soc Neurosci. 2012;32:4017–4031. doi: 10.1523/JNEUROSCI.5139-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA J Am Med Assoc. 2012;307:922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztein J, Represa A, Jorquera I, Ben-Ari Y, Crépel V. Recurrent mossy fibers establish aberrant kainate receptor-operated synapses on granule cells from epileptic rats. J Neurosci Off J Soc Neurosci. 2005;25:8229–8239. doi: 10.1523/JNEUROSCI.1469-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci Off J Soc Neurosci. 2004;24:9658–9668. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck JE, Schwartzkroin PA. Do kainate-lesioned hippocampi become epileptogenic? Brain Res. 1985;329:309–313. doi: 10.1016/0006-8993(85)90540-2. [DOI] [PubMed] [Google Scholar]
- French ED, Aldinio C, Schwarcz R. Intrahippocampal kainic acid, seizures and local neuronal degeneration: relationships assessed in unanesthetized rats. Neuroscience. 1982;7:2525–2536. doi: 10.1016/0306-4522(82)90212-3. [DOI] [PubMed] [Google Scholar]
- Friedman LK, Pellegrini-Giampietro DE, Sperber EF, Bennett MV, Moshé SL, Zukin RS. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: an in situ hybridization study. J Neurosci Off J Soc Neurosci. 1994;14:2697–2707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Rogawski MA, Braga MFM. Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis. Neuroscience. 2009;163:415–429. doi: 10.1016/j.neuroscience.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado M, de A, Braga GK, Oliveira JAC, Del Vecchio F, Garcia-Cairasco N. Behavioral, morphologic, and electroencephalographic evaluation of seizures induced by intrahippocampal microinjection of pilocarpine. Epilepsia. 2002;43(Suppl 5):37–39. doi: 10.1046/j.1528-1157.43.s.5.41.x. [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Malhotra S, Hasson H, Velísková J, Rosenbaum DM, Moshé SL. Effects of status epilepticus early in life on susceptibility to ischemic injury in adulthood. Epilepsia. 2005;46:490–498. doi: 10.1111/j.0013-9580.2005.42304.x. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. Oxford University Press; USA: 1997. [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Goffin K, Nissinen J, Van Laere K, Pitkänen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol. 2007;205:501–505. doi: 10.1016/j.expneurol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Golden GT, Smith GG, Ferraro TN, Reyes PF. Rat strain and age differences in kainic acid induced seizures. Epilepsy Res. 1995;20:151–159. doi: 10.1016/0920-1211(94)00079-c. [DOI] [PubMed] [Google Scholar]
- Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crépel V, Cossart R. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci Off J Soc Neurosci. 2007;27:9560–9572. doi: 10.1523/JNEUROSCI.1237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouder N, Fritschy JM, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia. 2003;44:877–885. doi: 10.1046/j.1528-1157.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- Gröticke I, Hoffmann K, Löscher W. Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp Neurol. 2008;213:71–83. doi: 10.1016/j.expneurol.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Gurbanova AA, Aker RG, Sirvanci S, Demiralp T, Onat FY. Intra-amygdaloid injection of kainic acid in rats with genetic absence epilepsy: the relationship of typical absence epilepsy and temporal lobe epilepsy. J Neurosci Off J Soc Neurosci. 2008;28:7828–7836. doi: 10.1523/JNEUROSCI.1097-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshé SL. Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- Hájos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Harroud A, Bouthillier A, Weil AG, Nguyen DK. Temporal lobe epilepsy surgery failures: a review. Epilepsy Res Treat. 2012 doi: 10.1155/2012/201651. [DOI] [PMC free article] [PubMed]
- Hasegawa D, Orima H, Fujita M, Hashizume K, Tanaka T. Complex partial status epilepticus induced by a microinjection of kainic acid into unilateral amygdala in dogs and its brain damage. Brain Res. 2002;955:174–182. doi: 10.1016/s0006-8993(02)03430-3. [DOI] [PubMed] [Google Scholar]
- Heggli DE, Malthe-Sørenssen D. Systemic injection of kainic acid: effect on neurotransmitter markers in piriform cortex, amygdaloid complex and hippocampus and protection by cortical lesioning and anticonvulsants. Neuroscience. 1982;7:1257–1264. doi: 10.1016/0306-4522(82)91132-0. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Chemoconvulsant model of chronic spontaneous seizures. In: Jacqueline N, Crawley Al, editors. Curr Protoc Neurosci. Unit 9.19. Chapter 9. 2005. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Thompson JL. Effects of kainic acid on seizure susceptibility in the developing brain. Brain Res. 1988;467:51–59. doi: 10.1016/0165-3806(88)90066-1. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869–1875. doi: 10.1212/wnl.40.12.1869. [DOI] [PubMed] [Google Scholar]
- Jin XT, Smith Y. Localization and functions of kainate receptors in the basal ganglia. Adv Exp Med Biol. 2011;717:27–37. doi: 10.1007/978-1-4419-9557-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinde S, Belforte JE, Yamamoto J, Wilson MA, Tonegawa S, Nakazawa K. Lack of kainic acid-induced gamma oscillations predicts subsequent CA1 excitotoxic cell death. Eur J Neurosci. 2009;30:1036–1055. doi: 10.1111/j.1460-9568.2009.06896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Kar S, Seto D, Doré S, Chabot JG, Quirion R. Systemic administration of kainic acid induces selective time dependent decrease in [125I]insulin-like growth factor I, [125I]insulin-like growth factor II and [125I]insulin receptor binding sites in adult rat hippocampal formation. Neuroscience. 1997;80:1041–1055. doi: 10.1016/s0306-4522(97)00185-1. [DOI] [PubMed] [Google Scholar]
- Kasugai M, Akaike K, Imamura S, Matsukubo H, Tojo H, Nakamura M, Tanaka S, Sano A. Differences in two mice strains on kainic acid-induced amygdalar seizures. Biochem Biophys Res Commun. 2007;357:1078–1083. doi: 10.1016/j.bbrc.2007.04.067. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci. 2003;6:1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JDB, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci Off J Soc Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado FA. Chronic bilateral stimulation of the anterior thalamus of kainate-treated rats increases seizure frequency. Epilepsia. 2006;47:27–32. doi: 10.1111/j.1528-1167.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Wheal HV. A comparative histological and electrophysiological study of some neurotoxins in the rat hippocampus. J Comp Neurol. 1982;211:105–114. doi: 10.1002/cne.902110202. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Wheal HV. Chronic failure of inhibition of the CA1 area of the hippocampus following kainic acid lesions of the CA3/4 area. Brain Res. 1984;295:317–324. doi: 10.1016/0006-8993(84)90980-6. [DOI] [PubMed] [Google Scholar]
- Leite JP, Babb TL, Pretorius JK, Kuhlman PA, Yeoman KM, Mathern GW. Neuron loss, mossy fiber sprouting, and interictal spikes after intrahippocampal kainate in developing rats. Epilepsy Res. 1996;26:219–231. doi: 10.1016/s0920-1211(96)00055-1. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Langlois JMP, Lema P, Courtemanche R, Bilodeau GA, Carmant L. Synchronized gamma oscillations (30–50 Hz) in the amygdalo-hippocampal network in relation with seizure propagation and severity. Neurobiol Dis. 2009;35:209–218. doi: 10.1016/j.nbd.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Bortel A, Gotman J, Avoli M. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2011;42:231–241. doi: 10.1016/j.nbd.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci Off J Soc Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JM, Zeng YJ, Peng F, Li L, Yang TH, Hong Z, Lei D, Chen Z, Zhou D. Aberrant glutamate receptor 5 expression in temporal lobe epilepsy lesions. Brain Res. 2010;1311:166–174. doi: 10.1016/j.brainres.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lytle N, Lan JQ, Sandau US, Boison D. Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: a first step in epileptogenesis? Glia. 2012;60:83–95. doi: 10.1002/glia.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo BM, Mello LE. Effect of long-term spontaneous recurrent seizures or reinduction of status epilepticus on the development of supragranular mossy fiber sprouting. Epilepsy Res. 1999;36:233–241. doi: 10.1016/s0920-1211(99)00054-6. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Collins RC, Ferrendelli JA. Kainic acid-induced limbic seizures: electrophysiologic studies. Neurology. 1981;31:806–812. doi: 10.1212/wnl.31.7.806. [DOI] [PubMed] [Google Scholar]
- Lu CB, Jefferys JGR, Toescu EC, Vreugdenhil M. In vitro hippocampal gamma oscillation power as an index of in vivo CA3 gamma oscillation strength and spatial reference memory. Neurobiol Learn Mem. 2011;95:221–230. doi: 10.1016/j.nlm.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Selective neuronal death in the contralateral hippocampus following unilateral kainate injections into the CA3 subfield. Neuroscience. 1993;56:317–335. doi: 10.1016/0306-4522(93)90334-c. [DOI] [PubMed] [Google Scholar]
- Marchi N, Oby E, Batra A, Uva L, De Curtis M, Hernandez N, Van Boxel-Dezaire A, Najm I, Janigro D. In vivo and in vitro effects of pilocarpine: relevance to ictogenesis. Epilepsia. 2007;48:1934–1946. doi: 10.1111/j.1528-1167.2007.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Tierney W, Alexopoulos AV, Puvenna V, Granata T, Janigro D. The etiological role of blood–brain barrier dysfunction in seizure disorders. Cardiovasc Psychiatry Neurol 2011. 2011 doi: 10.1155/2011/482415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Cifuentes F, Leite JP, Pretorius JK, Babb TL. Hippocampal EEG excitability and chronic spontaneous seizures are associated with aberrant synaptic reorganization in the rat intrahippocampal kainate model. Electroencephalogr Clin Neurophysiol. 1993;87:326–339. doi: 10.1016/0013-4694(93)90186-y. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Kornblum HI, Mendoza D, Lozada A, Leite JP, Chimelli L, Born DE, Fried I, Sakamoto AC, et al. Altered hippocampal kainate-receptor mRNA levels in temporal lobe epilepsy patients. Neurobiol Dis. 1998;5:151–176. doi: 10.1006/nbdi.1998.0200. [DOI] [PubMed] [Google Scholar]
- McNamara JO. Kindling: an animal model of complex partial epilepsy. Ann Neurol. 1984;16(Suppl):S72–S76. doi: 10.1002/ana.410160712. [DOI] [PubMed] [Google Scholar]
- Medvedev A, Mackenzie L, Hiscock JJ, Willoughby JO. Kainic acid induces distinct types of epileptiform discharge with differential involvement of hippocampus and neocortex. Brain Res Bull. 2000;52:89–98. doi: 10.1016/s0361-9230(00)00239-2. [DOI] [PubMed] [Google Scholar]
- Meier CL, Obenaus A, Dudek FE. Persistent hyperexcitability in isolated hippocampal CA1 of kainate-lesioned rats. J Neurophysiol. 1992;68:2120–2127. doi: 10.1152/jn.1992.68.6.2120. [DOI] [PubMed] [Google Scholar]
- Menini C, Meldrum BS, Riche D, Silva-Comte C, Stutzmann JM. Sustained limbic seizures induced by intraamygdaloid kainic acid in the baboon: symptomatology and neuropathological consequences. Ann Neurol. 1980;8:501–509. doi: 10.1002/ana.410080507. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Beales M, Meshul CK. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Exp Neurol. 2009;219:334–340. doi: 10.1016/j.expneurol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Werner S, Shalak L, Shehab L, Shamseddine A, Simaan E, Liu Z, Tarif S, Stafstrom C, Holmes G. Stages of status epilepticus in the developing brain. Epilepsy Res. 2003;55:9–19. doi: 10.1016/s0920-1211(03)00089-5. [DOI] [PubMed] [Google Scholar]
- Morin F, Beaulieu C, Lacaille JC. Selective loss of GABA neurons in area CA1 of the rat hippocampus after intraventricular kainate. Epilepsy Res. 1998;32:363–369. doi: 10.1016/s0920-1211(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Mouri G, Jimenez-Mateos E, Engel T, Dunleavy M, Hatazaki S, Paucard A, Matsushima S, Taki W, Henshall DC. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–151. doi: 10.1016/j.brainres.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Pérez-Otaño I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Murakami S, Takemoto T, Shimizu S. Studies on the effective principles of Diagenea simplex Aq. I Separation of the effective fraction by liquid chromatography. J Pharm Soc Jpn. 1953;73:1026–1028. [Google Scholar]
- Nadler JV. Kainic acid: neurophysiological and neurotoxic actions. Life Sci. 1979;24:289–299. doi: 10.1016/0024-3205(79)90325-4. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Cotman CW. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature. 1978;271:676–677. doi: 10.1038/271676a0. [DOI] [PubMed] [Google Scholar]
- Nadler JV. Minireview. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Cuthbertson GJ. Kainic acid neurotoxicity toward hippocampal formation: dependence on specific excitatory pathways. Brain Res. 1980;195:47–56. doi: 10.1016/0006-8993(80)90865-3. [DOI] [PubMed] [Google Scholar]
- Najm I, Jehi L, Palmini A, Gonzalez-Martinez J, Paglioli E, Bingaman W. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013;54:772–782. doi: 10.1111/epi.12152. [DOI] [PubMed] [Google Scholar]
- Nitecka L, Tremblay E, Charton G, Bouillot JP, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. II Histopathological sequelae. Neuroscience. 1984;13:1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Nadler JV. Protective effects of mossy fiber lesions against kainic acid-induced seizures and neuronal degeneration. Neuroscience. 1988;26:763–781. doi: 10.1016/0306-4522(88)90097-8. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Molnár P, Nadler JV. Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol. 1999;81:1645–1660. doi: 10.1152/jn.1999.81.4.1645. [DOI] [PubMed] [Google Scholar]
- Patel S, Meldrum BS, Collins JF. Distribution of [3H]kainic acid and binding sites in the rat brain: in vivo and in vitro receptor autoradiography. Neurosci Lett. 1986;70:301–307. doi: 10.1016/0304-3940(86)90569-0. [DOI] [PubMed] [Google Scholar]
- Pernot F, Heinrich C, Barbier L, Peinnequin A, Carpentier P, Dhote F, Baille V, Beaup C, Depaulis A, Dorandeu F. Inflammatory changes during epileptogenesis and spontaneous seizures in a mouse model of mesiotemporal lobe epilepsy. Epilepsia. 2011;52:2315–2325. doi: 10.1111/j.1528-1167.2011.03273.x. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Pont F, Collet A, Lallement G. Early and transient increase of rat hippocampal blood–brain barrier permeability to amino acids during kainic acid-induced seizures. Neurosci Lett. 1995;184:52–54. doi: 10.1016/0304-3940(94)11166-g. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raedt R, Van Dycke A, Van Melkebeke D, De Smedt T, Claeys P, Wyckhuys T, Vonck K, Wadman W, Boon P. Seizures in the intrahippocampal kainic acid epilepsy model: characterization using long-term video-EEG monitoring in the rat. Acta Neurol Scand. 2009;119:293–303. doi: 10.1111/j.1600-0404.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- Raol YH, Brooks-Kayal AR. Experimental models of seizures and epilepsies. Prog Mol Biol Transl Sci. 2012;105:57–82. doi: 10.1016/B978-0-12-394596-9.00003-2. [DOI] [PubMed] [Google Scholar]
- Redler RL, Dokholyan NV. The complex molecular biology of amyotrophic lateral sclerosis (ALS) Prog Mol Biol Transl Sci. 2012;107:215–262. doi: 10.1016/B978-0-12-385883-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J, Emond M, Meilleur S, Psarropoulou C, Carmant L. AIDA, a class I metabotropic glutamate-receptor antagonist limits kainate-induced hippocampal dysfunction. Epilepsia. 2002;43:1306–1317. doi: 10.1046/j.1528-1157.2002.10402.x. [DOI] [PubMed] [Google Scholar]
- Represa A, Tremblay E, Ben-Ari Y. Kainate binding sites in the hippocampal mossy fibers: localization and plasticity. Neuroscience. 1987;20:739–748. doi: 10.1016/0306-4522(87)90237-5. [DOI] [PubMed] [Google Scholar]
- Riban V, Bouilleret V, Pham-Lê BT, Fritschy JM, Marescaux C, Depaulis A. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience. 2002;112:101–111. doi: 10.1016/s0306-4522(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Gryder D, Castaneda D, Yonekawa W, Banks MK, Li He. GluR5 kainate receptors, seizures, and the amygdala. Ann NY Acad Sci. 2003;985:150–162. doi: 10.1111/j.1749-6632.2003.tb07079.x. [DOI] [PubMed] [Google Scholar]
- Rose Priel M, dos Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996;26:115–121. doi: 10.1016/s0920-1211(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Saija A, Princi P, Pisani A, Santoro G, De Pasquale R, Massi M, Costa G. Blood–brain barrier dysfunctions following systemic injection of kainic acid in the rat. Life Sci. 1992;51:467–477. doi: 10.1016/0024-3205(92)90023-i. [DOI] [PubMed] [Google Scholar]
- Sanon N, Carmant L, Emond M, Congar P, Lacaille JC. Short-term effects of kainic acid on CA1 hippocampal interneurons differentially vulnerable to excitotoxicity. Epilepsia. 2005;46:837–848. doi: 10.1111/j.1528-1167.2005.21404.x. [DOI] [PubMed] [Google Scholar]
- Sarkisian MR, Tandon P, Liu Z, Yang Y, Hori A, Holmes GL, Stafstrom CE. Multiple kainic acid seizures in the immature and adult brain: ictal manifestations and long-term effects on learning and memory. Epilepsia. 1997;38:1157–1166. doi: 10.1111/j.1528-1157.1997.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Fuller T, Price JL, Olney JW. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5:991–1014. doi: 10.1016/0306-4522(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35:984–999. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Jordan WH, Reams RY, Hall DG, Snyder PW. Temporal profile of clinical signs and histopathologic changes in an F-344 rat model of kainic acid-induced mesial temporal lobe epilepsy. Toxicol Pathol. 2008;36:932–943. doi: 10.1177/0192623308326093. [DOI] [PubMed] [Google Scholar]
- Sharman JL, Benson HE, Pawson AJ, Lukito V, Mpamhanga CP, Bombail V, Davenport AP, Peters JA, Spedding M, Harmar AJ, et al. IUPHAR-DB: updated database content and new features. Nucleic Acids Res. 2013;41:D1083–D1088. doi: 10.1093/nar/gks960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda S, Araki T, Lan JQ, Schindler CK, Simon RP, Taki W, Henshall DC. Development of a model of seizure-induced hippocampal injury with features of programmed cell death in the BALB/c mouse. J Neurosci Res. 2004;76:121–128. doi: 10.1002/jnr.20064. [DOI] [PubMed] [Google Scholar]
- Shinozaki H, Konishi S. Actions of several anthelmintics and insecticides on rat cortical neurones. Brain Res. 1970;24:368–371. doi: 10.1016/0006-8993(70)90122-8. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. C R Biol. 2005;328:143–153. doi: 10.1016/j.crvi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Damiano BP. On the relationship between kainic acid-induced epileptiform activity and hippocampal neuronal damage. Neuropharmacology. 1981;20:1003–1011. doi: 10.1016/0028-3908(81)90088-5. [DOI] [PubMed] [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal–hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Sperk G, Lassmann H, Baran H, Kish SJ, Seitelberger F, Hornykiewicz O. Kainic acid induced seizures: neurochemical and histopathological changes. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- Sperk G, Lassman H, Baran H, Seitelberger F, Hornykiewicz O. Kainic acid-induced seizures: dose-relationship of behavioural, neurochemical and histopathological changes. Brain Res. 1985;338:289–295. doi: 10.1016/0006-8993(85)90159-3. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Thompson JL, Holmes GL. Kainic acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Brain Res Dev Brain Res. 1992;65:227–236. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- Strain SM, Tasker RAR. Hippocampal damage produced by systemic injections of domoic acid in mice. Neuroscience. 1991;44:343–352. doi: 10.1016/0306-4522(91)90059-w. [DOI] [PubMed] [Google Scholar]
- Suarez LM, Cid E, Gal B, Inostroza M, Brotons-Mas JR, Gomez-Dominguez D, de la Prida LM, Solis JM. Systemic injection of kainic acid differently affects LTP magnitude depending on its epileptogenic efficiency. PLoS One. 2012;7:e48128. doi: 10.1371/journal.pone.0048128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb K, Boss-Williams KA, Weiss JM, Weinshenker D. Rats bred for susceptibility to depression-like phenotypes have higher kainic acid-induced seizure mortality than their depression-resistant counterparts. Epilepsy Res. 2007;74:140–146. doi: 10.1016/j.eplepsyres.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kaijima M, Daita G, Ohgami S, Yonemasu Y, Riche D. Electroclinical features of kainic acid-induced status epilepticus in freely moving cats. Microinjection into the dorsal hippocampus. Electroencephalogr Clin Neurophysiol. 1982;54:288–300. doi: 10.1016/0013-4694(82)90178-x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kaijima M, Yonemasu Y, Cepeda C. Spontaneous secondarily generalized seizures induced by a single microinjection of kainic acid into unilateral amygdala in cats. Electroencephalogr Clin Neurophysiol. 1985;61:422–429. doi: 10.1016/0013-4694(85)91034-x. [DOI] [PubMed] [Google Scholar]
- Telfeian AE, Federoff HJ, Leone P, During MJ, Williamson A. Overexpression of GluR6 in rat hippocampus produces seizures and spontaneous nonsynaptic bursting in vitro. Neurobiol Dis. 2000;7:362–374. doi: 10.1006/nbdi.2000.0294. [DOI] [PubMed] [Google Scholar]
- Théorêt Y, Earnhardt TS, Bouldin TW, Krigman MR. The neurotoxicity of intrahippocampal kainic acid injection in rats is not accompanied by a reduction of Timm stain. Brain Res. 1988;449:341–346. doi: 10.1016/0006-8993(88)91050-5. [DOI] [PubMed] [Google Scholar]
- Thorn M. Neuropathologic findings in postmortem studies of sudden death in epilepsy. Epilepsia. 1997;38:S32–S34. doi: 10.1111/j.1528-1157.1997.tb06123.x. [DOI] [PubMed] [Google Scholar]
- Tremblay E, Ottersen OP, Rovira C, Ben-Ari Y. Intra-amygdaloid injections of kainic acid: regional metabolic changes and their relation to the pathological alterations. Neuroscience. 1983;8:299–315. doi: 10.1016/0306-4522(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkänen A. Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur J Neurosci. 1996;8:2711–2725. doi: 10.1111/j.1460-9568.1996.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Uva L, Librizzi L, Marchi N, Noe F, Bongiovanni R, Vezzani A, Janigro D, de Curtis M. Acute induction of epileptiform discharges by pilocarpine in the in vitro isolated guinea-pig brain requires enhancement of blood–brain barrier permeability. Neuroscience. 2008;151:303–312. doi: 10.1016/j.neuroscience.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velísek L, Kubová H, Velísková J, Mares P, Ortová M. Action of antiepileptic drugs against kainic acid-induced seizures and automatisms during ontogenesis in rats. Epilepsia. 1992;33:987–993. doi: 10.1111/j.1528-1157.1992.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Vincent P, Mulle C. Kainate receptors in epilepsy and excitotoxicity. Neuroscience. 2009;158:309–323. doi: 10.1016/j.neuroscience.2008.02.066. [DOI] [PubMed] [Google Scholar]
- Werner P, Voigt M, Keinänen K, Wisden W, Seeburg PH. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991;351:742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- White A, Williams PA, Hellier JL, Clark S, Edward Dudek F, Staley KJ. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia. 2010;51:371–383. doi: 10.1111/j.1528-1167.2009.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P, White A, Ferraro D, Clark S, Staley K, Dudek FE. The use of radiotelemetry to evaluate electrographic seizures in rats with kainate-induced epilepsy. J Neurosci Methods. 2006;155:39–48. doi: 10.1016/j.jneumeth.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci Off J Soc Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. J Neurosci Off J Soc Neurosci. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Stewart GR, Miller JP, Olney JW. Age-related sensitivity to kainate neurotoxicity. Exp Neurol. 1991;114:250–253. doi: 10.1016/0014-4886(91)90042-b. [DOI] [PubMed] [Google Scholar]
- Wyler AR, Hermann BP, Somes G. Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery. 1995;37:982–990. doi: 10.1227/00006123-199511000-00019. discussion 990–991. [DOI] [PubMed] [Google Scholar]
- Ying Z, Babb TL, Comair YG, Bushey M, Touhalisky K. Increased densities of AMPA GluR1 subunit proteins and presynaptic mossy fiber sprouting in the fascia dentata of human hippocampal epilepsy. Brain Res. 1998;798:239–246. doi: 10.1016/s0006-8993(98)00421-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cui SS, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, Honer WG, Corcoran ME. Relations between brain pathology and temporal lobe epilepsy. J Neurosci Off J Soc Neurosci. 2002;22:6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker DK, Wooten GF, Lothman EW. Blood–brain barrier changes with kainic acid-induced limbic seizures. Exp Neurol. 1983;79:422–433. doi: 10.1016/0014-4886(83)90223-6. [DOI] [PubMed] [Google Scholar]