Abstract
The mechanism of action of several antiepileptic drugs (AEDs) rests on their ability to modulate the activity of voltage-gated sodium currents that are responsible for fast action potential generation. Recent data indicate that lacosamide (a compound with analgesic and anticonvulsant effects in animal models) shares a similar mechanism. When compared with other AEDs, lacosamide has the unique ability to interact with sodium channel slow inactivation without affecting fast inactivation. This article reviews these findings and discusses their relevance within the context of neuronal activity seen during epileptiform discharges generated by limbic neuronal networks in the presence of chemical convulsants. These seizure-like events are characterized by sustained discharges of sodium-dependent action potentials supported by robust depolarizations, thus providing synchronization within neuronal networks. Generally, AEDs such as phenytoin, carbamazepine and lamotrigine block sodium channels when activated. In contrast, lacosamide facilitates slow inactivation of sodium channels both in terms of kinetics and voltage dependency. This effect may be relatively selective for repeatedly depolarized neurons, such as those participating in seizure activity in which the persistence of sodium currents is more pronounced and promotes neuronal excitation.
The clinical effectiveness of lacosamide has been demonstrated in randomized, double-blind, parallel-group, placebo-controlled, adjunctive-therapy trials in patients with refractory partial seizures. Further studies should determine whether the effects of lacosamide in animal models and in clinical settings are fully explained by its selective action on sodium current slow inactivation or whether other effects (e.g. interactions with the collapsin-response mediator protein-2) play a contributory role.
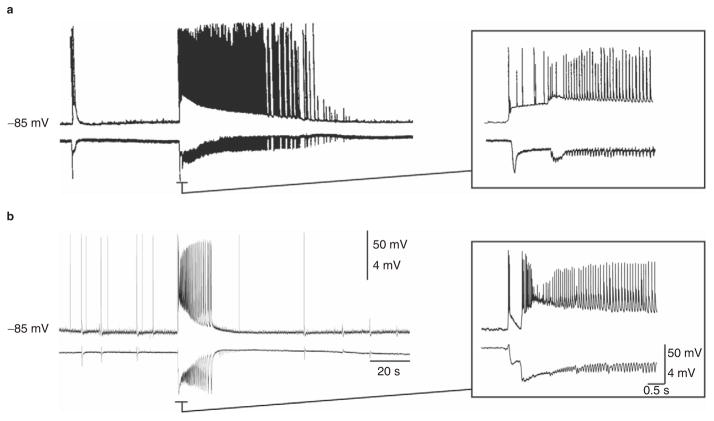
Epilepsy is a neurological disorder characterized by neuronal hyperexcitability associated with recurrent episodes of excessive synchronized neuronal activity manifesting phenotypically as seizures and, at times, as interictal electroencephalographic discharges without overt clinical manifestations. Similar patterns of neuronal activity are seen in animal models of epilepsy or when brain slices are treated with chemical convulsants in vitro. Intracellular recordings obtained from areas of the limbic system in rodents have firmly established that both focal and generalized epileptic discharges are characterized by sustained firing of sodium-dependent action potentials that ride on network-driven depolarizations, mainly reflecting ligand-gated, glutamatergic currents[1–3] (figure 1). In view of the involvement of sodium-dependent action potentials in the generation and propagation of epileptiform discharges, it is not surprising that several antiepileptic drugs (AEDs) target sodium channels.
Fig. 1.
Intracellular (top trace) and field (bottom trace) recordings from rat brain slices from the entorhinal cortex (a) and cingulate cortex (b) during application of fampridine (4-aminopyridine) 50 μmol/L. Ictal network discharges (on the right side of the trace) correspond to high frequency sodium-dependent action potentials that ride on network-driven depolarizations, while interictal events (on the left side of the trace) are associated with single action potentials or bursts of action potentials. Insets on the right are time expansions of the onset of ictal discharges and clearly show the very high frequency (up to 200 Hz) discharges of action potentials (panel [a] modified from Lopantsev and Avoli,[4] with permission).
Although less vigorously than in the 1990s, research aimed at discovering new pharmacological treatments for epileptic disorders continues to provide anticonvulsants with novel or more selective mechanisms.[5–9] The main rationale for this search rests on the fact that, in spite of the introduction of a dozen second-generation AEDs in the last 20 years, approximately one-third of patients with epilepsy fail to achieve complete seizure control with existing treatments.[10,11] In addition, currently available AEDs produce a wide range of adverse effects, some of which can be life threatening.[11,12] Newer AEDs with more specific targets are needed to increase the chance of controlling drug-resistant epileptic disorders, as well as to reduce the risk of adverse effects.
AEDs are discovered by screening procedures in animal models that invariably include the maximal electroshock (MES) and the subcutaneous pentetrazol (pentylenetetrazol; PTZ) tests in rodents.[13,14] Compounds found to be active in either the MES or PTZ tests are then investigated in a broader range of other experimental models of seizures and epilepsy before undergoing assessment in clinical trials. Screening in standard seizure models is relatively unbiased with respect to mechanisms of action, and has allowed identification of AEDs acting on novel targets, such as gabapentin[15] and pregabalin,[16] although levetiracetam is an exception to this rule in that it is inactive in the classical MES and PTZ tests.[17] Discovery of novel targets has also provided hints to understand the patho-physiological basis of some types of epilepsy.
In parallel, progress in genetic investigations has revealed that some epileptic syndromes are associated with mutations in the structure of ionic channel subunits, highlighting the need to target specific channel dysfunctions. This also applies to the family of voltage-gated sodium channels, for which genetically determined alterations have been identified in various epileptic disorders such as generalized epilepsy with febrile seizures plus (GEFS+) and severe myoclonic epilepsy of infancy.[18,19] In these conditions, some mutations (but not all of them) impair the mechanisms responsible for inactivation of sodium channels, leading to neuronal over-excitation.[18,20] An increase in low-threshold persistent sodium current has been described in the entorhinal cortex of pilocarpine-treated rats during the chronic epileptic stage, suggesting that the structure of voltage-gated sodium channels may also be altered by repeated exposure to the seizures themselves.[21] Similar changes have been described in a subset of subicular neurons isolated from resected tissue obtained from patients with drug-resistant temporal lobe epilepsy.[22]
Blockade of voltage-gated sodium channels is a primary mechanism of action for many AEDs commonly used to treat convulsive seizures.[23,24] Many of these drugs, which include carbamazepine, oxcarbazepine, phenytoin, lamotrigine, topiramate and felbamate, possess unrelated chemical structures, and some of them exhibit a clinical efficacy profile that extends beyond epilepsy, which has led to their effective use in the treatment of other disorders such as bipolar disorder, migraine and neuropathic pain.[25,26] Although additional mechanisms may contribute to these activities, blockade of sodium channels is also likely to contribute to therapeutic effects in non-epileptic disorders.[25] The way by which sodium channel-blocking AEDs modify the kinetics of voltage-gated sodium channels differs from one drug to another, and it is likely that such differences influence their clinical activity spectrum. However, for most AEDs acting on sodium channels, the main effects that have been documented include a reduction in peak amplitude of the current and an alteration in fast inactivation parameters (table I; see also Ragsdale and Avoli[23]).
Table I.
Effect of lacosamide (LCM), carbamazepine (CBZ), lamotrigine (LTG) and phenytoin (PHT) on sodium current parameters (all drugs at a concentration of 100 μmol/L). Lacosamide differs from the other antiepileptic drugs because of the lack of effects on fast inactivation and on time to recovery from fast activation
| Sodium current parameter | Antiepileptic drugs
|
References | |||
|---|---|---|---|---|---|
| CBZ | LTG | PHT | LCM | ||
| Reduction in the peak current | +++ | ++ | ++ | ++ | 27–29 |
| Depolarizing shift of V50 a activation | − | − | −b | − | 30–33 |
| Hyperpolarizing shift of V50a fast inactivation | ++++ | +++ | + | − | 28,33 |
| Hyperpolarizing shift of V50a slow inactivation | ++ | +++ | +++ | ++++ | 27,33 |
| 10 Hz frequency- dependent facilitation of block | + | + | + | − | 27,33 |
| Slowed recovery time from fast inactivation | ++ | ++ | ++ | − | 27,33 |
| Slowed recovery time from slow inactivation | − | NR | NR | − | 33 |
V50 indicates the membrane potential where 50% of the processes described (activation and fast or slow inactivation, respectively) are operating.
Effect with PHT 10 μmol/L.
NR = not reported; − indicates no effect (0–10% change; <3 mV); + indicates small effect (10–35% change; 3–7 mV); ++ indicates moderate effect (35–60% change; 8–11 mV); +++ indicates large effect (60–85% change; 12–16 mV): ++++ indicates almost complete effect (>85% change; >17 mV).
Recently, the new AED lacosamide ([R]-2-acetamido-N-benzyl-3-methoxypropionamide; also known as SPM-927, harkoseride or ADD-234037) was found to exert anticonvulsant effects through a new, stereoselective mode of interaction with sodium channels. In fact, this compound, which belongs to a novel class of anticonvulsant agents collectively referred to as functionalized amino acids, acts primarily by interfering with the slowly inactivating component of voltage-gated sodium currents, without affecting the fast component that is targeted by traditional sodium channel blockers.[29,33]
In this article, we discuss lacosamide and its particular mode of action in relation to its experimental and clinical activity profile. In doing so, we first summarize some functional and molecular characteristics of voltage-gated sodium channels and discuss the actions of available AEDs on sodium currents. Second, we review the studies demonstrating the activity of lacosamide in experimental models of seizures, epilepsy and neuropathic pain, as well as the clinical studies supporting its use in the treatment of partial-onset seizures. Third, we briefly discuss potential relationships between the ability of lacosamide to control seizures and neuropathic pain and its specific sodium-dependent mechanism. The information reviewed in this article was derived from the author’s files and from a literature search (with the latest update on 10 February 2009) using the online database, PubMed, and the keywords ‘lacosamide’, ‘harkoseride’, ‘SPM 927’, ‘ADD 234037’, ‘sodium channels and epilepsy’ and ‘sodium channels and slow inactivation’.
1. Voltage-Gated Sodium Channels as Targets for Lacosamide and Other Antiepileptic Drugs
The voltage-gated sodium channel is composed of α subunits that surround a central pore, which is relatively selective for sodium ions. Four accessory β subunits, which are not involved in forming the channel pore, have been shown to modulate the biophysical properties of the channel. To date, nine isoforms of the main subunit, α1−9, have been described, with a rather conserved structure represented by four domains, each consisting of six transmembrane segments and a re-entrant loop. In addition, some charged residues in the protein, responsible for the selectivity filter of the channel and for all the voltage-dependent mechanisms described below, are often well conserved.
Sodium channels occur physiologically in three different states (figure 2), and the transition among these states is voltage dependent. At membrane potentials close to the resting potential of the cell, sodium channels are mainly closed, and passage of ions across the membrane is not possible. When the membrane depolarizes, the channels switch to the open state through an ‘activation’ mechanism that develops within a few milliseconds.[34–36] The open state is the only conductive state of the channel, and sodium ions can pass through the channel by following their electrochemical gradient. The depolarizing stimulus (which can be applied through an amplifier during a voltage-clamp experiment or occurs as a result of a synaptic excitatory event in a physiological system) causes the opening of the channel.
Fig. 2.
Diagram illustrating the various states of the sodium channel. The sodium channel is in the closed state at resting potential (orange line in the current trace) and it switches to the open state after a depolarizing stimulus (activation, blue line in the current trace). The opposite mechanism (deactivation) occurs when the potential is repolarized. If the stimulus persists, the channel goes into the inactivated state through the mechanisms of fast and slow inactivation (pink line in the current trace). Repolarization of the membrane potential is also required to allow recovery from inactivation.
However, the same depolarization induces conformational changes in other portions of the channel; thus, a portion of protein located on the intracellular side (the so-called ball and chain) is attracted towards the channel pore within a few tens of milliseconds from the delivery of the depolarizing stimulus and, by obstructing the pore, triggers the fast inactivation process. If the depolarizing stimulus lasts longer, up to seconds, a slow inactivation mechanism also takes place. This is mediated by a portion of the protein located on the extracellular side that is also displaced by the depolarization but with slower kinetics. The two inactivated states of the channel are both non-conducting, but they are biophysically different from the closed state.[35,36] In fact, to allow re-opening of the channel, the portions of the protein responsible for the fast and slow inactivation have to be removed from the pore and the only way for this to occur is through hyperpolarization of the membrane potential. These processes, known as recovery from fast and slow inactivation, respectively, occur with time constants that are of the same magnitude as those needed to induce the inactivation process, i.e. milliseconds for recovery from fast inactivation and up to seconds for recovery from slow inactivation. The hyperpolarizing stimulus also restores the activation gate to its closed condition, a mechanism known as deactivation.
Inactivation of sodium channels is of paramount importance for the actions of AEDs. Many AEDs, in fact, act on both fast and slow inactivation processes, whereas lacosamide may be unique in that it facilitates slow inactivation without altering the fast component (table I). In other words, lacosamide is highly selective for the slow inactivation phase of sodium currents. Binding experiments have demonstrated that la-cosamide displaces [3H]-batrachotoxin bound to voltage-gated sodium channels, but does not compete with radioligands specific for amino-hydroxy-methyl-isoxazole propionic acid (AMPA), kainate, N-methyl-D-aspartate (NMDA), GABA, adenosine, muscarinic, histamine, dopamine, nor-adrenaline (norepinephrine) and other receptors, and it does not alter type N, P, L and T calcium channels, the delayed rectifier or the IA potassium current at concentrations up to 100 μmol/L.[29] In line with these findings, the passive membrane properties are not altered by lacosamide.
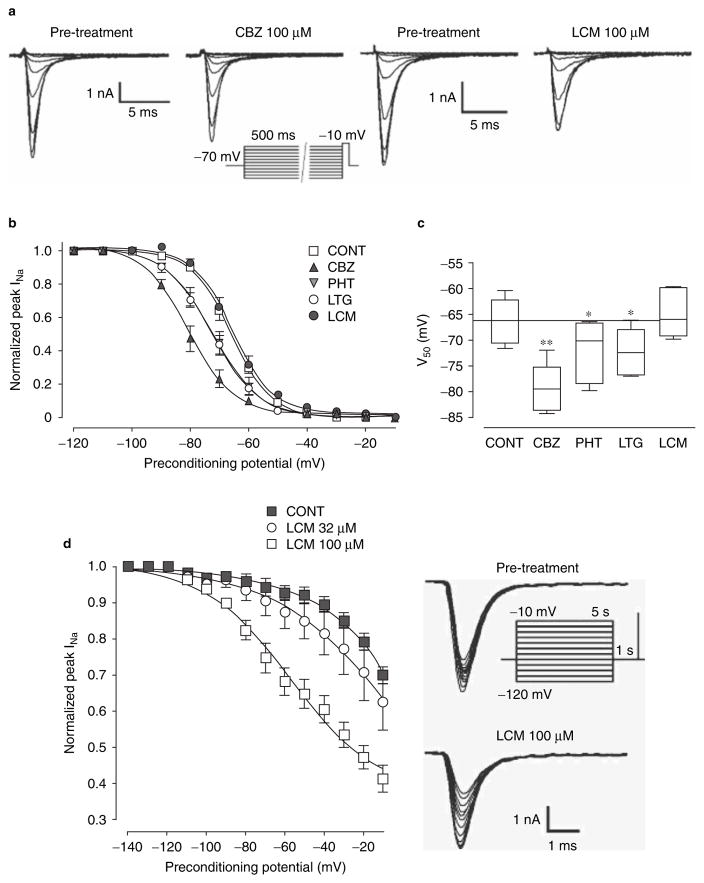
Patch-clamp experiments in the voltage-clamp configuration using mouse N1E-115 neuroblastoma cells, described by Errington et al.,[33] have also revealed that lacosamide decreases the amplitude of the sodium current at all membrane potentials tested, without affecting the potential at which 50% of the channels are activated (voltage of semi-activation, V50Act).[33] Fast inactivation (figure 3a–c), as well as the recovery from fast inactivation, is not altered by lacosamide in these experiments. However, lacosamide facilitates the switch of sodium channels to the non-conducting slow-inactivated state (time constant for entry, τ = 4.79 s vs 11.95 s in controls),[33] and shifts the voltage of semi-inactivation (V50Inact) for slow inactivation to more hyperpolarized potentials in a concentration-dependent manner (figure 3d). In contrast with observations made for sodium channels involved in pain (see section 4), no effect on the recovery from the slow inactivation has been observed in the study published by Errington et al.[33]
Fig. 3.
(a–c): Steady-state fast inactivation is modulated by carbamazepine (CBZ), phenytoin (PHT) and lamotrigine (LTG), but not by lacosamide (LCM) in mouse neuroblastoma cells. Sodium current tracings during the stimulus protocol (shown under CBZ traces) used to study steady-state fast inactivation are shown in (a). Note in (b) that the fast inactivation curve is not affected by lacosamide, while it is shifted to the left by carbamazepine, phenytoin and lamotrigine. Note also that the LTG curve is superimposed on that of PHT. Histograms shown in (c) summarize the half-inactivation potentials (V50; mean ± standard error of the mean [SEM]) and demonstrate that lacosamide does not alter fast inactivation, while the other drugs shift V50 potentials toward hyperpolarized values. (d) Slow inactivation is modulated by lacosamide. Note that lacosamide shifts the slow inactivation curve to the left in a concentration-dependent manner. Data are presented as means ± SEM. The stimulus protocol and the tracings used to create the slow inactivation curves are shown on the right (modified from Errington et al.,[33] with permission). CONT = control; INa = sodium current; * p < 0.05, ** p < 0.001.
The effects of lacosamide were also characterized in voltage clamp experiments in which stimulation trains (30 test pulses, 20 ms to 0 mV from holding potential of −80 mV) were applied to mouse N1E-115 neuroblastoma cells. In this experiment, peak current amplitude measured at the 30th pulse was markedly reduced by carbamazepine, lamotrigine and phenytoin, but not by lacosamide.[33] In another set of experiments, lacosamide only reduced the number of current transients evoked with a slow depolarizing voltage ramp (from −70 mV to +20 mV, 3 mV/s) in primary neocortical neurons obtained from Sprague-Dawley rats during embryonic days 16–18, whereas carbamazepine and phenytoin completely abolished them.[33] In the presence of a faster ramp (90 mV/s), spikes were almost insensitive to lacosamide, whereas sensitivity to carbamazepine and phenytoin, which act on fast inactivation, was fully retained.[33] In the experiments using the fast depolarizing ramp, the latency to the first spike was decreased by lacosamide, whereas it was increased by carbamaze-pine and phenytoin. In experiments in which 10 s sustained repetitive firing was evoked in current clamp, lacosamide did not affect the firing rate in the early phase of the burst, but attenuated it in the later stage. Conversely, carbamazepine, lamotrigine and phenytoin all produced a marked reduction in firing frequency within the first second of the burst.[33]
A relevant observation from the studies conducted by Errington et al.[33] is that the concentrations at which lacosamide was found to reduce sodium channel availability are comparable to those at which lacosamide inhibits ictal-like events in rodent brain slices (values for the concentration that produces a 50% effective response [EC50] of around 40 μmol/L for clonic and 70 μmol/L for tonic seizures, see figure 3b in Lees et al.[37]). These concentrations are also of a similar order of magnitude to those found in the plasma of patients who received lacosamide in clinical trials (10–60 μmol/L).[38,39]
2. Lacosamide Effects in In Vitro Seizure Models
In a neuronal cellular monolayer system in vitro, perfusion with lacosamide 100 μmol/L markedly reduced the rate of occurrence of spontaneous inhibitory and excitatory postsynaptic currents.[29] Lacosamide also decreased, in a concentration-dependent manner, the frequency of spontaneous action potentials and the spikes-per-burst frequency induced by depolarizing current steps in cultured neocortical cells.[29] Interestingly, lacosamide reduced and eventually abolished the ictal-like activity induced by fampridine (4-amino-pyridine) 100 μmol/L application in rat brain slices from the visual cortex. In this model, lacosamide mainly affected the tonic phase of ictal events, so that the residual discharges included only a clonic-like component. In agreement with these findings, lacosamide has also been shown to reduce the population spike frequency of epileptiform events elicited by high-frequency stimulation.[37] These effects were stereoselective, as the S-stereoisomer SPM-6953 was devoid of activity.
3. Studies in In Vivo Models of Seizures and Epilepsy
The first experiments with functionalized amino acids were performed in the MES-induced seizure model and demonstrated that a variety of compounds within this class are effective in suppressing seizures with EC50 values comparable to the EC50 of phenobarbital (22 mg/kg, intraperitoneal [IP]).[40] Lacosamide stood out among other functionalized amino acids for its greater potency (EC50 of 8.3 mg/kg IP, 3.9 mg/kg orally),[40,41] and was selected for further development. In additional experiments, lacosamide was found to be effective against sound-induced seizures in the genetically susceptible Frings mouse.[42] Although lacosamide is inactive in the subcutaneous (SC) PTZ seizure model, it is effective in increasing seizure threshold in animals given continuous intravenous (IV) infusion of PTZ.[42] In the latter model, lacosamide effects have been analyzed in relation to the behavioural sequence of motor seizures, and the drug was found to significantly increase the threshold of the first twitch and of the clonus induced by IV PTZ. Lacosamide does not affect the threshold of clonic seizures induced by SC bicuculline and picrotoxin in mice, but it prevents NMDA-induced seizures and mortality.[42] In the 6 Hz model of refractory psychomotor seizures, lacosamide (20 mg/kg), injected IP 30 minutes before electrical stimulation, completely antagonized both seizure occurrence and the seizure-induced increase in glucose uptake, without suppressing normal brain metabolic activity.[42,43] In the amygdala and hippocampal kindling models of focal epilepsy, pre-treatment with lacosamide has been found to delay the acquisition of kindling and to reduce after-discharge duration.[42,44]
Lacosamide has been found to be effective in animal models of status epilepticus (SE). In the cobalt/homocysteine SE model, lacosamide effectively blocked generalized tonic-clonic seizures, although less potently than in the kindling model. This effect was potentiated by the concomitant administration of diazepam.[42] In the perforant path model of self-sustained SE in rats, lacosamide also reduced cumulative seizure duration and SE-induced hippocampal damage.[38] The latter finding suggests that lacosamide may have neuroprotective activity, a possibility supported by evidence from other experimental models. A study conducted in the middle cerebral artery occlusion model of ischaemia in rats, in particular, found that lacosamide decreases infarct volume when administered 15 minutes before occlusion and for 4 hours post-infusion.[45] Additionally, lacosamide has been found to inhibit, in a concentration-dependent manner, the glutamate- and oxygen-glucose deprivation-induced apoptosis in organotypic rat hippocampal slice cultures.[45] However, lacosamide was ineffective in preventing brain damage or functional deficits in a rat model of traumatic brain injury.[45]
4. Effects in Non-Epilepsy Models
Lacosamide has been found to possess analgesic effects in several animal models, including the rat model of spinal cord or trigeminal nerve injury,[46] tumour- and chemotherapy-induced cancer pain,[47] muscle pain induced by tumour necrosis factor-α,[48] a rat model of osteoarthritis pain[49] and a rat model of painful diabetic neuropathy.[50] Lacosamide has also been found to reduce hyperalgesia when morphine fails to do so.[47]
The analgesic activity of lacosamide is likely to be mediated, at least in part, by the mechanisms responsible for its anticonvulsant effects, including facilitation of slow inactivation of voltage-gated sodium channels.[51] In line with this hypothesis, lacosamide has been tested on sodium channels NaV1.7 and NaV1.3 expressed in HEK293 cells, and on NaV1.8 in dorsal root ganglia neurons of adult Sprague-Dawley rats.[51] These types of sodium channels are known to be involved in human pain and hereditary painful neuropathies, pain following chronic inflammation and nerve injury, and inflammatory and neuropathic pain mechanisms, respectively. Lacosamide inhibited NaV1.7, NaV1.3 and NaV1.8 current amplitudes without affecting the voltage dependency of activation and fast inactivation, or recovery from fast inactivation. Channels in the inactivated state were more sensitive to lacosamide than channels in the resting state. Lacosamide shifted the voltage dependency for slow inactivation to more hyperpolarized potentials, and increased time to recovery from slow inactivation.[51]
5. Clinical Pharmacology
Following intake of single oral doses of up to 800 mg, lacosamide shows linear pharmaco-kinetics.[52] Absorption is rapid, with peak serum concentrations being achieved within 4 hours of administration, and oral bioavailability is virtually complete.[39,53] The bioavailability of lacosamide is not affected by concomitant intake of food.[54] With a twice-daily dosage regimen, steady state serum concentrations are achieved after 3 days.[55] A parenteral formulation has been developed, which is intended for use as intravenous replacement therapy in patients temporarily unable to take oral medication. When infused intravenously over 30 or 60 minutes, the parenteral formulation produces serum concentration profiles comparable to those obtained after intake of equivalent oral doses.[39,53]
Lacosamide is negligibly (<15%) bound to plasma proteins, has a volume of distribution of 0.5–0.8 L/kg and it is eliminated with a half-life of 12–16 hours.[45,52] Elimination occurs partly by urinary excretion in unchanged form (about 40% of an oral dose) and partly by metabolic biotransformation. The pharmacologically inactive O-demethylated derivative SPM-12809 is a primary metabolite and its recovery in urine accounts for about 30% of the administered dose.[55] The pharmacokinetics of lacosamide are influenced by renal function, and the serum concentrations of lacosamide and its main metabolite SPM-12809 increase with increasing degree of renal impairment. On average, serum lacosamide concentrations are increased by approximately 30% in patients with mild to moderate renal impairment and by 60% in patients with severe renal impairment (creatinine clearance <30 mL/min).[56]
Population pharmacokinetic studies in patients with epilepsy receiving adjunctive therapy with lacosamide suggested that serum lacosamide concentrations are reduced by approximately 25% in the presence of concomitant treatment with enzyme-inducing AEDs such as carbamaze-pine, phenytoin and phenobarbital.[57] However, in previous pharmacokinetic studies, lacosamide pharmacokinetics were unaffected by concomitant treatment with carbamazepine 400 mg/day, or by valproic acid 600 mg/day.[52] In other clinical studies, lacosamide has not been found to affect the plasma concentrations of carbamazepine, carbamazepine-10,11-epoxide, phenytoin, valproic acid, lamotrigine, monohydroxy-carbazepine, topiramate, zonisamide, levetiracetam, gabapentin, metformin, digoxin, omeprazole, ethinylestradiol and levonorgestrel.[57–59]
6. Efficacy Data in Patients with Epilepsy
The efficacy of lacosamide has been investigated in three randomized, double-blind, parallel-group, placebo-controlled, adjunctive-therapy trials in adults with partial-onset seizures, with or without secondary generalization, unresponsive to other AEDs.[58,60,61] The three trials used a similar design, which included an 8-week baseline period, a titration period in which the target dose of lacosamide was gradually achieved in 100 mg/day increments each week, and a 12-week maintenance period. In all trials, the daily dose was given in two divided administrations (twice daily).
In the first trial, 418 patients were randomized to receive placebo or lacosamide dosages of 200, 400 or 600 mg/day in addition to pre-existing medication with up to two AEDs.[58] A single back titration by 100 mg was allowed at the end of the 6-week titration period for those patients who could not tolerate the target dosage. The median percent reduction in seizure frequency from baseline to maintenance (primary endpoint) in the 200, 400 and 600 mg/day groups was 26%, 39% and 40%, respectively, compared with 10% in the placebo group. The difference versus placebo was statistically significant (p < 0.01) for the intermediate and the highest dosage groups. A ≥50% reduction in seizure frequency during the maintenance period compared with baseline was reported in 21.9% of patients randomized to placebo, and in 32.7%, 41.1% and 38.1% of those randomized to 200 mg/day (not statistically significant), 400 mg/day (p = 0.004) and 600 mg/day (p= 0.014), respectively.
In the second trial, 485 patients receiving up to three AEDs were randomized to placebo or lacosamide dosages of 200 and 400 mg/day.[60] Patients assigned to the lacosamide 200 and 400 mg/day groups had a median percent reduction in seizure frequency of 35.3% and 36.4%, respectively, compared with 20.5% for placebo (p < 0.05 for both dosages). A ≥50% reduction in seizure frequency occurred in 25.8% of patients randomized to placebo and in 35.0% and 40.5% of those randomized to 200 and 400 mg/day, respectively (p < 0.01 for the 400 mg/day group).
The third trial, which has been reported only in summary form, compared placebo with lacosamide dosages of 400 or 600 mg/day in 405 patients receiving up to three concomitant AEDs.[61] A ≥50% reduction in seizure frequency was reported in 38.3% of patients receiving 400 mg/day and 41.2% of patients receiving 600 mg/day, compared with 18.3% of patients in the placebo group (p < 0.001 for both dosages). Median percent reductions in seizure frequency were 37.3% for lacosamide 400 mg/day (p = 0.008) and 37.8% for lacosamide 600 mg/day (p = 0.006) compared with 20.8% for the placebo group.
A comparison of efficacy data in the three trials is reported in table II. A pooled analysis of data from the same trials showed that 2.7%, 3.3% and 4.8% of patients randomized to lacosamide 200, 400 and 600 mg/day, respectively, were completely free from seizures during the maintenance period, compared with a seizure-free rate of 0.9% for patients randomized to placebo.[9] Overall, the results of these studies indicate that lacosamide is effective in reducing the frequency of partial seizures in the 200–600 mg/day dosage range. However, the gain in efficacy at the 600 mg/day compared with the 400 mg/day dosage appears to be modest. In view of this result and because the highest dosage has a less favourable tolerability profile (see section 7), only the 200–400 mg/day dosage range has been approved by regulatory authorities in Europe and the US for use as adjunctive treatment of refractory partial-onset seizures in adults.
Table II.
Median percent reduction in seizure frequency and percentage of patients with at least 50% reduction in seizure frequency (vs baseline) in three double-blind, parallel-group, placebo-controlled, adjunctive-therapy trials of lacosamide (LCM) in patients with refractory partial-onset seizures
| Study | No. of patients | Duration of treatment (titration/maintenance) | Treatment | Median % reduction in seizure frequency vs baseline (p-value) | % of patients with ≥ 50% reduction in seizure frequency vs baseline (p-value) |
|---|---|---|---|---|---|
| Ben-Menachem et al.[58] | 418 | 6 wk/12 wk | Placebo | 10 | 21.9 |
| LCM 200 mg/day | 26 (NS) | 32.7 (NS) | |||
| LCM 400 mg/day | 39 (p = 0.002) | 41.1 (p = 0.004) | |||
| LCM 600 mg/day | 40 (p = 0.008) | 38.1 (p = 0.014) | |||
| Chung et al.[61] | 405 | 6 wk/12 wk | Placebo | 20.8 | 18.3 |
| LCM 400 mg/day | 37.3 (p = 0.008) | 38.3 (p = 0.0004) | |||
| LCM 600 mg/day | 37.8 (p = 0.006) | 41.2 (p = 0.0005) | |||
| Halász et al.[60] | 485 | 4 wk/12 wk | Placebo | 20.5 | 25.8 |
| LCM 200 mg/day | 35.3 (p = 0.02) | 35.0 (NS) | |||
| LCM 400 mg/day | 36.4 (p = 0.03) | 40.5 (p = 0.01) |
NS = not statistically significant (vs placebo).
Long-term follow-up studies suggest that, in patients who responded favourably to lacosamide, response is maintained over time.[62] Lacosamide has not been tested in seizure types other than partial-onset seizures, and its potential value in the treatment of generalized epilepsies is unknown.
7. Tolerability Profile in Patients with Epilepsy
Based on observations made in adjunctive-therapy trials in patients with refractory partial-onset seizures, the tolerability profile of lacosamide shows some similarities with that reported for traditional sodium channel-blocking agents such as carbamazepine and phenytoin. A pooled analysis of adverse effects reported in randomized, placebo-controlled epilepsy trials of lacosamide has been conducted recently.[63,64] The most common dose-related adverse event was dizziness, which occurred in 53%, 30% and 16% of patients receiving dosages of 600, 400 and 200 mg/day, respectively, compared with 8% for patients randomized to placebo. Other dose-related adverse events were nausea, vomiting, abnormal coordination, tremor, nystagmus, diplopia, blurred vision and, possibly, fatigue, but for none of these events was the incidence at the highest dosage >17%. Remarkably, somnolence was very uncommon, and very few patients reported cognitive difficulties.[57,63]
Overall discontinuation rates due to adverse events during the treatment period were 8%, 17% and 29% for lacosamide dosages of 200, 400 and 600 mg/day, compared with 5% for placebo.[9] CNS events, particularly dizziness, and gastrointestinal symptoms were the most common adverse events leading to discontinuation. The tolerability profile of the intravenous formulation appears to be similar to that of the oral preparation.[53]
Cardiac safety studies have shown that lacosamide does not affect corrected QT intervals. However, it causes a small dose-related prolongation of the PR interval.[64,65] The incidence of reported first-degree atrioventricular (AV) block in clinical trials in patients with epilepsy has been low (<1.0%), and there have been no reports of second- or higher degree AV block in patients treated with lacosamide in epilepsy studies.[64] However, caution is recommended before using this drug in patients with pre-existing cardiac disease and in those taking class I anti-arrhythmics or drugs known to cause PR interval prolongation.[65]
8. Conclusions
The functionalized amino acid lacosamide has been shown to inhibit repetitive firing of neuronal cells by interfering with voltage-gated sodium currents, a mechanism shared by established AEDs such as carbamazepine, lamotrigine and phenytoin. However, at variance with these AEDs, lacosamide appears to possess a peculiar interaction with voltage-gated sodium channels. In fact, while carbamazepine, phenytoin and lamotrigine affect both fast and slow inactivation currents, lacosamide is devoid of effects on the fast inactivation component. In addition, the enhancement of slow inactivation of sodium channels caused by lacosamide is not use dependent, unlike the effect produced by classical sodium channel-blocking AEDs. The precise molecular mechanisms by which lacosamide influences the function of sodium channels have not been defined, but they may involve an interaction with a novel binding site.[33]
The slow inactivation process, which is intrinsic to voltage-gated sodium channel functioning, is likely to play a major role during the paroxysmal depolarizing shifts associated with epileptic activity. Therefore, interference with this mechanism appears to be primarily responsible for the effectiveness of lacosamide in standard models of epilepsy, such as the MES test, and in less common paradigms such as the 6 Hz psychomotor model of treatment-resistant seizures.[38,43] These in vivo effects are consistent with observations made in vitro, demonstrating suppression of epileptic activity in systems such as fampridine-induced tonic discharges and spike frequency in stimulated neuronal cell cultures or slices. In in vitro seizure models, the effects of lacosamide appear to be similar to those of other AEDs, such as carbamazepine. Lacosamide is also active in animal models of neuropathic pain, and an important role of sodium channel blockade in this activity is suggested by in vitro findings on sodium channels known to be involved in mediating neuropathic pain responses.
To date, the clinical effectiveness of lacosamide has been demonstrated in three randomized, double-blind, parallel-group, placebo-controlled, adjunctive-therapy trials in patients with refractory partial-onset seizures. Positive results have also been reported in trials conducted in patients with distal diabetic painful neuropathy.[66,67] Overall, these findings support the role of slow inactivation of sodium currents as a target for clinically significant drug effects both in epilepsy and neuropathic pain. However, it should be stressed that other actions may contribute to the efficacy of lacosamide in these conditions. In particular, recent findings suggest that lacosamide may also interfere functionally with collapsin-response mediator protein-2 (CMRP-2), a protein that has been implicated in the pathogenesis of both epilepsy and neuropathic pain.[38] However, these data should be interpreted with caution because, to date, they have only been reported in abstract form.[68] While the bulk of the evidence points to sodium channel blockade as the primary mechanism for the actions of lacosamide, further studies are required to define the potential contributory role of additional mechanisms.
Acknowledgments
This review was supported by grants from the Italian Ministry of Education, University & Research (FIRB2001 RBNE01NR34_011, PRIN 2003060538), the Canadian Institutes of Health Research (CIHR; Grant MT-8109), the Savoy Foundation and the Pierfranco and Luisa Mariani Foundation (R-06-50). Dr Giulia Curia was supported by a postdoctoral fellowship from Fragile X Research Foundation of Canada (FXRFC) in partnership with the CIHR.
Footnotes
Dr Emilio Perucca has received speaker’s or consultancy fees and/or research grants from Novartis, Bial, Pfizer, Glaxo-SmithKline, UCB Pharma, Sanofi-aventis, Eisai, Johnson & Johnson and Valeant Pharmaceuticals. Dr Massimo Avoli has received grants from Pfizer and UCB Pharma. Drs Curia and Biagini have no conflicts of interest that are directly relevant to the current review.
References
- 1.Avoli M, D’Antuono M, Louvel J, et al. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- 2.Inaba Y, Biagini G, Avoli M. The H current blocker ZD7288 decreases epileptiform hyperexcitability in the rat neocortex by depressing synaptic transmission. Neuropharmacology. 2006;51:681–91. doi: 10.1016/j.neuropharm.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Panuccio G, Curia G, Colosimo A, et al. Epileptiform synchronization in the cingulate cortex. Epilepsia. 2009;50:521–36. doi: 10.1111/j.1528-1167.2008.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictal-like discharges in the rat entorhinal cortex. J Neurophysiol. 1998;79:352–60. doi: 10.1152/jn.1998.79.1.352. [DOI] [PubMed] [Google Scholar]
- 5.Detlev B. Cell and gene therapies for refractory epilepsy. Curr Neuropharmacol. 2007;5:115–25. doi: 10.2174/157015907780866938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwan P, Brodie MJ. Emerging drugs for epilepsy. Expert Opin Emerg Drugs. 2007;12:407–22. doi: 10.1517/14728214.12.3.407. [DOI] [PubMed] [Google Scholar]
- 7.Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4:18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perucca E, French J, Bialer M. Development of new anti-epileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 2007;6:793–804. doi: 10.1016/S1474-4422(07)70215-6. [DOI] [PubMed] [Google Scholar]
- 9.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX) Epilepsy Res. 2009;83:1–43. doi: 10.1016/j.eplepsyres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Spencer SS. When should temporal-lobe epilepsy be treated surgically? Lancet Neurol. 2002;1:375–82. doi: 10.1016/s1474-4422(02)00163-1. [DOI] [PubMed] [Google Scholar]
- 11.Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7:514–24. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- 12.Zaccara G, Franciotta D, Perucca E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007;48:1223–44. doi: 10.1111/j.1528-1167.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- 13.White HS, Johnson M, Wolf HH, et al. The early identification of anticonvulsant activity: role of the maximal electroshock and subcutaneous pentylenetetrazol seizure models. Ital J Neurol Sci. 1995;16:73–7. doi: 10.1007/BF02229077. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt D, Rogawski MA. New strategies for the identification of drugs to prevent the development or progression of epilepsy. Epilepsy Res. 2002;50:71–8. doi: 10.1016/s0920-1211(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 15.Bayer K, Ahmadi S, Zeilhofer HU. Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology. 2004;46:743–9. doi: 10.1016/j.neuropharm.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–36. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 17.Gillard M, Chatelain P, Fuks B. Binding characteristics of levetiracetam to synaptic vesicle protein 2A (SV2A) in human brain and in CHO cells expressing the human recombinant protein. Eur J Pharmacol. 2006;536:102–8. doi: 10.1016/j.ejphar.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest. 2005;115:2010–7. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkovic SF, Mulley JC, Scheffer IE, et al. Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 2006;29:391–7. doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Köhling R. Voltage-gated sodium channels in epilepsy. Epilepsia. 2002;43:1278–95. doi: 10.1046/j.1528-1157.2002.40501.x. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal N, Alonso A, Ragsdale DS. Increased persistent sodium currents in rat entorhinal cortex layer V neurons in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia. 2003;44:1601–4. doi: 10.1111/j.0013-9580.2003.23103.x. [DOI] [PubMed] [Google Scholar]
- 22.Vreugdenhil M, Hoogland G, van Veelen CW, et al. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19:2769–78. doi: 10.1111/j.1460-9568.2004.03400.x. [DOI] [PubMed] [Google Scholar]
- 23.Ragsdale DS, Avoli M. Sodium channels as molecular targets for antiepileptic drugs. Brain Res Rev. 1998;26:16–28. doi: 10.1016/s0165-0173(97)00054-4. [DOI] [PubMed] [Google Scholar]
- 24.Stafstrom CE. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007;7:15–22. doi: 10.1111/j.1535-7511.2007.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogawski MA, Löscher W. The neurobiology of anti-epileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10:685–92. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- 26.Spina E, Perugi GG. Antiepileptic drugs: indications other than epilepsy. Epileptic Disord. 2004;6:57–75. [PubMed] [Google Scholar]
- 27.Lang DG, Wang CM, Barrett RC. Lamotrigine, phenytoin and carbamazepine interactions on the sodium current present in N4TG1 mouse neuroblastoma cells. J Pharmacol Exp Ther. 1993;266:829–34. [PubMed] [Google Scholar]
- 28.Zona C, Avoli M. Lamotrigine reduces voltage-gated sodium currents in rat central neurons in culture. Epilepsia. 1997;38:522–5. doi: 10.1111/j.1528-1157.1997.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 29.Errington AC, Coyne L, Stohr T, et al. Seeking a mechanism of action for the novel anticonvulsant lacosamide. Neuropharmacology. 2006;50:1016–29. doi: 10.1016/j.neuropharm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Willow M, Gonoi T, Catterall WA, et al. Voltage-clamp analysis of the inhibitory actions of diphenylhydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. Mol Pharmacol. 1985;27:549–58. [PubMed] [Google Scholar]
- 31.Xie X, Lancaster B, Peakman T, et al. Interactions of the antiepileptic drug lamotrigine with recombinant rat brain type IIA Na channels and native Na channels in rat hippocampal neurons. Pflugers Arch. 1995;430:437–46. doi: 10.1007/BF00373920. [DOI] [PubMed] [Google Scholar]
- 32.Vreugdenhil M, Wadman WJ. Modulation of sodium currents in rat CA1 neurons by carbamazepine and valproate after kindling epileptogenesis. Epilepsia. 1999;40:1512–22. doi: 10.1111/j.1528-1157.1999.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 33.Errington AC, Stohr T, Heers C, et al. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol. 2008;73:157–69. doi: 10.1124/mol.107.039867. [DOI] [PubMed] [Google Scholar]
- 34.Fozzard HA, Hank DA. Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol Rev. 1996;76:887–926. doi: 10.1152/physrev.1996.76.3.887. [DOI] [PubMed] [Google Scholar]
- 35.Vassilev PM, Scheuer T, Catterall WA. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science. 1988;241:1658–61. doi: 10.1126/science.241.4873.1658. [DOI] [PubMed] [Google Scholar]
- 36.Stühmer W, Conti F, Suzuki H, et al. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- 37.Lees G, Stohr T, Errington AC. Stereoselective effects of the novel anticonvulsant lacosamide against 4-AP induced epileptiform activity in rat visual cortex in vitro. Neuropharmacology. 2006;50:98–110. doi: 10.1016/j.neuropharm.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Beyreuther BK, Freitag J, Heers C, et al. Lacosamide: a review of preclinical properties. CNS Drug Rev. 2007;13:21–42. doi: 10.1111/j.1527-3458.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doty P, Rudd GD, Stöhr T, et al. Lacosamide. Neurotherapeutics. 2007;4:145–8. doi: 10.1016/j.nurt.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi D, Stables JP, Kohn H. Synthesis and anticonvulsant activities of N-benzyl-2-acetamidopropionamide derivatives. J Med Chem. 1996;39:1907–16. doi: 10.1021/jm9508705. [DOI] [PubMed] [Google Scholar]
- 41.Choi D, Stables JP, Kohn H. The anticonvulsant activities of functionalized N-benzyl-2-acetamidoacetamides: the importance of the 2-acetamido substituent. Bioorg Med Chem. 1996;4:2105–14. doi: 10.1016/s0968-0896(96)00225-8. [DOI] [PubMed] [Google Scholar]
- 42.Stöhr T, Kupferberg HJ, Stables JP, et al. Lacosamide, a novel anti-convulsant drug, shows efficacy with a wide safety margin in rodents models for epilepsy. Epilepsy Res. 2007;74:147–54. doi: 10.1016/j.eplepsyres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Duncan GE, Kohn H. The novel antiepileptic drug lacosamide blocks behavioral and brain metabolic manifestations of seizure activity in the 6 Hz psychomotor seizure model. Epilepsy Res. 2005;67:81–7. doi: 10.1016/j.eplepsyres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Brandt C, Heile A, Potschka H, et al. Effects of the novel antiepileptic drug lacosamide on the development of amygdala kindling in rats. Epilepsia. 2006;47:1803–9. doi: 10.1111/j.1528-1167.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 45.White S, Perucca E, Privitera M. Investigational drugs. In: Engel J Jr, Pedley TA, Aicardi J, et al., editors. Epilepsy. a comprehensive textbook. Philadelphia (PA): Lippincott, Williams & Wilkins; 2007. pp. 1721–40. [Google Scholar]
- 46.Hao JX, Stöhr T, Selve N, et al. Lacosamide, a new anti-epileptic, alleviates neuropathic pain-like behaviors in rat models of spinal cord or trigeminal nerve injury. Eur J Pharmacol. 2006;553:135–40. doi: 10.1016/j.ejphar.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 47.Beyreuther BK, Callizot N, Brot MD, et al. Antinociceptive efficacy of lacosamide in rat models for tumor- and chemotherapy-induced cancer pain. Eur J Pharmacol. 2007;565:98–104. doi: 10.1016/j.ejphar.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 48.Beyreuther BK, Geis C, Stöhr T, et al. Antihyperalgesic efficacy of lacosamide in a rat model for muscle pain induced by TNF. Neuropharmacology. 2007;52:1312–7. doi: 10.1016/j.neuropharm.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Beyreuther B, Callizot N, Stöhr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain [abstract] Arthritis Res Ther. 2007;9:R14. doi: 10.1186/ar2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beyreuther B, Callizot N, Stöhr T. Antinociceptive efficacy of lacosamide in a rat model for painful diabetic neuropathy. Eur J Pharmacol. 2006;539:64–70. doi: 10.1016/j.ejphar.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Sheets PL, Heers C, Stoher T, et al. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide, lidocaine and carbamazepine. J Pharm Exp Ther. 2008;26:89–99. doi: 10.1124/jpet.107.133413. [DOI] [PubMed] [Google Scholar]
- 52.Kropeit D, Schiltmeyer B, Cawello W, et al. Bioequivalence of short-time infusions compared to oral administration of SPM 927. Epilepsia. 2004;45(Suppl 7):123–4. [Google Scholar]
- 53.Biton V, Rosenfeld WE, Whitesides J, et al. Intravenous lacosamide as replacement for oral lacosamide in patients with partial-onset seizures. Epilepsia. 2008;49:418–24. doi: 10.1111/j.1528-1167.2007.01317.x. [DOI] [PubMed] [Google Scholar]
- 54.Cawello W, Kropeit D, Schiltmeyer B, et al. Food does not affect the pharmacokinetics of SPM 927 [abstract] Epilepsia. 2004;45(Suppl 7):307. [Google Scholar]
- 55.Schiltmeyer B, Cawello W, Kropeit D, et al. Pharmaco-kinetics of the new antiepileptic drug SPM 927 in human subjects with different age and gender [abstract] Epilepsia. 2004;45(Suppl 7):313. [Google Scholar]
- 56.UCB Pharma. Vimpat (Lacosamide): summary of product characteristics. Brussels: UCB Pharma; 2008. Sep, [Google Scholar]
- 57.Sachdeo R. Lacosamide. In: Shorvon SD, Perucca E, Engel J Jr, editors. The treatment of epilepsy. 3. Oxford: J Wiley and Sons; 2009. pp. 527–34. [Google Scholar]
- 58.Ben-Menachem E, Biton V, Jatuzis D, et al. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48:1308–17. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 59.Bialer M, Johannessen SI, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the Eighth Eilat Conference (EILAT VIII) Epilepsy Res. 2007;73:1–52. doi: 10.1016/j.eplepsyres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Halász P, Kälviäinen R, Mazurkiewicz-Beldziñska M, et al. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50:443–53. doi: 10.1111/j.1528-1167.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 61.Chung SM, Sperling M, Biton V, et al. Lacosamide: efficacy and safety as oral adjunctive therapy in adults with partial-onset seizures [abstract] Epilepsia. 2007;48(Suppl 7):57. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 62.Rosenfeld W, Fountain NB, Kaubrys G, et al. Lacosamide: an interim evaluation of long-term safety and efficacy as oral adjunctive therapy in subjects with partial-onset seizures. Epilepsia. 2007;48(Suppl 6):318–9. [Google Scholar]
- 63.Biton V, Fountain N, Rosenow F, et al. Safety and tolerability of lacosamide: a summary of adverse events in epilepsy clinical trials [abstract] Ann Neurol. 2008;64(Suppl 12):S21. [Google Scholar]
- 64.Cawello W, Horstmann R, Doty P, et al. No influence of lacosamide on the ECG time intervals QTC and PR. 58th Annual American Epilepsy Society Meeting, Scientific Exhibit; 2004 Dec 3–7; New Orleans (LA). [Google Scholar]
- 65.European Medicines Agency (EMEA) [Accessed 2009 Mar 23];European Public Assessment Report-Vimpat [online] Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/vimpat/H-863-PI-en.pdf.
- 66.Wymer JP, Simpson J, Sen D, et al. Lacosamide SP742 Study Group. Efficacy and safety of lacosamide in diabetic neuropathic pain: an 18-week double-blind placebo-controlled trial of fixed-dose regimens. Clin J Pain. 2009;25:376–85. doi: 10.1097/AJP.0b013e318196d2b6. [DOI] [PubMed] [Google Scholar]
- 67.Shaibani A, Fares S, Selam JL, et al. Lacosamide in painful diabetic neuropathy: an 18-week double-blind placebo-controlled trial. Pain. doi: 10.1016/j.jpain.2009.01.322. Epub 2009 Apr 29. [DOI] [PubMed] [Google Scholar]
- 68.Freitag J, Beyreuther B, Heers C, et al. Lacosamide modulates collapsin response mediated protein 2 (CRMP-2) [abstract] Epilepsia. 2007;48(Suppl 6):320. [Google Scholar]