Abstract
Changes in GABAB receptor subunit expression have been recently reported in the neocortex of epileptic WAG/Rij rats that are genetically prone to experience absence seizures. These alterations may lead to hyperexcitability by down-regulating the function of presynaptic GABAB receptors in neocortical networks as suggested by a reduction in paired-pulse depression. Here, we tested further this hypothesis by analyzing the effects induced by the GABAB receptor agonist baclofen (0.1–10 μM) on the inhibitory events recorded in vitro from neocortical slices obtained from epileptic (>180 day-old) WAG/Rij and age-matched, non-epileptic control (NEC) rats. We found that higher doses of baclofen were required to depress pharmacologically isolated, stimulus-induced IPSPs generated by WAG/Rij neurons as compared to NEC. We also obtained similar evidence by comparing the effects of baclofen on the rate of occurrence of synchronous GABAergic events recorded by WAG/Rij and NEC neocortical slices treated with 4-aminopyridine + glutamatergic receptor antagonists. In conclusion, these data highlight a decreased function of presynaptic GABAB receptors in the WAG/Rij rat neocortex. We propose that this alteration may contribute to neocortical hyperexcitability and thus to absence seizures.
Keywords: Absence seizures, 4-Aminopyridine, Baclofen, GABAB receptor, WAG/Rij rats
Introduction
It is well established that generalized spike-and-wave (SW) discharges in normal animals treated with convulsants and in GAERS or WAG/Rij rats – which are strains genetically predisposed to produce absence seizures – initiate in the neocortex [1, 2]. Evidence obtained from these genetic rodent models indicates that changes in both intrinsic [3, 4] and synaptic mechanisms [5–8] are likely to contribute to neocortical hyperexcitability. Moreover, we have recently reported that the somatosensory cortex of epileptic WAG/Rij rats exhibits alterations in GABAB receptor subunit expression and localization [9]. GABAB receptors regulate neuronal excitability by causing a postsynaptic K+-dependent hyperpolarization and by modulating transmitter release at presynaptic terminals [10]. In particular, in neocortical neurons paired-pulse depression is contributed by presynaptic GABAB receptors [11]. Accordingly, we have found that paired-pulse depression of pharmacologically isolated excitatory and inhibitory responses is reduced in WAG/Rij rat neocortical neurons recorded in an in vitro slice preparation [9].
In order to extend the investigation on the defective GABAB-mediated mechanisms in absence seizures, we analyzed the effects induced by the GABAB receptor agonist baclofen, acting presynaptically on neocortical cells [11, 12], on pharmacologically isolated, stimulus-induced inhibitory postsynaptic potentials (IPSPs) recorded intracellularly from brain slices of the perioral area of the somatosensory cortex obtained from epileptic (>180 day-old) WAG/Rij and age-matched non-epileptic control (NEC) rats. The perioral area has been identified as the ‘consistent cortical focus’ of initiation for SW discharges in rodent genetic models [13–15], and it is thus reasonable to presume that it represents the region in which neuronal networks display the most pronounced changes in excitability. In addition, we used field potential recordings to assess in these two types of neocortical tissue the effects induced by baclofen on the glutamatergic-independent synchronous events generated during concomitant bath application of 4-aminopyridine (4AP) and ionotropic glutamatergic receptor antagonists [16]. The effects induced by baclofen in normal rodent cortical neurons have been described by several authors and are known to mainly reflect the activation of presynaptic GABAB receptors [17, 18].
Methods
Epileptic (>180 day-old) WAG/Rij (Harlan, Horst, The Netherlands) and age-matched NEC (Crl(WI)BR Wistar, Harlan) rats were used according to the procedures established by the Canadian and the European Union Councils of Animal Care. All efforts were made to minimize the number of animals used and their suffering. WAG/Rij rats housed before the experiment had frequent episodes of behavioral arrest (duration = up to 15 s) with mild myoclonic twitches. Previous in vivo studies have demonstrated that these clinical events are associated with bilaterally generalized SW discharges at 7–11 Hz [13]. Similar behavioral episodes were only rarely seen in NEC rats; these animals, identified as previously indicated [5, 9], were not used for the experiments reported here.
Neocortical slices (450 μm) were cut coronally with a vibratome from a region corresponding to the somatosensory cortex that matched the plates reported by Paxinos and Watson [19] at −0.3 to 0.7 mm from the bregma [cf. also 7, 14 ]. Slices were then transferred to a tissue chamber where they laid in an interface between oxygenated artificial cerebral spinal fluid (ACSF) and humidified gas (95% O2/5% CO2) at 32°C (pH = 7.4). ACSF composition was (in mM): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2 or 4, CaCl2 2, NaHCO3 26, and glucose 10. The ACSF also contained one or more of the following drugs: 4AP (50 μM), (RS)-baclofen (0.1–10 μM), 3-((±)-2-carboxy-piperazin-4-yl)-propyl-1-phosphonate (CPP, 20 μM), 6-cyano-7-nitroquino-xaline-2,3-dione (CNQX, 10 μM), (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenyl-methyl)phosphinic acid (CGP 55845, 10 μM) and picrotoxin (10 μM). Chemicals were acquired from Sigma (St. Louis, Mo., USA) with the exception of CGP 55845, CNQX and CPP that were obtained from Tocris Cookson (Langford, UK).
Field potential recordings were made from a region corresponding to the perioral somatosensory cortex with ACSF-filled glass pipettes (resistance = 2–10 MΩ) that were connected to high-impedance amplifiers. Sharp-electrode intracellular recordings were obtained from cells located in the supragranular layers (i.e., at approx. 500 μm from the pia) of this area with pipettes that were filled with 3 M K-acetate or with 3 M K-acetate/75 mM lidocaine, N-ethyl bromide (QX314) (tip resistance = 70–140 MΩ in both cases). Intracellular signals were fed to an Axoclamp 2A amplifier (Molecular Devices, Palo Alto, Calif., USA) with an internal bridge circuit for passing intracellular current. The bridge balance was routinely checked. Signals were fed to a computer interface (Digidata 1322A, Molecular Devices) for subsequent analysis with the Clampfit 9 software (Molecular Devices). The neuron resting membrane potential (RMP) was measured after withdrawal from the cell, while the membrane apparent input resistance (Ri) was calculated from the peak of voltage responses to hyperpolarizing current pulses (amplitude < 0.5 nA). Overshooting action potentials were generated by all cells recorded with K-acetate-filled electrodes. Table 1 summarizes the membrane properties measured in WAG/Rij and NEC slices; these values were not significantly different among the two groups.
Table 1.
Intrinsic membrane properties of neurons recorded in neocortical slices obtained from NEC and WAG/Rij rats during superfusion of control ACSF
| RMP, mV | Ri, MΩ | τ, ms | APA, mV | APD, ms | |
|---|---|---|---|---|---|
| NEC (n = 9) | −70.2±8.3 | 37.8±7.0 | 11.6±3.1 | 83.6±13.4 | 1.1±0.2 |
| WAG/Rij (n =10) | −69.4±5.5 | 39.6±9.4 | 12.0±2.5 | 83.3±5.9 | 1.2±0.2 |
Values represent means ± SD; n = indicates the number of recorded neurons obtained from 3 different NEC or WAG/Rij rats. RMP = Resting membrane potential; Ri = input resistance; τ = time constant; APA = action potential amplitude; APD = action potential duration.
A bipolar stainless steel electrode was used to deliver single-shock stimuli (100 μs; <300 μA; intertrial intervals = 20–30 s) within the neocortical layers at a distance that ranged 150–500 μm from the site of intracellular recording. Reversal potential values for the early and late components of the IPSPs were obtained from a series of responses evoked at membrane potentials set to different levels by intracellular current injection. Reversal potentials were computed from regression plots of response amplitude versus membrane potential. Increasing baclofen doses were applied for 30–40 min and electrophysiological measurements were obtained shortly before changing to the following solution. When analyzing the effects of baclofen on the stimulus-induced, pharmacologically isolated IPSP, intracellular current injection was used to maintain the membrane potential at values similar to those seen under control conditions.
Values throughout the text are expressed as mean ± SD and n indicates the number of cells or slices analyzed. Statistical analysis was made with the Student’s t test or analysis of variance using Statistical Package for the Social Sciences version 8.0 software (SPSS, Chicago, Ill., USA). Specifically, the effects of increasing baclofen doses were analyzed by a general factorial model in which rat strain was the fixed factor, and baclofen-increasing doses were instead the random factor. Post-hoc comparisons were made using the Fisher’s least significant difference (LSD) test. Data sets were considered significantly different if p < 0.05.
Results
Stimulus-Induced Synaptic Responses in WAG/Rij and NEC Slices
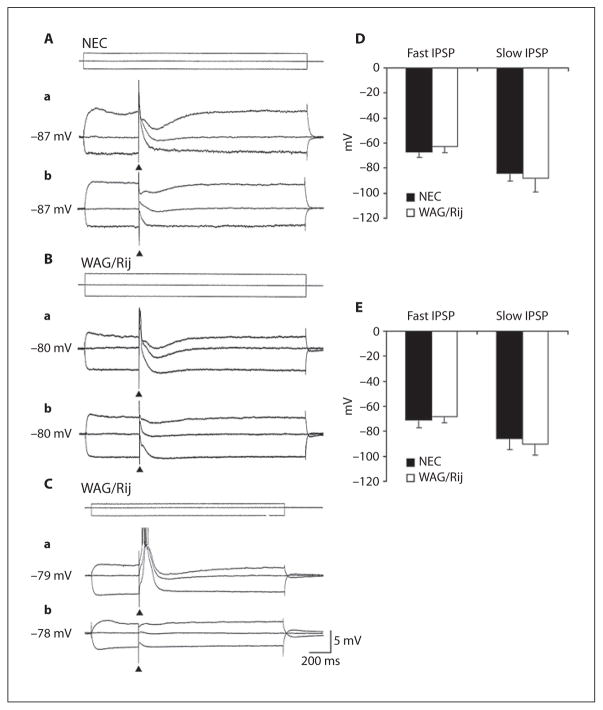
As illustrated in figure 1Aa and Ba, focal electrical stimuli delivered within the neocortical layers during application of control ACSF induced in both NEC (n = 9) and WAG/Rij (n = 7/10) neurons an EPSP that could trigger a single action potential (not illustrated). In addition, some WAG/Rij (n = 3) but no NEC cell could generate action potential bursts overriding the stimulus-induced depolarization (fig. 1Ca). At RMP either the initial EPSP or the burst response were followed by a slow hyperpolarization (overall duration = up to 380 ms; peak latency = 120–200 ms). As illustrated in figure 1Aa, Ba, and Ca, injection of depolarizing current modified the amplitude of these responses and disclosed a fast hyperpolarization (peak latency at around 20 ms from the stimulus) in all NEC neurons (fig. 1Aa) and in those WAG/Rij cells that generated an EPSP response (fig. 1Ba). Therefore, neurons recorded in both types of tissue could generate IPSPs that were characterized by a fast and a slow component. Injection of depolarizing and hyperpolarizing current demonstrated reversal potentials for the fast and slow components of these stimulus-induced IPSP with values that were similar in NEC (−67.3 ± 4.5 and −84.2 ± 6.5 mV, respectively, n = 9) and WAG/Rij (−62.8 ± 4.7 and −87.9 ± 11.1 mV, respectively, n = 7) neocortical cells (fig. 1D).
Fig. 1.
A–C Intracellular responses to focal electrical stimuli recorded in NEC and WAG/Rij neocortical cells in the presence of control ACSF (a) and during application of glutamatergic receptor antagonists (CNQX+CPP) (b). Samples were obtained at different membrane potentials by injecting pulses of intracellular current. Note that under control conditions the WAG/Rij cell shown in C generates a burst of action potentials as well as that during depolarizing commands the neurons in A and B produce a hyperpolarization characterized by fast and slow components. D, E Plots of the reversal potential values of IPSP fast and slow components recorded in control ACSF and in the presence of glutamatergic receptor antagonists from NEC (n = 9 and 10, respectively) and WAG/Rij (n = 7 and 12, respectively) cells. Statistical analysis of these values revealed no significant difference between the two types of tissue.
Application of medium containing the glutamatergic receptor antagonists CPP (20 μM) and CNQX (10 μM) abolished the stimulus-induced synaptic responses in both NEC (n = 5) and WAG/Rij cells (n = 6) (not shown). In addition, this pharmacological procedure blocked the stimulus-induced depolarization/action potential burst response generated in 3 WAG/Rij cells (fig. 1Cb). However, high-strength electrical stimuli delivered under these conditions could still elicit synaptic responses that included fast and slow hyperpolarizing components upon injection of depolarizing current (fig. 1Ab, Bb). As shown in figure 1Cb, the pharmacologically isolated responses generated by the WAG/Rij cells producing a burst response under control conditions were of small amplitude. The reversal potentials of the fast component of these glutamatergic-independent, presumptive IPSPs were analyzed in these neurons as well as in additional cells that were only recorded during superfusion of medium containing CPP+CNQX. As illustrated in figure 1E, the fast component of the pharmacologically isolated IPSP had reversal potentials of −71.0 ± 6.3 and −67.6 ± 5.3 mV in NEC (n = 10) and WAG/Rij (n = 12) neurons, respectively, while the IPSP slow component displayed reversal potential values of −85.6 ± 8.5 mV in NEC and −89.7 ± 8.8 mV in WAG/Rij cells. Statistical analysis of these values revealed no significant difference between the two types of tissue.
Baclofen Effects on Pharmacologically Isolated, Stimulus-Induced IPSPs
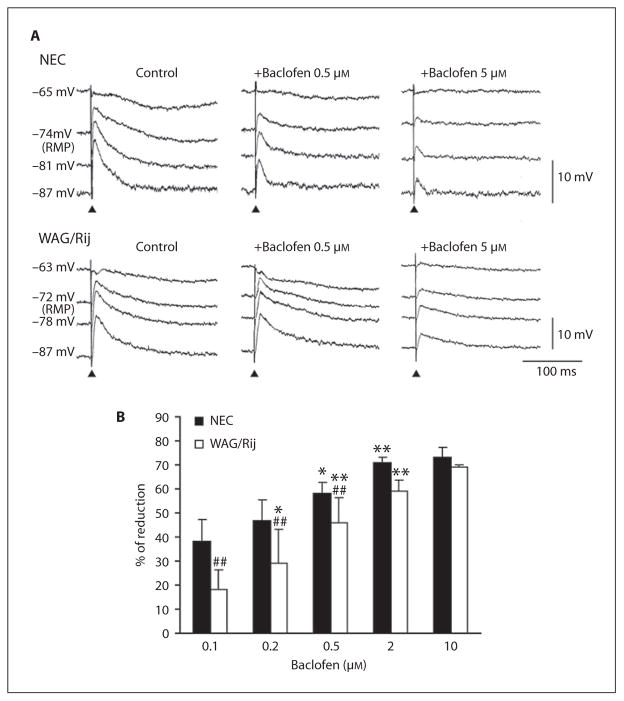
Next, we analyzed the effects induced by bath application of baclofen (0.1–10 μM) on the pharmacologically isolated IPSPs recorded intracellularly in NEC (n = 7) and WAG/Rij (n = 8) neurons following single-shock stimuli. As illustrated in figure 2A, these responses were reduced by baclofen in both types of tissue; however, the effects identified in WAG/Rij neurons were consistently less pronounced than in NECs. Similar conclusions could be drawn by analyzing the changes induced by this GABAB receptor agonist on the responses generated at any membrane potentials, but could be more easily appreciated when the stimulus-induced responses were ‘enhanced’ by injecting steady hyperpolarizing current (e.g., −87-mV traces in both NEC and WAG/Rij panels of fig. 2A). An increase of the slow hyperpolarization recorded during injection of depolarizing current was seen in 4 WAG/Rij neurons during application of 0.5 (fig. 2A, WAG/Rij panel) or 2 μM baclofen (not illustrated).
Fig. 2.
A Effects induced by baclofen (0.1–10 μM) on the pharmacologically isolated IPSPs recorded intracellularly from a NEC and from a WAG/Rij neuron following single-shock stimuli. Note that the effects exerted in the WAG/Rij cell are less pronounced than in NEC as well as that this difference can be better appreciated during injection of steady hyperpolarizing current. B Percentage of reduction induced by increasing doses of baclofen on the peak amplitude of the stimulus-induced responses measured in the two types of tissue during injection of hyperpolarizing current (membrane values = −84 to −90 mV). Note that values of reduction are significantly (p < 0.01) different in NEC and WAG/Rij rats at low baclofen doses, but they became similar when approaching the plateau (2 μM) of baclofen effect. * p <0.05, ** p < 0.01 vs. the preceding (lower) baclofen dose; ## p < 0.01 vs. the respective NEC group of baclofen treatment, Fisher’s LSD test for multiple comparisons.
The percentage of reduction induced by increasing doses of baclofen on the peak amplitude of the stimulus-induced responses measured in the two types of tissue during injection of hyperpolarizing current (membrane values ranging between −84 and −90 mV) are illustrated in figure 2B. Statistical analysis of IPSP reduction demonstrated the presence of significant effects of rat strain (F(1,43) = 22.6, p < 0.01) and baclofen-increasing doses (F(4,43) = 30.4, p < 0.01) without an interaction (F(4,43) = 1.6, not significant) between these two main factors. However, post-hoc comparisons with the Fisher’s LSD test showed a significantly (p < 0.01) progressive reduction in IPSPs that occurred in the presence of a maintained lower response in WAG/Rij up to 2–10 μM of baclofen, when a plateau was reached and the responses appeared to be similar in both rat strains.
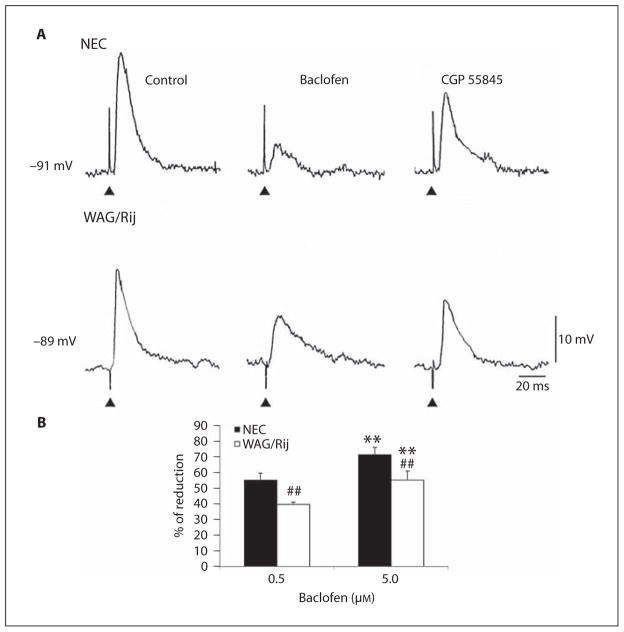
To establish whether the different efficacy of baclofen to depress pharmacologically isolated IPSPs in WAG/Rij tissue did reflect a presynaptic mechanism, we tested the effects induced by this GABAB receptor agonist on stimulus-induced responses recorded with K-acetate+QX-314-filled electrodes. In addition to reducing current through voltage-gated Na+ channels [20], QX-314 is known to block post-synaptic GABAB receptor-mediated conductances [21]. In these experiments, too, neurons were kept to hyperpolarized values (approx. −90 mV) by current injection in order to obtain depolarizing IPSPs. As shown in figure 3A, the reduction induced by 0.5 μM baclofen was more pronounced in NEC (n = 4) than in WAG/Rij (n = 3) cells and similar results were obtained by using 5 μM baclofen (n = 4 neurons in both cases; not illustrated). As illustrated in figure 3A, these effects were partially reversed by further addition of CGP 55845 (10 μM) in NEC (n = 4) and WAG/Rij neocortical cells (n = 3). In addition, these stimulus-induced intracellular responses were abolished by the GABAA receptor antagonist picrotoxin (10 μM) (not shown). Figure 3B summarizes the percentage of reduction induced by baclofen on the peak amplitude of the stimulus-induced responses measured in the two types of tissue. Statistical analysis of IPSP reduction demonstrated the presence of significant effects of rat strain (F(1,11) = 2,288, p < 0.05) and baclofen-increasing doses (F(1,11) = 2,288, p < 0.05) without an interaction (F(1,11) = 0.02, not significant) between the main factors. Post-hoc comparisons with the Fisher’s LSD test confirmed the significant (p < 0.01) reduction in IPSPs in both strains of animals with increasing baclofen doses. However, WAG/Rij rats maintained a significantly (p < 0.01) lower response also to 5 μM of baclofen, when compared with NEC exposed to the same drug dose.
Fig. 3.
A Effects induced by baclofen (0.5 μM) on the pharmacologically isolated IPSPs recorded intracellularly with K-acetate+QX314 filled electrodes from NEC and WAG/Rij neurons following single-shock stimuli. Note that the effects exerted in the WAG/Rij cell are less pronounced than in NEC and that these effects are reversed by application of the GABAB receptor antagonist CGP 55845; note also that the stimulus-induced event generated by the WAG/Rij neuron is abolished by bath application of picrotoxin. B Percentage of reduction induced by 0.5. and 5 μM of baclofen on the peak amplitude of the stimulus-induced responses measured in the two types of tissue. Note that values of reduction are significantly (## p < 0.01) different in NEC and WAG/Rij rats at both baclofen doses. ** p < 0.01 vs. baclofen 0.5 μM, Fisher’s LSD test for multiple comparisons.
Baclofen Effects on Network-Driven Synchronous IPSPs Induced by 4AP
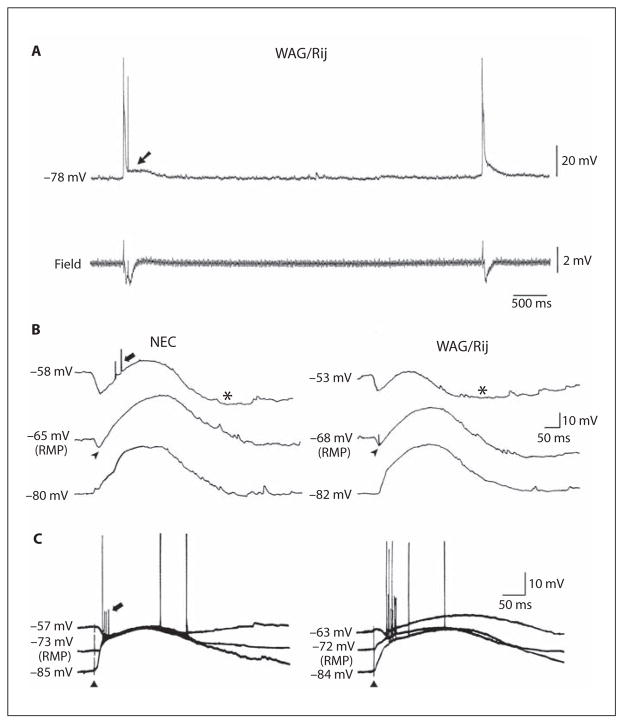
We further assessed the effects induced by baclofen on network-driven IPSPs by analyzing the changes induced by this GABAB receptor agonist on the synchronous activity disclosed by concomitant application of 4AP+CNQX+CPP. As reported in previous studies performed in rat [22] and human [16] neocortical tissue, these spontaneous field events occurred at intervals of 4.5–16 s (with values that were not different in NEC and epileptic WAG/Rij neocortical slices; n = 8 and 10, respectively). This type of synchronous activity was reduced and eventually abolished by application of the GABAA receptor antagonist picrotoxin (50 μM, n = 4; not illustrated) and was intracellularly mirrored by a long-lasting depolarization (fig. 4A, arrow) that could be preceded by an early hyperpolarization (fig. 4B, arrowheads) and followed by a slow hyperpolarization (fig. 4B, asterisks), depending upon the RMP of the neuron under study. Accordingly, the amplitude of these different components was changed by injection of intracellular current in a similar manner in both NEC and WAG/Rij neurons (fig. 4B). Intracellular responses with similar characteristics were generated by these cells following electrical stimuli (fig. 4C). This procedure allowed us to measure more precisely the reversal potentials of the different components of these intracellular responses in NEC and WAG/Rij slices; these data are summarized in table 2.
Fig. 4.
A Intracellular and field potential recordings of the spontaneous activity induced by application of 4AP+CNQX+CPP in a WAG/Rij neocortical slice. Note that the negative-going field events are mirrored by a long-lasting depolarization at the intracellular level (arrow). B Intracellular recordings of the spontaneous events recorded at different membrane potentials from NEC and WAG/Rij neocortical neurons during application of ACSF containing 4AP+CNQX+CPP. Note that the long-lasting depolarization is preceded by an early hyperpolarization (arrowheads) and followed by a slow hyperpolarization (asterisks). Note also that the amplitude of these different components is modified by injection of intracellular current in a similar manner in both NEC and WAG/Rij tissue. C Intracellular responses obtained following electrical stimuli demonstrate similar changes during intracellular injection of depolarizing and hyperpolarizing current. B, C Note that small amplitude action potentials, which may represent ectopic spikes, occur in both types of tissue during spontaneous and stimulus-induced network-driven IPSPs (thick arrows).
Table 2.
Reversal potentials of the different intracellular components of the synchronous events generated by neocortical neurons in NEC and WAG/Rij rat slices during superfusion of ACSF containing 4AP+CNQX+CPP
| Early hyperpolarization mV | Long-lasting depolarization mV | Late hyperpolarization mV | |
|---|---|---|---|
| NEC (n = 6) | −72.0±6.4 | −54.0±2.1 | −88±1.8 |
| WAG/Rij (n = 5) | −69.4±2.4 | −54.6±1.9 | −87±7.4 |
Values represent means ± SD and were obtained by measuring the three intracellular components at latencies of 25, 100 and 300–400 ms from the stimulus for the early hyperpolarization, long-lasting depolarization and late hyperpolarization, respectively.
Full-blown action potentials could often be generated at the peak of the long-lasting depolarization (fig. 4A, C). In addition, as reported in previous studies performed in cortical slices treated with 4AP [16, 23], small amplitude action potentials – presumably representing ectopic spikes – occurred in both types of tissue during both spontaneous and stimulus-induced network IPSPs (fig. 4B, C, thick arrows). As shown in detail in figure 4B, the small amplitude action potentials occurred more often during the early hyperpolarizing IPSP or during the rising phase of the long-lasting depolarization. These characteristics have been characterized in a previous study performed in hippocampal rodent neurons [23].
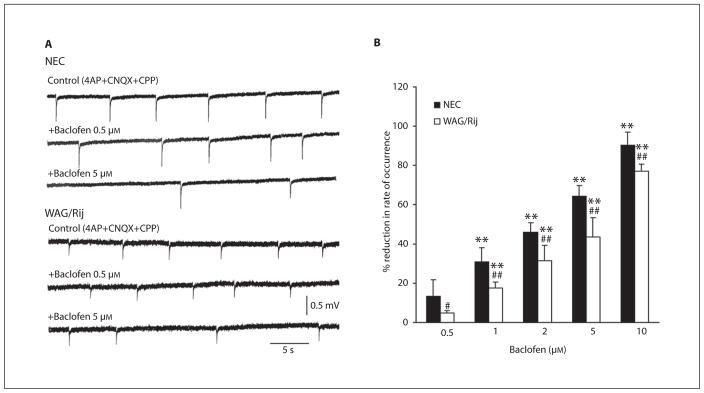
Application of baclofen (0.5–10 μM) reduced the rate of occurrence of these synchronous field potentials in NEC (n = 4–5) and epileptic WAG/Rij (n = 4–5) neocortical slices. However, these effects were more pronounced in NEC tissue; thus, as illustrated in figure 5A, 0.5 μM of this GABAB receptor agonist increased the interval of occurrence of these events in NEC tissue, but was unable to modify this parameter in the WAG/Rij slices. A reduced ability to influence the rate of occurrence of these network-driven field IPSPs in WAG/Rij slices was also seen with higher concentrations of baclofen (fig. 5A, 5-μM samples). These effects were decreased by further application of the GABAB receptor antagonist CGP 55845 (n = 3 NEC slices that have been pretreated with 5 μM of baclofen; not illustrated). In both types of tissue, baclofen did not consistently influence the amplitude of the field events generated in the presence of 4AP+CNQX+CPP.
Fig. 5.
Effects induced by baclofen on the rate of occurrence of the synchronous field potentials induced by application of 4AP+CNQX+CPP in NEC and WAG/Rij neocortical slices. A Field potential recordings obtained under control conditions and during application of 0.5 and 5 μM of the GABAB receptor agonist. Note that the effects are less pronounced in the WAG/Rij slice with both concentrations. B Percentage reduction in the occurrence of the synchronous field activity during increasing concentrations of baclofen. Note that values of reduction are significantly (p < 0.01) lower in NEC compared to WAG/Rij rats at every baclofen dose. ** p < 0.01 vs. the preceding (lower) baclofen dose; # p < 0.05, ## p < 0.01 vs. the respective NEC group of baclofen treatment, Fisher’s LSD test for multiple comparisons.
The percentage reduction exerted in the two strains by baclofen on the rate of occurrence of the network-driven field IPSPs is summarized in the histogram shown in figure 5B. Statistical analysis of reduction in the occurrence of the synchronous field activity during baclofen treatment showed main effects of rat strain (F(1,33) = 63.1, p < 0.01) and baclofen-increasing doses (F(4,33) = 222.7, p < 0.01) without interaction (F(4,33) = 1, not significant) between the main factors. In addition, post-hoc analysis with the Fisher’s LSD test demonstrated that the strain differences were significant at all the considered baclofen doses, as well as that the doses of this GABAB receptor agonist did not induce a ceiling effect in the range used.
Discussion
In this study we sought further evidence for a change in GABAB receptor-mediated function in the neocortex of WAG/Rij rats; these animals represent a genetic model of absence seizures in which SW discharges initiated in the neocortex are maintained through a cortico-thalamocortical loop [13]. A similar pathophysiological condition is encountered in patients presenting with absence epilepsy [1, 2]. Whereas dysfunction in thalamo-cortico-thalamic networks is contributed by a loss in the α3 GABAA receptor subunit in the thalamic reticular nucleus, GABAA receptors are preserved in the neocortex of WAG/Rij epileptic rats [24], which are defective in several GABAB1 receptor isoforms [9]. By performing intracellular recordings from the superficial layers of the perioral area of the somatosensory cortex in an in vitro slice preparation, we have found that: (i) neurons in WAG/Rij and NEC tissue have similar fundamental electrophysiological properties and similar reversal potentials for fast and slow IPSPs recorded during glutamatergic receptor blockade; (ii) higher doses of baclofen are required to depress pharmacologically isolated, stimulus-induced IPSPs generated by WAG/Rij neocortical neurons as compared to those recorded from NEC cells; (iii) a similar decreased sensitivity to baclofen of WAG/Rij neurons is evident when this GABAB receptor agonist is tested on the synchronous network-driven IPSPs generated by neocortical slices treated with 4AP + glutamatergic receptor antagonists.
Intrinsic and Synaptic Responses in NEC and Epileptic WAG/Rij Neocortical Cells
In line with evidence obtained in previous studies [5, 6], neurons recorded intracellularly from the superficial layers of neocortical slices obtained from NEC and epileptic WAG/Rij rats were characterized by similar fundamental electrophysiological properties. We have also found that focal electrical stimuli in the presence of control ACSF induced EPSPs that could trigger a single action potential in all NEC and in most WAG/Rij cells. Only few WAG/Rij neurons generated action potential bursts overriding the stimulus-induced depolarization; these burst discharges have been reported to be abolished by NMDA receptor antagonism [5], a finding that is in line with the ability of NMDA receptor antagonism to reduce the occurrence of absence seizures in epileptic WAG/Rij animals in vivo [25].
We also extended our electrophysiological analysis to the fast and slow IPSP generated by NEC and WAG/Rij neocortical neurons located in the superficial layers during application of control ACSF. By doing so, we identified reversal potentials that had similar values in the two strains. Similar conclusions could be drawn by studying the reversal potential values of the stimulus-induced IPSPs recorded during application of ionotropic glutamatergic receptor antagonists. Interestingly, fast IPSP were not seen in those WAG/Rij cells that generated action potential bursts when tested under control conditions; these cells produced small amplitude IPSPs following blockade of glutamatergic transmission. We have previously reported that WAG/Rij neocortical cells recorded in the deep layers generate pharmacologically isolated IPSPs with similar reversal potentials for fast and slow IPSPs [5]. Therefore, these data confirm that the overall efficacy of GABAergic postsynaptic inhibition is preserved in most of the neurons recorded in the WAG/Rij somatosensory cortex, and support the view that intracortical inhibition may play a critical role in the generation of the generalized SW discharge as originally proposed by Kostopoulos et al. [26] in the feline generalized epilepsy model.
GABAB Receptor Dysfunction in the Epileptic WAG/Rij Neocortex
We have recently reported that the somatosensory cortex of epileptic WAG/Rij rats exhibits lower mRNA levels for most GABAB(1) subunits when compared with NEC tissue as well as that GABAB(1) subunits fail to localize in the distal dendrites of WAG/Rij pyramidal cells, extending in the cortical superficial layers [9]. We also found in this study that paired-pulse depression of pharmacologically isolated excitatory and inhibitory responses is reduced in neurons recorded in the WAG/Rij so-matosensory cortex. It is, however, known that several mechanisms, in addition to activation of presynaptic GABAB receptors [18, 27], contribute to paired-pulse depression of neuronal responses [28]. Therefore, the functional meaning of the molecular changes in GABAB(1) subunits identified in the epileptic WAG/Rij rats remained unclear.
We sought conclusive evidence for a decreased function of GABAB receptors by employing the specific agonist baclofen. Our findings indicate that larger doses of baclofen are required to reduce the pharmacologically isolated, stimulus-induced IPSPs generated by epileptic WAG/Rij neurons as compared with age-matched NEC cells. In addition, we found a similar decrease in the ability of this GABAB receptor agonist to influence the occurrence of synchronous field potentials recorded from epileptic WAG/Rij neocortical slices during application of 4AP + glutamatergic receptor antagonists. We confirmed here that this field activity reflects network-driven IPSPs [cf. 16] since it was abolished by picrotoxin. In addition, by employing intracellular recordings we found that the different components of the intracellular activity associated with these field potentials were characterized by similar reversal potential values in both types of tissue. It should also be emphasized that the decreased ability of baclofen to reduce the rate of occurrence of these glutamatergic independent, network-driven IPSPs in epileptic WAG/Rij neocortical slices further highlights the reduced ability of GABAB receptor-mediated mechanisms to control network synchronization in the neocortex of epileptic WAG/Rij animals.
To which extent the altered expression and function of GABAB receptors contribute to the occurrence of absence seizures in vivo remains to be established. Two points must, however, be emphasized. First, it has been reported that GABAB(1) −/− mice exhibiting a loss of all biochemical and electrophysiological GABAB functions present with spontaneous generalized SW discharges [29, 30]. Second, an altered presynaptic release of neurotransmitters has been documented in animal models of temporal lobe epilepsy in which hyperexcitability of neuronal networks and abnormal epileptic synchronization occur [31–33]. It is conceivable that a hyperexcitable neocortex in epileptic WAG/Rij rats initiates SW discharges and entrains thalamic networks via corticothalamic inputs [34] into this rhythm as reported in several models of absence seizures [1, 2] and of Lennox-Gastaut syndrome [35].
Conclusions
Our study demonstrates that higher doses of the GABAB receptor agonist baclofen are required to depress stimulus-induced and network-driven IPSPs in the WAG/Rij neocortex, supporting the conclusion that GABAB receptors are dysfunctional in this genetic model of absence seizure. We anticipate that such a weakened presynaptic control should lead to an augmented release of inhibitory transmitter which in turn may depress interneuron excitability and thus cause disinhibition in the neocortex of epileptic WAG/Rij rats. In line with studies performed to date in this model [5, 6, 36], we propose that this condition is associated with, and perhaps it may cause, the functional overexpression of NMDA receptor-mediated mechanisms. In addition, as proposed for thalamocortical relay cells [37], an increased release of GABA should activate postsynaptic GABAB receptors, thus producing robust hyperpolarizing IPSPs that contribute to de-inactivate low-threshold Ca 2+ conductances, which in turn facilitate rhythmic bursting. In line with this view, injecting GABAB receptor antagonists, either into the thalamus or systemically, blocks absence seizures in both GAERS and WAG/Rij rats [38–40].
It remains unclear whether the changes in GABAB(1) subunit expression and thus the decreased function of GABAB receptors are genetically determined (and thus developmentally regulated) or whether they are the consequence of ongoing SW discharge activity. Interestingly, preliminary data [D. Merlo and M. Avoli, unpubl. data] indicate that the molecular changes identified by Merlo et al. [9] along with the functional alterations reported here are not seen in young (approx. 60-day-old) WAG/Rij rats that do not present as yet with SW discharges. Therefore, further experiments aimed at establishing whether antiepileptic drug pretreatment early in life can influence these changes are needed to answer this question.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research and the Savoy Foundation. We thank Ms. T. Papadopoulos for editorial assistance.
References
- 1.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 2.Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- 3.Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 4.Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- 5.D’Antuono M, Inaba Y, Biagini G, D’Arc-angelo G, Tancredi V, Avoli M. Synaptic hyperexcitability of deep layer neocortical cells in a genetic model of absence seizures. Genes Brain Behav. 2006;5:73–84. doi: 10.1111/j.1601-183X.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 6.Luhmann HJ, Mittmann T, van Luijtelaar G, Heinemann U. Impairment of intracortical GABAergic inhibition in a rat model of absence epilepsy. Epilepsy Res. 1995;22:43–51. doi: 10.1016/0920-1211(95)00032-6. [DOI] [PubMed] [Google Scholar]
- 7.Pinault D. Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5–9 Hz oscillations. J Physiol (Lond) 2003;552:881–905. doi: 10.1113/jphysiol.2003.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pumain R, Louvel J, Gastard M, Kurcewicz I, Vergnes M. Responses to N-methyl-D-aspartate are enhanced in rats with petit mal-like seizures. J Neural Transm Suppl. 1992;35:97–108. doi: 10.1007/978-3-7091-9206-1_7. [DOI] [PubMed] [Google Scholar]
- 9.Merlo D, Mollinari C, Inaba Y, Cardinale A, Rinaldi AM, D’Antuono M, D’Arcangelo G, Tancredi V, Ragsdale DA, Avoli M. Reduced GABAB receptor subunit expression and paired-pulse depression in a genetic model of absence seizures. Neurobiol Dis. 2007;25:631–641. doi: 10.1016/j.nbd.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Enna SJ, Bowery NG. GABAB receptor alterations as indicators of physiological and pharmacological function. Biochem Pharmacol. 2004;68:1541–1548. doi: 10.1016/j.bcp.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Deisz RA. The GABAB receptor antagonist CGP 55845A reduces presynaptic GABAB actions in neocortical neurons of the rat in vitro. Neuroscience. 1999;93:1241–1249. doi: 10.1016/s0306-4522(99)00203-1. [DOI] [PubMed] [Google Scholar]
- 12.Deisz RA. GABAB receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999;38:1755–1766. doi: 10.1016/s0028-3908(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 13.Meeren HK, Pijn JP, van Luijtelaar EL, Coenen AML, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning JPA, Richards DA, Leresche N, Crunelli V, Bowery NG. Cortical-area specific block of genetically determined absence seizures by ethosuximide. Neuroscience. 2004;123:5–9. doi: 10.1016/j.neuroscience.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Polack PO, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S. Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avoli M, Mattia D, Siniscalchi A, Perreault P, Tomaiuolo F. Pharmacology and electrophysiology of a synchronous GABA-mediated potential in the human neocortex. Neuroscience. 1994;62:655–666. doi: 10.1016/0306-4522(94)90467-7. [DOI] [PubMed] [Google Scholar]
- 17.Connors BW, Malenka RC, Silva LR. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol (Lond) 1988;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuro-modulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. San Diego: Academic Press; 1998. [Google Scholar]
- 20.Connors BW, Prince DA. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982;220:476–481. [PubMed] [Google Scholar]
- 21.Nathan T, Jensen MS, Lambert JD. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neurosci Lett. 1990;110:309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- 22.Mattia D, Hwa GGC, Avoli M. Epileptiform activity induced by 4-aminopyridine in guinea-pig and rat neocortices. Neurosci Lett. 1993;154:157–160. doi: 10.1016/0304-3940(93)90195-q. [DOI] [PubMed] [Google Scholar]
- 23.Avoli M, Methot M, Kawasaki H. GABA-dependent generation of ectopic action potentials in the rat hippocampus. Eur J Neurosci. 1998;10:2714–2722. doi: 10.1046/j.1460-9568.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu XB, Coble J, van Luijtelaar G, Jones EG. Reticular nucleus-specific changes in α3 subunit protein at GABA synapses in genetically epilepsyprone rats. Proc Natl Acad Sci USA. 2007;104:12512–12517. doi: 10.1073/pnas.0705320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peeters BWMM, van Rijn CM, Vossen JMH, Coenen AML. Involvement of NMDA receptors in non-convulsive epilepsy in WAG/Rij rats. Life Sci. 1990;47:523–529. doi: 10.1016/0024-3205(90)90612-u. [DOI] [PubMed] [Google Scholar]
- 26.Kostopoulos G, Avoli M, Gloor P. Participation of cortical recurrent inhibition in the genesis of spike and wave discharges in feline generalized penicillin epilepsy. Brain Res. 1983;267:101–112. doi: 10.1016/0006-8993(83)91043-0. [DOI] [PubMed] [Google Scholar]
- 27.Kang Y. Differential paired pulse depression of non-NMDA and NMDA currents in pyramidal cells of the rat frontal cortex. J Neurosci. 1995;15:8268–8280. doi: 10.1523/JNEUROSCI.15-12-08268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 29.Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA-B responses in mice lacking GABA-B1. Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- 30.Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epileptogenesis and enhanced prepulse inhibition in GABA-B1-deficient mice. Mol Cell Neurosci. 2001;17:1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- 31.Asprodini EK, Rainnie DG, Shinnick-Gallagher P. Epileptogenesis reduces the sensitivity of presynaptic γ-aminobutyric acid B receptors on glutamatergic afferents in the amygdala. J Pharmacol Exp Ther. 1992;262:1011–1021. [PubMed] [Google Scholar]
- 32.Benini R, Avoli M. Altered inhibition in lateral amygdala networks in a rat model of temporal lobe epilepsy. J Neurophysiol. 2006;95:2143–2154. doi: 10.1152/jn.01217.2005. [DOI] [PubMed] [Google Scholar]
- 33.Thompson SE, Ayman G, Woodhall GL, Jones RS. Depression of glutamate and GABA release by presynaptic GABAB receptors in the entorhinal cortex in normal and chronically epileptic rats. Neurosignals. 2006–2007;15:202–215. doi: 10.1159/000098515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deschênes M, Veinante P, Zhang ZW. The organization of corticothalamic projections: reciprocity versus parity. Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 35.Steriade M, Contreras D. Spike-wave complexes and fast components of cortically generated seizures. I. Role of neocortex and thalamus. J Neurophysiol. 1998;80:1439–1455. doi: 10.1152/jn.1998.80.3.1439. [DOI] [PubMed] [Google Scholar]
- 36.D’Arcangelo G, D’Antuono M, Biagini G, Warren R, Tancredi V, Avoli M. Thalamocortical oscillations in a genetic model of absence seizures. Eur J Neurosci. 2002;16:2383–2393. doi: 10.1046/j.1460-9568.2002.02411.x. [DOI] [PubMed] [Google Scholar]
- 37.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Vergnes M, Depaulis A, Marescaux C. Involvement of intrathalamic GABAB neurotransmission in the control of absence seizures in the rat. Neuroscience. 1992;48:87–93. doi: 10.1016/0306-4522(92)90340-8. [DOI] [PubMed] [Google Scholar]
- 39.Puigcerver A, van Luijtelaar EL, Drinkenburg WH, Coenen AL. Effects of the GABAB antagonist CGP 35348 on sleep-wake states, behaviour, and spike-wave discharges in old rats. Brain Res Bull. 1996;40:157–162. doi: 10.1016/0361-9230(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 40.Snead OC., 3rd Antiabsence seizure activity of specific GABAB and γ-hydroxybutyric acid receptor antagonists. Pharmacol Biochem Behav. 1996;53:73–79. doi: 10.1016/0091-3057(95)00200-6. [DOI] [PubMed] [Google Scholar]