Abstract
We analyzed with EEG-video monitoring the epileptic activity recorded during the latent and chronic periods in rats undergoing 30 or 120 min pilocarpine-induced convulsive status epilepticus (SE). Interictal discharges frequency in the entorhinal cortex (EC) of animals exposed to 120 min SE was significantly higher in the chronic than in the latent period. Following seizure appearance, interictal spikes diminished in duration in the CA3 of the 120 min SE group, and occurred at higher rates in the amygdala in all animals. Rats exposed to 120 min SE generated shorter seizures but presented twice as many non-convulsive seizures per day as the 30 min group. Finally, seizures most frequently initiated in CA3 in the 120 min SE group but had similar onset in CA3 and EC in the 30 min group. These findings indicate that convulsive SE duration influences the development of interictal and ictal activity, and that interictal discharges undergo structure-specific changes after seizure appearance.
Keywords: EEG, Interictal–ictal relation, Pilocarpine, Status epilepticus, Temporal lobe epilepsy
Introduction
Temporal lobe epilepsy (TLE) is a progressive disorder that results from brain insults such as status epilepticus (SE), febrile convulsions, encephalitis, or trauma early in life (French et al., 1993; Gloor, 1991; Nearing et al., 2007). The pre-epileptic state—which presumably reflects an epileptogenic process—is known as latent period while the epileptic condition is commonly defined as chronic period (Blume, 2006; Cavalheiro et al., 2006). TLE patients are often unresponsive to antiepileptic drugs (Wiebe et al., 2001), and present with a typical pattern of brain damage known as Ammon’s horn sclerosis (or mesial temporal sclerosis) that is characterized by neuronal loss in hippocampus and parahippocampal structures such as the entorhinal cortex (EC) and amygdala (Engel, 2001; Gloor, 1991).
Similar progressive changes occur in animals experiencing SE induced by injection of drugs such as pilocarpine (Turski et al., 1983; see for review: Cavalheiro et al., 2006; Curia et al., 2008) or kainate (Ben-Ari and Cossart, 2000; Bertram and Cornett, 1994) as well as by repetitive electrical stimulation of specific brain areas (Gorter et al., 2001; Mazarati et al., 2002). In these models, SE is defined as the acute period and may correspond in patients to the so-called “initial precipitating injury” (Mathern et al., 2002). The pilocarpine model of TLE is highly homologous to the human disease; animals present with spontaneous recurrent seizures that appear after the latent period, and they exhibit morphological damages in hippocampus and related brain regions (Biagini et al., 2008; Du et al., 1995; Turski et al., 1983). Moreover, seizures are poorly controlled by antiepileptic drugs (Glien et al., 2002).
To date, experiments in this model have been performed on rodents experiencing pilocarpine-induced SE of different durations. However, little effort (but see Biagini et al., 2006; Klitgaard et al., 2002) was made to address the question whether SE length does influence the epileptogenic process and/or the characteristics of the epileptic activity recorded during the chronic state. Moreover, none of the studies conducted to date in the pilocarpine or other animal models of TLE addressed the questions whether interictal activity is different during the latent and chronic period, and how this process might be influenced by SE length. Indeed, the role of interictal spikes in epileptogenesis or in seizure generation remains unclear (see Avoli et al. 2006; Staley and Dudek, 2006). Our experiments were, therefore, carried out to evaluate the influence exerted by the duration of the pilocarpine-induced SE on the characteristics of interictal spikes generated during the latent and chronic periods as well as on the features of the seizures observed during the chronic stage. To this aim, we performed continuous surface and depth brain EEG-video monitoring up to 20 days in rats that had experienced 30 or 120 min long convulsive SE following pilocarpine intraperitoneal (i.p.) injection.
Material and methods
Animal preparation
Experiments were carried out in adult male Sprague–Dawley rats (weighing 275–300 g) that were housed under controlled environmental conditions, at 22±2 °C and 12 h light/12 h dark cycle (lights on from 7:00 a.m. to 7:00 p.m.). They received food and water ad libitum. Rats were injected with scopolamine methylnitrate (1 mg/kg i.p.; Sigma-Aldrich, Canada) and 30 min later with a single dose of pilocarpine hydrochloride (380 mg/kg, i.p.; Sigma-Aldrich). Animals’ behavior after pilocarpine injection was scored according to the Racine’s scale (Racine, 1972). Rats were divided in two groups that included animals surviving either 30 min (n=10) or 120 min (n=10) of SE, defined as continuous stage 5 seizures. Pilocarpine-induced convulsions were terminated by double injection of diazepam (5 mg/kg, i.p.; Sandoz, Canada) and ketamine (50 mg/kg, i.p.; Wyeth, Canada) (Martin and Kapur, 2008). It is possible that non-convulsive seizures persisted beyond the time of treatment. The mortality rate was 38% and 44% respectively for rats experiencing 30 and 120 min long SE. Rats were then allowed to a post-SE recovery period of approx. 72 h during which they were video-monitored; none of the animals included in this study presented with behavioral seizures during this period. All procedures were approved by the Canadian Council of Animal Care and all efforts were made to minimize the number of animals used and their suffering.
Electrode implantation
Electrodes for EEG recordings were implanted on the third day after SE. Rats under deep anesthesia with isoflurane (3%) were placed in a stereotaxic frame. Skin covering the skull was incised to expose the skull plate. Four stainless steel screws (2.4 mm length) were fixed to the skull and up to four small holes were drilled to allow the implantation of bipolar electrodes (30–50 mm length) into the EC, the CA3 subfield of the hippocampus and the amygdala. Bipolar electrodes for depth recordings were custom made by gluing together two insulated wires (diameter=60 μm) with a distance between exposed tips of 500 μm. Depth electrodes were implanted according to the stereotaxic coordinates given by Paxinos and Watson (2005) into the right EC (AP −6.60 mm, ML ±4.00 mm, DV −8.80 mm), CA3 (AP −4.40 mm, ML ±4.00 mm, DV −8.00 mm) and amygdala (AP −2.00 mm, ML ± 4.50 mm, DV −9.00 mm). Screws and electrode pins were connected with a pin connector and fastened to the skull with dental cement. After surgery, animals received topic application of Chloramphenicol (Erfa, Canada) and Lidocain (5%; Odan, Canada) and were injected with Ketoprofen (5 mg/ kg s.c.; Merail, Canada), Buprenorphine (0.01–0.05 mg/kg s.c. repeated every 12 h if necessary; Schering-Plough, UK) and 2 ml of 0.9% sterile saline (s.c.).
EEG-video monitoring
After surgery, animals were transferred to modified cages (30×30×40 cm) and allowed to habituate to the environment for 24 h. They were then connected with multichannel cables and electrical swivels (Slip ring T13EEG, Air Precision, France; or Commutator SL 18C, HRS Scientific, Canada) and continuous EEG-video monitoring (24 h per day) was performed. Throughout the recordings, animals were housed one per cage under controlled environmental conditions (22±2 °C with a 12 h light/12 h dark cycle) and received food and water ad libitum.
EEG signals were amplified via an interface kit (Mobile 36ch LTM ProAmp, Stellate, Canada), filtered at 60 Hz and sampled at 200 Hz per channel. Simultaneously infrared camera was used to record day/ night video files that were time-stamped for integration with the electrophysiological data using monitoring software (Harmonie, Stellate). EEG recordings were reviewed afterwards and seizures quantified by visual inspection of the EEG records. Each electrographic seizure was verified with the video recordings and the behavioral severity was classified according to Racine’s scale. Seizures were further divided in two categories: non-convulsive seizures (scores 1–2) and convulsive seizures (scores 3–5). EEG-video monitoring was performed up to 20 days after pilocarpine treatment because after this time 90% of rats lost their connectors.
At the end of each recording session, histology was performed to confirm electrodes locations. To this aim, rats were decapitated under isoflurane anaesthesia and brains were extracted and post-fixed with formaldehyde (Sigma-Aldrich) for 24 h. Only rats with the expected location of electrodes were included in this study.
Analysis of the latent period
To estimate the length of the latent period we measured the time from the end of the pilocarpine-induced SE to the appearance of the first electrographic seizure. To obtain quantitative comparison of the interictal discharge duration between animals that experienced 30 or 120 min SE, EEG traces with at least 80 interictal spikes were taken for the analysis and were selected from a period that ended at least 3 h from the first seizure. Interictal discharge duration was calculated from the beginning of each event to the return to baseline. The same epochs were also used for evaluating interictal discharge distribution and frequency. Interictal frequency was represented as the number of interictal discharges per 1 s.
Analysis of the chronic period
To calculate the distribution, duration and frequency of interictal discharges during the chronic period, an interval of continuous EEG was chosen randomly, with at least 80 interictal events, starting on day 8 after SE since at this time all animals exhibited seizures (see Results). To average interictal discharges, intervals of EEG recordings were selected in a period ending at least 2 h from an ictal discharge. The first day of the chronic period is representing a consecutive calendar day after SE onset when the first seizure was observed. The number of seizures was measured and represented as seizure frequency (number of seizures per 24 h). Seizure duration and rate of occurrence were evaluated from the entire observation period for all rats. Then, the compressed spectral array transforms were performed and the graphic displays of the changes in frequency and amplitude were used to estimate, by visual inspection, the channel and timing of seizure origin in different brain areas.
Statistical analysis
Values in this study are expressed as a mean±SEM. Normally distributed values referred to a single factor affecting two or more different groups were compared with the Student’s t-test or one-way ANOVA. A 2×2 design, considering 30 and 120 min of SE duration as between factor and latent and chronic periods as within factor, was used to analyze data on interictal events. After significant ANOVA, groups were compared by the Fisher least-significant-difference (LSD) test. Each experimental group consisted of 10 animals that experienced either 30 or 120 min SE; n indicates the number of rats studied under each type of measurements. Some experiments were not included in the measurements due to bad electrode connection, movement artifacts or erroneous position of one of the depth electrodes. Differences with p<0.05 were considered statistically significant.
Results
Interictal discharges during the latent periods following 30 or 120 min long SE
The first spontaneous electrographic seizure in rats experiencing 30 min SE (n=10) occurred 4.3±0.4 days after SE while those surviving 120 min SE (n=10) had the first spontaneous electrographic seizures 5.2±0.5 days after SE. The first convulsive seizure (stages 3–5 of Racine’s scale) in these animals was seen 4.8±0.4 and 5.4±0.4 days after SEs lasting 30 and 120 min, respectively. These differences were, however, not statistically significant.
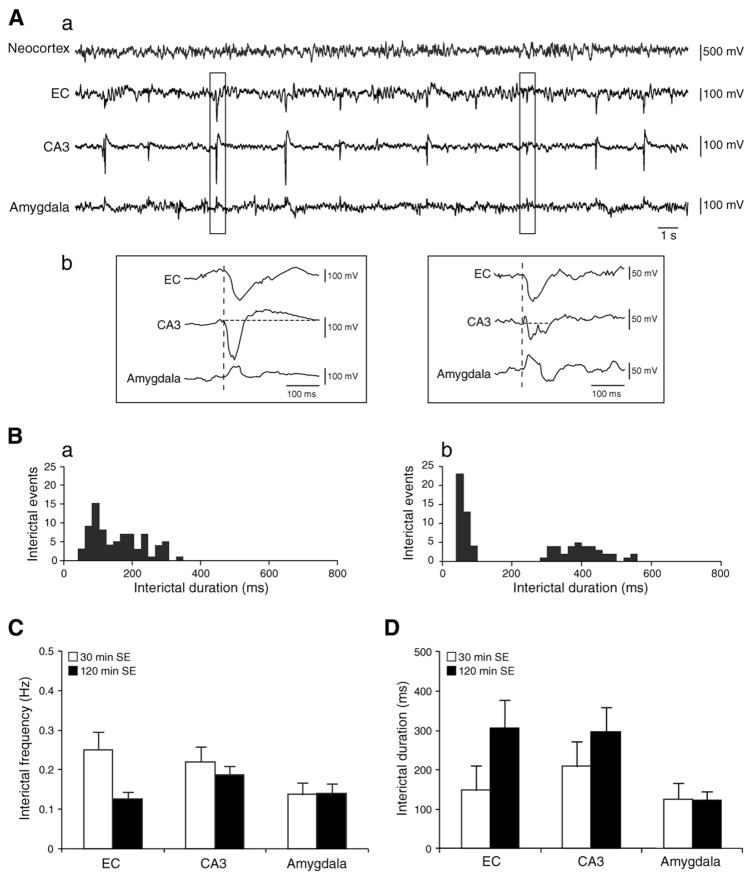
The EEG obtained from both animal groups during the latent period demonstrated interictal spikes that could occur in any of the brain areas analyzed (Fig. 1A), and were either monophasic or biphasic characterized by an initial, high amplitude fast component followed by a small amplitude slow component (Fig. 1Ab). When interictal discharges were recorded from at least two structures, the onset of each spike did not differ between EC, CA3 and amygdala (dashed line in Fig. 1Ab). The analysis of interictal duration suggested the existence of two types of interictal events, but tails of the two respective population curves often superimposed as suggested by the histogram distributions (Fig. 1Ba and b). Two-way ANOVA did not reveal significant differences in frequency and duration of the considered interictal events. As illustrated in Fig. 1C and D, interictal discharge frequencies and durations were comparable in the EC, CA3 and amygdala of rats experiencing 30 min (n=6) or 120 min SE (n=7).
Fig. 1.
A. EEG recordings illustrating interictal activity in a rat that survived 30 min SE induced by pilocarpine injection. A similar EEG pattern was observed in animals that experienced 120 min SE. a. EEG obtained from the neocortex, EC, CA3 and amygdala showing interictal discharges during the latent period. b. Expanded traces of the region marked by the rectangles showing monophasic and biphasic interictal events. Vertical dashed lines show that interictal activity starts at the same time in all recorded brain areas. B. Histograms representing the distribution of interictal spikes in CA3 in a. rat exposed to 30 min and b. 120 min SE; the distribution suggests two types of interictal discharges that were observed in all recorded brain areas. C. Bar graph representing interictal activity frequency during the latent period. Results are expressed as mean±SEM. D. Duration of interictal discharges measured in EC, CA3 and amygdala for rats experiencing 30 min (n=6) and 120 min (n=7) SE. Results are expressed as a mean±SEM.
Interictal activity recorded during the chronic period
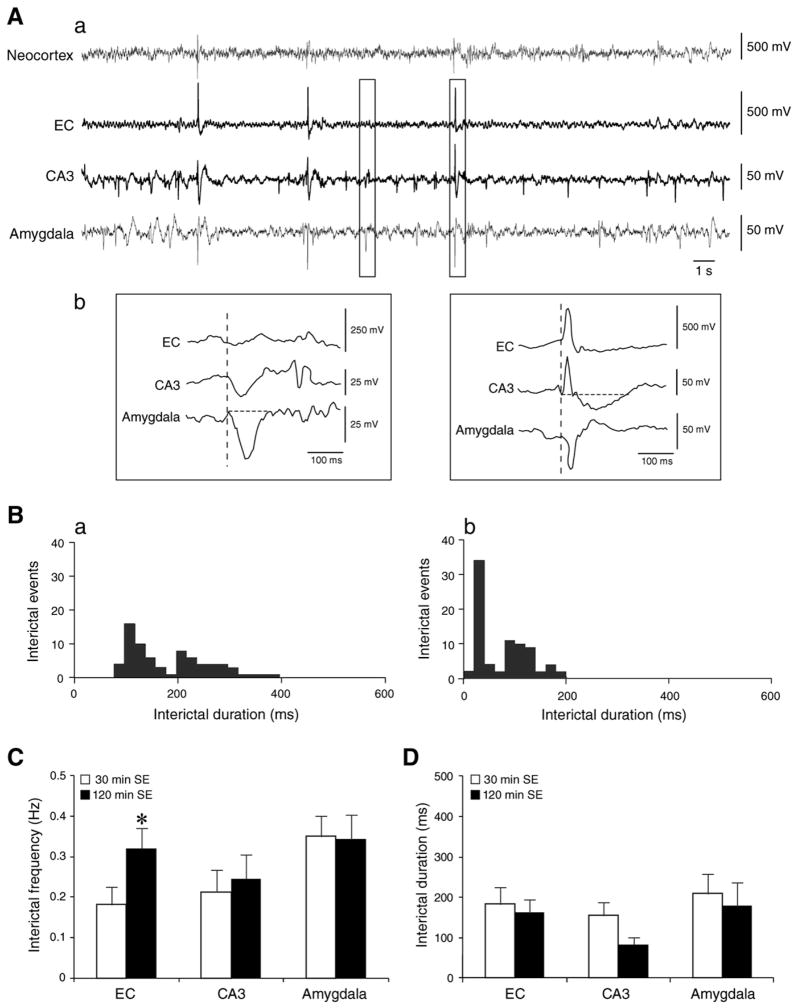
Interictal activity recorded during the chronic period was also characterized by two types of monophasic and/or biphasic events (Fig. 2Ab). these interictal spikes had similar onset time in EC, CA3 and amygdala (Fig. 2Ab). In addition, interictal event duration was similar in epileptic rats that experienced 30 min (n=6) and 120 min (n=7) SE (Fig. 2D), but contrary to what was observed during the latent period, the two-way ANOVA identified for interictal frequency in the EC a significant interaction (p<0.01) between the different SE lengths (between factor) and the epileptic condition (the within factor). Thus, post hoc analysis showed that the frequency of interictal discharges recorded in EC was higher (p<0.05, LSD test) in epileptic animals exposed to 120 min SE than in those with 30 min SE (Fig. 2C).
Fig. 2.
A. EEG recordings obtained from the chronic period representing interictal activity in a rat that experienced 30 min SE. The same EEG pattern was recorded in animals experiencing 120 min SE. a. EEG traces obtained from the neocortex, EC, CA3 and amygdala showing that interictal discharges occur at irregular intervals. b. Expanded traces of the region marked by the rectangles representing monophasic and biphasic interictal events. Note that vertical dashed lines show that interictal events have the same onset time in all brain areas. B. Histograms showing interictal discharge distributions in CA3 in rat exposed to a. 30 min and to b. 120 min SE. The distribution suggests two types of interictal events that were observed in all recorded brain areas and in all animals. C. The frequency of interictal discharges from rats in the 30 and 120 min SE group during the chronic period. Note that interictal events are more frequent in EC in animals that survived longer SE. Results are expressed as a mean±SEM, *p<0.05, LSD test after significant two-way ANOVA. D. Bar graph representing interictal discharges duration for rats that experienced 30 min (n=6) and 120 min (n=7) SE. Note that there are no statistically significant differences between the two groups.
Comparison of interictal discharges during the latent and chronic period
Comparing the recordings obtained during the latent and chronic periods with two-way analysis, we found structure-specific changes in the frequency and duration of interictal activity. First, a significant (p<0.01) increase in rate of occurrence was identified in the amygdala of both groups of animals during the chronic period as compared to the latent period. In addition, interictal spike frequency was increased (p<0.01) in the EC of epileptic rats exposed to 120 min SE, with respect to values observed in the same animals during the latent period. No significant changes could, however, be identified in the CA3 for both groups (Table 1). Analysis of the interictal discharge duration revealed a significant (p<0.01) decrease in the CA3 of rats exposed to 120 min SE during the chronic period as compared with the latent stage whereas a trend toward a decrease was seen in the 30 min group (Table 2).
Table 1.
Frequency (values are expressed in Hz) of the interictal discharges recorded during the latent and chronic periods in animals exposed to 30 min (n=6) and 120 min (n=7) SE.
| 30 min SE
|
120 min SE
|
|||
|---|---|---|---|---|
| Latent period | Chronic period | Latent period | Chronic period | |
| EC | 0.250±0.045 | 0.181±0.045 | 0.125±0.017 | 0.320±0.050**,# |
| CA3 | 0.220±0.038 | 0.213±0.055 | 0.186±0.024 | 0.245±0.059 |
| Amygdala | 0.137±0.030 | 0.350±0.050** | 0.139±0.026 | 0.342±0.059** |
Data represent mean±SEM;
p<0.05 between 30 and 120 min SE groups considered during chronic period,
p<0.01 between latent period versus chronic period; LSD test after significant two-way ANOVA.
Table 2.
Duration (values are expressed in ms) of the interictal discharges recorded during the latent and chronic periods in animals exposed to 30 min (n=6) and 120 min (n=7) SE.
| 30 min SE
|
120 min SE
|
|||
|---|---|---|---|---|
| Latent period | Chronic period | Latent period | Chronic period | |
| EC | 148.3±64.5 | 184.2±42.3 | 306.5±72.4 | 162.0±33.7 |
| CA3 | 209.3±64.5 | 156.7±32.2 | 296.8±61.0 | 84.1±18.3** |
| Amygdala | 126.3±40.4 | 209.0±50.2 | 123.0±22.1 | 179.2±57.2 |
Data represent mean±SEM;
p<0.01 between latent period versus chronic period. LSD test after significant two-way ANOVA.
Seizures during the chronic period
Seizures were observed and recorded in all animals experiencing 30 and 120 min SE. The first spontaneous seizure was non-convulsive (stages 1–2 of Racine’s scale) in 70% of animals in both groups. It was characterized by a frozen stare that was followed by stereotyped chewing movements. Convulsive seizures at stages 3–5 of Racine’s scale were observed in the remaining animals. One rat from the 30 min SE group and two rats from 120 min SE group exhibited only non-convulsive seizures through the entire recording period.
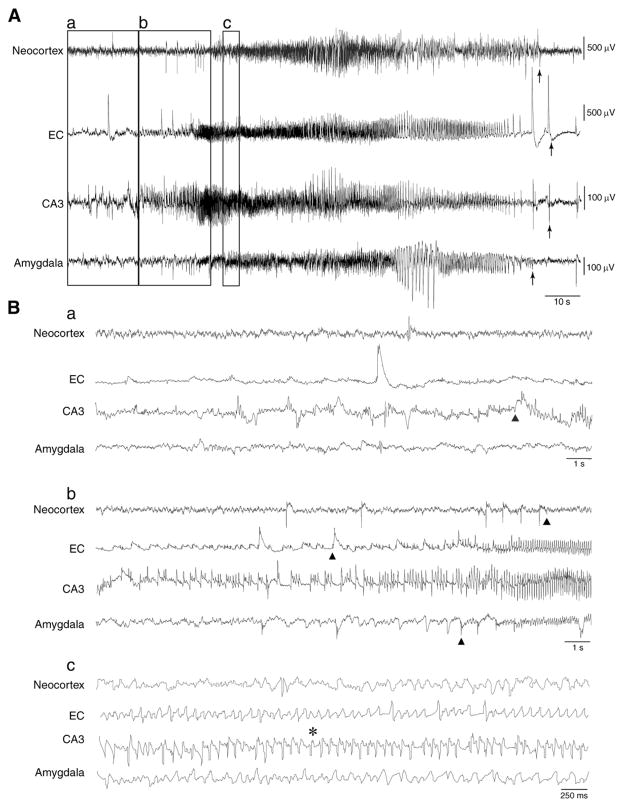
The electrographic activities recorded during non-convulsive and convulsive seizures were not different in rats experiencing 30 or 120 min SE. As illustrated in Fig. 3A, the onset of a stage 2 seizure (marked in each recorded brain area by triangles) consisted of high-frequency, negative or positive-going spikes depending on the recorded area (Fig. 3Ba and b). These events were later replaced by spikes with higher frequency and larger amplitude extending above and below the baseline. Behavioral changes such as eye blinking, chewing, head nodding or movement arrest were associated with a decrease in spike amplitude (Fig. 3Bc, pointed by an asterisk). The end of seizure in each brain structure was marked by arrows (Fig. 3A).
Fig. 3.
A spontaneous non-convulsive seizure (stage 2 of Racine’s scale) after pilocarpine treatment in a rat that experienced a 30 min SE. A. EEG recordings representing the electrographic seizure in the neocortex, EC, CA3 and amygdala. Arrows indicate the end of ictal discharges. B. Expanded traces of an ictal discharge showing a. and b. seizure initiation marked in each recorded brain area by a triangle and c. the first behavioral sign of stage 2 pointed by an asterisk.
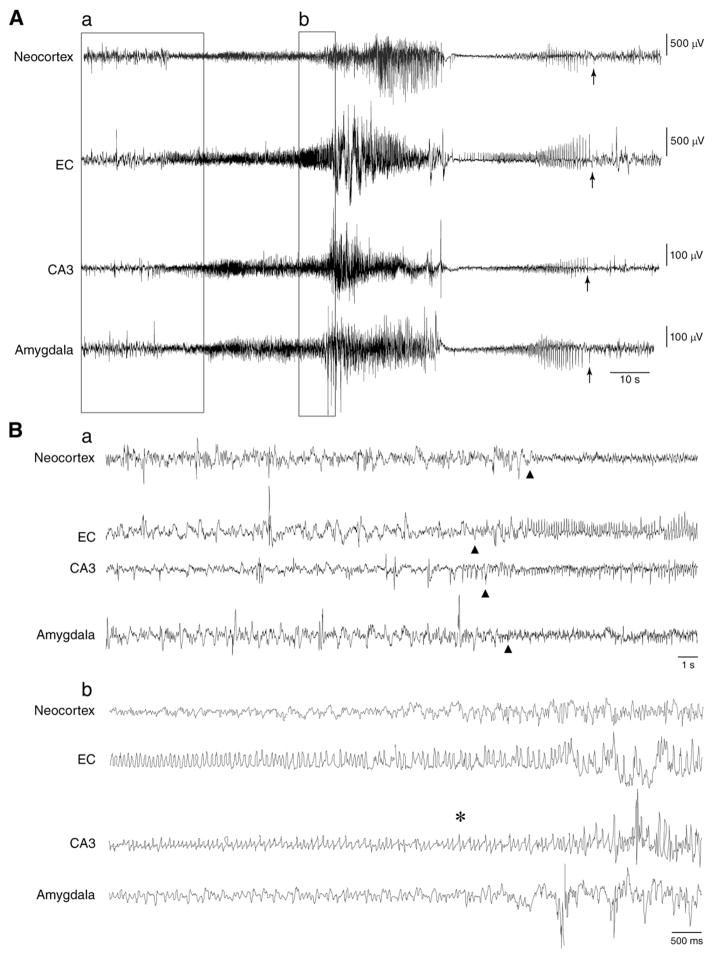
Stage 5 seizure onset, represented by triangles, usually consisted of high-frequency, low-amplitude events (Fig. 4Ba) that preceded the behavioral manifestations characterized by rearing and falling along with generalized tonic–clonic convulsions associated with loss of posture (asterisk in Fig. 4Bb). Large-amplitude spikes characterized these late behavioral manifestations. A transient EEG depression, from which negative/positive-multiple spikes at low-frequency could emerge, ended the seizure (Fig. 4A). Seizure end is pointed by arrows in Fig. 4A.
Fig. 4.
EEG traces obtained from a rat that experienced 30 min SE showing a spontaneous convulsive seizure at stage 5 of Racine’s scale. A. EEG recordings representing the pattern of a seizure in the neocortex, EC, CA3 and amygdala. The end of the seizure is marked by arrows in each brain structure. B. Expanded traces of the ictal discharge in all recorded brain areas showing a. seizure initiation (the onset of the seizure is marked with triangles) and b. the first convulsive manifestation of stage 5, marked with an asterisk, that appears right before the high-amplitude events.
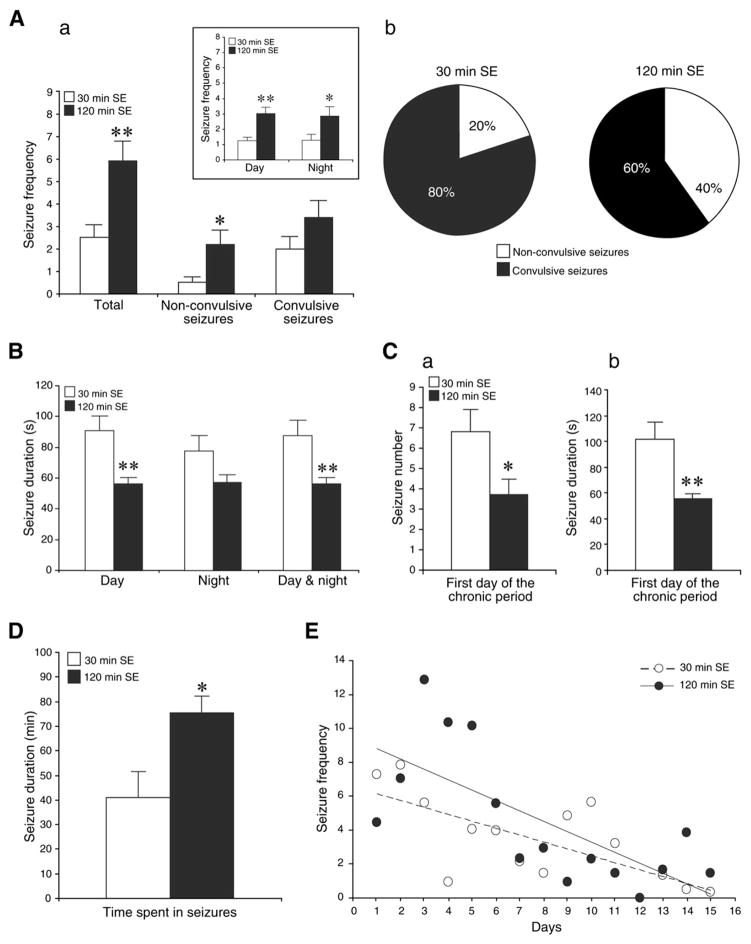
The average number of seizures per day experienced by rats subjected to 30 or 120 min SE are shown in Fig. 5Aa. Animals subjected to 120 min SE presented with more frequent seizures that those undergoing 30 min SE. This difference could still be appreciated by analyzing the day and night periods (Table 3 and Fig. 5Aa, insert). Non-convulsive seizures in the animals exposed to 120 min SE appeared to be the main contributor to this difference. This point is further emphasized in the plot of Fig. 5Ab in which the percentage of convulsive and non-convulsive seizures is shown: 20% of seizures in animals undergoing 30 min SE were non-convulsive while rats surviving 120 min SE presented with 40% of non-convulsive seizures (p<0.05). The duration of seizures in animals that underwent 30 min SE was 89.4±9.9 s (n=307 seizures) while in rats experiencing 120 min SE it was 57.3±3.9 s (n=733 seizures) (p<0.01) (Fig. 5B). There was no difference in seizure duration between day and night.
Fig. 5.
A. a. Non-convulsive and convulsive seizure frequency for rats experiencing 30 and 120 min SE. For each group n=10 and *p<0.05, **p<0.01, Student’s t-test. Insert: bar graph showing the number of seizures per day and night periods. Note that animals that survived longer SE exhibited much more seizures per any time period. b. Percentage of convulsive and non-convulsive seizures for rats that experienced 30 and 120 min SE. Significance with *p<0.05 for non-convulsive seizures between two groups of rats (Student’s t-test). B. Histogram showing the average seizure duration per day, night and 24 h for rats that experienced 30 min (n=10) and 120 min SE (n=10) induced by pilocarpine injection. Results are expressed as a mean±SEM; **p<0.01. C. First day of the chronic period. Bar graphs showing a. seizure number and b. seizure duration in animals exposed to 30 min (n=10) and 120 min SE (n=10) induced by pilocarpine injection. Values represent mean±SEM; *p<0.05, **p<0.01. D. Bar graph showing for both groups of rats the total time spent in seizures during the entire recording period; *p<0.05. E. Histogram showing the number of seizures per rat per day. Note that the number of seizures tends to decrease over time for animals exposed to 30 and 120 min SE. First day on x-axis represents the first day of the chronic period.
Table 3.
Number of seizures per day and night periods for each rat experiencing 30 and 120 min SE.
| Rat number
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Day | 30 min SE | 1.7 | 1.5 | 2 | 0.3 | 1.9 | 0.4 | 1.3 | 0.5 | 9.6 | 0.7 |
| 120 min SE | 4.4 | 1.1 | 2.6 | 1.8 | 0.4 | 4.0 | 3.0 | 3.7 | 3.8 | 11.2 | |
| Night | 30 min SE | 0.8 | 3.4 | 2.2 | 0.2 | 1.4 | 0.1 | 1.7 | 0.3 | 5.6 | 0.3 |
| 120 min SE | 4.7 | 0.6 | 5.4 | 0.8 | 0.3 | 2.4 | 4.1 | 2.8 | 2.2 | 8.3 | |
As illustrated in Fig. 5C, animals subjected to a 30 min SE (n=10) exhibited 6.8±1.1 seizures on the first day of the chronic period and these seizures lasted 101.4±13.8 s (n=68 seizures). At variance, rats exposed to 120 min SE (n=10) presented 3.7±0.8 seizures (p<0.05) on the first day of the chronic period; these seizures had duration of 55.3±4.3 s (n=44) (p<0.01). The total time spent in seizures during the entire recording period was 40.8±10.8 min (n=10) for animals that experienced 30 min SE and 75.5±6.7 min (n=10) for rats that underwent 120 min SE (Fig. 5D). Moreover, seizure frequency decreased over time in both 30 and 120 min SE animals (Fig. 5E).
Seizure onset and clustering
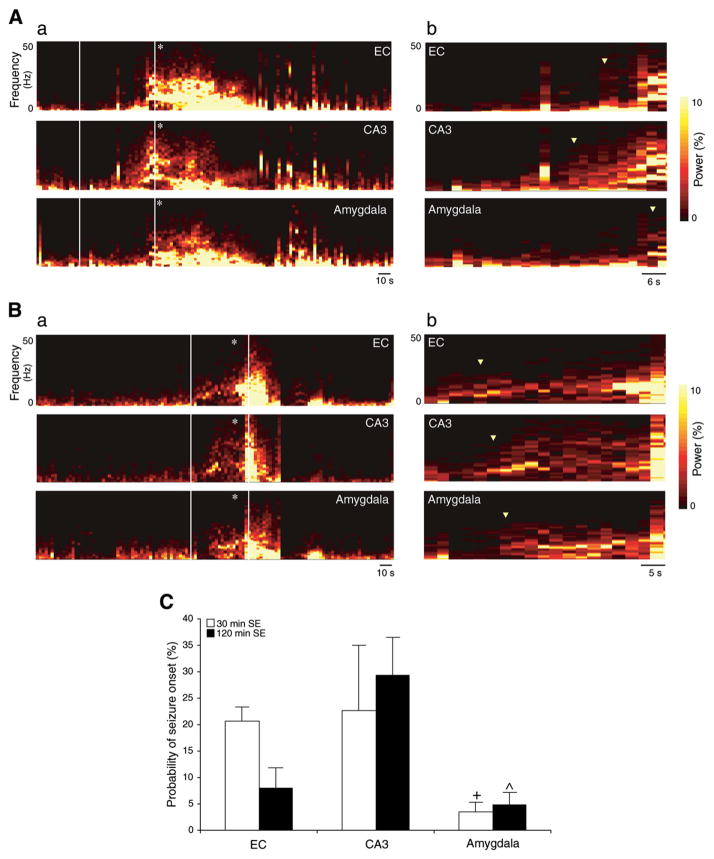
In approximately 50% of cases for both animal groups seizure onset was synchronous in the three limbic areas as well as in the neocortex. In the other half, the electrographic pattern of ictal discharge was characterized by a different time of onset in the three recorded limbic structures. This can be appreciated by examining the compressed spectral array of the EEG (Fig. 6A and B). Fig. 6A shows a non-convulsive seizure in a rat (EEG trace in Fig. 3) from the 30 min SE group: seizure activity appears first in CA3, then in EC and finally in amygdala (Fig. 6Ab). In contrast, a convulsive seizure also from an animal exposed to 30 min SE (Fig. 6B) shows the onset in EC and propagation to CA3 and amygdala (original EEG in Fig. 4). On average, in rats exposed to 30 min SE (n=8), seizures were initially recorded most often in CA3 or EC, (23±12% and in 21±3% of cases, respectively), while onset was observed in amygdala in 4±2% of cases. In animals that experienced 120 min SE (n=8), seizures were initially recorded in CA3 in 29±7% of cases, while they were less common in EC and amygdala (8 ± 4% and 5 ± 2% of events, respectively) (Fig. 6C).
Fig. 6.
A. a. Compressed spectral array display showing non-convulsive seizure at stage 2 in all recorded brain areas. The first behavioral sign observed in animal experiencing stage 2 is pointed with an asterisk. b. Expanded spectral array display showing onset of the non-convulsive seizure marked with triangles. B. a. Compressed spectral array display representing convulsive seizure at stage 5 in all recorded brain structures. The first convulsive manifestation of stage 5 is pointed with an asterisk. b. Expanded spectral array display showing initiation of convulsive seizure. Note that the seizure onset is marked with triangles C. Onset time of electrographic seizures. Values refer to the percentage of seizures that have origin in EC, CA3 or amygdala in rats that experienced 30 min (n=8) and 120 min (n=8) SE. Note that for rats in the 30 min SE group seizures start in EC and CA3, while for the 120 min SE group seizures were first observed in CA3. Data represent mean±SEM; ^p<0.05 between amygdala vs. CA3 in rats experiencing 120 min SE, +p<0.05 between amygdala vs. EC in animals that survived 30 min SE; Fisher (LSD) test.
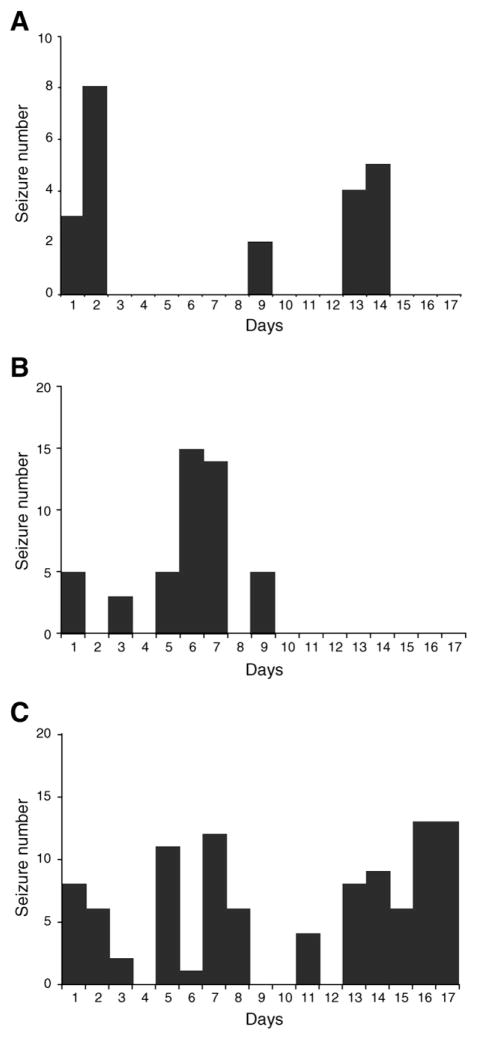
We also observed seizure clustering in animals experiencing 30 or 120 min SE. Clusters were defined as consisting of at least two seizures per day. As shown in Fig. 7, seizure clusters were more or less periodic, with no difference in their pattern in the two groups of rats. In 12/20 rats (60%) seizures occurred in clusters (Fig. 7A). In other five rats (25%), after the first clusters of non-convulsive and/or convulsive seizures, we did not observe any other electrographic seizure until the end of the recordings (Fig. 7B). At variance, in three rats (15%) we noticed a gradual increase in the number of daily seizures up to the end of recordings (Fig. 7C). The intervals between clusters of seizures were 2.3±0.5 days (n=10) and 2.4±0.6 days (n=10) for rats experiencing 30 and 120 min SE, respectively. These intervals were more than twice shorter than the interval between pilocarpine-induced SE and the appearance of the first spontaneous seizure.
Fig. 7.
Number of spontaneous seizures per day during the chronic period in animals treated with pilocarpine. ‘1’ on x-axis represents the first day of the appearance of electrographic seizures. The same percentage of rats experiencing 30 and 120 min SE exhibited A. a cyclic pattern of seizures, B. the first cluster of seizures up to the ninth day and then any ictal discharge up to the third week of recordings and C. the gradual increase of seizures up to the seventeenth day of the chronic period. Each bar graph represents data obtained from one experiment.
Discussion
The main results reported in this study can be summarized as follows. First, we found that the rate of occurrence of interictal discharges in the EC of rats exposed to 120 min SE significantly increases in the chronic compared with the latent period. This change was accompanied by a trend toward a decrease in duration of interictal events in EC of the same group. Second, we identified a decrease in duration for interictal spikes in the CA3 subfield in animals of both groups following seizure appearance, but in this area there was no significant change in frequency of occurrence. Third, interictal discharges increased in frequency in the amygdala of both groups during the chronic period. Fourth, rats exposed to 120 min SE generated shorter seizures when compared with animals undergoing 30 min SE. Fifth, rats surviving 120 min SE presented almost twice as many non-convulsive seizures per day compared with the 30 min group and consequently spent almost twice as long in the ictal state. Sixth, seizures most frequently initiated in the CA3 hippocampal area in the 120 min SE group, while having similar onset in CA3 and EC in the 30 min group. We have also confirmed that in both experimental groups non-convulsive seizures occur earlier than convulsive seizures (Goffin et al. 2007).
Latency to the first spontaneous seizure
It has been shown that pilocarpine-induced SE causes in the rodent brain a sequence of functional and morphological changes leading to the development of spontaneously recurrent seizures, the so-called chronic period (Cavalheiro et al., 2006; Leite et al., 1990). However, the characteristics of both latent and chronic period are still a matter of debate. Thus, in both pilocarpine and kainate TLE models the latent period has usually been considered to vary between 1 and 8 weeks (Bragin et al., 2004; Turski et al., 1983); however, this view has been disputed in other studies (Bumanglag and Sloviter, 2008; Williams et al., 2009). For instance, Williams et al. (2009) have proposed that the latent period may not be long enough to be associated with epileptogenesis and that the time occurring between SE and the appearance of the first spontaneous seizure is analogous to the interval between seizures. In our experiment, rats after 30 and 120 min long SE developed spontaneous seizures during the first week after the pilocarpine injection and the following seizure intervals were on average half the latent period. This evidence supports the view that the interval between pilocarpine-induced SE and first spontaneous seizure reflects epileptogenesis. Similar latent period durations have been observed in rats experiencing 2 or 3 h long pilocarpine-induced SE (Bumanglag and Sloviter, 2008; El-Hassar et al., 2007; Goffin et al., 2007).
Interictal activity
Our EEG recordings confirm that interictal activity is the first event to appear in animals committed to become epileptic (El-Hassar et al., 2007; Scorza et al., 2009). All animals from both groups presented with interictal discharges. However, the manifestation of interictal activity was: (i) different between the two animal groups, (ii) area specific, and (iii) dependent on whether interictal events occurred during the latent or the chronic period.
The frequency of interictal discharges in the EC in rats that experienced 120 min SE, became more frequent when spontaneous seizures recurred (i.e., during the chronic period). In addition, both groups of animals exhibited higher frequency of interictal discharges in the amygdala, compared to the latent period. In contrast, only a decrease in duration was identified in the hippocampus. These findings therefore suggest that changes in interictal activity may lead to ictogenesis. Interestingly, in a simplified system of epilepti-form synchronization such as the in vitro brain slice preparation, blocking the propagation of fast, CA3-driven interictal-like events to the EC causes the appearance of ictal-like discharges in the latter limbic area (Bragdon et al., 1992; Barbarosie and Avoli, 1997; Barbarosie et al., 2000; Benini et al., 2003). In addition, it has been shown in these studies these ictal-like events appear to be initiated by slow interictal events that are locally generated. Finally, we have reported that slices obtained from pilocarpine-treated epileptic rodents generate during application of 4-aminopyridine CA3-driven interictal-like events that have different duration and frequency when compared with those recorded in tissue slices from non-epileptic control animals (Nagao et al., 1994; Köhling et al., 1995; D’Antuono et al., 2002). Overall, this early in vitro evidence along with the in vivo data reported here, suggest that changes in interictal activity may intimately be involved in seizure generation.
Non-convulsive and convulsive seizures and SE length
The characteristics of the EEG activity at seizure onset were in agreement with previous experiments (Leite et al., 1990). The first spontaneous seizures observed in animals experiencing 30 and 120 min SE presented with minimal behavioral changes (stages 1–2 of the Racine’s scale). The following ictal discharges were accompanied with clonic convulsions corresponding to the stages 3–5 of Racine’s scale. These observations are in line with previous findings (Bumanglag and Sloviter, 2008; Goffin et al., 2007; Leite et al., 1990). Moreover, 20% and 40% of spontaneous seizures observed respectively in rats with 30 and 120 min SE were non-convulsive. Therefore, experiments based on behavioral monitoring may under-evaluate seizure activity in rats exposed to prolonged SE.
Interestingly, both groups presented with a similar occurrence of motor seizures. However, only in rats exposed to 120 min SE, and characterized by more frequent interictal events in the EC, non-convulsive seizures were particularly frequent. The parahippocampal cortex has been associated with generation of “dreamy states” (reminiscence of scenes or déjà vu), emotions and visceral responses in TLE patients, especially in response to stimulation applied directly in the EC (Bartolomei et al., 2004).
Origin of spontaneous seizures and their characterization
In approximately 50% of seizures observed in each group of animals exposed to 30 and 120 min SE, the onset time of ictal discharges was the same for EC, CA3 and amygdala. In the remaining 50% of seizures from animals that experienced 120 min SE, CA3 was the region in which ictal discharges were initially observed. At variance, rats exposed to 30 min SE evenly exhibited CA3 and EC as the region of seizure onset. On one hand, this fact confirms that pilocarpine-treated epileptic rats present spontaneous recurrent seizures that can originate in some cases from parahippocampal regions, a phenomenon that was previously unrecognized in many experiments (Harvey and Sloviter, 2005; Schwarcz et al., 2002). On the other hand, this finding suggests that the changes observed selectively in the EC, in rats exposed to 120 min SE, could be associated with the more frequent onset of seizures in CA3 observed in this group.
Animals that experienced 30 min pilocarpine-induced SE exhibited longer seizure duration and lower seizure frequency than those with 120 min SE. This finding is in line with what reported by Lemos and Cavalheiro (1995) who demonstrated that shorter pilocarpine-induced SE is associated with lower seizure frequency. Nevertheless, more recent investigations based on the impact of different pilocarpine-induced SE durations on seizure frequency have revealed that SE shorter than 30 min does not result in spontaneous recurrent seizures, and that animals that underwent exactly 30 min SE were seizing more frequently than rats after 120 min SE three weeks after the pilocarpine injection (Klitgaard et al., 2002). However, this study was performed on discontinuous EEG recordings and by analyzing only 72 h long EEG segments on the third week after SE induction. In addition, in the experiments performed by Klitgaard et al. (2002), seizures induced by pilocarpine were quelled by injecting diazepam while we used diazepam along with ketamine (Martin and Kapur, 2008).
The findings reported here are in agreement with previous studies that addressed the effects of variable SE duration and epileptogenesis in the pilocarpine model (Biagini et al., 2006, 2008). It was reported there that by quelling convulsions with diazepam after a 30 min SE, the latent period was significantly shortened and brain damage was smaller than that found in rats exposed to a SE lasting 180 min. In the present experiment, diazepam combined with ketamine was more effective in quelling motor seizures, as originally described by Martin and Kapur (2008). However, we cannot exclude that non-convulsive seizures could have continued after cessation of behavioral convulsions in both groups. In any case, the different lengths of convulsive SE certainly affected the extent of hippocampal and parahippocampal damage as reported in several studies (Biagini et al., 2008; Klitgaard et al., 2002; Lemos and Cavalheiro, 1995; Pitkänen et al., 2005). Indeed, diazepam is per se able to limit damage by acting on seizure activity, both by neuroprotection (Pitkänen et al., 2005; Qashu et al., 2010) and by shifting SE from motor to non-convulsive seizures (Goffin et al., 2007). Ketamine is also neuroprotective when administered after the SE onset (Fujikawa, 1995). Thus, the significantly prolonged ictal discharges found in rats exposed to 30 min SE could be explained by the better preserved neuronal networks, which are required for seizures to generalize.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (CIHR; grant 8109) and the Savoy Foundation.
References
- Avoli M, Biagini G, de Curtis M. Do interictal spikes sustain seizures and epileptogenesis? Epilepsy Curr. 2006;6:203–207. doi: 10.1111/j.1535-7511.2006.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal–entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, Kurcewicz I, Avoli M. CA3-released entorhinal seizures disclose dentate gyrus epileptogenicity and unmask a temporoammonic pathway. J Neurophysiol. 2000;83:1115–1124. doi: 10.1152/jn.2000.83.3.1115. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Barbeau E, Gavaret M, Guye M, McGonigal A, Régis J, et al. Cortical stimulation study of the role of rhinal cortex in déjà vu and reminiscence of memories. Neurology. 2004;63:858–864. doi: 10.1212/01.wnl.0000137037.56916.3f. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Benini R, D’Antuono M, Pralong E, Avoli M. Involvement of amygdala networks in epileptiform synchronization in vitro. Neuroscience. 2003;120:75–84. doi: 10.1016/s0306-4522(03)00262-8. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Cornett JF. The evolution of a rat model of chronic spontaneous limbic seizures. Brain Res. 1994;661:157–162. doi: 10.1016/0006-8993(94)91192-4. [DOI] [PubMed] [Google Scholar]
- Biagini G, Baldelli E, Longo D, Pradelli L, Zini I, Rogawski MA, et al. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp Neurol. 2006;201:519–524. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Biagini G, Baldelli E, Longo D, Contri MB, Guerrini U, Sironi L, et al. Proepileptic influence of a focal vascular lesion affecting entorhinal cortex–CA3 connections after status epilepticus. J Neuropathol Exp Neurol. 2008;67:687–701. doi: 10.1097/NEN.0b013e318181b8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume WT. The progression of epilepsy. Epilepsia. 2006;47:71–78. doi: 10.1111/j.1528-1167.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- Bragdon AC, Kojima H, Wilson WA. Suppression of interictal bursting in hippocampus unleashes seizures in entorhinal cortex: a proepileptic effect of lowering [K+]o and raising [Ca2+]o. Brain Res. 1992;590:128–135. doi: 10.1016/0006-8993(92)91088-v. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bumanglag AV, Sloviter RS. Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats. J Comp Neurol. 2008;510:561–580. doi: 10.1002/cne.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Naffah-Mazzacoratti MG, Mello LE, Laite JP. The pilocarpine model of seizures. In: Pitkänen A, Schwartzkroin PA, Moshé SL, editors. Models of Seizures and Epilepsy. Elsevier Academic Press; Amsterdam: 2006. pp. 433–448. [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antuono M, Benini R, Biagini G, D’Arcangelo G, Barbarosie M, Tancredi V, et al. Limbic network interactions leading to hyperexcitability in a model of temporal lobe epilepsy. J Neurophysiol. 2002;87:634–639. doi: 10.1152/jn.00351.2001. [DOI] [PubMed] [Google Scholar]
- Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol. 2007;578:193–211. doi: 10.1113/jphysiol.2006.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr Mesial temporal lobe epilepsy: what have we learned? Neuroscientist. 2001;7:340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG. Neuroprotective effect of ketamine administered after status epilepticus onset. Epilepsia. 1995;36:186–195. doi: 10.1111/j.1528-1157.1995.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Löscher W. Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia. 2002;43:350–357. doi: 10.1046/j.1528-1157.2002.18101.x. [DOI] [PubMed] [Google Scholar]
- Gloor P. Mesial temporal sclerosis: historical background and an overview from a modern perspective. In: Lüders H, editor. Epilepsy Surgery. Raven Press; New York: 1991. pp. 689–703. [Google Scholar]
- Goffin K, Nissinen J, Van Laere K, Pitkänen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol. 2007;205:501–505. doi: 10.1016/j.expneurol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Harvey BD, Sloviter RS. Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats: implications for hippocampal epileptogenesis. J Comp Neurol. 2005;488:442–463. doi: 10.1002/cne.20594. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Vanneste-Goemaere J, Margineanu DG. Pilocarpine-induced epileptogenesis in the rat: impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Res. 2002;51:93–107. doi: 10.1016/s0920-1211(02)00099-2. [DOI] [PubMed] [Google Scholar]
- Köhling R, Lücke A, Nagao T, Speckmann EJ, Avoli M. Extracellular potassium elevations in the hippocampus of rats with long-term pilocarpine seizures. Neurosci Lett. 1995;201:87–91. doi: 10.1016/0304-3940(95)12136-r. [DOI] [PubMed] [Google Scholar]
- Leite JP, Bortolotto ZA, Cavalheiro EA. Spontaneous recurrent seizures in rats: an experimental model of partial epilepsy. Neurosci Biobehav Rev. 1990;14:511–517. doi: 10.1016/s0149-7634(05)80076-4. [DOI] [PubMed] [Google Scholar]
- Lemos T, Cavalheiro EA. Suppression of pilocarpine-induced status epilepticus and the late development of epilepsy in rats. Exp Brain Res. 1995;102:423–428. doi: 10.1007/BF00230647. [DOI] [PubMed] [Google Scholar]
- Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49:248–255. doi: 10.1111/j.1528-1167.2007.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer’s hypothesis revisited. Prog Brain Res. 2002;135:237–251. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Bragin A, Baldwin R, Shin D, Wilson C, Sankar R, et al. Epileptogenesis after self-sustaining status epilepticus. Epilepsia. 2002;43:74–80. doi: 10.1046/j.1528-1157.43.s.5.25.x. [DOI] [PubMed] [Google Scholar]
- Nagao T, Avoli M, Gloor P. Interictal discharges in the hippocampus of rats with long-term pilocarpine seizures. Neurosci Lett. 1994;174:160–164. doi: 10.1016/0304-3940(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Nearing K, Madhavan D, Devinsky O. Temporal lobe epilepsy: a progressive disorder? Rev Neurol Dis. 2007;4:122–127. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Elsevier Academic Press; Boston: 2005. [Google Scholar]
- Pitkänen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res. 2005;63:27–42. doi: 10.1016/j.eplepsyres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Apland JP, Braga MF. Diazepam administration after prolonged status epilepticus reduces neurodegeneration in the amygdala but not in the hippocampus during epileptogenesis. Amino Acids. 2010;38:189–197. doi: 10.1007/s00726-008-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroenceph Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Scharfman HE, Bertram EH. Temporal lobe epilepsy: renewed emphasis on extrahippocampal areas. In: Davis KE, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott, Williams and Wilkins; Philadelphia: 2002. pp. 1843–1856. [Google Scholar]
- Scorza FA, Arida RM, da Naffah-Mazzacoratti MG, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: what have we learned. An Acad Bras Cienc. 2009;81:345–365. doi: 10.1590/s0001-37652009000300003. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr. 2006;6:199–202. doi: 10.1111/j.1535-7511.2006.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioral, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, et al. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]